Abstract
The risk of infant exposure to dextromethorphan (DM) and its active metabolite, dextrorphan (DX), through breast milk has not been evaluated. In this study, bound and unbound DM and DX concentrations in breast milk and plasma at 2 h post-dose were measured in 20 lactating women (n=20) following a single 30 mg oral dose of DM. The DM and DX concentrations in breast milk were positively correlated with their respective plasma concentrations. The breast milk-to-plasma (M/P) ratios of 1.0 and 1.6 and the unbound M/P ratios of 1.1 and 2.0 for DM and DX, respectively, suggested that DM and DX are extensively distributed into breast milk. The infant exposure following a single dose of 30 mg DM was estimated using the breast milk concentrations to be 0.33 ± 0.32 μg/kg/day and 1.8 ± 1.0 μg/kg/day for DM and DX, respectively. The steady-state infant exposure was estimated using the M/P ratios and previously reported AUC of DM and DX following repeated dosing of DM 60 mg orally twice daily to be 0.64 ± 0.22 μg/kg/day and 1.23 ± 0.38 μg/kg/day, respectively. Based on these estimated infant doses, the relative infant doses (RIDs) were estimated to be <1%, suggesting the infant is only exposed to a minor fraction of adult dose through breast milk; however, one nursing infant developed an erythematous rash during this study which warrants additional research to fully elucidate the risks of infant exposure to DM and DX through breast milk.
Keywords: Dextromethorphan, dextrorphan, infant exposure, drug safety, drug distribution into breast milk
Introduction
Dextromethorphan (DM) is a commonly used antitussive drug that is included as an active ingredient in more than 140 over-the-counter (OTC) cough and cold remedies in the United States. Although the mechanism of action of DM is not fully understood, DM has been shown to interact with a number of central nervous system receptors and transporters including N-methyl-D-aspartate (NMDA) receptors and serotonin transporters.1 DM generally has low toxicity, but has been reported to induce psychosis at high doses (>1500 mg/day).2 Despite their common use in adults, cough and cold medication use by young children has been restricted due to lack of evidence to support effectiveness and serious side effects including CNS excitation, ataxia, palpitations, and even death have been reported in young children.3–5 In one study, accidental overdoses with dextromethorphan in children under 5 have been associated with lethargy (20.4%), which included one patient requiring hospitalization.6 In a second study predominantly involving dextromethorphan overdose in pediatric patients, adverse effects included ataxia, tachycardia, flushing and/or urticarial rash and dystonia.7 Since breast milk is a possible route of DM exposure for infants,8 it is scientifically relevant to evaluate the infant DM exposure through breast milk.
DM is readily absorbed and undergoes substantial first pass metabolism by the liver following oral dosing.9 It is extensively metabolized by CYP2D6 to dextrorphan (DX) and by CYP3A4 to 3-methoxymorphinan (3MM).10 DX is further metabolized to dextrorphan-glucuronide (DG) by UGT2B and to 3-hydroxymorphinan (3HM) by CYP3A4, while 3MM is further metabolized to 3HM by CYP2D6.11 The circulating concentration of DX and DG are ~5-fold and ~200-fold greater than DM, respectively, following oral administration in CYP2D6 extensive metabolizers.10,12,13 DM and DX are extensively distributed to the brain. The brain-to-plasma ratios of DM and DX have been shown to be 8.1 and 4.3, respectively, in rats following intraperitoneal (IP) administration.9 In contrast, DG was not detected in the brain despite the high circulating concentration. This suggests limited partitioning of DG to the brain. In addition to the high circulating and brain concentrations of DX, DX has been shown to interact with N-methyl-D-aspartate (NMDA) receptors as noncompetitive antagonist similar to DM.1,14,15 These results collectively suggest that DX is an active metabolite of DM and may contribute to the pharmacological effects of DM. Hence, it is important to also evaluate the infant DX exposure through breast milk following maternal use of DM.
DM’s and DX’s distributions into breast milk depend on their pharmacokinetic characteristics, mammary gland physiology and the physiochemical properties of DM and DX. During gestation, blood supply to the mammary gland is increased and the mammary epithelial cells (MECs) grow and differentiate to form new alveolar structures in the breasts to prepare for milk production and storage. These matured milk-secreting luminal MECs form a semipermeable lipid layer which controls the distribution of nutrients and other essential elements from blood into breast milk.16–18 While the secreted milk is sitting in the alveolar lumen before it is emptied, it acts as a transient distribution compartment where circulating DM and DX can be distributed to and from the breast milk.8 Based on the lipophilic nature of DM (logP: 4.1) and DX (logP: 3.5),19 it is expected that both DM and DX will be readily distributed into breast milk. Surprisingly, no information on infant exposure to DM and DX through breast milk is available to provide evidence for safe use of DM in breastfeeding women.20 To fill this key knowledge gap, we report the DM and DX concentration in breast milk and plasma following a single oral dose of DM to lactating women. Infant exposure to DM and DX via breast milk was estimated using the measured breast milk concentrations.
Methods
Subjects
All enrolled subjects were between 18–50 years of age and CYP2D6 extensive metabolizers (genotyped as described below). Subjects provided written informed consent to participate in this study. The study protocol was approved by the institutional review board at the University of Washington and conducted in accordance with its guidelines.
CYP2D6 genotyping
Buccal swabs were collected from consented subjects for CYP2D6 genotyping. Genomic DNA was extracted from the buccal swab using the Qiagen QIAamp DNA Mini Kit (Germantown, MD). CYP2D6 genotype assays were performed on a StepOnePlus (Applied Biosystems) instrument following the manufacturer’s recommended protocols. Samples were genotyped for nine core single nucleotide polymorphisms (SNPs) to identify common CYP2D6 variant alleles using TaqMan Genotyping Master Mix and SNP genotyping assays (ThermoFisher, Waltham, MA) for the following: *2 (2851C>T; C_27102425_10), *3 (2550delA; C_32407232_50), *4 (1847G>A; C_27102431_D0), *6 (1708delT;, C_32407243_20), *9 (2616delAAG; C_32407229_60), *10 (100C>T; C_11484460_40), *17 (1022C>T; C_2222771_A0), *35 (31G>A; C_27102444_F0), and *41 (2989G>A; C_34816116_20). SNP genotyping assays were qualitative and considered homozygous for an allele if a signal was observed for either VIC or FAM reporter dyes and heterozygous if both reporter dyes were measured for a given assay. CYP2D6 copy number was determined using the TaqMan copy number assay targeting exon 9 (Hs00010001_cn) compared to the reference housekeeping gene assay for RNAseP (ThermoFisher cat. 4403326) present in two copies. All assays were performed as technical replicates. SNP assays yielding differences between technical replicates or unclear reporter dye signals were repeated on a separate day along with previously genotyped samples serving as positive and negative controls for the targeted assays. Copy number assays yielding discrepancy between the assigned alleles and measured copy number were repeated on a separate day along with previously genotyped samples serving as calibrator samples and analyzed using the 2−ΔΔCT method.21
The CYP2D6 genotype was assigned to each allele based on a previously published genotyping strategy.22 For samples with only 1 copy number, the *5 allele (deletion) was assigned. For all other alleles, the decision tree shown in Fig 1 was used. For alleles negative for *2 and all subsequent assays lacking the *2 SNP, the genotype was assigned at *1. Likewise, for alleles containing the *2 SNP, but lacking the sequential SNPs tested, the *2 allele was assigned. An activity score was assigned to each CYP2D6 variant allele (Fig 1) based on the previously published phenotype for each genotype.23 The CYP2D6 activity score for each subject was calculated by summation of the activity score of all alleles. Only subjects with CYP2D6 activity score between 1 and 2 (extensive metabolizers) were included in the study. Subjects with an activity score < 1 (intermediate and poor metabolizers) or > 2 (ultra-rapid metabolizers) were excluded from the study.
Figure 1:
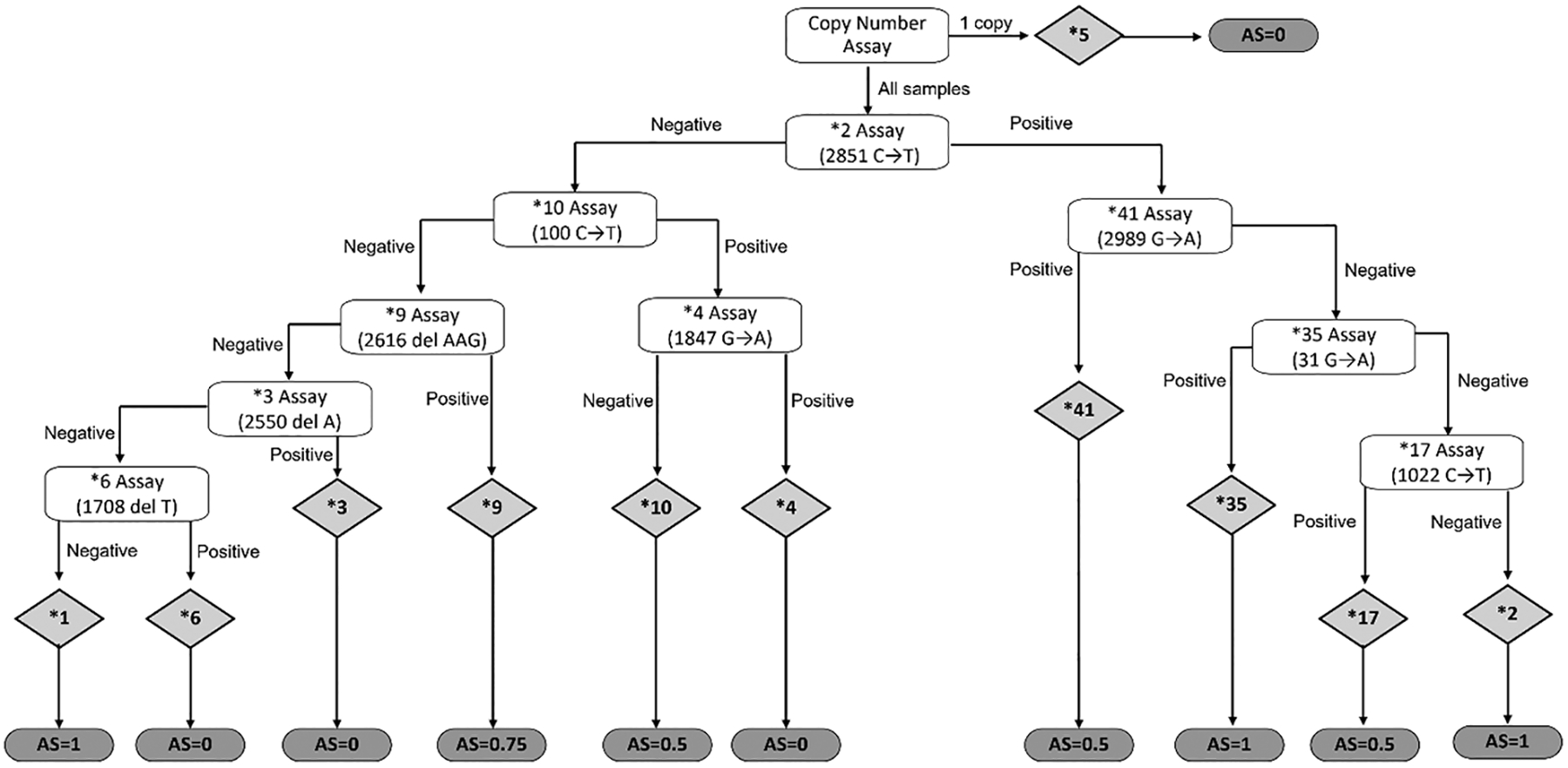
Decision tree for the assignment of genotype and activity score (AS). Each maternal DNA sample was analyzed using real-time PCR and TaqMan genotyping assays for *2, *3, *4, *6, *9, *10, *17, *35, and *41 alleles. A copy number assay was also performed and a genotype of *5 was assigned if only 1 allele was determined to be present. A *1 was assigned for alleles negative for all assays lacking *2 allele SNP. White boxes represent the assay performed for a given allele, grey diamonds represent the resulting allele assigned, and the grey ovals represent the activity score assigned to the corresponding allele.
Lactation study day
This lactation study is an ongoing research protocol taking advantage of the opportunity to evaluate medications in the breastmilk from lactating women already receiving a wide range of medications. In this case, we were able to study a series of lactating women receiving a single 30 mg dose of dextromethorphan as part of another research protocol. This provided us the opportunity to collect breast milk and plasma for this study. The subjects were studied ≥ 3 months postpartum as that is when they were to receive the dextromethorphan. On the study day, the subjects took a single 30 mg oral dose of DM and had a planned single blood sample collection 2 hours post-dose for the research protocol. The collection of the breast milk sample for the lactation study was performed just prior to the blood sample collection.
Subjects were asked to fast (except for clear liquids) for ≥ 4 hours prior to the study. The single dose of dextromethorphan (DM) was administered with water. The blood sample was collected using aluminum foil-wrapped vacutainer tubes containing K2 EDTA as the anti-coagulant. Blood samples were immediately placed on wet ice and then within 10 minutes, centrifuged at 3,000 g for 10 min for plasma separation. Breast milk was collected by entirely emptying one breast of milk by pumping for 10 minutes or until no more milk could be produced, using a Medela Pump in Style® breast pump (McHenry, IL) just prior to blood sample collection (except in one subject for whom the breast milk sample collection was initiated 9 min after blood sampling). All breast milk from the single breast collection for each subject was combined, an aliquot was collected for analysis and the remaining breast milk was returned to the mother. The single sample approach with a complete emptying of the breast of milk to collect the sample was selected for multiple reasons. First, this approach was chosen to get the best estimate of milk concentration by including all milk in the collection. This is important as foremilk and hindmilk can have differing concentrations. Second, this approach improved study feasibility and enhanced subject recruitment. Lastly, this approach limited the impact on infant feeding and reduced the number of times bottle feeding or other strategies to get the breast milk back to the infant needed to be employed. The plasma and breast milk samples were stored at −80° C until analysis.
HPLC-MS/MS analysis
Total and unbound DM and DX concentrations in breast milk and plasma were determined using a previously published HPLC-MS/MS method with slight modifications.24 Briefly, aliquots of 100 μL of breast milk or plasma were ultracentrifuged at 453,630 g for 90 min at 37° C to measure the unbound DM and DX concentration while a separate set of aliquots were incubated at 37° C for 90 min to measure the total DM and DX concentration in breast milk and plasma. After ultracentrifugation, the supernatant was collected and analyzed together with the incubated samples. Standard curve and spiked QCs were prepared in naïve plasma and breast milk (Mother’s Milk Bank of Montana, Missoula MT). Two parts of acetonitrile containing internal standard (DM-d3 and DX-d3) were added to each sample or standard. The precipitated samples were then centrifuged at 3,500 g for 30 min at 4° C and the supernatant was collected for HPLC-MS/MS analysis. To monitor for the analytes’ stability in breast milk and plasma during the above incubation period, additional breast milk and plasma samples were prepared without incubation at 37° C. The processed samples were analyzed using the Sciex 5500 QTRAP mass spectrometer (AB Sciex; Foster City, CA) connected in line with an Agilent 1290 UHPLC (Agilent; Santa Clara, CA) equipped with a Kinetex EVO C18 column (2.1 × 100 mm, 2.6 um) and guard column from Phenomenex (Torrance, CA). Gradient elution of aqueous 20 mM ammonium formate (mobile phase A) and 50:50 (%v/v) acetonitrile:methanol (mobile phase B) with a flow rate at 0.5 mL/min was used. The gradient was initiated at 5% B, gradually increased to 100% B over 2 min., continued at 100% B for 0.5 min. before returning to the initial conditions and held for an additional 2 min. The analytes were detected by electrospray ionization operated in positive ion mode. One MS/MS transition was monitored for each analyte for quantification (DM: m/z 272 > 215, DX: 258 > 157, DM-d3: m/z 275 > 215, and DX-d3: m/z 261 > 157). The lower limit of quantitation (LLOQ) for DM and DX were 0.25 ng/mL and 1.5 ng/mL in plasma and 0.5 ng/mL and 1.5 ng/mL in breastmilk, respectively.
Data analysis
The total and unbound DM and DX concentrations in breast milk and plasma, the % bound in breast milk and plasma, and the breast milk to plasma concentration ratios were reported as the mean, standard deviation (SD), and 95% confidence interval (CI) in all subjects (n=20) except in one subject from whom the unbound DM and DX concentrations in plasma were not measured due to insufficient sample volume. The % bound in breast milk and plasma was calculated using eq. 1:
| eq.1 |
where unbound concentration is the concentration of the ultracentrifuged sample and total concentration is the concentration of the same sample following incubation at 37° C without ultracentrifugation as described above. The breast milk to plasma concentration ratios were calculated by dividing the total concentration in breast milk by the total concentration in plasma. The DM and DX stability in breast milk and plasma following incubation at 37° C was determined by calculating the % recovery using eq. 2:
| eq.2 |
and the analytes were determined to be stable if % recovery was between 80% and 120%. The correlations for total and unbound DM and DX concentrations in breast milk and plasma were determined by simple linear regression. All calculations were performed using Microsoft Excel in Microsoft 365 (Redmond, WA). Graphical representation of results and linear regression were done using GraphPad Prism version 8 (San Diego, CA).
Daily infant exposure and relative infant dose (RID) estimations
Daily infant exposure by bodyweight and the bodyweight-adjusted relative infant dose (RID) were estimated as previously described.8,25 Infant exposure was estimated using two approaches. The first was to estimate the infant exposure to DM and DX via breast milk following a single 30 mg oral DM dose using eq. 3:
| eq.3 |
where Cmilk is the breast milk concentration of DM or DX at 2 h post-dose (estimated Tmax) from each subject (n=20) and Vmilk is the estimated volume of milk consumed by an average infant (Vmilk = 150 mL/kg/day) over 5 half-lives of DM and DX (half-lives ~4 h)26 which equals to 125 mL/kg/20 h.
The second approach to estimate infant exposure to DM and DX via breast milk was designed to determine the average steady state infant exposure if the mother was taking DM 60 mg orally every 12 hours using eq. 4:
| eq.4 |
where Css is the average DM or DX concentration calculated using the previously published AUC0-τ following repeated 60 mg DM oral dose every 12 hours at steady-state divided by the dosing interval (τ = 12h),27 BM/P is the average DM or DX breast milk to plasma concentration ratio measured in this study (n=20), and Vmilk is the estimated daily volume of milk consumed by an infant (Vmilk = 150 mL/kg/day). The RID was calculated by eq. 5:
| eq.5 |
where daily infant exposure was calculated as described in eq. 3 (single dose) or eq. 4 (steady state), and the maternal dosage was calculated by dividing 30 mg (single dose) or 120 mg (steady state) by the average weight of 66 kg to be 455 μg/kg or 1818 μg/kg. To calculate the RID of DX, the daily maternal dosage was calculated by dividing the molecular weight-corrected dose of 28.5 mg (single dose) or 113.8 mg (steady state) of DX by the average weight of 66 kg to be 431 μg/kg or 1724 μg/kg. The infant exposure and RID were reported as the mean, SD, and 95% CI of all subjects (n=20).
Results
Subject demographics
Subject demographic information (n=20) is listed in Table 1. All 20 subjects enrolled in this study completed the study and the data for all subjects were included in the analysis. All subjects were CYP2D6 extensive metabolizers, 9 subjects had activity scores of 2, 5 subjects had activity scores between 1.5 and 1.75, and 6 subjects had activity scores of 1.
Table 1.
Demographics.
| Maternal characteristics (n=20) | |
|
Age (yr)
Mean ± SD (range) |
32.8 ± 4.0 (27 – 42) |
|
Weight (kg)
Mean ± SD (range) |
65.9 ± 9.2 (42 – 81) |
|
Race
(No. of subjects) |
White (15), Black (2), Asian (2), Pacific Islander (1) |
|
CYP2D6 genotype
(No. of subjects) |
*1/*1 (4), *1/*2 (4), *2/*35 (1), *1/*9 (1), *1/*41 (1), *2/*10 (1), *2/*17 (1), *2/*41 (1), *1/*3 (1), *2/*4 (3), *2/*5 (1), *4/*35 (1) |
| Neonatal characteristics (n=20) | |
|
Age (wk)
Mean ± SD (range) |
16.2 ± 5.1 (13 – 37) |
|
Weight (kg)
Mean ± SD (range) |
6.1 ± 0.9 (5.2 – 8.3) |
Dextromethorphan (DM) and Dextrorphan (DX) concentration in plasma and breast milk
The plasma and breast milk concentrations of DM and its active metabolite, DX, at 2 h following an oral dose of 30 mg of DM (n=20) are listed in Table 2. The total and unbound DM concentrations in breast milk were similar to the respective concentrations in plasma. In addition, the protein binding of DM was similar in breast milk and plasma. The mean total and unbound breast milk to plasma DM concentration ratios were 1.0 and 1.1, respectively. (Table 2). In contrast, both total and unbound DX concentrations in breast milk were higher than the respective concentrations in plasma. In addition, the protein binding of DX in breast milk was about 50% of that in plasma. The mean total and unbound breast milk to plasma DX concentration ratios were 1.6 and 2.0, respectively. (Table 2). The total and unbound breast milk DM and DX concentrations positively correlated with the respective plasma concentrations as shown in Fig 2.
Table 2.
Total and unbound dextromethorphan (DM) and dextrorphan (DX) concentrations in plasma and breast milk at 2 h following a 30 mg oral dextromethorphan dose in lactating women (n=20).
| Total Mean ± SD (95% CI) |
Unbound Mean ± SD (95% CI) |
%Bound Mean ± SD (95% CI) |
|
|---|---|---|---|
|
DM plasma
(ng/mL) |
3.0 ± 2.8 (1.7, 4.3) |
*1.7 ± 1.8 (0.9, 2.6) |
*40 ± 17 (32, 48) |
|
DM breast milk
(ng/mL) |
2.7 ± 2.5 (1.5, 3.9) |
1.6 ± 1.4 (0.9, 2.2) |
40 ± 11 (35, 46) |
| DM breast milk/plasma ratio | 1.0 ± 0.3 (0.8, 1.1) |
*1.1 ± 0.7 (0.7, 1.4) |
not applicable |
|
DX plasma
(ng/mL) |
8.6 ± 3.1 (7.2, 10.1) |
*6.0 ± 2.5 (4.8, 7.2) |
*32 ± 11 (27, 38) |
|
DX breast milk
(ng/mL) |
14.0 ± 8.3 (10.1, 17.9) |
11.7 ± 5.6 (9.0, 14.3) |
14 ± 9 (10, 18) |
| DX breast milk/plasma ratio | 1.6 ± 0.5 (1.4, 1.8) |
*2.0 ± 0.5 (1.8, 2.3) |
not applicable |
Data reported are the arithmetic mean ± standard deviation (SD) and the 95% confidence interval (95% CI) of data from all subjects. The unbound DM and DX concentrations were measured following ultracentrifugation at 37° C for 90 min. and both DM and DX were shown to be stable at 37° C within the timeframe (data not shown).
n=19
Figure 2.
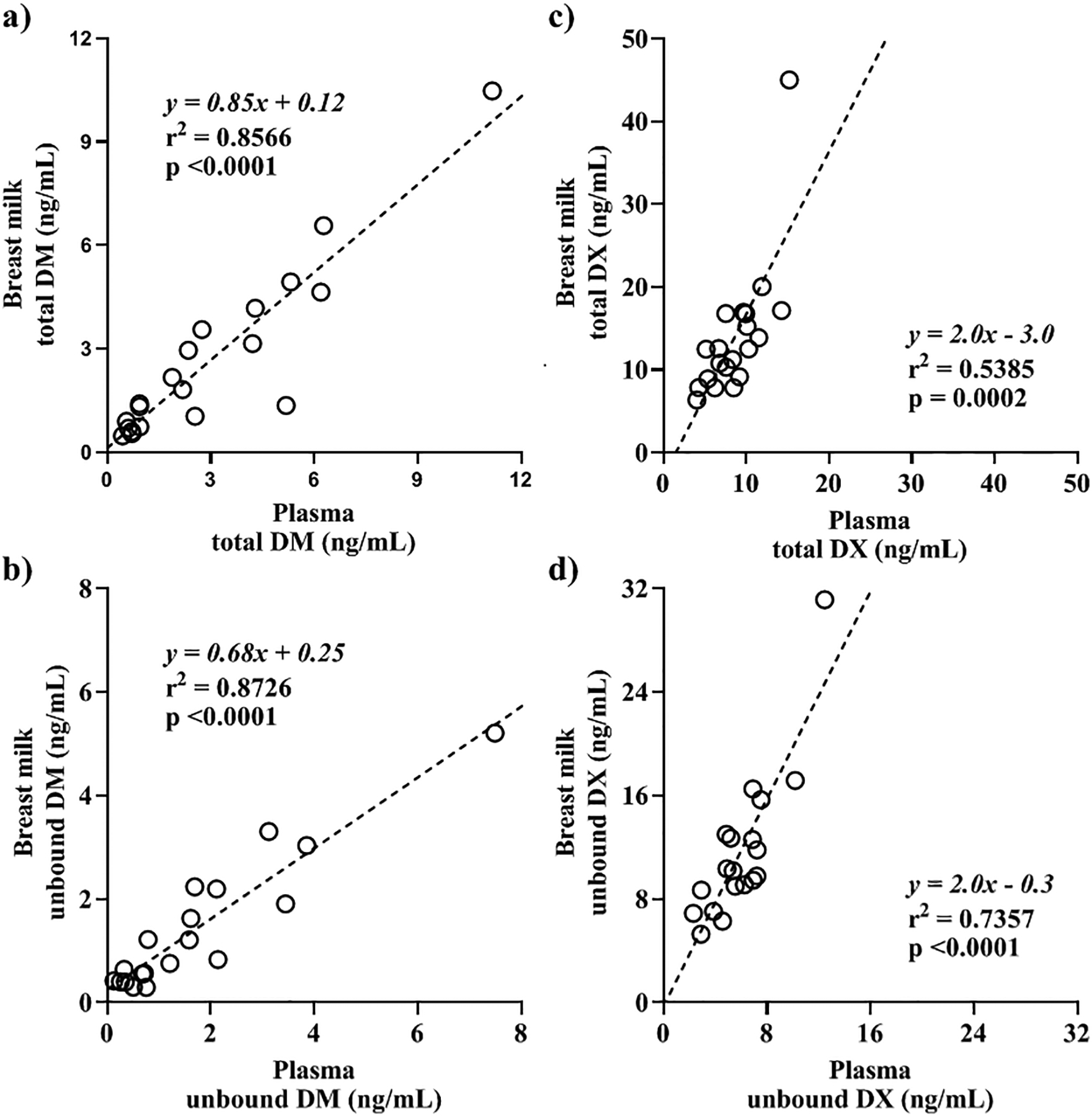
Breast milk concentration versus plasma concentration at 2 h following a 30 mg oral dose of dextromethorphan in lactating women, ≥ 3 months postpartum. a) Total dextromethorphan (DM) (n=20), b) unbound DM (n=19), c) total dextrorphan (DX) (n=20), and d) unbound DX concentration (n=19) in breast milk (y-axis) and plasma (x-axis). Open circles represent the observed breast milk and plasma concentrations of each subject and the dashed lines represent the best-fitted linear regression line. The equation, R-squared (R2) value, and the p-value of slope deviation from zero of the simple linear regression are reported on each respective graph.
Estimated infant exposure and relative infant dose (RID)
The infant exposure to DM and DX through breast milk following a single maternal oral dose of 30 mg DM was 0.33 ± 0.32 μg/kg/day and 1.8 ± 1.0 μg/kg/day, respectively. The relative infant doses (RID) following a single dose were 0.07 ± 0.07 % for DM and 0.41 ± 0.24 % for DX. The steady-state infant exposure of DM and DX through breast milk following 60 mg BID dosing was 0.64 ± 0.22 μg/kg/day and 1.23 ± 0.38 μg/kg/day for DM and DX, respectively. The RID at steady-state were 0.04 ± 0.01 % for DM and 0.07 ± 0.02 % for DX.
Nursing Infant Adverse Events
One adverse event was reported in a neonate who developed an erythematous rash on the body and face. The rash resolved without treatment in five days.
Discussion
The objectives of this study were to describe the transfer of DM and DX into breast milk and to estimate the infant DM and DX exposure through breast milk following maternal DM administration. Our results show that both DM and DX partition into breast milk and the breast milk concentrations positively correlate with the maternal plasma concentrations. These correlations demonstrate that the plasma concentration is one factor driving the distribution of DM and DX into the breast milk. Additionally, the breast milk to plasma (M/P) ratios of DM (M/P ratio = 1) and DX (M/P ratio = 2) at 2 h following an oral dose indicate that both DM and DX are extensively distributed from plasma into breast milk. The efficient transfer of DM and DX into breast milk is expected based on the lipophilicity of DM (logP: 4.1) and DX (logP: 3.5),19 the increased ionization of DM (pKa: 9.8) and DX (pKa: 9.7) in breast milk (pH = 7.2), and the increased mammary blood flow during lactation to support milk production.16 In addition, drug distribution into breast milk is governed by other factors such as protein binding in breast milk and plasma, interactions with drug transporters, and metabolism by drug metabolizing enzymes expressed in the MECs.8,28
To examine the effect of protein binding on the M/P ratios of DM and DX, the unbound concentrations of DM and DX in plasma and breast milk were measured. The results show that the fraction unbound (fu) of DM was similar between plasma and breast milk resulting in similar total (M/P ratio = 1) and unbound M/P ratios (unbound M/P ratio = 1.1). If protein binding was the only factor involved in DX distribution into breast milk, we would expect that the lower protein binding in breast milk than in plasma would result in total DX M/P ratio being less than 1 and unbound DX M/P ratio would equal 1. However, total DX M/P ratio was 1.6. and unbound DX MP ratio was 2.0 suggesting other factors are likely contributing to the distribution of DX into breast milk. These other factors are likely the active transport of DX by drug transporters and metabolism of DM to DX by drug metabolizing enzymes expressed in the MECs.29,30 DM has been shown to be a substrate of P-glycoprotein (P-gp) efflux transporter31 and both DM and DX have been shown to be transported by an unidentified transporter.32 Moreover, gene expression of multiple drug transporters including P-gp and organic cation transporters (OCTs) have been detected in the MECs28,29,33 suggesting that drug transporters may play an important role in the distribution of DM and DX into breast milk. Furthermore, metabolism by CYP enzymes expressed in the MECs including CYP3A4 and CYP2D630 might decrease the concentration of DM while increasing the concentration of DX in breast milk.
The nursing infant exposure to DM and DX through breast milk following a single oral dose of 30 mg DM to lactating women were estimated using the breast milk DM and DX concentrations at 2 h post-dose. In addition, we assumed that all the DM and DX would be gone after 5 half-lives and that the total volume of milk consumed (125 mL/kg/20 h) contained the same concentration. This approach is expected to overestimate the actual infant exposure (i.e. worst case scenario) following a single dose of DM by the mother. Nevertheless, the low RID (<1%) suggests that infant exposure is minimal. Additionally, the nursing infant exposure to DM and DX through breast milk at steady state following repeated oral doses of 60 mg DM every 12 hours were estimated using a previously reported steady state DM and DX AUC in adults and the observed M/P ratios of DM and DX in this study. In contrast to the single dose infant exposure, the steady state infant exposure is a more realistic estimation of the actual infant exposure because it was calculated using the steady state concentration (Css). The estimated low RID (<0.1%) suggests that infant exposure is negligible even with DM and DX readily transferring into breast milk.33 The high maternal clearances of DM and DX decrease the amount of DM and DX distributed into the breast milk, which also limits infant exposure.
It is important to note a few limitations of this study. First, the breast milk and plasma DM and DX concentrations were measured at a single time point (previously reported plasma Tmax).10,34 Since the DM and DX concentration-time profiles in breast milk are not known, it is possible that there is a delay in the distribution of DM and/or DX into breast milk, which could delay the breast milk Tmax and the 2 hour post dose sampling resulting in missing the true breast milk Cmax. The underestimated Cmax in breast milk may lead to underestimation of infant exposure following a single dose of DM by the mother. However, the RID was estimated using the milk concentrations at 2 h post-dose across 5 plasma half-lives, hence, the estimated RID should still exceed the actual infant exposure even if we missed the true Cmax in breast milk. Similarly, the M/P ratios reported in this study were calculated using the concentrations at a single time point instead of using the area under the concentration-time curve (AUC). In the ideal situation, the M/P ratio would be calculated using the AUC for milk and plasma. Because the M/P concentration ratio is affected by the distribution kinetics and changes over time until distribution equilibrium is reached, M/P AUC ratio is not affected by the distribution kinetics, whereas a single time point M/P ratio is.33 The over- or underestimated M/P ratio may lead to over- or underestimation of infant exposure following repeated dose of DM by the mother. Nevertheless, even if the actual M/P ratio at peak breast milk concentration is somewhat different than we report here, the RID in our steady-state DM and DX estimation is so low that the difference is unlikely to result in clinically important differences.
Second, the feasibility to extrapolate the results to the general population may be limited by enrolling only CYP2D6 EM subjects. DM is metabolized by CYP2D6 to DX, a major and active metabolite, and hence, DM and DX disposition and breast milk concentration are expected to be different among CYP2D6 ultra metabolizers (UM), EM, and poor metabolizers (PM). For example, the plasma DM concentration is expected to be higher in PMs but lower in UMs while the plasma DX concentration is expected to be higher in UMs but lower in PMs when compared to EMs because of their phenotypic CYP2D6 activities. Plasma DM concentration have been reported to be 8-fold higher in CYP2D6 PMs compared to EMs.35 However, since the estimated infant exposure to DM is very low for EMs (RIDs < 0.1%), the infant exposure is likely to be minimal even with the observed significant higher DM concentration in PMs. Conversely, a similar urinary DM/DX ratio has been reported in UMs when compared to EMs22,36, indicating similar plasma DM and DX exposure between EMs and UMs. Based on this observation, the infant exposure to DM and DX is not expected to be clinically different between CYP2D6 UMs and EMs.
Third, the infant DM and DX plasma concentrations were not measured in this study. The infant plasma concentrations could confirm not only infant dose through breast milk but also the infant’s ability to metabolize DM and DX. The protein expression of CYP3A4, CYP2D6, and UGTs in infants less than a year of age is 30–70% that of adults and is highly variable among individuals.37 Therefore, an infant might have higher DM and DX plasma concentrations than would be expected if drug metabolizing enzyme activity were fully mature at birth. However, given the amount of these compounds in the breast milk, having significant accumulation of DM and DX in nursing infants is extremely unlikely. One infant of a mother participating in this study developed an erythematous rash on the day after the study day. It is not clear whether this adverse event was drug related as infants often develop transient rashes, although temporally it corresponded with the study. The estimated DM and DX exposures through breast milk for this infant were less than the reported means, but low doses of medications can still cause allergic reactions. Additional studies are needed to fully determine the clinical risks of infant exposure to DM and DX through breast milk.
In conclusion, DM and its active metabolite, DX, are rapidly and extensively distributed into the breast milk. The high unbound M/P ratio of DX indicated that drug transporters and drug metabolizing enzymes expressed in the MECs may significantly contribute to the distribution of DX into breast milk. Using the breast milk concentration and M/P concentration ratio measured in this study, the estimated RIDs for DM and DX suggest that infants are exposed to a minor fraction of the maternal dose through breast milk. However, given the limitations of the study design, additional studies are warrant to fully elucidate the clinical risks of infant exposure to DM and DX through breast milk.
Acknowledgement:
This research was supported in part by the National Institute of General Medical Sciences grant # R01GM124264. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Footnotes
Disclosure: NI reports consultancy agreements with Boehringer-Ingelheim and Johnson and Johnson. None of the other authors have conflicts of interest.
Data Sharing:
The authors of this manuscript conform to NIH requirements for data sharing. For questions regarding data sharing related to the data in this manuscript, please contact Mary F. Hebert, PharmD, FCCP at mhebert@uw.edu.
References
- 1.Taylor CP, Traynelis SF, Siffert J, Pope LE, Matsumoto RR. Pharmacology of dextromethorphan: Relevance to dextromethorphan/quinidine (Nuedexta®) clinical use. Pharmacol Ther. 2016;164:170–182. [DOI] [PubMed] [Google Scholar]
- 2.Spangler DC, Loyd CM, Skor EE. Dextromethorphan: A case study on addressing abuse of a safe and effective drug. Subst Abus Treat Prev Policy. 2016;11(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Food and Drug Administration. Use caution when giving cough and cold products to kids. https://www.fda.gov/drugs/special-features/use-caution-when-giving-cough-and-cold-products-kids. Published 2018.
- 4.Isbister GK, Prior F, Kilham HA. Restricting cough and cold medicines in children. J Paediatr Child Health. 2012;48(2):91–98. [DOI] [PubMed] [Google Scholar]
- 5.Dolansky G, Rieder M, Ontario W. Over-the-Counter Cough and Cold Preparations for. Paediatr Child Heal Vol. 2008;13(2):125–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LoVecchio F, Pizon A, Matesick L, O’Patry S. Accidental dextromethorphan ingestions in children less than 5 years old. J Med Toxicol. 2008;4(4):251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paul IM, Reynolds KM, Kauffman RE, et al. Adverse events associated with pediatric exposures to dextromethorphan. Clin Toxicol. 2017;55(1):25–32. [DOI] [PubMed] [Google Scholar]
- 8.Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100(1):42–52. [DOI] [PubMed] [Google Scholar]
- 9.Wu D, Otton SV., Kalow W, Sellers EM. Effects of route of administration on dextromethorphan pharmacokinetics and behavioral response in the rat. J Pharmacol Exp Ther. 1995;274(3):1431–1437. [PubMed] [Google Scholar]
- 10.Capon DA, Bochner F, Kerry N, Mikus G, Danz C, Somogyi AA. The influence of CYP2D6 polymorphism and quinidine on the disposition and antitussive effect of dextromethorphan in humans. Clin Pharmacol Ther. 1996;60(3):295–307. [DOI] [PubMed] [Google Scholar]
- 11.Lutz JD, Isoherranen N. Prediction of relative in vivo metabolite exposure from in vitro data using two model drugs: Dextromethorphan and omeprazole. Drug Metab Dispos. 2012;40(1):159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schadel M, Wu D, Otton SV., Kalow W, Sellers EM. Pharmacokinetics of dextromethorphan and metabolites in humans: Influence of the CYP2D6 phenotype and quinidine inhibition. J Clin Psychopharmacol. 1995;15(4):263–269. [DOI] [PubMed] [Google Scholar]
- 13.Donato JL, Koizumi F, Pereira AS, Mendes GD, De Nucci G. Simultaneous determination of dextromethorphan, dextrorphan and doxylamine in human plasma by HPLC coupled to electrospray ionization tandem mass spectrometry: Application to a pharmacokinetic study. J Chromatogr B Anal Technol Biomed Life Sci. 2012;899:46–56. [DOI] [PubMed] [Google Scholar]
- 14.Roth JE, Murray TF, Franklin PH. Regional distribution and characterization of [3H]dextrorphan binding sites in rat brain determined by quantitative autoradiography. J Pharmacol Exp Ther. 1996;277(3):1823–1836. [PubMed] [Google Scholar]
- 15.Nguyen L, Thomas KL, Lucke-Wold BP, Cavendish JZ, Crowe MS, Matsumoto RR. Dextromethorphan: An update on its utility for neurological and neuropsychiatric disorders. Pharmacol Ther. 2016;159:1–22. [DOI] [PubMed] [Google Scholar]
- 16.Truchet S, Honvo-Houéto E. Physiology of milk secretion. Best Pract Res Clin Endocrinol Metab. 2017;31(4):367–384. [DOI] [PubMed] [Google Scholar]
- 17.Obermeier S, Huselweh B, Tinel H, Kinne RHK, Kunz C. Expression of glucose transporters in lactating human mammary gland epithelial cells. Eur J Nutr. 2000;39(5):194–200. [DOI] [PubMed] [Google Scholar]
- 18.Cai C, Eck P, Friel JK. Gene expression profiles suggest iron transport pathway in the lactating human epithelial cell. J Pediatr Gastroenterol Nutr. 2017;64(3):460–464. [DOI] [PubMed] [Google Scholar]
- 19.Silva AR, Dinis-Oliveira RJ. Pharmacokinetics and pharmacodynamics of dextromethorphan: clinical and forensic aspects. Drug Metab Rev. 2020;52(2):258–282. [DOI] [PubMed] [Google Scholar]
- 20.Drugs and Lactation Database (LactMed) [Internet]. Dextromethorphan. Bethesda (MD): National Library of Medicine (US); 2006-. Tetracycline. [Updated 2018 Oct 31]. https://www.ncbi.nlm.nih.gov/books/. Published 2019. [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 22.Gaedigk A, Gotschall RR, Forbes NS, Simon SD, Kearns GL, Leeder JS. Optimization of cytochrome P4502D6 (CYP2D6) phenotype assignment using a genotyping algorithm based on allele frequency data. Pharmacogenetics. 1999;9:669–682. [DOI] [PubMed] [Google Scholar]
- 23.Hicks JK, Swen JJ, Thorn CF, et al. Clinical pharmacogenetics implementation consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin Pharmacol Ther. 2013;93(5):402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevison F, Kosaka M, Kenny JR, et al. Does In Vitro Cytochrome P450 Downregulation Translate to In Vivo Drug-Drug Interactions? Preclinical and Clinical Studies With 13-cis-Retinoic Acid. Clin Transl Sci. 2019;12(4):350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sachs HC. The transfer of drugs and therapeutics into human breast milk: An update on selected topics. Pediatrics. 2013;132(3). [DOI] [PubMed] [Google Scholar]
- 26.Silvasti M, Karttunen P, Tukiainen H, Kokkonen P, Hanninen U, Nykanen S. Pharmacokinetics of dextromethorphan and dextrorphan: a single dose comparison of three preparations in human volunteers. Int J Clin Pharmacol. 1987;25(9):493–497. [PubMed] [Google Scholar]
- 27.Pope LE, Khalil MH, Berg JE, Stiles M, Yakatan GJ, Sellers EM. Pharmacokinetics of dextromethorphan after single or multiple dosing in combination with quinidine in extensive and poor metabolizers. J Clin Pharmacol. 2004;44(10):1132–1142. [DOI] [PubMed] [Google Scholar]
- 28.Kimura S, Morimoto K, Okamoto H, et al. Development of a human mammary epithelial cell culture model for evaluation of drug transfer into milk. Arch Pharm Res. 2006;29(5):424–429. [DOI] [PubMed] [Google Scholar]
- 29.Alcorn J, Lu X, Moscow JA, McNamara PJ. Transporter gene expression in lactating and nonlactating human mammary epithelial cells using real-time reverse transcription-polymerase chain reaction. J Pharmacol Exp Ther. 2002;303(2):487–496. [DOI] [PubMed] [Google Scholar]
- 30.Hellmold H, Rylander T, Magnusson M, Reihnér E, Warner M, Gustafsson JÅ. Characterization of cytochrome P450 enzymes in human breast tissue from reduction mammaplasties. J Clin Endocrinol Metab. 1998;83(3):886–895. [DOI] [PubMed] [Google Scholar]
- 31.Uhr M, Namendorf C, Grauer MT, Rosenhagen M, Ebinger M. P-glycoprotein is a factor in the uptake of dextromethorphan, but not of melperone, into the mouse brain: Evidence for an overlap in substrate specificity between P-gp and CYP2D6. J Psychopharmacol. 2004;18(4):509–515. [DOI] [PubMed] [Google Scholar]
- 32.Kanaan M, Daali Y, Dayer P, Desmeules J. Lack of Interaction of the NMDA Receptor Antagonists Dextromethorphan and Dextrorphan with P-Glycoprotein. Curr Drug Metab. 2008;9(2):144–151. [DOI] [PubMed] [Google Scholar]
- 33.Ito S Opioids in Breast Milk: Pharmacokinetic Principles and Clinical Implications. J Clin Pharmacol. 2018;58(February):S151–S163. [DOI] [PubMed] [Google Scholar]
- 34.Abdul Manap R, Wright CE, Gregory A, et al. The antitussive effect of dextromethorphan in relation to CYP2D6 activity. Br J Clin Pharmacol. 1999;48(3):382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chládek J, Zimová G, Beránek M, Martínková J. In-vivo indices of CYP2D6 activity: Comparison of dextromethorphan metabolic ratios in 4-h urine and 3-h plasma. Eur J Clin Pharmacol. 2000;56(9–10):651–657. [DOI] [PubMed] [Google Scholar]
- 36.Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder J. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. 2007;83(2):234–242. [DOI] [PubMed] [Google Scholar]
- 37.Hines RN. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther. 2008;118(2):250–267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors of this manuscript conform to NIH requirements for data sharing. For questions regarding data sharing related to the data in this manuscript, please contact Mary F. Hebert, PharmD, FCCP at mhebert@uw.edu.


