Abstract
Glioblastoma is the most aggressive primary brain tumor with a poor prognosis. The 2021 WHO CNS5 classification has further stressed the importance of molecular signatures in diagnosis although therapeutic breakthroughs are still lacking. In this review article, updates on the current and novel therapies in IDH-wildtype GBM will be discussed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-022-01251-6.
Keywords: Glioblastoma, IDH-wildtype, Targeted therapy, Precision medicine, Clinical trials
Introduction
Glioblastoma (GBM), a subtype of adult diffuse glioma, is a primary central nervous system (CNS) tumor presumed to arise from neuroglial stem cells or their progenitors in the subventricular zone [1–3]. There has been a recent paradigm shift, with increasing reliance on molecular information for diagnostic classification and prognostication within gliomas, as seen in the most recent World Health Organization (WHO) classification of CNS tumors [3]. This update further incorporated molecular markers in addition to histology for an integrated diagnosis, resulting in a clearer separation of adult and pediatric-type gliomas. As a result, GBM now refers specifically to the most aggressive form of isocitrate dehydrogenase (IDH)-wildtype diffuse adult-type astrocytoma, for which O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status is prognostic. Despite increased understanding of the molecular evolution of GBM, it continues to be an incurable disease with poor survival. The standard multimodal therapies of maximal safe resection and radiation therapy (RT) with concomitant and adjuvant temozolomide (TMZ) remain the backbone of treatment. Clinical trials continue to expand on novel approaches such as targeted agents and immunotherapy. This review will provide an overview of current updates in the classification, diagnosis, and management of GBM.
Epidemiology and Risk Factors
Glioblastoma accounts for 14.5% of all primary CNS tumors and 48.6% of all malignant primary CNS tumors, with an annual age-adjusted incidence rate of 3.23 per 100,000 population [4]. Age, sex, and race/ethnicity influence the incidence rate which exponentially increases beyond 40 years of age, with a mean age of diagnosis at 65, and peaking between 75 and 84 years at 15.30 per 100,000 population. GBM is 1.59 times more common in males and 1.99 times more common in Caucasians compared to African-American patients [3–5].
Multiple studies have tried to delineate whether environmental risk factors could be linked to GBM, but only ionizing radiation in medium-to-high doses, particularly in children, was consistently found to be a risk factor (e.g., atomic bomb survivors, therapeutic radiation cohorts) [6]. In case–control studies, the odds of identifying a family history of primary brain tumor are 2.3 times more likely in patients with adult gliomas. Although 5% of gliomas are familial, a Mendelian inherited syndrome (e.g., Lynch syndrome, neurofibromatosis, tuberous sclerosis), which commonly harbors loss-of-function mutations in tumor suppressor genes, can only be pinpointed in approximately 1–2% and 4% of adult and pediatric gliomas, respectively [6–9]. Conversely, case–control studies have also shown that a history of atopic diseases (e.g., eczema, allergies) decreases the risk of glioma by 30% [10].
Imaging Techniques to Aid in Diagnosis
Combined with the appropriate clinical history, imaging can help achieve an accurate pre-operative diagnosis. Structural magnetic resonance imaging (MRI) with gadolinium remains the gold standard diagnostic modality for GBM evaluation. GBM has a predilection of involving the cerebral hemispheres, with the frontal and temporal lobes most involved. Typical findings are that of an infiltrative mass with poorly delineated margins that exhibits mixed signal intensities on T1-weighted imaging (T1-WI). Heterogeneous hyperintensities are also evident on T2/fluid-attenuated inversion recovery (FLAIR)-WI, which also demonstrate indistinct margins that blend in with extensive vasogenic edema. Cysts, hemorrhage at various stages of evolution, fluid/debris levels, and “flow voids” are common findings. Radiologically, the majority of glioblastomas appear as a unifocal mass; approximately 20% are multifocal/multicentric [11–13]. In contrast to multifocal GBM which refers to enhancing foci that are embedded within the same abnormal high FLAIR signal region, the less common multicentric pattern demonstrates enhancing foci that are spatially (commonly occurring in regions separated by a tentorium) and temporally separated as most of them arise metachronously [14].
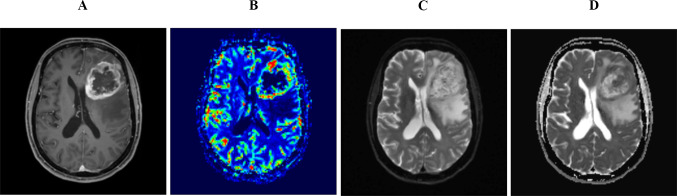
Advanced imaging techniques can help differentiate GBM from other diagnoses. These include diffusion-weighted imaging (DWI) and perfusion-weighted imaging (PWI). Contrary to PWI, which reflects the state of the tumor vascular bed, DWI can be used to calculate apparent diffusion coefficient (ADC) values, which can be used to indirectly assess tumor cellularity through measuring water diffusivity. GBM has a significantly lower ADC value as compared to gliomas of lower grades [15], while primary CNS lymphoma has lower ADC values than GBM [16]. Figure 1 shows a classic appearance of GBM on post-contrast T1-WI, MR perfusion, and DWI as well as ADC.
Fig. 1.
A 70-year-old woman who presented with headache and behavioral changes and was found to have an IDH-wildtype GBM over the left frontal region causing minimal mass effect. Contrast-enhanced T1 image shows a large necrotic mass over the left frontal region with irregular thick rind (A). Elevated CBV on MR perfusion correlates to areas of enhancement (B). Compared to DWI map (C), cellular heterogeneity is evident within the tumor fields as more hypercellular areas demonstrates higher signal drop on ADC map (D)
Dynamic susceptibility contrast MRI measures cerebral blood volume (CBV), a surrogate marker of total tumor vascularity. In contrast, dynamic contrast-enhanced MRI measures the permeability surface area which reflects the leakiness of vasculature [17]. Both parameters may differentiate GBM from lower-grade gliomas [18]. Compared to IDH-mutant tumors, IDH-wildtype tumors are associated with significantly higher relative CBV values [19, 20]. Several studies have assessed whether certain imaging signatures can be predictive of genotypic or epigenetic signatures such as IDH status and MGMT promoter methylation, respectively [21–24].
Metabolic alterations within GBM can be detected by MR spectroscopy [25]. Accelerated cellular proliferation, neuronal death, and necrosis are reflected through an elevated choline peak, suppressed N-acetyl aspartate (NAA)/creatine peaks, and increased lactate/lipid peaks, respectively. Compared to lower grades, higher grade gliomas demonstrate lower NAA and creatine levels, higher lipid/lactate levels, and higher choline/NAA and choline/creatine ratios [26, 27]. Chemical exchange saturation transfer (CEST) is an MRI technique that relies on the amide proton exchange rate between proteins and bulk water, which is inversely correlated with the tissue pH [28, 29]. A recent study including IDH-wildtype GBM found a diagnostic sensitivity and specificity of 90% when incorporating additional physiologic (ADC values) and metabolic (CEST) parameters into the model [30].
Positron emission tomography (PET) uses radiolabeled molecules to gain insight into neoplastic tissue biology. Glucose transporters are upregulated in gliomas and exhibit a high affinity to [18F]-2-fluoro-2-deoxy-d-glucose (FDG) tracers. Due to the similar affinity seen with inflammatory entities and the physiologic uptake by cerebral parenchyma, FDG tracers have low diagnostic specificity and low signal/noise ratio hindering their ability to delineate gliomas [31–33]. To overcome these issues, radiolabeled amino acid tracers are preferred, and the repertoire of such molecules continues to expand [34–37]. Clinical applicability includes the delineation of tumor extent, especially in normal-appearing tissue where it can aid in radiotherapy and surgical planning [38]. Assessment of treatment response by amino acid PET is gaining interest. Decreased 18F-fluoro-ethyl-tyrosine (FET) uptake 6–8 weeks following chemoradiation is associated with improved outcome compared to non-responders [39, 40].
Response Assessment in Neuro-oncology
The Response Assessment in Neuro-Oncology (RANO) criteria for high-grade gliomas (WHO grade 3 and 4) is the established standard for evaluation of response or progression in GBM clinical trials [41]. To address the challenges of response interpretation in the context of changes due to disease biology and treatment effect, the RANO criteria evolved from the previously adopted Macdonald et al. criteria [42], and other response assessment frameworks [42, 43]. The modified RANO criteria for high-grade gliomas (mRANO) was later developed and uses the post-radiotherapy MRI baseline for these newly diagnosed gliomas [43]. In addition, confirmation of progression is recommended in both newly diagnosed and recurrent high-grade gliomas after the preliminary progressive disease is observed on an initial MRI scan. The RANO criteria use post-contrast two-dimensional tumor measurements based on the standardized Brain Tumor Imaging Protocol [44]. Beyond MRI-guided response assessment, other response assessment modalities are being investigated. These include the Neurologic Assessment in Neuro-Oncology, which integrates clinical response assessment [45]; immunotherapy RANO (iRANO), which addresses possible pseudo-progression related to immunotherapies [46]; and PET-RANO, which leverages metabolic imaging for response assessment in glioma [47]. Future directions in RANO include leveraging advances in imaging technology and artificial intelligence for automated volumetric measurements and response assessments [48, 49]. Additional factors that need to be weighed are the changing landscape of therapies (particularly immunotherapy) and the revised classification of gliomas. The RANO group is currently reviewing its criteria with revised criteria scheduled to be proposed later in 2022.
Pathology, Molecular, and Genomic Signatures, and Recent Updates in Classification
Glioma classification has relied on histopathology, with the hallmarks of GBM as an astroglial tumor with features of microvascular proliferation and/or necrosis. With integrated molecular classification, updated in the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy (c-IMPACT NOW) and the more recent WHO CNS5 classification, diffuse astrocytic gliomas have now been subdivided based on molecular lineage. A mutation in isocitrate dehydrogenase 1 or 2 (IDH1/2) has become a defining branch point in adult-type diffuse glioma diagnosis [50]. The most common IDH variant in glioma is the IDH1 R132H mutation, for which there is a rapid immunohistochemistry stain [51]. The yield of non-canonical IDH mutations identified with sequencing is age-dependent. Compared to the 6.3% rate in patients younger than 55 years, only 0.9% are identified in older patients with glioblastoma. This low yield has raised concerns regarding the cost-effectiveness of sequencing in that age group [52].
The distinction between IDH-mutant vs wildtype gliomas has also become clearer, with the omission of “secondary” or IDH-mutant GBM in favor of the term diffuse IDH-mutant astrocytoma, WHO grade 4. Diffuse gliomas that are IDH-wildtype and have molecular features of GBM can now be classified as the latter, even when histological high-grade features are absent [3, 53]. These molecular features include the combination of gain of chromosome 7 and loss of chromosome 10, TERT promoter mutations, or EGFR amplification.
Pediatric-type diffuse high-grade gliomas possess distinct mutations from their adult counterparts [3]. Notably, diffuse midline gliomas can carry an H3 K27 alteration and are now recognized as a separate entity from GBM. Similarly, diffuse hemispheric gliomas with an H3 G34 mutation are a separate entity found in adolescents and young adults.
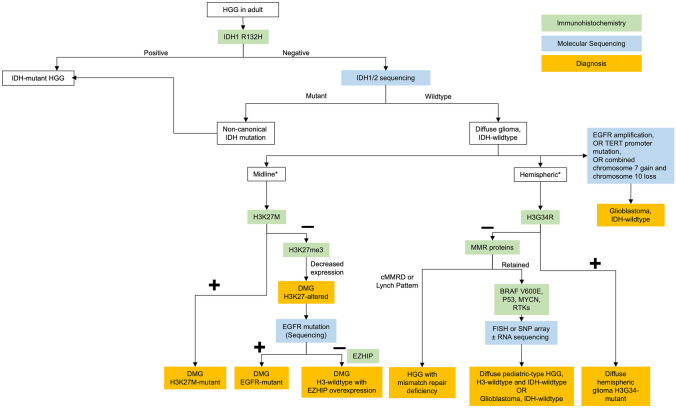
Of the IDH-wildtype and H3-wildtype high-grade gliomas, there are other mutations that have distinct prognostic and therapeutic implications. These include BRAF V600E mutations, FGFR alterations, or MYB or MYBL1 rearrangement [54]. GBM with BRAF V600E mutations has been described as epithelioid GBM [55], especially with a co-occurring mutation in the TERT promoter and homozygous deletion of CDKN2A/2B [56]. While rare (<2% of GBM) [57] and difficult to distinguish from pleomorphic xanthoastrocytoma [58], recognizing the molecular, clinical, and radiological features of this subtype is important [58] due to the observed efficacy of combination BRAF/MEK inhibition in gliomas with BRAF V600E mutation [59]. Figure 2 provides a proposed sequential approach to molecular testing in gliomas, although many centers now opt for targeted next-generation sequencing panels, which allows for the testing of all relevant molecular markers of GBM in one custom assay. The clinical utility of molecular testing through targeted sequencing panels remains limited beyond diagnostic confirmation and prognosis [60].
Fig. 2.
Proposed molecular testing algorithm for HGG. *Consider testing in young adults. Abbreviations: HGG, high-grade gliomas; IDH, isocitrate dehydrogenase; FISH, fluorescence in situ hybridization; SNP, single nucleotide polymorphism; WHO, World Health Organization; DMG, diffuse midline glioma; EGFR, epidermal growth factor receptor; RNA, ribonucleic acid; EZHIP, EZH Inhibitory Protein; MMR, mismatch repair; cMMRD, constitutional mismatch repair deficiency; BRAF, B-Raf; MYCN, N-myc proto oncogene; RTK, receptor tyrosine kinase; NTRK, neurotrophic tyrosine receptor kinase; TERT, telomerase reverse transcriptase
Advances in sequencing technology have led to the identification of specific somatic alterations in gliomagenesis [61, 62]. Three specific GBM subgroups with distinct somatic alteration signatures have been identified: the proneural type with cyclin-dependent kinase 4 (CDK4) and platelet-derived growth factor alpha (PDGFRɑ) amplification; mesenchymal type with neurofibromatosis type 1 (NF1) loss; and classical type with epidermal growth factor receptor (EGFR) amplifications and homozygous loss of CDKN2A/B [57, 63]. Beyond genomic alterations, profiling the epigenome and methylome has provided a more accurate classification of GBM. DNA methylation profiling has identified 7 distinct entities within GBM [64]. However, knowledge of these distinct subtypes has not yet translated into clinical utility.
Current Approach to Newly Diagnosed GBM
The well-known standard of care for newly diagnosed GBM is maximal safe surgical resection followed by concurrent chemoradiation with TMZ, and adjuvant TMZ [65–68]. Attempts to improve the efficacy of chemoradiation for GBM have been disappointing. Alternative TMZ dosing regimens aimed to exhaust MGMT reservoirs; for example, the RTOG 0525 trial compared adjuvant dose-dense (DD) TMZ (75–100 mg/m2/day for 21 days in a 28-day cycle) to conventional TMZ. Even for patients with MGMT methylated tumors, DD TMZ did not improve outcomes and was associated with more toxicity [69]. Similarly, extending the conventional TMZ regimen beyond 6 cycles did not change survival outcomes in a randomized open-label trial (GEINO14-01) [70]. This lack of benefit even in patients with a methylated MGMT promoter was also corroborated by non-randomized studies [71, 72]. Prolonged TMZ therapy has a cumulative negative impact on bone marrow reserves [73, 74] and a cumulative increase in treatment-related fatigue [75, 76], and is not recommended in routine practice.
Tumor-treating fields (TTF) are wearable scalp transducers that deliver local low-intensity, intermediate-frequency (200 kHz) alternating electrical fields that have an anti-mitotic effect, and work synergistically with concurrent chemotherapies [77, 78]. The EF-14 trial was an open-label, non-sham-controlled randomized phase III trial investigating the addition of TTF to standard therapy and demonstrated an improvement in median OS (20.9 versus 16 months) and PFS (6.7 versus 4 months) without compromising the health-related quality of life (HRQoL) [79–81]. However, compliance with TTF might be a limiting factor for its implementation in real-world practice [82]. A sub-group analysis from the same trial [83] has shown that the minimum average compliance rate threshold is 50% for a clinical gain in PFS (HR 0.70, 95% CI 0.47–1.05) and OS (HR 0.67, 95% CI 0.45–0.99) to be attained with a stepwise increase in both outcomes with higher compliance rates. A cutoff compliance rate of at least 75% per day was found to be prognostic of improved survival that is independent of age, performance status, the extent of resection, and MGMT methylation status (HR 0.78; p = 0.031). Other limitations have also deterred the widespread adoption of TTF despite its endorsement by the NCCN guidelines [84]. An ongoing randomized study of TTF concomitant with RT and TMZ is underway (EF-32-NCT04471844).
The addition of bevacizumab (BEV), a humanized monoclonal VEGF antibody, to the standard of care was studied in two phase III double-blind randomized trials [85, 86]. Both trials showed a statistically significant advantage of 3 months in PFS with the addition of BEV but no improvement in OS. While RTOG 0825 reported worsening quality of life and neurocognitive functions, the AVAglio trial reported a longer global health status deterioration-free survival. It may be that the effects of BEV upon vascular permeability may have reduced the effective concentration or penetration of TMZ in tumor tissue. The addition of BEV to the standard of care therapy is therefore not routinely implemented.
Approach to Elderly Patients with GBM
Patients with unfavorable prognostic markers such as advanced age or poor performance status have a worse prognosis and are vulnerable to treatment toxicities [87, 88]. Several RCTs have addressed the optimal strategy to manage these patients who cannot tolerate standard 6-week chemoradiation. It is clear that RT provides better outcomes than supportive care alone [89], while early randomized studies suggested that there was no survival disadvantage to shorter radiation schedules such as 40 Gy in 15 fractions [90] or 25 Gy in 5 fractions [91]. In larger RCTs such as the NORDIC study, there is evidence that conventional 6-week radiation may lead to worse outcomes than hypofractionated schedules [92]. These data lead to the phase III CCTG CE.6/EORTC 26,062 study which randomized 562 patients above the age of 65 years to short-course RT (40 Gy in 15 fractions) alone or with concurrent and adjuvant TMZ. Adding TMZ resulted in an improvement in median OS with most of the benefit seen in the methylated MGMT promoter group [93]. Although there has not been a study comparing 60 Gy/30 with TMZ to 40 Gy/15 with TMZ, the latter has become a common standard of care, particularly for patients over 70 years of age.
In patients where RT may be impractical (for example distance to a treatment facility, neurological impairment), TMZ alone can be considered for those harboring a methylated MGMT promoter. In relatively fit older patients, the updated NOA-08 study results show an impressive median OS of 18.4 months for those treated with TMZ alone. These results set the stage for consideration of a study using lower RT doses, or perhaps omitting RT, in elderly patients with MGMT promoter methylated GBM [94].
MGMT Promoter Methylation as a Predictive and Prognostic Biomarker
MGMT promoter methylation status is the most predictive and prognostic molecular biomarker and roughly 40% of IDH-wildtype glioblastomas will be methylated [95]. From a modern pooled analysis of 5 phase III trials, the median OS in MGMT methylated patients is approximately 24 months compared to 14 months in their unmethylated counterparts [96]. The optimal CpG island methylation detection assays are unclear; however, methylation-specific polymerase chain reaction (MSP) has been widely used in clinical trials [97]. A pitfall of reporting methylation status in a binary fashion using a qualitative MSP is a potential underestimation of TMZ benefit for patients categorized as unmethylated. Quantitative MSP can further refine the methylation score into high range (highly methylated), intermediate range (partially methylated or gray zone), and low range (truly unmethylated) [98]. The partially methylated group, which constitutes 10% of the “unmethylated” cohort, has a sensitivity to TMZ with a significantly improved OS (HR 0.58, 95% CI 0.43–0.78; p < 0.001) compared to “truly unmethylated” patients [99].
MGMT methylation predicts response to standard of care therapy with TMZ with a 50% increase in median OS [69, 100]. The benefit of the addition of TMZ in unmethylated patients is less clear [98, 101–103]. In these patients, there is an urgent unmet need for effective systemic therapy. In the setting of clinical trials, it is now widely accepted that TMZ can be omitted from the standard of care; doing so optimizes the assessment of the efficacy and toxicity of new agents and eliminates the need for pharmacokinetic and dose-finding evaluations of new agents combined with TMZ.
Current Approach to Recurrent GBM
The vast majority of all GBM recur with a median survival time of 6.2 months after the first progression [104]. There is no universally accepted salvage therapy, and treatment for recurrent GBM (rGBM) requires individualized consideration of re-operation, re-irradiation, and systemic therapy options. Clinical trial involvement is critical for drug discovery in these patients.
Surgical resection is feasible in 20–30% of rGBM patients and is indicated to reduce the mass effect and provide pathological evidence of disease recurrence versus radionecrosis. Early true progression is an indicator of aggressive tumor biology and may mitigate the advantages of a second surgery [67]. The extent of resection (EOR) in rGBM was evaluated in the DIRECTOR trial which enrolled patients with a median age of 55 years with a relatively small volume of enhancing lesion (< 10 cm3) and the majority had a KPS of 70 or more. Complete resection of the enhancing tumor was associated with an improvement in post-recurrence PFS (3.5 versus 1.9 months) and survival (12.9 versus 6.5 months) [105]. However, the generalizability of the trial results is limited as the cohort was highly selective for patients with favorable prognostic factors.
Since most GBM recur locally and in the high-dose radiation volume, the benefit of re-irradiation strategies can be limited by dose constraints and the risk of radiation necrosis. Doses in the range of 30–35 Gy delivered in 5–15 fractions are well-tolerated with an acceptable toxicity profile [106, 107]. Small tumor volumes can be managed with radiosurgery [108, 109]. Radiation for recurrence is aimed at local disease control and has a primarily symptomatic/palliative role [110]. BEV may be useful to treat radionecrosis or to mitigate the development of radionecrosis with re-irradiation. In one randomized study, re-irradiation combined with BEV compared to BEV alone was associated with an improved PFS (7.1 versus 3.8 months; p = 0.05) but no survival benefit [111]. A better understanding of patient selection factors will be key to the development of safe strategies for retreatment.
There are no attractive systemic therapies for rGBM. Nitrosoureas are commonly used, with lomustine (CCNU) preferred for its oral formulation, less frequent administration, and tolerability [112]. No novel agents tested in randomized trials have proven superior to lomustine [113]. Overall, in phase III trials, CCNU confers a benchmark of 20% 6-month PFS and 7–10 months of overall survival from the time of progression [114, 115]. A recent phase II randomized study detected improved survival with regorafenib, an oral multikinase inhibitor, compared to CCNU in rGBM; however, the findings are limited by imbalances in prognostic factors and evaluation in phase III is ongoing [116]. It is humbling to reflect upon the lack of progress beyond CCNU in rGBM, especially when one acknowledges that the 6-month PFS associated with CCNU may be no better than supportive care alone.
As in the upfront setting, there have been attempts to optimize TMZ dosing and schedule to leverage the depletion of MGMT by TMZ. Multiple prospective studies used the surrogate marker of MGMT activity in peripheral blood mononuclear cell (PBMC) levels and suggest depletion of MGMT with extended or dose-dense schedules of TMZ [117–119]. The DIRECTOR trial [120] demonstrated similar efficacy between a 1 week on/1 week off schedule compared to a 21-day regimen. The RESCUE study [121] was an uncontrolled single-arm study that used a continuous 28/28 regimen (50 mg/m2 daily) at the time of disease progression following standard chemoradiation. Patients who were rechallenged at least 2 months after 6 cycles of adjuvant TMZ had a PFS at 6 months of 35.7% MGMT status was not predictive of outcome, perhaps suggesting that a continuous schedule of TMZ may overcome the disadvantage of unmethylated disease.
BEV dramatically alters vascular permeability and causes rapid reductions in cerebral edema and an apparent reduction in the contrast-enhancing volume of rGBM. This was the basis for its approval in some countries; however, it has not been evaluated in a controlled trial. It can be useful to improve clinical and radiological deterioration [122], and symptomatic radiation-necrosis [123], and aid with corticosteroid tapering but the survival benefits are unclear. No trials have yet compared BEV to placebo and combination treatments with CCNU [115, 124] or irinotecan [125, 126] have not shown any survival benefit. Although the usual administered dose is 10 mg/kg every 2 weeks, lower doses have been anecdotally reported to be as effective [127, 128].
Newer Approaches to Treatment
Surgery and Surgical Approaches
Cytoreductive surgery likely prevents neurological deterioration and improves survival but is of course noncurative as tumor cells migrate to regions distant from the contrast-enhanced (CE) lesion [129]. Earlier studies focused on concepts of gross-total resection (GTR) and subtotal resection (STR) of the CE lesion to define the extent of resection (EOR) thresholds (or its inverse correlate, residual volume) where survival benefit becomes significant. Prospective and retrospective studies have shown that there is an incremental gain in survival with an EOR of at least 78% or RV below 2–5 mm3 [130–132]. In a 2016 meta-analysis [133], compared to STR, those who underwent a GTR had a superior outcome and STR, compared to biopsy, was also associated with a statistically significant, clinically meaningful improvement albeit less profound and less durable. Since most tumors recur within 2 cm of the enhanced lesion [134], the surgical concept of maximal safe resection replaced the earlier rigid EOR thresholds [135]. A large retrospective cohort of 1229 patients (median age, 55.7 years) found that extending the resection into the FLAIR regions resulted in improved survival. Compared to less extensive resections, resection of ≥ 53.21% of the surrounding non-contrast enhancing (NCE) abnormality was associated with a significant prolongation of median OS (20.7 versus 15.5 months) [136]. Age and molecular diagnosis, independent of MGMT status, seemed to impact the benefits attained from maximal safe resection as well [65]. In patients <65 years, GTR of the CE lesion and maximal resection of NCE regions resulted in a similar median OS independent of IDH status. However, older IDH-wildtype patients did not have additional survival benefits beyond the GTR of the CE lesion. The Glioma Supra Marginal Incision Trial (G-SUMIT; NCT04737577) is a phase II pilot randomized control trial that aims to assess whether there is a survival benefit in extending the resection 1 cm beyond the CE lesion in patients with high-grade gliomas.
Laser interstitial thermal therapy (LITT) is an emerging minimally invasive cytoreductive technique that relies on heating the tumor tissue with laser light delivered through a stereotactically positioned optic fiber over a short period of time while simultaneously monitoring the tissue temperature with an intra-operative MRI. LITT techniques have gained interest for ablating small, well-circumscribed, and deep-seated lesions with an oblong morphology in both newly diagnosed and recurrent GBM. However, randomized clinical trials have not been forthcoming considering its null effect on PFS and OS from retrospective studies and the associated high rate of adverse events [137].
Different techniques have been developed to maximize the EOR while minimizing new neurologic deficits. 5-Aminolevulinic acid (5-ALA) is administered orally causing fluorescence in malignant tissue with reported specificity and sensitivity of 100% and 85%, respectively [138–140]. Compared to white light, fluorescence-guided surgery nearly doubled the rates of complete resection of CE lesions (65% versus 36%) and PFS at 6 months (41% vs. 21.1%) [141]. In experienced centers, a minimum EOR of 90% can be achieved [142]. Reliance on neuronavigation can be difficult due to the invariable intra-operative brain shift, but this can be overcome with intraoperative MRI (iMRI). In addition, iMRI provides near real-time imaging thus eliminating the risk of incomplete resections and re-operating. Complete CE lesion resection is achievable in nearly 96% of the patients with iMRI [143]. Compared to 5-ALA, iMRI is a superior surgical adjunct [144]. Multiple studies have found that combining iMRI with 5-ALA has an even higher rate of achieving GTR [145–147]. Intraoperative ultrasound is an inexpensive alternative to iMRI with GTR rates of 77%; however, resection beyond the CE lesion is limited [148]. Although pre-operative tractography and functional MRI can aid with surgical planning, intraoperative cortical mapping (e.g., awake craniotomy) is pivotal for supramaximal resection of tumors in proximity to eloquent cortices [149–152].
Radiotherapy
It was previously presumed that after surgery, any gross residual tumor and the surgical cavity remained relatively static throughout a 3- or 6-week course of RT. A relatively large expansion of 1.5 to 3 cm around the GTV, called the clinical target volume (CTV), is used to encompass microscopic disease not visible on MRI. However, recent data evaluated volume changes and migration distances of tumors during RT and reported shifts in tumor location of over 5 mm in half of the patients and over 10 mm in 20% of patients [153]. Others have found an increase in the size of GTV by 25% over RT, resulting in underdosing of the RT target [154–158]. Conversely, a reduction in the tumor volume or surgical cavity during RT may allow for a smaller margin but adaptive RT (whereby GTV and CTV volumes are adjusted based on updated imaging) reduces the toxicity by limiting RT dose to normal surrounding brain tissue. One form of MRI-guided Radiation Therapy uses an MR-Linac (Unity, Elekta) that combines a linear accelerator with an MRI that affords daily MRI just prior to RT delivery and allows for adaptation to interfraction changes. The UNITED study (UNIty-Based MR-Linac Guided AdapTive RadiothErapy for High GraDe Glioma; NCT04726397) is an ongoing phase II trial testing this approach in newly diagnosed GBM.
Protons have a unique depth-dose (energy-dependent) characteristic which leads to reduced dose deposition in normal tissue proximal and distal to the target volume. In addition, higher doses can theoretically be given safely, potentially improving local control and survival while at the same time reducing toxicity and improving quality of life [159]. Proton radiotherapy (PT) was compared to intensity-modulated radiotherapy (IMRT) in a small cohort phase II unblinded trial [160]. The primary endpoint, time to cognitive failure, was similar in both groups. Compared to IMRT, PT patients had less fatigue and lower rates of grade ≥ 2 toxicities but no difference in PFS was seen. The clinical benefit of PT, compared to standard photon RT, in GBM remains unclear.
Updates in Systemic Therapies
There are over 100 systemic therapies currently under investigation for GBM [161]. Broad strategies include drug repurposing, use of targeted therapies (to intrinsic GBM targets or microenvironmental targets), metabolic therapies, immunotherapies, and viral therapies. Several investigational agents utilize more than one of these strategies, and our review will focus on a subset of these therapies.
Chemotherapy
While chronotherapy has been most extensively studied in other solid cancers, its role in GBM is emerging. Gene transcription [162], cell cycle regulation [163], metabolism [164], and DNA repair [165] are dynamic processes that fluctuate throughout the day and appear to be paced by the hypothalamic suprachiasmatic nucleus, known as the “circadian rhythm pacemaker.” Based on data from non-CNS tumors [166–168] and the particularly relevant findings of diurnal fluctuations in MGMT activity [169, 170], Dalmato et al. retrospectively analyzed OS in 166 patients according to the timing of TMZ (AM versus PM) [171]. AM dosing was associated with longer survival than PM dosing with a more pronounced effect in MGMT methylated patients. A phase II trial randomizing patients to either AM (before 10) or PM (after 8) (NCT02781792) is ongoing.
Microtubule-targeting agents (MTAs) disturb microtubule function resulting in mitotic arrest and cell death through the spindle assembly-checkpoint pathway. In addition, cells in mitotic arrest have a heightened sensitivity to radiotherapy [172, 173]. Avanbulin (BAL27862) is a synthetic microtubule-destabilizing drug with a similar binding site to colchicine [174] and is active in GBM stem-like cells resistant to other MTAs [175]. Lisavanbulin (BAL101553) is a water-soluble lysine prodrug of avanbulin. In GBM patient-derived xenograft models, it improved survival as a monotherapy and showed a synergistic effect with RT/TMZ independent of MGMT status [176]. Lisavanbulin has already entered phase I/IIa clinical trials for the treatment of non-CNS solid tumors [177]. A phase I trial to establish the maximally tolerated dose in unmethylated GBM in combination with RT is in progress (NCT03250299).
The formation of O6-methylguanine, if not removed by MGMT, has a cytotoxic effect facilitated by the DNA mismatch repair (MMR) pathway. However, 90% of TMZ methylation occurs at N7-guanine and N3-adenine sites which are efficiently repaired by the base excision (BER) pathway [178, 179]. VAL 083 (dianhydrogalactitol) is an orphan molecule that readily crosses the blood–brain barrier (BBB) and has a long half-life [180]. It is a bifunctional alkylating agent that introduces interstrand-crosslinks at the N7-guanine sites that are resistant to MGMT and induces cytotoxicity independent of the MMR pathway [181–184]. Dose-escalation data from a phase I/II clinical trial [185, 186] have shown that doses in the range of 30–40 mg/m2/day (IV for 3 days in a 21-day cycle) are well tolerated and active in both methylated and unmethylated disease. Currently, it is being evaluated in the GBM AGILE trial (see later) in newly diagnosed methylated and unmethylated patients as well as in recurrent disease.
Focused ultrasound (FUS) causes intravenously administered microbubbles to oscillate in the applied low-energy ultrasound field, thereby increasing the BBB permeability by the shear stress mechanism. This modality is gaining interest because it can allow chemotherapeutic agents, especially those with a large molecular weight, to achieve higher concentrations in the tumor tissue [187]. The safety and feasibility of FUS were first established in a study of 5 patients with high-grade gliomas. The study revealed a 15 to 50% increase in enhancement on T1-WI that was durable up to 20 h and an increase of TMZ tissue concentration with sonification [188]. Multiple studies followed and confirmed the safety and feasibility of delivering different therapeutic agents in recurrent and newly diagnosed GBM with promising results regarding survival [189–193]. Multiple open-label phase I/II studies are currently ongoing to better characterize its safety and clinical efficacy.
Targeted Therapies and Precision Oncology
With increase in access to sequencing panels at some institutions, and its value in diagnostic confirmation [194], targeted gene testing has become prevalent in clinical practice with some optimized workflows reporting final gene panels in less than 1 week from tissue biopsy [195]. In addition, some centers have the capacity of integrating whole-exome sequencing (WES) or whole-genome sequencing for deeper molecular profiling. While knowledge of the evolving molecular landscape in glioma has grown exponentially [196–198], targeted therapy options remain limited and have not significantly changed the overall outcome in GBM. A selected review of published trials investigating some intrinsic GBM targets is summarized below.
There have been a number of clinical trials with selection based on molecular biomarkers, the most notable of which is EGFR amplification or EGFRvIII mutation, which is commonly found in GBM. Strategies targeting EGFR have unfortunately fallen short, including the EGFR inhibitor erlotinib [199], the EGFRvIII targeted neoantigen peptide vaccine rindopepimut [200], and the antibody–drug conjugate (depatuximab mafotidin) [201–203]. Clinical trials investigating EGFRvIII-directed chimeric antigen receptor (CAR) T cells in recurrent GBM are currently ongoing [204, 205].
TERT promoter mutations are one of the most common alterations (~80%) [206], and targeting telomerase is an attractive strategy in GBM [207]. Inhibition of telomerase in vivo has been shown to reduce cell proliferation in GBM xenografts [208]; however, effective translation to patients with GBM has so far been unsuccessful. Novel strategies could include the use of small molecules such as the purine nucleoside analogue 6-thio-2′-deoxyguanosine (6-thio-dG) to induce alteration of the structure of telomeres leading to cell death [209]. Eribulin, a mitotic inhibitor with activity against TERT promoter-mutant lines [210], may also represent another opportunity to target telomerase and is currently under investigation.
Targeting alterations in FGFR have also been of interest in GBM, where about 3% of tumors can harbor an oncogenic fusion with the coding domains of TACC 1 or TACC 3 [211]. The use of the FGFR inhibitor erdafitinib has been reported to inhibit glioma cells harboring FGFR3-TACC3 fusion in vitro and in vivo and has shown some activity in two reported patients [212]. The pan-FGFR kinase inhibitor infigratinib is currently being investigated in an early-phase clinical trial in recurrent GBM (NCT04424966).
About 2% of GBM harbor a mutation in BRAF V600E. While previous strategies have included monotherapy with RAF inhibitors such as vemurafenib [213], with a modest response seen in high-grade gliomas, the ongoing ROAR trial (NCT02034110), a phase II open-label multicenter study now closed to accrual, investigated the role of dual BRAF/MEK with dabrafenib and trametinib in gliomas (WHO grade 1–4) harboring a BRAF V600E mutation. There was clinically meaningful activity seen in both low- and high-grade glioma patients (31 of which were GBM patients) with the latter showing a 33% objective response rate [59].
Another rare but druggable target in GBM is the alterations in the neurotrophic tyrosine receptor kinase (NTRK) genes. Mutations in NTRK1, NTRK2, and NTRK3 have been described in solid tumors and genomic rearrangement and fusions trigger oncogenic signaling [214]. Larotrectinib has received tissue-agnostic FDA approval in 2018 for adult and pediatric solid tumors with NTRK gene fusions. However, NTRK fusions are most commonly found in pediatric gliomas and are present in molecularly diverse gliomas [215], and their incidence remains relatively low in adult GBM (~ 1%).
MET fusions occur in about 3% of gliomas [215]. These mutations have been targeted with the drug crizotinib in pediatric GBM [216]; however, significant response has not been observed in adults. Another target of interest in GBM is the PI3K/AKT/mTOR pathway; however, studies with the mTOR inhibitor CCL-779 [217] and the pan-PI3K inhibitor buparlisib [218] showed a lack of activity of both agents. Targets to the retinoblastoma (pRB) pathway have also been investigated, for example, a phase II trial of palbociclib in recurrent RB1-positive GBM [219] which did not show a survival benefit. A phase II trial of abemaciclib in patients with recurrent RB-wildtype GBM with CDKN2A/2B activation or CDK4 or CDK6 amplification is currently accruing (NCT02981940).
Clinical trial designs exploring molecularly matched therapies include the open-label multicenter phase I/IIa trial N2M2 [220], which stratifies patents with MGMT unmethylated GBM to their respective targeted agents based on matching alterations. In addition, this trial randomizes patients without matching alterations among some of the treatment arms to aid in the development of predictive biomarkers. While targeting specific mutations is one proposed strategy, assessing other molecular biomarkers as potential predictors of response may be helpful. This is being explored in two different large-scale adaptive Bayesian multi-arm platform trials, the Individualized Screening Trial of Innovative Glioblastoma Therapy (INSIGhT) trial (NCT02977780) [221] and the global multi-arm phase II/III GBM Adaptive Global Innovative Learning Environment (GBM AGILE) trial (NCT03970447) [222]. In these designs, patients are initially randomized to multiple experimental arms with a targeted agent vs a control arm, and patient subtypes including biomarkers are identified with adaptive randomization based on cumulative real-time efficacy data. Table 1 summarizes the experimental drugs used in the beforementioned trials.
Table 1.
Summary of the drugs used in the three ongoing trials for GBM as of 2022
| Trial Overview | Adjuvant Experimental drugs | Mechanism of action |
|---|---|---|
| N2M2 (NOA-20; NCT03158389) [220] | ||
|
Germany; multi-center, open-label, parallel group, nonrandomized phase I/IIa trial for newly diagnosed GBM MGMT promoter: Unmethylated Drug assignment: Molecular matching |
Alectinib | Oral ALK inhibitor |
| Idasanutlin | Oral MDM2 inhibitor | |
|
Palbociclib (concurrent and adjuvant) |
Oral CDK4/6 inhibitor | |
| Vismodegib | Oral SHH inhibitor | |
| Temsirolimus | IV mTOR inhibitor | |
| INSIGhT (NCT02977780) [221] | ||
|
USA; multi-center, open-label, multi-arm, randomized phase II trial for newly diagnosed GBM MGMT promoter: unmethylated Drug assignment: adaptive randomization |
Neratinib | Oral pan-ErbB tyrosine kinase inhibitor |
|
CC-115 (concurrent and adjuvant) |
Oral mTOR and DNA-PK inhibitor; radiosensitizer | |
| Abemaciclib | Oral CDK4/6 inhibitor | |
| GBM AGILE (NCT03970447) [222] | ||
|
Global; open-label, multi-arm, randomized phase II/III trial for newly diagnosed and recurrent GBM MGMT promoter: unmethylated and methylated Drug assignment: adaptive randomization |
Regorafenib | Oral multikinase inhibitor |
| Paxalisib | Oral PI3K inhibitor | |
| VAL-083 | IV Bi-functional alkylating agent | |
| Troriluzole | Oral modulator of glutamate metabolism | |
| VT1021 | First-in-class compound that blocks the CD46 immune checkpoint and influences CD36 activity | |
SHH sonic hedgehog, ALK anaplastic lymphoma kinase, MDM2 mouse double minute 2 homolog, CDK cyclin-dependent kinases, mTOR mechanistic target of rapamycin, DNA-PK DNA-dependent protein kinase, ErbB Erythroblastic leukemia viral oncogene homolog, CD cluster of differentiation, PI3K phosphoinositide 3-kinase
There are many barriers to the development of effective molecular therapies in GBM including intra- and inter-tumor molecular heterogeneity [57], redundant signaling pathways, and a lack of active and brain-penetrant targeted therapies [223]. Combinatorial approaches based on molecular biomarkers studies and/or the use of modalities aimed at increasing BBB penetration may represent future directions to fulfill the potential of targeted therapies.
Immunotherapies
Tumor eradication through cancer immunotherapy has garnered great interest in GBM, and the immunosuppressive paradigm in GBM has been well described [224]. There are several challenges to the development of effective immunotherapies in GBM including a paucity of tumor infiltrating effector lymphocytes and their exhaustion, predominantly related to the immunosuppressive microenvironment induced by the tumoral cells’ surface proteins and secreted cytokines. In addition, the immunosuppressive nature of standard therapies and the frequent use of corticosteroids further complicate the effectiveness of immunotherapies.
Immunotherapy in GBM has thus far been disappointing [225] but could be a result of a lack of proper patient selection, microenvironment inhibition or specific immunogenicity assessments [161]. Immune checkpoint inhibitors (ICIs) such as anti-programmed cell death protein-1 (PD-1)/programmed death ligand-1 (PD-L1) and anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) have been investigated in GBM. Unfortunately, these did not show any survival benefit in newly diagnosed [226] or recurrent GBM [226, 227]. Despite these discouraging results, there may be a role for ICI in the neoadjuvant setting or in combination with other therapies. For example, a study in which 35 patients with recurrent surgically resectable GBM were given pembrolizumab prior to and/or following surgical resection showed that neoadjuvant PD-1 blockade was associated with significantly longer overall survival [228]. Another subset of GBM patients who may benefit from ICI are patients with hypermutated tumors resulting from a germline DNA MMR deficiency [229, 230] as they may exhibit tumor-associated neoantigens that trigger an immune response. The genomic signatures associated with PD-1 response in pediatric patients with germline DNA replication repair deficiency including CNS tumors were recently described [231].
Neoantigen vaccines as a means to induce immune surveillance against glioma cells through vaccination are also being explored. Previous trials utilizing this strategy included the ACT IV study, where the addition of rindopepimut, a peptide vaccine targeting EGFRvIII to the standard of care chemoradiation, did not improve survival in newly diagnosed GBM [200]. There are many possible reasons for the failure of this approach, and other strategies have included multi-epitope personalized neoantigen GBM vaccines that were found to generate a neo-antigen specific T cell response [232]. Personalized neoantigens derived from next-generation sequencing analyses of individual GBM transcriptomes and immunopeptidomes have therefore been studied in small patient cohorts with good feasibility data [233]. Larger trials are required using this approach and in combination with other immunotherapy strategies and a few are under way (NCT04015700, NCT02287428).
Viral therapies have been investigated in GBM in the form of oncolytic viruses (invading and/or replicating selectively in tumor cells) or gene therapy (delivery of anticancer cDNA into tumor cells) [234, 235]. Both strategies thereafter activate an immune response through cytotoxicity and the release of tumor antigens and are therefore theoretically further enhanced with the addition of ICIs. Examples of such approaches include the convection-enhanced delivery of an engineered poliovirus (PVSRIPO; NCT02986178) [236], the combination of gene-mediated cytotoxic immunotherapy (GMCI-AdV-Tk) with valcyclovir and chemoradiation in newly diagnosed GBM (NCT03576612) [237], and Toca 511, an intracavitary injection of a retrovirus that delivers a cytosine deaminase cDNA that provides chemosensitivity to 5-fluorocytosine (NCT02414165). The primary endpoint of the phase III Toca 5 trial was however not met as Toca 511 and Toca FC did not improve OS compared to standard therapy in recurrent high-grade gliomas [238].
CAR T cells are engineered T cells that express antigen-binding receptors that lead to target cell killing of tumor cells. There are a number of current efforts in GBM to exploit this immunotherapy strategy [205, 239], including targeting multiple neoantigens, in combination with ICI or through direct intratumoral delivery.
Future directions in immunotherapy could include more personalized and precise approaches based on genomic or immunogenic biomarkers, neoadjuvant approaches, and a combination of immunotherapy with local therapies such as radiosurgery or local chemotherapy as well as multi-modality approaches (ICI in combination with vaccine therapy/viral therapy/cellular therapy) [240].
Drug Repurposing
Repurposing approved drugs as cancer-directed therapies has been of great interest to the oncology community. Selected drugs that have been investigated in this context include antiseizure medications, as up to 60% of patients with high-grade gliomas have seizures. Valproic acid (VPA) has histone deacetylase inhibitor activity at high concentrations in vitro and has been studied for its impact in GBM. While post hoc analysis of the Stupp trial data indicated longer survival for patients on VPA [241], this was not confirmed in a larger analysis [242] although smaller prospective studies indicate a potential benefit in younger patients [243]. Recently, two separate studies demonstrated that neuron-glioma synapses could be activated through the AMPA receptor contributing to glioma proliferation [244, 245]. In a study looking at four different anticonvulsants (levetiracetam, VPA, carbamazepine, and perampanel), only perampanel was found to have antitumoral effects [246]. Two clinical trials testing the AMPA receptor blocker talampanel showed contrasting results [247, 248] and larger randomized controlled trials targeting AMPA receptors are needed to explore the possible benefits of this strategy in GBM.
Another example of drug repurposing was the recent discovery in vitro that the highly CNS penetrant selective serotonin reuptake inhibitor antidepressant fluoxetine could inhibit sphingomyelin phosphodiesterase 1 resulting in dose-dependent GBM cell death through inhibition of EGFR signaling [249]. Clinical studies are however required to validate these findings in patients with GBM.
Anti-glycemic agents targeting the Warburg effect have been of interest in GBM [250]. The effect of metformin, in particular, has been investigated in pooled retrospective analyses of clinical trial data [251], with negative results.
Combinations of multiple approved drugs in specific “treatment protocols” such as CUSP9 (a combination of aprepitant, artesunate, auranofin, captopril, copper gluconate, disulfiram, ketoconazole, nelfinavir, sertraline) have also been used anecdotally [252] but beyond in vitro studies [253] remain of questionable efficacy and are being further validated in an ongoing clinical trial [254].
In the future, drug repurposing could be made more efficient by machine learning and artificial intelligence strategies [255] to identify matches based on large data repositories and therefore accelerate validation of preliminary data through GBM-specific clinical trials.
Future Directions
The history of neuro-oncology has not been kind to recurrent glioblastoma. Our field has failed to develop a meaningful improvement in therapy beyond older alkylating agents, which in themselves may have little to no activity. Some two to three decades ago, the failure of phase III clinical trials was felt to be the result of selection bias in earlier studies. Indeed, even well-known prognostic factors were poorly accounted for in phase I and II trials, leading to over-estimation of clinical efficacy, and failure in later randomized trials. As a next step, learning from the significant influence of selection bias, attempts were made to use well-matched historical controls in phase II work. Even relatively recent negative phase III studies such as the CENTRIC study [256] were based upon promising non-randomized data. These well-intentioned efforts consumed large financial and patient resources with a drug development cycle approximately a decade long from concept to drug failure. Advances in imaging, surgery, and radiation delivery likely account for the modest shift in the overall survival seen in completed phase III studies; for example, the historical median survival of 14.3 months in the pivotal Stupp study has shifted now to 18 months in more recent trials [257]. In many instances, the use of historical controls has not adapted well and often the older survival data are still inappropriately used as comparators.
Such shortcomings in clinical trial design and therapeutic evaluation should now be behind us, and the stage is ready for more rapid drug discovery in “window of opportunity” studies and adaptive platform clinical trial designs. It remains implausible to think that one single targeted therapy will move the needle in the overall survival from recurrent GBM [258]; but our field has made little effort to understand whether specifically defined subgroups of patients may demonstrate benefit despite negative phase III results. The proverbial “throwing out the baby with the bathwater” approach to failed clinical trials should not be an acceptable outcome of these efforts. Clinicians, together with our patients, would highly value an effective treatment, even if it only worked in a fraction of all cases (assuming that fraction can be identified a priori). We need to encourage the drug development industry to pay attention to these small victories. While a fractional share of a rare disease provides little commercial incentive, we feel that the most likely way forward is a series of cumulative successes in clinical trials enriched for patients most likely to respond to novel therapies.
Having said that, it is noteworthy that not only the quantity but also the “quality” of the diseased patients influences the research question of clinical trials. Inequalities and inequities spanning the spectrum of social determinants of health such as race, ethnicity, and socioeconomic status [259, 260] are front and center targets that stakeholders should address to ensure equitable access [261]. Tele-health modalities are quickly taking over the post-pandemic healthcare scene and these may provide the ultimate solution for the inclusion of the abovementioned population. Thus, contextualizing patient care into clinical trials can be easily and cost-effectively secured.
Conclusions
The most significant recent advances in GBM have been in our understanding of the molecular pathways, oncogenic drivers, and tumor microenvironment distinguishing these tumors from other CNS neoplasms. Future clinical trials leveraging this newly gained knowledge and correlative scientific efforts to understand patterns of response and resistance will hopefully translate into improved outcomes for this population.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Aimee Chan for her editorial assistance.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mary Jane Lim-Fat and James R. Perry contributed equally.
References
- 1.Canoll P, Goldman JE. The interface between glial progenitors and gliomas. Acta Neuropathol. 2008;116(5):465–477. doi: 10.1007/s00401-008-0432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JH, Lee JE, Kahng JY, Kim SH, Park JS, Yoon SJ, et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature. 2018;560(7717):243–247. doi: 10.1038/s41586-018-0389-3. [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro Oncol. 2020;22(Supplement_1):iv1–iv96. doi: 10.1093/neuonc/noaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrom QT, Cote DJ, Ascha M, Kruchko C, Barnholtz-Sloan JS. Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol. 2018;4(9):1254–1262. doi: 10.1001/jamaoncol.2018.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostrom QT, Adel Fahmideh M, Cote DJ, Muskens IS, Schraw JM, Scheurer ME, et al. Risk factors for childhood and adult primary brain tumors. Neuro Oncol. 2019;21(11):1357–1375. doi: 10.1093/neuonc/noz123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wrensch M, Lee M, Miike R, Newman B, Barger G, Davis R, et al. Familial and personal medical history of cancer and nervous system conditions among adults with glioma and controls. Am J Epidemiol. 1997;145(7):581–593. doi: 10.1093/oxfordjournals.aje.a009154. [DOI] [PubMed] [Google Scholar]
- 8.Ranger AM, Patel YK, Chaudhary N, Anantha RV. Familial syndromes associated with intracranial tumours: a review. Childs Nerv Syst. 2014;30(1):47–64. doi: 10.1007/s00381-013-2309-z. [DOI] [PubMed] [Google Scholar]
- 9.Vijapura C, Saad Aldin E, Capizzano AA, Policeni B, Sato Y, Moritani T. Genetic syndromes associated with central nervous system tumors. Radiographics. 2017;37(1):258–280. doi: 10.1148/rg.2017160057. [DOI] [PubMed] [Google Scholar]
- 10.Amirian ES, Zhou R, Wrensch MR, Olson SH, Scheurer ME, Il’yasova D, et al. Approaching a scientific consensus on the association between allergies and glioma risk: a report from the glioma international case-control study. Cancer Epidemiol Biomarkers Prev. 2016;25(2):282–290. doi: 10.1158/1055-9965.EPI-15-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas RP, Xu LW, Lober RM, Li G, Nagpal S. The incidence and significance of multiple lesions in glioblastoma. J Neurooncol. 2013;112(1):91–97. doi: 10.1007/s11060-012-1030-1. [DOI] [PubMed] [Google Scholar]
- 12.Lasocki A, Gaillard F, Tacey M, Drummond K, Stuckey S. Multifocal and multicentric glioblastoma: improved characterisation with FLAIR imaging and prognostic implications. J Clin Neurosci. 2016;31:92–98. doi: 10.1016/j.jocn.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Haque W, Thong Y, Verma V, Rostomily R, Brian Butler E, Teh BS. Patterns of management and outcomes of unifocal versus multifocal glioblastoma. J Clin Neurosci. 2020;74:155–159. doi: 10.1016/j.jocn.2020.01.086. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Zhang ZX, Huang GH, Xiang Y, Yang L, Pei YC, et al. A systematic review of multifocal and multicentric glioblastoma. J Clin Neurosci. 2021;83:71–76. doi: 10.1016/j.jocn.2020.11.025. [DOI] [PubMed] [Google Scholar]
- 15.Higano S, Yun X, Kumabe T, Watanabe M, Mugikura S, Umetsu A, et al. Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology. 2006;241(3):839–846. doi: 10.1148/radiol.2413051276. [DOI] [PubMed] [Google Scholar]
- 16.Lu X, Xu W, Wei Y, Li T, Gao L, Fu X, et al. Diagnostic performance of DWI for differentiating primary central nervous system lymphoma from glioblastoma: a systematic review and meta-analysis. Neurol Sci. 2019;40(5):947–956. doi: 10.1007/s10072-019-03732-7. [DOI] [PubMed] [Google Scholar]
- 17.van Dijken BRJ, van Laar PJ, Smits M, Dankbaar JW, Enting RH, van der Hoorn A. Perfusion MRI in treatment evaluation of glioblastomas: clinical relevance of current and future techniques. J Magn Reson Imaging. 2019;49(1):11–22. doi: 10.1002/jmri.26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain R, Griffith B, Alotaibi F, Zagzag D, Fine H, Golfinos J, et al. Glioma angiogenesis and perfusion imaging: understanding the relationship between tumor blood volume and leakiness with increasing glioma grade. AJNR Am J Neuroradiol. 2015;36(11):2030–2035. doi: 10.3174/ajnr.A4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Álvarez-Torres MDM, Fuster-García E, Juan-Albarracín J, Reynés G, Aparici-Robles F, Ferrer-Lozano J, et al. Local detection of microvessels in IDH-wildtype glioblastoma using relative cerebral blood volume: an imaging marker useful for astrocytoma grade 4 classification. BMC Cancer. 2022;22(1):40. doi: 10.1186/s12885-021-09117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu H, Tong H, Du X, Guo H, Ma Q, Zhang Y, et al. Vascular habitat analysis based on dynamic susceptibility contrast perfusion MRI predicts IDH mutation status and prognosis in high-grade gliomas. Eur Radiol. 2020;30(6):3254–3265. doi: 10.1007/s00330-020-06702-2. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita K, Hiwatashi A, Togao O, Kikuchi K, Hatae R, Yoshimoto K, et al. MR imaging-based analysis of glioblastoma multiforme: estimation of IDH1 mutation status. AJNR Am J Neuroradiol. 2016;37(1):58–65. doi: 10.3174/ajnr.A4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan W, Xiong J, Huang W, Wu J, Zhan S, Geng D. Noninvasively detecting isocitrate dehydrogenase 1 gene status in astrocytoma by dynamic susceptibility contrast MRI. J Magn Reson Imaging. 2017;45(2):492–499. doi: 10.1002/jmri.25358. [DOI] [PubMed] [Google Scholar]
- 23.Rundle-Thiele D, Day B, Stringer B, Fay M, Martin J, Jeffree RL, et al. Using the apparent diffusion coefficient to identifying MGMT promoter methylation status early in glioblastoma: importance of analytical method. J Med Radiat Sci. 2015;62(2):92–98. doi: 10.1002/jmrs.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romano A, Calabria LF, Tavanti F, Minniti G, Rossi-Espagnet MC, Coppola V, et al. Apparent diffusion coefficient obtained by magnetic resonance imaging as a prognostic marker in glioblastomas: correlation with MGMT promoter methylation status. Eur Radiol. 2013;23(2):513–520. doi: 10.1007/s00330-012-2601-4. [DOI] [PubMed] [Google Scholar]
- 25.Oz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270(3):658–679. doi: 10.1148/radiol.13130531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Costanzo A, Scarabino T, Trojsi F, Giannatempo GM, Popolizio T, Catapano D, et al. Multiparametric 3T MR approach to the assessment of cerebral gliomas: tumor extent and malignancy. Neuroradiology. 2006;48(9):622–631. doi: 10.1007/s00234-006-0102-3. [DOI] [PubMed] [Google Scholar]
- 27.Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003;24(10):1989–1998. [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PCM. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med. 2003;50(6):1120–1126. doi: 10.1002/mrm.10651. [DOI] [PubMed] [Google Scholar]
- 29.Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PCM. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9(8):1085–1090. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 30.Hagiwara A, Tatekawa H, Yao J, Raymond C, Everson R, Patel K, et al. Visualization of tumor heterogeneity and prediction of isocitrate dehydrogenase mutation status for human gliomas using multiparametric physiologic and metabolic MRI. Sci Rep. 2022;12(1):1078. doi: 10.1038/s41598-022-05077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galldiks N, Lohmann P, Albert NL, Tonn JC, Langen KJ. Current status of PET imaging in neuro-oncology. Neurooncol Adv. 2019;1(1):vdz010. doi: 10.1093/noajnl/vdz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langen KJ, Galldiks N, Hattingen E, Shah NJ. Advances in neuro-oncology imaging. Nat Rev Neurol. 2017;13(5):279–289. doi: 10.1038/nrneurol.2017.44. [DOI] [PubMed] [Google Scholar]
- 33.Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18(9):1199–1208. doi: 10.1093/neuonc/now058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drake LR, Hillmer AT, Cai Z. Approaches to PET imaging of glioblastoma. Molecules. 2020;25(3):E568. doi: 10.3390/molecules25030568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao C, Zhang Y, Wang J. A meta-analysis on the diagnostic performance of (18)F-FDG and (11)C-methionine PET for differentiating brain tumors. AJNR Am J Neuroradiol. 2014;35(6):1058–1065. doi: 10.3174/ajnr.A3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunet V, Rossier C, Buck A, Stupp R, Prior JO. Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: a systematic review and Metaanalysis. J Nucl Med. 2012;53(2):207–214. doi: 10.2967/jnumed.111.096859. [DOI] [PubMed] [Google Scholar]
- 37.Law I, Albert NL, Arbizu J, Boellaard R, Drzezga A, Galldiks N, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540–557. doi: 10.1007/s00259-018-4207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suchorska B, Albert NL, Bauer EK, Tonn JC, Galldiks N. The role of amino-acid PET in the light of the new WHO classification 2016 for brain tumors. Q J Nucl Med Mol Imaging. 2018;62(3):267–271. doi: 10.23736/S1824-4785.18.03090-X. [DOI] [PubMed] [Google Scholar]
- 39.Galldiks N, Langen KJ, Holy R, Pinkawa M, Stoffels G, Nolte KW, et al. Assessment of treatment response in patients with glioblastoma using O-(2–18F-fluoroethyl)-L-tyrosine PET in comparison to MRI. J Nucl Med. 2012;53(7):1048–1057. doi: 10.2967/jnumed.111.098590. [DOI] [PubMed] [Google Scholar]
- 40.Piroth MD, Pinkawa M, Holy R, Klotz J, Nussen S, Stoffels G, et al. Prognostic value of early [18F]fluoroethyltyrosine positron emission tomography after radiochemotherapy in glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2011;80(1):176–184. doi: 10.1016/j.ijrobp.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 41.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 42.Macdonald DR, Cascino TL, Schold SC, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 43.Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017;14(2):307–320. doi: 10.1007/s13311-016-0507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellingson BM, Bendszus M, Boxerman J, Barboriak D, Erickson BJ, Smits M, et al. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol. 2015;17(9):1188–1198. doi: 10.1093/neuonc/nov095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nayak L, DeAngelis LM, Brandes AA, Peereboom DM, Galanis E, Lin NU, et al. The Neurologic Assessment in Neuro-Oncology (NANO) scale: a tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology (RANO) criteria. Neuro Oncol. 2017;19(5):625–635. doi: 10.1093/neuonc/nox029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–e542. doi: 10.1016/S1470-2045(15)00088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galldiks N, Niyazi M, Grosu AL, Kocher M, Langen KJ, Law I, et al. Contribution of PET imaging to radiotherapy planning and monitoring in glioma patients - a report of the PET/RANO group. Neuro Oncol. 2021;23(6):881–893. doi: 10.1093/neuonc/noab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kickingereder P, Isensee F, Tursunova I, Petersen J, Neuberger U, Bonekamp D, et al. Automated quantitative tumour response assessment of MRI in neuro-oncology with artificial neural networks: a multicentre, retrospective study. Lancet Oncol. 2019;20(5):728–740. doi: 10.1016/S1470-2045(19)30098-1. [DOI] [PubMed] [Google Scholar]
- 49.Chang K, Beers AL, Bai HX, Brown JM, Ly KI, Li X, et al. Automatic assessment of glioma burden: a deep learning algorithm for fully automated volumetric and bidimensional measurement. Neuro Oncol. 2019;21(11):1412–1422. doi: 10.1093/neuonc/noz106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Capper D, Weissert S, Balss J, Habel A, Meyer J, Jäger D, et al. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 2010;20(1):245–254. doi: 10.1111/j.1750-3639.2009.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeWitt JC, Jordan JT, Frosch MP, Samore WR, Iafrate AJ, Louis DN, et al. Cost-effectiveness of IDH testing in diffuse gliomas according to the 2016 WHO classification of tumors of the central nervous system recommendations. Neuro Oncol. 2017;19(12):1640–1650. doi: 10.1093/neuonc/nox120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brat DJ, Aldape K, Colman H, Holland EC, Louis DN, Jenkins RB, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018;136(5):805–810. doi: 10.1007/s00401-018-1913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellison DW, Hawkins C, Jones DTW, Onar-Thomas A, Pfister SM, Reifenberger G, et al. cIMPACT-NOW update 4: diffuse gliomas characterized by MYB, MYBL1, or FGFR1 alterations or BRAFV600E mutation. Acta Neuropathol. 2019;137(4):683–687. doi: 10.1007/s00401-019-01987-0. [DOI] [PubMed] [Google Scholar]
- 55.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 56.Nakajima N, Nobusawa S, Nakata S, Nakada M, Yamazaki T, Matsumura N, et al. BRAF V600E, TERT promoter mutations and CDKN2A/B homozygous deletions are frequent in epithelioid glioblastomas: a histological and molecular analysis focusing on intratumoral heterogeneity. Brain Pathol. 2018;28(5):663–673. doi: 10.1111/bpa.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brennan CW, Verhaak RGW, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Korshunov A, Chavez L, Sharma T, Ryzhova M, Schrimpf D, Stichel D, et al. Epithelioid glioblastomas stratify into established diagnostic subsets upon integrated molecular analysis. Brain Pathol. 2018;28(5):656–662. doi: 10.1111/bpa.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wen PY, Stein A, van den Bent M, De Greve J, Wick A, de Vos FYFL, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutant low-grade and high-grade glioma (ROAR): a multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 2022;23(1):53–64. doi: 10.1016/S1470-2045(21)00578-7. [DOI] [PubMed] [Google Scholar]
- 60.Lim-Fat MJ, Youssef GC, Touat M, Iorgulescu JB, Whorral S, Allen M, et al. Clinical utility of targeted next generation sequencing assay in IDH-wildtype glioblastoma for therapy decision-making. Neuro Oncol. 2021;noab282. [DOI] [PMC free article] [PubMed]
- 61.Parsons DW, Jones S, Zhang X, Lin JCH, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cancer Genome Atlas Research Network. Brat DJ, Verhaak RGW, Aldape KD, Yung WKA, Salama SR, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–563. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. doi: 10.1038/nature26000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Molinaro AM, Hervey-Jumper S, Morshed RA, Young J, Han SJ, Chunduru P, et al. Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol. 2020;6(4):495–503. doi: 10.1001/jamaoncol.2019.6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vuorinen V, Hinkka S, Färkkilä M, Jääskeläinen J. Debulking or biopsy of malignant glioma in elderly people - a randomised study. Acta Neurochir (Wien) 2003;145(1):5–10. doi: 10.1007/s00701-002-1030-6. [DOI] [PubMed] [Google Scholar]
- 67.Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–186. doi: 10.1038/s41571-020-00447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 69.Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Balana C, Vaz MA, Manuel Sepúlveda J, Mesia C, Del Barco S, Pineda E, et al. A phase II randomized, multicenter, open-label trial of continuing adjuvant temozolomide beyond 6 cycles in patients with glioblastoma (GEINO 14–01) Neuro Oncol. 2020;22(12):1851–1861. doi: 10.1093/neuonc/noaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gramatzki D, Kickingereder P, Hentschel B, Felsberg J, Herrlinger U, Schackert G, et al. Limited role for extended maintenance temozolomide for newly diagnosed glioblastoma. Neurology. 2017;88(15):1422–1430. doi: 10.1212/WNL.0000000000003809. [DOI] [PubMed] [Google Scholar]
- 72.Blumenthal DT, Gorlia T, Gilbert MR, Kim MM, Burt Nabors L, Mason WP, et al. Is more better? The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma: a secondary analysis of EORTC and NRG Oncology/RTOG. Neuro Oncol. 2017;19(8):1119–1126. doi: 10.1093/neuonc/nox025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su YB, Sohn S, Krown SE, Livingston PO, Wolchok JD, Quinn C, et al. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22(4):610–616. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 74.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pouratian N, Gasco J, Sherman JH, Shaffrey ME, Schiff D. Toxicity and efficacy of protracted low dose temozolomide for the treatment of low grade gliomas. J Neurooncol. 2007;82(3):281–288. doi: 10.1007/s11060-006-9280-4. [DOI] [PubMed] [Google Scholar]
- 76.Bae SH, Park MJ, Lee MM, Kim TM, Lee SH, Cho SY, et al. Toxicity profile of temozolomide in the treatment of 300 malignant glioma patients in Korea. J Korean Med Sci. 2014;29(7):980–984. doi: 10.3346/jkms.2014.29.7.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kirson ED, Dbalý V, Tovarys F, Vymazal J, Soustiel JF, Itzhaki A, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007;104(24):10152–10157. doi: 10.1073/pnas.0702916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kirson ED, Schneiderman RS, Dbalý V, Tovarys F, Vymazal J, Itzhaki A, et al. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields) BMC Med Phys. 2009;8(9):1. doi: 10.1186/1756-6649-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 80.Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. doi: 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taphoorn MJB, Dirven L, Kanner AA, Lavy-Shahaf G, Weinberg U, Taillibert S, et al. Influence of treatment with tumor-treating fields on health-related quality of life of patients with newly diagnosed glioblastoma: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(4):495–504. doi: 10.1001/jamaoncol.2017.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Regev O, Merkin V, Blumenthal DT, Melamed I, Kaisman-Elbaz T. Tumor-Treating Fields for the treatment of glioblastoma: a systematic review and meta-analysis. Neurooncol Pract. 2021;8(4):426–440. doi: 10.1093/nop/npab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Toms SA, Kim CY, Nicholas G, Ram Z. Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: a subgroup analysis of the EF-14 phase III trial. J Neurooncol. 2019;141(2):467–473. doi: 10.1007/s11060-018-03057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cloughesy TF, Lassman AB. NovoTTF: where to go from here? Neuro Oncol. 2017;19(5):605–608. doi: 10.1093/neuonc/nox014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 86.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wick A, Kessler T, Elia AEH, Winkler F, Batchelor TT, Platten M, et al. Glioblastoma in elderly patients: solid conclusions built on shifting sand? Neuro Oncol. 2018;20(2):174–183. doi: 10.1093/neuonc/nox133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sijben AE, McIntyre JB, Roldán GB, Easaw JC, Yan E, Forsyth PA, et al. Toxicity from chemoradiotherapy in older patients with glioblastoma multiforme. J Neurooncol. 2008;89(1):97–103. doi: 10.1007/s11060-008-9593-6. [DOI] [PubMed] [Google Scholar]
- 89.Keime-Guibert F, Chinot O, Taillandier L, Cartalat-Carel S, Frenay M, Kantor G, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356(15):1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 90.Roa W, Brasher PMA, Bauman G, Anthes M, Bruera E, Chan A, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–1588. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 91.Roa W, Kepka L, Kumar N, Sinaika V, Matiello J, Lomidze D, et al. International atomic energy agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2015;33(35):4145–4150. doi: 10.1200/JCO.2015.62.6606. [DOI] [PubMed] [Google Scholar]
- 92.Malmström A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 93.Perry JR, Laperriere N, O’Callaghan CJ, Brandes AA, Menten J, Phillips C, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. doi: 10.1056/NEJMoa1611977. [DOI] [PubMed] [Google Scholar]
- 94.Wick A, Kessler T, Platten M, Meisner C, Bamberg M, Herrlinger U, et al. Superiority of temozolomide over radiotherapy for elderly patients with RTK II methylation class, MGMT promoter methylated malignant astrocytoma. Neuro Oncol. 2020;22(8):1162–1172. doi: 10.1093/neuonc/noaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR. Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol. 2019;15(7):405–417. doi: 10.1038/s41582-019-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alnahhas I, Alsawas M, Rayi A, Palmer JD, Raval R, Ong S, et al. Characterizing benefit from temozolomide in MGMT promoter unmethylated and methylated glioblastoma: a systematic review and meta-analysis. Neurooncol Adv. 2020;2(1):vdaa082. doi: 10.1093/noajnl/vdaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mansouri A, Hachem LD, Mansouri S, Nassiri F, Laperriere NJ, Xia D, et al. MGMT promoter methylation status testing to guide therapy for glioblastoma: refining the approach based on emerging evidence and current challenges. Neuro Oncol. 2019;21(2):167–178. doi: 10.1093/neuonc/noy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kamson DO, Grossman SA. The role of temozolomide in patients with newly diagnosed wild-type IDH, unmethylated MGMTp glioblastoma during the COVID-19 pandemic. JAMA Oncol. 2021;7(5):675–676. doi: 10.1001/jamaoncol.2020.6732. [DOI] [PubMed] [Google Scholar]
- 99.Hegi ME, Genbrugge E, Gorlia T, Stupp R, Gilbert MR, Chinot OL, et al. MGMT promoter methylation cutoff with safety margin for selecting glioblastoma patients into trials omitting temozolomide: a pooled analysis of four clinical trials. Clin Cancer Res. 2019;25(6):1809–1816. doi: 10.1158/1078-0432.CCR-18-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 101.Weller M. Where does O6 -methylguanine DNA methyltransferase promoter methylation assessment place temozolomide in the future standards of care for glioblastoma? Cancer. 2018;124(7):1316–1318. doi: 10.1002/cncr.31244. [DOI] [PubMed] [Google Scholar]
- 102.Hegi ME, Stupp R. Withholding temozolomide in glioblastoma patients with unmethylated MGMT promoter–still a dilemma? Neuro Oncol. 2015;17(11):1425–1427. doi: 10.1093/neuonc/nov198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gállego Pérez-Larraya J, Ducray F, Chinot O, Catry-Thomas I, Taillandier L, Guillamo JS, et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol. 2011;29(22):3050–3055. doi: 10.1200/JCO.2011.34.8086. [DOI] [PubMed] [Google Scholar]
- 104.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 105.Suchorska B, Weller M, Tabatabai G, Senft C, Hau P, Sabel MC, et al. Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro Oncol. 2016;18(4):549–556. doi: 10.1093/neuonc/nov326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ryu S, Buatti JM, Morris A, Kalkanis SN, Ryken TC, Olson JJ, et al. The role of radiotherapy in the management of progressive glioblastoma : a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014;118(3):489–499. doi: 10.1007/s11060-013-1337-6. [DOI] [PubMed] [Google Scholar]
- 107.Straube C, Kessel KA, Zimmer C, Schmidt-Graf F, Schlegel J, Gempt J, et al. A second course of radiotherapy in patients with recurrent malignant gliomas: clinical data on re-irradiation, prognostic factors, and usefulness of digital biomarkers. Curr Treat Options Oncol. 2019;20(9):71. doi: 10.1007/s11864-019-0673-y. [DOI] [PubMed] [Google Scholar]
- 108.Scoccianti S, Francolini G, Carta GA, Greto D, Detti B, Simontacchi G, et al. Re-irradiation as salvage treatment in recurrent glioblastoma: a comprehensive literature review to provide practical answers to frequently asked questions. Crit Rev Oncol Hematol. 2018;126:80–91. doi: 10.1016/j.critrevonc.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 109.Minniti G, Niyazi M, Alongi F, Navarria P, Belka C. Current status and recent advances in reirradiation of glioblastoma. Radiat Oncol. 2021;16(1):36. doi: 10.1186/s13014-021-01767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shi W, Scannell Bryan M, Gilbert MR, Mehta MP, Blumenthal DT, Brown PD, et al. Investigating the effect of reirradiation or systemic therapy in patients with glioblastoma after tumor progression: a secondary analysis of NRG Oncology/Radiation Therapy Oncology Group Trial 0525. Int J Radiat Oncol Biol Phys. 2018;100(1):38–44. doi: 10.1016/j.ijrobp.2017.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsien C, Pugh S, Dicker A, Raizer J, Matuszak M, Lallana E, et al. ACTR-32. NRG oncology RTOG 1205: randomized phase II trial of concurrent bevacizumab and re-irradiation vs. bevacizumab alone as treatment for recurrent glioblastoma. Neuro Oncol. 2019;21(Suppl 6):vi20–vi20. doi: 10.1093/neuonc/noz175.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brandes AA, Bartolotti M, Tosoni A, Franceschi E. Nitrosoureas in the management of malignant gliomas. Curr Neurol Neurosci Rep. 2016;16(2):13. doi: 10.1007/s11910-015-0611-8. [DOI] [PubMed] [Google Scholar]
- 113.Weller M, Le Rhun E. How did lomustine become standard of care in recurrent glioblastoma? Cancer Treat Rev. 2020;87:102029. doi: 10.1016/j.ctrv.2020.102029. [DOI] [PubMed] [Google Scholar]
- 114.Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. doi: 10.1200/JCO.2012.47.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. doi: 10.1056/NEJMoa1707358. [DOI] [PubMed] [Google Scholar]
- 116.Lombardi G, De Salvo GL, Brandes AA, Eoli M, Rudà R, Faedi M, et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019;20(1):110–119. doi: 10.1016/S1470-2045(18)30675-2. [DOI] [PubMed] [Google Scholar]
- 117.Tolcher AW, Gerson SL, Denis L, Geyer C, Hammond LA, Patnaik A, et al. Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer. 2003;88(7):1004–1011. doi: 10.1038/sj.bjc.6600827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26(25):4189–4199. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 119.Nagane M. Dose-dense temozolomide: is it still promising? Neurol Med Chir (Tokyo) 2015;55(Suppl 1):38–49. doi: 10.2176/nmc.ra.2014-0277. [DOI] [PubMed] [Google Scholar]
- 120.Weller M, Tabatabai G, Kästner B, Felsberg J, Steinbach JP, Wick A, et al. MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin Cancer Res. 2015;21(9):2057–2064. doi: 10.1158/1078-0432.CCR-14-2737. [DOI] [PubMed] [Google Scholar]
- 121.Perry JR, Bélanger K, Mason WP, Fulton D, Kavan P, Easaw J, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28(12):2051–2057. doi: 10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 122.Lim Fat MJ, Maurice C, Maganti M, Mason WP. Bevacizumab in recurrent high-grade gliomas: a Canadian retrospective study. Can J Neurol Sci. 2018;45(1):56–61. doi: 10.1017/cjn.2017.248. [DOI] [PubMed] [Google Scholar]
- 123.Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Taal W, Oosterkamp HM, Walenkamp AME, Dubbink HJ, Beerepoot LV, Hanse MCJ, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. doi: 10.1016/S1470-2045(14)70314-6. [DOI] [PubMed] [Google Scholar]
- 125.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 127.Raizer JJ, Grimm S, Chamberlain MC, Nicholas MK, Chandler JP, Muro K, et al. A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer. 2010;116(22):5297–5305. doi: 10.1002/cncr.25462. [DOI] [PubMed] [Google Scholar]
- 128.Wong ET, Gautam S, Malchow C, Lun M, Pan E, Brem S. Bevacizumab for recurrent glioblastoma multiforme: a meta-analysis. J Natl Compr Canc Netw. 2011;9(4):403–407. doi: 10.6004/jnccn.2011.0037. [DOI] [PubMed] [Google Scholar]
- 129.Sahm F, Capper D, Jeibmann A, Habel A, Paulus W, Troost D, et al. Addressing diffuse glioma as a systemic brain disease with single-cell analysis. Arch Neurol. 2012;69(4):523–526. doi: 10.1001/archneurol.2011.2910. [DOI] [PubMed] [Google Scholar]
- 130.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 131.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. doi: 10.3171/2011.2.JNS10998. [DOI] [PubMed] [Google Scholar]
- 132.Grabowski MM, Recinos PF, Nowacki AS, Schroeder JL, Angelov L, Barnett GH, et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121(5):1115–1123. doi: 10.3171/2014.7.JNS132449. [DOI] [PubMed] [Google Scholar]
- 133.Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1460–1469. doi: 10.1001/jamaoncol.2016.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sherriff J, Tamangani J, Senthil L, Cruickshank G, Spooner D, Jones B, et al. Patterns of relapse in glioblastoma multiforme following concomitant chemoradiotherapy with temozolomide. Br J Radiol. 2013;86(1022):20120414. doi: 10.1259/bjr.20120414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE. Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol. 2014;32(8):774–782. doi: 10.1200/JCO.2013.51.8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg. 2016;124(4):977–988. doi: 10.3171/2015.5.JNS142087. [DOI] [PubMed] [Google Scholar]
- 137.van Solinge TS, Nieland L, Chiocca EA, Broekman MLD. Advances in local therapy for glioblastoma - taking the fight to the tumour. Nat Rev Neurol. 2022;18(4):221–236. doi: 10.1038/s41582-022-00621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, Goetz C, et al. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery. 1998;42(3):518–526. doi: 10.1097/00006123-199803000-00017. [DOI] [PubMed] [Google Scholar]
- 139.Okuda T, Yoshioka H, Kato A. Fluorescence-guided surgery for glioblastoma multiforme using high-dose fluorescein sodium with excitation and barrier filters. J Clin Neurosci. 2012;19(12):1719–1722. doi: 10.1016/j.jocn.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 140.Hadjipanayis CG, Widhalm G, Stummer W. What is the surgical benefit of utilizing 5-aminolevulinic acid for fluorescence-guided surgery of malignant gliomas? Neurosurgery. 2015;77(5):663–673. doi: 10.1227/NEU.0000000000000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 142.Diez Valle R, Tejada Solis S, Idoate Gastearena MA, de Garcia Eulate R, Dominguez Echavarri P, Aristu MJ. Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: volumetric analysis of extent of resection in single-center experience. J Neuro-Oncol. 2011;102(1):105–113. doi: 10.1007/s11060-010-0296-4. [DOI] [PubMed] [Google Scholar]
- 143.Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 2011;12(11):997–1003. doi: 10.1016/S1470-2045(11)70196-6. [DOI] [PubMed] [Google Scholar]
- 144.Roder C, Bisdas S, Ebner FH, Honegger J, Naegele T, Ernemann U, et al. Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: high-field iMRI versus conventional and 5-ALA-assisted surgery. Eur J Surg Oncol. 2014;40(3):297–304. doi: 10.1016/j.ejso.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 145.Coburger J, Engelke J, Scheuerle A, Thal DR, Hlavac M, Wirtz CR, et al. Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: a prospective study based on histopathological assessment. Neurosurg Focus. 2014;36(2):E3. doi: 10.3171/2013.11.FOCUS13463. [DOI] [PubMed] [Google Scholar]
- 146.Eyüpoglu IY, Hore N, Merkel A, Buslei R, Buchfelder M, Savaskan N. Supra-complete surgery via dual intraoperative visualization approach (DiVA) prolongs patient survival in glioblastoma. Oncotarget. 2016;7(18):25755–25768. doi: 10.18632/oncotarget.8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Quick-Weller J, Lescher S, Forster MT, Konczalla J, Seifert V, Senft C. Combination of 5-ALA and iMRI in re-resection of recurrent glioblastoma. Br J Neurosurg. 2016;30(3):313–317. doi: 10.3109/02688697.2015.1119242. [DOI] [PubMed] [Google Scholar]
- 148.Mahboob S, McPhillips R, Qiu Z, Jiang Y, Meggs C, Schiavone G, et al. Intraoperative ultrasound-guided resection of gliomas: a meta-analysis and review of the literature. World Neurosurg. 2016;92:255–263. doi: 10.1016/j.wneu.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 149.Schucht P, Beck J, Abu-Isa J, Andereggen L, Murek M, Seidel K, et al. Gross total resection rates in contemporary glioblastoma surgery: results of an institutional protocol combining 5-aminolevulinic acid intraoperative fluorescence imaging and brain mapping. Neurosurgery. 2012;71(5):927–936. doi: 10.1227/NEU.0b013e31826d1e6b. [DOI] [PubMed] [Google Scholar]
- 150.De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30(20):2559–2565. doi: 10.1200/JCO.2011.38.4818. [DOI] [PubMed] [Google Scholar]
- 151.Eseonu CI, Rincon-Torroella J, ReFaey K, Lee YM, Nangiana J, Vivas-Buitrago T, et al. Awake craniotomy vs craniotomy under general anesthesia for perirolandic gliomas: evaluating perioperative complications and extent of resection. Neurosurgery. 2017;81(3):481–489. doi: 10.1093/neuros/nyx023. [DOI] [PubMed] [Google Scholar]
- 152.Lau D, Hervey-Jumper SL, Han SJ, Berger MS. Intraoperative perception and estimates on extent of resection during awake glioma surgery: overcoming the learning curve. J Neurosurg. 2018;128(5):1410–1418. doi: 10.3171/2017.1.JNS161811. [DOI] [PubMed] [Google Scholar]
- 153.Stewart J, Sahgal A, Lee Y, Soliman H, Tseng CL, Detsky J, et al. Quantitating interfraction target dynamics during concurrent chemoradiation for glioblastoma: a prospective serial imaging study. Int J Radiat Oncol Biol Phys. 2021;109(3):736–746. doi: 10.1016/j.ijrobp.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 154.Kim TG, Lim DH. Interfractional variation of radiation target and adaptive radiotherapy for totally resected glioblastoma. J Korean Med Sci. 2013;28(8):1233–1237. doi: 10.3346/jkms.2013.28.8.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Manon R, Hui S, Chinnaiyan P, Suh J, Chang E, Timmerman R, et al. The impact of mid-treatment MRI on defining boost volumes in the radiation treatment of glioblastoma multiforme. Technol Cancer Res Treat. 2004;3(3):303–307. doi: 10.1177/153303460400300308. [DOI] [PubMed] [Google Scholar]
- 156.Tsien C, Gomez-Hassan D, Ten Haken RK, Tatro D, Junck L, Chenevert TL, et al. Evaluating changes in tumor volume using magnetic resonance imaging during the course of radiotherapy treatment of high-grade gliomas: Implications for conformal dose-escalation studies. Int J Radiat Oncol Biol Phys. 2005;62(2):328–332. doi: 10.1016/j.ijrobp.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 157.Hamstra DA, Galbán CJ, Meyer CR, Johnson TD, Sundgren PC, Tsien C, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol. 2008;26(20):3387–3394. doi: 10.1200/JCO.2007.15.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mehta S, Gajjar SR, Padgett KR, Asher D, Stoyanova R, Ford JC, et al. Daily tracking of glioblastoma resection cavity, cerebral edema, and tumor volume with MRI-guided radiation therapy. Cureus. 2018;10(3):e2346. doi: 10.7759/cureus.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mohan R, Grosshans D. Proton therapy - present and future. Adv Drug Deliv Rev. 2017;15(109):26–44. doi: 10.1016/j.addr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brown PD, Chung C, Liu DD, McAvoy S, Grosshans D, Al Feghali K, et al. A prospective phase II randomized trial of proton radiotherapy vs intensity-modulated radiotherapy for patients with newly diagnosed glioblastoma. Neuro Oncol. 2021;23(8):1337–1347. doi: 10.1093/neuonc/noab040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073–1113. doi: 10.1093/neuonc/noaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ruben MD, Wu G, Smith DF, Schmidt RE, Francey LJ, Lee YY, et al. A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. Sci Transl Med. 2018;10(458):eaat8806. doi: 10.1126/scitranslmed.aat8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Khapre RV, Samsa WE, Kondratov RV. Circadian regulation of cell cycle: molecular connections between aging and the circadian clock. Ann Med. 2010;42(6):404–415. doi: 10.3109/07853890.2010.499134. [DOI] [PubMed] [Google Scholar]
- 164.Dong D, Yang D, Lin L, Wang S, Wu B. Circadian rhythm in pharmacokinetics and its relevance to chronotherapy. Biochem Pharmacol. 2020;178:114045. doi: 10.1016/j.bcp.2020.114045. [DOI] [PubMed] [Google Scholar]
- 165.Yang Y, Adebali O, Wu G, Selby CP, Chiou YY, Rashid N, et al. Cisplatin-DNA adduct repair of transcribed genes is controlled by two circadian programs in mouse tissues. Proc Natl Acad Sci U S A. 2018;115(21):E4777–E4785. doi: 10.1073/pnas.1804493115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Innominato PF, Giacchetti S, Moreau T, Smaaland R, Focan C, Bjarnason GA, et al. Prediction of survival by neutropenia according to delivery schedule of oxaliplatin-5-Fluorouracil-leucovorin for metastatic colorectal cancer in a randomized international trial (EORTC 05963) Chronobiol Int. 2011;28(7):586–600. doi: 10.3109/07420528.2011.597532. [DOI] [PubMed] [Google Scholar]
- 167.Lin HH, Farkas ME. Altered circadian rhythms and breast cancer: from the human to the molecular level. Front Endocrinol (Lausanne) 2018;9:219. doi: 10.3389/fendo.2018.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rivard GE, Infante-Rivard C, Dresse MF, Leclerc JM, Champagne J. Circadian time-dependent response of childhood lymphoblastic leukemia to chemotherapy: a long-term follow-up study of survival. Chronobiol Int. 1993;10(3):201–204. doi: 10.3109/07420529309073888. [DOI] [PubMed] [Google Scholar]
- 169.Marchenay C, Cellarier E, Lévi F, Rolhion C, Kwiatkowski F, Claustrat B, et al. Circadian variation in O6-alkylguanine-DNA alkyltransferase activity in circulating blood mononuclear cells of healthy human subjects. Int J Cancer. 2001;91(1):60–66. doi: 10.1002/1097-0215(20010101)91:1<60::AID-IJC1010>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 170.Martineau-Pivoteau N, Cussac-Buchdahl C, Chollet P, Rolhion C, Debiton E, Rapp M, et al. Circadian variation in O6-methylguanine-DNA methyltransferase activity in mouse liver. Anticancer Drugs. 1996;7(6):703–709. doi: 10.1097/00001813-199608000-00012. [DOI] [PubMed] [Google Scholar]
- 171.Damato AR, Luo J, Katumba RGN, Talcott GR, Rubin JB, Herzog ED, et al. Temozolomide chronotherapy in patients with glioblastoma: a retrospective single-institute study. Neurooncol Adv. 2021;3(1):vdab041. doi: 10.1093/noajnl/vdab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Čermák V, Dostál V, Jelínek M, Libusová L, Kovář J, Rösel D, et al. Microtubule-targeting agents and their impact on cancer treatment. Eur J Cell Biol. 2020;99(4):151075. doi: 10.1016/j.ejcb.2020.151075. [DOI] [PubMed] [Google Scholar]
- 173.Charest A. Optimizing an effective combination of the new microtubule targeting agent Lisavanbulin with standard of care therapy for glioblastoma in patient-derived xenograft pre-clinical models. Neuro Oncol. 2021;noab278. [DOI] [PMC free article] [PubMed]
- 174.Prota AE, Danel F, Bachmann F, Bargsten K, Buey RM, Pohlmann J, et al. The novel microtubule-destabilizing drug BAL27862 binds to the colchicine site of tubulin with distinct effects on microtubule organization. J Mol Biol. 2014;426(8):1848–1860. doi: 10.1016/j.jmb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 175.Bergès R, Tchoghandjian A, Honoré S, Estève MA, Figarella-Branger D, Bachmann F, et al. The novel tubulin-binding checkpoint activator BAL101553 inhibits EB1-dependent migration and invasion and promotes differentiation of glioblastoma stem-like cells. Mol Cancer Ther. 2016;15(11):2740–2749. doi: 10.1158/1535-7163.MCT-16-0252. [DOI] [PubMed] [Google Scholar]
- 176.Burgenske DM, Talele S, Pokorny JL, Mladek AC, Bakken KK, Carlson BL, et al. Preclinical modeling in GBM PDX xenografts to guide clinical development of lisavanbulin - a novel tumor checkpoint controller targeting microtubules. Neuro Oncol. 2021;noab162. [DOI] [PMC free article] [PubMed]
- 177.Joerger M, Stathis A, Metaxas Y, Hess D, Mantiero M, Mark M, et al. A Phase 1 study of BAL101553, a novel tumor checkpoint controller targeting microtubules, administered as 48-h infusion in adult patients with advanced solid tumors. Invest New Drugs. 2020;38(4):1067–1076. doi: 10.1007/s10637-019-00850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Trivedi RN, Almeida KH, Fornsaglio JL, Schamus S, Sobol RW. The role of base excision repair in the sensitivity and resistance to temozolomide-mediated cell death. Cancer Res. 2005;65(14):6394–6400. doi: 10.1158/0008-5472.CAN-05-0715. [DOI] [PubMed] [Google Scholar]
- 179.Zhang J, Stevens MFG, Bradshaw TD. Temozolomide: mechanisms of action, repair and resistance. Curr Mol Pharmacol. 2012;5(1):102–114. doi: 10.2174/1874467211205010102. [DOI] [PubMed] [Google Scholar]
- 180.Levin VA, Freeman-Dove MA, Maroten CE. Dianhydrogalactitol (NSC-132313): pharmacokinetics in normal and tumor-bearing rat brain and antitumor activity against three intracerebral rodent tumors. J Natl Cancer Inst. 1976;56(3):535–539. doi: 10.1093/jnci/56.3.535. [DOI] [PubMed] [Google Scholar]
- 181.Jiménez-Alcázar M, Curiel-García Á, Nogales P, Perales-Patón J, Schuhmacher AJ, Galán-Ganga M, et al. Dianhydrogalactitol overcomes multiple temozolomide resistance mechanisms in glioblastoma. Mol Cancer Ther. 2021;20(6):1029–1038. doi: 10.1158/1535-7163.MCT-20-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Németh L, Institóris L, Somfai S, Gál F, Pályi I, Sugár J, et al. Pharmacologic and antitumor effects of 1,2:5,6-dianhydrogalactitol (NSC-132313) Cancer Chemother Rep. 1972;56(5):593–602. [PubMed] [Google Scholar]
- 183.Institóris E, Tamás J. Alkylation by 1,2:5,6-dianhydrogalactitol of deoxyribonucleic acid and guanosine. Biochem J. 1980;185(3):659–666. doi: 10.1042/bj1850659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhai B, Steinø A, Bacha J, Brown D, Daugaard M. Dianhydrogalactitol induces replication-dependent DNA damage in tumor cells preferentially resolved by homologous recombination. Cell Death Dis. 2018;9(10):1016. doi: 10.1038/s41419-018-1069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen ZP, Guo C, Yang QY, Li JW, Wu SX, Bacha J, et al. ACTR-06. Clinical trial of VAL-083 in newly diagnosed MGMT-unmethylated GBM: half-way report. Neuro Oncol. 2019;21(Suppl 6):vi13–vi13. doi: 10.1093/neuonc/noz175.050. [DOI] [Google Scholar]
- 186.Bacha JA, Steino A, Schwartz R, Langlands J, Kanekal S, Lopez LM, et al. P09.57 Clinical trials of VAL-083 in patients with chemoresistant glioblastoma. Neuro Oncol. 2017;19(Suppl 3):iii83–iii83. doi: 10.1093/neuonc/nox036.312. [DOI] [Google Scholar]
- 187.Meng Y, Pople CB, Budiansky D, Li D, Suppiah S, Lim-Fat MJ, et al. Current state of therapeutic focused ultrasound applications in neuro-oncology. J Neurooncol. 2022;156(1):49–59. doi: 10.1007/s11060-021-03861-0. [DOI] [PubMed] [Google Scholar]
- 188.Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, et al. Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci Rep. 2019;9(1):321. doi: 10.1038/s41598-018-36340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Idbaih A, Canney M, Belin L, Desseaux C, Vignot A, Bouchoux G, et al. Safety and feasibility of repeated and transient blood-brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin Cancer Res. 2019;25(13):3793–3801. doi: 10.1158/1078-0432.CCR-18-3643. [DOI] [PubMed] [Google Scholar]
- 190.Chen KT, Chai WY, Lin YJ, Lin CJ, Chen PY, Tsai HC, et al. Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumors. Sci Adv. 2021;7(6):eabd0772. doi: 10.1126/sciadv.abd0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C, et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med. 2016;8(343):343re2. doi: 10.1126/scitranslmed.aaf6086. [DOI] [PubMed] [Google Scholar]
- 192.Park SH, Kim MJ, Jung HH, Chang WS, Choi HS, Rachmilevitch I, et al. Safety and feasibility of multiple blood-brain barrier disruptions for the treatment of glioblastoma in patients undergoing standard adjuvant chemotherapy. J Neurosurg. 2020;3:1–9. doi: 10.3171/2019.10.JNS192206. [DOI] [PubMed] [Google Scholar]
- 193.Park SH, Kim MJ, Jung HH, Chang WS, Choi HS, Rachmilevitch I, et al. One-year outcome of multiple blood-brain barrier disruptions with temozolomide for the treatment of glioblastoma. Front Oncol. 2020;10:1663. doi: 10.3389/fonc.2020.01663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Horbinski C, Ligon KL, Brastianos P, Huse JT, Venere M, Chang S, et al. The medical necessity of advanced molecular testing in the diagnosis and treatment of brain tumor patients. Neuro Oncol. 2019;21(12):1498–1508. doi: 10.1093/neuonc/noz119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sahm F, Schrimpf D, Jones DTW, Meyer J, Kratz A, Reuss D, et al. Next-generation sequencing in routine brain tumor diagnostics enables an integrated diagnosis and identifies actionable targets. Acta Neuropathol. 2016;131(6):903–910. doi: 10.1007/s00401-015-1519-8. [DOI] [PubMed] [Google Scholar]
- 196.Barthel FP, Johnson KC, Varn FS, Moskalik AD, Tanner G, Kocakavuk E, et al. Longitudinal molecular trajectories of diffuse glioma in adults. Nature. 2019;576(7785):112–120. doi: 10.1038/s41586-019-1775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Verhaak RGW, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.van den Bent MJ, Brandes AA, Rampling R, Kouwenhoven MCM, Kros JM, Carpentier AF, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27(8):1268–1274. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. doi: 10.1016/S1470-2045(17)30517-X. [DOI] [PubMed] [Google Scholar]
- 201.Reardon DA, Lassman AB, van den Bent M, Kumthekar P, Merrell R, Scott AM, et al. Efficacy and safety results of ABT-414 in combination with radiation and temozolomide in newly diagnosed glioblastoma. Neuro Oncol. 2017;19(7):965–975. doi: 10.1093/neuonc/now257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.van den Bent M, Gan HK, Lassman AB, Kumthekar P, Merrell R, Butowski N, et al. Efficacy of depatuxizumab mafodotin (ABT-414) monotherapy in patients with EGFR-amplified, recurrent glioblastoma: results from a multi-center, international study. Cancer Chemother Pharmacol. 2017;80(6):1209–1217. doi: 10.1007/s00280-017-3451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Van Den Bent M, Eoli M, Sepulveda JM, Smits M, Walenkamp A, Frenel JS, et al. INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFR amplified glioblastoma. Neuro Oncol. 2020;22(5):684–693. doi: 10.1093/neuonc/noz222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399):eaaa0984. doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bagley SJ, Desai AS, Linette GP, June CH, O’Rourke DM. CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges. Neuro Oncol. 2018;20(11):1429–1438. doi: 10.1093/neuonc/noy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Aquilanti E, Kageler L, Wen PY, Meyerson M. Telomerase as a therapeutic target in glioblastoma. Neuro Oncol. 2021;23(12):2004–2013. doi: 10.1093/neuonc/noab203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li X, Qian X, Wang B, Xia Y, Zheng Y, Du L, et al. Programmable base editing of mutated TERT promoter inhibits brain tumour growth. Nat Cell Biol. 2020;22(3):282–288. doi: 10.1038/s41556-020-0471-6. [DOI] [PubMed] [Google Scholar]
- 209.Mender I, Gryaznov S, Dikmen ZG, Wright WE, Shay JW. Induction of telomere dysfunction mediated by the telomerase substrate precursor 6-thio-2’-deoxyguanosine. Cancer Discov. 2015;5(1):82–95. doi: 10.1158/2159-8290.CD-14-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Takahashi M, Miki S, Fujimoto K, Fukuoka K, Matsushita Y, Maida Y, et al. Eribulin penetrates brain tumor tissue and prolongs survival of mice harboring intracerebral glioblastoma xenografts. Cancer Sci. 2019;110(7):2247–2257. doi: 10.1111/cas.14067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Singh D, Chan JM, Zoppoli P, Niola F, Sullivan R, Castano A, et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science. 2012;337(6099):1231–1235. doi: 10.1126/science.1220834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Di Stefano AL, Fucci A, Frattini V, Labussiere M, Mokhtari K, Zoppoli P, et al. Detection, characterization, and inhibition of FGFR-TACC fusions in IDH wild-type glioma. Clin Cancer Res. 2015;21(14):3307–3317. doi: 10.1158/1078-0432.CCR-14-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kaley T, Touat M, Subbiah V, Hollebecque A, Rodon J, Lockhart AC, et al. BRAF Inhibition in BRAFV600-mutant gliomas: results from the VE-BASKET study. J Clin Oncol. 2018;36(35):3477–3484. doi: 10.1200/JCO.2018.78.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gatalica Z, Xiu J, Swensen J, Vranic S. Molecular characterization of cancers with NTRK gene fusions. Mod Pathol. 2019;32(1):147–153. doi: 10.1038/s41379-018-0118-3. [DOI] [PubMed] [Google Scholar]
- 215.Torre M, Vasudevaraja V, Serrano J, DeLorenzo M, Malinowski S, Blandin AF, et al. Molecular and clinicopathologic features of gliomas harboring NTRK fusions. Acta Neuropathol Commun. 2020;8(1):107. doi: 10.1186/s40478-020-00980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.International Cancer Genome Consortium PedBrain Tumor Project Recurrent MET fusion genes represent a drug target in pediatric glioblastoma. Nat Med. 2016;22(11):1314–1320. doi: 10.1038/nm.4204. [DOI] [PubMed] [Google Scholar]
- 217.Chang SM, Wen P, Cloughesy T, Greenberg H, Schiff D, Conrad C, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23(4):357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 218.Wen PY, Touat M, Alexander BM, Mellinghoff IK, Ramkissoon S, McCluskey CS, et al. Buparlisib in patients with recurrent glioblastoma harboring phosphatidylinositol 3-kinase pathway activation: an open-label, multicenter, multi-arm, phase II trial. J Clin Oncol. 2019;37(9):741–750. doi: 10.1200/JCO.18.01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Taylor JW, Parikh M, Phillips JJ, James CD, Molinaro AM, Butowski NA, et al. Phase-2 trial of palbociclib in adult patients with recurrent RB1-positive glioblastoma. J Neurooncol. 2018;140(2):477–483. doi: 10.1007/s11060-018-2977-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wick W, Dettmer S, Berberich A, Kessler T, Karapanagiotou-Schenkel I, Wick A, et al. N2M2 (NOA-20) phase I/II trial of molecularly matched targeted therapies plus radiotherapy in patients with newly diagnosed non-MGMT hypermethylated glioblastoma. Neuro Oncol. 2019;21(1):95–105. doi: 10.1093/neuonc/noy161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Alexander BM, Trippa L, Gaffey S, Arrillaga-Romany IC, Lee EQ, Rinne ML, et al. Individualized Screening Trial of Innovative Glioblastoma Therapy (INSIGhT): a Bayesian adaptive platform trial to develop precision medicines for patients with glioblastoma. JCO Precis Oncol. 2019;3:1–13. doi: 10.1200/PO.18.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Alexander BM, Ba S, Berger MS, Berry DA, Cavenee WK, Chang SM, et al. Adaptive global innovative learning environment for glioblastoma: GBM AGILE. Clin Cancer Res. 2018;24(4):737–743. doi: 10.1158/1078-0432.CCR-17-0764. [DOI] [PubMed] [Google Scholar]
- 223.Touat M, Idbaih A, Sanson M, Ligon KL. Glioblastoma targeted therapy: updated approaches from recent biological insights. Ann Oncol. 2017;28(7):1457–1472. doi: 10.1093/annonc/mdx106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nduom EK, Weller M, Heimberger AB. Immunosuppressive mechanisms in glioblastoma. Neuro Oncol. 2015;17(Suppl 7):vii9–vii14. doi: 10.1093/neuonc/nov151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Medikonda R, Dunn G, Rahman M, Fecci P, Lim M. A review of glioblastoma immunotherapy. J Neurooncol. 2021;151(1):41–53. doi: 10.1007/s11060-020-03448-1. [DOI] [PubMed] [Google Scholar]
- 226.Bristol-Myers Squibb. Bristol-Myers Squibb Announces Phase 3 CheckMate -498 Study Did Not Meet Primary Endpoint of Overall Survival with Opdivo (nivolumab) Plus Radiation in Patients with Newly Diagnosed MGMT-Unmethylated Glioblastoma Multiforme. 2019. Available from: https://news.bms.com/news/corporate-financial/2019/Bristol-Myers-Squibb-Announces-Phase-3-CheckMate--498-Study-Did-Not-Meet-Primary-Endpoint-of-Overall-Survival-with-Opdivo-nivolumab-Plus-Radiation-in-Patients-with-Newly-Diagnosed-MGMT-Unmethylated-Glioblastoma-Multiforme/default.aspx. 10.1093/neuonc/noac099.
- 227.Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. doi: 10.1001/jamaoncol.2020.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. doi: 10.1038/s41591-018-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bouffet E, Larouche V, Campbell BB, Merico D, de Borja R, Aronson M, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016;34(19):2206–2211. doi: 10.1200/JCO.2016.66.6552. [DOI] [PubMed] [Google Scholar]
- 230.Johanns TM, Miller CA, Dorward IG, Tsien C, Chang E, Perry A, et al. Immunogenomics of hypermutated glioblastoma: a patient with germline POLE deficiency treated with checkpoint blockade immunotherapy. Cancer Discov. 2016;6(11):1230–1236. doi: 10.1158/2159-8290.CD-16-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Das A, Sudhaman S, Morgenstern D, Coblentz A, Chung J, Stone SC, et al. Genomic predictors of response to PD-1 inhibition in children with germline DNA replication repair deficiency. Nat Med. 2022;28(1):125–135. doi: 10.1038/s41591-021-01581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanović S, Gouttefangeas C, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 234.Lawler SE, Speranza MC, Cho CF, Chiocca EA. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017;3(6):841–849. doi: 10.1001/jamaoncol.2016.2064. [DOI] [PubMed] [Google Scholar]
- 235.Lawler SE, Chiocca EA. Oncolytic virus-mediated immunotherapy: a combinatorial approach for cancer treatment. J Clin Oncol. 2015;33(25):2812–2814. doi: 10.1200/JCO.2015.62.5244. [DOI] [PubMed] [Google Scholar]
- 236.Desjardins A, Gromeier M, Herndon JE, Beaubier N, Bolognesi DP, Friedman AH, et al. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med. 2018;379(2):150–161. doi: 10.1056/NEJMoa1716435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wheeler LA, Manzanera AG, Bell SD, Cavaliere R, McGregor JM, Grecula JC, et al. Phase II multicenter study of gene-mediated cytotoxic immunotherapy as adjuvant to surgical resection for newly diagnosed malignant glioma. Neuro Oncol. 2016;18(8):1137–1145. doi: 10.1093/neuonc/now002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.PR Newswire. Tocagen reports results of Toca 5 phase 3 trial in recurrent brain cancer. 2019. Available from: https://www.bloomberg.com/press-releases/2019-09-12/tocagen-reports-results-of-toca-5-phase-3-trial-in-recurrent-brain-cancer. PMID: 33119048 PMCID: PMC7596685 10.1001/jamaoncol.2020.3161.
- 239.Yoo HJ, Harapan BN. Chimeric antigen receptor (CAR) immunotherapy: basic principles, current advances, and future prospects in neuro-oncology. Immunol Res. 2021;69(6):471–486. doi: 10.1007/s12026-021-09236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol. 2018;15(7):422–442. doi: 10.1038/s41571-018-0003-5. [DOI] [PubMed] [Google Scholar]
- 241.Weller M, Gorlia T, Cairncross JG, van den Bent MJ, Mason W, Belanger K, et al. Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology. 2011;77(12):1156–1164. doi: 10.1212/WNL.0b013e31822f02e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Happold C, Gorlia T, Chinot O, Gilbert MR, Nabors LB, Wick W, et al. Does valproic acid or levetiracetam improve survival in glioblastoma? A pooled analysis of prospective clinical trials in newly diagnosed glioblastoma. J Clin Oncol. 2016;34(7):731–739. doi: 10.1200/JCO.2015.63.6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Krauze AV, Myrehaug SD, Chang MG, Holdford DJ, Smith S, Shih J, et al. A phase 2 study of concurrent radiation therapy, temozolomide, and the histone deacetylase inhibitor valproic acid for patients with glioblastoma. Int J Radiat Oncol Biol Phys. 2015;92(5):986–992. doi: 10.1016/j.ijrobp.2015.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Venkatesh HS, Morishita W, Geraghty AC, Silverbush D, Gillespie SM, Arzt M, et al. Electrical and synaptic integration of glioma into neural circuits. Nature. 2019;573(7775):539–545. doi: 10.1038/s41586-019-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Venkataramani V, Tanev DI, Strahle C, Studier-Fischer A, Fankhauser L, Kessler T, et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature. 2019;573(7775):532–538. doi: 10.1038/s41586-019-1564-x. [DOI] [PubMed] [Google Scholar]
- 246.Lange F, Weßlau K, Porath K, Hörnschemeyer J, Bergner C, Krause BJ, et al. AMPA receptor antagonist perampanel affects glioblastoma cell growth and glutamate release in vitro. PLoS ONE. 2019;14(2):e0211644. doi: 10.1371/journal.pone.0211644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Grossman SA, Ye X, Chamberlain M, Mikkelsen T, Batchelor T, Desideri S, et al. Talampanel with standard radiation and temozolomide in patients with newly diagnosed glioblastoma: a multicenter phase II trial. J Clin Oncol. 2009;27(25):4155–4161. doi: 10.1200/JCO.2008.21.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Iwamoto FM, Kreisl TN, Kim L, Duic JP, Butman JA, Albert PS, et al. Phase 2 trial of talampanel, a glutamate receptor inhibitor, for adults with recurrent malignant gliomas. Cancer. 2010;116(7):1776–1782. doi: 10.1002/cncr.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Bi J, Khan A, Tang J, Armando AM, Wu S, Zhang W, et al. Targeting glioblastoma signaling and metabolism with a re-purposed brain-penetrant drug. Cell Rep. 2021;37(5):109957. doi: 10.1016/j.celrep.2021.109957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wolf A, Agnihotri S, Guha A. Targeting metabolic remodeling in glioblastoma multiforme. Oncotarget. 2010;1(7):552–562. doi: 10.18632/oncotarget.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Seliger C, Genbrugge E, Gorlia T, Chinot O, Stupp R, Nabors B, et al. Use of metformin and outcome of patients with newly diagnosed glioblastoma: pooled analysis. Int J Cancer. 2020;146(3):803–809. doi: 10.1002/ijc.32337. [DOI] [PubMed] [Google Scholar]
- 252.Kast RE, Karpel-Massler G, Halatsch ME. CUSP9* treatment protocol for recurrent glioblastoma: aprepitant, artesunate, auranofin, captopril, celecoxib, disulfiram, itraconazole, ritonavir, sertraline augmenting continuous low dose temozolomide. Oncotarget. 2014;5(18):8052–8082. doi: 10.18632/oncotarget.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Skaga E, Skaga IØ, Grieg Z, Sandberg CJ, Langmoen IA, Vik-Mo EO. The efficacy of a coordinated pharmacological blockade in glioblastoma stem cells with nine repurposed drugs using the CUSP9 strategy. J Cancer Res Clin Oncol. 2019;145(6):1495–1507. doi: 10.1007/s00432-019-02920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Halatsch ME, Kast RE, Karpel-Massler G, Mayer B, Zolk O, Schmitz B, et al. A phase Ib/IIa trial of 9 repurposed drugs combined with temozolomide for the treatment of recurrent glioblastoma: CUSP9v3. Neurooncol Adv. 2021;3(1):vdab075. doi: 10.1093/noajnl/vdab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tanoli Z, Vähä-Koskela M, Aittokallio T. Artificial intelligence, machine learning, and drug repurposing in cancer. Expert Opin Drug Discov. 2021;16(9):977–989. doi: 10.1080/17460441.2021.1883585. [DOI] [PubMed] [Google Scholar]
- 256.Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071–22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100–1108. doi: 10.1016/S1470-2045(14)70379-1. [DOI] [PubMed] [Google Scholar]
- 257.Taslimi S, Ye VC, Zadeh G. Lessons learned from contemporary glioblastoma randomized clinical trials through systematic review and network meta-analysis: part 2 newly diagnosed disease. Neurooncol Adv. 2021;3(1):vdab028. doi: 10.1093/noajnl/vdab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.McBain C, Lawrie TA, Rogozińska E, Kernohan A, Robinson T, Jefferies S. Treatment options for progression or recurrence of glioblastoma: a network meta-analysis. Cochrane Database Syst Rev. 2021;5:CD013579. doi: 10.1002/14651858.CD013579.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Chukwueke UN, Hervey-Jumper S, Porter A. Disparities and inequities among patients with central nervous system tumor. Hematol Oncol Clin North Am. 2022;36(1):e1–8. doi: 10.1016/j.hoc.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 260.Lee EQ, Chukwueke UN, Hervey-Jumper SL, de Groot JF, Leone JP, Armstrong TS, et al. Barriers to accrual and enrollment in brain tumor trials. Neuro Oncol. 2019;21(9):1100–1117. doi: 10.1093/neuonc/noz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Porter AB, Chukwueke UN, Mammoser AG, Friday B, Hervey-Jumper S. Delivering equitable care to underserved neuro-oncology populations. Am Soc Clin Oncol Educ Book. 2021;41:1–9. doi: 10.1200/EDBK_320803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.