Abstract
Purpose:
Dexamethasone is commonly given during radiotherapy (RT) to manage toxicities. Our study examines if dexamethasone co-administration with RT inhibits the RT-induced anti-tumor T cell response in mouse.
Materials and Methods:
Intramuscularly implanted MC38 tumors were irradiated with 15 Gy after establishing for 7 days. Tumor bearing mice were administered dexamethasone using multiple schedules and doses. Peripheral lymphocyte reduction was monitored by complete blood count and intratumoral and tumor draining lymph node (tdLN) populations by flow cytometry. Effector phenotype and function of ex vivo stimulated tumor-infiltrating lymphocytes (TILs) and naïve splenocytes as well as in vivo TILs with or without dexamethasone were monitored by flow cytometry and ELISA.
Results:
Long course high dose, short course high dose, and short course human equivalent (HE) dose dexamethasone reduced peripheral lymphocytes yet did not inhibit survival following irradiation. Short course high dose administration decreased TIL and tdLN lymphocyte activation as well as tdLN mass but did not affect TIL frequencies or change tdLN cell population composition. Dexamethasone inhibited effector function of ex vivo stimulated naïve splenocytes and TILs, but magnitude of IFN-γ secretion was consistently higher in TILs regardless of dexamethasone dose. In vivo analysis of TILs following irradiation and HE dexamethasone treatment showed that TILs had a similar effector phenotype compared to vehicle controls.
Conclusions:
Dexamethasone reduces blood and tdLN lymphocytes. Dexamethasone also suppresses TIL activation/effector function yet does not affect survival in irradiated MC38 tumor bearing mice, which depend on RT-induced immune responses for therapy efficacy. Additional study in human subjects is warranted.
Keywords: ablative tumor irradiation, dexamethasone, corticosteroids, CD8 T cell, CD4 T cell IFN-γ, tumor microenvironment, peripheral blood
Introduction
Improvements in imaging, motion management and treatment planning have improved the therapeutic ratio of radiation therapy. However, toxicities such as inflammation, edema, nausea, and anorexia are still common during RT e.g. for primary and metastatic brain tumors. (1, 2). One method to combat RT-induced side effects is co-administration of corticosteroids, such as dexamethasone.
The precise mechanism by which dexamethasone exerts its effects are unclear, but glucocorticoid receptor binding is essential for restoring blood brain barrier function of brain capillary endothelial cells (3), thus preventing edema, and for inhibition of inflammatory gene expression, which can occur through induction of immune suppressive genes and inhibition of pro-inflammatory transcription factors (4).
However, the immune response is also essential for RT efficacy, as cells killed by RT release damage associated molecular patterns and tumor antigen which activate dendritic cells that cross-present antigen to CD8 T cells (5, 6), which then migrate to the tumor microenvironment (TME) and reduce tumor burden primarily through secretion of IFN-γ (7,8). Consequently, although dexamethasone reduces RT side effects, its immunosuppressive properties may inhibit the RT response.
Some studies have shown that glioblastoma patients treated with combined RT and chemotherapy have reduced progression free survival when receiving dexamethasone, and steroid administration is associated with worse outcomes in patients receiving different schedules of fractionated RT (2, 9). Moreover, analysis of cancer patients treated with single agent PD-L1 blocking antibodies to improve T cell response showed that co-administration of high dose prednisone negatively correlated with survival, which was most prevalent when corticosteroid administration overlapped with initiation of immune checkpoint blockade (ICB) (10). These studies suggest that dexamethasone may inhibit RT efficacy, potentially through inhibiting the RT-induced anti-tumor T cell response.
Our study utilized the MC38 murine adenocarcinoma tumor model. Previous analysis of this model has demonstrated that local ablative RT (15 Gy) causes a robust CD8 T cell and IFN-γ dependent anti-tumor immune response (8). We analyzed whether co-administration of dexamethasone can inhibit this response.
Methods
Mice and cell lines
C57BL/6 mice purchased from the Jackson Laboratory were treated in accordance with guidelines approved by the University Committee on Animal Resources. MC38 cells were obtained from Dr. Edward Brown and maintained in MAT/P medium containing 100 U/mL penicillin, 100 mg/mL streptomycin, and 2% fetal calf serum, never exceeding 10 consecutive in vitro passages. Cells used for experiments tested negative for mycoplasma.
Tumor implantation and growth monitoring
For primary tumor challenge, 6- to 8-week-old mice were injected with 1 × 105 MC38 cells intramuscularly in the hind limb and left unirradiated or given local ablative radiation (15 Gy) targeting the mouse calf (leg) on day 7 post implantation (PI) via a cesium source irradiator. Starting on day 7 post implantation and continuing every 2 days until sacrifice, tumor bearing legs were measured in two dimensions by calipers and the geometric mean (GM) was calculated to monitor tumor growth. Mice were sacrificed at the indicated time points or when GM of leg diameter was ≥ 13 mm.
Flow cytometry
Tumors were cut into 1–2 mm pieces and incubated in collagenase solution at 100 mg tissue/1 mL collagenase (2 mg/mL, Sigma) for 30 minutes at 37°C. Digested tumor was filtered through a 40 μm mesh to obtain a single cell suspension (SCS). Lymph nodes and spleens were mechanically processed with frosted microscope slides and filtered through a 40 μm mesh to obtain a SCS. Cells were incubated with Fc block (clone 24G2) for 10 minutes at 4°C, stained with antibodies in phosphate buffered saline (PBS) solution containing 1% (w/v) bovine serum albumin and 0.1% (w/v) NaN3 for 30 minutes at 4°C, and fixed with BD Cytoperm/CytoFix solution (BD Biosciences) for 20 minutes at 4°C. For intracellular staining, tumors were prepared as described previously except for the addition of GolgiPlug (BD Biosciences) during collagenase digestion and washing steps. Prior to surface staining, cells were stained with Ghost dye 510 (Tonbo Biosciences). After surface staining and fixation, cells were washed twice in permeabilization buffer (BD Biosciences) and then stained for intracellular markers for 30 min at room temperature. The following antibodies were used for analysis of cell populations: CD45 BUV395, Ly6C APC-Cy7, Ly6G BV605, CD4 APC-Cy7, NK1.1 PE-Cy7, NK1.1 PECF594, CD44 FITC, CD62L PE, CD4 BV605, CD44 BV786, CD45 PerCP-Cy5.5, CD8 PE-Cy5, CD69 APC, IFN-γPE and CD19 BV510 from BD Biosciences; CD11b eFluor450, F4/80 APC, CD8a eFluor450, and PD-1 PE-Cy7 from eBioscience; CD69 APC, CD107a PE/Dazzle594, and Granzyme B Pacific Blue (Biolegend); Tim3 AF488 (R&D Systems). All samples were run on BD LSR II or BD LSR Fortessa flow cytometers (BD Biosciences) in the university’s flow cytometry core facility.
Dexamethasone administration
For long course high dose dexamethasone administration, unirradiated mice and irradiated mice were injected subcutaneously with 4 mg/kg dexamethasone or vehicle in 100 μL from day −1 to day 11 PI. Mouse mass was measured at days 10 and 12 PI to monitor toxicity. For short course high dose dexamethasone administration, mice were injected with equivalent doses in an equivalent manner to long course therapy, but injections started on day 6 and continued until day 11. For short course intermediate and human equivalent dose administration, mice were injected subcutaneously from day 6 to day 11 with 0.66 mg/kg, 0.114 mg/kg dexamethasone, or vehicle in 100 μL.
Magnetic sorting and in vitro activation of T cells
Tumors and spleens were processed to obtain SCS as described above. SCS were sorted for T cells using a T cell negative selection kit (STEMCELL) according to the manufacturer’s instructions. Following isolation, cells were plated in a 96 well round bottom plate. Stimulated cells received anti-CD3/anti-CD28 magnetic stimulation beads (GIBCO) at a 1:1 bead to cell ratio and cultured for 2 days at 37°C prior to further analysis. After 2 days, supernatants were harvested for ELISA analysis and activation beads were magnetically separated from cells prior to flow cytometry analysis.
Interferon gamma ELISA of tumor homogenates
Untreated and irradiated MC38 tumors treated with human equivalent dose dexamethasone or vehicle control were harvested at day nine post tumor implantation (two days post radiotherapy). Tumors were suspended in lysis buffer 11 solution at 400 mg/mL and snap frozen in liquid nitrogen. Snap frozen tumors were thawed on ice and homogenized for 30 seconds on ice using a Bio-Gen PRO200 Homogenizer (PRO Scientific), centrifuged to remove cellular debris, and supernatants were used in bicinchoninic acid assays (Thermo Scientific) and IFN-γ ELISAs (Invitrogen) according to the manufacturers’ instructions. Intratumoral IFN-γ concentration was calculated by dividing pg IFN-γ as determined by ELISA by mg total protein as determined by BCA. All BCA and ELISA sample plates were read on a Gen5 plate reader (BioTek).
Complete blood count analysis
MC38 tumor bearing mice were bled (max 250 μL) via tail vein into microtainer tubes (BD Biosciences) containing EDTA on days 5 and/or day 10 PI. Samples were run on a HemaTrue veterinary hematology analyzer (HESKA).
Flow cytometry and statistical analysis
All flow cytometry data were analyzed using Flowjo (BD Biosciences) and all statistical analyses were performed with GraphPad Prism (Graphpad Software Inc.). For comparisons between three or more groups, one-way ANOVAs with Bonferonni multiple comparisons tests were used. Survival curves were compared using Log-rank tests and corrected for multiple comparisons using the Bonferroni method. Error bars indicate standard deviation. (* = p<0.05, ** = p<0.01, *** = p<0.001, **** = p<0.00001).
Results
Long course high dose dexamethasone does not inhibit the efficacy of RT
To determine if dexamethasone inhibits the RT-induced anti-tumor immune response, we first examined the effects of long-term dexamethasone in irradiated tumor bearing mice. When clinically administered, dexamethasone is typically administered twice daily in 4 mg doses (1). In contrast, most murine studies use daily doses ranging from 1–10 mg/kg delivered intraperitoneally or orally. Importantly, previous work in murine models has demonstrated that subcutaneous administration is more effective than intraperitoneal injection (11). Thus, we used 4 mg/kg doses delivered subcutaneously once daily.
Mice were administered long course high dose (LCHD) dexamethasone and irradiated as shown in Fig.1A. Tail vein blood was collected prior to and post RT on days 5 and 10, respectively, for complete blood count (CBC). Prior to RT, dexamethasone lowered lymphocyte numbers in both the unirradiated (UI) and irradiated (IR) groups, but this trend was not significant and other cell types were largely unaffected (Fig.S1A-D). Post RT, dexamethasone significantly reduced lymphocyte numbers in unirradiated and irradiated mice (Fig.1B). Monocyte numbers decreased slightly following combination therapy but were not significantly affected by monotherapies (Fig.1C). Granulocyte and platelet numbers did not show any significant differences between groups (Fig.1D, 1E). Altogether, these data suggest that LCHD dexamethasone could specifically reduce lymphocytes, but not other immune cells, in peripheral blood, similar to clinical administration.
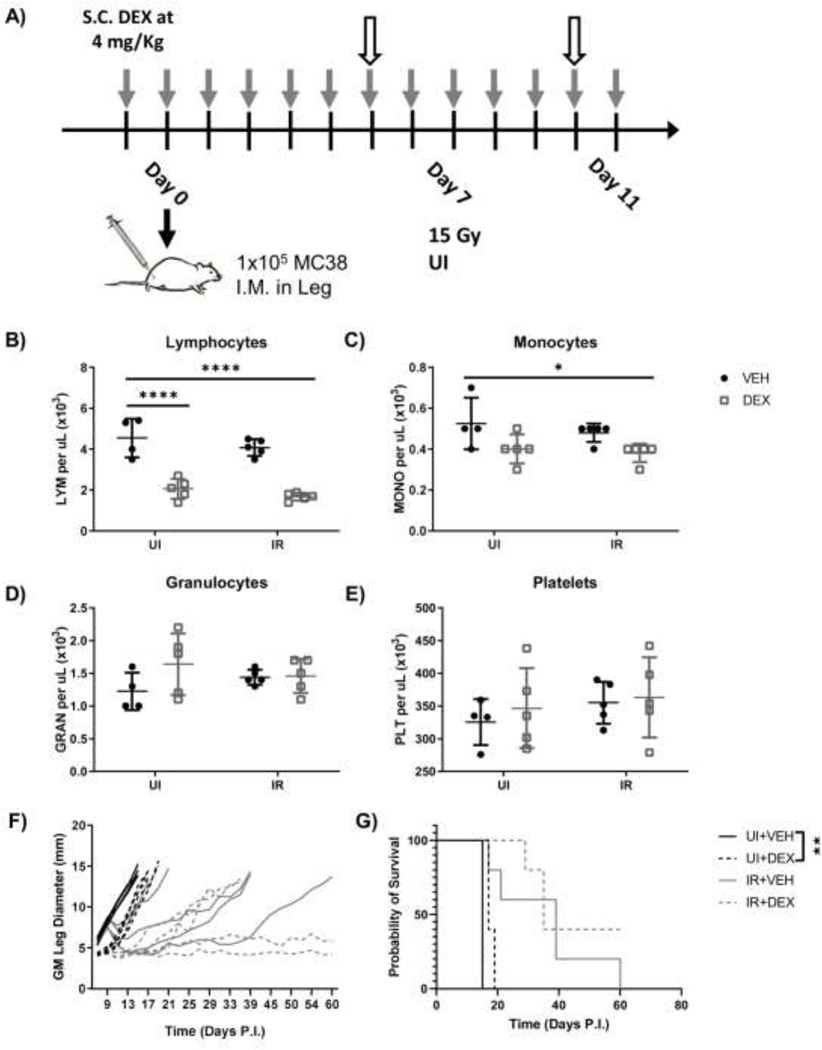
Figure 1.
LCHD dexamethasone reduces PB lymphocytes but does not affect RT response. (A) Experimental design for LCHD dexamethasone administration in unirradiated (UI) and irradiated (IR) mice bearing MC38 tumors. Gray arrows indicate timepoints for dexamethasone administration. White arrows indicate blood draws. (B-E) Mice were bled via tail vein on day 10 PI and peripheral blood lymphocyte (B), monocyte (C), granulocyte (D), and platelet (E) numbers were enumerated via CBC analysis. Tumor growth (F) and survival curves (G) for unirradiated or irradiated mice medicated with LCHD dexamethasone. (n=5 per group).
High dose and/or chronic dexamethasone administration can be toxic, thus we monitored mouse body mass as a surrogate measure for toxicity immediately after RT. On days 10 and 12, mice medicated with dexamethasone alone or combined with RT showed decreased body masses, and RT alone trended similarly (Fig.S1E, S1F). We did not observe weight gain in dexamethasone-medicated mice, as is sometimes seen in clinical patients (1). This suggests that LCHD dexamethasone displays some toxicity in mice, though it is similar in severity to RT alone and does not exacerbate toxicity when combined with RT.
Having observed expected lymphocyte reduction, we then monitored the impact of dexamethasone on tumor growth. RT decreased tumor growth and improved survival regardless of dexamethasone administration (Fig.1F, 1G). Unexpectedly, minor but significant delayed growth kinetics were observed in RT naïve dexamethasone-medicated mice compared to vehicle controls (Fig.1F). It was unclear if the delay in growth kinetics was due to dexamethasone-mediated inhibition of tumor cells or reduction in inflammation at the site of tumor implantation, the latter of which could artificially lengthen time to endpoint without a decrease in tumor cell burden. Dexamethasone did not significantly delay growth kinetics (Fig.1F) or affect survival (Fig.1G) in irradiated mice. In summary, these results indicate that LCHD dexamethasone administration does not inhibit RT response, despite reduction of peripheral blood (PB) lymphocytes.
Short course high dose dexamethasone does not significantly alter tumor growth after RT
To decouple the effects of dexamethasone on early tumor growth PI, as well as the RT-induced immune response, we altered our administration scheme as shown in Fig.2A. As before, we isolated peripheral blood to examine lymphocyte reduction by dexamethasone. On day 10, short course high dose (SCHD) dexamethasone therapy reduced PB lymphocytes (Fig.2B). Dexamethasone also reduced peripheral blood monocytes with and without RT (Fig.2C), while having no effect on granulocytes (Fig.2D). Platelet numbers were consistent between groups (Fig.2E). Interestingly, radiation alone also reduced lymphocytes, granulocytes, and monocytes (Fig.2B-D), an effect which has been reported previously (12). These results differed from LCHD dexamethasone regarding myeloid populations but confirmed that SCHD dexamethasone administration reduces PB lymphocytes.
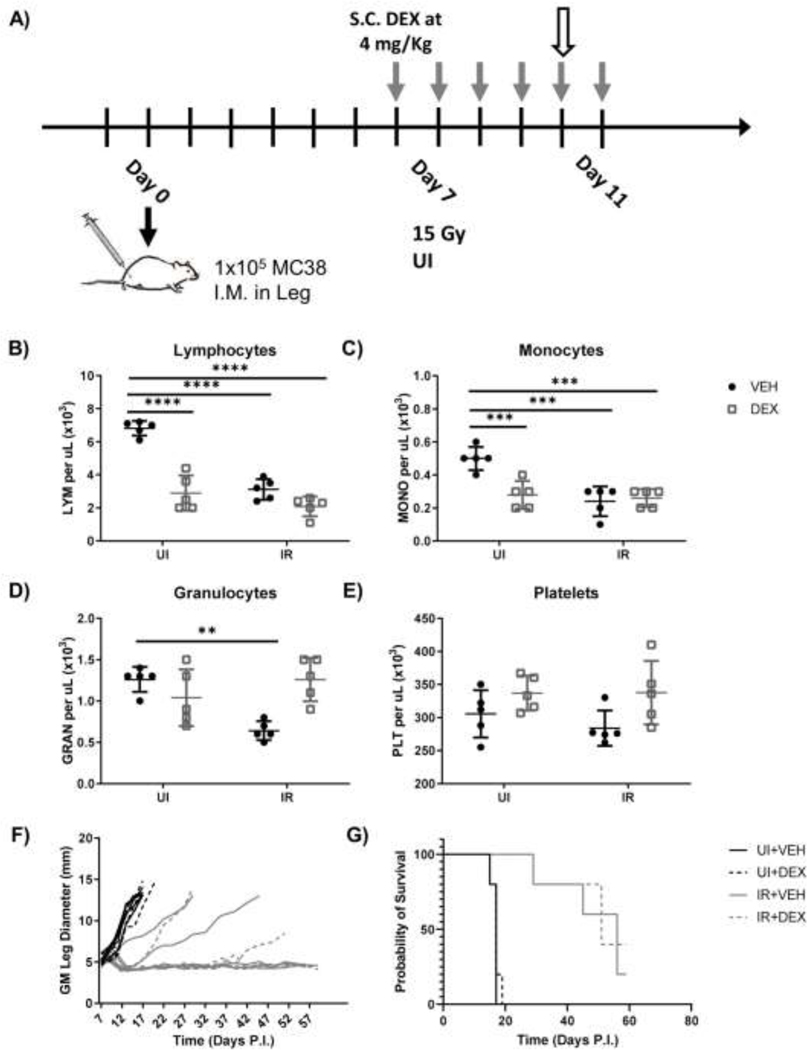
Figure 2.
SCHD dexamethasone reduces PB lymphocytes but does not affect RT response. (A) Experimental design for short course high dose dexamethasone administration in unirradiated (UI) and irradiated (IR) mice bearing MC38 tumors. Gray arrows indicate timepoints for dexamethasone administration. White arrow indicates timepoint for blood draw. (B-E) Mice were bled via tail vein on day 10 PI and peripheral blood lymphocyte (B), monocyte (C), granulocyte (D), and platelet (E) numbers were enumerated via CBC analysis. Growth (F) and survival curves (G) for unirradiated or irradiated mice medicated with SCHD dexamethasone. (n=5 per group).
Growth curve comparison between dexamethasone-medicated and vehicle control mice showed remarkable similarities, regardless of exposure to radiation (Fig.2F). Unlike LCHD dexamethasone administration, SCHD dexamethasone did not inhibit tumor growth in RT naïve mice. Furthermore, tumor growth inhibition and mouse survival were similar in irradiated mice receiving dexamethasone or vehicle (Fig.2F, 2G).
SCHD dexamethasone alters immune cell populations in the tumor and tumor draining lymph nodes
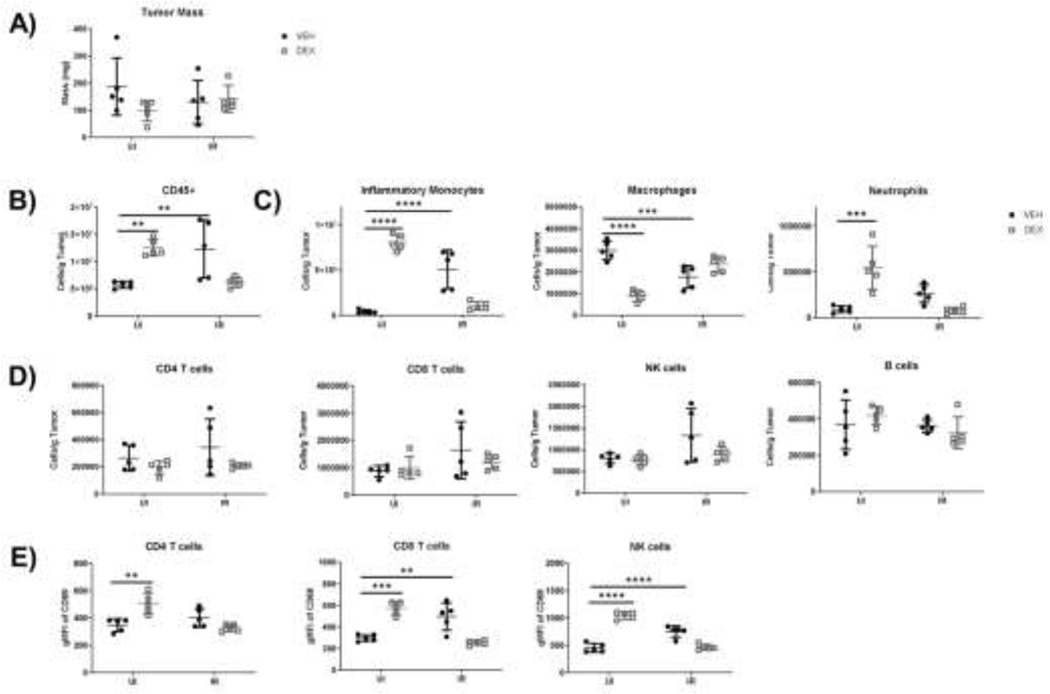
To determine if SCHD dexamethasone reduced lymphocytes in the tumor and tumor draining lymph nodes (tdLNs) as well as PB lymphocytes we analyzed these compartments at day 9 PI (2 days post RT). Neither dexamethasone, RT, nor the combination of the two resulted in different tumor masses at day 9 (Fig.3A). Although tumor mass was unaffected, intratumoral immune cell frequencies were drastically altered by dexamethasone alone and RT alone (Fig. 3B-D). Dexamethasone alone and RT alone increased recruitment of CD45+ immune cells, which was not observed when dexamethasone was combined with RT. Most of the increased immune infiltrate in both monotherapy groups resulted from increased inflammatory monocyte (IM) recruitment (Fig. 3C). Dexamethasone alossne decreased macrophage frequencies and increased neutrophil frequencies in the TME, whereas RT alone slightly decreased macrophage populations but did not affect neutrophils. Combined RT and dexamethasone tumors had myeloid populations comparable to unirradiated vehicle controls. Monotherapies and combined therapies did not alter lymphocyte frequencies in the TME (Fig.3D), but effector lymphocyte activation, measured by CD69 expression, differed based on treatment group (Fig.3E). Surprisingly, dexamethasone alone significantly upregulated CD69 in CD4 T cells, CD8 T cells, and NK cells in the TME. Radiation alone also increased CD69 expression, although only in CD8 T cells and NK cells. Importantly, combined irradiation and dexamethasone administration did not significantly increase CD69 on any lymphocyte subpopulations. Taken together, these results suggest that combined dexamethasone and irradiation decrease inflammation, decrease IM recruitment, and decrease CD69 expression on intratumoral lymphocytic effector cells compared to either dexamethasone or RT alone.
Figure 3.
SCHD dexamethasone does not reduce intratumoral effector cell populations but does inhibit their activation. Tumors were harvested at day 9 PI and analyzed by flow cytometry. (A) Masses of MC38 tumors in unirradiated (UI) and irradiated (IR) mice medicated with SCHD dexamethasone. (B) Frequency of immune infiltrate (CD45+ cells). (C) Frequency of intratumoral IMs (CD45+ CD11b+ Ly6Chi Ly6G-) (CD45+ CD11b+ Ly6Chi Ly6G-), macrophages (CD45+ CD11b+ Ly6Clo Ly6G- F4/80+), and neutrophils (CD45+ CD11b+ Ly6C- Ly6G+). (D) Frequency of intratumoral CD4 T cells (CD45+ CD4+ CD8α-), CD8 T Cells (CD45+ CD4- CD8α+), NK cells (CD45+ CD4- CD8α- NK1.1+), and B cells (CD45+ CD4- CD8α- NK1.1- CD19+). (E) Expression of short-term activation marker CD69 on intratumoral effector cell populations. (n=5 per group).
RT is thought to cause T cell priming in the tdLNs; thus, we analyzed tdLNs outside the irradiation field to determine if activation was inhibited by dexamethasone. Dexamethasone significantly reduced tdLN mass in irradiated mice at day 9, indicative of decreased lymphocyte numbers (Fig.S2A). Myeloid populations in the dLN were largely unaffected by different treatment modalities, although dexamethasone alone did significantly increase IMs (Fig.S2B). Although absolute numbers of lymphocytes were decreased due to smaller tdLN mass, CD4 T cell, CD8 T cell, NK cell, and B cell frequencies were similar amongst all groups (Fig.S2C). In contrast to the TME, dexamethasone alone or combined with RT did not affect CD4 T cell activation (Fig.S2D). Conversely, dexamethasone prevented RT-mediated increase in CD8 T cell activation. tdLN NK cells mirrored their TME counterparts in showing increased CD69 expression in dexamethasone and RT monotherapy groups, but again combination therapy inhibited this response. In summary, SCHD dexamethasone decreased tdLN mass without altering frequencies of remaining immune cell populations, but combined RT and dexamethasone decreased RT-induced T cell activation similar to our observations in the TME.
Short course human equivalent dexamethasone administration does not significantly alter tumor growth kinetics
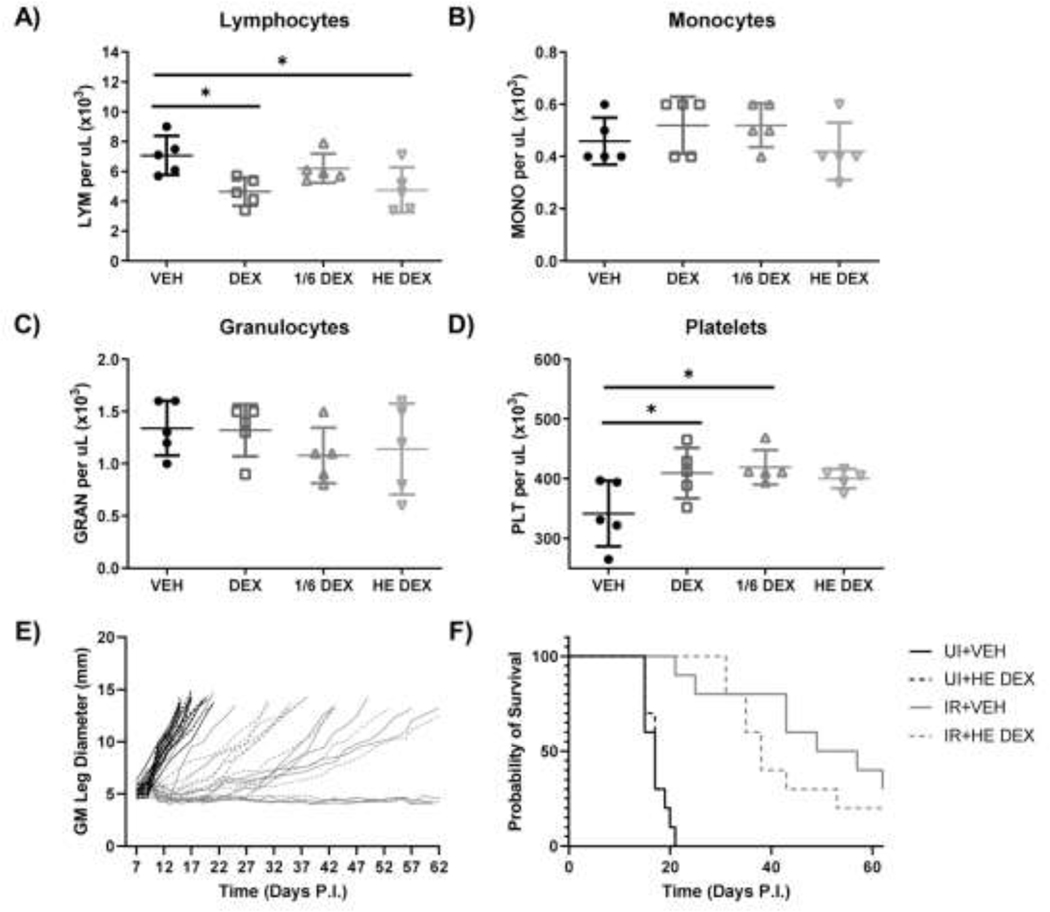
SCHD dexamethasone did not affect survival compared to vehicle control mice, regardless of RT exposure. Dexamethasone can reduce lymphocytes, but it also has demonstrable cytotoxic activity against myeloma cells (13) as well as colorectal cancer cells (Fig.S3), and has been shown to reduce tumor growth in breast tumor xenografts (14). To determine if the negative effects of dexamethasone-mediated peripheral lymphocyte reduction and inhibition of tumor infiltrating lymphocytes (TILs) activation are counteracted by beneficial dexamethasone-mediated tumor cell killing, we titrated our dexamethasone dosing. In doing so, we sought a lower dexamethasone dose to limit tumoricidal activity while retaining PB lymphocyte reduction. Mice were inoculated with tumor and medicated with short course dexamethasone as described above with three dose levels: 4 mg/kg (high dose), 0.66 mg/kg (1/6 of the high dose), or 0.114 mg/kg human equivalent dose (HE, 4 mg twice daily per 70 kg). At day 10 PI, high dose (4 mg/kg daily) and HE dose (0.114 mg/kg) dexamethasone reduced lymphocytes, whereas monocytes and granulocytes remained unaffected (Fig.4A-C). High and intermediate dose dexamethasone marginally increased platelet numbers, which was not seen in the HE dose (Fig.4D). Due to the lymphocyte reduction observed with HE dose dexamethasone, we medicated unirradiated and irradiated tumor bearing mice with this dosage in a short course schedule, as described previously. Short course HE dexamethasone trended towards decreased survival in the RT groups, but was not statistically significant, and dexamethasone did not affect survival in unirradiated mice (Fig.4E, 5F). Thus, despite using a much lower dose that still reduces PB lymphocytes, dexamethasone did not inhibit the RT-induced anti-tumor immune response.
Figure 4.
HE dexamethasone doses reduce PB lymphocytes but do not affect RT response. Mice were injected subcutaneously with 4 mg/kg (DEX), 0.66 mg/kg (1/6 DEX), or 0.114 mg/kg (HE DEX) dexamethasone or vehicle starting on day 6 and continuing until day 11. (A-E) Mice were bled via tail vein on day 10 PI and peripheral blood lymphocyte (A), monocyte (B), granulocyte (C), and platelet (D) numbers were enumerated via CBC analysis. Growth curves (E) and survival curves (F) for unirradiated (UI) or irradiated (IR) mice medicated with short course HE dose (0.114 mg/kg) dexamethasone (combined data from two experiments). (n=5 for CBC, n=10 for tumor growth and survival).
Figure 5.
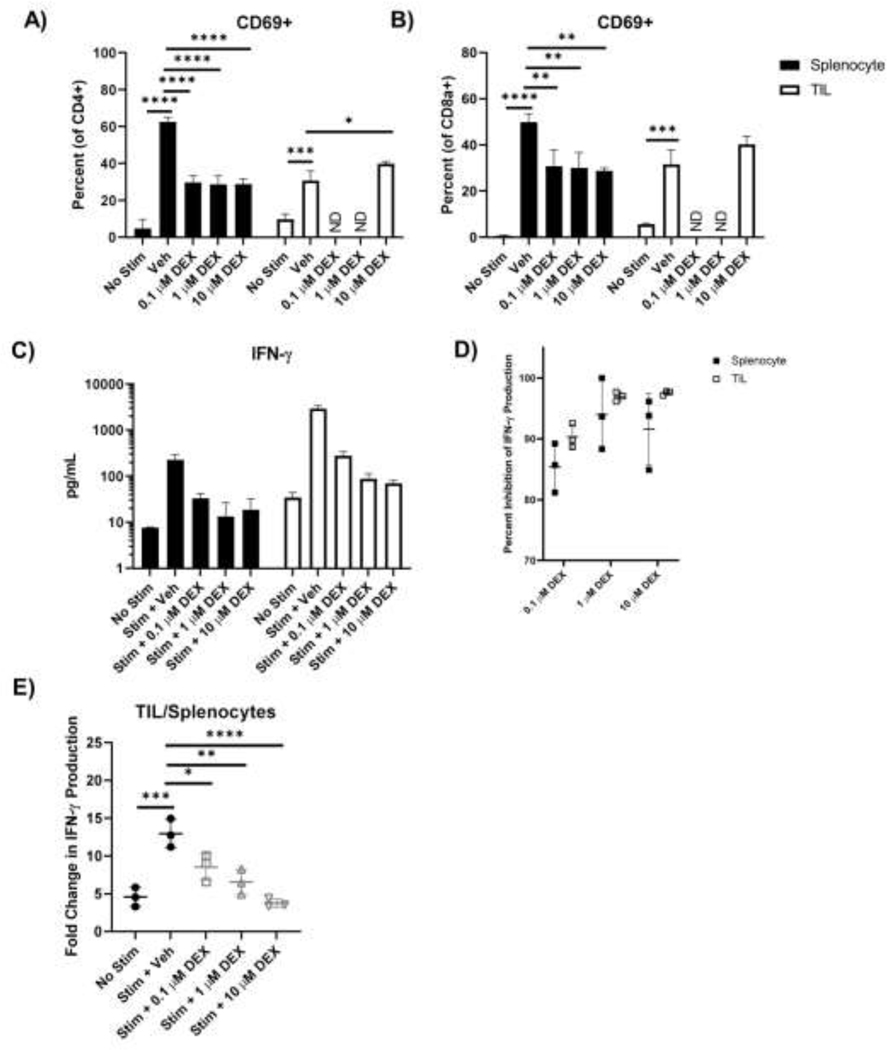
Dexamethasone inhibits activation and IFN-γ production more substantially in ex vivo stimulated naïve splenocytes than TILs. Unirradiated tumors and spleens were isolated, processed, and magnetically sorted for T cells using negative selection on day 7 PI. For TILs, samples were pooled for further analyses. Following isolation, cells were left unstimulated or stimulated with anti-CD3/anti-CD28 beads as well as dexamethasone at 0 μM, 0.1 μM, 1 μM, and 10 μM concentrations for 48 hours. Expression of short-term activation marker CD69 on resting or stimulated splenic and intratumoral CD4 (A) and CD8 (B) T cells was determined by flow cytometry. (C) Concentration of IFN-γ in the supernatant of rested and stimulated splenic and intratumoral T cells as determined by ELISA. (D) Percent inhibition of IFN-γ production by T cells cultured in different concentrations of dexamethasone. (E) Fold change in IFN-γ production by intratumoral T cells compared to splenic T cells cultured in different concentrations of dexamethasone. (ND = not determined). (Data analyzed by comparing each group to stimulated vehicle controls). (n=3 per group). (For all comparisons to stimulated controls with vehicle in (C), p< 0.0001).
Dexamethasone inhibits TIL function in vitro, though TILs retain heightened activity in comparison to naïve T cells
Neither high nor HE dose dexamethasone significantly altered RT response, yet both reduced PB lymphocytes. Although TILs were not reduced by SCHD dexamethasone (Fig.3D), we sought to determine if dexamethasone could inhibit TIL effector activity. Naïve splenocytes and TILs were isolated from tumor bearing mice 7 days PI. Cells were restimulated in vitro with anti-CD3/anti-CD28 beads and assessed by flow cytometry and ELISA for activation and effector status, respectively. In contrast to naïve splenocytes, TILs predominantly exhibited effector and effector memory cell phenotypes (Fig.S4). Dexamethasone inhibited CD69 upregulation in naïve CD4 and CD8 T cell splenocytes in vitro (Fig.5A, 6B). Despite observable naïve T cell inhibition, 10 μM dexamethasone had no effect on TIL CD69 expression. Dexamethasone did decrease IFN-γ secreted by in vitro stimulated naïve splenocytes and TILs(Fig.5C). Though percent inhibition by dexamethasone was similar between naïve splenocytes and TILs at each tested dexamethasone dose (Fig.5D), the magnitude of IFN-γ production between these two populations differed significantly. TILs stimulated without dexamethasone produced more than 12X the amount of IFN-γ compared to naïve splenocytes (Fig.5E). Dexamethasone administration decreased this fold-change in a dose dependent manner, but even at 10 μM dexamethasone, TILs produced more than 4X as much IFN-γ as naïve splenocytes (Fig.5E), an amount similar in magnitude to naïve splenocytes stimulated in 0.1 μM dexamethasone (Fig.5C). These data suggest that dexamethasone inhibits TIL effector function, but the magnitude of the basal TIL response may be enough to overcome the detrimental effects of dexamethasone.
Figure 6.
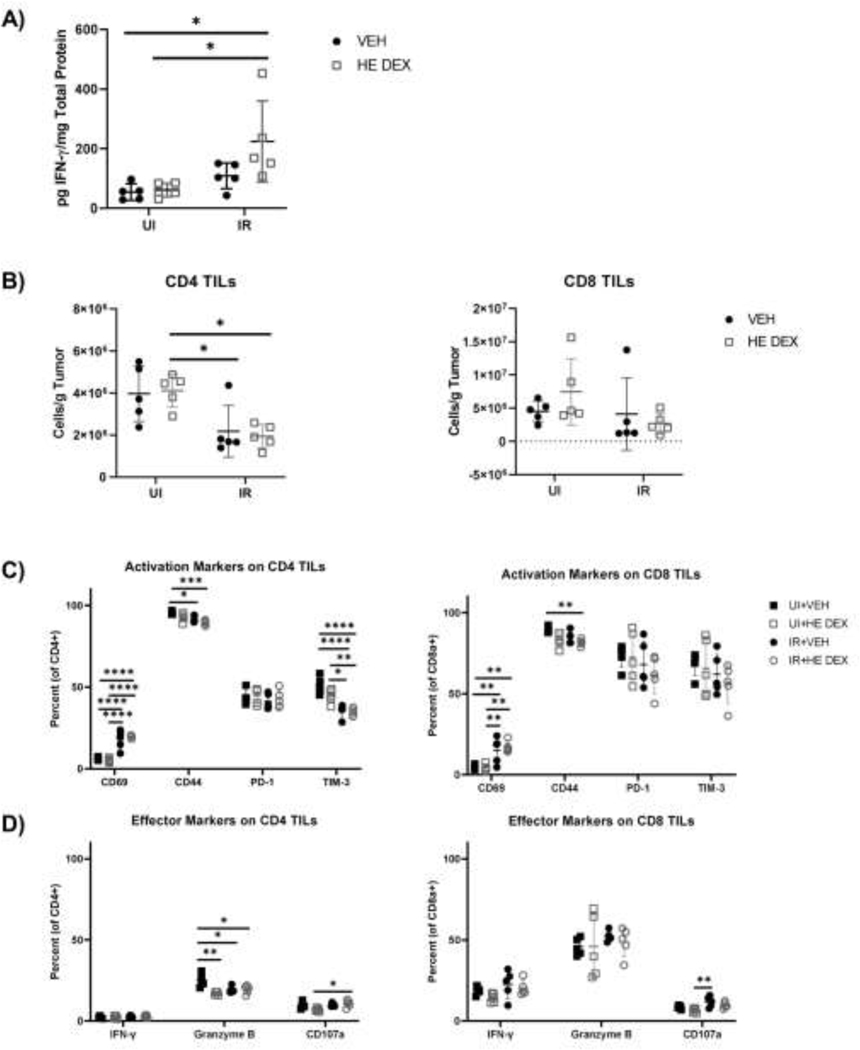
HE dexamethasone does not significantly alter effector molecule expression as well as CD4 and CD8 TILs in vivo compared to vehicle controls. Tumor bearing mice were treated with short course HE dexamethasone or vehicle control and left unirradiated or irradiated with 15 Gy. (A) At day 9 PI tumors were harvested and analyzed for total IFN-γ levels normalized to total protein. (B) CD4 and CD8 TIL numbers at day 9 PI were analyzed by flow cytometry. (C) Analysis of the activation markers CD69, CD44, PD-1, and TIM-3 in CD4 and CD8 TILs on day 9 PI. (D) Analysis of effector and cytotoxicity markers IFN-γ, granzyme B, and CD07a in CD4 and CD8 TILs on day 9 PI. (n=5 per group).
HE Dexamethasone does not significantly alter TIL numbers and expression of effector molecules in vivo
We previously observed that HE dexamethasone significantly reduced peripheral lymphocytes in vivo (Fig.4) and low dose dexamethasone limited effector function of ex vivo activated TILs, although at this dose TILs still produced more IFN-γ than counterpart ex vivo naïve splenocytes. To determine if HE dexamethasone significantly inhibited the RT-elicited anti-tumor response in vivo, we monitored the effector response in unirradiated and irradiated tumor bearing mice treated with short course HE dexamethasone or vehicle. Surprisingly, we observed that total intratumoral IFN-γ was increased in irradiated mice treated with HE dexamethasone compared to unirradiated and vehicle controls (Fig.6A). Radiation decreased CD4 TIL numbers, but dexamethasone had no significant effect on CD4 or CD8 TIL numbers compared to vehicle controls (Fig.6B). HE dexamethasone did not significantly alter activation marker expression in CD4 and CD8 TILs compared to vehicle controls (Fig.6C). RT increased CD69 expression in both CD4 and CD8 TILs, with comparable increased CD69 positive cells in HE dexamethasone and vehicle controls. Radiation also decreased CD44 and TIM-3 expression in CD4 TILs, regardless of exposure to HE dexamethasone. HE dexamethasone only significantly decreased CD44 expression in CD8 TILs in irradiated mice compared to unirradiated vehicle controls, but expression of CD44 was similar between HE dexamethasone and vehicle treated irradiated groups. Expression of the effector and cytotoxicity markers IFN-γ, granzyme B, and CD107a did not differ between HE dexamethasone groups and vehicle control groups except for granzyme B expression in CD4 TILs of unirradiated mice (Fig.6D), which are less abundant in the MC38 tumors and have lower expression of granzyme B compared to CD8 T cells in all groups examined. Altogether, these datsa suggest that HE dexamethasone, which can reduce peripheral lymphocyte numbers, does not significantly alter CD4 and CD8 TIL numbers or phenotype following radiation.
Discussion
We analyzed the effect of dexamethasone on the RT response using the MC38 tumor model, in which efficacy of RT response is dependent on CD8 T cells that secrete IFN-γ (8). LCHD dexamethasone reduced PB lymphocytes and slightly improved survival in unirradiated mice but did not affect survival after RT, although this was likely due to decreased inflammation in the irradiated leg, resulting in artificially increased time to endpoint. To account for potential direct cytotoxicity resulting from LCHD dexamethasone, we also tested short course high dose and HE dexamethasone starting one day prior to RT. However, we still did not observe significant differences in survival following RT, though PB lymphocyte reduction was evident.
SCHD dexamethasone reduced tdLN mass in irradiated mice, indicative of reduced lymphocyte numbers, but did not reduce TILs. Dexamethasone alone, like RT alone, increased CD69 expression on tdLN lymphocytes and TILs. Although unexpected, dexamethasone treatment has been shown to increase CD69 on immature NK cells and skew T cells to an effector phenotype in treatment of multiple myeloma with an anti-CD38 antibody (15). Importantly, SCHD dexamethasone did reduce expression of the activation marker CD69 on TILs and lymphocytes in irradiated tumors and corresponding tdLNs, suggesting some impairment of RT-induced activation. Combined dexamethasone and RT also decreased intratumoral myeloid cells compared to RT alone. Intratumoral myeloid cells can inhibit the effector function of T cells (16), therefore dexamethasone-mediated decrease in intratumoral myeloid cells may indirectly alleviate immune suppression of TILs while simultaneously directly inhibiting T cells. Dexamethasone did not affect CD69 expression in ex vivo restimulated TILs, but did inhibit IFN-γ production, although this inhibition still resulted in IFN-γ production that was more robust than similarly stimulated naïve T cells. Furthermore, in vivo analysis of CD4 and CD8 TILs in unirradiated and irradiated mice demonstrated that HE dexamethasone does not significantly alter TIL phenotype in comparison to vehicle treated controls. Specifically, IFN-γ, which was decreased in ex vivo stimulated TILs cultured with dexamethasone compared to vehicle controls, was similarly expressed in TILs from tumors treated with HE dexamethasone or vehicle control. Interestingly, total intratumoral IFN-γ was highest in irradiated tumor treated with HE dexamethasone. It is possible that even at low doses such as our human equivalent schedule, dexamethasone is still toxic to tumor cells and their resulting death increases T cell and other immune cell IFN-γ secretion over vehicle treated controls. Alternatively, it is possible that dexamethasone may decrease expression of the IFN-γ receptor on target cells (or modulate ligand/receptor binding activity), leading to accumulation of intratumoral IFN-γ. Further studies are needed to clarify this observation. Altogether, these data argue that dexamethasone can inhibit TILs, but the lack of effect on tumor growth curves and survival curves suggests that this inhibition is not enough to overcome the immunostimulatory effect of RT.
These results contradict our expectations given dexamethasone’s immunosuppressive nature. Clinical analysis of glioblastoma treatment supports our data, as only RT combined with chemotherapy showed significant negative effects of dexamethasone co-administration that were not seen in patients receiving RT alone (2). Perhaps combined toxicity of chemotherapy and dexamethasone-mediated inhibition of lymphocytes is enough to inhibit the RT response whereas dexamethasone alone cannot, although this result may be confounded by the fact that patients with greater tumor burden are more likely to receive dexamethasone. Another possible explanation for the lack of inhibition by dexamethasone in our model may be due to the acute activity of dexamethasone. Hong et al. observed that a single dexamethasone dose at 5 mg/kg transiently inhibited cytokine and adhesion molecule expression in murine lungs following high dose RT, but these inflammatory markers rebounded after 24 hours (17). In our model, dexamethasone was administered until at most three days post conclusion of RT, allowing for rebound of the RT-elicited immune response after this time point. It is possible that prolonged dexamethasone exposure following RT might have a more drastic effect on survival, but corticosteroid administration is often limited temporally, as prolonged usage causes adrenal suppression (18).
Most work studying dexamethasone has focused on the dexamethasone-mediated inhibition of effector T cells, but recent work has suggested that dexamethasone may act through T regulatory cells (Tregs). Although others have noted that dexamethasone does not affect Treg numbers (19), Kim et al. argue that Tregs are required for dexamethasone-mediated immune suppression in multiple models of inflammation (20). Importantly, dexamethasone does not increase Treg frequency, but instead increases suppressive capability by inhibiting mTORC2 signaling. We have observed few intratumoral Tregs following RT in MC38 tumors, though we have seen that fractionated RT increases intratumoral Tregs more so than ablative RT (unpublished observations). Thus, the effects of dexamethasone combined with fractionated RT require further study. Moreover, the use of tumor models with greater intratumoral Treg frequencies, such as breast cancer, melanoma, and head and neck squamous cell carcinoma (21–24), may further delineate potential immunosuppressive effects of dexamethasone combined with RT.
In all tested administration schedules, dexamethasone decreased PB lymphocytes while having no significant effect on survival following RT. Clinically, dexamethasone administration in glioblastoma patients results in reduction of PB lymphocytes (25), mirroring our data. Interestingly, dexamethasone has been shown to increase granulocytes and have no effect on monocytes in human patients (25), whereas we observe decreased monocytes and no effect on granulocytes in our model, indicating differences in response to dexamethasone in mouse and human that must be taken into consideration when extrapolating from our data. Most analyses of dexamethasone use in tumor treatment focus on peripheral blood T cell counts, with little consideration of the TME. Notably, SCHD dexamethasone did not affect TIL frequency in our model. In a phase 1 clinical trial examining neoantigen vaccine in glioblastoma, patients receiving dexamethasone and RT had decreased peripheral blood T cells while TIL numbers were not decreased (26). Instead, dexamethasone only inhibited the recruitment of additional T cells to the TME from the periphery. This result is not entirely surprising, as dexamethasone administration reduces adhesion molecules on murine endothelial cells following irradiation (27), likely reducing extravasation of PB lymphocytes into the tumor. In addition, TILs often exhibit effector/memory cell phenotypes (Fig.S4), especially in transplantable tumor models (28), and this subset is more resistant to dexamethasone inhibition (19, 29).
Although direct tumoricidal activity of RT is localized to targeted tissue, RT can elicit abscopal effects at non-irradiated metastatic sites. Pre-clinical studies as well as clinical observations suggest that abscopal responses are achieved less with RT alone, but more frequently in combination with immunotherapy, such as ICB (22, 30–32). Importantly, dexamethasone has been shown to inhibit response to anti-CTLA-4 and anti-PD-L1 therapy (10, 33). Efficacy of both anti-CTLA-4 and anti-PD-1 therapy depends on de novo generation of tumor specific T cells rather than just enhancement of extant TILs (34, 35). Thus, although RT response in the primary tumor is minimally affected by dexamethasone, the detrimental effects of dexamethasone may become apparent in the context of localized radiotherapy combined with systemic immunotherapy like ICB that reduces metastatic burden as well as primary tumor size through recruitment of peripheral T cells.
Clinical and pre-clinical studies by others have implied that corticosteroids inhibit immunotherapies such as ICB. Although unable to examine the effect of dexamethasone administration on TILs, Arbour et al. found that dexamethasone administration did correlate with decreased survival following PD-1/PD-L1 blockade (10). The authors do note that their study was limited by examining a small cohort of patients that received corticosteroid around ICB initiation. Additionally, the prognostic value of dexamethasone administration on survival is unclear, as high dose corticosteroid administration might simply identify patients with more severe disease at immunotherapy onset. Dexamethasone also correlated with decreased survival in patients receiving high dose corticosteroids combined with tumor treating electric field therapy (36). Again, this study may simply identify that patients with greater tumor burden are more likely to receive dexamethasone. Furthermore, this study compared patients receiving different dexamethasone doses, but lacked comparison to dexamethasone naïve patients, therefore it is unclear if low dose dexamethasone negatively affects response. Additionally, the authors were unable to examine TILs and instead relied on analysis of PB lymphocytes to monitor immune suppression. Regardless of these limitations, it is possible that the negative effect of corticosteroids is due to inhibition of low affinity T cells, which are more susceptible to corticosteroids and are more prevalent in patients with low mutational burden (33). Neither forementioned study monitored mutational burdens in their patients, so it is unclear if this factor determines the inhibitory capacity of corticosteroids.
Altogether, our observations and those of others suggest that dexamethasone reduces PB lymphocytes but spares TILs at the time of irradiation. Nevertheless, the question remains as to whether TILs retain enough functional capacity to reduce tumor burden without support from peripherally activated T cells. We noted that dexamethasone partially reduced effector function of intratumoral T cells ex vivo, but this inhibition did not significantly reduce survival following RT with any dexamethasone schedule tested. These data indicate that partial inhibition of TILs by dexamethasone results in robust enough effector function to facilitate an effective RT response. This was exemplified in our analysis of CD4 and CD8 TILs following RT in mice treated with HE dexamethasone or vehicle control, in which we found that dexamethasone treated TILs had similar activation/effector phenotypes compared to vehicle treated TILs in vivo. Furthermore, although these cells are likely exposed to RT, tumor resident T cells are highly radioresistant and can mediate an RT-induced immune response without PB lymphocyte aid (12). Thus, TILs may exert effector function that mediates a beneficial RT response despite exposure to inhibitory and cytotoxic dexamethasone and RT, although further analysis in other tumor models with less robust RT-elicited immune responses is warranted.
In conclusion, our data suggest that dexamethasone can reduce PB lymphocytes and inhibit the function of TILs, but TILs still respond to radiation-induced damage and elicit tumor control similar to vehicle controls. The resistance of TILs to dexamethasone administration is likely due to their high affinity memory cell phenotype, thus careful consideration must still be paid to dexamethasone co-administration in patients lacking an existing intratumoral T cell infiltrate, patients exhibiting low tumor mutational burden, and when the efficacy of cotherapies, such as ICB, are dependent on stimulation of peripheral T cells.
Supplementary Material
Acknowledgements:
We would like to thank Dr. John Frelinger (University of Rochester Medical Center, Rochester, NY, USA) for recommendations on study design and directions and Jeremy Oulhen for generously providing artwork for experimental design schemes.
Funding Statement:
This work was supported by the University of Rochester Sproull Fellowship, National Cancer Institute Grants R01CA028332 and R01CA230277, and the University of Rochester Immunology training grant (T32AI007285) from the National Institute of Allergy and Infectious Diseases.
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability:
Data in this manuscript are available upon reasonable request.
References
- 1.Chow E, Meyer RM, Ding K, et al. Dexamethasone in the prophylaxis of radiation-induced pain flare after palliative radiotherapy for bone metastases : a double-blind, randomised placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16(15):1463–72. doi: 10.1016/S1470-2045(15)00199-0 [DOI] [PubMed] [Google Scholar]
- 2.Pitter KL, Tamagno I, Alikhanyan K, et al. Corticosteroids compromise survival in glioblastoma. Brain. 2016;139(Pt 5):1458–71. doi: 10.1093/brain/aww046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotsarini C, Griffiths PD, Wilkinson ID, et al. A systematic review of the literature on the effects of dexamethasone on the brain from in vivo human-based studies: Implications for physiological brain imaging of patients with intracranial tumors. Neurosurgery. 2010;67(6):1799–1815. doi: 10.1227/NEU.0b013e3181fa775b [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Brit J Pharmacol. 2006;148(3):245–54. doi: 10.1038/sj.bjp.0706736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filatenkov A, Baker J, Mueller AMS, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res. 2015;21(16):3727–39. doi: 10.1158/1078-0432.CCR-14-2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lugade AA, Moran JP, Gerber SA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–23. doi: 10.4049/jimmunol.174.12.7516 [DOI] [PubMed] [Google Scholar]
- 7.Lugade AA, Sorensen EW, Gerber SA, et al. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180(5):3132–9. doi: 10.4049/jimmunol.180.5.3132 [DOI] [PubMed] [Google Scholar]
- 8.Gerber SA, Sedlacek AL, Cron KR, et al. IFN-γ mediates the antitumor effects of radiation therapy in a murine colon tumor. Am J Pathol. 2013;182(6):2345–54. doi: 10.1016/j.ajpath.2013.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewitzki V, Klement RJ, Kosmala R, et al. Accelerated hyperfractionated radiochemotherapy with temozolomide is equivalent to normofractionated radiochemotherapy in a retrospective analysis of patients with glioblastoma. Radiat Oncol. 2019;14(1):227. doi: 10.1186/s13014-019-1427-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non– small-cell lung cancer. J Clin Oncol. 2018;36(28):2872–78. doi: 10.1200/JCO.2018.79.0006 [DOI] [PubMed] [Google Scholar]
- 11.Weichhart T, Brandt O, Lassnig C, et al. The anti-inflammatory potency of dexamethasone is determined by the route of application in vivo. Immunol Lett. 2010;129(1):50–2. doi: 10.1016/j.imlet.2009.12.025 [DOI] [PubMed] [Google Scholar]
- 12.Arina A, Beckett M, Fernandez C, et al. Tumor-reprogrammed resident T cells resist radiation to control tumors. Nat Commun. 2019;10(1):3959. doi: 10.1038/s41467-019-11906-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bera S, Greiner S, Choudhury A, et al. Dexamethasone-induced oxidative stress enhances myeloma cell radiosensitization while sparing normal bone marrow hematopoiesis. Neoplasia. 2010;12(12):980–92. doi: 10.1593/neo.101146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Chen R, Yao Q-Y, et al. Time-dependent pharmacokinetics of dexamethasone and its efficacy in human breast cancer xenograft mice: a semi-mechanism-based pharmacokinetic/pharmacodynamic model. Acta Pharm Sinic. 2018;39(3):472–81. doi: 10.1038/aps.2017.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casneuf T, Adams HC III, van de Donk NWCJ, et al. Deep immune profiling of patients with or without daratumumab. Leukemia. 2020. doi: 10.1038/s41375-020-0855-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connolly KA, Belt BA, Figueroa NM, et al. Increasing the efficacy of radiotherapy by modulating the CCR2/CCR5 chemokine axes. Oncotarget. 2016;7(52):86522–35. doi: 10.18632/oncotarget.13287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong JH, Chiang CS, Tsao CY, et al. Can short-term administration of dexamethasone abrogate radiation-induced acute cytokine gene response in lung and modify subsequent molecular responses? Int J Radiat Oncol. 2001;51(2):296–303. doi: 10.1016/s0360-3016(01)01702-3 [DOI] [PubMed] [Google Scholar]
- 18.Williams DM. Clinical pharmacology of corticosteroids. Resp Care. 2018;63(6):655–70. doi: 10.4187/respcare.06314 [DOI] [PubMed] [Google Scholar]
- 19.Giles AJ, Hutchinson MND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1):51. doi: 10.1186/s40425-018-0371-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D, Nguyen QT, Lee J, et al. Anti-inflammatory roles of glucocorticoids are mediated by Foxp3+ regulatory T cells via a miR-342-dependent mechanism. Immunity. 2020;53(3):581–96.e5. doi: 10.1016/j.immuni.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127(4):759–67. doi: 10.1002/ijc.25429 [DOI] [PubMed] [Google Scholar]
- 22.Schrand B, Verma B, Levay A, et al. Radiation-induced enhancement of antitumor T cell immunity by VEGF-targeted 4–1BB costimulation. Cancer Res. 2017;77(6):1310–21. doi: 10.1158/0008-5472.CAN-16-2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muroyama Y, Nirschl TR, Kochel CM, et al. Stereotactic radiotherapy increases functionally suppressive regulatory T cells in the tumor microenvironment. Cancer Immunol Res. 2017;5(11):002–1004. doi: 10.1158/2326-6066.CIR-17-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oweida A, Hararah MK, Phan A, et al. Resistance to radiotherapy and PD-L1 blockade is mediated by TIM-3 upregulation and regulatory T-cell infiltration. Clin Cancer Res. 2018;24(21):5368–80. doi: 10.1158/1078-0432.CCR-18-1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gustafson MP, Lin Y, New KC, et al. Systemic immune suppression in glioblastoma : the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010;12(7):631–44. doi: 10.1093/neuonc/noq001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keskin DB, Anandappa AJ, Sun J, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–9. doi: 10.1038/s41586-018-0792-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan H, Goetz DJ, Gaber MW, et al. Radiation-induced up-regulation of adhesion molecules in brain microvasculature and their modulation by dexamethasone. Radiat Res. 2005;163(5):544–51. doi: 10.1667/rr3361 [DOI] [PubMed] [Google Scholar]
- 28.Crittenden MR, Zebertavage L, Kramer G, et al. Tumor cure by radiation therapy and checkpoint inhibitors depends on pre-existing immunity. Sci Rep-UK. 2018;8(1):7012. doi: 10.1038/s41598-018-25482-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar D, Sehrawat S. Divergent effects of a transient corticosteroid therapy on virusspecific quiescent and effector CD8+T Cells. Front Immunol. 2019;10:1521. doi: 10.3389/fimmu.2019.01521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Niedermann G. Abscopal effects with hypofractionated schedules extending into the effector phase of the tumor-specific T-cell response. Int J Radiat Oncol. 2018;101(1):63–73. doi: 10.1016/j.ijrobp.2018.01.094 [DOI] [PubMed] [Google Scholar]
- 31.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Postow MA, Callahan MK, Barker CA, et al. Abscopal effect in a patient with melanoma. New Engl J Med. 2012;366(10):925–31. doi: 10.1056/NEJMoa1112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tokunaga A, Sugiyama D, Maeda Y, et al. Selective inhibition of low-affinity memory CD8 + T cells by corticosteroids. J Exp Med. 2019;216(12):2701–13. doi: 10.1084/jem.20190738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spitzer MH, Carmi Y, Reticker-Flynn NE, et al. Systemic immunity is required for effective cancer immunotherapy. Cell. 2017;168(3):487–502.e15. doi: 10.1016/j.cell.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kvistborg P, Philips D, Kelderman S, et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med. 2014;6(254):254ra128. doi: 10.1126/scitranslmed.3008918 [DOI] [PubMed] [Google Scholar]
- 36.Wong ET, Lok E, Gautam S, et al. Dexamethasone exerts profound immunologic interference on treatment efficacy for recurrent glioblastoma. Brit J Cancer. 2015;113(11):1642. doi: 10.1038/bjc.2015.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data in this manuscript are available upon reasonable request.