Abstract
Introduction
We hypothesized that subclinical disruption in blood pressure (BP) dynamics, captured by lower complexity and higher variability, may contribute to dementia risk, above and beyond BP levels.
Methods
This prospective cohort study followed 1,835 older adults from 1997-2016, with BP complexity quantified by sample entropy and BP variability quantified by coefficient of variation using beat-to-beat BP measured at baseline.
Results
334 developed dementia over 20 years. Reduced systolic BP (SBP) complexity was associated with a higher risk of dementia (hazard ratio [HR] comparing extreme quintiles: 1.55; 95% confidence interval [CI]: 1.09-2.20). Higher SBP variability was also associated with a higher risk of dementia (HR comparing extreme quintiles: 1.57; 95% CI: 1.11-2.220. These findings were observed after adjusting for age, sex, APOE genotype, mean SBP and other confounding variables.
Discussions
Our findings suggest that lower complexity and higher variability of SBP are potential novel risk factors or biomarkers for dementia.
Keywords: blood pressure, blood pressure variability, blood pressure complexity, dementia, Alzheimer’s disease, prospective cohort
Introduction
Dementia is the most common neurodegenerative disease in older people, leading to loss of functional independence and disability.[1] Hypertension and other vascular risk factors are major modifiable contributors in the multifactorial etiology of dementia.[2], but the relationship between blood pressure (BP) and dementia is still not fully understood.[3] Most studies and clinical guidelines focus on the control of usual BP levels as a stable marker, but BP regulation is, in fact, highly dynamic.[3–5] Conventional BP levels may thus fail to capture the subclinical changes in numerous neural and hormonal feedback mechanisms involved in real-time BP regulation.
It has been reported that excessive BP variability over periods of days or years is associated with elevated risk of dementia, independent of mean BP level,[6–8] but the clinical translation of such measures of long-term BP variability faces practical challenges, such as measuring BP reliably and accurately in clinical settings over multiple days or years. Nowadays the measurement of BP variability over shorter periods (e.g., seconds) has been made feasible with the advances in wearable devices. Short-term BP variability may capture subtle physiological changes in BP regulation that differ from chronic variation spanning years, but its relation to dementia risk remains unknown.[9] Additionally, variability measures assess the amplitude of BP dynamic processes, and therefore may not adequately quantify the complex dynamics of BP regulation, which consists of numerous neural and hormonal feedback mechanisms that are interacting with each other over different scales of time and space.[10] Entropy, as a well-accepted measure of complexity of dynamic systems, provides a new way to quantify the adaptive capacity (i.e. complexity) of dynamic BP processes.[11, 12] Previous studies have linked lower complexity in the dynamics of given physiological signals (e.g., heart rate, standing postural sway) to aging and age-related adverse events (e.g., frailty and falls). [13–18] The complexity of BP dynamics is thought to be due to the interaction of multiple blood pressure regulatory processes operating on different time scales, including baroreflex sensitivity, which adjusts SBP on a beat-to-beat basis, and arterial stiffness, which affects baroreflex sensitivity, pulse pressure, and intravascular volume regulation over the longer term [19–22]. Alterations in each of these mechanisms may affect BP complexity and variability, which could contribute to dementia risk through, for example, impairing cerebral autoregulation, threatening cerebral blood flow, and causing ischemic damage to the brain. Alternatively, the alterations in BP dynamics may also result from neurodegenerative processes in autonomic control centers of the brain. We therefore hypothesized that higher variability and lower complexity in BP dynamics are associated with higher risk of dementia. We tested this hypothesis in the population-based Rotterdam Study, leveraging its prospective collection of unique continuous beat-to-beat BP measurements and the subsequent follow-up of incident dementia for up to 20 years.[23]
Methods
Study Population
This study is embedded in the Rotterdam Study, a prospective cohort study underway since 1989 in the Ommoord District in the city of Rotterdam, The Netherlands.[23] Briefly, 7,983 participants (out of 10,215 invitees) aged ≥55 years have been followed for 26 years (since July 27, 1989, through January 1, 2016), with the first through sixth examination cycles performed in 1989–1993, 1993–1995, 1997–1999, 2002–2004, 2009–2011 and 2014-2015. In a subset of 2,531 random participants, continuous beat-to-beat BP measurements were measured during the third examination between March 1997 and December 1999. Among these 2,531 participants, individuals with any of the following conditions were excluded: 1) clinical diagnosis of dementia before and at the third examination (n =60); 2) continuous BP measurement ≤ 300 heartbeats (n=195); and 3) history of cardiovascular disease (CVD) including stroke, coronary heart disease, and atrial fibrillation, reported and verified before and at the third examination (n = 441). Ultimately, 1,835 eligible participants were included in the current study. The Rotterdam Study has been approved by the institutional review board (Medical Ethics Committee) of the Erasmus Medical Center and by the review board of The Netherlands Ministry of Health, Welfare and Sports, and written informed consent has been obtained from all participants.
BP Measurement
During the third examination between 1997 and 1999 (i.e. baseline of the current study), continuous beat-to-beat finger BP was measured noninvasively using Finapres (TNO BioMedical Instrumentation, Amsterdam, The Netherlands) at the middle finger of the left hand when the participant was in the supine position. The participant was asked to refrain from speaking during the measurement and was explained that movements of hand and fingers, laughing and coughing may affect BP measurement. Beat-to-beat BP and inter-beat interval were recorded continuously for at least 5 minutes and were sampled at 100 Hz. The BeatScope software package (TNO BioMedical Instrumentation, Amsterdam, The Netherlands) was used to calculate continuous beat-to-beat systolic BP (SBP), diastolic BP (DBP) and mean arterial pressure (MAP). The assessment of finger BP using Finapres has been validated against brachial BP in previous studies.[24] Our analysis used BP series of the first 300 continuous beats of stable BP signals as detected by Finapres.
Assessment of BP Complexity and BP Variability
The BP complexity was quantified using sample entropy, a well-established measure of the non-linear dynamics (i.e. complexity) of physiological time-series.[12, 25] Briefly, sample entropy is defined as the negative natural logarithm of the conditional probability that a time-series, having repeated itself within the similarity criteria of r for m points, will also repeat itself for m+1 points, without including self-matches in the probability calculation.[12] As an a priori procedure, we set m (length of the BP segment being compared) as 2 following recommendations for short time series data and r as 0.15 after examining sample entropy using multiple values of r (i.e., 0.1, 0.15, 0.2, 0.25 and 0.3) based on the criterion that optimizes both the accuracy and discrimination capability of sample entropy estimates.[26, 27] In our study, sample entropy explicitly calculates the probability that patterns within the BP series repeating themselves for any two consecutive SBP points, would also repeat themselves for any three consecutive points, within a defined distance of 0.15 times the standard deviation (SD) of the entire BP series (i.e., r=0.15*SD). Lower sample entropy indicates less complexity in BP dynamics, i.e. reduced adaptive capacity to maintain optimal BP regulation (detailed methods in the Supplement).
Beat-to-beat BP variability was quantified using coefficient of variation, defined as SD/mean of the continuous beat-to-beat BP series. Figure 1 illustrates individual examples with similar mean SBP but different complexity and variability levels, with hypothetical interpretation provided. To further assess the joint role of BP complexity and variability, we constructed an interaction term that identified individuals with both high complexity and low variability and individuals with both low complexity and high variability in BP, where “high” and “low” were pre-specified as within the top and bottom quintile of the corresponding measure, respectively.
Figure 1. Illustration of beat-to-beat SBP measurements with the same mean but different levels of complexity and variability.
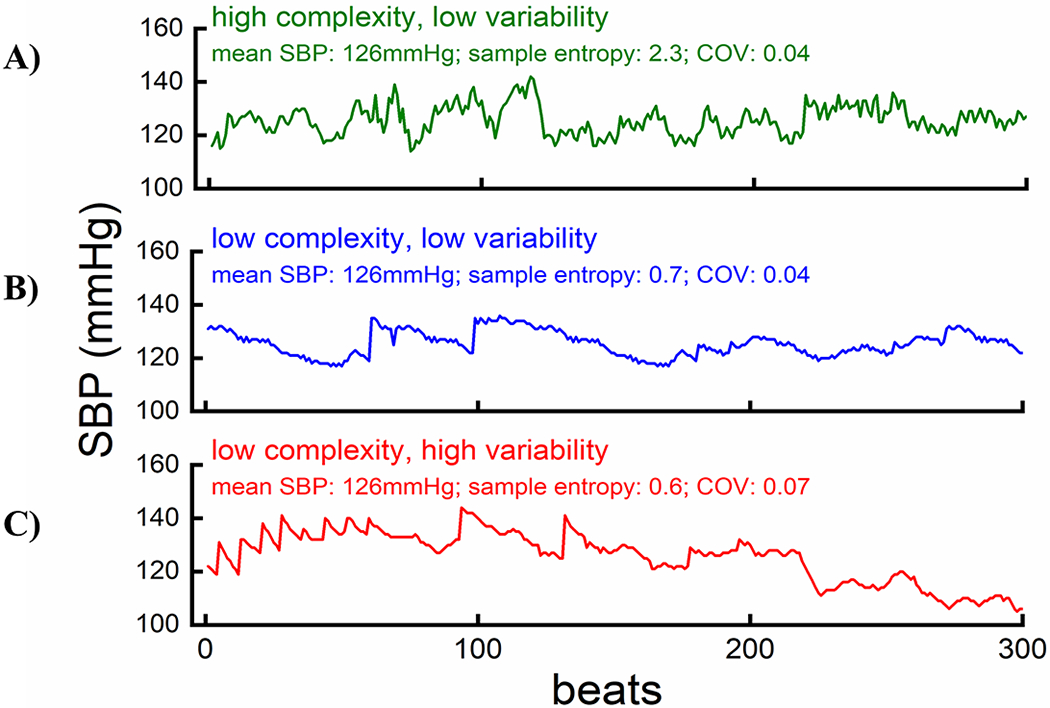
Hypothetical Interpretation: Panel A visualizes the scenario of intact BP regulation, in which numerous and hormonal feedback mechanisms are making flexible adaptations sensitively, manifested as many small disturbances on the waveform, to minimize BP variability (high complexity and low variability). Panel B visualizes the scenario in which the complex interactions between multiple regulatory feedback loops are partially impaired, but BP variability can still be largely limited through compensatory mechanism (low complexity and low variability). Panel C visualizes the scenario in which the complex feature of BP regulation is further impaired and BP variability cannot be effectively buffered by compensatory mechanism (low complexity and high variability).
Ascertainment of Dementia
Dementia cases were ascertained throughout the study using a standardized protocol.[28] Specifically, all participants were screened for dementia at baseline and subsequent visits using the Mini-Mental State Examination and the Geriatric Mental Schedule.[28] Participants having a Mini-Mental State Examination score < 26 or Geriatric Mental State Schedule organic level > 0 underwent further examination and informant interview with the Cambridge Examination for Mental Disorders of the Elderly.[28] Participants who were suspected of having dementia underwent extra neuropsychological testing if necessary. Of all 1,835 participants, 412 (22%) did not return to the research center for in-person follow-up examinations for dementia assessment. Participants who did not return for follow-up were less healthy at baseline, with a lower baseline MMSE score. Because the entire cohort was continuously monitored for dementia through electronic linkage with medical records from general practitioners and the regional institute for outpatient mental healthcare, dementia status for non-visitors were also recorded. Available information on cognitive testing and clinical neuroimaging was used when required for diagnosis of dementia subtype. Based on all the above information, a consensus panel led by a consultant neurologist established the final diagnosis according to standard criteria for dementia (the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised; DSM-III-R). For consistency throughout the study period, Alzheimer’s disease was defined according to the clinical NINCDS-ADRDA-criteria.[28] As of January 1, 2016, follow-up for dementia was complete for 98% of potential person-years.
Arterial Stiffness and Cardiovagal Baroreflex Function
Arterial stiffness was assessed by carotid-femoral pulse wave velocity using an automatic device at the same time with continuous Finapres BP measurement.[29, 30] A higher value of pulse wave velocity indicates elevated arterial stiffness. Baroreflex sensitivity was calculated from same beat-to-beat measurements using methods applied to the Rotterdam Study previously.[31, 32] Baroreflex sensitivity was defined as the slope of linear regression of the change in inter-beat interval on the change in SBP, with a higher value indicating better baroreflex sensitivity.[32] Further details on both measures are provided (detailed methods in the Supplement).
Other Measurements
Information on demographic characteristics was collected at the first examination after cohort entry in 1989. The apolipoprotein E (APOE) genotype was determined using polymerase chain reaction on coded genomic DNA samples. During each follow-up exam, smoking habits, alcohol consumption, medication use, body mass index, total cholesterol, high-density lipoprotein cholesterol, and diabetes mellitus were assessed with standardized protocols. Cardiovascular disease, including coronary heart disease (i.e. myocardial infarction, percutaneous coronary intervention or coronary artery bypass grafting) stroke and atrial fibrillation, was assessed via interviews and verified by medical records. The diagnosis of atrial fibrillation was further verified by a 12-lead resting electrocardiogram test.
Statistical Analyses
Primary Analyses
Our primary analysis focused on the association of SBP complexity and variability with incident dementia, and hazard ratios (HR) were estimated by Cox proportional hazards models. Person-time accrued from the third examination (time of beat-to-beat BP measurement) until the date of dementia diagnosis, date of death, date of loss to follow-up, or censoring at the end of study on January 1, 2016, whichever came first. We estimated the cumulative incidence of dementia while accounting for the competing risk of death.[33] Differences in curves across quintiles of SBP complexity were tested using Gray’s test for equality of cumulative incidence functions.[34] We assessed SBP complexity on both continuous and categorical scales. As the clinical cut-offs for SBP complexity have yet to be established, we used pre-specified quintile-based categories, with the reference group defined as the highest quintile for SBP complexity (i.e. high complexity). Testing for linear trends across quintiles of SBP complexity was performed by entering a single ordinal term into the model. To control for possible confounding, Cox models were built with the adjustment for the following covariates in three ways: 1) adjustment for age, sex in model 1; 2) additional adjustment for mean SBP level in model 2; and 3) further adjusting for education level (primary, intermediate, higher education), APOE ε4 carrier status, smoking habits (never, past, current), body mass index (<24.9, 25-29.9, ≥30kg/m2), lipid levels (quartiles), history of diabetes and antihypertensive medication use in the final model. The proportional hazard assumptions were tested by including an interaction term with time in the model, and the assumptions were also verified. To provide causal relative risk estimates in the presence of competing risk of death, cause-specific HRs were computed.[35] All covariates, except SBP level and age, were categorical, and missing data were handled by adding an additional category indicating missing values (<10%) to the model. We also used a multiple-imputation approach with five imputations for missing data in our sensitivity analysis. We assessed the association of SBP variability with dementia risk using the same approach, except that the reference group was defined as the lowest quintile of SBP variability. We also estimated dementia risk in relation to the interaction term of complexity and variability using the same methods.
Secondary Analyses
We repeated the analyses described above for the complexity and variability in DBP, pulse pressure (PP) and MAP respectively. To identify potential effect modification, we stratified the analyses by age, sex, APOE genotype, antihypertensive treatment and SBP level at baseline. Interaction was formally tested on a multiplicative scale by adding a product term to the model. To explore potential mechanisms, we further stratified the association by pulse wave velocity, the most common indicator of arterial stiffness[29] and by baroreflex sensitivity, an established assessment of autonomic control of the cardiovascular system[36]. We additionally adjusted for pulse wave velocity and baroreflex sensitivity to test whether these two markers could be potential mediators. Finally, to inform the relationship between short-term beat-to-beat BP variability and long-term visit-to-visit BP variability, we further derived visit-to-visit BP variability using coefficient of variation of BP measured at the first three examinations (in 1989–1993, 1993–1995, 1997–1999), and assessed its correlation with beat-to-beat BP variability and its association with dementia risk using methods consistent with our primary analyses.
Sensitivity Analyses
To test the robustness of the main findings, we performed the following analyses: (1) repeating analyses for clinical Alzheimer’s disease as the outcome (analyses for vascular dementia were not performed due to the small number of cases); (2) censoring dementia cases ascertained within 5 years of BP measurements to reduce potential issue of reverse causation; (3) censoring participants at the time of stroke; (4) imputing missing data using a multiple-imputation approach; (5) including the 441participants with pre-existing cardiovascular disease; (6) adjusting for covariates updated at follow-up examinations using time-varying Cox models; and (7) additionally adjusting for baseline cognitive function.
All effect estimates are given with corresponding 95% confidence intervals (CIs). All P values presented are two sided, and a P value of 0.05 or less was considered statistically significant for all the analyses, except that the significance level for an interaction effect was set as 0.1 or less to increase the statistical power to detect biologically meaningful interactions. Statistical analyses were performed using SAS version 9.4 (SAS Institute), R version 3.6.2 (R Foundation, ggplot2 package) and Matlab (2019b, The MathWorks, Inc).
Results
Among the 1,835 participants (63% women) with mean age of 71.0 (6.4) years, 334 participants developed dementia during a median follow-up of 14.5 years (interquartile range 8.6–16.4), including 271 (81.1%) with clinical diagnosis of Alzheimer’s disease and 15 (4.5%) with pure vascular dementia. Table 1 and Table 2 describe the characteristics of the participants across quintiles of SBP complexity and variability, respectively. SBP complexity and variability were inversely correlated (r=−0.49, P<0.001), while SBP complexity only weakly correlated with mean SBP (r=−0.08) and SBP variability did not correlate with mean SBP (r=0.01) (Supplementary eFigure 1).
Table 1.
Basic characteristics of the study population across quintiles of SBP complexity
| Characteristics a | Overall |
Quintiles of SBP complexity |
|||||
|---|---|---|---|---|---|---|---|
| (n=1,835) | Q1 (n=367) | Q2 (n=367) | Q3 (n=367) | Q4 (n=367) | Q5 (n=367) | P value | |
| Age at baseline, years | 71.0 (6.4) | 70.7 (6.2) | 71.3 (6.3) | 71.4 (6.7) | 70.9 (6.5) | 70.6 (6.1) | 0.61 |
| Women, % | 62.5 | 58.0 | 63.2 | 68.1 | 59.9 | 63.2 | 0.38 |
| SBP (mmHg) | 144 (21) | 143 (21) | 146 (21) | 147 (21) | 143 (21) | 140 (19) | 0.001 |
| DBP (mmHg) | 76 (11) | 76 (11) | 77 (12) | 77 (11) | 77 (11) | 75 (10) | 0.09 |
| Total cholesterol, mg/dL | 5.9 (0.9) | 6.0 (0.9) | 5.9 (1.0) | 6.0 (1.0) | 5.9 (0.9) | 5.8 (0.9) | 0.07 |
| HDL cholesterol, mg/dL | 1.4 (0.4) | 1.5 (0.4) | 1.4 (0.4) | 1.4 (0.4) | 1.4 (0.4) | 1.4 (0.4) | 0.01 |
| APOE genotype, % | 0.93 | ||||||
| ε3/ε3 | 59.1 | 59.8 | 58.6 | 59.6 | 59.7 | 58.0 | - |
| ε2/ε2 or ε2/ε3 | 13.8 | 11.8 | 16.0 | 12.6 | 12.8 | 15.7 | - |
| ε2/ε4 or ε3/ε4 | 25.0 | 25.9 | 23.5 | 25.8 | 25.6 | 24.0 | - |
| ε4/ε4 | 2.1 | 2.5 | 1.9 | 1.9 | 1.9 | 2.2 | - |
| Education, % | 0.21 | ||||||
| Primary/intermediate | 89.3 | 86.5 | 89.6 | 91.0 | 89.5 | 89.8 | - |
| Higher education | 10.7 | 13.5 | 10.4 | 9.0 | 10.5 | 10.2 | - |
| Smoking status, % | 0.04 | ||||||
| Never | 34.4 | 33.0 | 40.1 | 36.4 | 30.6 | 31.7 | - |
| Past | 47.7 | 49.2 | 48.9 | 43.8 | 49.6 | 47.1 | - |
| Current | 17.9 | 17.9 | 11.0 | 19.7 | 19.8 | 21.2 | - |
| Weight status, % b | <0.001 | ||||||
| Normal weight | 34.2 | 45.4 | 36.6 | 27.9 | 31.2 | 29.8 | - |
| Overweight | 46.6 | 44.8 | 47.5 | 50.0 | 45.5 | 45.2 | - |
| Obese | 19.2 | 9.8 | 15.8 | 22.1 | 23.3 | 25.1 | - |
| History of Diabetes, % | 12.0 | 8.9 | 10.7 | 13.7 | 12.7 | 13.8 | 0.03 |
| History of Hypertension, % | 65.8 | 61.0 | 71.7 | 70.8 | 64.3 | 61.1 | 0.32 |
| Antihypertensive medication use, % | 31.2 | 27.7 | 29.8 | 32.7 | 31.8 | 33.9 | 0.06 |
Abbreviations: SBP=systolic blood pressure; DBP=diastolic blood pressure; HDL=high-density lipoproteins; APOE= Apolipoprotein E. Data are shown in the format of means (SD) or percentages.
Characteristics at baseline (i.e. the third examination between 1997 and 1999 when continuous BP measurements were taken).
Weight status was assessed by body mass index (BMI), with overweight defined as 25≤ BMI <30 kg/m2 and obesity defined as BMI ≥30 kg/m2.
Table 2.
Basic characteristics of the study population across quintiles of SBP variability
| Characteristics a | Overall |
Quintile of SBP variability |
|||||
|---|---|---|---|---|---|---|---|
| (n=1,835) | Q1 (n=367) | Q2 (n=366) | Q3 (n=367) | Q4 (n=368) | Q5 (n=367) | P value | |
| Age at baseline, years | 71.0 (6.4) | 70.4 (6.2) | 70.1 (6.0) | 71.1 (6.4) | 71.4 (6.5) | 72.0 (6.5) | <0.001 |
| Women, % | 62.5 | 56.1 | 62.0 | 64.0 | 62.8 | 67.5 | 0.003 |
| SBP (mmHg) | 144 (21) | 146 (21) | 142 (19) | 142 (19) | 144 (22) | 144 (21) | 0.96 |
| DBP (mmHg) | 76 (11) | 79 (12) | 76 (10) | 76 (10) | 76 (11) | 75 (11) | <0.001 |
| Total cholesterol, mmol/L | 5.9 (0.9) | 5.9(1.0) | 6.0(1.0) | 5.9(0.9) | 5.9(1.0) | 6.0(0.9) | 0.85 |
| HDL cholesterol, mmol/L | 1.4 (0.4) | 1.4(0.4) | 1.4(0.4) | 1.4(0.4) | 1.4(0.4) | 1.4(0.4) | 0.20 |
| APOEgenotype, % | 0.91 | ||||||
| ε3/ε3 | 59.1 | 58.6 | 58.1 | 59.6 | 61.8 | 57.5 | - |
| ε2/ε2 or ε2/ε3 | 13.8 | 14.6 | 14.8 | 14.2 | 13.5 | 11.9 | - |
| ε2/ε4 or ε3/ε4 | 25.0 | 25.7 | 23.7 | 24.0 | 23.1 | 28.3 | - |
| ε4/ε4 | 2.1 | 1.1 | 3.4 | 2.2 | 1.6 | 2.2 | - |
| Education, % | 0.03 | ||||||
| Primary/intermediate | 89.3 | 87.7 | 88.4 | 89.0 | 87.6 | 93.6 | - |
| Higher education | 10.7 | 12.3 | 11.6 | 11.0 | 12.4 | 6.4 | - |
| Smoking status, % | 0.63 | ||||||
| Never | 34.4 | 33.5 | 34.3 | 39.9 | 27.5 | 36.6 | - |
| Past | 47.7 | 48.4 | 49.7 | 42.6 | 54.5 | 43.3 | - |
| Current | 17.9 | 18.1 | 16.0 | 17.5 | 17.9 | 20.1 | - |
| Weight status, % b | 0.25 | ||||||
| Normal weight | 34.2 | 31.1 | 33.1 | 35.8 | 33.2 | 37.8 | - |
| Overweight | 46.6 | 51.0 | 43.9 | 44.3 | 46.6 | 47.1 | - |
| Obese | 19.2 | 18.0 | 23.1 | 19.9 | 20.2 | 15.1 | - |
| History of Diabetes, % | 12.0 | 12.4 | 13.3 | 9.2 | 14.0 | 10.9 | 0.70 |
| History of Hypertension, % | 65.8 | 70.5 | 62.6 | 63.8 | 64.1 | 67.8 | 0.60 |
| Antihypertensive medication, % | 31.2 | 35.2 | 26.8 | 31.1 | 31.0 | 31.8 | 0.73 |
Abbreviations: SBP=systolic blood pressure; DBP=diastolic blood pressure; HDL=high-density lipoproteins; APOE= Apolipoprotein E. Data are shown in the format of means (SD) or percentages.
Characteristics at baseline (i.e. the third examination between 1997 and 1999 when continuous BP measurements were taken).
Weight status was assessed by body mass index (BMI), with overweight defined as 25≤ BMI <30 kg/m2 and obesity defined as BMI ≥30 kg/m2.
BP Complexity, Variability and Dementia Risk
Lower SBP complexity was associated with higher risk of all-cause dementia. As shown in Figure 2A, the incidence rate of all-cause dementia was higher with lower SBP complexity across quintiles of SBP complexity, after accounting for the competing risk of death (P=0.013). Table 3 further shows the cause-specific HRs of dementia by quintiles of SBP complexity. The risk of dementia was higher with lower SBP complexity, and the HR was 1.59 (comparing lowest versus highest quintile; 95% CI: 1.13–2.24, P for trend=0.003) after adjusting for age and sex. The association remained statistically significant after further adjusting mean SBP, education, APOE genotype, antihypertensive medication use and traditional vascular risk factors (HR:1.55; 95%CI: 1.09-2.20; P for trend=0.007). Association estimates for covariates in the final model were also provided (Supplementary eTable 1). Dementia risk did not change significantly with the complexity in DBP, PP or MAP (Supplementary eTable 2).
Figure 2. Cumulative incidence of all-cause dementia across quintiles of SBP complexity (A) and variability (B).
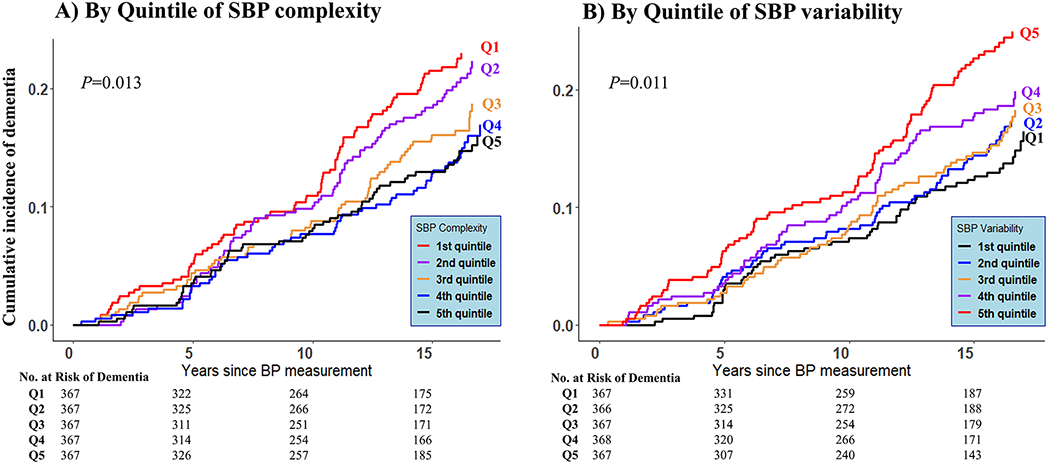
*SBP = Systolic Bloood Pressure; Q1-Q5 represent 1st~5th quintiles of the corresponding measures; Q1 is the bottom quintile and Q5 is the top quintile.
Table 3.
Beat-to-beat SBP complexity and risk of all-cause dementia
| Risk by quintiles of BP complexity |
Per SD decrease | P values | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st quintile (<0.94) | 2nd quintile (0.94~1.15) | 3rd quintile (1.15~1.38) | 4th quintile (1.38~1.76) | 5th quintile (1.76~3.23) | P for trend | |||
| Events/Participants at risk | 83/367 | 79/367 | 63/367 | 55/367 | 54/367 | - | - | - |
| HR (Model 1) (95%CI) | 1.59 (1.13, 2.24) | 1.35 (0.95, 1.91) | 1.19 (0.83, 1.71) | 1.08 (0.74, 1.57) | 1 (reference) | 0.003 | 1.18 (1.06, 1.33) | 0.004 |
| HR (Model 2) (95%CI) | 1.58 (1.12, 2.22) | 1.32 (0.93, 1.88) | 1.18 (0.82, 1.70) | 1.07 (0.74, 1.56) | 1 (reference) | 0.004 | 1.18 (1.05, 1.33) | 0.005 |
| HR (Model 3) (95%CI) | 1.55 (1.09, 2.20) | 1.32 (0.92, 1.88) | 1.13 (0.78, 1.63) | 1.09 (0.75, 1.60) | 1 (reference) | 0.007 | 1.17 (1.04, 1.31) | 0.008 |
Model 1: adjusting for age and sex;
Model 2: further adjustment for mean SBP in addition to model 1;
Model 3: further adjustment for APOE genotype, education, total and HDL cholesterol levels, smoking habits, weight status, history of diabetes and antihypertensive medication use in addition to model 2.
Excessive SBP variability was associated with a higher risk of all-cause dementia. Figure 2B shows higher incidence rate of all-cause dementia among those with larger SBP variability (P=0.011). Table 4 shows the HRs of dementia by quintiles of SBP variability. Higher SBP variability measured by coefficient of variation was associated with a higher risk of dementia after adjusting for age and sex, mean SBP, education, APOE genotype and traditional vascular risk factors. The HR of all-cause dementia for large SBP variability at the highest (vs lowest) quintile was 1.57 (95% CI: 1.11-2.22, P for trend=0.017). Association estimates for covariates in the final model were also provided (Supplementary eTable 3). Consistent associations with dementia risk were observed for variability in DBP and MAP, though the association was less consistent for PP variability (Supplementary eTable 4).
Table 4.
Beat-to-beat SBP variability and risk of all-cause dementia
| Risk by quintiles of BP complexity |
Per SD increase | P values | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st quintile (<0.05) | 2nd quintile (0.05~0.06) | 3rd quintile (0.06~0.07) | 4th quintile (0.07~0.09) | 5th quintile (0.09~0.22) | P for trend | |||
| Events/Participants at risk | 53/367 | 60/366 | 62/367 | 70/368 | 89/366 | - | - | - |
| HR (Model 1) (95%CI) | 1 (reference) | 1.23 (0.85, 1.78) | 1.18 (0.81, 1.70) | 1.24 (0.87, 1.77) | 1.70 (1.21, 2.39) | 0.004 | 1.15 (1.04, 1.27) | 0.004 |
| HR (Model 2) (95%CI) | 1 (reference) | 1.21 (0.84, 1.75) | 1.16 (0.80, 1.67) | 1.22 (0.85, 1.75) | 1.65 (1.18, 2.33) | 0.007 | 1.14 (1.04, 1.26) | 0.006 |
| HR (Model 3) (95%CI) | 1 (reference) | 1.30 (0.90, 1.89) | 1.37 (0.94, 1.99) | 1.35 (0.94, 1.95) | 1.57 (1.11, 2.22) | 0.017 | 1.12 (1.01, 1.23) | 0.026 |
Model 1: adjusting for age and sex;
Model 2: further adjustment for mean SBP in addition to model 1;
Model 3: further adjustment for APOE genotype, education, total and HDL cholesterol levels, smoking habits, weight status, history of diabetes and antihypertensive medication use in addition to model 2.
When SBP variability and complexity were considered jointly, individuals with both low complexity (at the bottom quintile) and high variability (at the top quintile) had a significantly higher risk of developing dementia compared with those of both high complexity (at the top quintile) and low variability (at the bottom quintile) in SBP (HR: 2.22; 95%CI: 1.31-3.76, P for trend=0.002; Supplementary eTable 5).
Secondary Analysis
The association of SBP complexity with dementia risk was significant for individuals with both low and elevated (defined as >75th percentile of the measure) levels of arterial stiffness, but tended to be stronger in the presence of elevated pulse wave velocity (adjusted P for interaction=0.07; Supplementary eFigure 2 ). The magnitude of the association also appeared to be slightly (statistically non-significant) larger with lower baroreflex sensitivity; the associations did not differ significantly by antihypertensive medication, age, sex, or APOE ε4 carrier status. (Supplementary eTable 6). The association with SBP variability also did not differ significantly across these subgroups, and the association patterns stratified by arterial stiffness and baroreflex sensitivity were similar to those for SBP complexity (Supplementary eTable 7). The association remained similar with additional adjustment for pulse wave velocity and baroreflex sensitivity (Supplementary eTable 8). Additionally, short-term beat-to-beat BPV was not correlated with long-term visit-to-visit BPV (r=0.02, P=0.35). When both short-term and long-term SBP variability were assessed in the same model, they both were significantly associated with dementia risk (Supplementary eTable 9).
Sensitivity Analysis
Risk estimates of SBP complexity and variability were similar for all-cause dementia and the clinical diagnosis of Alzheimer’s disease (Supplementary eTables 10–11). The association of SBP complexity with dementia risk remained essentially the same after removing dementia cases that occurred during the first five years of follow-up, using multiple imputation, adjusting for time-varying covariates, and additionally adjusting for baseline cognitive function. The association was slightly attenuated after censoring the 35 post-stroke dementia cases and without excluding pre-existing cardiovascular cases at baseline (Supplementary eTable 12). Similar results were observed for SBP variability (data not shown).
Discussion
In this population-based prospective cohort study among older adults, we observed that both lower complexity and higher variability of beat-to-beat SBP were associated with elevated long-term dementia risk, after adjusting for age, sex, mean SBP and other potential confounding.
Our study provides novel evidence that lower SBP complexity was associated with increased dementia risk in older adults. The “complexity theory of aging” postulates that aging and age-related diseases alter dynamic physiological feedback loops and their structural and functional connectivity, contributing to reduced system functionality and impairment in an organism’s ability to adapt to stress, i.e. loss of complexity.[13] Previous studies showed the relationship between SBP complexity and other diseases. For example, a case-control study showed that SBP complexity was lower in patients with type I diabetes mellitus.[37] Similarly, in pediatric patients with acute brain injury reduced SBP complexity was associated with a higher risk of death.[38] The findings on SBP complexity and risk of dementia in our study further demonstrates that lower physiological complexity of SBP regulation may capture subtle abnormalities in the central nervous and cardiovascular systems that occur at the early subclinical stage of dementia.
In terms of BP variability, it has been linked to coronary heart disease, stroke and renal disease.[5, 39] Both higher day-to-day and visit-to-visit BP variability over years were also associated with higher dementia risk.[6, 7, 40] Our study adds new evidence by showing that excessive beat-to-beat SBP and DBP variability over a short period of minutes were associated with the elevated risk of dementia. Interestingly, the association of DBP variability with dementia risk was slightly stronger than that of SBP variability. Given that SBP is mathematically composed of DBP and PP, the findings suggest that DBP may be more relevant to dementia etiology than SBP as a whole or PP. Additionally, and importantly, we observed that beat-to-beat SBP variability was not correlated with long-term visit-to-visit BP variability and that both were associated with elevated risk of dementia with mutual adjustment, suggesting that the underlying mechanisms of BP variability over different time periods may vary. Notably, compared to BP variability measured spanning years or days, beat-to-beat BP can be measured more conveniently in clinical settings, making short-term BP dynamic measures potentially more useful in practice. Incorporating variability and complexity measures of different BP components over different timescales into prediction models for dementia might also help improve dementia risk stratification.
The relationship between the complexity and variability of SBP and risk of dementia was observed in older adults free of major cardiovascular events after adjusting for traditional vascular risk factors, suggesting that SBP complexity and variability may capture important early subclinical changes in BP regulation that contribute to dementia through pathways independent of traditional vascular risk factors. We observed similar association after censoring dementia cases occurring after incident stroke. This observation suggests that BP complexity and variability may be linked to dementia risk mainly through the accumulation of chronic subclinical vascular pathology, instead of pathways mediated by major sudden vascular events such as stroke. It thereby indicates the importance of vascular pathology, assessed by BP complexity and variability, in the early-stage etiology of dementia.
The biological explanations of SBP complexity and variability are largely unknown. Numerous neural and hormonal feedback mechanisms operate simultaneously to precisely maintain the oscillation of BP within a certain range.[10] This complex rhythm of the real time BP regulation may decrease if the underlying feedback loops cannot communicate and respond efficiently, giving rise to excessive BP variability. This speculation is in concert with the observed inverse correlation between SBP complexity and variability. The association of SBP complexity and variability with dementia appeared stronger in the presence of arterial stiffness and impaired baroreflex sensitivity, which is in line with a previous report,[7] although most of these subgroup differences did not reach statistical significance. It also concurs with an animal study showing that the denervation of baroreceptors led to less complex and more variable BP[22] and evidence suggesting that the primary function of the baroreceptor reflex was not to set the BP level, but, instead, to minimize variations in systemic arterial BP.[41] Taken together, these data suggest that lower complexity and higher variability of BP may reflect subtle subclinical vascular impairment and autonomic dysfunction that contribute to the disturbances of dynamic BP regulation. For the observed associations of BP complexity and variability with dementia, there could be several explanations. First, a causal relationship is possible. The brain receives high-volume blood flow and the disturbance in BP dynamics may expose cerebral vessel walls to wider pressure fluctuation, especially when the cushioning function of large vessels is impaired, as occurs with the arterial stiffness.[42] Extreme low and high BP levels resulting from excessive variability may escape cerebral autoregulation limits and contribute to vasculature impairment, ultimately leading to dementia.[43–45] Alternatively, BP variability and complexity could also be preclinical markers of dementia if neurodegenerative changes of prodromal dementia, which affect neural regulation of BP, occur long (e.g., more than one decade) before the diagnosis of dementia. Also, a non-causal explanation cannot be ruled out given that baroreceptor insensitivity, arterial stiffness and cognitive impairment could all represent a general phenomenon of aging that may occur simultaneously. Further studies are needed to confirm the “directionality” of the observed associations, such as employing neuroimaging techniques. Additionally, SBP complexity and SBP variability demonstrated similar associations with dementia, suggesting that they could be markers of the same pathophysiological processes. By contrast, DBP variability was associated with dementia risk but DBP complexity was not, possibly because DBP complexity reflects different physiological processes or that sample entropy captures less pathophysiological signals of DBP regulation.
Several limitations need to be noted. First, although our analyses assessing BP complexity using sample entropy have demonstrated robust associations, continuous BP series in this study was of relatively short length, thus limiting the use of multiscale entropy to quantify BP complexity over broader scales of time. Despite the careful implementation of a standard protocol, random measurement error in beat-to-beat BP series is still possible, which may have led to conservative association estimates. Second, our study was based upon an older population mainly comprised of white participants free of major cardiovascular events at baseline, the generalizability of our results to younger, sicker, or other racial/ethnic populations therefore warrant further investigation. Third, as an observational study, we cannot rule out potential residual confounding, e.g. subclinical cerebrovascular or neurodegenerative changes preceding beat-to-beat BP measurement, although we have comprehensively adjusted for traditional vascular factors. Fourth, beat-to-beat BP was only measured at baseline, precluding us from further assessing the longitudinal changes in BP variability and complexity with the development of dementia. Additionally, the observational nature also limited our ability to identify factors that may be intervened upon to modify SBP complexity and variability. This important evidence can be obtained, for instance, through incorporating continuous BP measurements (possibly using wearable device) into a randomized trial and assessing which types of antihypertensive medication optimize both static BP levels and variability (or complexity).
Our study also has several strengths. It provides novel evidence on the association of both beat-to-beat BP complexity and variability with dementia risk in a generally healthy population. Notably, although sample entropy has been a well-established concept widely used in many fields such as physics and physiology, it has been rarely implemented in epidemiological studies and clinical practice. Our study provides a novel perspective to understand how various dynamic physiological processes may contribute to aging and age-related disease. Additionally, our study is embedded in a well-established ongoing cohort with continuous follow-up of dementia over 20 years, making our findings more robust to potential reverse causation.
Conclusions
This study shows that lower complexity and higher variability in continuous beat-to-beat SBP measures are associated with a higher risk of dementia. These findings suggest that lower complexity and higher variability of beat-to-beat systolic BP are potential novel risk factors or biomarkers for dementia. Further investigations are needed to test whether the observed associations are causal.
Supplementary Material
Acknowledgements
We gratefully acknowledge the dedication, commitment, and contribution of inhabitants, general practitioners, and pharmacists of the Ommoord district to the Rotterdam Study. We thank Dr. Lori B. Chibnik (Harvard T. H. School of Public Health) for her helpful advice on statistical analyses.
Funding Sources
The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam; Netherlands Organization for the Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly (RIDE); the Ministry of Education, Culture and Science; the Ministry for Health, Welfare and Sports; the European Commission (DG XII), and the Municipality of Rotterdam. This work was partially supported by an unrestricted grant from the Janssen Prevention Center. This study was partly funded by ZonMW Memorabel (projectnr 73305095005) and Alzheimer Nederland through the Netherlands Consortium of Dementia Cohorts (NCDC) in the context of Deltaplan Dementie. Further funding was obtained from the Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation (CVON 2018-28 Heart Brain Connection Cross-roads), Dutch Federation of University Medical Centres , the Netherlands Organisation for Health Research and Development and the Royal Netherlands Academy of Sciences. This work was also supported by the American Heart Association Grant #20POST35120057(Dr. Yuan Ma). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflicts of interest: None.
References
- [1].Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–734. [DOI] [PubMed] [Google Scholar]
- [2].Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–94. [DOI] [PubMed] [Google Scholar]
- [3].Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–99. [DOI] [PubMed] [Google Scholar]
- [4].Sprint Mind Investigators for the SPRINT Research Group. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA. 2019;321:553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–48. [DOI] [PubMed] [Google Scholar]
- [6].Alperovitch A, Blachier M, Soumare A, Ritchie K, Dartigues JF, Richard-Harston S, et al. Blood pressure variability and risk of dementia in an elderly cohort, the Three-City Study. Alzheimers Dement. 2014;10:S330–7. [DOI] [PubMed] [Google Scholar]
- [7].Ma Y, Wolters FJ, Chibnik LB, Licher S, Ikram MA, Hofman A, et al. Variation in blood pressure and long-term risk of dementia: A population-based cohort study. PLoS medicine. 2019;16:e1002933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].van Middelaar T, van Dalen JW, van Gool WA, van den Born BH, van Vught LA, Moll van Charante EP, et al. Visit-To-Visit Blood Pressure Variability and the Risk of Dementia in Older People. J Alzheimers Dis. 2018;62:727–35. [DOI] [PubMed] [Google Scholar]
- [9].Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nature reviews Cardiology. 2013;10:143–55. [DOI] [PubMed] [Google Scholar]
- [10].Glass L, Beuter A, Larocque D. Time delays, oscillations, and chaos in physiological control systems. Mathematical Biosciences. 1988;90:111–25. [Google Scholar]
- [11].Pincus SM. Approximate entropy as a measure of system complexity. Proceedings of the National Academy of Sciences. 1991;88:2297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. American Journal of Physiology-Heart and Circulatory Physiology. 2000;278:H2039–H49. [DOI] [PubMed] [Google Scholar]
- [13].Lipsitz LA, Goldberger AL. Loss of ‘Complexity’ and Aging: Potential Applications of Fractals and Chaos Theory to Senescence. JAMA. 1992;267:1806–9. [PubMed] [Google Scholar]
- [14].Goldberger AL. Non-linear dynamics for clinicians: chaos theory, fractals, and complexity at the bedside. Lancet. 1996;347:1312–4. [DOI] [PubMed] [Google Scholar]
- [15].Goldberger AL, Amaral LA, Hausdorff JM, Ivanov P, Peng CK, Stanley HE. Fractal dynamics in physiology: alterations with disease and aging. Proceedings of the National Academy of Sciences of the United States of America. 2002;99 Suppl 1:2466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chaves PH, Varadhan R, Lipsitz LA, Stein PK, Windham BG, Tian J, et al. Physiological complexity underlying heart rate dynamics and frailty status in community-dwelling older women. J Am Geriatr Soc. 2008;56:1698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhou J, Habtemariam D, Iloputaife I, Lipsitz LA, Manor B. The Complexity of Standing Postural Sway Associates with Future Falls in Community-Dwelling Older Adults: The MOBILIZE Boston Study. Scientific reports. 2017;7:2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li P, Yu L, Lim ASP, Buchman AS, Scheer F, Shea SA, et al. Fractal regulation and incident Alzheimer’s disease in elderly individuals. Alzheimers Dement. 2018;14:1114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tedla YG, Yano Y, Carnethon M, Greenland P. Association Between Long-Term Blood Pressure Variability and 10-Year Progression in Arterial Stiffness. Hypertension. 2017;69:118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mancia G, Parati G, Pomidossi G, Casadei R, Di Rienzo M, Zanchetti A. Arterial baroreflexes and blood pressure and heart rate variabilities in humans. Hypertension. 1986;8:147–53. [DOI] [PubMed] [Google Scholar]
- [21].Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. American Journal of Physiology-Heart and Circulatory Physiology. 2001;281:H284–H9. [DOI] [PubMed] [Google Scholar]
- [22].Wagner CD, Mrowka R, Nafz B, Persson PB. Complexity and “chaos” in blood pressure after baroreceptor denervation of conscious dogs. The American journal of physiology. 1995;269:H1760–6. [DOI] [PubMed] [Google Scholar]
- [23].Ikram MA, Brusselle GGO, Murad SD, van Duijn CM, Franco OH, Goedegebure A, et al. The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol. 2017;32:807–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Guelen I, Westerhof BE, van der Sar GL, van Montfrans GA, Kiemeneij F, Wesseling KH, et al. Validation of brachial artery pressure reconstruction from finger arterial pressure. J Hypertens. 2008;26:1321–7. [DOI] [PubMed] [Google Scholar]
- [25].Delgado-Bonal A, Marshak A. Approximate Entropy and Sample Entropy: A Comprehensive Tutorial. Entropy. 2019;21:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yentes JM, Hunt N, Schmid KK, Kaipust JP, McGrath D, Stergiou N. The appropriate use of approximate entropy and sample entropy with short data sets. Annals of biomedical engineering. 2013;41:349–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lake DE, Richman JS, Griffin MP, Moorman JR. Sample entropy analysis of neonatal heart rate variability. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2002;283:R789–R97. [DOI] [PubMed] [Google Scholar]
- [28].de Bruijn RF, Bos MJ, Portegies ML, Hofman A, Franco OH, Koudstaal PJ, et al. The potential for prevention of dementia across two decades: the prospective, population-based Rotterdam Study. BMC medicine. 2015;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. European heart journal. 2006;27:2588–605. [DOI] [PubMed] [Google Scholar]
- [30].Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–63. [DOI] [PubMed] [Google Scholar]
- [31].Westerhof BE, Gisolf J, Stok WJ, Wesseling KH, Karemaker JM. Time-domain cross-correlation baroreflex sensitivity. 2004;22:1371–80. [DOI] [PubMed] [Google Scholar]
- [32].Mattace-Raso FU, van den Meiracker AH, Bos WJ, van der Cammen TJ, Westerhof BE, Elias-Smale S, et al. Arterial stiffness, cardiovagal baroreflex sensitivity and postural blood pressure changes in older adults: the Rotterdam Study. J Hypertens. 2007;25:1421–6. [DOI] [PubMed] [Google Scholar]
- [33].Lin G, So Y, Johnston G. Analyzing survival data with competing risks using SAS® software. Citeseer. [Google Scholar]
- [34].Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. The Annals of statistics. 1988;16:1141–54. [Google Scholar]
- [35].Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016;133:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].La Rovere MT, Pinna GD, Raczak G. Baroreflex Sensitivity: Measurement and Clinical Implications. Annals of Noninvasive Electrocardiology. 2008;13:191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Trunkvalterova Z, Javorka M, Tonhajzerova I, Javorkova J, Lazarova Z, Javorka K, et al. Reduced short-term complexity of heart rate and blood pressure dynamics in patients with diabetes mellitus type 1: multiscale entropy analysis. Physiol Meas. 2008;29:817–28. [DOI] [PubMed] [Google Scholar]
- [38].Papaioannou V, Giannakou M, Maglaveras N, Sofianos E, Giala M. Investigation of heart rate and blood pressure variability, baroreflex sensitivity, and approximate entropy in acute brain injury patients. J Crit Care. 2008;23:380–6. [DOI] [PubMed] [Google Scholar]
- [39].Gosmanova EO, Mikkelsen MK, Molnar MZ, Lu JL, Yessayan LT, Kalantar-Zadeh K, et al. Association of Systolic Blood Pressure Variability With Mortality, Coronary Heart Disease, Stroke, and Renal Disease. Journal of the American College of Cardiology. 2016;68:1375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Oishi E, Ohara T, Sakata S, Fukuhara M, Hata J, Yoshida D, et al. Day-to-Day Blood Pressure Variability and Risk of Dementia in a General Japanese Elderly Population: The Hisayama Study. Circulation. 2017;136:516–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cowley AW, Liard JF, Guyton AC. Role of the Baroreceptor Reflex in Daily Control of Arterial Blood Pressure and Other Variables in Dogs. Circulation research. 1973;32:564–76. [DOI] [PubMed] [Google Scholar]
- [42].Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility--Reykjavik study. Brain. 2011;134:3398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Strandgaard S, Olesen J, Skinhøj E, Lassen NA. Autoregulation of Brain Circulation in Severe Arterial Hypertension. British Medical Journal. 1973;1:507–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ma Y, Song A, Viswanathan A, Blacker D, Vernooij MW, Hofman A, et al. Blood Pressure Variability and Cerebral Small Vessel Disease: A Systematic Review and Meta-Analysis of Population-Based Cohorts. Stroke. 2020;51:82–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ma Y, Yilmaz P, Bos D, Blacker D, Viswanathan A, Ikram MA, et al. Blood Pressure Variation and Subclinical Brain Disease. Journal of the American College of Cardiology. 2020;75:2387–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


