Abstract
Background
This study aimed to investigate the effects of hypoxia and normoxia preconditioning in rabbit intervertebral disc-derived stem cells (IVDSCs) and discus-derived conditioned medium (DD-CM)/secretomes in vitro. Transforming growth factor (TGF)-β1, platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF) have a role in the proliferation, development, differentiation, and migration of MSCs.
Materials and Methods
Intervertebral discs were isolated from rabbit and incubated in normoxia and hypoxia 1%, 3%, and 5% (hypoxia groups) condition. Cell counting was performed after 24 hours of manipulation, then analyzed using one-way ANOVA. TGF-β1, PDGF, FGF, and VEGF were measured using the ELISA.
Results
The highest number of cells was in the hypoxia 3% preconditioning compared to the normoxia, hypoxia 1%, and hypoxia 5% groups. Hypoxia 3% also had the highest increase in PDGF protein production compared to normoxia, with hypoxia 1% and 5%. Among hypoxia groups, the highest secretions of VEGF and FGF proteins were in the hypoxia 3% group. Based on TGF-β1 protein measurement, the hypoxia 1% group was the highest increase in this protein compared to other groups.
Conclusion
Oxygen level in hypoxia preconditioning has a role in the preparation of IVDSCs and secretome preparation in vitro. The highest cell numbers were found in the treatment group with 3% hypoxia, and 3% hypoxia was significantly related to support IVDSCs preparation. Preconditioning with 3% hypoxia had higher PDGF and VEGF levels than other hypoxia groups.
Keywords: intervertebral disc-derived stem cells, secretomes, growth factors, hypoxia, normoxia
Introduction
Low back pain (LBP) is a common musculoskeletal disorder that affects socio-economic aspects, both directly and indirectly.1–3 This is the most common chief complaint, with an estimate that more than 50% of adults have complained about LBP throughout their life.2,4 The most common cause in LBP is intervertebral disc degeneration (IDD). IDD is a degenerative skeletal disorder that can be natural or pathological process in the human spine.1,4,5 A previous study estimated that the prevalence of IDD reaches 266 million people worldwide.3
IDD management has been very varied, and generally only relieved pain complaints in patients.5–8 The most common initial management used is physical therapy, education, and pain medication.4,7,8 Surgery is advanced management in IDD, by disc excision or arthrodesis procedures. These treatments only focus on relieving pain complaints without regenerating the disc structure or function.1,4,9 The side effects of these treatments may accelerate the degenerative process.9
One of the latest methods that are developing and quite promising in the management of IDD is trigger regeneration in IDD with mesenchymal stem cells (MSCs).1,5,10,11 The transplanted MSCs are expected to repair, maintain, and increase the ability of regeneration so it could stop or even reverse the degeneration process.1,4,5,9 One of the MSCs developed is intervertebral disc-derived stem cells (IVDSCs). IVDSCs are resident SCs in normal or degenerated IVD.1,12 Research on various test animals has shown good results in IVD regeneration.13 IVDSCs can differentiate into various cytotypes belonging to osteogenic and chondrogenic.12 In addition, IVDSCs have a good ability to withstand IDD extreme microenvironment conditions.14
The ability of MSCs in regenerative medicine is influenced by many factors, secretomes in the form of growth factors (GFs) are one of them.15 MSCs secrete secretomes in the form of GFs that are pro-angiogenic such as transforming growth factor (TGF)-β1, platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF).4,15,16 The increase of these secretomes can enhance the therapeutic effects.15 MSCs secretomes play a role in immunomodulation, anti-inflammatory, inhibiting catabolic activity, neuroprotective, neurotrophic, anti-apoptotic, stimulating extracellular matrix production, and angiogenesis regulation.16–18 Those mechanisms trigger IVD regeneration by modulating nucleus pulposus gene expression, stimulating the IVD progenitor cell differentiation, and increasing disc cell viability.17 These secretomes help the proliferation, development, differentiation, and migration of MSCs.16,18 Without exogenous manipulation, MSCs only secrete a limited amount of GFs and will not have the maximum effect because of their poor survival.15
The key to the success of MSCs, so they can be used in the regeneration process, depends on the survivability and proliferation of the MSCs.15 The original microenvironment of IVD with low oxygen levels, low nutrition, and a heavy mechanical burden is a challenge in the use of MSCs in IDD.6 The IDD microenvironment is more extreme than healthy IVD, which makes it a challenge for transplanted MSCs.19 Preconditioning of MSCs has a role in regulating the proliferation of secretome secretion. One of the most effective and widely used preconditioning is to manipulate the oxygen condition into hypoxia because it can increase secretion and proliferation.16 MSCs cultured under hypoxia (2–3%) conditions showed an increase in differentiation.4 This method also can increase angiogenesis and decrease apoptosis, which has a role in the MSCs survival.20
The therapeutic efficacy of MSCs, including IVDSCs, depends on the number of implanted cells and secretomes secreted by MSCs.15 As one of the MSCs, preconditioning with hypoxia is expected to affect IVDSCs. The study on the effect of hypoxia preconditioning on IVDSCs is limited compared to MSCs from other sources.1 This study aimed to investigate the effects of hypoxia and normoxia preconditioning on rabbit IVDSCs and discus-derived conditioned medium (DD-CM)/secretome in vitro.
Materials and Methods
This study was carried out following the guidelines for medical ethics and research of the Animal Care and Use Committee, Faculty of Veterinary Medicine, Airlangga University, Indonesia, No. 2.KE.098.11.2020.
Rabbit Intervertebral Disc Cells Culture
We used a single rabbit and took the annulus fibrosus tissue for intervertebral disc cell isolation, then we cultured it. Rabbit intervertebral disc was harvested from a rabbit lumbar disc, in a Dulbecco’s phosphate-buffered saline (DPBS) with 1% antibiotic antimycotic (Gibco, Thermo Fisher Scientific, USA), then the intervertebral disc was washed with DPBS in a sterile petri dish in a bio-safety locker. The middle-third of the discs were raked carefully using a bard parker’s blade number 15 into a sterile petri-dish. The tissues collected were examined and crumbled into 1 mm3 when required. Freshly prepared 3 mg/mL collagenase type I solution (Gibco, Thermo Fisher Scientific, USA) and 4 mg/mL of dispase II (Gibco) were used for the enzymatic process with incubation at 37° C in 5% carbon dioxide for 45 minutes. An equal amount of Alfa modified eagle’s medium (Alfa MEM, Gibco, USA) was added to neutralize the action of the enzyme collagenase I. To prepare a single-cell suspension, the mixture was passed through a 100 mmeter strainer (NEST, China), then it was transferred to a 100 mm petri dish (Iwaki, USA) containing 1% amphotericin B (Gibco, USA), Alfa modified eagle’s medium (Alfa MEM, Gibco, USA), 1% penicillin-streptomycin (Gibco, USA), and 20% fetal bovine serum (Gibco, USA). The petri dish contained cultured cells was conditioned in a humidified atmosphere of 5% carbon dioxide and 37°C for two weeks. The cultured cells were analyzed using a microscope (Olympus CKX53, Japan) at 4x magnification and the medium was replaced every three days. After being conditioned, the cell has raised to 80% of confluence, and the cells were passaged. The research was carried out using passage 4 cells for all groups.
Hypoxia Manipulation
The petri dishes containing cultured cells were moved to an airtight incubator (Esco Celculture CO2 Incubator, Singapore). The setting of the incubator was a water-saturated gas mixture of 1%, 3%, and 5% oxygen (according to the group), 5% carbon dioxide, and 94% nitrogen, which was used to simulate hypoxia conditions in the hypoxia group. The incubator was set at 37°C. The incubator was set to maintain these conditions automatically. After 24 hours, the medium was gathered for cell counting.
Cells Counting
Cells counting was performed after 24 hours of manipulation of every petri dish of normoxia and 1%, 3%, 5% hypoxia. The cells were counted using an automatic cell counter TC20 (Bio-Rad, USA) to confirm total live cells and cell viability.
VEGF, FGF, TGF-β1, and PDGF Measurement
Measurement of secretomes (TGF-β1, VEGF, FGF, and PDGF)/growth factor level in DD-CM were performed in each group using ELISA Assay (Bioassay Technology Laboratory, E0026Rb (VEGF); E0227Rb (FGF); E0133Rb (TGF-β1); E0052Rb (PDGF)). DD-CM was collected using sterile tubes. The cell culture supernatant was obtained from DD-CM, which had been centrifuged at 2000–3000 rpm for 20 minutes. Incubation was carried out for 60 minutes at 37°C using sample wells. The composition was a 40μL sample with 10μL of anti-secretome antibody, then 50μL of streptavidin-HRP. The next step was washing the palate using the 0.35 mL wash buffer for 30–60 seconds. This step was repeated 5 times. For automated washing, aspirate all wells and wash 5 times with a wash buffer. The wells were overfilled with a wash buffer. The plate was dried up with absorbent material like paper towels. Each well was given 50μL of the substrate solution A and then combined with 50μL substrate solution B. The plates were incubated for 10 minutes at 37°C in the dark. After incubation, 50μL Stop Solution was added to each well. Then set up Optical Density (OD value) of every well directly with a microplate reader set to 450 nm within 10 minutes (Bioassay Technology Laboratory, China).
Statistical Analysis
Analyses were performed using SPSS version 26.0 (IBM Corp., Armonk, NY). A one-way analysis of variance (ANOVA) was used to analyze the differences among groups with the significance level set at p < 0.05 and 95% confidence interval (CI), along with Tukey post hoc test and Bonferroni test. The dependent variable used in the analysis is the number of cells.
Results
Proliferation Rate
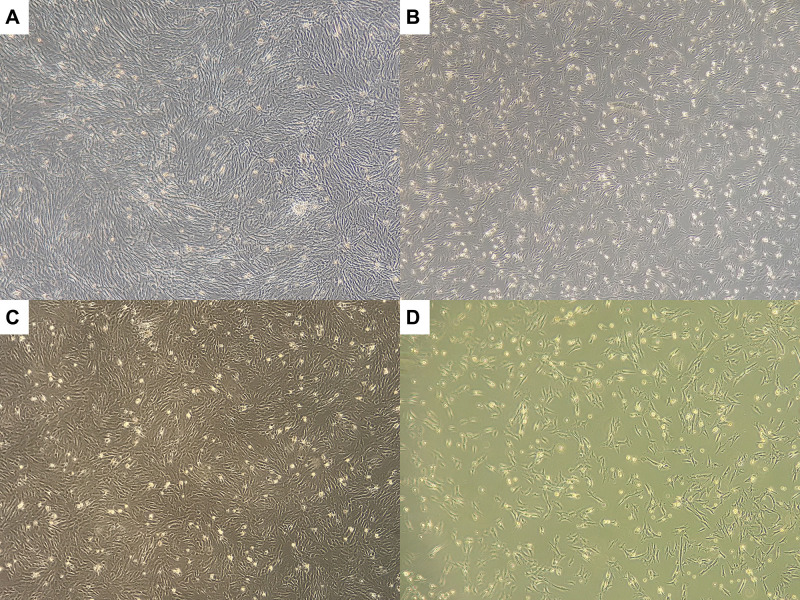
Analysis of the cell proliferation in each group was presented. The highest mean proliferation rate was found in the hypoxia 3% group compared with normoxia, hypoxia 1%, and 5% groups (Figure 1 and Table 1). The increase in the mean proliferation rate in the hypoxia group 3% was significantly different compared to the other groups (Table 2).
Figure 1.
The cell proliferation in each group. (A) Normoxia. (B) Hypoxia 1%. (C) Hypoxia 3% (D) Hypoxia 5%.
Table 1.
One Way ANOVA for Cell Count Data
| Group | Cells Number (Mean ± SD) |
|---|---|
| Normoxia | 2.20 ± 0.09 x 106 |
| Hypoxia 5% | 1.39 ± 0.13 x 106 |
| Hypoxia 3% | 2.94 ± 0.67 x 106 |
| Hypoxia 1% | 2.07 ± 0.58 x 106 |
Note: Mean ± SD.
Table 2.
One Way ANOVA Analysis for Cell Proliferation
| (I) Group | (J) Group | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| Tukey HSD | Normoxia | Hypoxia 5% | 0.81000* | 0.20863 | 0.020 | 0.1419 | 1.4781 |
| Hypoxia 3% | −0.74000* | 0.20863 | 0.031 | −1.4081 | −0.0719 | ||
| Hypoxia 1% | 0.70000* | 0.20863 | 0.040 | 0.0319 | 1.3681 | ||
| Hypoxia 5% | Normoxia | −0.81000* | 0.20863 | 0.020 | −1.4781 | −0.1419 | |
| Hypoxia 3% | −1.55000* | 0.25551 | 0.001 | −2.3682 | −0.7318 | ||
| Hypoxia 1% | −0.11000 | 0.25551 | 0.972 | −0.9282 | 0.7082 | ||
| Hypoxia 3% | Normoxia | 0.74000* | 0.20863 | 0.031 | 0.0719 | 1.4081 | |
| Hypoxia 5% | 1.55000* | 0.25551 | 0.001 | 0.7318 | 2.3682 | ||
| Hypoxia 1% | 1.44000* | 0.25551 | 0.002 | 0.6218 | 2.2582 | ||
| Hypoxia 1% | Normoxia | −0.70000* | 0.20863 | 0.040 | −1.3681 | −0.0319 | |
| Hypoxia 5% | 0.11000 | 0.25551 | 0.972 | −0.7082 | 0.9282 | ||
| Hypoxia 3% | −1.44000* | 0.25551 | 0.002 | −2.2582 | −0.6218 | ||
| Bonferroni | Normoxia | Hypoxia 5% | 0.81000* | 0.20863 | 0.028 | 0.0842 | 1.5358 |
| Hypoxia 3% | −0.74000* | 0.20863 | 0.045 | −1.4658 | −0.0142 | ||
| Hypoxia 1% | 0.70000 | 0.20863 | 0.060 | −0.0258 | 1.4258 | ||
| Hypoxia 5% | Normoxia | −0.81000* | 0.20863 | 0.028 | −1.5358 | −0.0842 | |
| Hypoxia 3% | −1.55000* | 0.25551 | 0.002 | −2.4389 | −0.6611 | ||
| Hypoxia 1% | −0.11000 | 0.25551 | 1.000 | −0.9989 | 0.7789 | ||
| Hypoxia 3% | Normoxia | 0.74000* | 0.20863 | 0.045 | 0.0142 | 1.4658 | |
| Hypoxia 5% | 1.55000* | 0.25551 | 0.002 | 0.6611 | 2.4389 | ||
| Hypoxia 1% | 1.44000* | 0.25551 | 0.003 | 0.5511 | 2.3289 | ||
| Hypoxia 1% | Normoxia | −0.70000 | 0.20863 | 0.060 | −1.4258 | 0.0258 | |
| Hypoxia 5% | 0.11000 | 0.25551 | 1.000 | −0.7789 | 0.9989 | ||
| Hypoxia 3% | −1.44000* | 0.25551 | 0.003 | −2.3289 | −0.5511 | ||
Note: * The mean difference is significant at the 0.05 level.
FGF, PDGF, VEGF, and TGFβ-1 Measurement
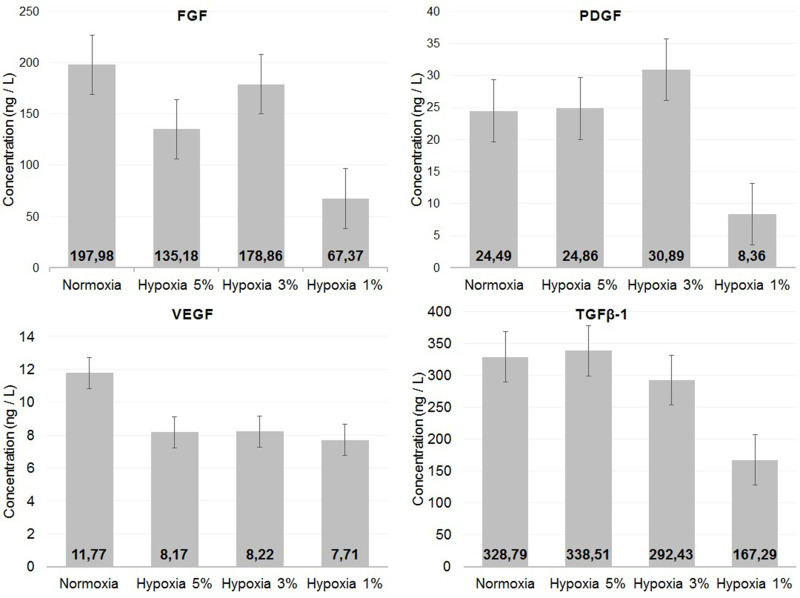
Figure 2 illustrates the average concentration levels of secretome measurement. The normoxia group had the highest concentration levels of FGF (197.98 ng/L) and VEGF (11.77 ng/L). When compared to other hypoxia groups, the hypoxia 3% group had higher FGF (178.86 ng/L) and VEGF (8.22 ng/L) levels. The highest average levels of PDGF were the hypoxia 3% group (30.89 ng/L). In TGFβ-1 measurements, the highest concentration level was the hypoxia 5% group (338.51 ng/L).
Figure 2.
The results of FGF, PDGF, VEGF, and TGFβ-1 measurement.
Discussion
The novelty of this study was determining the effective oxygen level in the preconditioning of IVDSCs using hypoxia preconditioning in vitro. Previous studies have stated that hypoxia preconditioning affects the proliferation of nucleus pulposus-derived mesenchymal stem cells.14 This research was conducted using a method similar to previous studies by using cultured cells, which had reached 80% confluence21 and hypoxia treatment according to the groups for 24 hours.22 The findings from this study present the role of oxygen at different levels in increasing in vitro replication of IVDSCs cultures and DD-CM in preparation for further studies.
Generally, MSCs were studied in a normoxia culture condition.23,24 MSCs study with hypoxia preconditioning has developed in the last few decades and has proven useful.23 Hypoxia preconditioning can increase proliferation rate, proliferative lifespan, and differentiation.23,25 This method is also able to reduce genetic instability, which plays a role in tumorigenesis.25 Preconditioning with this method can increase the secretion of cytokines and secretomes that affect the development of MSCs.16 Reactive oxygen species (ROS) formation can be suppressed, and oxidative stress can be prevented by this method. Hypoxia preconditioning also can provide antioxidant effects, and it will optimize self-renewal ability.20
The average oxygen level in the human body is 4% to 7%, and in IDD conditions it can decrease up to 1% (hypoxia).14 By using hypoxia preconditioning at a 3% oxygen level, it multiplies the number of MSCs from the first five passages.25 In hypoxia conditions, IVDSCs will experience an increase in chondrogenic and proliferative abilities compared to normoxia conditions.1 The results of this study showed that hypoxia preconditioning with 3% oxygen levels could boost the proliferation rate of IVDSCs. These results can be seen from the highest cell counts found in the hypoxia group 3%. The number of cells in the hypoxia 5% group was the lowest among other groups. Results from previous studies used hypoxia 3% culture medium can increase the proliferation of MSCs and it decreases in hypoxia 1% culture medium.25 Another study showed that preconditioning with 2% hypoxia inhibited the growth of stem cells and increased the percentage of cells that had apoptosis and necrosis.14
The secretome secretion from MSCs is a key for the regeneration ability of MSCs.15 Secretomes have a role in the development and differentiation of MSCs.18 TGF-β1, PDGF, FGF, and VEGF were secretomes that had those functions.4,23 FGF is a polypeptide that plays a role in neovascularization in wound healing and embryogenesis.18 TGF-β1 has a role in MSCs survival, differentiation and increases the regeneration ability of cartilage tissue.15 TGF-β1 stimulating extracellular matrix production and inhibiting IL-1 catabolic activity.18 TGF-β1 also has angiogenic potential so that it can induce blood vessels.4 VEGF and PDGF play a role in vascular formation and stability.16 PDGF plays a role in wound healing and extracellular matrix production, which plays a role in tissue engineering and repair.26
Preconditioning controls secretome production, so it can be used to increase the secretomes or decrease it to prevent toxicity due to increased cytokines.16,23 The results of this study showed an increase in PDGF secretion compared to other groups. Previous studies with bone marrow mesenchymal stem cells (BM-MSCs) in rats showed the highest levels of VEGF and FGF secretion was a group cultured in hypoxia 2% and PDGF under hypoxia 5%.27 There were differences with previous studies on other stem cells, which showed preconditioning with 3% hypoxia increased the secretion of VEGF compared to normoxia.14,28
The therapeutic efficacy of MSCs in regenerative therapy, including IVDSCs, depends on the number, survival ability and secretomes secreted by implanted cells.14,15 This is necessary for MSCs to be able to reach the site of damage and survive for the regenerative process.19,29 Hypoxia preconditioning with certain oxygen levels can increase the number of cells and secretomes so it is possible to survive in the IDD environment.4,16,20 Increased secretion of secretomes (VEGF, FGF, TGF-β1, and PDGF) can be maximized with hypoxia preconditioning so it can increase the regenerative ability of MSCs by triggering chondrogenesis and osteogenesis.12,29
The results of this study showed oxygen level in hypoxia preconditioning has a role in the preparation of IVDSCs and DD-CM/secretomes preparation in vitro. Further study is needed to evaluate the effect in vivo and other factors that play a role in the preparation of IVDSCs and secretomes.
Conclusions
Oxygen level in hypoxia preconditioning has a role in the preparation of intervertebral disc-derived stem cells (IVDSCs) and discus-derived conditioned medium (DD-CM)/secretome preparation in vitro. The highest cell numbers were found in the treatment group with 3% hypoxia, and 3% hypoxia was significantly related to support IVDSCs preparation. Preconditioning with 3% hypoxia had higher PDGF and VEGF levels than other hypoxia groups. Preconditioning with 3% hypoxia had higher PDGF and VEGF levels than other hypoxia groups.
Abbreviations
ANOVA, one-way analysis of variance; BM-MSCs, bone marrow-mesenchymal stem cells; DD-CM, Discus-Derived Conditioned Medium; DPBS, Dulbecco’s Phosphate-Buffered Saline; FGF, fibroblast growth factor; GFs, growth factors; IDD, intervertebral disc degeneration; IVD, intervertebral disc; IVDSCs, intervertebral disc-derived stem cells; LBP, low back pain; MSCs, mesenchymal stem cells; OD, optical density; PDGF, platelet-derived growth factor; ROS, reactive oxygen species; TGF-β1, transforming growth factor; VEGF, vascular endothelial growth factor.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
- 1.Hu B, He R, Ma K, et al. Intervertebral disc-derived stem/progenitor cells as a promising cell source for intervertebral disc regeneration. Stem Cells Int. 2018;2018:1–11. doi: 10.1155/2018/7412304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall JA, Konstantinou K, Lewis M, Oppong R, Ogollah R, Jowett S. Systematic review of decision analytic modelling in economic evaluations of low back pain and sciatica. Appl Health Econ Health Policy. 2019;17(4):467–491. doi: 10.1007/s40258-019-00471-w [DOI] [PubMed] [Google Scholar]
- 3.Ravindra VM, Senglaub SS, Rattani A, et al. Degenerative lumbar spine disease: estimating global incidence and worldwide volume. Glob Spine J. 2018;8(8):784–794. doi: 10.1177/2192568218770769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Feng C, Liu H, Yang Y, Huang B. Growth and differentiation factor-5 contributes to the structural and functional maintenance of the intervertebral disc. Cell Physiol Biochem. 2015;35(1):1–16. doi: 10.1159/000369670 [DOI] [PubMed] [Google Scholar]
- 5.Kos N, Gradisnik L, Velnar T, Brief A. Review of the degenerative intervertebral disc disease. Med Arch. 2019;73(6):421–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loibl M, Wuertz‐Kozak K, Vadala G, Lang S, Fairbank J, Urban JP. Controversies in regenerative medicine: should intervertebral disc degeneration be treated with mesenchymal stem cells? JOR SPINE. 2019;2(1):e1043. doi: 10.1002/jsp2.1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishiguro H, Kaito T, Yarimitsu S, et al. Intervertebral disc regeneration with an adipose mesenchymal stem cell-derived tissue-engineered construct in a rat nucleotomy model. Acta Biomater. 2019;15(87):118–129. doi: 10.1016/j.actbio.2019.01.050 [DOI] [PubMed] [Google Scholar]
- 8.Casiano VE, Dydyk AM, Varacallo M. Back pain. In: StatPearls. StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 9.Peletti-Figueiró M, Da Silva PG, De Souza OE, et al. Stem-cell treatment in disc degeneration: what is the evidence? Coluna/ Columna. 2013;12(1):61–63. doi: 10.1590/S1808-18512013000100013 [DOI] [Google Scholar]
- 10.Hoogendoorn RJW, Lu ZF, Kroeze RJ, Bank RA, Wuisman PI, Helder MN. Adipose stem cells for intervertebral disc regeneration: current status and concepts for the future: tissue Engineering Review Series. J Cell Mol Med. 2008;12(6A):2205–2216. doi: 10.1111/j.1582-4934.2008.00291.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huri PY, Hamsici S, Ergene E, Huri G, Doral MN. Infrapatellar fat pad-derived stem cell-based regenerative strategies in orthopedic surgery. Knee Surg Relat Res. 2018;30(3):179–186. doi: 10.5792/ksrr.17.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vadalà G, Russo F, Ambrosio L, Loppini M, Denaro V. Stem cells sources for intervertebral disc regeneration. World J Stem Cells. 2016;8(5):185–201. doi: 10.4252/wjsc.v8.i5.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakai D, Andersson GBJ. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11(4):243–256. doi: 10.1038/nrrheum.2015.13 [DOI] [PubMed] [Google Scholar]
- 14.Li H, Tao Y, Liang C, et al. Influence of hypoxia in the intervertebral disc on the biological behaviors of rat adipose-and nucleus pulposus-derived mesenchymal stem cells. Cells Tissues Organs. 2014;198(4):266–277. doi: 10.1159/000356505 [DOI] [PubMed] [Google Scholar]
- 15.Nie WB, Zhang D, Wang LS. Growth factor gene-modified mesenchymal stem cells in tissue regeneration. Drug Des Devel Ther. 2020;14:1241–1256. doi: 10.2147/DDDT.S243944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahangar P, Mills SJ, Cowin AJ. Mesenchymal stem cell secretome as an emerging cell-free alternative for improving wound repair. Int J Mol Sci. 2020;21(19):1–15. doi: 10.3390/ijms21197038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prakoeswa CRS, Tinduh D, Notobroto HB, et al. The potential of mesenchymal stem‐cell secretome for regeneration of intervertebral disc: a review article. Indones J Biotechnol. 2021;26(2):61–75. doi: 10.22146/ijbiotech.63318 [DOI] [Google Scholar]
- 18.Pourmollaabbassi B, Hashemibeni B, Esfandiari E. A Review Study: Effect of growth factors on human mesenchymal stem cells differentiation into cartilage tissue. J Iran Anat Sci. 2015;12(4):183–190. [Google Scholar]
- 19.Vadalà G, Ambrosio L, Russo F, Papalia R, Denaro V. Interaction between mesenchymal stem cells and intervertebral disc microenvironment: from cell therapy to tissue engineering. Stem Cells Int. 2019;2019:1–15. doi: 10.1155/2019/2376172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mas-Bargues C, Sanz-Ros J, Román-Domínguez A, et al. Relevance of oxygen concentration in stem cell culture for regenerative medicine. Int J Mol Sci. 2019;20(5):1195. doi: 10.3390/ijms20051195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Cao H, Yang Y, et al. Effects of vascular endothelial cells on osteogenic differentiation of noncontact co-cultured periodontal ligament stem cells under hypoxia. J Periodontal Res. 2013;48(1):52–65. doi: 10.1111/j.1600-0765.2012.01503.x [DOI] [PubMed] [Google Scholar]
- 22.Kifune T, Ito H, Ishiyama M, et al. Hypoxia-induced upregulation of angiogenic factors in immortalized human periodontal ligament fibroblasts. J Oral Sci. 2018;60(4):519–525. doi: 10.2334/josnusd.17-0441 [DOI] [PubMed] [Google Scholar]
- 23.Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Gonçalves RM. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol. 2018;9. doi: 10.3389/fimmu.2018.02837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang YC, Leung VYL, Lu WW, Luk KDK. The effects of microenvironment in mesenchymal stem cell-based regeneration of intervertebral disc. Spine J. 2013;13(3):352–362. doi: 10.1016/j.spinee.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 25.Haque N, Rahman MT, Abu Kasim NH, Alabsi AM. Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. Sci World J. 2013;2013:12. doi: 10.1155/2013/632972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen M, Guo W, Gao S, et al. Biochemical stimulus-based strategies for meniscus tissue engineering and regeneration. Biomed Res Int. 2018;2018. doi: 10.1155/2018/8472309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Hao H, Xia L, et al. Hypoxia pretreatment of bone marrow mesenchymal stem cells facilitates angiogenesis by improving the function of endothelial cells in diabetic rats with lower ischemia. PLoS One. 2015;10(5):e0126715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han YU, Kuang S-Z, Gomer A, Ramirez-Bergeron DL. TISSUE-SPECIFIC STEM CELLS hypoxia influences the vascular expansion and differentiation of embryonic stem cell cultures through the temporal expression of vascular endothelial growth Factor receptors in an ARNT-dependent manner. Stem Cells. 2010;28(4):799–809. doi: 10.1002/stem.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahyudin F, Sigit Prakoeswa CR, Notobroto HB, et al. An update of current therapeutic approach for Intervertebral disc degeneration: a review article. Ann Med Surg. 2022;77:103619. doi: 10.1016/j.amsu.2022.103619 [DOI] [PMC free article] [PubMed] [Google Scholar]