Abstract
Socioeconomic disadvantage has been linked to increased stress exposure in children and adults. Exposure to stress in childhood has been associated with deleterious effects on cognitive development and well-being throughout the lifespan. Further, exposure to stress has been associated with differences in brain development in children, both in cortical and subcortical gray matter. However, less is known about the associations among socioeconomic disadvantage, stress, and children’s white matter development. In this study, we investigated whether socioeconomic disparities would be associated with differences in white matter microstructure in the cingulum bundle, as has been previously reported. We additionally investigated whether any such differences could be explained by differences in stress exposure and/or physiological stress levels. White matter tracts were measured via diffusion tensor imaging in 58 children aged 5–9 years. Results indicated that greater exposure to stressful life events was associated with higher child hair cortisol concentrations. Further, physiological stress, as indexed by hair cortisol concentrations, were associated with higher fractional anisotropy in the cingulum bundle. These results have implications for better understanding how perceived and physiological stress may alter neural development during childhood.
Keywords: child, cortisol, diffusion-weighted imaging, neurodevelopment, socioeconomic status, stress
1 |. INTRODUCTION
1.1 |. Socioeconomic disadvantage and stress exposure
Socioeconomic status (SES) is a multidimensional construct that includes measures of economic resources as well as social factors such as educational attainment, occupational prestige and social status (Hackman & Farah, 2009). Most commonly characterized by family income and parental education, socioeconomic disparities have been associated with differences in stress exposure in children and adults (Luby et al., 2013). For example, socioeconomic disadvantage has been associated with an increased risk of experiencing chronic stressors such as neighborhood violence, uncertainty about material resources, family conflict, and household chaos (Brooks-Gunn & Duncan, 1997; Evans, 2004). Further, children from socioeconomically disadvantaged backgrounds may experience multiple stressors at once, potentially exacerbating the effects of stress. Indeed, increased exposure to stress may be an important mediating mechanism linking SES to children’s outcomes (Evans & Kim, 2013).
1.2 |. Stress exposure and cortisol
Stress exposure reflects the type and degree of stressors that children experience or encounter in everyday life. At the physiological level, chronic stress exposure has been linked to dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis stress–response system (Gunnar & Donzella, 2001). This dysregulation is one mechanism through which stress exposure may impact developmental outcomes.
Dysregulation of the HPA axis is typically observed through changes in glucocorticoid hormone levels, namely cortisol. Recent studies have measured hair cortisol concentration (HCC) in humans, which indexes average cortisol levels over the prior several months and is less sensitive to situational factors than other cortisol metrics (Flom et al., 2017). Further, recent work has linked HCC to stress exposure in adults (O’Brien et al., 2013), as well as to stress exposure (Vanaelst et al., 2013) and SES (Ursache et al., 2017; Vliegentart et al., 2016) in children. As such, alterations in stress physiology in response to stress may represent one mechanism by which socioeconomic disadvantage may “get under the skin” and influence outcomes (Kim et al., 2017).
1.3 |. Socioeconomic disadvantage, stress exposure, and brain structure
Both stress exposure and SES have been associated with differences in cortical and subcortical gray matter in the hippocampus (Humphreys et al., 2019; Noble et al., 2015), the amygdala (Merz et al., 2018), and the prefrontal cortex (Lawson et al., 2013). While work has suggested that stress exposure may serve as a mediating factor between SES and gray matter (Luby et al., 2013; Merz et al., 2019), few studies have examined whether the same holds true for white matter.
White matter plays a critical role in neural signaling and connections between different brain regions (Fields, 2010). Fractional anisotropy (FA), a measure of white matter microstructure, reflects the degree of directionality of water diffusion in white matter tracts, which indicates the microstructural properties of white matter tracts and in turn the capacity for functional communication between connected brain regions (Beaulieu, 2002; Thomason & Thompson, 2011). Given the ability for white matter development to be environmentally influenced (Fields, 2008), understanding how environmental circumstances impact the development of specific white matter tracts may help us better understand brain development in children.
The cingulum bundle is a frontolimbic white matter tract that might be expected to be particularly susceptible to differences in both socioeconomic circumstance and stress exposure. This tract assists with communication between components of the limbic system and the cingulate gyrus, two regions which have high concentrations of glucocorticoid receptors (Patel et al., 2008) and which have been individually associated with both socioeconomic factors and stress (Hanson et al., 2015; McDermott et al., 2019; Merz et al, 2019). Additionally, the cingulum has been implicated in executive function and emotion regulation (dorsal cingulum), as well as memory processes (parahippocampal cingulum) (Bathelt et al., 2019; Bubb et al., 2018; Catani, 2006), cognitive processes that have consistently been linked to both socioeconomic factors and stress (Evans & Schamberg, 2009; Noble et al., 2005; Raver et al., 2013; Ursache et al., 2015). Finally, the cingulum undergoes a protracted period of development, with typically developing individuals showing increases in FA throughout childhood and young to middle adulthood (Lebel & Beaulieu, 2011; Lebel et al., 2017), suggesting that it may be particularly susceptible to postnatal differences in socioeconomic circumstances, stress exposure, and alterations in stress physiology in childhood (Peters et al., 2014; Tamnes et al., 2010).
Although a few studies have reported associations between the cingulum bundle microstructure and socioeconomic factors and/or stress in children and young adults (see Noble & Giebler, 2020 for a review), results have been mixed, with some studies reporting that socioeconomic disadvantage (Dufford & Kim, 2017; Ursache & Noble, 2016) and stress (Dufford & Kim, 2017) are associated with lower cingulum FA, while others reporting that socioeconomic disadvantage (Noble et al., 2013) and stress (Weis et al., 2018) are associated with higher FA in this tract. These discrepancies may be due to differences in how stress exposure and/or SES were measured. Further, only one study (Dufford & Kim, 2017) looked concurrently at SES and stress in relation to the cingulum bundle, leaving it unclear whether increased stress exposure may mediate any relationship between SES and the cingulum.
1.4 |. Cortisol and brain structure
Previous studies have reported associations between cortisol and brain structure (Chen et al., 2016; Merz et al., 2019). For example, higher HCC has been associated with smaller hippocampal volume (Chen et al., 2016; Merz et al., 2019) and greater cortical thickness in the anterior cingulate cortex (ACC) in children (Merz et al., 2019).
Although white matter tracts such as the cingulum bundle, which connects regions with an abundance of glucocorticoid receptors, might be expected to be particularly vulnerable to increased cortisol levels, few studies have examined links between cortisol and white matter. One study of girls reported that high salivary cortisol reactivity was associated with lower white matter FA in several tracts, including the cingulum (Sheikh et al., 2014). Another study found that decreased cortisol production in response to a stressor was related to increased FA in the uncinate fasciculus (though the cingulum was not examined) (Kircanski et al., 2019). To our knowledge, no study has investigated associations between HCC and white matter microstructure in children.
1.5 |. The current study
As reviewed above, previous studies have investigated links between SES and white matter microstructure in children (Dufford & Kim, 2017; Ursache & Noble, 2016). However, the extent to which these links may be mediated by stress exposure, and/or alterations in stress physiology, has not been examined. Previous work in the present sample investigated whether socioeconomic factors were associated with differences in hair cortisol, and in turn whether HCC was associated with differences in hippocampal and ACC structure (Merz et al., 2019). Building on that work, we hypothesized that socioeconomic factors would also be associated with differences in cingulum microstructure—the white matter tract that communicates between the limbic system and the cingulate gyrus—and that these associations would be mediated by differences in stress exposure and/or stress physiology.
We had four main hypotheses: (1) Socioeconomic disadvantage would be associated with lower FA in the cingulum bundle, (2) greater stress exposure would be associated with lower FA in the cingulum, (3) higher physiological stress, as operationalized by children’s HCC, would be associated with lower FA in the cingulum, and (4) physiological stress would mediate the link between stress exposure and cingulum FA. Exploratory post hoc analyses examined these questions in relation to FA of different segments of the cingulum bundle.
2 |. METHOD
2.1 |. Participants
Participants were recruited in the New York City area by posting flyers and meeting families at local community events. Children aged 5–9 years (N = 94; 61% female) and their parents participated in this study. Children were included if they were primarily English speakers in the home, born from a singleton pregnancy, and had no history of premature birth (< 37 weeks), or medical or psychiatric problems. Children with MRI contraindications were also excluded from the study. Families were socioeconomically diverse, with average parental education ranging from 7 to 20 years and family income-to-needs (ITN) ratio ranging from 0.17 to 15.21 (see Table 1 for sample characteristics).
TABLE 1.
Descriptive statistics for sample characteristics (N = 94)
| M | SD | |
|---|---|---|
| Child age (years) | 7.03 | 1.29 |
| % | n | |
| Child sex (female) | 60.64 | 57 |
| Child race/ethnicity | ||
| African American, non-Hispanic/Latino | 30.85 | 29 |
| Hispanic/Latino | 50.00 | 47 |
| European American, non-Hispanic/Latino | 13.83 | 13 |
| Other | 5.32 | 5 |
| Family income below U.S. poverty threshold | 29.79 | 28 |
At the first study visit, all parents (N = 94) completed questionnaires regarding socioeconomic factors and exposure to stress. Hair samples were collected for a total of 67 children (77% female), with some children unable to provide a sample because their hair was too short (n = 24) or because their parents declined to have a sample taken (n = 3). Of the 67 hair samples collected, hair cortisol data were available for 65 children. The children who comprised the final sample of hair cortisol data differed significantly from the full sample in regards to sex (t(93) = −5.263, p < .0001), as boys were less likely to have hair long enough (at least 3 cm in length) to participate. During the first study visit, participants were also offered the opportunity to participate in the MRI portion of the study.
Eighty-five children were enrolled in the MRI portion of the study. Out of these 85, 66 children participated in an actual MRI scanning session. Scans were completed for a total of 61 children, with 58 children having usable diffusion-weighted imaging (DTI) scans. The remaining children were either unwilling or unable to participate in the MRI portion of the study after mock scanning (n = 12) or began to attempt scanning but were afraid, fidgety, or uninterested in completing the session before the session started (n = 7). Furthermore, some children opted to stop the session early before DTI data could be collected (n = 5). Finally, some participants’ data were dropped due to excessive head motion upon visual inspection (n = 3), leading us to our final sample of 58 children with usable DTI data.
The children who completed the MRI portion of the study did not differ from the larger sample in terms of age, sex, race/ethnicity, family income, or parental education. However, the subsample with usable DTI data was generally older (t(92) = −4.545, p < .01), as older children were more likely to complete the mock scan and entire MRI scan. In total, 38 children had full hair cortisol, DTI, and questionnaire data.
2.2 |. Procedure
Parents and children visited the lab on two separate occasions within 1 month. During the first visit, hair samples were collected and parents completed questionnaires. Children then completed a mock MRI session in order to gain familiarity with the conditions of MRI scanning. During the second visit, children participated in an MRI scan which included a diffusion-weighted sequence. Informed consent/assent was provided by all families, and all study procedures were approved by the New York State Psychiatric Institute and Teachers College, Columbia University Institutional Review Boards.
2.3 |. DTI acquisition and processing
MRI data were acquired on a 3-Tesla General Electric (GE) MR750 scanner with a 32-channel head coil. Whole brain DTI data were acquired with the following parameters: 60 axial slices were collected (posterior to anterior) each at 2.5 mm thickness, TR = 15,700 ms, TE = 86.4 ms, FOV (x) = 24 mm, FOV (y) = 24 mm, matrix size = 132 × 128 (machine-interpolated to 256 × 256 for postprocessing), and voxel size = 0.94 mm × 0.94 mm × 2.5 mm. The diffusion-weighted images were acquired along 15 noncollinear directions, with a b value of 1000 s/mm2, and three baseline images with b = 0 s/mm2. Total scan duration for DTI was 9 min, 57 s (number of excitation = 2). All images were visually inspected by two trained research assistants, independently. Research assistants visually checked the raw diffusion-weighted images, eddy current corrected diffusion-weighted images, the color-encoded FA images, and each participant’s FA image after alignment to potentially exclude participants who had motion-corrupted DTI data (n = 3).
Images were processed using the Oxford Center for Functional MRI of the Brain’s (FMRIB) Software (FSL version 5.0.11) (Smith et al., 2004). DWI acquisitions were corrected for subject motion and Eddy current-induced distortion using FSL Eddy (Andersson et al., 2017). The FSL DTIFIT was used to fit diffusion tensors at each voxel, and FA image was derived from the fitted diffusion tensors. The Johns Hopkins University White Matter Tractography Atlas (Mori et al., 2008; Wakana et al., 2007) was used to extract mean FA values for white matter tracts of interest after running tract-based spatial statistics (TBSS) (Smith et al., 2006). Due to the age of the sample, the most representative subject was automatically identified as the target image from all subjects using TBSS script (tbss_2_reg) and the target was then affine-aligned into MNI152 standard space. Each participant’s FA image was then transformed into standard space by applying the nonlinear transform to the target FA image, followed by the affine transform from that target to the standard space. A skeleton of white matter tracts that were common to all participants was then created by thinning the mean FA image using a threshold of 0.2. This value was chosen to exclude FA values from gray matter or cerebrospinal fluid. Aligned FA image of each subject was then projected onto the skeleton and mean FA values were extracted from region of interest tracts used for statistical analyses. After projecting the aligned FA image onto the skeleton, the skeletonized FA image for each subject was visually inspected for quality control. For the current study, mean FA values of the cingulum bundle were extracted.
2.4 |. Measures
2.4.1 |. Socioeconomic factors
Parents reported their total annual household income and the number of adults and children in the household. A family ITN ratio was calculated for each participant by dividing the total household income by the nationally set poverty threshold for a family of that size. Family ITN was log-transformed in all analyses to correct for positive skew. Parents also reported their years of educational attainment, which were then averaged across all parents.
2.4.2 |. Stress exposure
Life experiences survey
The Life Experiences Survey (LES; Sarason et al., 1978) is a measure of the quantity and impact of life events. Parents indicated whether a series of 44 events occurred within the last year. Parents rated the impact of events that occurred on a 7-point scale from −3 (extremely negative) to +3 (extremely positive). If the event did not occur or occurred but was rated as neutral, the item was scored as 0. A negative life experiences score was computed as the absolute value of the sum of the impact of events rated as negative. The LES is a reliable measure of recent life changes and their impact, with well-established internal consistency (α = .77), test–retest reliability, and validity (Sarason et al., 1978).
Perceived stress scale
The Perceived Stress Scale (PSS) (Cohen et al., 1983) is a 14-item scale that asked parents about their feelings and thoughts about their stress and ability to control their stress during the last month). For each item, they were asked to mark how often they felt or thought a certain way on a 5-point scale ranging from 0 (never) to 4 (very often). Items were reverse coded when appropriate and then summed to create a total score (α = .84).
Material deprivation scale
In the Material Deprivation Scale (Pilkauskas et al., 2012) parents were asked a series of 14 questions about possible hardships in bills, utilities, food, medical care, and housing insecurity. Parents were asked if they experienced hardships in these areas within the past year and provided a dichotomous answer (yes or no). All affirmative answers were recorded and summed to create a total score (α = .77).
2.4.3 |. Hair cortisol concentration
A trained research staff member cut a small section of hair proximal to the posterior vertex of the participant’s scalp. Each hair sample weighed at least 15 mg and was approximately 3 cm long in order to account for cortisol deposited during roughly the 3 months prior to collection. Samples were stored at −20°C until being sent to the University of Massachusetts for analysis. Samples were processed and analyzed using previously validated methods, described in detail elsewhere (Meyer et al., 2014).
Briefly, each sample was weighed, washed twice in isopropanol to remove external contaminants, ground to a fine powder, and extracted with methanol. The methanol extract was evaporated, redissolved in assay buffer, and analyzed in duplicate along with standards and quality controls by a sensitive and specific enzyme-linked immunosorbent assay (Arbor Assays, Ann Arbor, MI). Assay readout was converted to pg cortisol per mg dry hair weight. Intra- and inter-assay coefficients of variation for this assay are <10%. Hair cortisol values outside of 3 standard deviations from the mean were examined and ultimately excluded from the final analyses, due to their values being biologically implausible (n = 5), and hair cortisol data were log-transformed to correct for skew, similar to previous methods (Chen et al., 2016). There were no significant associations between hair cortisol and potential confounds, including hair washing frequency, steroid medication use, and hair dye use.
2.5 |. Statistical plan
Descriptive statistics and preliminary analyses were conducted in SPSS (version 25). Subsequent analyses were conducted in R (version 3.5.1). Principal component analysis was used to assess the extent to which the LES, the PSS, and Material Deprivation Scale loaded onto a single “parental perceived stress” factor. Multiple linear regression analyses were used to examine associations between socioeconomic factors, parental perceived stress, HCC, and white matter microstructure (FA) in the cingulum bundle. Covariates included child age, sex, and race/ethnicity. Race/ethnicity was not significant in any of the regression models and did not improve model fit and was therefore dropped from final analyses. Measures with outliers more than 3 SD from the mean were Winsorized; in addition, models were run both with and without any outliers to investigate any substantive differences. Thirty-eight participants had completed DTI data, hair cortisol data, and survey data (i.e., stress and SES measures). For models examining DTI data and survey data, 58 participants had complete data. For models examining hair cortisol and survey data, 60 participants had complete data. All tests were two-tailed, α = 0.05. To account for multiple comparisons, false discovery rate corrections were applied (via p.adjust in R) to analyses. Uncorrected p-values are reported in regard to exploratory post hoc analyses.
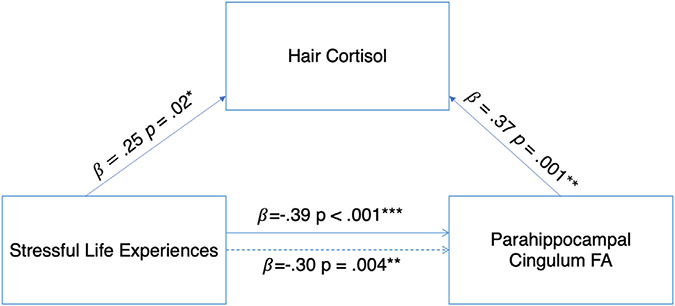
To test the significance of indirect effects when a and b paths were supported in mediation analyses (see Figure 2), bias-corrected bootstrapping via the lavaan library in R was conducted using a 95% confidence interval (Rosseel, 2012). In mediation analyses, Full Information Maximum Likelihood Estimation was employed to account for missing data and produce unbiased parameter estimates and standard errors (Enders & Bandalos, 2001).
FIGURE 2.
Hair cortisol significantly mediated the association between exposure to stressful life experiences and FA in the parahippocampal cingulum. Higher negative life experience scores were associated with higher HCC, which in turn was associated with higher FA in the parahippocampal cingulum. The dotted line between exposure to stressful life experiences and parahippocampal cingulum FA represents the total effect (c), while the solid line represented the direct effect after accounting for the mediated or indirect effect (c’). FA, fractional anisotropy * p < .05 ** p < .01 *** p < .001
3 |. RESULTS
Descriptive statistics are provided in Table 2. Two LES scores were outliers (3.20 and 4.07 standard deviations from the mean), as was as was one participant’s FA value (3.07 standard deviations from the mean). These scores were Winsorized in models below. Results were also examined when outliers were excluded, as discussed below.
TABLE 2.
Descriptive Statistics for socioeconomic factors, stress exposure, HCC, and white matter microstructure
| N | M | SD | Range | |
|---|---|---|---|---|
| Parental education (years) | 94 | 14.14 | 2.64 | 7–20 |
| Income-to-needs Ratio | 94 | 2.68 | 2.79 | 0–15 |
| Perceived stress exposure Score | 94 | −0.01 | 0.99 | −2.08 to 2.98 |
| Hair cortisol concentration (pg/mg) | 60 | 25.20 | 25.34 | 1–104 |
| Parahippocampal cingulum FA | 58 | 0.53 | 0.04 | 0.43–0.61 |
| Dorsal cingulum FA | 58 | 0.59 | 0.03 | 0.50–0.66 |
Abbreviation: FA, fractional anisotropy; M, mean; SD, standard deviation.
Note. Parental education reflects educational attainment averaged across parents in the household.
3.1 |. Parent perceived stress
As described previously (Merz et al., 2019), principal component analysis was used to assess the extent to which the LES, the PSS, and Material Deprivation Scale loaded onto the same construct. A single factor with an eigenvalue greater than 1.0 (1.69) was extracted, explaining 56.4% of the total variance. Factor loadings ranged from 0.63 to 0.85. This factor score was termed “parent perceived stress.”
3.2 |. Socioeconomic circumstance and white matter microstructure
Neither family ITN nor parental education was significantly associated with FA in the cingulum when controlling for child age and sex (all corrected ps > .05).
3.3 |. Stress exposure and white matter microstructure
Parent perceived stress was not associated with FA in the cingulum bundle when controlling for child age and sex (p > .05). We reasoned that perhaps parental perception of stress was too indirect a measure of the child’s exposure to stress. We further reasoned that perhaps exposure to explicit stressful life experiences, as measured by the Life Experiences Scale, would more proximally reflect the child’s own experiences over the last year (acknowledging that, while the LES may be more likely to capture children’s own exposure to stress than measures specifically focused on parental perception, it is still a parent-report measure). We therefore explored, post hoc, whether greater exposure to stressful life experiences, as measured by a higher LES negative life experience score, was associated with white matter microstructure. Indeed, greater exposure to stressful life events was associated with lower FA in the cingulum (β = −.25, p = .03).
When results were rerun without the LES and FA outliers, results remained similar (β = −.24, p = .04).
3.4 |. Hair cortisol and white matter microstructure
Higher child HCC was significantly associated with higher FA in the cingulum (β = .35, p = .007) when controlling for child age and sex. This result remained consistent when removing the outlying FA value (β = .29, p = .04).
3.5 |. Stress exposure, hair cortisol, and white matter microstructure
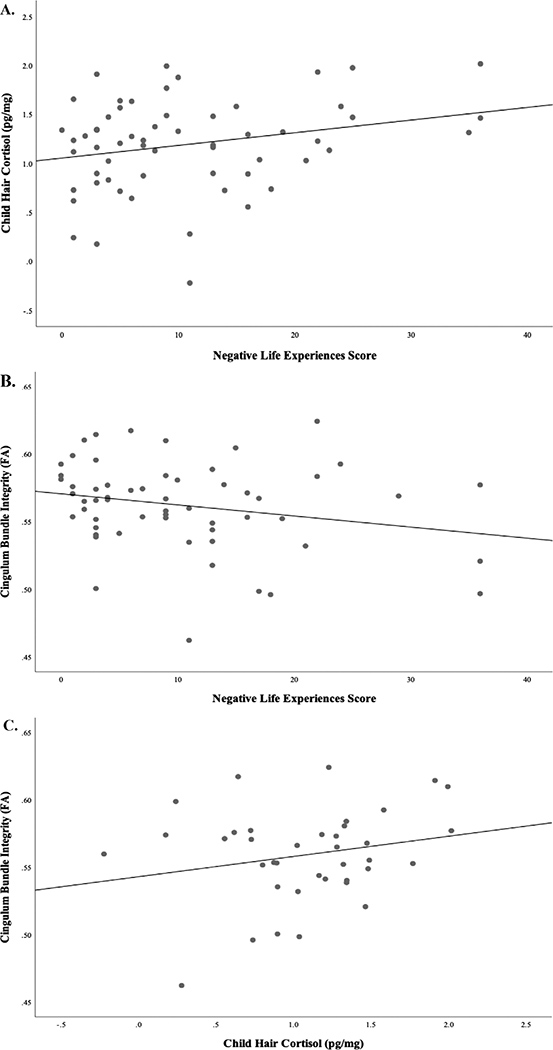
The composite of parent perceived stress was not associated with children’s HCC. We then examined, post hoc, whether greater exposure to stressful events, as measured by LES, was significantly associated with higher child HCC. Indeed, we found that greater exposure to stressful life events was associated with higher HCC in children, controlling for child age and sex (β = .25, p = .03) (see Figure 1). This result remained similar when removing the two outlying LES scores, although results were no longer statistically significant (β = .23, p = .13).
FIGURE 1.
(a) Higher parent-reported negative life experiences scores were significantly associated with higher levels of (logarithmically transformed) hair cortisol in children. (b) Negative life experiences scores were significantly associated with lower FA in the cingulum bundle. (c) HCCs (logarithmically transformed) were significantly associated with higher FA in the cingulum bundle. Child age and sex were included as covariates in these models
HCC did not significantly mediate the association between stressful life experiences and FA of the cingulum.
3.6 |. Post hoc analyses: cingulum segments
To further understand whether the current findings were specific to different segments of the cingulum bundle, we conducted exploratory analyses using extracted mean FA values of the parahippocampal and cingulate gyrus (henceforth referred to as the dorsal cingulum) segments of the cingulum. Greater socioeconomic disadvantage was significantly associated with higher FA in the dorsal cingulum (family ITN: β = −.27, p = .03; parental education: β = −.28, p = .02), but not with the parahippocampal cingulum. In contrast, higher parental perceived stress was neither associated with FA in the parahippocampal cingulum (β = −.22, p = .06) nor with the dorsal cingulum (β = .07, p = .48). Higher levels of parent-reported stressful life events over the past year, as measured by the LES, were significantly associated with lower FA in the parahippocampal cingulum (β = −.30, p = .005), but not the dorsal cingulum. These results remained similar when removing the outlying LES value (parahippocampal cingulum: β = −0.28; p = 0.03). Finally, higher HCC was significantly associated with higher FA in both the dorsal and parahippocampal cingulum (dorsal cingulum: β = .39, p = .04; parahippocampal cingulum β = .31, p = .02).
Based on these results, exposure to stressful life experiences was not further considered as a mediator linking socioeconomic disadvantage to FA in either segment of the cingulum. On the other hand, both socioeconomic disadvantage and HCC were associated with increased FA in the dorsal cingulum. HCC was thus probed as a potential mediator of the link between socioeconomic disparities and dorsal cingulum FA. Hair cortisol did not mediate the relationship between parental education and dorsal cingulum FA, however, as the association between cortisol and the dorsal cingulum (b path) was no longer statistically significant after parent education was added to the model. Since ITN was not significantly associated with hair cortisol in this sample (Merz et al., 2019), we did not explore ITN in association with dorsal cingulum FA and hair cortisol.
We then tested whether HCC mediated the association between exposure to stressful life experiences and parahippocampal cingulum FA and found a significant indirect effect (indirect effect = 0.09, 95% CI [0.003, 0.180]. Greater exposure to stressful life experiences was related to higher HCC, which in turn was associated with higher FA in the parahippocampal cingulum (see Figure 2).
4 |. DISCUSSION
The goal of this study was to build upon previous work by examining associations among socioeconomic factors, stress exposure, HCC, and white matter microstructure in children. Our first hypothesis was that socioeconomic disadvantage would be associated with lower FA in the cingulum bundle. Contrary to predictions, parental education and family ITN were not associated with cingulum bundle FA. Our second hypothesis was that greater parent perceived stress would be associated with lower FA in the cingulum bundle. While a composite of parental perceived stress was not linked with cingulum FA, greater exposure to stressful life experiences—arguably a more proximal measure of the child’s own experiences of stress, and therefore perhaps more relevant for brain development—was significantly associated with lower FA in the cingulum bundle. Third, we hypothesized that higher physiological stress, as operationalized by children’s HCC, would also be associated with lower FA in the cingulum bundle. Counter to expectations, higher HCC was associated with higher FA in the cingulum bundle. Finally, we hypothesized that HCC would mediate the link between exposure to stressful life experiences and cingulum FA. This hypothesis was not supported, as the indirect effect was not significant.
Exploratory analyses separately examined the individual segments of the cingulum bundle. Results revealed that socioeconomic disadvantage was associated with higher FA in the dorsal segment of the cingulum. In contrast, greater exposure to stressful life events was associated with lower FA in the parahippocampal cingulum. Moreover, hair cortisol was significantly associated with higher FA in both segments of the cingulum. Lastly, these analyses revealed a significant indirect effect linking exposure to stressful life events to greater parahippocampal cingulum FA via HCC. While exploratory and requiring replication, these results provide some evidence of physiological stress as a mediating factor between stress exposure and white matter microstructure in children.
4.1 |. Socioeconomic disadvantage and cingulum bundle FA
Higher family ITN and higher parental education were not associated with FA in the total cingulum. However, exploratory analyses revealed that higher ITN and parental education were both associated with lower FA in the dorsal cingulum. While one study has reported a similar relationship in young adults (Noble et al., 2013), other findings have been mixed. In contrast with these results, some studies among children have reported associations between higher family income and higher FA in the cingulum (Dufford & Kim, 2017; Ursache & Noble, 2016), whereas others have found no associations between SES and white matter microstructure in children (Jednorog et al., 2012). Additional work is needed to better understand possible moderating factors that may account for these differences.
4.2 |. HCC is associated with higher cingulum FA
Contrary to our hypothesis that higher HCC would be associated with reduced FA in the cingulum bundle, we found that higher HCC was associated with higher FA. Further, exploratory analyses found that this association held in both segments of the cingulum. One possible explanation for these findings may stem from the “stress-acceleration hypothesis” (Callaghan & Tottenham, 2016), which posits that chronic stress may induce earlier and more rapid maturation of stress-sensitive brain regions as an adaptive mechanism (Belsky, 2019; Callaghan & Tottenham, 2016). Indeed, animal work suggests that increased exposure to stress may lead to increased FA in several white matter tracts. For example, one study in rodents found that precocious weaning led to earlier and more rapid myelination in the brain (Ono et al., 2008). Another study found that increases in stress and glucocorticoid levels led to increases in oligodendrogenesis in rats, a key process that precedes changes in myelination and white matter structure (Chetty et al., 2014). One possibility is therefore that chronically elevated cortisol in childhood may lead to accelerated myelination in certain white matter tracts. Indeed, some work has linked higher cortisol to increased FA in other white matter tracts such as the uncinate fasciculus (Kircanski et al., 2019; Dennison et al., 2019; though see Lichtin et al., 2021, which linked material deprivation to lower FA in this tract). In the future, longitudinal research is needed to elucidate developmental mechanisms and discern how stress physiology relates to changes in white matter organization over time.
While it is typically hypothesized that physiological measures such as cortisol affect brain structure, the cross-sectional nature of these data precludes us from making causal inferences as to the directionality of this relationship. Another possible explanation for these results is therefore that higher FA in the cingulum, a white matter tract associated with stress and limbic system communications, may contribute to differences in physiological stress levels in children, such as higher levels of cortisol being released over time in response to stress exposure.
4.3 |. Socioeconomic disadvantage, stress exposure, hair cortisol, and cingulum FA
We examined, post hoc, whether hair cortisol mediated the relationship between socioeconomic disadvantage and dorsal cingulum FA. Despite the associations between parental education and hair cortisol, and between hair cortisol and dorsal cingulum FA, hair cortisol did not statistically mediate the association between parent education and dorsal cingulum FA. It is possible that an indirect effect may exist, but that we did not have the statistical power to detect such an effect, as our sample size with all three measures in this model was small (n = 38). As such, more research with larger sample sizes is needed in order to better understand whether hair cortisol may serve as a mediator between socioeconomic factors and white matter microstructure.
4.4 |. Exposure to stressful life experiences is associated with hair cortisol and cingulum FA
We found no link between a composite of parental perceived stress and children’s hair cortisol. However, post hoc analyses revealed that child exposure to stressful life experiences (measured using the LES) was linked with higher hair cortisol levels. Though this finding requires replication, it suggests that greater exposure to explicit stressful events—as opposed to parental perceptions of stress—may be more closely tied to children’s stress physiology and brain development. Further, while our parent perceived stress composite was not significantly associated with white matter microstructure, our exploratory analyses revealed that the experience of more stressful life events was significantly associated with lower FA in the parahippocampal cingulum. This finding is consistent with some previous studies examining stress exposure and white matter microstructure, (Dufford & Kim, 2017; Hanson et al., 2013).
Importantly, although greater exposure to stressful life events was associated with reduced parahippocampal cingulum FA, we found a significant positive indirect effect when testing whether HCC mediated the relationship between stressful life events and white matter microstructure. That is, more stressful life events were associated with higher HCC, which in turn was associated with higher parahippocampal cingulum FA. This difference in directionality between the indirect and total effect is referred to as “competitive mediation” (Zhao et al., 2010). This pattern may be likened to the classically cited example of stress, coping, and mood: Stress worsens mood, but stress is also associated with coping strategies, which in turn increase mood (MacKinnon et al., 2000). In the context of the current study, it is possible that while exposure to stressful life events may generally lead to lower FA in the parahippocampal cingulum, stress-related increases in cortisol may suppress the relationship between exposure to stress and decreased parahippocampal FA. These results may indicate that the cortisol response may be involved in adaptive white matter development in response to exposure to stress. Indeed, other studies have suggested cortisol may play the role of a suppressor variable in the context of stress exposure and neural outcomes (Oei et al., 2014).
5 |. CONCLUSION
Our results may help disentangle inconsistencies in previously reported findings between stress exposure and white matter microstructure in children. When not accounting for cortisol, our results show that exposure to stress is predictive of lower FA in the cingulum, in concordance with previous studies. At the same time, HCC was associated with higher FA in the parahippocampal cingulum bundle, which is a relatively novel finding that may be supported by research-linking exposure to physiological stress to accelerated neurodevelopment.
While studies generally suggest that higher FA is associated with higher performance on a number of outcomes, some studies have reported that higher FA is associated with worse performance, or in some cases, associated with developmental disorders (Bashat, et al., 2007; Hoeft, et al., 2007). To better understand the long-term development of white matter in response to physiological stress and stress exposure, future work should examine brain development longitudinally in order to examine the extent to which stress may alter white matter microstructural development. Such research will be needed in order to better understand the relationship between stress, both perceived and physiological, and brain development in children.
Several limitations should be taken into account when interpreting these findings. First, the cross-sectional and correlational design of this study precludes the ability to make causal inferences. Second, this study had a small sample, precluding the statistical power to detect small effects, indicating the need for future examinations of these questions with larger sample sizes. Additionally, our exploratory results require replication and should be interpreted with caution, as they were not subject to any type of correction for multiple comparisons. Further, while the number of stressful life events may be more proximal to the child’s experiences than a survey asking about parental perception of stress, it still relies on parent report. In addition, while this measure is reliable and has been used as a measure of stress exposure in children in previous work (e.g., Cohen et al., 1987), it may not account for all the stressful events that a child may experience in their day-to-day lives. With regard to our measure of white matter organization, future studies should use additional measures in addition to FA, such as medial diffusivity. Further, while we examined here one form of physiologic stress, it is worth noting that other indices of physiologic stress need to be examined in order to elucidate the potential effects of increased physiologic stress on neural development. Lastly, we had significantly fewer hair samples from boys than from girls, as boys’ hair was often too short to provide an adequate sample.
Despite these limitations, this is among the first studies to examine associations among socioeconomic circumstance, exposure to stress, hair cortisol, and white matter microstructure in children. Taken together, these results contribute to our understanding of how socioeconomic disadvantage and exposure to stress may “get under the skin” and contribute to developmental outcomes. Replicating these effects in a larger sample is a necessary next step to strengthen confidence in many of the exploratory findings discussed here. Building on this work has the potential to lead to more effective prevention and intervention strategies, increasing the odds that children’s developmental trajectories lead to positive academic and mental health outcomes.
ACKNOWLEDGMENTS
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant numbers UL1TR001873 and UL1RR024156. Additional funding was provided by the Russell Sage Foundation; the Gertrude H. Sergievsky Center, Columbia University Medical Center; Teachers College, Columbia University; and a National Institute of Mental Health training grant (T32MH13043). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other funders. We are grateful to the families who participated in this study. We also thank Elaine Maskus, Rehan Rehman, Rachel RouChen Lin, Charles Sisk, Mayra Lemus Rangel, Lexi Paul, Samantha Moffett, Julissa Veras, and Victor Issa Garcia for assisting with data collection.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Andersson JLR, Graham MS, Drobnjak I, Zhang H, Filippini N, & Bastiani M (2017). Towards a comprehensive framework for movement and distortion correction of diffusion MR images: Within volume movement. NeuroImage, 152, 450–466. 10.1016/j.neuroimage.2017.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathelt J, Johnson A, Zhang M, & Astle DE (2019). The cingulum as a marker of individual differences in neurocognitive development. Scientific Reports, 9(1), 10.1038/s41598-019-38894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C (2002). The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in Biomedicine, 15(7–8), 435–455. 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Belsky J (2019). Early-life adversity accelerates child and adolescent development. Current Directions in Psychological Science, 28(3), 241–246. 10.1177/0963721419837670 [DOI] [Google Scholar]
- Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, Even A, Levy Y, & Ben Sira L (2007). Accelerated maturation of white matter in young children with autism: A high b value DWI study. NeuroImage, 37(1), 40–47. 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, & Duncan GJ (1997). The effects of poverty on children. The Future of Children, 7(2), 55–71. 10.2307/1602387 [DOI] [PubMed] [Google Scholar]
- Bubb EJ, Metzler-Baddeley C, & Aggleton JP (2018). The cingulum bundle: Anatomy, function, and dysfunction. Neuroscience & Biobehavioral Reviews, 92, 104–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, & Tottenham N (2016). The stress acceleration hypothesis: Effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences, 7, 76–81. 10.1016/j.cobeha.2015.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M (2006). Diffusion tensor magnetic resonance imaging tractography in cognitive disorders. Current Opinion in Neurology, 19, 599–606 10.1097/01.wco.0000247610.44106.3f [DOI] [PubMed] [Google Scholar]
- Chen R, Muetzel RL, El Marroun H, Noppe G, van Rossum EFC, Jaddoe VW, Verhulst FC, White T, Fang F, & Tiemeier H (2016). No association between hair cortisol or cortisone and brain morphology in children. Psychoneuroendocrinology, 74, 101–110. 10.1016/j.psyneuen.2016.08.023. [DOI] [PubMed] [Google Scholar]
- Chetty S, Friedman AR, Taravosh-Lahn K, Kirby ED, Mirescu C, Guo F, Krupik D, Nicholas A, Geraghty A, Krishnamurthy A, Tsai MK, Covarrubias D, Wong A, Francis D, Sapolsky RM, Palmer TD, Pleasure D, & Kaufer D (2014). Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Molecular psychiatry, 19(12), 1275–1283. 10.1038/mp.2013.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LH, Burt CE, & Bjorck JP (1987). Life stress and adjustment: Effects of life events experienced by young adolescents and their parents. Developmental Psychology, 23, 583–592 10.1037/0012-1649.23.4.583 [DOI] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983): A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- Dennison MJ, Rosen ML, Sambrook KA, Jenness JL, Sheridan MA, & McLaughlin KA (2019). Differential Associations of Distinct Forms of Childhood Adversity With Neurobehavioral Measures of Reward Processing: A Developmental Pathway to Depression. Child Development, 90(1), 10.1111/cdev.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufford AJ, & Kim P (2017). family income, cumulative risk exposure, and white matter structure in middle childhood. Frontiers in Human Neuroscience, 11, 547. 10.3389/fnhum.2017.00547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK, & Bandalos DL (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling, 8(3), 430–457. 10.1207/S15328007SEM0803_5 [DOI] [Google Scholar]
- Evans GW (2004). The Environment of Childhood Poverty. American Psychologist, 59(2), 77–92. 10.1037/0003-066x.59.2.77. [DOI] [PubMed] [Google Scholar]
- Evans GW, & Schamberg MA (2009). Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences, 106(16), 6545–6549. 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, & Kim P (2013). Childhood poverty, chronic stress, self-regulation, and coping. Child Development Perspectives, 7(1), 43–48. 10.1111/cdep.12013 [DOI] [Google Scholar]
- Fields RD (2008). White matter in learning, cognition and psychiatric disorders. Trends in Neurosciences, 31(7), 361–370. 10.1016/j.tins.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD (2010). Change in the Brain’s White Matter. Science, 330(6005), 768–769. 10.1126/science.1199139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flom M, John AM, Meyer JS, & Tarullo AR (2017). Infant hair cortisol: associations with salivary cortisol and environmental context. Developmental Psychobiology, 59(1), 26–38. 10.1002/dev.21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, & Donzella B (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27(1–2), 199–220. 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Hackman DA, & Farah MJ (2009). Socioeconomic status and the developing brain. Trends in Cognitive Sciences, 13(2), 65–73. 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Adluru N, Chung MK, Alexander AL, Davidson RJ, & Pollak SD (2013). Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Development, 84, 1566–1578. 10.1111/cdev.12069pmid:23480812 PMID: 23480812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, Shirtcliff EA, Pollak SD, & Davidson RJ (2015). Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biological Psychiatry, 77(4), 314–323. 10.1016/j.biopsych.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Barnea-Goraly N, Haas BW, Golarai G, Ng D, Mills D, Korenberg J, Bellugi U, Galaburda A, & Reiss AL (2007). More Is Not Always Better: Increased Fractional Anisotropy of Superior Longitudinal Fasciculus Associated with Poor Visuospatial Abilities in Williams Syndrome. Journal of Neuroscience, 27(44), 11960–11965. 10.1523/jneurosci.3591-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, King LS, Sacchet MD, Camacho MC, Colich NL, Ordaz SJ, Ho TC, & Gotlib IH (2019). Evidence for a sensitive period in the effects of early life stress on hippocampal volume. Developmental Science, 22(3), e12775. 10.1111/desc.12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jednoróg K, Altarelli I, Monzalvo K, Fluss J, Dubois J, Billard C, Dehaene-Lambertz G, & Ramus F (2012). The Influence of Socioeconomic Status on Children’s Brain Structure. PLoS ONE, 7(8), e42486. 10.1371/journal.pone.0042486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Evans GW, Chen E, Miller G, & Seeman T (2017). How socioeconomic disadvantages get under the skin and into the brain to influence health development across the lifespan. In Halfon N, Forrest CB, Lerner RM, & Faustman E (Eds.), Handbook of life course health development (pp. 463–497). Springer. [PubMed] [Google Scholar]
- Kircanski K, Sisk L, Ho T, Humphreys K, King L, Colich N, Ordaz SJ, & Gotlib I (2019). Early life stress, cortisol, frontolimbic connectivity, and depressive symptoms during puberty. Development and Psychopathology, 31(3), 1011–1022. 10.1017/S0954579419000555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson GM, Duda JT, Avants BB, Wu J, & Farah MJ (2013). Associations between children’s socioeconomic status and prefrontal cortical thickness. Developmental Science, 16(5), 641–652. 10.1111/desc.12096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, & Beaulieu C (2011). Longitudinal Development of Human Brain Wiring Continues from Childhood into Adulthood. Journal of Neuroscience, 31(30), 10937–10947. 10.1523/jneurosci.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Treit S, & Beaulieu C (2019). A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR in Biomedicine, 32(4), e3778. 10.1002/nbm.3778. [DOI] [PubMed] [Google Scholar]
- Lichtin RD, Merz EC, He X, Desai PM, Simon KR, Melvin SA, Maskus EA, & Noble KG (2021). Material hardship, prefrontal cortex–amygdala structure, and internalizing symptoms in children. Developmental Psychobiology, 63(2), 364–377. 10.1002/dev.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, Nishino T, & Barch D (2013). The Effects of Poverty on Childhood Brain Development. JAMA Pediatrics, 167(12), 1135. 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP (2000). Prevention Science, 1(4), 173–181. 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott CL, Seidlitz J, Nadig A, Liu S, Clasen LS, Blumenthal JD, Reardon PK, Lalonde F, Greenstein D, Patel R, Chakravarty MM, Lerch JP, & Raznahan A (2019). Longitudinally mapping childhood socioeconomic status associations with cortical and subcortical morphology. The Journal of Neuroscience, 39(8), 1365–1373. 10.1523/JNEUROSCI.1808-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC, Desai PM, Maskus EA, Melvin SA, Rehan Rehman SD, Meyer J, He X, & Noble KG (2019). Socioeconomic Disparities in Chronic Physiologic Stress Are Associated With Brain Structure in Children. Biological Psychiatry, 86(12), 921–929. 10.1016/j.biopsych.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC, Tottenham N, & Noble KG (2018). Socioeconomic Status, Amygdala Volume, and Internalizing Symptoms in Children and Adolescents. Journal of Clinical Child & Adolescent Psychology, 47(2), 312–323. 10.1080/15374416.2017.1326122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J, Novak M, Hamel A, & Rosenberg K (2014). Extraction and Analysis of Cortisol from Human and Monkey Hair. Journal of Visualized Experiments, (83), 10.3791/50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, & Mazziotta J (2008). Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage, 40(2), 570–582. 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Norman MF, & Farah MJ (2005). Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science, 8(1), 74–87. PMID: 15647068 10.1111/j.1467-7687.2005.00394.x [DOI] [PubMed] [Google Scholar]
- Noble KG, Korgaonkar MS, Grieve SM, & Brickman AM (2013). Higher education is an age-independent predictor of white matter integrity and cognitive control in late adolescence. Developmental Science, 16(5), 653–664 10.1111/desc.12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, Schork NJ, Murray SS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kennedy DN, Van Zijl P, Mostofsky S, Kaufmann WE, Kenet T, Dale AM, Jernigan TL, & Sowell ER (2015). Family income, parental education and brain structure in children and adolescents. Nature Neuroscience, 18(5), 773–778. 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, & Giebler MA (2020). The neuroscience of socioeconomic inequality. Current Opinion in Behavioral Sciences, 36, 23–28. 10.1016/j.cobeha.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KM, Tronick EZ, & Moore CL (2013). Relationship between Hair Cortisol and Perceived Chronic Stress in a Diverse Sample. Stress and Health, 29(4), 337–344. 10.1002/smi.2475. [DOI] [PubMed] [Google Scholar]
- Oei NYL, Both S, van Heemst D, & van der Grond J (2014). Acute stress-induced cortisol elevations mediate reward system activity during subconscious processing of sexual stimuli. Psychoneuroendocrinology, 39, 111–120. 10.1016/j.psyneuen.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Ono M, Kikusui T, Sasaki N, Ichikawa M, Mori Y, & Murakami-Murofushi K (2008). Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male Balb/c mice. Neuroscience, 156(4), 1103–1110. 10.1016/j.neuroscience.2008.07.078 [DOI] [PubMed] [Google Scholar]
- Patel PD, Katz M, Karssen AM, & Lyons DM (2008). Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology, 33(3), 360–367. 10.1016/j.psyneuen.2007.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, Ikuta T, DeRosse P, John M, Burdick KE, Gruner P, Prendergast DM, Szeszko PR, & Malhotra AK (2014). Age-related differences in white matter tract microstructure are associated with cognitive performance from childhood to adulthood. Biological Psychiatry, 75(3), 248–256. 10.1016/j.biopsych.2013.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkauskas NV, Currie JM, & Garfinkel I (2012). The Great Recession, Public Transfers, and Material Hardship. Social Service Review, 86(3), 401–427. 10.1086/667993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver CC, Blair C, & Willoughby M (2013). Poverty as a predictor of 4-year-olds’ executive function: New perspectives on models of differential susceptibility. Developmental Psychology, 49(2), 292–304. 10.1037/a0028343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y (2012). lavaan: An R package for structural equation modeling. Journal of Statistical Software, 48(2), 1–36. URL: https://www.jstatsoft.org/v48/i02/, [Google Scholar]
- Sarason IG, Johnson JH, & Siegel JM (1978). Assessing the impact of life changes: Development of the Life Experiences Survey. Journal of Consulting and Clinical Psychology, 46, 932–946. 10.1037/0022-006X.46.5.932 [DOI] [PubMed] [Google Scholar]
- Sheikh HI, Joanisse MF, Mackrell SM, Kryski KR, Smith HJ, Singh SM, & Hayden EP (2014). Links between white matter microstructure and cortisol reactivity to stress in early childhood: Evidence for moderation by parenting. NeuroImage: Clinical, 6, 77–85. 10.1016/j.nicl.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, & Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, S208–S219. 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, & Behrens TE (2006). Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage, 31, 1487–1505. 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due-Tønnessen P, & Walhovd KB (2010). Brain Maturation in Adolescence and Young Adulthood: Regional Age-Related Changes in Cortical Thickness and White Matter Volume and Microstructure. Cerebral Cortex, 20(3), 534–548. 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Thomason ME, & Thompson PM (2011). Diffusion Imaging, White Matter, and Psychopathology. Annual Review of Clinical Psychology, 7(1), 63–85. 10.1146/annurev-clinpsy-032210-104507. [DOI] [PubMed] [Google Scholar]
- Ursache A, Noble KG, & Blair C (2015). Socioeconomic status, subjective social status, and perceived stress: Associations with stress physiology and executive functioning. Behavioral Medicine, 41(3), 145–154. 10.1080/08964289.2015.1024604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursache A, & Noble KG, (2016). Socioeconomic status, white matter, and executive function in children. Brain and Behavior, 6(10), e00531. 10.1002/brb3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursache A Merz EC, Melvin S, Meyer J, & Noble KG (2017). Socioeconomic Status, hair cortisol and internalizing symptoms in parents and children. Psychoneuroendocrinology, 78, 142–150. 10.1016/j.psyneuen.2017.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanaelst B, Michels N, De Vriendt T, Huybrechts I, Vyncke K, Sioen I, Bammann K, Rivet N, Raul JS, Molnar D, & De Henauw S (2013). Cortisone in hair of elementary school girls and its relationship with childhood stress. European Journal of Pediatrics, 172(6), 843–846. 10.1007/s00431-013-1955-1 [DOI] [PubMed] [Google Scholar]
- Vliegenthart J, Noppe G, van Rossum EFC, Koper JW, Raat H, & van den Akker ELT (2016). Socioeconomic status in children is associated with hair cortisol levels as a biological measure of chronic stress. Psychoneuroendocrinology, 65, 9–14. 10.1016/j.psyneuen.2015.11.022. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, & Mori S (2007). Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage, 36(3), 630–644. 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis CN, Belleau EL, Pedersen WS, Miskovich TA, & Larson CL (2018). Structural Connectivity of the Posterior Cingulum Is Related to Reexperiencing Symptoms in Posttraumatic Stress Disorder. Chronic Stress, 2, 247054701880713. 10.1177/2470547018807134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Lynch J, & Chen Q, & John Deighton served as editor and Gavan Fitzsimons served as associate editor for this article. (2010). Reconsidering Baron and Kenny: Myths and truths about mediation analysis. Journal of Consumer Research, 37(2), 197–206. 10.1086/651257 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.