Abstract
Earthworms (Aporrectodea caliginosa, Lumbricus rubellus, and Octolasion lacteum) obtained from nitrous oxide (N2O)-emitting garden soils emitted 0.14 to 0.87 nmol of N2O h−1 g (fresh weight)−1 under in vivo conditions. L. rubellus obtained from N2O-emitting forest soil also emitted N2O, which confirmed previous observations (G. R. Karsten and H. L. Drake, Appl. Environ. Microbiol. 63:1878–1882, 1997). In contrast, commercially obtained Lumbricus terrestris did not emit N2O; however, such worms emitted N2O when they were fed (i.e., preincubated in) garden soils. A. caliginosa, L. rubellus, and O. lacteum substantially increased the rates of N2O emission of garden soil columns and microcosms. Extrapolation of the data to in situ conditions indicated that N2O emission by earthworms accounted for approximately 33% of the N2O emitted by garden soils. In vivo emission of N2O by earthworms obtained from both garden and forest soils was greatly stimulated when worms were moistened with sterile solutions of nitrate or nitrite; in contrast, ammonium did not stimulate in vivo emission of N2O. In the presence of nitrate, acetylene increased the N2O emission rates of earthworms; in contrast, in the presence of nitrite, acetylene had little or no effect on emission of N2O. In vivo emission of N2O decreased by 80% when earthworms were preincubated in soil supplemented with streptomycin and tetracycline. On a fresh weight basis, the rates of N2O emission of dissected earthworm gut sections were substantially higher than the rates of N2O emission of dissected worms lacking gut sections, indicating that N2O production occurred in the gut rather than on the worm surface. In contrast to living earthworms and gut sections that produced N2O under oxic conditions (i.e., in the presence of air), fresh casts (feces) from N2O-emitting earthworms produced N2O only under anoxic conditions. Collectively, these results indicate that gut-associated denitrifying bacteria are responsible for the in vivo emission of N2O by earthworms and contribute to the N2O that is emitted from certain terrestrial ecosystems.
Biological production of the greenhouse gas nitrous oxide (N2O) is primarily mediated by microorganisms (8, 16). Soils account for 60 to 80% of the global emission of N2O (4, 5, 8–10, 21). Although abiotic processes can contribute, microbial processes are primarily responsible for the formation of N2O in soils (8, 9). The net emission of N2O at the soil surface depends on (i) production of N2O by soil microorganisms, (ii) consumption of N2O by soil denitrifying bacteria, and (iii) physical transport of N2O through the soil column. Denitrification and nitrification are the main microbial processes involved in emission of N2O by soils (5, 8–10, 16). However, alternative microbial N2O-producing processes, such as dissimilatory reduction of nitrate or nitrite to ammonium and assimilatory reduction of nitrate for biomass synthesis, might also contribute to N2O emission (3, 19, 20, 29, 30).
It was recently demonstrated that earthworms from forest soil emit N2O under in vivo conditions (18), which suggested that earthworms are a mobile N2O-producing microsite in forest soils. The general occurrence and significance of N2O-emitting earthworms in terrestrial ecosystems, as well as the process(es) responsible for the production of N2O by earthworms, have not been determined. The two main objectives of the present study were (i) to evaluate the in vivo emission of N2O by earthworms from two contrasting, earthworm-containing soils (namely, garden and forest soils) and (ii) to determine if a gut-associated microbial process is involved in the in vivo emission of N2O by earthworms.
MATERIALS AND METHODS
Field sites and collection of earthworms.
Earthworms (Aporrectodea caliginosa Savingny, Octolasion lacteum Örley, and Lumbricus rubellus Hoffmeister) were collected in the summer of 1997 from tilled soils (sandy loam soils) in two gardens (Heinersreuth and Weidenberg) in the vicinity of Bayreuth, Germany; properties of these soils are outlined in Table 1. The average soil dry weights of the garden soils were 78.2% ± 2.4% (Heinersreuth) and 76.2% ± 6.2% (Weidenberg). L. rubellus Hoffmeister earthworms were collected in the summer of 1996 from beech forest soils at Geisberger Forst in Germany; this site has been described previously (22). The earthworms were transported and stored in the dark in aseptic beakers containing soil or soil and litter at 10 or 15°C (depending on the in situ soil temperature) until they were used. Lumbricus terrestris L. was obtained from a local store and was stored at 4°C (the temperature used during commercial storage). The earthworms were stored for a maximum of 2 weeks before they were used and were identified by using standard protocols (28).
TABLE 1.
Characteristics of garden soils (Hortisols)a
| Site | pH (CaCl2) | Organic C concn (g kg−1)b | Total N concn (g kg−1)b | C/N ratio | NH4+ concn (mg kg−1)b | NO3− concn (mg kg−1)b |
|---|---|---|---|---|---|---|
| Heinersreuth | 7.1 (0.2) | 35.6 (5.4) | 2.2 (0.8) | 17.3 (2.6) | 1.4 (0.5) | 105.7 (82.6) |
| Weidenberg | 6.9 (0.2) | 44.2 (25.8) | 4.0 (2.0) | 10.0 (2.2) | 1.5 (0.9) | 30.1 (14.6) |
The values in parentheses are standard deviations based on a minimum of five replicate analyses.
The values are based on soil dry weight.
In situ emission of N2O.
To evaluate N2O emission at the soil surface, the concentrations of N2O inside gas-tight static chambers were determined over a period of several hours. Stainless steel rings were driven about 3 cm into the ground. Plexiglas chamber tops (height, 6.5 cm; diameter, 17 cm) were placed inside the rings and sealed with large rubber seals. Gas samples were withdrawn through a rubber-stoppered port with syringes at various times after closure and were injected into evacuated, rubber stopper-sealed vials.
N2O emission by earthworms.
In vivo emission of N2O by earthworms was evaluated by using aerobic microcosms that did not contain soil. Each microcosm consisted of an aseptic 38-ml serum vial that contained one living earthworm that had been washed with sterile water to remove soil particles; excess moisture was removed by blotting the earthworm with sterile tissue paper. For L. terrestris (a very large worm), 150-ml infusion flasks were utilized. The vials and flasks were closed with rubber stoppers and seals; the gas phase was air. To evaluate the effects of mineral salts, earthworms were moistened with 0.4 ml (1.0 ml for L. terrestris) of sterile mineral salt solutions or water (control) as indicated below. The microcosms were incubated at 20°C in the dark. The procedures used did not appear to affect the activity of the earthworms; i.e., the earthworms behaved normally until the end of the experiment. Gas samples were withdrawn with sterile syringes and were analyzed by gas chromatography over a period of approximately 8 h. The N2O emission rate of an individual earthworm was determined during the initial period of emission.
Soil columns and soil microcosms.
Each soil column consisted of a Plexiglas cylinder (height, 20 cm; diameter, 17 cm) that had a ceramic plate at the bottom and contained 2.8 kg (fresh weight) of garden soil. To assess the emission of N2O from soil columns, columns were closed with Plexiglas covers (height, 5.6 cm; diameter, 17 cm) and gas-tight rubber seals for 26 h. Each soil microcosm was constructed by placing 30 g (fresh weight) of soil into a 150-ml infusion flask, which was then sealed with a rubber stopper and a metal screw cap. Earthworms were added at a density of one worm per microcosm or eight worms (four A. caliginosa, three L. rubellus, and one O. lacteum) per soil column. The soil columns and microcosms were incubated at 15°C; the gas phase was air. Gas samples were withdrawn with syringes at different times after closure.
Preincubation of earthworms in soil supplemented with antibiotics.
To evaluate the effect of antibiotics on in vivo emission of N2O, four earthworms (L. rubellus) that were obtained from forest soil were preincubated for 3 days at 10°C in 80 g of forest soil supplemented with streptomycin and tetracycline (each at a concentration of 10 mg g [dry weight] of soil−1).
Preparation of gut sections.
Freshly collected earthworms were narcotized with 100% CO2 prior to dissection. Earthworm gut sections (posterior to the gizzard) were dissected out at a lab bench under air. The dissected gut sections and the remaining worm material were washed with sterile water to remove gut debris and then placed into 38-ml serum vials. The vials were sealed, incubated in the dark at 20°C, and analyzed for N2O.
Preparation of microcosms with fresh casts from N2O-emitting earthworms.
Washed earthworms were moistened with 0.4 ml of 2 mM potassium nitrate and incubated in microcosms under air as described above. The earthworms were removed from the microcosms after they produced casts. The microcosms containing the fresh casts were then supplemented with 0.4 ml of 2 mM potassium nitrate, resealed, and analyzed for N2O under either oxic (air) or anoxic (100% argon) conditions.
Analytical procedures.
N2O was analyzed with a Hewlett-Packard model 5890 series II gas chromatograph equipped with a Porapak Q column and an electron capture detector (18). Acetylene was generated from calcium carbide (CaC2) and water in a gas formation flask immediately before it was used. All chemicals and gases were of the highest purity available. Unless otherwise indicated, the data are means based on four replicates.
RESULTS
In situ emission of N2O at field sites.
The field sites emitted N2O under in situ conditions during the summer months when earthworms were collected. The rates of in situ emission of N2O at the garden sites ranged from 71 to 714 nmol of N2O h−1 m−2 (2 to 20 μg of N2O N h−1 m−2). The rates of in situ emission of N2O at the beech forest site ranged from 7 to 54 nmol of N2O h−1 m−2 (0.2 to 1.5 μg of N2O N h−1 m−2). These in situ emission rates were approximately the same as those of similar terrestrial ecosystems (4).
In vivo emission of N2O by earthworms from garden soils and other sources.
A. caliginosa, O. lacteum, and L. rubellus obtained from garden soils emitted N2O under in vivo conditions (Table 2), and emission was relatively linear over a 3- to 6-h period (data not shown). The mean N2O emission rates ranged from 0.14 to 0.87 nmol h−1 g (fresh weight)−1 (Table 2). L. rubellus obtained from beech forest soils also emitted N2O under in vivo conditions (Table 2), which confirmed previous observations (18). Some individual earthworms did not emit N2O, and the rates of emission of N2O by earthworms were highly variable (Table 2). The emission of N2O by commercially obtained L. terrestris worms was negligible; however, such worms emitted low amounts of N2O when they were preincubated in garden soils (Table 2).
TABLE 2.
In vivo emission of N2O by earthworms obtained from various sources
| Earthworm source | Earthworm species | No. of replicates | Mean N2O emission rate (nmol h−1 g [fresh wt]−1)a |
|---|---|---|---|
| Garden soil (Heinersreuth) | A. caliginosa | 7 | 0.64 (0.13) |
| L. rubellus | 2 | 0.54 (NA) | |
| O. lacteum | 1 | 0.29 (NA) | |
| Total | 10 | 0.58 (0.17) | |
| Garden soil (Weidenberg) | A. caliginosa | 15 | 0.27 (0.27) |
| L. rubellus | 1 | 0.87 (NA) | |
| O. lacteum | 5 | 0.14 (0.10) | |
| Total | 21 | 0.24 (0.25) | |
| Beech forest soil (Geisberg) | L. rubellus | 4 | 0.43 (0.08) |
| Commercial | L. terrestris | 11 | 0.00 (0.01) |
| Commercialb | L. terrestris | 11 | 0.08 (0.06) |
The values in parentheses are standard deviations. NA, not applicable.
Earthworms were incubated in fresh garden soil for 2.5 days prior to analysis.
Although the vegetation of the garden soils and the vegetation of the forest soils were different, the rates of N2O emission by the earthworms obtained from these soils were relatively similar. Thus, the theoretical quality of the soil organic carbon (decomposed beech litter in the forest soils and decomposed crops in tilled soils) did not appear to significantly affect the capacity of earthworms to emit N2O. Other soil characteristics may more directly influence N turnover processes in earthworms. When the two garden sites were compared, the highest mean in vivo N2O emission rates were obtained with earthworms collected from the Heinersreuth site, which contained the highest amount of soil nitrate and had the highest soil C/N ratio (Tables 1 and 2).
Emission of N2O by garden soil columns and microcosms.
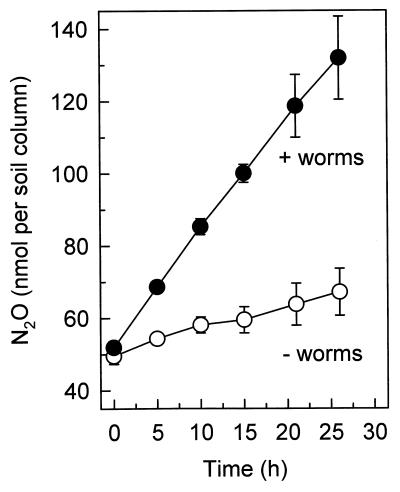
The emission of N2O by earthworm-supplemented garden soil columns was significantly greater than the emission of N2O by garden soil columns lacking earthworms (Fig. 1). The emission of N2O by soil columns containing or lacking earthworms was relatively linear for extended periods of time (Fig. 1). The N2O emission rates were 1.4 ± 0.2 pmol h−1 g (dry weight) of soil−1 for columns containing both garden soil and worms and 0.3 ± 0.1 pmol h−1 g (dry weight) of soil−1 for columns containing garden soil alone. With garden soil microcosms, N2O emission was also relatively linear (data not shown), and the emission rates were 7.6 ± 4.8 pmol h−1 g (dry weight) of soil−1 for microcosms containing both garden soil and worms and 0.3 ± 0.4 pmol h−1 g (dry weight) of soil−1 for microcosms containing garden soil alone. The calculated mean N2O emission rates for the earthworms in soil columns (0.40 nmol h−1 g [fresh weight]−1) and microcosms (0.17 nmol h−1 g [fresh weight]−1) were similar to the in vivo emission rates obtained with individual worms (Table 2).
FIG. 1.
Emission of N2O by garden soil columns containing (●) or lacking (○) earthworms. Experiments were performed in triplicate.
Effect of mineral salts on in vivo emission of N2O.
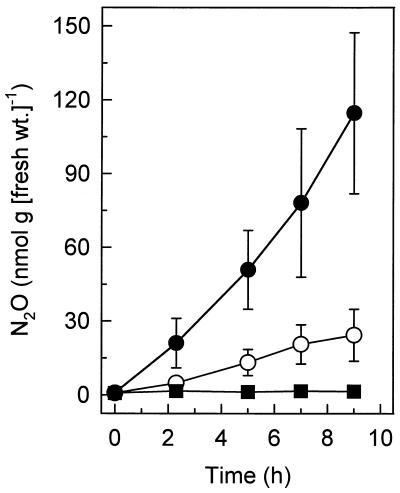
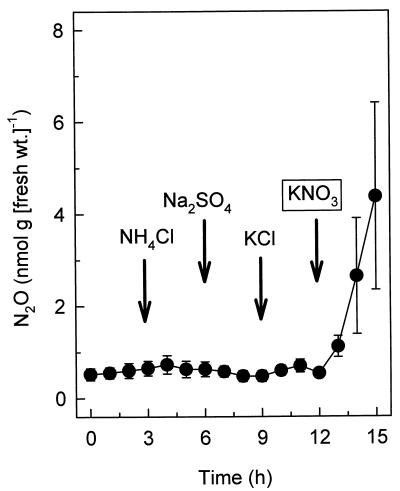
In vivo emission of N2O was greatly stimulated when earthworms were moistened with small amounts of potassium nitrate or potassium nitrite (Fig. 2). Such stimulation was not observed with potassium chloride, ammonium chloride, or sodium sulfate (Fig. 3). Nitrate also rapidly stimulated the emission of N2O after earthworms were preincubated for 12 h in the absence of soil (Fig. 3; data not shown). The N2O emission rates increased with increasing nitrate concentrations from 0.025 to 2 mM, and the N2O emission rates were similar with 2 and 10 mM nitrate (data not shown). In all cases, the N2O emission rates obtained with nitrite were substantially greater than the N2O emission rates obtained with nitrate, and ammonium did not stimulate emission of N2O (Table 3).
FIG. 2.
Effects of 2 mM potassium nitrate (○) and potassium nitrite (●) on in vivo emission of N2O by L. rubellus obtained from forest soil. Water was added instead of mineral salts to the controls (■).
FIG. 3.
Effects of various mineral salts (10 mM each) (arrows) on in vivo emission of N2O by L. rubellus obtained from forest soil.
TABLE 3.
In vivo emission of N2O by earthworms moistened with 2 mM potassium nitrate, potassium nitrite, or ammonium chloride
| Earthworm source | Earthworm species | Mean N2O emission rate (nmol h−1 g [fresh wt]−1) in the presence of:
|
|||
|---|---|---|---|---|---|
| Nitrate | Nitrite | Ammonium | H2O | ||
| Garden soil (Heinersreuth) | A. caliginosa, O. lacteum | 0.62 | 5.83 | 0.25 | 0.27 |
| Garden soil (Weidenberg) | A. caliginosa, O. lacteum | 0.55 | 13.24 | 0.01 | 0.02 |
| Beech forest soil | L. rubellus | 2.77 | 12.44 | 0.00 | 0.06 |
Effect of antibiotics on in vivo emission of N2O.
The in vivo emission of N2O by L. rubellus obtained from forest soil (1.95 ± 0.59 nmol h−1 g [fresh weight]−1) decreased by approximately 80% (to 0.42 ± 0.06 nmol h−1 g [fresh weight]−1) when earthworms were preincubated for 3 days in soil containing streptomycin and tetracycline. These results indicate that bacterial processes were involved in the production of N2O. The earthworms had a normal appearance and behaved normally after 3 days of preincubation in the antibiotic-containing soil, which indicated that the general health of the earthworms was not affected by this treatment.
Anatomical site of N2O production.
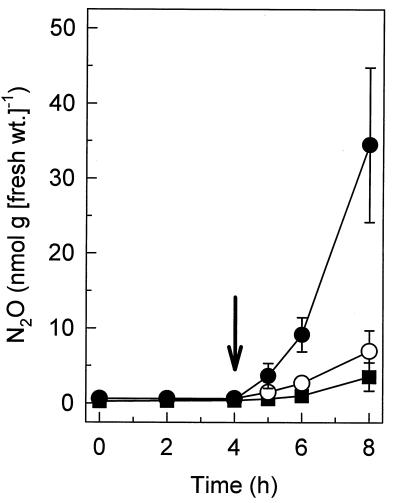
Under oxic conditions, the capacity of nitrate-supplemented earthworm gut sections to produce N2O was substantially greater than the capacity of dissected worms lacking gut sections to produce N2O (Fig. 4), which indicated that the N2O that was produced under in vivo conditions originated in the gut rather than on the surface of the earthworm. On a fresh weight basis, the initial capacity of gut sections to produce N2O in response to supplemental nitrate was substantially greater than the capacity of living earthworms to produce N2O under the same conditions (Fig. 4).
FIG. 4.
Emission of N2O by L. rubellus gut sections (●), degutted earthworms (■), and living earthworms (○) obtained from forest soil. The gas phase was air, and the arrow indicates when potassium nitrate (2 mM) was added.
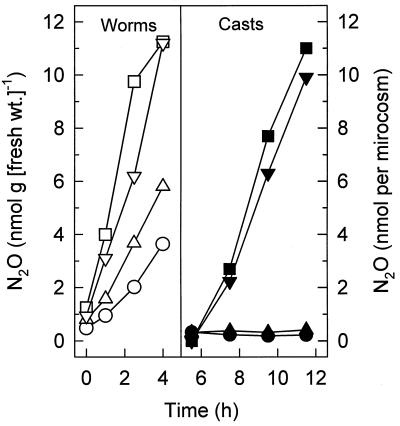
Effect of O2 on emission of N2O by casts.
Fresh casts from N2O-emitting earthworms produced negligible amounts of N2O under oxic conditions (i.e., in the presence of air) (Fig. 5). However, under anoxic conditions, such casts rapidly produced N2O (Fig. 5). These results indicated that the microbial process responsible for the formation of N2O in the earthworm gut was sensitive to O2.
FIG. 5.
Effects of oxic (▴ and ●) and anoxic (■ and ▾) conditions on emission of N2O by fresh casts produced by four N2O-emitting earthworms (L. rubellus) (▵, ○, □, and ▿) obtained from forest soil.
Effect of acetylene on in vivo emission of N2O.
Collectively, the findings described above indicated that nitrate-reducing bacteria in the gut were primarily responsible for emission of N2O by earthworms. Acetylene is an inhibitor of nitrous oxide reductase and increases the production of N2O by denitrifiers by blocking the last reductive step in denitrification (37). In contrast, acetylene inhibits, rather than enhances, the production of N2O during dissimilatory reduction of nitrite to ammonium (20).
Acetylene increased the mean N2O emission rates of earthworms that were moistened with potassium nitrate (Table 4). The increase was statistically significant for garden soil earthworms and commercially obtained, garden soil-fed L. terrestris. In contrast, acetylene had little or no effect on the mean N2O emission rates of earthworms that were moistened with potassium nitrite (data not shown).
TABLE 4.
Effect of acetylene (10 kPa) on in vivo emission of N2O by earthworms moistened with 2 mM potassium nitrate
| Earthworm source | Earthworm species | Mean N2O emission rate (nmol h−1 g [fresh wt]−1)a
|
N2O emission rate with C2H2/N2O emission rate without C2H2 | |
|---|---|---|---|---|
| Without C2H2 | With C2H2 | |||
| Garden soil | A. caliginosa, O. lacteum | 1.78 (0.96) | 4.93 (0.44) | 2.77b |
| Beech forest soil | L. rubellus | 1.64 (1.25) | 4.21 (2.19) | 2.57 |
| Commercialc | L. terrestris | 0.16 (0.07) | 1.15 (0.32) | 7.18b |
The values in parentheses are standard deviations.
These ratios reflect statistically significant differences (P < 0.05, as determined by the Mann-Whitney U test) in the values obtained with and without acetylene.
Earthworms were incubated in fresh garden soil for 2.5 days prior to analysis.
DISCUSSION
Garden and forest soils that emitted N2O under in situ conditions contained earthworms that emitted N2O under in vivo conditions. To estimate the contribution of earthworms to the in situ emission of N2O at the garden sites, we assumed that (i) the zone of earthworm activity in the soil was 20 cm deep, (ii) the in situ earthworm population density was 100 worms per m2 (an average population density for soils of central and northern Europe [27]), and (iii) the density of the garden soils was 1,200 kg (fresh weight) m−3 (this value was the mean of values from 10 analyses). When these assumptions were used, the mean N2O emission rates of garden soil columns with and without earthworms were approximately 2 and 1.3 μg of N2O N h−1 m−2, respectively. Thus, based on these calculations, the contribution of earthworms to the total N2O emitted from garden soils under field conditions was approximately 33%. Similar values were obtained when the data from soil microcosms were extrapolated to in situ earthworm population densities. Our estimates are higher than the values calculated previously for forest soils (18) and indicate that earthworms can contribute to N2O emission in certain tilled soils. Since agricultural soils can have earthworm population densities as high as 400 worms per m2 (11), the contribution might be even higher under certain conditions. The nitrate- and nitrite-dependent stimulation of in vivo production of N2O which has been observed suggests that fertilization of soil might trigger a short-term increase in the in situ emission of N2O by earthworms. Soil parameters (e.g., water content and C/N ratio) and processes (e.g., competing processes in the turnover of soil nitrogen) might also affect the in situ emission of N2O by earthworms.
N2O can be produced during nitrification, denitrification, and dissimilatory and assimilatory reduction of nitrate (5, 9). The enhancement of in vivo emission of N2O by nitrate but not by ammonium which was observed indicated that nitrate-reducing processes, rather than nitrification, were primarily responsible for the emission of N2O by the earthworms examined in this study. Since acetylene did not inhibit emission of N2O by nitrite-treated earthworms, it seems unlikely that production of N2O was coupled to dissimilatory reduction of nitrite to ammonium (20).
N2O is an intermediate in the reduction of nitrate to N2 by respiratory denitrifying bacteria (34), and N2O is often a product of denitrification in soils (6, 14, 32). The acetylene-dependent increase in the emission of N2O from garden and forest soil earthworms implied that denitrifying bacteria are involved in the emission of N2O by earthworms. Certain denitrifying bacteria produce various amounts of N2O in addition to N2 or produce N2O as an intermediate prior to production of N2 (2, 34). Since the rate constants for the sequential steps in the reduction of nitrate to N2 are probably not equivalent, the relative amounts of N2O and N2 may depend on whether nitrate or nitrite is utilized during denitrification (2). Thus, differences in the flow of reductant might account for the high N2O emission rates observed with nitrite (Fig. 2 and Table 3). In a number of denitrifying bacteria, nitrous oxide reductase is absent (31). In these bacteria, N2O is the end product of denitrification, and acetylene probably has little effect on N2O production. The effect of acetylene on N2O emission therefore probably depends on the composition of the resident N2O-producing gut microflora. The apparent nitrate- and nitrite-dependent stimulation of N2O emission may have involved nonrespiratory denitrification. In general, nonrespiratory denitrification (i) does not couple the reduction of nitrogen oxides to electron transport phosphorylation, (ii) does not yield N2, (iii) is facilitated by a number of bacteria, including propionibacteria, and (iv) can yield large amounts of N2O via the reduction of nitrate or nitrite (1, 19, 35).
Based on the results obtained with dissected earthworms, the N2O that was emitted by earthworms originated from gut-associated microorganisms. Since nitrate and nitrite significantly stimulated in vivo emission of N2O, it seems likely that supplemental nitrate or nitrite was transported into the gut, where denitrifying bacteria (18) and production of N2O were localized. Uptake of water or dissolved salts by earthworms usually does not involve oral ingestion of fluids (26). Thus, it is likely that nitrate and nitrite were translocated through the body wall and into the gut via either passive diffusion or active transport (23, 26). Differences in the efficiencies by which nitrate and nitrite were transported into the gut might be partially responsible for the differences observed in the N2O emission rates of earthworms treated with nitrate and nitrite. The nitrate- and nitrite-dependent stimulation of N2O emission by earthworms and gut sections was rapid and occurred without an appreciable delay, indicating that (i) the source of reductant used for the production of N2O was not limiting and (ii) the N2O-producing microflora of the gut was poised to respond quickly to nitrate and nitrite.
In contrast to living earthworms and gut sections that produced N2O under oxic conditions (i.e., in the presence of air), fresh casts from N2O-emitting earthworms produced N2O only under anoxic conditions. These results indicate that the N2O production process in the earthworm gut occurs optimally under anoxic conditions and, furthermore, suggests that the interior of the earthworm gut provides an anoxic (or partially anoxic) habitat for microbial production of N2O. The microflora of the earthworm gut is enriched with bacteria capable of anaerobic growth and activity (17), and several strictly anaerobic bacteria, as well as facultatively anaerobic bacteria, have been obtained from the earthworm gut and earthworm casts and characterized (7, 15, 18, 24). Denitrifiers have been observed in earthworm casts collected from agricultural soils (12, 13, 25, 33) and have been enumerated from the gut material of earthworms collected from forest soil (18). Commercially obtained earthworms emitted N2O only when they were preincubated in fresh soil (Table 2), indicating that (i) N2O-producing microbes in the soil were ingested or (ii) ingested soil provided essential nutrients to the N2O-producing microflora of the gut. Characterization of the N2O-producing bacteria of the earthworm gut is currently under way.
The activities of gastrointestinal microfloras are linked to the greenhouse gas budget. For example, it has been estimated that the gut microfloras of livestock and termites are responsible for approximately 30% of the global methane budget (36) and that the gut microflora of cattle is responsible for approximately 2% of the global N2O budget (i.e., 0.5 Tg year−1) (21). Our findings indicate that the gastrointestinal microflora of the earthworm contributes to the global N2O budget. Determining the extent of this contribution will require extensive evaluation of the diverse terrestrial ecosystems in which earthworms are endemic.
ACKNOWLEDGMENTS
We thank Kirsten Küsel for reviewing the manuscript and Anita Gößner and Andreas Popp for technical assistance.
This study was supported by grant PT BEO 51-0339476B from the German Ministry of Education, Science, Research and Technology.
REFERENCES
- 1.Allison C, Macfarlane G T. Dissimilatory nitrate reduction by Propionibacterium acnes. Appl Environ Microbiol. 1989;55:2899–2903. doi: 10.1128/aem.55.11.2899-2903.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betlach M R, Tiedje J M. A kinetic explanation for accumulation of nitrite, nitric oxide, and nitrous oxide during bacterial denitrification. Appl Environ Microbiol. 1981;42:1074–1084. doi: 10.1128/aem.42.6.1074-1084.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleakley B H, Tiedje J M. Nitrous oxide production by organisms other than nitrifiers or denitrifiers. Appl Environ Microbiol. 1982;44:1342–1348. doi: 10.1128/aem.44.6.1342-1348.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouwman A F. Exchange of greenhouse gases between terrestrial ecosystems and the atmosphere. In: Bouwman A F, editor. Soils and the greenhouse effect. New York, N.Y: John Wiley & Sons Ltd.; 1990. pp. 61–127. [Google Scholar]
- 5.Bremner J M. Sources of nitrous oxide in soils. Nutr Cycl Agroecosyst. 1997;49:7–16. [Google Scholar]
- 6.Burth I, Ottow J C G. Influence of pH on the production of N2O and N2 by different denitrifying bacteria and Fusarium solani. Ecol Bull. 1983;35:207–215. [Google Scholar]
- 7.Citernesi U, Neglia R, Seritti A, Lepidi A A, Filippi C, Bagnoli G, Nuti M P, Galluzzi R. Nitrogen fixation in the gastro-enteric cavity of soil animals. Soil Biol Biochem. 1977;9:71–72. [Google Scholar]
- 8.Conrad R. Soil microbial processes involved in production and consumption of atmospheric trace gases. In: Jones J G, editor. Advances in microbial ecology. Vol. 14. New York, N.Y: Plenum Press; 1995. pp. 207–250. [Google Scholar]
- 9.Conrad R. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO) Microbiol Rev. 1996;60:609–640. doi: 10.1128/mr.60.4.609-640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson E A. Fluxes of nitrous oxide and nitric oxide from terrestrial ecosystems. In: Rogers J E, Whitman W B, editors. Microbial production and consumption of greenhouse gases: methane, nitrogen oxides, and halomethanes. Washington, D.C: American Society for Microbiology; 1991. pp. 219–235. [Google Scholar]
- 11.Edwards C A, Bohlen P J. Biology and ecology of earthworms. London, United Kingdom: Chapman and Hall; 1996. [Google Scholar]
- 12.Elliot P W, Knight D, Anderson J M. Denitrification in earthworm casts and soil from pastures under different fertilizer and drainage regimes. Soil Biol Biochem. 1990;22:601–605. [Google Scholar]
- 13.Elliot P W, Knight D, Anderson J M. Variables controlling denitrification from earthworm casts and soil in permanent pastures. Biol Fertil Soils. 1991;11:24–29. [Google Scholar]
- 14.Firestone M K, Firestone R B, Tiedje J M. Nitrous oxide from soil denitrification: factors controlling its biological production. Science. 1980;208:749–751. doi: 10.1126/science.208.4445.749. [DOI] [PubMed] [Google Scholar]
- 15.Furlong M A, Whitman W B, Coleman D C. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Phenotypic comparison of bacterial isolates obtained from drilosphere soil, bulk soil and earthworm guts, abstr. N-10; p. 367. [Google Scholar]
- 16.Hutchinson G L. Biosphere-atmosphere exchange of gaseous N oxides. In: Lal R, Kimble J, Levine E, Stewart B A, editors. Soils and global change. Boca Raton, Fla: CRC Press, Inc.; 1995. pp. 219–236. [Google Scholar]
- 17.Karsten G R, Drake H L. Comparative assessment of the aerobic and anaerobic microflora of earthworm guts and forest soils. Appl Environ Microbiol. 1995;61:1039–1044. doi: 10.1128/aem.61.3.1039-1044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karsten G R, Drake H L. Denitrifying bacteria in the earthworm gastrointestinal tract and in vivo emission of nitrous oxide (N2O) by earthworms. Appl Environ Microbiol. 1997;63:1878–1882. doi: 10.1128/aem.63.5.1878-1882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaspar H F. Nitrite reduction to nitrous oxide by propionibacteria: detoxication mechanism. Arch Microbiol. 1982;133:126–130. [Google Scholar]
- 20.Kaspar H F, Tiedje J M. Dissimilatory reduction of nitrate and nitrite in the bovine rumen: nitrous oxide production and effect of acetylene. Appl Environ Microbiol. 1981;41:705–709. doi: 10.1128/aem.41.3.705-709.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalil M A K, Rasmussen R A. The global sources of nitrous oxide. J Geophys Res. 1992;97:14651–14660. [Google Scholar]
- 22.Küsel K, Drake H L. Effects of environmental parameters on the formation and turnover of acetate by forest soils. Appl Environ Microbiol. 1995;61:3667–3675. doi: 10.1128/aem.61.10.3667-3675.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K E. Earthworms—their ecology and relationships with soils and land use. North Ryde, Australia: Academic Press; 1985. pp. 56–66. [Google Scholar]
- 24.Márialigeti K. On the community-structure of the gut-microbiota of Eisenia lucens (Annelida, Oligochaeta) Pedobiologia. 1979;19:213–220. [Google Scholar]
- 25.Mulongoy K, Bedoret A. Properties of worm casts and surface soils under various plant covers in the humid tropics. Soil Biol Biochem. 1989;21:197–203. [Google Scholar]
- 26.Oglesby L C. Salt and water balance. In: Mill P J, editor. Physiology of annelids. London, United Kingdom: Academic Press; 1978. pp. 555–658. [Google Scholar]
- 27.Schachtschabel P, Blume H-P, Brümmer G, Hartge K-H, Schwertmann U. Scheffer/Schachtschabel—Lehrbuch der Bodenkunde. 13th ed. Stuttgart, Germany: Ferdinand Enke Verlag; 1992. [Google Scholar]
- 28.Schaefer M. Brohmer—Fauna von Deutschland. Heidelberg, Germany: Quelle & Meyer; 1992. [Google Scholar]
- 29.Smith M S. Dissimilatory reduction of nitrite to ammonium and nitrous oxide by a soil Citrobacter sp. Appl Environ Microbiol. 1982;43:854–860. doi: 10.1128/aem.43.4.854-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith M S, Zimmermann K. Nitrous oxide production by nondenitrifying soil nitrate reducers. Soil Sci Soc Am J. 1981;45:865–871. [Google Scholar]
- 31.Stouthamer A H. Dissimilatory reduction of oxidized nitrogen compounds. In: Zehnder A J B, editor. Biology of anaerobic microorganisms. New York, N.Y: John Wiley & Sons, Inc.; 1988. pp. 245–303. [Google Scholar]
- 32.Struwe S, Kjøller A. Potential for N2O production from beech (Fagus silvaticus) forest soils with varying pH. Soil Biol Biochem. 1994;26:1003–1009. [Google Scholar]
- 33.Svensson B H, Boström U, Klemedtson L. Potential for higher rates of denitrification in earthworm casts than in the surrounding soil. Biol Fertil Soils. 1986;2:147–149. [Google Scholar]
- 34.Tiedje J M. Denitrification. In: Page A L, Miller R H, Keeney D R, editors. Methods of soil analysis, part 2. Chemical and microbiological properties. Agronomy Monograph no. 9. Madison, Wis: American Society of Agronomy; 1982. pp. 1011–1026. [Google Scholar]
- 35.Tiedje J M. Ecology of denitrification and dissimilatory nitrate reduction to ammonium. In: Zehnder A J B, editor. Biology of anaerobic microorganisms. New York, N.Y: John Wiley & Sons, Inc.; 1988. pp. 179–244. [Google Scholar]
- 36.Tyler S C. The global methane budget. In: Rogers J E, Whitman W B, editors. Microbial production and consumption of greenhouse gases: methane, nitrogen oxides, and halomethanes. Washington, D.C: American Society for Microbiology; 1991. pp. 7–38. [Google Scholar]
- 37.Yoshinari T, Knowles R. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem Biophys Res Commun. 1976;69:705–710. doi: 10.1016/0006-291x(76)90932-3. [DOI] [PubMed] [Google Scholar]