Summary
Cytoplasmic polyadenylation has an essential role in activating maternal mRNA translation during early development. In vertebrates, the reaction requires CPEB, a RNA binding protein and the poly(A) polymerase GLD-2. GLD-2-type poly(A) polymerases form a family clearly identifiable from canonical poly(A) polymerases (PAP). In Drosophila, canonical PAP is involved in cytoplasmic polyadenylation with Orb, the Drosophila CPEB, during mid-oogenesis. We show that the female germline GLD-2 is encoded by wispy. Wispy acts as a poly(A) polymerase in a tethering assay and in vivo for cytoplasmic polyadenylation of specific mRNA targets during late oogenesis and early embryogenesis. wispy function is required at the latest stage of oogenesis for metaphase of meiosis I arrest and for progression beyond this stage. In contrast, canonical PAP acts with Orb for the earliest steps of oogenesis. Both Wispy and PAP interact with Orb genetically and physically in an ovarian complex. We conclude that two distinct poly(A) polymerases have a role in cytoplasmic polyadenylation in the female germline, each of them being specifically required for different steps of oogenesis.
Keywords: Cytoplasmic polyadenylation, Drosophila, GLD2, Meiosis, Metaphase I, Translational control
Introduction
In many species, the oocyte and early embryo develop in the absence of transcription, therefore, the first steps of development depend on maternal mRNAs and on their regulation at the level of translation, stability and localization. Regulation of mRNA poly(A) tail length is a common mechanism of translational control. Deadenylation or poly(A) tail shortening results in mRNA decay or translational repression. Conversely, poly(A) tail elongation by cytoplasmic polyadenylation results in translational activation (Richter, 2000; Wickens et al., 2000). How the poly(A) tail length of a particular mRNA and consequently its level of translation is determined has been a matter of investigations for many years. It is becoming clear that poly(A) tail length results from a balance between concomitant deadenylation and polyadenylation (Kim and Richter, 2006).
The molecular mechanisms of cytoplasmic polyadenylation have been investigated in Xenopus oocytes. The specific RNA binding protein in the reaction is CPEB (Cytoplasmic Polyadenylation Element Binding Protein) which binds CPE in the 3’-UTR of regulated mRNAs. Two other factors, CPSF (Cleavage and Polyadenylation Specificity Factor) and Symplekin, in addition to a poly(A) polymerase are required (Barnard et al., 2004; Richter, 2007). Before meiotic maturation the polyadenylation complex also contains PARN, a deadenylase whose activity counteracts poly(A) tail elongation (Kim and Richter, 2006). At meiotic maturation, CPEB phosphorylation results in the release of PARN from the complex, thus leading to polyadenylation and translational activation.
CPSF and Symplekin are also required for nuclear polyadenylation, a cotranscriptional reaction which leads to the synthesis of a poly(A) tail at the 3’ end of all mRNAs (Edmonds, 2002). A canonical poly(A) polymerase (PAP) is responsible for poly(A) tail synthesis during nuclear polyadenylation. Particular isoforms of PAP were first thought to be required for cytoplasmic polyadenylation (Ballantyne et al., 1995). Moreover, TPAP, a testis specific PAP in mouse, is cytoplasmic in spermatogenic cells and was shown to be required, using Tpap KO, for cytoplasmic polyadenylation of specific mRNAs and for spermiogenesis (Kashiwabara et al., 2002; Zhuang et al., 2004). More recently a new family of atypical poly(A) polymerases, the GLD-2 family, has been characterized, with a first member identified in C. elegans (Wang et al., 2002). GLD-2-type proteins exist in all eukaryotes and have different functions (Buhler et al., 2007; Kwak and Wickens, 2007; Rissland et al., 2007).
In C. elegans, GLD-2 is required for entry into meiosis from the mitotic cycle in the gonad and for meiosis I progression (Kadyk and Kimble, 1998). C. elegans GLD-2 has a poly(A) polymerase activity in vitro (Wang et al., 2002) and in vivo (Suh et al., 2006). In Xenopus oocytes, GLD-2 is found in the cytoplasmic polyadenylation complex where it directly interacts with CPEB and CPSF, and it has a poly(A) polymerase activity in vitro in the presence of the other factors of the complex (Barnard et al., 2004). GLD-2 is in complexes with mRNAs regulated by cytoplasmic polyadenylation such as cyc B1 and mos (Rouhana et al., 2005). It is thus very likely that GLD-2 plays a role in cytoplasmic polyadenylation during Xenopus meiotic maturation. However, although cytoplasmic polyadenylation of mos and cyc B1 mRNAs is required for meiotic maturation (Sheets et al., 1995; Stebbins-Boaz et al., 1996), the functional role of Xenopus GLD-2 in meiotic maturation has not been addressed. Unexpectedly, although mouse GLD-2 is found in oocytes at metaphases I and II, a recent study shows that oocyte maturation in gld-2 KO mice is not altered, demonstrating that if GLD-2 acts as a poly(A) polymerase at this stage, another protein acts redundantly (Nakanishi et al., 2006; Nakanishi et al., 2007).
In Drosophila, poly(A) tail regulation by deadenylation and cytoplasmic polyadenylation is essential for controlling mRNAs involved in axis patterning and other aspects in early development (Benoit et al., 2005; Castagnetti and Ephrussi, 2003; Kadyrova et al., 2007; Morris et al., 2005; Semotok et al., 2005; Vardy and Orr-Weaver, 2007; Zaessinger et al., 2006). In ovaries, cytoplasmic polyadenylation regulates translation of oskar (osk), the posterior determinant and of cyc B mRNAs, and depends on Orb, the Drosophila homologue of CPEB (Benoit et al., 2005; Castagnetti and Ephrussi, 2003; Chang et al., 1999; Juge et al., 2002). Orb is required at the earliest steps of oogenesis, for the regulation of the synchronous divisions of a cystoblast which lead to the production of sixteen germ cells per cyst, and for the restriction of meiosis to one oocyte (Huynh and St Johnston, 2000). A single gene, hiiragi (hrg) which encodes one isoform of canonical PAP exists in the Drosophila genome (Juge et al., 2002; Murata et al., 2001). Genetic interactions have implicated orb and hrg in cytoplasmic polyadenylation of osk mRNA and accumulation of Osk protein at the posterior pole of the oocyte during mid-oogenesis. This led to the conclusion that canonical PAP has a role in cytoplasmic polyadenylation at this stage (Juge et al., 2002).
Cytoplasmic poly(A) tail elongation is also crucial in early embryos to activate translation of mRNAs, including bicoid (bcd) which encodes the anterior morphogen (Salles et al., 1994). Polyadenylation and translation occur upon egg activation, a process that also induces meiosis resumption from the metaphase I arrest in mature oocytes, and which is triggered by egg laying, the passage of the egg through the oviduct (Heifetz et al., 2001). A link has been established between cytoplasmic polyadenylation and meiotic progression at egg activation, because mutants defective for meiotic progression are also defective for poly(A) tail elongation (Horner et al., 2006; Lieberfarb et al., 1996; Page and Orr-Weaver, 1996).
Here, we analyse the function of Drosophila GLD-2 in the female germline. We show that this protein is encoded by wispy (wisp), a gene previously identified genetically (Brent et al., 2000), and we therefore refer to this protein as Wisp. We find that Wisp has a poly(A) polymerase activity in vitro and in vivo, and that it is required for poly(A) tail elongation of maternal mRNAs during late oogenesis and early embryogenesis. Wisp is required for meiotic progression in mature oocytes. A key target of Wisp during this process is cortex (cort) mRNA which encodes a meiosis-specific activator of the Anaphase Promoting Complex (APC). This demonstrates the role of polyadenylation and translational activation in meiotic progression. In addition, we investigate the respective roles of conventional PAP and of Wisp in oogenesis and show that PAP and Orb are involved earlier than Wisp and Orb. Our results establish the requirement of two poly(A) polymerases in cytoplasmic polyadenylation for different steps of oogenesis.
Materials and methods
Drosophila stocks and genetics
The w1118 stock was used as control. The wispKG5287 (previously called CG15737KG5287) mutant was from BDGP. The P-element (SUPor-P) was mobilized in the presence of P transposase and internal deletions of the P-element were recovered. Several female fertile revertants were also recovered among which two were sequenced and had retained a small portion of P-element ends (33 nt and 39 nt respectively). As the insertion point is downstream of the start codon and the remaining short insertions are coding and in frame with Wisp in both revertants, we believe these revertants produce slightly longer functional Wisp proteins. A 1.7 kb genomic region overlapping the conserved domains in wisp12−3147 was PCR amplified and sequenced. Two point mutations, T1151I and I1292V were found. The stock y1 fs(1)K104 cv1 v 1 f1/FM0 that was generated in the same mutagenesis as wisp12−3147 (Mohler, 1977) was used as a source of parental chromosome, and the same region of wisp was sequenced in this stock. The conservative I1292V mutation was present in this control stock, whereas T1151I was not.
RNA
PAT assays and RT-PCR were performed as reported previously (Benoit et al., 2005; Zaessinger et al., 2006). RNA preparations were from 20 embryos, 10 ovaries, and from dissected germarium-to-stage 8, stage 9–10 and stage 14 egg chambers from 10 ovaries. RT-PCR were with the same RNA preparations used for PAT assays and were performed on serial dilutions of the cDNAs. Dilutions 1:10 are shown. Oligos for PAT assays were: osk 5’AAGCGCTTGTTTGTAGCACA, cyc B 5’GCTGGCCGAACACATCGGCG, nos 5’TTTTGTTTACCATTGATCAATTTTTC, bcd 5’CATTTGCGCATTCTTTGACC, cort 5’GGCCAAGGACAAGTGCAGCTC, sop 5’GGATTGCTACACCTCGGCCCGT. Oligos for RT-PCR were: osk 5’GCCATATTGCTGAGCCACGCCC and 5’CCAGTAGCGTGGAGGTGCTCG, nos 5’CGATCCTTGAAAATCTTTGCGCAGGT and 5’TCGTTGTTATTCTCACAAAAGACGCA, bcd 5’CTGGGTCGACCAATGTCAATGGCG and 5’GCTCTTGTCCAGACCCTTCAAAGG, sop 5’CACCCCAATAAAGTTGATAGACCT and 5’ATCTCGAACTCTTTGATGGGAAGC. Whole-mount RNA in situ hybridizations were performed by standard methods. The probes were RNA antisense made from pKSbcdwt (bcd), pN5 (nos) and osk cDNA clones.
Poly(A) polymerase assays in Xenopus oocytes
To express MS2 fusion proteins in Xenopus oocytes, the C-terminal half of Wisp (residues 702–1373) and full length HsGLD-2 were cloned into pCSMS2 vector using NheI and XhoI (Rouhana et al., 2005). Wisp D1031A and HsGLD-2 D215A mutations were created by site-directed mutagenesis. For in vitro transcription, Wisp and HsGLD-2 clones were linearized with XbaI and NotI, respectively and transcribed using SP6 Megascript kit (Ambion). Xenopus oocyte manipulation, injection, luciferase assays and labelled RNA analysis were performed as previously described (Dickson et al., 1999; Kwak et al., 2004). To test MS2 fusion protein expression, oocytes were harvested 6h after mRNA injection and analyzed by western blotting using α-HA11 antibody (1:2000) (Covance). Two oocytes were loaded per lane.
GST pull-down assays
GST recombinant proteins were produced by cloning the C-terminal half of Wisp (residues 702–1373) into pBAH vector using EcoRI and XhoI, and the N-terminal region of Wisp (residues 11–547) digested with BglII into pGEX-5X-2 vector digested with BamHI. In vitro interactions were performed as described (Benoit et al., 2002), in the presence of 0.2 μg/μl RNase A. Orb was in vitro translated from the orb D5 cDNA cloned into pBluescript using EcoRI and HindIII.
Antibodies, western blots, immunostaining
Antibodies against Wisp were obtained by cloning a portion of wisp cDNA LD18468 encoding residues 702 to 1373 into pMAL vector. The Wisp-MBP fusion protein was expressed in E.coli and purified using amylose beads (NEB). The purified fusion protein was injected to Guinea pigs. Western blots and immunostaining were performed as reported (Benoit et al., 2005; Benoit et al., 1999). Antibody dilutions for western blots were: anti-Wisp 1:3000, rabbit anti-Bic-C (Saffman et al., 1998) 1:1000, rat anti-PAP (Juge et al., 2002) 1:500, anti-Orb 6H4 (Developmental Studies Hybridoma Bank) 1:20, rabbit anti-Cyclin A 1:10000 (Whitfield et al., 1990), anti-Cort 1:2000 (Pesin and Orr-Weaver, 2007), anti-α-tubulin (Sigma T5168) 1:10000. Dilutions for immunostaining were: anti-Wisp 1:2000, anti-Osk (Kim-Ha et al., 1995) 1:500, anti-Nos (gift from A. Nakamura) 1:1000, anti-Bcd (Kosman et al., 1998) 1:200, mouse anti-C(3)G (Anderson et al., 2005) 1:500. To visualize meiotic or mitotic spindles in embryos, methanol fixation was performed as reported (Brent et al., 2000) and dilution of anti-α-tubulin (Sigma T9026) was 1:200. Meiotic spindles in stage 14 oocytes were visualized as previously described (Endow and Komma, 1997) using FITC-conjugated anti-α-tubulin (Sigma F2168).
Immunoprecipitations
Immunoprecipitations were as described previously (Zaessinger et al., 2006). Each immunoprecipitation was with 60 ovaries of well fed 3–4-day-old females and 5 μl of serum (immune or pre-immune), or 10 ml of hybridoma supernatant (Orb 6H4 or irrelevant 12CA5).
Results
The female germline GLD-2 poly(A) polymerase in Drosophila
Two genes, GC5732 and CG15737, encoding GLD-2 homologues are present in the Drosophila genome. The corresponding proteins share the characteristics of GLD-2 family members in other species. They have a catalytic DNA polymerase β-like nucleotidyltransferase domain containing three conserved aspartic acid residues, which is included in a larger conserved central domain, a PAP/25A associated domain, and they lack a RNA binding domain (Fig. 1A). The region which is N-terminal to the central domain is variable in size and non-conserved in the Drosophila GLD-2. Several CG5732 cDNAs described in FlyBase are from adult testis indicating that CG5732 is expressed in this tissue. We verified by RT-PCR that CG5732 is not expressed in ovaries (data not shown). We focused on CG15737 (Fig. 1B) which was expressed in ovaries.
Figure 1:
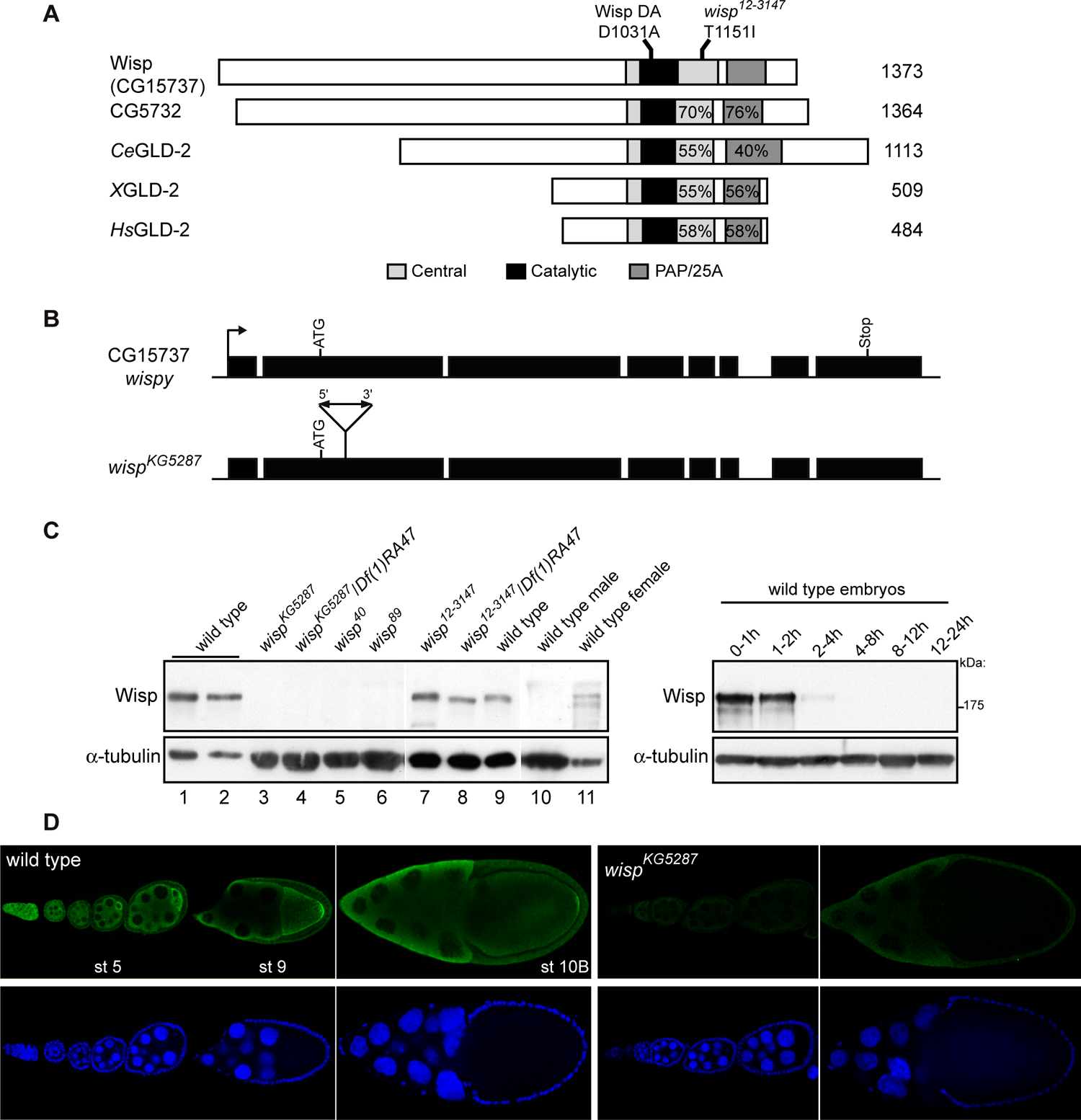
GLD-2 encoding genes and proteins in Drosophila
(A) Schematic representation of GLD-2 proteins from different species. Accession numbers are NP_572766 for Wisp, NP_651012 for CG5732 encoded protein, NP_491842 for C. elegans, AAT98005 for Xenopus and NP_776158 for human. The regions showing homology are in grey and black; percentages of similarity with Wisp in the central (including catalytic) domain, and in the PAP/25A domain are indicated. The mutation D1031A in the catalytic domain which precludes poly(A) polymerase activity and the point mutation in wisp12–3147 are shown.
(B) Schematic representation of wisp (CG15737) locus and mutant. Black boxes are exons. The arrow indicates the transcription start site. Three cDNAs, LD18468, RE03648 and RE14825 were sequenced. RE03648 and RE14825 are full-length, they start and end at identical nucleotides, LD18468 is incomplete at both ends. The P-element (not drawn to scale) in wispKG5287 is shown. The insertion site was verified by sequencing.
(C) Western blots revealed with anti-Wisp showing Wisp expression in females and ovaries and the lack of protein in wispKG5287, wisp40 and wisp89 mutant ovaries. Protein extracts were from 0.4 (lane 1) or 0.2 (lanes 2, 9) wild-type ovary, from one (lanes 3–6) or 0.2 (lanes 7, 8) mutant ovary, 0.3 male (lane 10) and 0.1 female (lane 11), and from 20 wild-type embryos (right panel). α–tubulin was used as a loading control.
(D) Immunostaining of ovaries with anti-Wisp antibody showing cytoplasmic expression (green) throughout oogenesis and the lack of expression in wispKG5287 mutant ovaries. Nuclei are visualized with DAPI (blue). Anterior is oriented towards the left.
An antibody against the C-terminal half of the protein (residues 702–1373) was developed. In western blots, this antibody revealed a band of 180 kDa in adult females and ovaries, that was absent in adult males (Fig. 1C). This band corresponds to the protein encoded by CG15737 as it is completely lacking in mutants of this gene (see below). In embryos, expression of the protein peaked at 0–2 h of development, decreased at 2–4 h and was undetected in later stages, consistent with a maternal expression. Immunostaining of ovaries showed that the protein is expressed throughout oogenesis (Fig. 1D). It was cytoplasmic, present both in nurse cells and the oocyte and accumulated in the oocyte from stage 5 onwards. In stages 9 and 10, protein accumulation was visible at the posterior pole of the oocyte. Staining of ovaries mutant for CG15737 was reduced to background levels, indicating that the antibody specifically recognized the protein encoded by this gene.
The female germline GLD-2 poly(A) polymerase is encoded by wispy and is required for metaphase of meiosis I
A P-element insertion in CG15737 (CG15737KG5287) was available at the Berkeley Drosophila Genome Project (BDGP), in which the insertion occurred 36 residues downstream of the initiation codon (Fig. 1B). Homozygous CG15737KG5287 males were viable and fertile, whereas homozygous CG15737KG5287 females were viable and sterile. Oogenesis did not appear affected and these females laid a normal number of eggs, but 100% of embryos from mutant females crossed with wild-type males showed no development (Fig. 2A, B). Because the protein encoded by CG15737 was lacking in ovaries from CG15737KG5287 homozygous females (Fig. 1C, D) and the phenotypes of CG15737KG5287 homozygous were identical to those of CG15737KG5287/Df(1)RA47 (a deficiency overlapping the region) females, we believe that CG15737KG5287 is a null allele. Mobilization of the P-element in CG15737KG5287 generated new mutant alleles (CG1573740 and CG1573789) corresponding to internal deletions of the P-element, and allowed to show that a nearly complete deletion of the P-element restored female fertility. A close examination of CG15737KG5287 phenotypes showed that the gene is required at metaphase of meiosis I.
Figure 2:
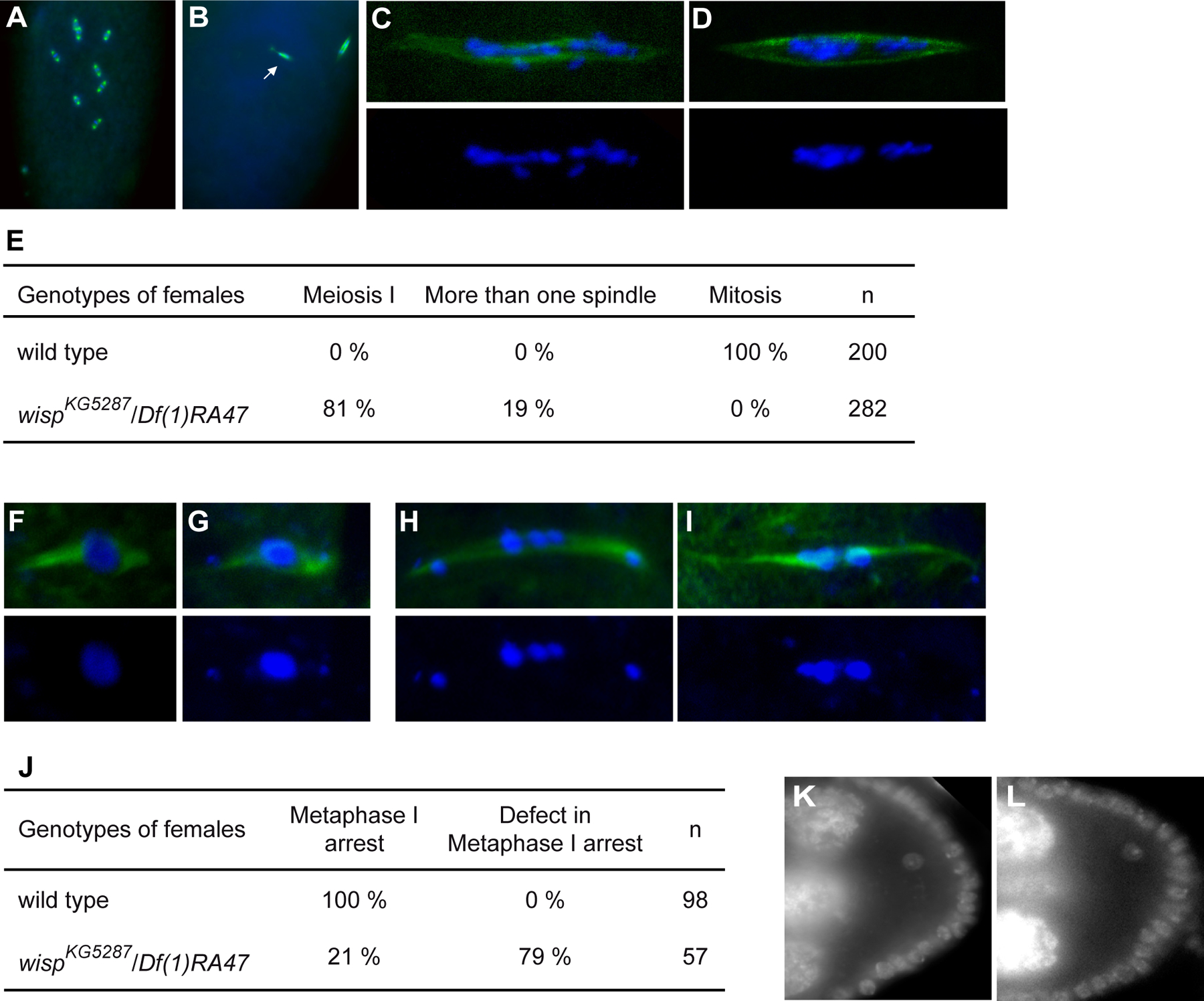
wisp function in the germline
(A-E) Meiotic arrests visualized in embryos. Immunostaining of 0–20 min wild-type embryos and 0–2-h wispKG5287/Df(1)RA47 embryos with anti-α-tubulin (green) and DAPI (blue). (A) Wild-type embryo showing mitoses. (B) wispKG5287/Df(1)RA47 embryo showing one metaphase I anastral female spindle and one mitotic-like spindle with a centrosome, associated with the male pronucleus (arrow). (C, D) Examples of meiosis I spindle in wispKG5287/Df(1)RA47 embryos showing scattered chromosomes along the spindle (C), and asymmetric pools of chromosomes (D). DAPI alone is in bottom panels. (E) Meiotic arrest visualized with anti-α-tubulin and DAPI staining were scored.
(F-J) Meiotic defects in stage 14 oocytes visualized with anti-α-tubulin (green) and DAPI (blue). (F) Prometaphase I in wild-type stage 14 oocyte. (G) Wild-type-like metaphase I in wispKG5287/Df(1)RA47 embryo, the fourth chromosomes are separated from the main chromosome pool (Metaphase I arrest in J). (H, I) Abnormal metaphase I spindles in wispKG5287/Df(1)RA47 oocytes. DAPI alone is in bottom panels. (J) Meiotic figures visualized with anti-α-tubulin and DAPI were scored.
(K, L) DAPI staining of stage 8 oocytes showing that the karyosome is not affected in the mutant. (K) wild-type, (L) wispKG5287/Df(1)RA47.
The phenotypes were similar to those of wisp mutants, a gene genetically identified earlier and which is located in the same chromosome region as CG15737 (Brent et al., 2000; Mohler, 1977). Complementation tests between CG15737KG5287 and wisp12–3147 showed that CG15737 is wisp. Sequencing of the conserved domains in wisp12–3147 identified a point mutation, T1151I in the central domain of the protein, changing a residue that is conserved in the other species (Fig. 1A). This mutation did not prevent the production of Wisp in ovaries (Fig. 1C).
Staining of embryos from wispKG5287 or wispKG5287/Df(1)RA47 females (thereafter called wisp embryos) with anti-α-tubulin and DAPI to visualize meiotic figures showed that these embryos did not complete meiosis (Fig. 2A–E). In most embryos (74%), a single meiotic spindle was visible, which could be thin or distorted and on which the chromosomes were scattered or separated in asymmetric pools (Fig. 2C, D). A second small spindle could be present which was nucleated by one or several lost chromosomes (8% of embryos). These figures (81%, Fig. 2E) correspond to a block in meiosis I. Among the remaining embryos, 15% could be identified as blocked in meiosis II by visualization of two meiotic spindles, which were in most cases abnormally arranged. This phenotype is stronger than that of wisp12–3147/Df(1)RA47 embryos, most of which were arrested at or after metaphase II (Brent et al., 2000). Meiotic figures in wisp mutant embryos correspond to either abnormal metaphase I or anaphase I. In the wild type, a meiotic arrest occurs at metaphase I in mature oocytes (Fig. 2F, G). Meiosis I resumes at egg activation, driven by egg laying, and meiosis II is rapidly completed without further arrest.
Meiotic figures were analysed in wispKG5287/Df(1)RA47 mature (stage 14) oocytes and we found that in most oocytes (79%), metaphase I arrest was not maintained properly (Fig. 2H–J). The chromosomes separated along the spindle and did so asymmetrically, the fourth chromosomes often migrated out of the spindle. The spindle appeared thin or irregular (Fig. 2H–I). Therefore, wisp mutants showed a defect in metaphase I arrest and meiosis is then blocked at this stage as abnormal meiotic figures are similar after egg activation in wisp mutant embryos. Because a defect in metaphase I arrest could result from a defect earlier in meiosis, we analysed earlier aspects of meiosis. We found that the karyosome in wispKG5287/Df(1)RA47 stage 8 oocytes appeared wild type (Fig. 2K, L). Entry into meiosis, as well as meiosis restriction to one oocyte in the germarium, visualized by the formation of synaptonemal complex using anti-C(3)G antibody (Anderson et al., 2005) were also as in the wild type (data not shown).
We conclude that the GLD-2-type poly(A) polymerase in the female germline is encoded by wisp and is required for metaphase I arrest and for progression of meiosis after this stage.
Wisp is a poly(A) polymerase and is involved in poly(A) tail elongation during late oogenesis
A tethering assay was previously developed in Xenopus oocytes to analyse the poly(A) polymerase activity of candidate proteins (Kwak et al., 2004). The tested protein is tethered to mRNAs through MS2, an exogenous RNA binding protein, and poly(A) addition as well as translational activation of the targeted mRNA is assayed. A chimeric mRNA encoding a MS2-Wisp fusion protein was injected into Xenopus oocytes. Two reporter mRNAs: luciferase with MS2 binding sites and β-galactosidase lacking MS2 binding sites were then coinjected. Translational activation was calculated by comparing luciferase activity to β-galactosidase activity (Fig. 3A). HsGLD-2 was used as a positive control and induced translational activation that was abolished when the catalytic site was destroyed by mutation of an essential aspartic acid residue (HsGLD-2 DA). Wisp protein strongly stimulated translation of the reporter RNA and the stimulatory effect was abolished by a point mutation in the catalytic domain (Wisp DA) (Fig. 1A). Poly(A) tail addition to a short RNA containing MS2 binding sites was measured and a robust polyadenylation which depended on the catalytic domain integrity was observed, in the presence of either MS2-HsGLD-2 or MS2-Wisp (Fig. 3B). These results demonstrate that Wisp is a poly(A) polymerase.
Figure 3:
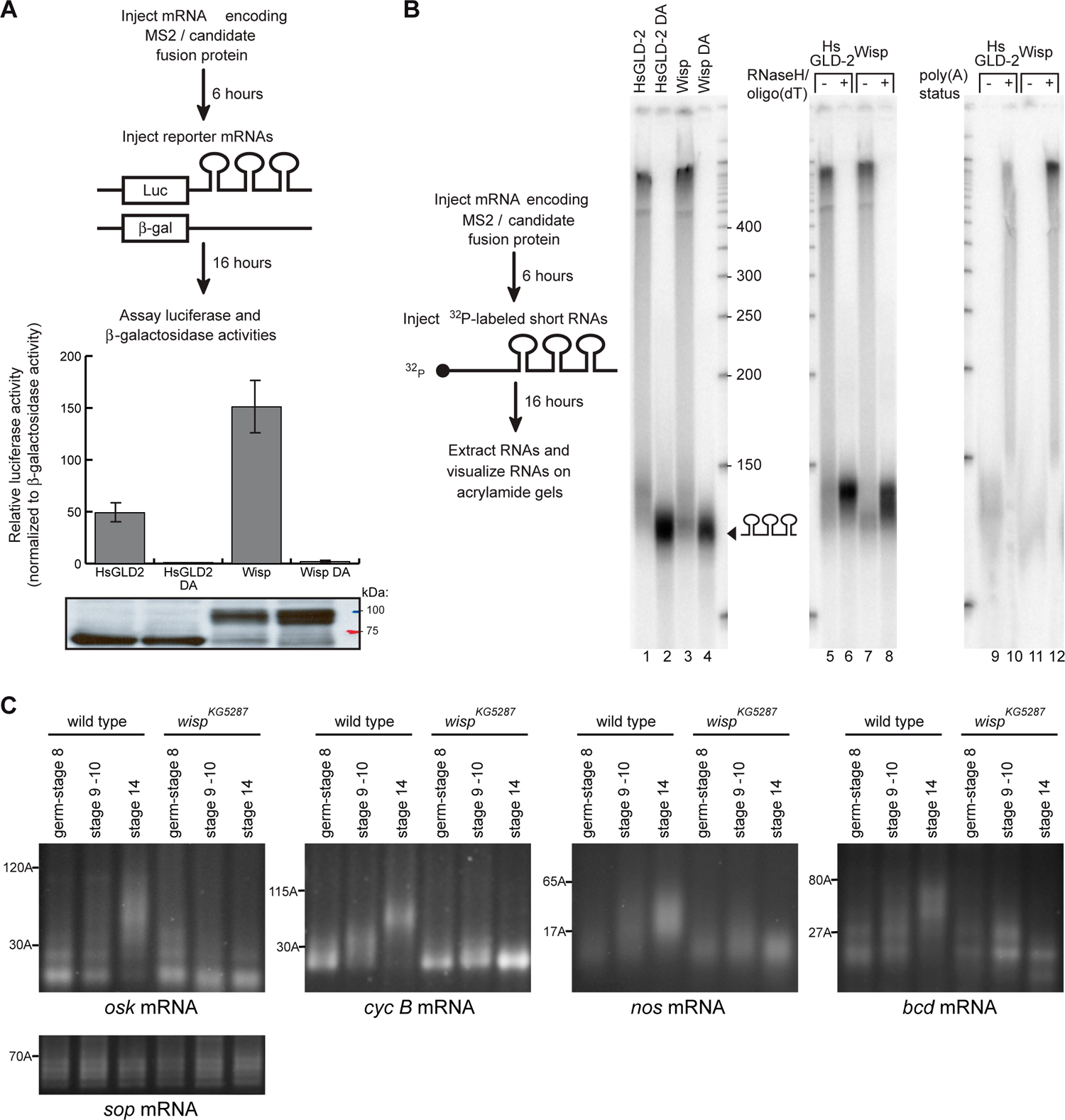
Poly(A) polymerase activity of Wisp in a tethering assay and during oogenesis (A, B) Tethering assay in Xenopus oocytes. (A) Wisp and a control protein, HsGLD2, were tethered to luciferase reporter mRNA using MS2. β-galactosidase mRNA lacking MS2 binding site was used as an internal control. Translational stimulation was assayed by measuring luciferase activity. Wild-type and point mutant proteins (DA) were expressed at similar levels, as determined by western blotting with anti-HA11 (bottom). (B) 130nt-long, 32 P-labeled RNA was injected and then purified from oocytes. Lanes 1–4: Tethered HsGLD-2 and Wisp added long tails onto labelled RNA. Active site mutations disrupted elongation. Lanes 5–8: Tails added by wild-type Wisp were removed by RNaseH/oligo(dT) treatment, confirming that they were poly(A). −, RNaseH only; +, RNAseH plus oligo(dT). Lanes 9–12: RNAs elongated by Wisp were bound to an oligo(dT) column. −, RNAs that did not bind; +, RNAs that bound.
(C) PAT assays measuring osk, cyc B, and nos and bcd poly(A) tail lengths in egg chambers of different stages (germarium to stage 8, stages 9 and 10, and stage 14 oocytes) from wild-type and wispKG5287 ovaries. sop mRNA which encodes a ribosomal protein and is not regulated by cytoplasmic polyadenylation was used as a control.
We confirmed the role of Wisp in poly(A) tail elongation in vivo, using PAT assays, a RT-PCR based technique that allows measurements of poly(A) tail lengths. Cytoplasmic polyadenylation of osk and cyc B mRNAs during oogenesis has been reported and depends on Orb (Benoit et al., 2005; Castagnetti and Ephrussi, 2003). We determined whether it also depended on the Wisp poly(A) polymerase. Cytoplasmic polyadenylation of nanos (nos) and bcd mRNAs was also analysed. Egg chambers of progressive stages were dissected and poly(A) tails were measured (Fig. 3C). For all tested mRNAs, poly(A) tails lengthened during oogenesis in the wild type, with a moderate lengthening between early stages (germarium to stage 8) and stages 9–10, and a pronounced elongation between stages 9–10 and stage 14 oocytes. For bcd mRNA, this lengthening is not sufficient for translational activation which occurs after egg activation (Salles et al., 1994). In wispKG5287 egg chambers, the lengthening at stage 9–10 was slightly affected and the strong elongation at stage 14 was completely abolished.
To determine whether Wisp has a general role in regulating many mRNAs, we analysed the poly(A) status of 30 mRNAs that we have identified to be regulated by cytoplasmic polyadenylation and which poly(A) tail undergo a robust lengthening in mature oocytes (I. Busseau and M.S. unpublished). For all tested mRNAs, the poly(A) tail lengthening in wispKG5287 mature oocytes was impaired (Supplementary Fig. 1).
Together, these data show that Wisp is the poly(A) polymerase responsible for cytoplasmic polyadenylation of mRNA targets during late oogenesis.
cort mRNA is a meiotic target of Wisp
To determine if the meiotic defect observed in wisp mutants resulted from defects in mRNA polyadenylation and translational activation, we identified a Wisp target required for meiotic progression. cort encodes a female meiosis-specific activator of the APC, necessary for female meiosis (Chu et al., 2001). Eggs from cort mutant females show aberrant chromosome segregation in meiosis I and eventually arrest in metaphase II (Lieberfarb et al., 1996; Page and Orr-Weaver, 1996). Cort is required for sequential degradation of Cyc A, B and B3 during meiosis, with Cyc A being degraded earlier, prior to metaphase I arrest (Pesin and Orr-Weaver, 2007; Swan and Schupbach, 2007). Cort protein expression was shown to peak during oocyte maturation (stages 13 and 14) and was correlated with cort mRNA poly(A) tail lengthening (Pesin and Orr-Weaver, 2007). We found that cort poly(A) tail elongation was abolished in wispKG5287 stage 14 oocytes (Fig. 4A). This led to a defect in Cort protein accumulation at this stage (Fig. 4B). The earliest target of Cort is Cyc A, the degradation of which fails in cort mutants by metaphase I arrest (Pesin and Orr-Weaver, 2007). We verified that the lack of Cort accumulation in wispKG5287 mature oocytes resulted in a defect of Cyc A destruction. Cyc A levels were elevated in wispKG5287 mature oocytes, consistent with impaired destruction (Fig. 4C).
Figure 4:
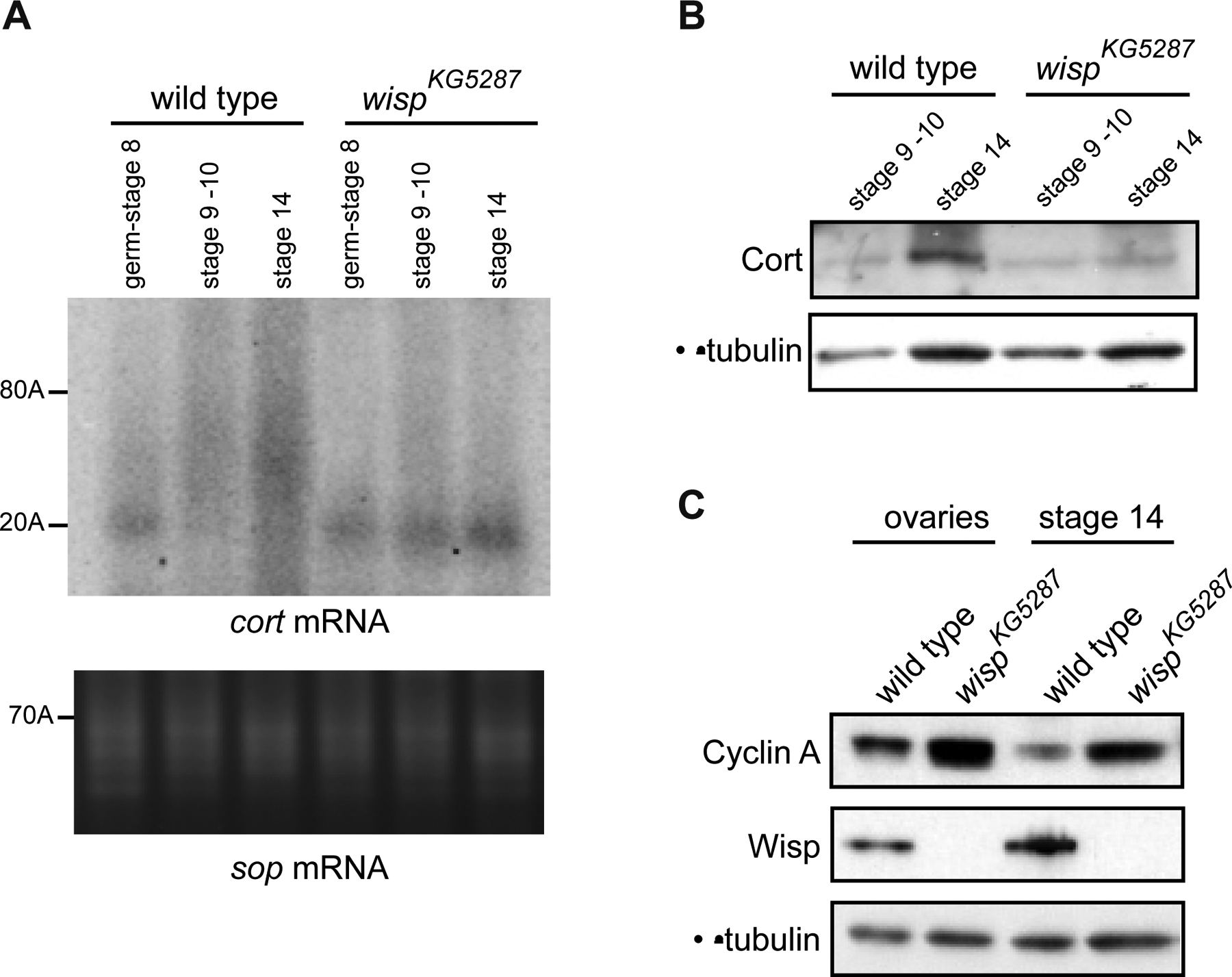
cort mRNA is a meiotic target of Wisp
(A) PAT assays measuring cort poly(A) tail lengths in egg chambers of progressive stages from wild-type and wispKG5287 ovaries. sop mRNA was used as a control.
(B) Western blots showing that Cort protein levels are reduced in wispKG5287 stage 14 oocytes compared to wild type. Protein extracts were from 30 stage 9–10 and 15 stage 14 oocytes.
(C) Western blots showing that Cyc A levels are higher than wild type in wispKG5287 ovaries and stage 14 oocytes. Protein extracts were from 0.5 ovary and 20 stage 14 oocytes. α-tubulin was used as a loading control in B and C.
These results identify cort, an mRNA required for meiotic progression, as a Wisp target which poly(A) tail elongation and translation depend on Wisp. They strongly suggest that the poly(A) polymerase function of Wisp is required during progression of meiosis.
Wisp and PAP poly(A) polymerases are both in a cytoplasmic polyadenylation complex with Orb
Cytoplasmic polyadenylation of osk and cyc B mRNAs during oogenesis is Orb-dependent, and GLD-2 is in complex with CPEB in Xenopus oocytes (Barnard et al., 2004; Rouhana et al., 2005). We, therefore, analysed the presence of Wisp and Orb in a complex, by co-immunoprecipitations in ovary extracts. Orb was able to coprecipitate with Wisp and this interaction was RNA-independent (Fig. 5A). Conversely, Wisp coprecipitated with Orb, and the interaction again was RNA-independent (Fig. 5B). A direct interaction between Wisp and Orb was confirmed by in vitro binding assays, in which in vitro translated Orb bound to recombinant GST-Wisp(702–1373), but not to GST-Wisp(11–547) or to GST alone (Fig. 5E).
Figure 5:
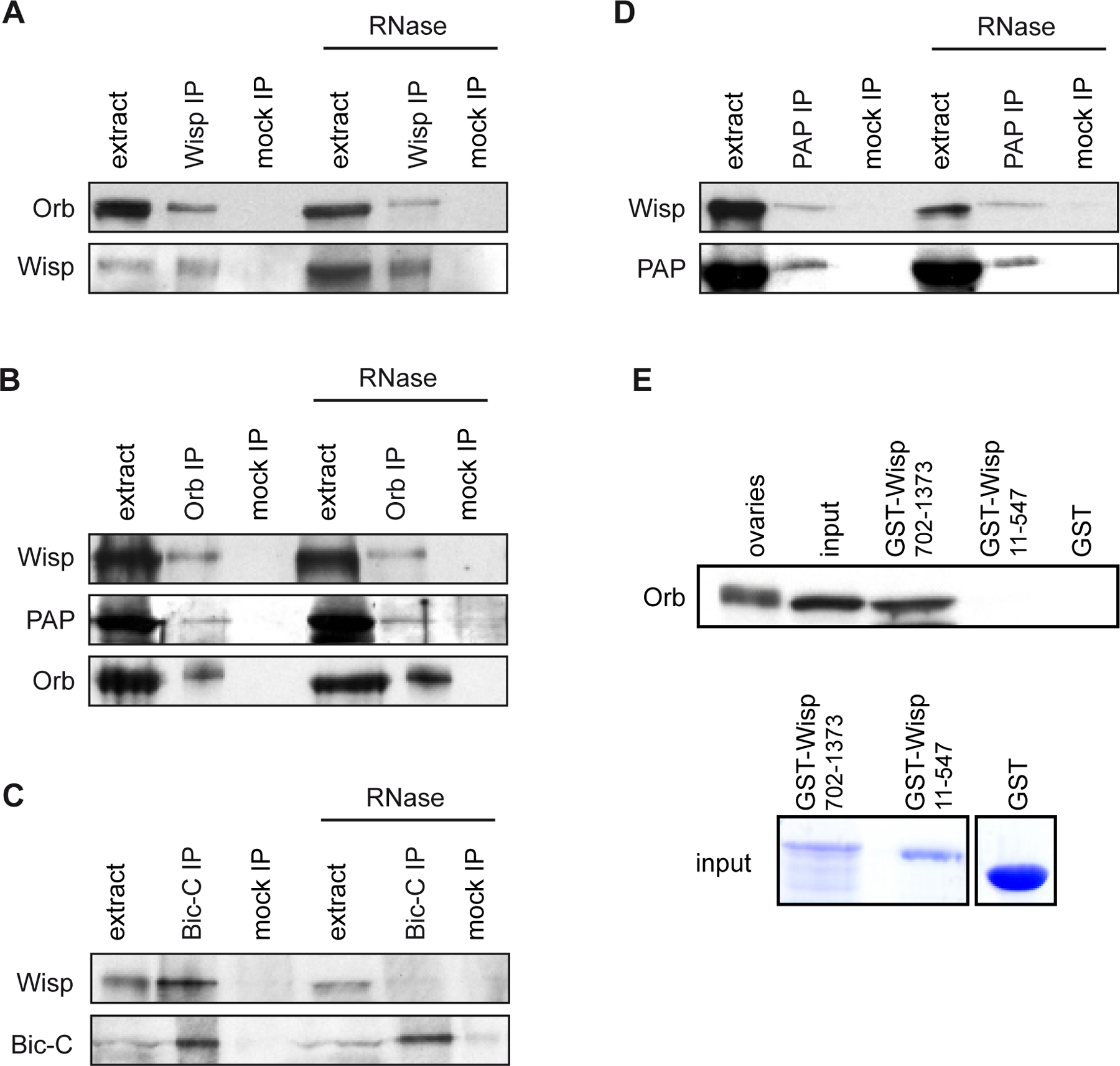
Cytoplasmic polyadenylation complexes in ovaries
(A-D) Immunoprecipitations (IP) were performed in ovary extracts either in the presence of RNase inhibitor or in the presence of RNase A (RNase). Co-immunoprecipitated proteins were identified by western blots. Mock IPs were with preimmune serum for Wisp and PAP IPs, with rabbit serum for Bic-C IP and with an irrelevant monoclonal antibody for Orb IP. Extract (1/20) prior to IP was loaded.
(E) In vitro interaction assays showing that Orb directly interacts with recombinant GST-Wisp(702–1373), but not with GST-Wisp(11–547) or GST alone. Orb was revealed by western blot. 1/10 of in vitro synthesized Orb before the interaction assay was loaded (input). Protein extract of 0.5 ovary was also loaded. Recombinant and GST proteins are shown (input).
In C. elegans, GLD-2 directly interacts with the RNA binding protein GLD-3 (Wang et al., 2002), a homologue of Drosophila Bic-C. In co-immunoprecipitation between Wisp and Bic-C, Wisp coprecipitated with Bic-C. This interaction depended on the presence of RNA, suggesting that Bic-C and Wisp can be present in common RNP complexes, but do not interact directly (Fig. 5C).
We reported previously that poly(A) tail elongation of osk mRNA in ovaries, and Osk protein accumulation at the posterior pole of oocytes depended on Orb and the canonical PAP (Juge et al., 2002). We now find that cytoplasmic polyadenylation of osk mRNA requires Wisp, another poly(A) polymerase. To understand the role of two different poly(A) polymerases in poly(A) tail lengthening of the same targets, we investigated the presence of PAP in the cytoplasmic polyadenylation complex. PAP co-precipitated with Orb in ovary extract, independently of the presence of RNA (Fig. 5B). More strikingly, Wisp co-precipitated with PAP and the interaction was maintained in the absence of RNA (Fig. 5D).
These data show that Wisp is recruited to mRNAs through a direct interaction with Orb, and they are consistent with the role of both PAP and Wisp in cytoplasmic polyadenylation with Orb.
Functions of PAP and Wisp during oogenesis
To investigate the respective roles of PAP and Wisp in oogenesis, we analysed genetic interactions between orb and wisp and between orb and the PAP encoding gene, hrg. Strong hrg mutants are lethal and do not produce late egg chambers in germline clones (Juge et al., 2002). Therefore, the implication of PAP and Orb in cytoplasmic polyadenylation had been inferred from strong genetic interactions between heterozygous hrg alleles and the homozygous weak orb allele, orbmel. The combination of both mutants strongly reduced female fertility and oogenesis stopped around stage 8 in a number of ovarioles (Juge et al., 2002).
Because the phenotype of orbmel mutant tended to become stronger with time, we backcrossed orbmel in a new background (Canton S) to produce a weaker phenotype. In this background, orbmel females produced a low level of maternal effect embryonic lethality (15%), which was increased to 31 and 45% in the hrg−/+; orbmel combinations, consistent with the previously described interactions (Fig. 6A). Embryonic lethality from wisp−/+; orbmel females also increased to similar levels, 35 to 50%. These genetic interactions corroborate the physical interactions between Wisp and Orb (Fig. 5) and provide additional support for the proposed role of Wisp and Orb together in cytoplasmic polyadenylation. A number of embryos from orbmel females are ventralized due to defects in gurken (grk) mRNA localization and translation during mid-oogenesis (Chang et al., 2001). Whereas the percentages of ventralized embryos did not increase in interactions between wisp and orb, they were enhanced for embryos from hrg−/+; orbmel females (26 to 31%) (Fig. 6A). These results suggest that hrg and orb interact during early or mid-oogenesis (before or during stages 9–10), whereas wisp and orb interact later (after stage 10).
Figure 6:
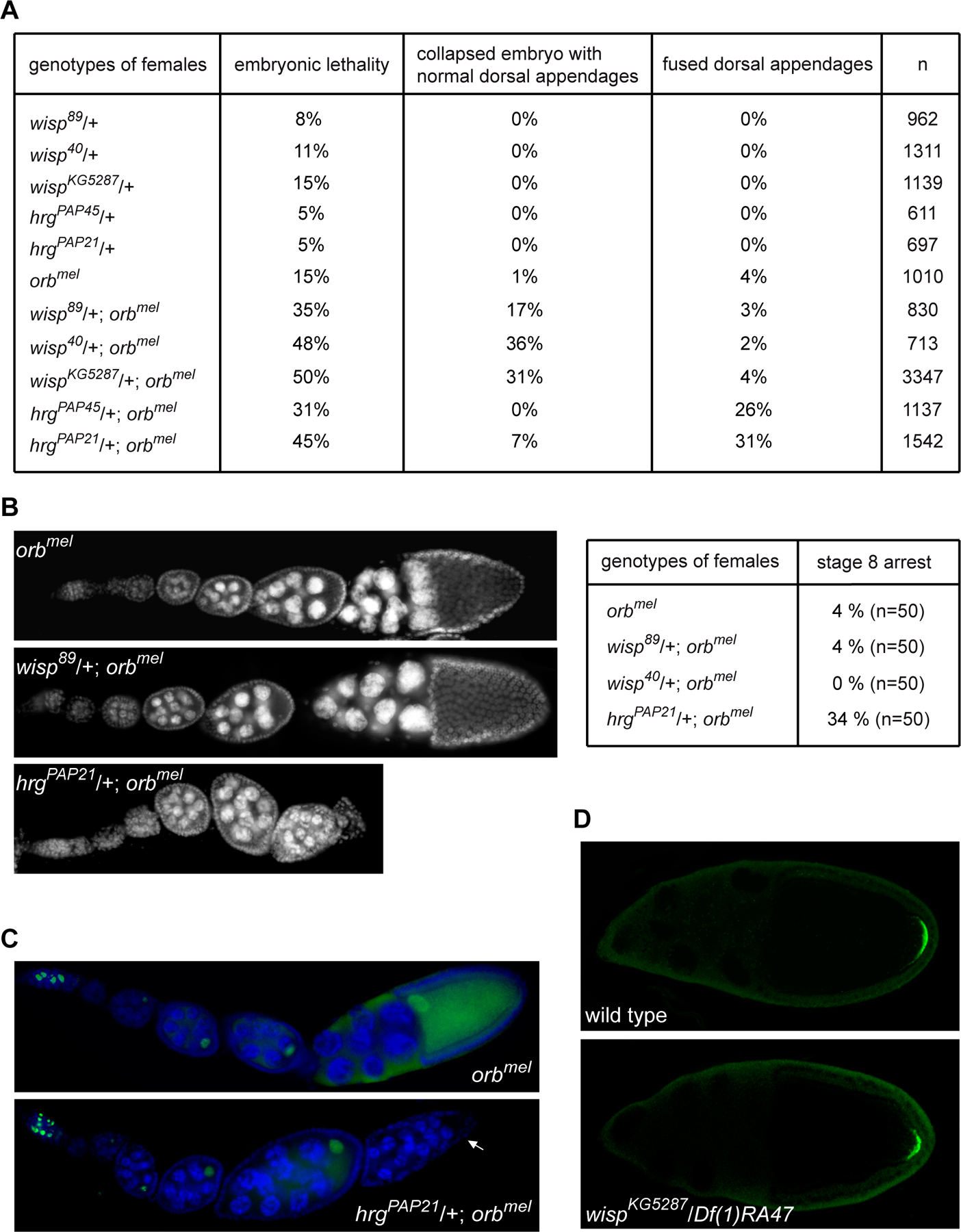
PAP has a earlier role in oogenesis than Wisp
(A) Genetic interactions between orb and wisp and between orb and hrg. Females of the indicated genotype were crossed with wild-type males and embryonic lethality of their progeny was scored. The percentage of embryonic lethality from orbmel mothers is increased in the presence of both heterozygous wisp or hrg mutants. hrg mutations only are able to dominantly increase the ventralization phenotype of orbmel. Note that collapsed embryos with normal dorsal appendages is a common phenotype of wisp mutant embryos. This phenotype suggests defect in vitelline membrane cross-linking, an early event at egg activation.
(B) Ovaries of orbmel single mutant females, or in the presence of wisp−/+ or hrg−/+ mutant visualized with DAPI. Oogenesis progresses normally in orbmel, wisp89/+; orbmel and wisp40/+; orbmel females, but is blocked at stage 8 in hrgPAP21/+; orbmel ovaries. 50 ovarioles of the indicated genotype were scored and the percentage of abnormal egg chamber arrested at stage 8 is indicated. For stage 8 arrests that were scored in orbmel or wisp−/+; orbmel ovaries, the oocyte was present.
(C) Characterization of stage 8 arrest in hrgPAP21/+; orbmel ovaries. Immunostaining of ovaries with anti-C(3)G antibody (green) and DAPI (blue), showing the presence of the oocyte in orbmel and its absence in arrested hrgPAP21/+; orbmel egg chamber (arrow).
(D) Osk protein accumulation is not affected in wisp mutant oocytes during mid-oogenesis. Immunostaining of wild-type and wispKG5287/Df(1)RA47 mutant ovaries with anti-Osk antibody showing that Osk accumulation at the posterior pole is similar in wild-type and wisp mutant stage 10 oocytes.
We then compared the phenotypes of genetic interactions between orb and hrg and between orb and wisp during oogenesis. Oogenesis stopped at stage 8 in a number of ovarioles from hrgPAP21/+; orbmel females (34%) (Fig. 6B). Staining with anti-C(3)G to visualize the oocyte was impaired in arrested hrgPAP21/+; orbmel egg chambers, indicating either degeneration or the lack of oocyte determination (Fig. 6C). Lack of oocyte determination was confirmed by the presence of sixteen nurse cells (10% of ovarioles). Consistent with the possible function of PAP with Orb during early oogenesis, PAP and Orb expressions overlap at these stages (Supplementary Fig. 2). Note that these data do not preclude a role of PAP later in oogenesis.
In contrast, early defects did not appear in wisp−/+; orbmel ovaries (Fig. 6B). Moreover, translation of osk mRNA was quantified previously in the orbmel mutant and found to be strongly affected in stage 9–10 oocytes (Castagnetti and Ephrussi, 2003). We found that Osk protein accumulation was not affected in wispKG5287/Df(1)RA47 oocytes at these stages (Fig. 6D) (n=128).
We conclude that PAP is required with Orb during early oogenesis, whereas Wisp has an essential function with Orb after stage 10 of oogenesis.
Wisp is required for cytoplasmic polyadenylation in early embryos
Upon egg activation, cytoplasmic polyadenylation leads to a robust poly(A) tail elongation and translation of a number of maternal mRNAs, including bcd. In wisp mutant embryos, poly(A) tail elongation of bcd mRNA was abolished (Fig. 7A). This short poly(A) tail was stable during the first three hours of embryogenesis and consistently bcd mRNA was not completely destabilized. bcd mRNA was also detected in embryos by in situ hybridizations, however, the transcript was delocalized in the anterior region and to a lesser extent in the rest of the embryo (Fig. 7B). Maternal mRNA delocalization in wisp mutant embryos had been reported, but was weaker, probably due to the utilization of weaker alleles (Brent et al., 2000). Consistent with previous data establishing that poly(A) tail elongation of bcd mRNA is necessary and sufficient for its translation in embryos (Salles et al., 1994), the lack of poly(A) lengthening in wisp mutant embryos prevented Bcd protein accumulation (Fig. 7C, Supplementary Fig. 3).
Figure 7:
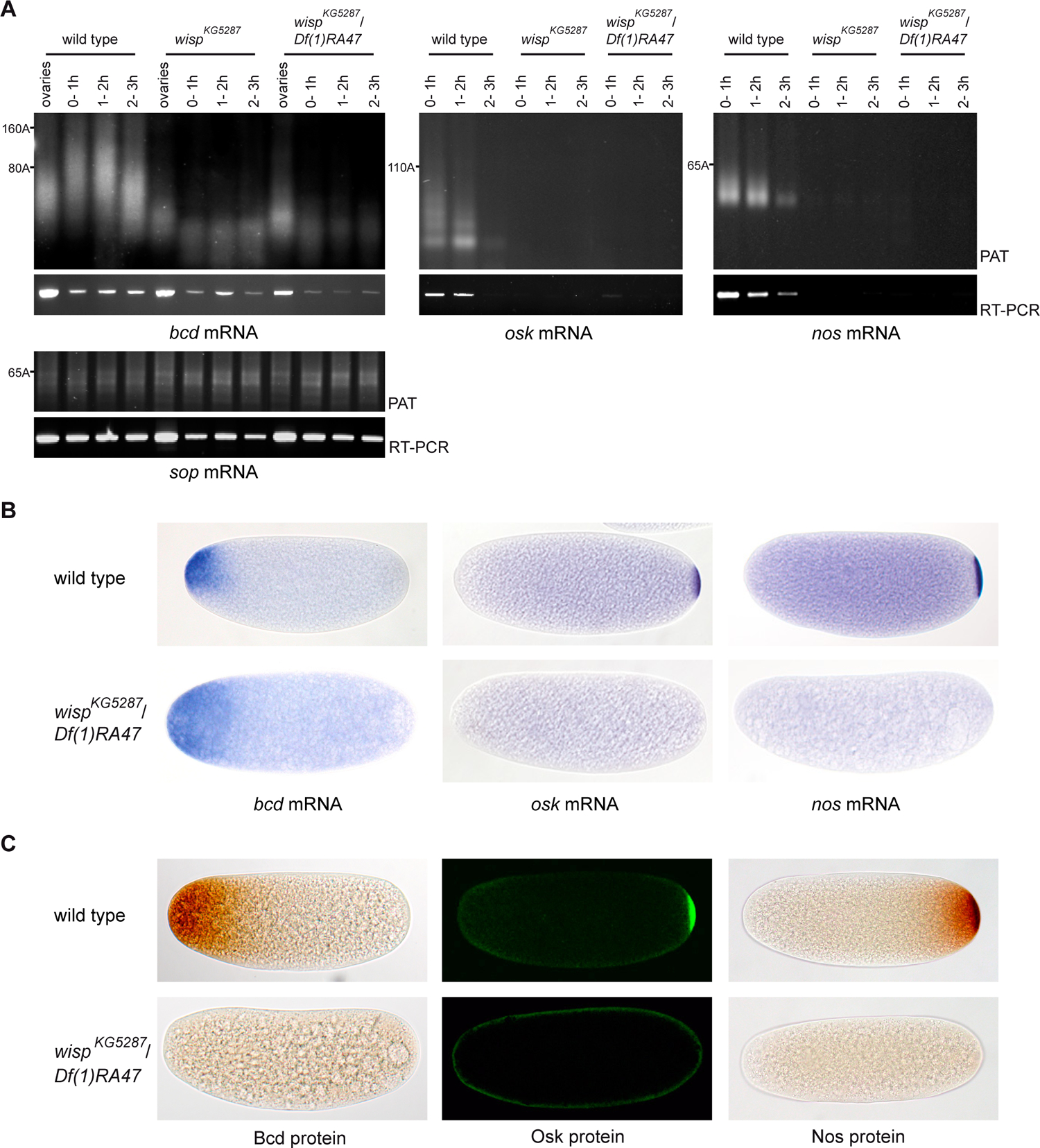
Role of Wisp in cytoplasmic polyadenylation in early embryos
(A) PAT assays and RT-PCR of bcd, osk and nos mRNAs in embryos from 0–1 h, 1–2 h and 2–3 h of development, from wild-type, wispKG5287 or wispKG5287/Df(1)RA47 females. In the absence of Wisp, osk and nos mRNAs are destabilized and the poly(A) tails of bcd mRNA are not elongated in embryos. sop mRNA was used as a control. The sop RT-PCR is the loading control for bcd, osk and nos RT-PCR. For bcd mRNA, PAT assays from ovaries were loaded to show the poly(A) tail elongation between ovaries and early embryos in the wild type.
(B) In situ hybridizations of 0–1 h embryos showing bcd, osk and nos mRNA.
(C) Immunostaining of 0–1 h embryos with anti-Bcd, anti-Nos and anti-Osk antibodies showing that the lack of poly(A) tail elongation in wispKG5287/Df(1)RA47 mutant embryos, leading or not to mRNA decay, results in the lack of corresponding proteins. Note that the lack of nos mRNA/protein at the posterior pole could also result from the lack of Osk protein as Osk is required for nos mRNA stabilization.
We measured poly(A) tail lengths of osk and nos mRNAs in embryos. In wild-type embryos, pools of osk and nos mRNAs are localized at the posterior pole, but another fraction of these mRNAs is widespread in the bulk cytoplasm (Bergsten and Gavis, 1999) and then degraded. In agreement with this, both osk and nos mRNAs analysed by PAT assays and RT-PCR were deadenylated and destabilized during the first three hours in wild-type embryos (Fig. 7A). We have shown previously that nos mRNA destabilization in the bulk cytoplasm of the embryo depended on deadenylation by CCR4 (Zaessinger et al., 2006). In wisp mutant embryos, osk and nos mRNAs were destabilized prematurely in the first hour of embryogenesis and pools of these mRNAs were not stabilized at the posterior pole (Fig. 7A, B) (see Supplementary Fig. 3B, C). Accordingly, Osk and Nos proteins were completely lacking in wisp mutant embryos (Fig. 7C). We propose that premature destabilization of osk and nos mRNAs in wisp mutant embryos results from increased shortening of poly(A) tails in the absence of poly(A) tail elongation by Wisp. As Smaug protein is required for nos mRNA destabilization, through the recruitment of the deadenylation complex (Zaessinger et al., 2006), we verified that Smaug levels were unaffected in wisp mutant embryos (data not shown).
These results demonstrate that Wisp is responsible for cytoplasmic polyadenylation in early embryos and that poly(A) tail elongation is required for the production of the major determinants in embryo antero-posterior patterning.
Discussion
We have characterized Wisp, one of the two GLD-2-type poly(A) polymerases in Drosophila, which has a function in the female germline. We show that this protein is a bona fide poly(A) polymerase: it has a poly(A) polymerase activity in a tethering assay which depends on a conserved residue in the catalytic domain. Wisp is required for poly(A) tail lengthening of a pool of mRNAs in late stages of oogenesis. GLD-2 poly(A) polymerases do not have a RNA binding domain, they interact with RNA through their association with RNA binding proteins. In Xenopus oocytes, GLD-2 interacts with CPEB in a complex that is active in cytoplasmic polyadenylation (Barnard et al., 2004; Rouhana et al., 2005). We find that Wisp interacts directly with Orb. Consistent with a role of Wisp and Orb together in an ovarian cytoplasmic polyadenylation complex, wisp mutants are dominant enhancers of a orb weak allele. In C. elegans, GLD-2 was reported to interact with the KH domain RNA binding protein GLD-3, which has homology with Drosophila Bic-C. Although we find Wisp and Bic-C together in an ovarian RNP complex, their association is mediated by RNA, suggesting that the proteins do not interact directly. We have reported recently Bic-C function in deadenylation; Bic-C recruits the CCR4-NOT deadenylase complex to mRNAs (Chicoine et al., 2007). However, we also found a role of Bic-C in poly(A) tail elongation during oogenesis (Chicoine et al., 2007).
In addition to its function in oogenesis, Wisp-dependent cytoplasmic polyadenylation is required for translation of essential determinants of the antero-posterior patterning of the embryo. bcd mRNA poly(A) tail elongation was known to be required for the deployment of Bcd gradient from the anterior pole of the embryo (Salles et al., 1994). We now show that Osk and Nos accumulation at the posterior pole also depends on Wisp. This highlights the general role of poly(A) tail length regulation in Drosophila early development.
Meiotic progression and translational control
In Drosophila, meiosis starts in the germarium where several cells per germline cyst enter meiotic prophase. Meiosis is then restricted to a single oocyte that remains in prophase I during most oogenesis. Progression to metaphase I (oocyte maturation) occurs in stage 13, with maintenance of metaphase I arrest in mature stage 14 oocytes (King, 1970). Arrested oocytes are then activated by egg laying which induces meiosis resumption (Heifetz et al., 2001).
The earliest phenotypes in wisp null mutant are defects in metaphase I arrest and in the progression beyond this stage. This suggests that Wisp-dependent cytoplasmic polyadenylation and translational activation are essential for meiosis during and after metaphase I (but not for oocyte maturation). Consistent with this, massive translation appears to be dispensable for the completion of meiosis (Page and Orr-Weaver, 1997), but translational activation of specific mRNAs, at least cort, is required (Pesin and Orr-Weaver, 2007). We identify cort as a Wisp target: cort poly(A) tail elongation and Cort accumulation in mature oocytes require Wisp. Moreover, defects in Cort accumulation in wisp mutant oocytes result in impaired Cyc A destruction, an event thought to be critical for meiotic progression (Pesin and Orr-Weaver, 2007; Swan and Schupbach, 2007). We find that Wisp regulates many mRNAs at oocyte maturation, several of which might be involved at various steps of meiosis. Identification of these specific targets will be necessary to fully unravel the role of Wisp during meiosis.
Cytoplasmic polyadenylation has been linked to meiotic progression at egg activation because some maternal mRNAs undergo poly(A) tail elongation at egg activation (Benoit et al., 2005; Salles et al., 1994; Vardy and Orr-Weaver, 2007). Moreover, bcd polyadenylation is affected in mutants that are defective in meiosis such as cort (Lieberfarb et al., 1996). It has been proposed that the link between cytoplasmic polyadenylation and egg activation resulted from the inactivation of canonical PAP activity by phosphorylation via the MPF (Mitotic Promoting Factor: Cdc2/Cyc B) (Chu et al., 2001). Cyc B degradation by APC-Cort would both induce meiotic progression and release PAP inactivation, leading to polyadenylation.
This model can be adapted with results presented here and in the recent literature (Pesin and Orr-Weaver, 2007; Vardy and Orr-Weaver, 2007). Two waves of cytoplasmic polyadenylation occur successively, one during oocyte maturation and one at egg activation. They both depend on Wisp poly(A) polymerase. The first wave is Orb-dependent and the pathway that triggers its activation is unknown. This polyadenylation induces the synthesis of Cort (and probably other proteins), which in turn is required for the second wave of cytoplasmic polyadenylation at egg activation. Cort could act in this process through the destruction of cyclins or of other proteins more specifically involved in the regulation of the polyadenylation machinery.
Two poly(A) polymerases function in translational control during oogenesis
The more striking result in this paper is the requirement of two poly(A) polymerases for cytoplasmic polyadenylation during oogenesis. Since the discovery of GLD-2 poly(A) polymerases it has been assumed that these proteins were responsible for cytoplasmic polyadenylation. Our data show a higher level of complexity of this regulation. The phenotypes of wisp mutants indicate a function of Wisp late in oogenesis. We find that entry into meiosis and restriction of meiosis to one oocyte, as well as DNA condensation in the karyosome are unaffected in wisp mutants. In strong contrast, orb null mutants arrest oogenesis in the germarium with defects in the synchronous mitoses of cystoblasts and in the restriction of meiosis to one oocyte (Huynh and St Johnston, 2000), I. Busseau and M.S. unpublished). We find that orb phenotypes corresponding to early defects in oogenesis, including oocyte determination and dorso-ventral patterning, are dominantly enhanced by hrg mutants, strongly suggesting that canonical PAP and Orb act together in cytoplasmic polyadenylation during the first steps of oogenesis. Because Orb is in complex with both PAP and Wisp, the same pools of mRNAs can be regulated by the two different complexes, at different steps of oogenesis. The implication of one or the other poly(A) polymerase could allow for different types of regulation. In addition, it is possible that the presence of both poly(A) polymerases together in the complex could be required for some step of oogenesis.
In Xenopus, GLD2 catalyzes polyadenylation during oocyte maturation (Barnard et al., 2004; Rouhana et al., 2005), but the enzymes involved after fertilization have not been identified. Moreover, polyadenylation at earlier stages of oogenesis is unexplored.
CPEB function has been addressed genetically in mouse and the defect in the female germline of CPEB KO mice was found to be during prophase I (Tay and Richter, 2001). In contrast, GLD-2 expression in the oocytes appears to start at metaphase I (Nakanishi et al., 2006). Moreover, no female germline defective phenotype were observed in GLD-2 KO mice (Nakanishi et al., 2007). This demonstrates some level of redundancy in poly(A) polymerase function in mouse female meiosis and indicates that the involvement of different types of poly(A) polymerase for translational activation in oogenesis and meiotic progression is common to other species.
Supplementary Material
Acknowledgments
We are grateful to A. Nakamura, P. Macdonald, P. Lasko, J. Reinitz, S. Hawley, D. Glover, J. Pesin and T. Orr-Weaver for gifts of antibodies and to the Bloomington Stock Center for Drosophila stocks. We thank M. Wolfner for sharing unpublished data and I. Busseau for comments on the manuscript. This work was supported by the CNRS UPR1142, “ANR Blanche” (ANR-06-BLAN-0343) and FRM (Equipe FRM 2007) to MS, and by NIH (GM31892 and 50942) grants to MW. PB held awards from the Ministère de la Recherche and from the Association pour la Recherche sur le Cancer. JK held a Mary Shine Peterson fellowship.
References
- Anderson LK, Royer SM, Page SL, McKim KS, Lai A, Lilly MA and Hawley RS (2005). Juxtaposition of C(2)M and the transverse filament protein C(3)G within the central region of Drosophila synaptonemal complex. Proc Natl Acad Sci U S A 102, 4482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne S, Bilger A, Astrom J, Virtanen A and Wickens M (1995). Poly (A) polymerases in the nucleus and cytoplasm of frog oocytes: dynamic changes during oocyte maturation and early development. Rna 1, 64–78. [PMC free article] [PubMed] [Google Scholar]
- Barnard DC, Ryan K, Manley JL and Richter JD (2004). Symplekin and xGLD-2 are required for CPEB-mediated cytoplasmic polyadenylation. Cell 119, 641–51. [DOI] [PubMed] [Google Scholar]
- Benoit B, Juge F, Iral F, Audibert A and Simonelig M (2002). Chimeric human CstF-77/Drosophila Suppressor of forked proteins rescue suppressor of forked mutant lethality and mRNA 3’-end processing in Drosophila. Proc. Natl. Acad. Sci. USA 99, 10593–10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit B, Mitou G, Chartier A, Temme C, Zaessinger S, Wahle E, Busseau I and Simonelig M (2005). An essential cytoplasmic function for the nuclear poly(A) binding protein, PABP2, in poly(A) tail length control and early development in Drosophila. Dev Cell 9, 511–522. [DOI] [PubMed] [Google Scholar]
- Benoit B, Nemeth A, Aulner N, Kühn U, Simonelig M, Wahle E and Bourbon HM (1999). The Drosophila poly(A)-binding protein II is ubiquitous throughout Drosophila development and has the same function in mRNA polyadenylation as its bovine homolog in vitro. Nucleic Acids Res. 27, 3771–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsten SE and Gavis ER (1999). Role for mRNA localization in translational activation but not spatial restriction of nanos RNA. Development 126, 659–69. [DOI] [PubMed] [Google Scholar]
- Brent AE, MacQueen A and Hazelrigg T (2000). The Drosophila wispy gene is required for RNA localization and other microtubule-based events of meiosis and early embryogenesis. Genetics 154, 1649–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler M, Haas W, Gygi SP and Moazed D (2007). RNAi-dependent and - independent RNA turnover mechanisms contribute to heterochromatic gene silencing. Cell 129, 707–21. [DOI] [PubMed] [Google Scholar]
- Castagnetti S and Ephrussi A (2003). Orb and a long poly(A) tail are required for efficient oskar translation at the posterior pole of the Drosophila oocyte. Development 130, 835–843. [DOI] [PubMed] [Google Scholar]
- Chang JS, Tan L and Schedl P (1999). The Drosophila CPEB homolog, Orb, is required for Oskar protein expression in oocytes. Dev. Biol 215, 91–106. [DOI] [PubMed] [Google Scholar]
- Chang JS, Tan L, Wolf MR and Schedl P (2001). Functioning of the Drosophila orb gene in gurken mRNA localization and translation. Development 128, 3169–77. [DOI] [PubMed] [Google Scholar]
- Chicoine J, Benoit P, Gamberi C, Paliouras M, Simonelig M and Lasko P (2007). Bicaudal-C Recruits CCR4-NOT Deadenylase to Target mRNAs and Regulates Oogenesis, Cytoskeletal Organization, and Its Own Expression. Dev Cell 13, 691–704. [DOI] [PubMed] [Google Scholar]
- Chu T, Henrion G, Haegeli V and Strickland S (2001). Cortex, a Drosophila gene required to complete oocyte meiosis, is a member of the Cdc20/fizzy protein family. Genesis 29, 141–52. [DOI] [PubMed] [Google Scholar]
- Dickson KS, Bilger A, Ballantyne S and Wickens MP (1999). The cleavage and polyadenylation specificity factor in Xenopus laevis oocytes is a cytoplasmic factor involved in regulated polyadenylation. Mol. Cell. Biol 19, 5707–5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M (2002). A history of poly A sequences: from formation to factors to function. Prog Nucleic Acid Res Mol Biol 71, 285–389. [DOI] [PubMed] [Google Scholar]
- Endow SA and Komma DJ (1997). Spindle dynamics during meiosis in Drosophila oocytes. J Cell Biol 137, 1321–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz Y, Yu J and Wolfner MF (2001). Ovulation triggers activation of Drosophila oocytes. Dev Biol 234, 416–24. [DOI] [PubMed] [Google Scholar]
- Horner VL, Czank A, Jang JK, Singh N, Williams BC, Puro J, Kubli E, Hanes SD, McKim KS, Wolfner MF et al. (2006). The Drosophila calcipressin sarah is required for several aspects of egg activation. Curr Biol 16, 1441–6. [DOI] [PubMed] [Google Scholar]
- Huynh JR and St Johnston D (2000). The role of BicD, Egl, Orb and the microtubules in the restriction of meiosis to the Drosophila oocyte. Development 127, 2785–94. [DOI] [PubMed] [Google Scholar]
- Juge F, Zaessinger S, Temme C, Wahle E and Simonelig M (2002). Control of poly(A) polymerase level is essential to cytoplasmic polyadenylation and early development in Drosophila. EMBO J. 21, 6603–6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadyk LC and Kimble J (1998). Genetic regulation of entry into meiosis in Caenorhabditis elegans. Development 125, 1803–13. [DOI] [PubMed] [Google Scholar]
- Kadyrova LY, Habara Y, Lee TH and Wharton RP (2007). Translational control of maternal Cyclin B mRNA by Nanos in the Drosophila germline. Development 134, 1519–27. [DOI] [PubMed] [Google Scholar]
- Kashiwabara S, Noguchi J, Zhuang T, Ohmura K, Honda A, Sugiura S, Miyamoto K, Takahashi S, Inoue K, Ogura A et al. (2002). Regulation of spermatogenesis by testis-specific, cytoplasmic poly(A) polymerase TPAP. Science 298, 1999–2002. [DOI] [PubMed] [Google Scholar]
- Kim JH and Richter JD (2006). Opposing polymerase-deadenylase activities regulate cytoplasmic polyadenylation. Mol Cell 24, 173–83. [DOI] [PubMed] [Google Scholar]
- Kim-Ha J, Kerr K and Macdonald PM (1995). Translational regulation of oskar mRNA by bruno, an ovarian RNA-binding protein, is essential. Cell 81, 403–12. [DOI] [PubMed] [Google Scholar]
- King RC (1970). Ovarian development in Drosophila melanogaster. New York: Academic press. [Google Scholar]
- Kosman D, Small S and Reinitz J (1998). Rapid preparation of a panel of polyclonal antibodies to Drosophila segmentation proteins. Dev. Genes Evol 208, 290–294. [DOI] [PubMed] [Google Scholar]
- Kwak JE, Wang L, Ballantyne S, Kimble J and Wickens M (2004). Mammalian GLD-2 homologs are poly(A) polymerases. Proc Natl Acad Sci U S A 101, 4407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JE and Wickens M (2007). A family of poly(U) polymerases. Rna 13, 860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberfarb ME, Chu T, Wreden C, Theurkauf W, Gerden JP and Strickland S (1996). Mutation that perturb poly(A)-dependent maternal mRNA activation block the initiation of development. Development 122, 579–588. [DOI] [PubMed] [Google Scholar]
- Mohler JD (1977). Developmental genetics of the Drosophila egg. I. Identification of 59 sex-linked cistrons with maternal effects on embryonic development. Genetics 85, 259–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JZ, Hong A, Lilly MA and Lehmann R (2005). twin, a CCR4 homolog, regulates cyclin poly(A) tail length to permit Drosophila oogenesis. Development 132, 1165–74. [DOI] [PubMed] [Google Scholar]
- Murata T, Nagaso H, Kashiwabara S, Baba T, Okano H and Yokoyama KK (2001). The hiiragi gene encodes a poly(A) polymerase, which controls the formation of the wing margin in Drosophila melanogaster. Dev. Biol 233, 137–147. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Kubota H, Ishibashi N, Kumagai S, Watanabe H, Yamashita M, Kashiwabara S, Miyado K and Baba T (2006). Possible role of mouse poly(A) polymerase mGLD-2 during oocyte maturation. Dev Biol 289, 115–26. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Kumagai S, Kimura M, Watanabe H, Sakurai T, Kashiwabara S and Baba T (2007). Disruption of mouse poly(A) polymerase mGLD-2 does not alter polyadenylation status in oocytes and somatic cells. Biochem Biophys Res Commun 364, 14–9. [DOI] [PubMed] [Google Scholar]
- Page AW and Orr-Weaver TL (1996). The Drosophila genes grauzone and cortex are necessary for proper female meiosis. J Cell Sci 109, 1707–15. [DOI] [PubMed] [Google Scholar]
- Page AW and Orr-Weaver TL (1997). Activation of the meiotic divisions in Drosophila oocytes. Dev Biol 183, 195–207. [DOI] [PubMed] [Google Scholar]
- Pesin JA and Orr-Weaver TL (2007). Developmental Role and Regulation of cortex, a Meiosis-Specific Anaphase-Promoting Complex/Cyclosome Activator. PLoS Genet 3, e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JD (2000). The influence of polyadenylation-induced translation on metazoan development and neuronal synaptic function. In Translational Control of Gene Expression, (ed. Hershey JWB Mathews MB and Sonenberg N), pp. 785–806. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Richter JD (2007). CPEB: a life in translation. Trends Biochem Sci 32, 279–85. [DOI] [PubMed] [Google Scholar]
- Rissland OS, Mikulasova A and Norbury CJ (2007). Efficient RNA polyuridylation by noncanonical poly(A) polymerases. Mol Cell Biol 27, 3612–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhana L, Wang L, Buter N, Kwak JE, Schiltz CA, Gonzalez T, Kelley AE, Landry CF and Wickens M (2005). Vertebrate GLD2 poly(A) polymerases in the germline and the brain. Rna 11, 1117–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffman EE, Styhler S, Rother K, Li W, Richard S and Lasko P (1998). Premature translation of oskar in oocytes lacking the RNA-binding protein bicaudal-C. Mol Cell Biol 18, 4855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles FJ, Lieberfarb ME, Wreden C, Gergen JP and Strickland S (1994). Coordinate initiation of Drosophila development by regulated polyadenylation of maternal messenger RNAs. Science 266, 1996–1999. [DOI] [PubMed] [Google Scholar]
- Semotok JL, Cooperstock RL, Pinder BD, Vari HK, Lipshitz HD and Smibert CA (2005). Smaug recruits the CCR4/POP2/NOT deadenylase complex to trigger maternal transcript localization in the early Drosophila embryo. Curr Biol 15, 284–94. [DOI] [PubMed] [Google Scholar]
- Sheets MD, Wu M and Wickens M (1995). Polyadenylation of c-mos mRNA as a control point in xenopus meiotic maturation. Nature 374, 511–516. [DOI] [PubMed] [Google Scholar]
- Stebbins-Boaz B, Hake LE and Richter JD (1996). CPEB controls the cytoplasmic polyadenylation of cyclin, Cdk2 and c-mos mRNAs and is necessary for oocyte maturation in Xenopus. EMBO J. 15, 2582–2592. [PMC free article] [PubMed] [Google Scholar]
- Suh N, Jedamzik B, Eckmann CR, Wickens M and Kimble J (2006). The GLD-2 poly(A) polymerase activates gld-1 mRNA in the Caenorhabditis elegans germ line. Proc Natl Acad Sci U S A 103, 15108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan A and Schupbach T (2007). The Cdc20 (Fzy)/Cdh1-related protein, Cort, cooperates with Fzy in cyclin destruction and anaphase progression in meiosis I and II in Drosophila. Development 134, 891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay J and Richter JD (2001). Germ cell differentiation and synaptonemal complex formation are disrupted in CPEB knockout mice. Dev. Cell 1, 201–213. [DOI] [PubMed] [Google Scholar]
- Vardy L and Orr-Weaver TL (2007). The Drosophila PNG kinase complex regulates the translation of cyclin B. Dev Cell 12, 157–66. [DOI] [PubMed] [Google Scholar]
- Wang L, Eckmann CR, Kadyk LC, Wickens M and Kimble J (2002). A regulatory cytoplasmic poly(A) polymerase in Caenorhabditis elegans. Nature 419, 312–6. [DOI] [PubMed] [Google Scholar]
- Whitfield WGF, Gonzalez C, Maldonado-Colina G and Glover DM (1990). The A- and B-type cyclins of Drosophila are accumulated and destroyed in temporally distinct events that define separable phases of the G2-M transition. EMBO journal 9, 2563–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M, Goodwin EB, Kimble J, Strickland S and Hentze M (2000). Translational control of developmental decisions. In Translational Control of Gene Expression, (ed. Hershey JWB Mathews MB and Sonenberg N), pp. 295–370. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Zaessinger S, Busseau I and Simonelig M (2006). Oskar allows nanos mRNA translation in Drosophila embryos by preventing its deadenylation by Smaug/CCR4. Development 133, 4573–83. [DOI] [PubMed] [Google Scholar]
- Zhuang T, Kashiwabara S, Noguchi J and Baba T (2004). Transgenic expression of testis-specific poly(A) polymerase TPAP in wild-type and TPAP-deficient mice. J Reprod Dev 50, 207–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


