Abstract
Debaryomyces hansenii is a yeast species that is known for its halotolerance. This organism has seldom been mentioned as a pentose consumer. In the present work, a strain of this species was investigated with respect to the utilization of pentoses and hexoses in mixtures and as single carbon sources. Growth parameters were calculated for batch aerobic cultures containing pentoses, hexoses, and mixtures of both types of sugars. Growth on pentoses was slower than growth on hexoses, but the values obtained for biomass yields were very similar with the two types of sugars. Furthermore, when mixtures of two sugars were used, a preference for one carbon source did not inhibit consumption of the other. Glucose and xylose were transported by cells grown on glucose via a specific low-affinity facilitated diffusion system. Cells derepressed by growth on xylose had two distinct high-affinity transport systems for glucose and xylose. The sensitivity of labeled glucose and xylose transport to dissipation of the transmembrane proton gradient by the protonophore carbonyl cyanide m-chlorophenylhydrazone allowed us to consider these transport systems as proton symports, although the cells displayed sugar-associated proton uptake exclusively in the presence of NaCl or KCl. When the Vmax values of transport systems for glucose and xylose were compared with glucose- and xylose-specific consumption rates during growth on either sugar, it appeared that transport did not limit the growth rate.
Debaryomyces hansenii is a xylose-utilizing yeast that exhibits an industrially interesting xylitol/ethanol production ratio (17, 20). Xylose fermentation and subsequent ethanol production have been studied in several other yeast species, including Pichia stipitis, Candida shehatae, and Pachysolen tannophilus, but the ethanol productivity of these organisms compares very poorly with that of Saccharomyces cerevisiae when glucose-based substrates are used (6, 18, 22, 26, 27). This is due to several factors, including the low resistance to ethanol stress of the yeasts (25). Optimization of biomass and xylitol production from xylose by yeasts has been studied by using batch and continuous cultures (3, 20). However, industrial substrates, such as the hemicellulose hydrolysates obtained from several sources, are usually mixtures of hexoses and pentoses. Upon hydrolysis, hemicellulose yields d-xylose as the major component, as well as other sugars, including d-glucose, d-mannose, d-galactose, and l-arabinose in variable combinations depending on the source of the raw material. Utilization of mixed substrates by yeasts has so far received little attention; the only exceptions have been studies of ethanol production from glucose-xylose mixtures by P. tannophilus (9) and consumption of xylose in complex sugar mixtures by several strains of S. cerevisiae (26). On the other hand, D. hansenii, which is an osmotolerant yeast, may be a very attractive microorganism for polyol production (1). The purpose of the present work was to evaluate growth parameters in mixed sugar cultures and to elucidate the underlying transport systems for the sugars and the corresponding regulation in D. hansenii INETI CL18.
MATERIALS AND METHODS
Microorganism and media.
D. hansenii INETI CL18, which was obtained from the Instituto Nacional de Engenharia e Tecnologia Industrial, Portugal, was originally isolated from sugarcane. It was maintained at 4°C in solid medium containing 2% (wt/vol) glucose, 2% (wt/vol) peptone, and 1% (wt/vol) yeast extract. Cells were cultivated in liquid mineral medium by using 0.5% (wt/vol) ammonium sulfate as the nitrogen source (23) and different carbon sources (d-glucose, d-galactose, d-mannose, d-xylose, and l-arabinose), as indicated below.
Culture conditions.
Batch cultures were grown with a liquid/air ratio of 1:5 and were incubated at 30°C with orbital shaking at 160 rpm (Certomat HK; B. Braun, Melsungen, Germany). Growth was monitored by measuring the optical density (absorbance at 640 nm) with a spectrophotometer (Spectronic 21; Bausch & Lomb) and by determining dry weight. The experiments were performed by taking 10-ml samples which were filtered through ME 25/41 ST mixed ester membranes (Schleicher and Schuell, Dassel, Germany) and then washed with an identical volume of distilled water and dried at 80°C overnight. The specific growth rates (μmax) during the exponential phase of growth were determined either by measuring the optical density or by determining the dry weight. Yield coefficients (YX/S) were based on dry weights and substrate concentrations in the stationary phase. Specific consumption rates for glucose or xylose were calculated by determining μmax/YX/S.
Estimating sugar concentrations in growth media.
Sugar concentrations in growth media were determined by high-performance liquid chromatography. The system used included a model 307 pump (Gilson, Villiers le Bel, France) and a model 132 RI detector (Gilson). Compounds were separated by using a Merck Polyspher OA KC column (catalog no. 51270) at 50°C; 1 mM sulfuric acid at a flow rate of 0.5 ml min−1 was the eluent. Quantification was performed by the internal standard method.
Measuring initial uptake rates.
Cells were harvested in the exponential phase of growth (optical density at 640 nm, 0.6 to 0.7) by centrifugation with a model 4K10 centrifuge (B. Braun, Osterod Harz, Germany), washed twice with 200 ml of ice-cold distilled water (5 min at 12,200 × g each time), and suspended at a final concentration of 20 to 25 mg (dry weight) ml−1 in ice-cold distilled water. To estimate the initial rates of uptake of labeled glucose and xylose at pH 5.0, we used a previously described method (14) and aqueous solutions of [U-14C]glucose and [U-14C]xylose having specific activities of 8.5 and 7.4 MBq mmol−1, respectively (3% ethanolic solutions; Amersham, Buckinghamshire, England). The concentration of the final cell suspension was 8 to 10 mg (dry weight) ml−1. The sampling times used were 0, 5, and/or 10 s; each experiment was repeated three times (the linearity of uptake was maintained for up to 20 s). Kinetic constants were estimated from Eadie-Hostee plots and were confirmed by a computer nonlinear regression analysis performed with GraphPad PRISM (GraphPad Software, Inc.). No quenching effects were observed in uptake experiments, even in the presence of high concentrations of NaCl.
The method used to estimate the initial rates of proton uptake after glucose or xylose was added in the absence of NaCl or in the presence of several NaCl concentrations was the method described previously (10). All of the experiments were performed at 30°C.
The effects of other sugars on uptake of glucose or xylose (14) were determined by using each sugar at concentrations of 200 and 20 mM to study inhibition of the low-affinity and high-affinity uptake systems, respectively. The effect of ethanol on sugar transport was determined, and the exponential inhibition constant (ki) (24, 25) and MIC of ethanol (Cmin) (24, 25) were also determined. To do this, cells were incubated for 2 min in the presence of different concentrations of ethanol (5 to 15%, vol/vol), after which uptake was assayed. The same method was used to determine the effect of the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP) (concentration in the assay mixture, 50 μM) on sugar transport. The effect of starvation was investigated by incubating the cells in mineral medium without a carbon source at 30°C for different periods of time. Samples were centrifuged, washed twice in ice-cold distilled water, and assayed as described above. A cycloheximide MIC of 200 μg ml−1 was used to prevent protein synthesis during starvation in controls.
Reproducibility of the results.
All of the experiments were repeated at least three times with independent batches of cells. The standard deviations are presented below.
RESULTS
Growth in batch culture.
D. hansenii was grown on pentoses or hexoses as single carbon and energy sources, and growth parameters (μmax, YX/S, μmax/YX/S) were calculated (Table 1). Growth on glucose and growth on mannose resulted in similar growth rates, and growth on galactose resulted in a slightly lower value. Growth on xylose or arabinose was slower than growth on hexoses. In spite of the differences, the final biomass yields for all of the sugars assayed were similar.
TABLE 1.
Growth parameters for D. hansenii grown on single and mixed carbon sources (hexoses and pentoses)
| Carbon source(s)a | μmax (h−1) | YX/S (g · g−1) | μmax/YX/S (mmol · h−1 · g−1) |
|---|---|---|---|
| Single carbon sources | |||
| Glucose | 0.447 ± 0.047 (4)b | 0.448 ± 0.093 (3) | 5.519 |
| Mannose | 0.466 ± 0.030 (4) | 0.477 ± 0.054 (4) | 5.419 |
| Galactose | 0.369 ± 0.020 (4) | 0.437 ± 0.004 (4) | 4.671 |
| Xylose | 0.279 ± 0.022 (4) | 0.451 ± 0.062 (4) | 4.103 |
| Arabinose | 0.270 ± 0.022 (4) | 0.459 ± 0.119 (4) | 3.857 |
| Mixed carbon sources | |||
| Glucose-mannose | 0.404 ± 0.023 (4) | 0.323 ± 0.061 (3) | NDc |
| Xylose-arabinose | 0.334 ± 0.027 (3) | 0.469 ± 0.050 (3) | ND |
| Glucose-xylose | 0.405 ± 0.032 (4) | 0.368 ± 0.035 (4) | ND |
The initial concentration of each sugar was 10 g liter−1.
The numbers in parentheses are the numbers of independent experiments performed.
ND, not determined.
Growth on mixtures of two sugars (1% [wt/vol] each) was investigated by using all of the possible combinations of the hexoses and pentoses mentioned above. Representative results obtained with mixtures of two hexoses, mixtures of two pentoses, and mixtures of one hexose and one pentose are presented in Table 1. The biomass yields obtained with hexose and hexose-pentose mixtures were slightly lower than the yields obtained with the same amount of sugar alone. Diauxic growth with similar growth parameters was observed when glucose was mixed with either mannose or galactose, and the glucose was consumed first. With all of the other mixtures, consumption of the two sugars was nondiauxic; the beginning of consumption of the second substrate generally followed a lag phase that was longer than the lag phase for the first substrate, after which consumption of the two sugars proceeded simultaneously. For example, in the case of the glucose-xylose mixture, xylose consumption began only when the glucose concentration was less than 20% of the original concentration. However, the same μmax was observed in both phases of growth (Table 1). The experiments were repeated with a lower concentration (0.1%, wt/vol) of each sugar, but still no distinct μmax value was determined during the second growth phase. Similar results were obtained for all of the other mixtures examined. Utilization of hexoses was preferred to utilization of pentoses, in the following order: glucose, mannose, galactose, xylose, arabinose.
With hexose-arabinose or pentose-arabinose mixtures, no consumption of arabinose was observed unless the medium pH was readjusted from pH 2.2 to 2.5 to pH 5.5 (initial pH of the growth medium) after the first carbon source was consumed. The influence of the initial pH on consumption of arabinose as a single carbon source was examined at pH values between pH 1.8 and 7.3 in approximately 0.5-pH unit increments. The μmax varied, and the optimum μmax was observed when the initial pH was 5.2. At initial pH values below 2.6 no growth was detected.
Glucose and xylose transport on glucose-grown cells.
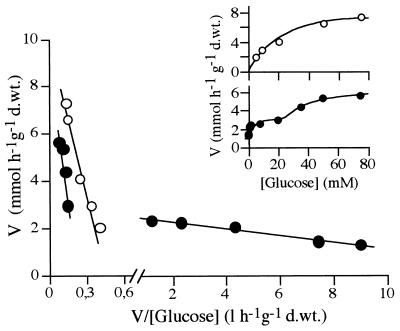
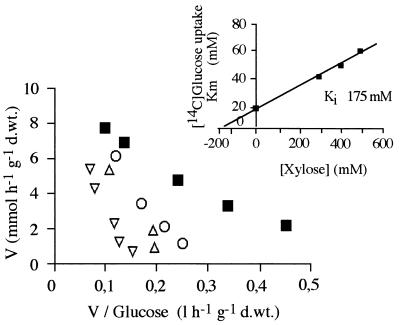
Uptake of glucose (Fig. 1) and uptake of xylose (data not shown) by cells of D. hansenii growing on glucose and collected in the mid-exponential phase exhibited Michaelis-Menten kinetics. Both transport systems had low affinities for their substrates; the Km for glucose was approximately eight times lower than the Km for xylose (Table 2), whereas the Vmax values for the two sugar transport systems were very similar. Xylose inhibited glucose uptake competitively (Fig. 2), with a Ki of 175 mM. Galactose, arabinose, mannose, and 2-deoxyglucose were also tested as potential inhibitors of glucose transport but had no effect.
FIG. 1.
Eadie-Hofstee plot and direct plot (inset) of initial rates of uptake of labeled glucose in glucose-grown cells (○) and xylose-grown cells (●). d.wt., dry weight.
TABLE 2.
Kinetic parameters for glucose and xylose transport systems in D. hansenii grown on glucose or xylose
| Carbon source for growth |
14C-labeled substrate uptake parameters
|
|||
|---|---|---|---|---|
| Glucose
|
Xylose
|
|||
| Km (mM) | Vmax (mmol · h−1 · g [dry wt]−1) | Km (mM) | Vmax (mmol · h−1 · g [dry wt]−1) | |
| Glucose | 18.5 ± 2.3 (4)a | 8.6 ± 0.7 (4) | 140.0 ± 17.0 (3) | 10.4 ± 1.7 (4) |
| Xylose | 0.2 ± 0.03 (4) | 2.2 ± 0.4 (4) | 0.8 ± 0.2 (4) | 1.4 ± 0.3 (4) |
| 25.0 (2) | 7.6 (2) | NDb | 10.7 (2) | |
The numbers in parentheses are the numbers of independent experiments performed.
ND, not determined.
FIG. 2.
Inhibition of low-affinity glucose transport in glucose-grown cells by xylose. Symbols: ■, no xylose; ○, 300 mM xylose; ▵, 400 mM xylose; ▿, 500 mM xylose. (Inset) Effect of xylose concentration on Km for glucose. d.wt., dry weight.
The initial glucose uptake rates were approximately constant and did not exhibit any definite variation at external pH values ranging from 3.0 to 7.0, the Vmax value being 8.86 ± 1.31 mmol h−1 g (dry weight)−1. Furthermore, when uptake was measured in the presence of the protonophore CCCP, the Vmax values were about the same as the Vmax values in the absence of CCCP (data not shown). Ethanol inhibited the initial rates of uptake of glucose and xylose in a noncompetitive way. Vmax decreased exponentially with the ethanol concentration, which is consistent with the equation obtained for other mediated transport systems (24, 25). On the basis of the results of these experiments, a ki value for ethanol of 0.6 M−1 was estimated, the ethanol Cmin being approximately zero.
Glucose and xylose transport on xylose-grown cells.
We also measured transport of glucose and xylose in cells of D. hansenii growing exponentially on xylose. With these cells, the Eadie-Hofstee plots of the initial rates of glucose uptake (Fig. 1) and xylose uptake (data not shown) were biphasic. The lower-affinity component had kinetic parameters similar to the parameters obtained for the low-affinity glucose-xylose uptake observed with glucose-grown cells (Table 2). In addition to the low-affinity component, a higher-affinity system for glucose seemed to operate in xylose-grown cells. Similar results were obtained for xylose transport. The kinetic parameters estimated for these systems are shown in Table 2. The Km and Vmax values for the higher-affinity transport of glucose were different from the values for xylose uptake. Mannose competitively inhibited high-affinity glucose transport (Ki, 0.38 mM), whereas galactose, xylose, and arabinose did not. On the other hand, xylose uptake was not inhibited by any of these sugars (data not shown).
The Km values for the high-affinity glucose and xylose transport systems were not affected by the extracellular pH (the pH was varied from 3.0 to 7.0), while the Vmax for either glucose or xylose uptake decreased slightly at pH values below 5.0 (data not shown). Both the glucose transport system and the xylose transport system were strongly inhibited by the protonophore CCCP (the Vmax values decreased 82 and 67%, respectively). Both glucose uptake and xylose uptake were inhibited by ethanol in a noncompetitive way. As observed with glucose-grown cells, the Vmax values decreased exponentially with the ethanol concentration; in these experiments the ki values for ethanol were 0.98 and 0.80 M−1 for glucose transport and xylose transport, respectively, and the ethanol Cmin values were 860 mM and almost zero for glucose transport and xylose transport, respectively.
Regulation of glucose and xylose transport systems.
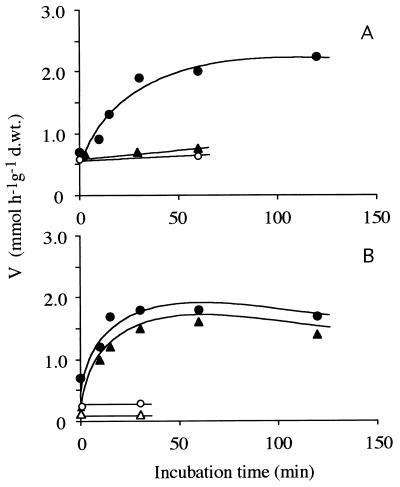
Carbon source starvation of glucose-grown cells in mineral medium for 2 h resulted in a gradual increase in the activity of the high-affinity transport system for glucose (Fig. 3), which was prevented by the presence of cycloheximide. Transfer of glucose-grown cells to mineral medium containing 2% xylose resulted (within 10 min) in the formation of both the high-affinity transport system for glucose and the high-affinity transport system for xylose (Fig. 3), which was again prevented by cycloheximide.
FIG. 3.
(A) Effect of starving glucose-grown cells in mineral medium without a carbon source on the formation of the high-affinity transport system for glucose. Symbols: ● and ○, [U-14C]glucose; ▴, [U-14C]xylose. (B) Appearance of the high-affinity transport systems for glucose and xylose: Vmax values for [U-14C]glucose (● and ○) and [U-14C]xylose (▴ and ▵) after glucose-grown cells were transferred to medium containing 2% xylose. Open symbols, cell suspensions incubated in the presence of cycloheximide; solid symbols, cell suspensions incubated in the absence of cycloheximide. d.wt., dry weight.
H+ movements associated with sugar uptake.
In many cases, when the mechanism of sugar transport in yeasts is an H+ symport, transient alkalinization of an aqueous cell suspension occurs during initial uptake of the substrate (13). In cells of D. hansenii grown on any of the hexoses or pentoses mentioned above as a single carbon source, addition of glucose, mannose, galactose, xylose, or arabinose did not result in extracellular alkalinization. However, in xylose-grown cells incubated for 2 min in the presence of 1 M NaCl or 1 M KCl (but not in the presence of 1 M LiCl, 1 M MgCl2, or 1 M CaCl2), addition of glucose, mannose, galactose, or xylose elicited alkalinization. The initial proton uptake rates followed saturation kinetics, and the corresponding Km and Vmax values for glucose and xylose, calculated from Eadie-Hofstee plots, are presented in Table 3. The Km values obtained were identical to the corresponding Km values estimated with labeled sugars, but the Vmax values were considerably lower than the values shown in Table 2. Labeled glucose and xylose uptake parameters were reestimated by incubating cell suspensions in buffer containing 1 M NaCl (Table 3). The Km values obtained in this way did not differ from the Km values determined in the absence of NaCl (Table 2), but the Vmax values decreased, reaching levels close to those for proton uptake. Hence, one proton per glucose or xylose molecule was transported in the presence of 1 M NaCl.
TABLE 3.
Kinetic parameters for proton and sugar uptake rates in D. hansenii
| Salt (1 M) | Proton uptake elicited by the addition of:
|
14C-sugar uptake
|
||||||
|---|---|---|---|---|---|---|---|---|
| Glucose
|
Xylose
|
Glucose
|
Xylose
|
|||||
| Km (mM) | Vmax (mmol · h−1 · g [dry wt]−1) | Km (mM) | Vmax (mmol · h−1 · g [dry wt]−1) | Km (mM) | Vmax (mmol · h−1 · g [dry wt]−1) | Km (mM) | Vmax (mmol · h−1 · g [dry wt]−1) | |
| NaCl | 0.12 ± 0.04 (7)a | 1.08 ± 0.21 (7) | 0.78 ± 0.18 (5) | 0.82 ± 0.17 (8) | 0.16 ± 0.04 (3) | 1.00 ± 0.20 (3) | 0.11 ± 0.21 (3) | 0.70 ± 0.07 (3) |
| KCl | 0.26 ± 0.07 (4) | 1.28 ± 0.11 (5) | 0.85 ± 0.08 (3) | 0.86 ± 0.21 (5) | NDb | ND | ND | ND |
The numbers in parentheses are the numbers of independent experiments performed.
ND, not determined.
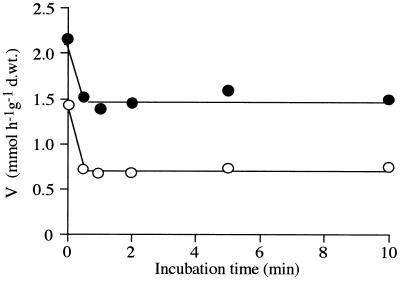
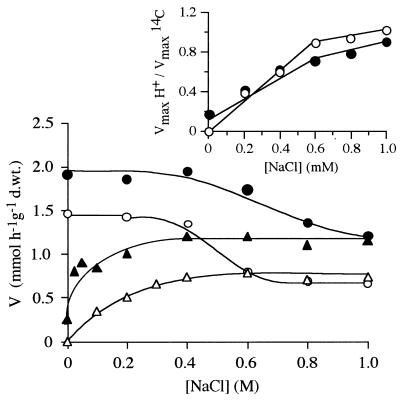
The minimum incubation period in the presence of 1 M NaCl for detection of a lower Vmax assayed, 30 s, was still long enough to determine the observed decrease in the Vmax, as shown in Fig. 4. The Vmax of proton uptake increased with increasing salt concentration (Fig. 5). The 1:1 proton-sugar stoichiometry mentioned above was valid only for salt concentrations greater than 600 to 800 mM (Fig. 5).
FIG. 4.
Effects of incubation with 1 M NaCl on the Vmax values of the high-affinity transport systems for radiolabeled glucose (●) and xylose (○). d.wt., dry weight.
FIG. 5.
Vmax values for [U-14C]glucose (●) and [U-14C]xylose (○) and proton uptake after glucose (▴) and xylose (▵) were added as a function of NaCl concentration in suspensions of xylose-grown cells. (Inset) Ratio between Vmax from proton uptake and labeled glucose (●) or xylose (○) uptake as a function of NaCl concentration in the assay mixture. d.wt., dry weight.
No extracellular alkalinization elicited by glucose or xylose was detected in glucose-grown cell suspensions incubated in the absence and in the presence of NaCl or KCl.
DISCUSSION
Our data show that growth of D. hansenii on glucose or mannose resulted in approximately the same μmax and the same biomass yield. On the other hand, the growth rate on xylose or arabinose was lower, whereas similar biomass yields were obtained. With sugar mixtures, either diauxic or nondiauxic sugar consumption was observed. The same type of nondiauxic growth of P. tannophilus was observed when a glucose-xylose mixture was used (9). In D. hansenii, pentose metabolism and hexose metabolism proceed without any particular drawbacks, in contrast to what has been described previously for other yeasts (2, 18, 22, 26), since, disregarding the type of consumption of each sugar mixture, the yields are rather similar. Our data suggest that mixtures of hexoses and pentoses, such as those present in hemicellulose hydrolysates, are probably completely consumed by D. hansenii as long as pH of the medium can be maintained close to 4 to 5. Hemicellulose extracts used industrially usually undergo acid hydrolysis, but the pH of the solution is normally neutralized with CaCO3.
When D. hansenii was grown on glucose, it exhibited a low-affinity glucose transport system. The absence of simultaneous proton uptake, the insensitivity of glucose uptake to CCCP and to changes in the external pH, and the relatively low level of inhibition by ethanol led us to conclude that glucose uptake occurs by facilitated diffusion. Xylose competitively inhibited glucose transport and had a Ki identical to its transport Km, which suggests that xylose shares glucose-facilitated diffusion with even lower affinity.
In contrast, D. hansenii cells induced by growth on xylose were different. Radiolabeled glucose and xylose had uptake kinetic parameters whose affinity was much higher than the affinity in glucose-grown cells and did not act as mutual inhibitors, which indicated that these sugars are probably transported by different permeases. Both sugar transport systems in these cells were inhibited by the protonophore CCCP. The inhibition by ethanol was characterized by ki values higher than the values determined for facilitated diffusion in glucose-grown cells but lower than the previously published values for active transporters of the proton symport type (21, 25). This finding is probably related to the fact that in xylose-grown cells glucose and xylose are taken up by two transport systems that have different sensitivities for ethanol, the reason why the ki for ethanol has an intermediate value. This is not a result that allows one to distinguish easily between facilitated diffusion and active transport. However, it reinforces the conclusion that permeases are involved in sugar uptake and corroborates other data.
Uptake of mannose also occurred via the glucose transport system, while the xylose transport system did not transport any of the other monosaccharides and thus was apparently specific for this sugar. Also, in C. shehatae, facilitated diffusion and sugar proton symports have distinct specificities for different pentoses and hexoses (14).
The specific rate of consumption of glucose by D. hansenii was lower than the glucose transport capacity (facilitated diffusion Vmax) (Tables 1 and 2), suggesting that glucose transport does not limit growth on this sugar. For cells growing on xylose, the specific rate of consumption of this sugar was considerably higher than the Vmax of the high-affinity transport system, indicating that glucose-xylose-facilitated diffusion could also play an important role in sustaining growth on xylose. Consistent with these conclusions is the finding that growth in mixtures of glucose and xylose was nondiauxic; this means that as soon as a low concentration of glucose in the growth medium is reached, xylose may compete with glucose transport by the facilitated diffusion system. This should enable induction (in the presence of glucose) of xylose high-affinity transport, as well as the xylose catabolism-specific enzymes (5, 12).
The concentrative monosaccharide transport systems usually have been described as proton symports that are driven by the proton motive force generated by the plasma membrane H+ ATPase (for example, the H+-xylose symport described in E. coli [11] and the H+-glucose and H+-xylose symports in different yeasts [4, 7, 13, 14]). Surprisingly, in D. hansenii, no proton uptake was detected when glucose or xylose was added to xylose-grown cell suspensions. If we take into consideration (i) the fact that D. hansenii is a halotolerant yeast (1, 19), (ii) the fact that it has been postulated that a Na+-glycerol symport occurs in this yeast (15), and (iii) the fact that this yeast has been described as an organism that regulates K+ and Na+ intracellular contents as an even interchange, substituting one ion for the other and generating ion potential from high intracellular sodium contents (16, 19), it is not unlikely that glucose and xylose high-affinity transport systems are affected by a salt gradient over the plasma membrane. Proton uptake was detected exclusively in the presence of salt, and at salt concentrations above a certain level stoichiometry was determined. The results favored recognition of the glucose and xylose high-affinity transport systems as proton symports that may indirectly depend on the presence of salt to determine sensible variations in the proton motive force, which can be critical for proton uptake detection.
Starvation resulted in gradual induction of the high-affinity glucose-proton symport, whereas transfer of glucose-grown cells to xylose-containing medium resulted in the gradual appearance of high-affinity glucose- and xylose-proton symports. On the basis of these results we concluded that the glucose-proton symport was subject to glucose repression, while the xylose-proton symport requires induction by the substrate. This type of transport regulation is similar to what has been described previously for glucose and xylose transport in C. shehatae (14) and P. stipitis (7), as well as for xylose transport in Candida utilis (8). Furthermore, the results obtained in the transport studies performed with D. hansenii were consistent with the pattern observed for consumption of mixed substrates. In conclusion, we stress that the results obtained in this study support the hypothesis that D. hansenii is a good candidate for biodegradation of hemicellulose hydrolysates and, therefore, for further biochemical engineering in which the goal is improving xylose consumption and xylitol production.
ACKNOWLEDGMENTS
This work was financed in part by European Union project BIOTECH PL 95016. A. P. Nobre was the recipient of Ph.D. grant PRAXIS XXI/BD/3488/94.
REFERENCES
- 1.Adler L, Gustafsson L. Polyhydric alcohol production and intracellular amino acid pool in relation to halotolerance of the yeast Debaryomyces hansenii. Arch Microbiol. 1980;124:123–130. [Google Scholar]
- 2.Delgenes J P, Moletta R, Navarro J M. Fermentation of d-xylose, d-glucose and l-arabinose mixture by Pichia stipitis Y 7124: sugar tolerance. Appl Microbiol Biotechnol. 1988;29:155–161. [Google Scholar]
- 3.Duarte L C, Nobre A P, Gírio F M, Amaral-Collaço M T. Determination of the kinetic parameters in continuous cultivation by Debaryomyces hansenii grown in d-xylose. Biotechnol Tech. 1994;8:859–864. [Google Scholar]
- 4.Gasnier B. Characterisation of low- and high-affinity glucose transports in the yeast Kluyveromyces marxianus. Biochim Biophys Acta. 1987;903:425–433. doi: 10.1016/0005-2736(87)90049-6. [DOI] [PubMed] [Google Scholar]
- 5.Gírio F M, Pelica F, Amaral-Collaço M T. Characterization of xylitol dehydrogenase from Debaryomyces hansenii. Appl Biochem Biotechnol. 1996;56:7987. [Google Scholar]
- 6.Hahn-Hägerdal B, Jeppsson H, Skoog K, Prior B. Biochemistry and physiology of xylose fermentation by yeasts. Enzyme Microb Technol. 1994;16:933–943. [Google Scholar]
- 7.Kilian S G, van Uden N. Transport of xylose and glucose in the xylose-fermenting yeast Pichia stipitis. Appl Microbiol Biotechnol. 1988;27:545–548. [Google Scholar]
- 8.Kilian S G, Prior B A, du Preez J C. The kinetics and regulation of d-xylose transport in Candida utilis. World J Microbiol Biotechnol. 1993;9:357–360. doi: 10.1007/BF00383080. [DOI] [PubMed] [Google Scholar]
- 9.Kruse B, Schügerl K. Investigation of ethanol formation by Pachysolen tannophilus from xylose and glucose/xylose co-substrates. Process Biochem. 1996;31:389–407. [Google Scholar]
- 10.Lages F, Lucas C. Characterization of a glycerol/H+ symport in the halotolerant yeast Pichia sorbitophila. Yeast. 1995;11:111–119. doi: 10.1002/yea.320110203. [DOI] [PubMed] [Google Scholar]
- 11.Lam V M S, Daruwalla K R, Henderson P J F, Jones-Mortimer M C. Proton-linked d-xylose transport in Escherichia coli. J Bacteriol. 1980;143:396–402. doi: 10.1128/jb.143.1.396-402.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H, Sopher C R, Yau K Y F. Induction of xylose reductase and xylitol dehydrogenase activities on mixed sugars in Candida guilliermondii. J Chem Tech Biotechnol. 1996;66:375–379. [Google Scholar]
- 13.Loureiro-Dias M C. Movements of protons coupled to glucose transport in yeasts. A comparative study among 248 yeast strains. Antonie Leeuwenhoek. 1988;54:331–343. doi: 10.1007/BF00393524. [DOI] [PubMed] [Google Scholar]
- 14.Lucas C, van Uden N. Transport of hemicellulose monomers in the xylose-fermenting yeast Candida shehatae. Appl Microbiol Biotechnol. 1986;23:491–495. [Google Scholar]
- 15.Lucas C, da Costa M, van Uden N. Osmoregulatory active sodium-glycerol co-transport in the halotolerant yeast Debaryomyces hansenii. Yeast. 1990;6:187–191. [Google Scholar]
- 16.Neves M L, Oliveira R P, Lucas C M. Metabolic flux response to salt-induced stress in the halotolerant yeast Debaryomyces hansenii. Microbiology. 1997;143:1133–1139. doi: 10.1099/00221287-143-4-1133. [DOI] [PubMed] [Google Scholar]
- 17.Parajó J C, Dominguez H, Dominguez J M. Production of xylitol from raw wood hydrolysates by Debaryomyces hansenii NRRL Y-7426. Bioprocess Eng. 1995;13:125–131. [Google Scholar]
- 18.Prior B A, Kilian S G, du Preez J C. Fermentation of d-xylose by the yeasts Candida shehatae and Pichia stipitis. Process Biochem. 1989;89:21–32. [Google Scholar]
- 19.Prista C, Almagro A, Loureiro-Dias M C, Ramos J. Physiological basis for high salt tolerance of Debaryomyces hansenii. Appl Environ Microbiol. 1997;63:4005–4009. doi: 10.1128/aem.63.10.4005-4009.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roseiro J C, Peito M A, Amaral-Collaço M T. The effects of the oxygen transfer coefficient and substrate concentration on the xylose fermentation by Debaryomyces hansenii. Arch Microbiol. 1991;156:484–490. [Google Scholar]
- 21.Spencer-Martins I. Transport of sugars in yeasts: implications in the fermentation of lignocellulosic materials. Bioresource Technol. 1994;50:51–57. [Google Scholar]
- 22.van Dijken J P, Scheffers W A. Redox balances in the metabolism of sugars by yeasts. FEMS Microbiol Rev. 1986;32:199–224. [Google Scholar]
- 23.van Uden N. Transport limited fermentation and growth of Saccharomyces cerevisiae and its competitive inhibition. Arch Microbiol. 1967;58:155–168. doi: 10.1007/BF00406676. [DOI] [PubMed] [Google Scholar]
- 24.van Uden N. Ethanol toxicity and ethanol tolerance in yeasts. Annu Rep Ferment Processes. 1985;8:11–58. [Google Scholar]
- 25.van Uden N. Alcohol toxicity in yeasts and bacteria. New York, N.Y: CRC Press, Inc.; 1989. [Google Scholar]
- 26.van Zyl C, Prior B A, Kilian S G, Kock J L. d-Xylose utilization by Saccharomyces cerevisiae. J Gen Microbiol. 1989;135:2791–2798. doi: 10.1099/00221287-135-11-2791. [DOI] [PubMed] [Google Scholar]
- 27.van Zyl C, Prior B A, Kilian S G, Brandt V. Role of d-ribose in d-xylose metabolism by Saccharomyces cerevisiae. Appl Environ Microbiol. 1993;59:1487–1494. doi: 10.1128/aem.59.5.1487-1494.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]