Abstract
Background:
The “crescent sign” is a hyperattenuating crescent-shaped region on CT within the mural thrombus or wall of an aortic aneurysm. Although it has previously been associated with aneurysm instability or impending rupture, the literature is largely based on retrospective analyses of urgently repaired aneurysms. We strove to more rigorously assess the association between an isolated “crescent sign” and risk of impending aortic rupture.
Methods:
Patients were identified by querying a single health system PACS database for radiology reports noting a crescent sign. Adult patients with a CT demonstrating a descending thoracic, thoracoabdominal, or abdominal aortic aneurysm and “crescent sign” between 2004 and 2019 were included, with exclusion of those showing definitive signs of aortic rupture on imaging.
Results:
A total of 82 patients were identified. Aneurysm size was 7.1 ± 2.0 cm. Thirty patients had emergent or urgent repairs during their index admission (37%), 19 had elective repairs at a later date (23%), and 33 patients had no intervention due to either patient choice or prohibitive medical comorbidities (40%). Patients without intervention had a median follow up of 275 days before death or loss to follow up. In patients undergoing elective intervention, 6,968 patient-days elapsed between presentation and repair, with zero episodes of acute rupture (median 105 days). Patients undergoing elective repair had smaller aneurysms compared to those who underwent emergent/urgent repair (6.2 ± 1.3 vs. 7.7 ± 2.1 cm, P = 0.008). No surgical candidate with an aneurysm smaller than 8 cm ruptured. There were 31 patients with previous axial imaging within 2 years prior to presentation with a “crescent sign,” with mean aneurysm growth rate of 0.85 ± 0.62 cm per 6 months [median 0.65, range 0–2.6]. Those with aneurysms sized below 5.5 cm displayed decreased aneurysm growth compared to patients with aneurysm’s sized 5.5–6.5 cm or patients with aneurysms greater than 6.5 cm (0.12 vs. 0.64 vs. 1.16 cm per 6 months, P=0.002).
Conclusions:
The finding of an isolated radiographic “crescent sign” without other signs of definitive aortic rupture (i.e., hemothorax, aortic wall disruption, retroperitoneal bleeding) is not necessarily an indicator of impending aortic rupture, but may be found in the setting of rapid aneurysm growth. Many factors, including other associated radiographic findings, aneurysm size and growth rate, and patient symptomatology, should guide aneurysm management in these patients. We found that patients with minimal symptoms, aneurysm sizes below 6.5 cm, and no further imaging findings of aneurysm instability, such as periaortic fat stranding, can be successfully managed with elective intervention after optimization of comorbid factors with no evidence of adverse outcomes.
INTRODUCTION
The prevalence of asymptomatic aortic aneurysms (AAAs) has increased as noninvasive screening modalities have become more widely available. Aortic diseases, including aortic aneurysms, are the 12th leading cause of death in the United States.1 AAA rupture is a major source of morbidity and mortality, with reported mortality rates of 40–50%.2 While aneurysm size is known to be the most important predictor of aneurysm rupture, other characteristics such as patient gender, comorbidities, wall stress, and aneurysm morphology are also thought to be important contributors 1,3–5 Acute aortic rupture can readily be diagnosed by computed tomography (CT) when there are findings of retroperitoneal hematoma, hemothorax, focal aortic wall discontinuity, or unstable patient hemodynamics.6–8 Several findings on CT scans of intact aneurysm have previously been postulated to be associated with “imminent” or “impending” aortic rupture even in otherwise asymptomatic patients, such as aortic bulging, draping of the aorta over the spine, or the presence of periaortic fat stranding.6–9
The “crescent sign” is a hyperattenuating crescent-shaped region on CT within the mural thrombus or wall of an aortic aneurysm; previous literature has suggested that this radiographic finding is associated with aneurysm intability.10–13 Initial retrospective studies proposed a sensitivity for “crescent sign” of 77% in detecting frank or contained aortic rupture, specificity of 93%, and positive predictive value of 53%, and therefore suggested that patients with this sign should be referred for urgent surgery.11 While previously described only in noncontrast enhanced CT scans, the “crescent sign” has also been well described in contrast enhanced CT imaging (Fig. 1).12 More recent studies continue to advocate that the “crescent sign” is associated with aortic rupture, even without other radiographic findings of rupture, and suggest that this sign should be an indication for urgent surgical repair.14,15 Prior literature investigating the “crescent sign” matched patients with clear signs of aortic rupture undergoing surgical repair to control groups without aortic rupture and assessed imaging findings found in the rupture cohort to those found in the nonrupture cohort, with limited investigation of patients with isolated “crescent signs” without clear evidence of rupture.11–13,15 A “crescent sign” can be found incidentally in asymptomatic patients, where an urgent intervention may be ill-advised due to patient comorbidities, and while “imminent” or “impending” rupture are often described concepts, the time to rupture and optimal timing of intervention has not been fully elucidated. Elective intervention in select patients with aortic aneurysms presenting with a “crescent sign” and without other clear signs of aneurysm rupture may benefit from preoperative cardiopulmonary optimization or evaluation for endovascular options, which may result in improved outcomes. We strove to more rigorously assess whether patients presenting with the “crescent sign” without other radiographic evidence of aneurysm rupture have aortic instability, with the hypothesis that the “crescent sign” can often be incidentally seen in large or growing aneurysms, but in itself is not indicative of imminent aortic rupture and need for emergent aneurysm intervention.
Fig. 1.
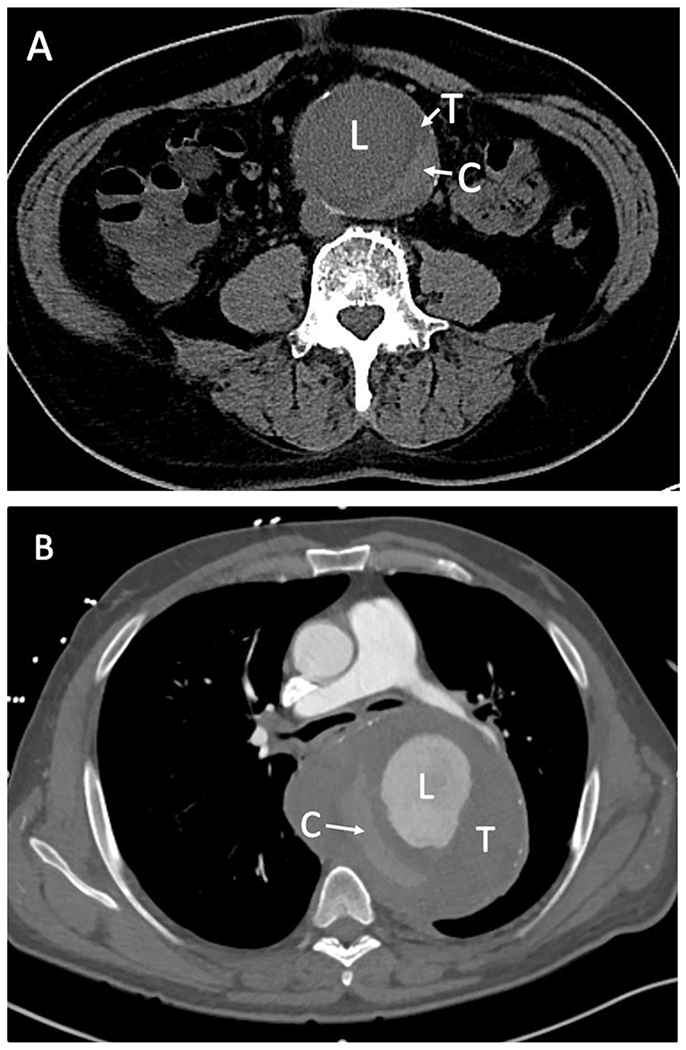
CT scans showing a “crescent sign” (C) in a contrast unenhanced scan (A) and in a contrast enhanced scan (B). C, Hyperattenuating “crescent sign”; L, Lumen; T, intraluminal thrombus.
METHODS
Patients were identified by querying the University of Pennsylvania Health System PACS database for CT radiology reports with keywords associated with the “crescent sign” using Nuance mPower from 2004 to 2019. The included keywords were “crescent” “crescentic” “hyperattenuating crescent” and “crescent sign” which resulted in identification of 2,033 reports during the study period. Patients with a CT report demonstrating a descending thoracic, thoracoabdominal, or abdominal aortic aneurysm and terminology indicating a “crescent sign” were included. Both contrast enhanced and unenhanced CT chest, abdomen, and pelvis reports were included. Individual CT films were reviewed when available. Exclusion criteria included: (1) patients with a “crescent sign” in the setting of an ascending aortic aneurysm; (2) patients with a “crescent sign” in the setting of definitive imaging findings of aortic rupture, including hemothorax, retroperitoneal bleeding, or focal aortic wall disruption; (3) patients with a history of aortic dissection; and (4) patients with inadequate information for complete chart review. Patients with previously defined radiographic signs of aortic instability were included, such as: (1) draping of the aorta over the spine (draped aorta sign); (2) focal aortic bulging; and (3) periaortic fat stranding. Two physician reviewed all imaging and radiology reports to confirm findings for patient inclusion or exclusion.
Medical records were reviewed for patient demographics, comorbidities, presence of aneurysm related symptoms, aneurysm characteristics, aneurysm management, and outcomes. We classified urgent/emergent interventions as those patients that underwent aneurysm repair during the same hospitalization as their “crescent sign” diagnoses, and elective interventions as those patients that underwent repair at a separate hospitalization. Time to aneurysm repair was measured from radiographic diagnosis with a “crescent sign” to aneurysm intervention. Patient medical records were also searched for cross sectional imaging performed prior to their presentation with a “crescent signs” in order to characterize aneurysm growth rate prior to “crescent sign” presentation. Patient deaths were confirmed using the medical record, social security database, or obituary information. Patient demographics were compared to historical values reported in the literature. The χ2 test and Fisher exact test were used to compare categorical variables, and t-tests used for continuous variables. Logistic regression was used to determine risk factors for rapid aneurysm growth, considered to be growth greater than 0.5 cm per 6-month period. Statistical analysis was performed using STATA version 15 (College Station, TX), with a P value cutoff of <0.05 considered statistically significant. The study was approved by the University of Pennsylvania Institutional Review Board.
RESULTS
From 2004 to 2019, a total of 2,033 radiographic reports were identified on database query and underwent physician review, with 179 determined to be true “crescent signs.” Details regarding report review and inclusion are shown in Figure 2. A total 102 patients were identified as having CT reports indicating the presence of a DTA, TAAA, or AAA with a “crescent sign.” Chart review was completed on 82 patients, with 20 patients excluded due to insufficient records. Baseline demographics for the cohort are listed in Table I. Mean aortic aneurysm size was 7.1 ± 2.0 cm with a median size of 7.0 cm (IQR 5.7–8.0 cm), there were 19 patients (23%) with aortic aneurysm sizes below 5.5 cm. In the 82 patients, there were 70 reports (85%) describing “risk of imminent aortic rupture” in association with the “crescent sign” findings. There were 44 aneurysms limited to the abdominal aorta (54%), 20 limited to the descending thoracic aorta (24%), and 18 classified as thoracoabdominal (22%). CT scans were acquired in the emergency room or inpatient setting for 67 patients (82%) and 15 obtained in the outpatient setting (18%). Of the fifteen patients diagnosed on outpatient imaging, eleven were subsequently admitted for observation. On presentation, 54 patients (66%) reported chest and/or abdominal pain as their chief complaint and all symptomatic patients were admitted. After diagnosis of the “crescent sign,” 30 patients had emergent or urgent repairs during their index admission (37%), 19 had elective repairs at a later date (23%), and 33 patients had no intervention (40%), due to either prohibitive medical comorbidities or patient choice. In the thirty patients who underwent urgent/emergent intervention, 20 (67%) had AAAs and 10 had interventions within 36 hr. There were 16 patients who presented with aneurysms sized below 5.5 cm, 9 with AAAs, 4 with DTAs, and 3 with TAAAs. One patient with aneurysm below 5.5. cm and persistent abdominal pain underwent urgent repair (AAA), 5 patients (4 AAA, 1 TAAA) underwent elective repair, and 10 underwent surveillance alone. There were 13 patients (15.9%) with a “crescent sign” and additional radiographic signs of aneurysm instability (draped aorta sign or periaortic fat stranding). Those patients with additional radiographic evidence of aneurysm instability were significantly more likely to undergo emergent aneurysm repair compared to elective repair or surveillance (76% vs. 8% vs. 16% respectively, P= 0.004). Outcomes of patients by intervention acuity are noted in Table II. Thirty-eight patients presenting with the “crescent sign” were female (46%), and when compared to the historically reported gender distribution for aortic aneurysm disease, female gender found to be associated with the “crescent sign” in those with AAA (P= 0.009) and TAAA (P= 0.004) as shown in Table III.1
Fig. 2.
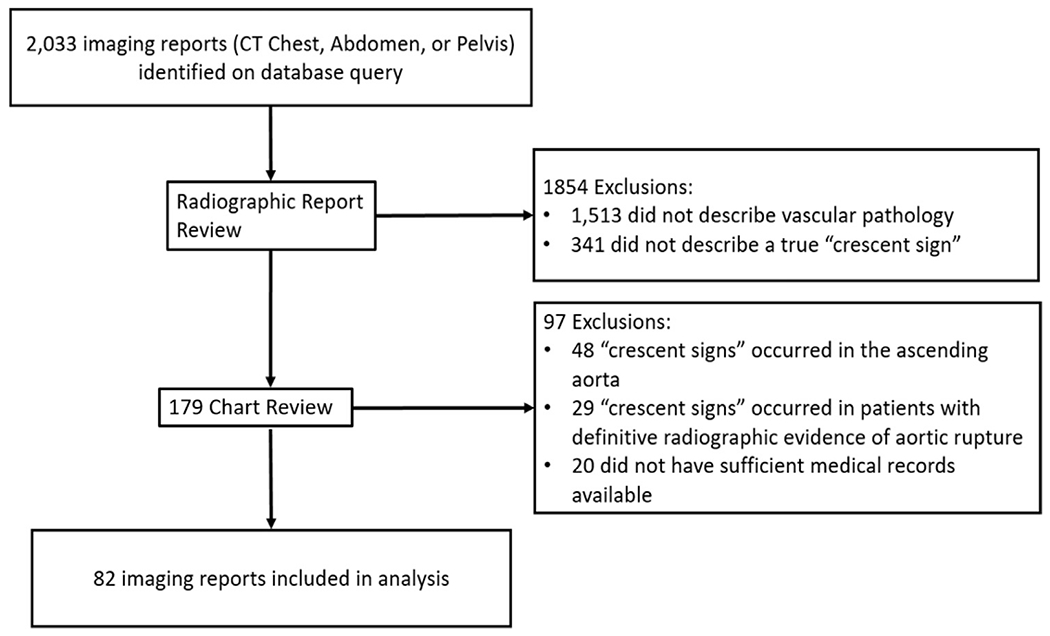
Flow diagram describing identification and inclusion of patients for analysis.
Table I.
Patient demographics of those presenting with the hyperattenuating crescent sign
| Covariate | ||
|---|---|---|
| Patient Age in Years, mean ± SD | 71.5 ± 13.4 | |
| Patient Gender, n (%) | Female | 38 (46%) |
| Male | 44 (54%) | |
| Aneurysm Location, n (%) | AAA | 44 (54%) |
| DTA | 20 (24%) | |
| TAAA | 18 (22%) | |
| Aneurysm Size in cm, mean ± SD | 7.1 ± 2.0 | |
| Associated Pain, n (%) | 54 (66%) | |
| Patient Location, n (%) | Emergency | 15 (18%) |
| Inpatient | 52 (63%) | |
| Outpatient | 15 (18%) | |
| Treatment Type, n (%) | Surveillance | 33 (40%) |
| Elective Repair | 19 (23%) | |
| Urg/Emergent Repair | 30 (37%) | |
| Aneurysm Rupture Related Death Within 30-Days of Presentation, n (%) | 3 (4%) | |
| Aneurysm Rupture or Intervention Related Death Within 30-Days of Presentation, n (%) | 9 (11%) |
DTA, Descending thoracic aneurysm; AAA, Abdominal aortic aneurysm; TAAA, Thoracoabdominal aortic aneurysm.
Table II.
Patient clinical characteristics and outcomes stratified by intervention acuity
| Urgent/emergent repair (n = 30) | Elective repair (n = 19) | No intervention (n = 33) | P value | ||
|---|---|---|---|---|---|
| Age (years) | 68.1 ± 12.9 | 69.1 ± 15.5 | 77.4 ± 10.5 | <0.01 | |
| Male sex, n (%) | 19 (63%) | 14 (74%) | 11 (33%) | 0.01 | |
| Aneurysm Location, n (%) | DTA | 6 (20%) | 4 (21%) | 10 (30%) | 0.39 |
| AAA | 20 (67%) | 10 (53%) | 14 (42%) | ||
| TAAA | 4 (13%) | 5 (26%) | 9 (27%) | ||
| Aneurysm Size (cm), mean ± SD | 7.7 ± 2.1 | 6.2 ± 1.3 | 6.7 ± 2.1 | <0.01 | |
| Prior Axial Imaging, n (%) | 9 (30%) | 7 (37%) | 16 (48%) | ||
| Aneurysm Growth Rate (cm/6months)a, mean ± SD | 1.4 ± 1.4 | 0.6 ± 0.4 | 1.4 ± 3.2 | 0.74 | |
| Associated Pain, n (%) | 24 (80%) | 8 (42%) | 17 (52%) | 0.01 | |
| Additional Evidence of Aneurysm Instability on Imaging, n (%) | 10 (33%) | 1 (5%) | 2 (6%) | 0.004 | |
| Repair Type, n (%) | Endo | 14 (47%) | 12 (63%) | 0.24 | |
| Open | 16 (53%) | 7 (37%) | |||
| Days to Repair, median (IQR) | 2 (0.5–7) | 105 (25–360) | 0.001 | ||
| 30-Day Mortality, n (%) | 6 (20%)b | 0 (0%) | 0.048 | ||
DTA, Descending thoracic aneurysm; AAA, Abdominal aortic aneurysm; TAAA, Thoracoabdominal aortic aneurysm, Bold Value indicate statistical signficance (P < 0.05).
Aneurysm growth rate in 6-months leading up to presentation with the “crescent sign.”
Two of these patients were noted to have aneurysm rupture prior to intervention.
Table III.
Gender distribution by aneurysm location in those presenting with the “crescent sign” compared to historically reported data
| Historical data | “Crescent sign” cohort | P value | ||
|---|---|---|---|---|
| Female Gender | AAA | 20% 15 | 16/44 (36%) | 0.007 |
| DTA | 35% 15 | 10/20 (50%) | 0.160 | |
| TAAA | 37% 15 | 12/18 (67%) | 0.009 |
AAA, Abdominal aortic aneurysm; DTA, Descending thoracic aneurysm; TAAA, Thoracoabdominal aortic aneurysm. Bold Value indicate statistical signficance (P < 0.05).
In the 19 patients undergoing elective repair, a total of 6,968 patient-days elapsed between “crescent sign” presentation and aneurysm repair with no episodes of acute aneurysm rupture occurring (median 105 days). Patients undergoing elective repair had smaller mean aneurysm size compared to those undergoing emergent/urgent repair, (6.2 ± 1.3 vs. 7.7 ± 2.1 cm, P= 0.008). For the 33 patients who did not undergo intervention, 21 (64%) were deemed to not be surgical candidates and the remaining 12 (36%) refused surgical intervention. Patients who did not undergo any intervention were significantly older (77.4 vs. 67.6 years, P= 0.001), more likely to be female (67% vs. 33%, P= 0.002), but had no difference in aneurysm size (6.7 vs. 7.3 cm, P= 0.14) compared to those who underwent any intervention. Those who did not undergo intervention showed a trend towards increased likelihood of TAAA or DTA (57.5% vs. 38.8%, P= 0.094). The 21 patients deemed to not be surgical candidates survived a median of 196 days after discharge (IQR 24–353 days), a total of 7,039 patient-days. In patients deemed to not be surgical candidates, 11 had advanced cardiopulmonary disease (52%) and 7 had advanced cancer (33%).
There were 5 patients (6.1%) that were clinically diagnosed with acute aortic aneurysm rupture shortly after presentation due to worsening symptoms, hemorrhage, or hemodynamic instability. Aneurysm sizes in these patients were 8.0 cm, 8.1 cm, 10.3 cm, 11.3 cm, and 12.0 cm, with 3 of these patients expiring due to their disease. Four of these patients had additional imaging findings associated with aneurysm instability (3 with periaortic fat stranding). One death occurred in a patient with an 8.1 cm aneurysm planned for urgent repair who experienced acute rupture before transfer to the operating room and expired shortly after endovascular repair. A second patient with an aneurysm size of 10.3 cm underwent emergent endovascular repair and developed decompensated heart failure in the postoperative setting from massive resuscitation resulting in patient death. The last patient death due to aneurysm rupture occurred in a poor surgical candidate due to medical comorbidities with an 11.3 cm aneurysm who elected to go on hospice instead of pursuing intervention and experienced in hospital death secondary to hypotension-associated mesenteric ischemia. The other 2 patients who developed acute rupture after presentation underwent successful emergent intervention with no 30-day mortality. With exclusion of the 5 patients who experience aneurysm rupture, there were 4 deaths in those who underwent emergent or urgent aneurysm intervention (3 had interventions within 36 hr of crescent time diagnosis). Those undergoing nonelective intervention experienced a higher 30-day mortality rate compared to patients who underwent elective repair (18% vs. 0%, P= 0.048).
There were 31 patients presenting with the “crescent sign” with prior axial imaging within 2 years that was available for review, mean aneurysm size was 6.9 ± 1.7 cm [median 6.85 cm, range 4.1–9.7] and mean aneurysm growth rate prior to presenting with the “crescent sign” was 0.85 ± 0.62 cm per 6 months [median 0.65 cm per 6 months, range 0–2.6]. Eighteen of these 31 patients (58%) had growth rates above 0.5 cm per 6-months, classified as rapid growth. On multivariable logistic regression, with adjustment for patient age, gender, aneurysm location, aneurysm size, and presence of chest/abdomen pain at presentation, only increasing aneurysm size was associated with rapid aneurysm growth (OR 2.0 per cm, 95% CI 1.1–3.5, P= 0.039, ROC = 0.78). Seven patients presenting with a “crescent sign” in an aneurysm sized 5.5 cm or below had prior axial imaging and a none displayed rapid growth. Those with aneurysms sized below 5.5 cm had lower growth rates compared to those with those with aneurysms sized between 5.5–6.5 cm (0.12 ± 0.10 vs. 0.64 ± 0.16 cm per 6 months, P=0.001), and those with aneurysms sized between 5.5 and 6.5 cm displayed lower growth rates than patients with aneurysms sizes greater than 6.5 cm (0.64 ± 0.16 vs. 1.57 ± 1.16 cm per 6 months, P=0.038).
DISCUSSION
Our findings support the hypothesis that the radiographic finding of a “crescent sign,” in the absence of additional well established radiographic characteristics of aortic rupture, does not necessarily indicate impending aortic rupture and the need for urgent or emergent aortic intervention. In this study, only 6% of those patients presenting with a “crescent sign” in an intact aneurysm experienced subsequent acute aneurysm rupture, and all of those patients had extremely large aneurysms and many with additional signs of aneurysm instability such as periaortic fat stranding. We found that patients with minimal symptoms and aneurysm size below 6.5 cm can be successfully managed with elective intervention after optimization of comorbid factors, with no adverse events occurring in this subset of patients in this study. We document that delaying intervention is safe in appropriately selected patients. No patients selected for elective repair experienced adverse events in the interim between presentation with the “crescent sign” and eventual repair, a total of 6,968 patient-days. No patient presenting with a “crescent sign” in an aneurysm below 8 cm in size, with no other definitive radiographic findings of aneurysm rupture, experienced aneurysm rupture. Furthermore, continued surveillance in patients with a “crescent sign” and maximum aneurysm diameter less than 5.5 cm may be reasonable in certain clinical scenarios, given that no patients in this group ruptured and none demonstrated rapid growth. While in this study we cannot calculate sensitivity and specificity of the “crescent sign” for predicting impending aortic rupture, they are likely significantly lower than the previously described rates of 77% and 93%, respectively. Given that abdominal aortic aneurysmal disease has a strong male predominance, women may be more susceptible to developing a “crescent sign” given that nearly 50% of those in this study were female.5
As demonstrated in prior reports, the findings of a “crescent sign” is associated with large aneurysm size, with 50% of patients identified having aneurysms greater than 7.0 cm in size.11,12 Our findings differ from prior literature that suggest the radiographic “crescent sign” is a risk for aneurysm rupture.11–13,15 Prior reports which matched those who definitively experience aneurysm rupture to those that did not, and note that the “crescent sign” is more often seen in ruptured aneurysms.11–13,15 This does not necessarily indicate that the “crescent sign” precedes aneurysm rupture, just that in the setting of aneurysm rupture a crescent sign can be seen. We found that the “crescent sign” when identified in the absence of additional signs of either aneurysm instability or definitive rupture, may appear in the setting of rapid aneurysm growth, especially in those with larger aneurysms. Aneurysm growth in this cohort presenting with the “crescent sign” was 0.85 cm per 6 months, which well exceeds the observed growth rate in those with familial aneurysmal disease or those with aneurysmal degeneration after aortic dissection, previously reported as 0.5 cm per 6 months.16,17 This supports the prior hypothesis that the “crescent sign” occurs due to fissuring of the mural thrombus during rapid aneurysm wall expansion.12,14 Those with an aneurysm sizes between 5.5 cm and 6.5 cm had increased growth rates compared to those with aneurysm sizes below 5.5 cm, but lower than those demonstrated in patients with aneurysms greater than 6.5 cm in size. Given this concern that the “crescent sign” is a sequelae of rapid growth, we advocate that patients presenting with a “crescent sign” and aneurysms 5.5 cm or larger should undergo intervention, but many can be managed akin to patients with rapidly expanding aneurysms rather than requiring emergent intervention.
This retrospective review has several limitations. Patients were identified by searching radiographic reports at a single health system over a 15-year period for inclusion keywords of interest, with only 60% of the images available for review to confirm radiographic findings. Therefore, strict uniform criteria to define a “crescent sign” cannot be confirmed in all cases. Misclassification bias may be present in that the diagnosis of a “crescent sign” is not consistently defined in the literature, with previous reports varying on its exact definition between the acute mural thrombus, acute intramural hematoma, and localized mural thrombus hyperattenuation. While this introduces heterogeneity in this study, all patients included were identified on radiographic report as having an aortic aneurysm related “crescent sign” with 85% of the reports containing language indicating “aortic instability or impending rupture.” Given that many patients underwent urgent intervention after presentation and were never given an opportunity to experience aneurysm rupture, it is possible that we are underestimating the rate of rupture after presenting with the “crescent sign.” This does not change the conclusion that while the “crescent sign” can occur in the setting of imminent or acute aortic rupture, a significant number of patients presented with the “crescent sign” but never went on to develop aneurysm rupture.
CONCLUSION
The finding of an isolated radiographic “crescent sign” without other signs of definitive aortic rupture (i.e., hemothorax, aortic wall disruption, retroperitoneal bleeding) is not necessarily an indicator of impending aortic rupture and may be seen in the setting of rapid aneurysm growth. Many factors including other associated radiographic findings, aneurysm size and growth rate, and patient symptomatology should guide management of these patients after presentation. We found that patients with minimal symptoms, aneurysm sizes below 6.5 cm, and no further imaging findings of aneurysm instability, such as periaortic fat stranding, can be successfully managed with elective intervention after optimization of comorbid factors.
Footnotes
No authors have conflicts to declare.
Oral presentation at Society for Clinical Vascular Surgery 48th Annual Symposium, March 13–17, 2021 in Miami Beach, FL.
REFERENCES
- 1.Beckman JA, Creager MA, Dzau VJ, et al. Aortic aneurysms: pathophysiology, epidemiology and prognosis. Vascular medicine, Saunders Elsevier Inc, Philadelphia, PA. 2006. [Google Scholar]
- 2.Schermerhorn ML, Bensley RP, Giles KA. Changes in abdominal aortic aneurysm rupture and short-term mortality, 1995-2008: a retrospective observational study. Ann Surg. 2012;256:651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fillinger MF, Marra SP, Raghavan ML, et al. Prediction of rupture risk in abdominal aortic aneurysm during observation: wall stress versus diameter. J Vasc Surg. 2003;37:724–32. [DOI] [PubMed] [Google Scholar]
- 4.Fillinger MF, Racusin J, Baker RK. Anatomic characteristics of ruptured abdominal aortic aneurysm on conventional CT scans: implications for rupture risk. J Vasc Surg. 2004;39:1243–52. [DOI] [PubMed] [Google Scholar]
- 5.Pair A, McCann M, Bradshaw B, et al. Thrombus volume is associated with cardiovascular events and aneurysm growth in patients who have abdominal aortic aneurysms. J Vasc Surg.2011; 53: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boules TN, Compton CN, Stanziale SF. Can computed tomography scan findings predict “impending” aneurysm rupture? Vasc Endovasc Surg. 2006;40:41–7. [DOI] [PubMed] [Google Scholar]
- 7.Vu KN, Kaitoukov Y, Morin-Roy F. Rupture signs on computed tomography, treatment, and outcome of abdominal aortic aneurysms. Insights Imaging. 2014;5:281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf YG, Thomas WS, Brennan FJ, et al. Computed tomography scanning findings associated with rapid expansion of abdominal aortic aneurysms. J Vasc Surg. 1994; 20: 529–35; discussion 535-538. [DOI] [PubMed] [Google Scholar]
- 9.Chao CP, Walker TG, Kalva SP. Natural history and CT appearances of aortic intramural hematoma. Radiographics 2009;29:791–804. [DOI] [PubMed] [Google Scholar]
- 10.Maraj R, Rerkpattanapipat P, Jacobs LE, et al. Meta-analysis of 143 reported cases of aortic intramural hematoma. Am J Cardiol. 2000;86:664–8. [DOI] [PubMed] [Google Scholar]
- 11.Siegel CL, Cohan RH, Korobkin M, et al. Abdominal aortic aneurysm morphology: CT features in patients with ruptured and nonruptured aneurysms. AJR Am J Roentgenol. 1994;163:1123–9. [DOI] [PubMed] [Google Scholar]
- 12.Roy J, Labruto F, Beckman MO, et al. Bleeding into the intraluminal thrombus in abdominal aortic aneurysms is associated with rupture. J Vasc Surg. 2008:48:1108–13. [DOI] [PubMed] [Google Scholar]
- 13.Arita T, Matsunaga N, Takano K. Abdominal aortic aneurysm: rupture associated with the high-attenuating crescent sign. Radiology. 1997;204:765–8. [DOI] [PubMed] [Google Scholar]
- 14.Labruto F, Blomqvist L, Swedenborg J. Imaging the intraluminal thrombus of abdominal aortic aneurysms: techniques, findings, and clinical implications. J Vasc Interv Radiol. 2011; 22: 1069–75; quiz 1075. [DOI] [PubMed] [Google Scholar]
- 15.Lorandon F, Salomon du Mont L, Puyraveau M. Scannographic study of risk factors of abdominal aortic aneurysm rupture. Ann Vasc Surg. 2021;73:27–36. [DOI] [PubMed] [Google Scholar]
- 16.Albornoz G, Coady MA, Roberts M. Familial thoracic aortic aneurysms and dissections–incidence, modes of inheritance, and phenotypic patterns. Ann Thorac Surg. 2006;82:1400–5. [DOI] [PubMed] [Google Scholar]
- 17.Loeys BL, Schwarze U, Holm T. Aneurysm syndromes caused by mutations in the TGF-β receptor. N Engl J Med. 2006;355:788–98. [DOI] [PubMed] [Google Scholar]


