To the editor:
Internal tandem duplication (ITD) mutations of the FMS-like tyrosine kinase (FLT3) gene are seen in approximately 25% of the patients with acute myeloid leukemia (AML) and are associated with a high risk of relapse and poor survival [1]. However, FLT3-ITD allelic ratio (AR), calculated as the ratio of mutated FLT3-ITD divided by wild type FLT3 alleles (using DNA fragment analysis), less than 0.5, especially with concurrent NPM1 mutations, have been categorized as a lower-risk disease by the European Leukemia Network (2017) guidelines [2]. The use of FLT3 inhibitors (FLT3i), such as sorafenib, midostaurin, gilteritinib, and quizartinib, has improved survival in patients with FLT3-mutated AML in the frontline, relapsed/refractory (R/R), and post-transplant maintenance settings. FLT3i’s appear to be effective irrespective of the FLT3-ITD ARs, but recent analysis have suggested a clearer benefit for second-generation FLT3i’s compared with salvage chemotherapy in patients with R/R AML who had higher FLT3 ARs [3]. Furthermore, the phase III FLT3i trials, RATIFY and ADMIRAL, excluded patients with FLT3 AR <0.05 [4, 5]. The role and impact of FLT3i’s, allogeneic stem cell transplantation (ASCT) and NPM1 co-mutation, in patients with very low allelic burden FLT3 mutations is unclear, often debated, and is the focus of this analysis.
In our institution, FLT3 assays are reported as allelic frequency (AF) rather than AR. AF is calculated as the ratio of mutated FLT3-ITD divided by wild type plus mutated FLT3. In this study, baseline FLT3-ITD AFs ≤ 0.1 (equivalent to FLT3 ARs ≤0.11) were defined as very low level. We retrospectively reviewed patients who received therapy for newly diagnosed FLT3-mutated AML (excluding core-binding factor AML and acute promyelocytic leukemia) between 2012–2020 at our institution. We identified 50 patients with FLT3-ITD AF ≤0.1 (at diagnosis). A polymerase chain reaction (PCR) based DNA analysis followed by capillary electrophoresis was utilized to detect FLT3-ITD and codon 835/836 point mutation at diagnosis and relapse as described previously. This study excluded patients with isolated FLT3-D835 mutations at diagnosis. All patients were treated with intensive (cytarabine and anthracycline-based) or low-intensity (hypomethylating agents or low-dose cytarabine based) regimens with or without a FLT3i.
Patients were analyzed in two cohorts depending on receipt of a FLT3i with frontline chemotherapy. Categorical variables were compared for statistical significance using the chi-square or Fisher’s exact test, and continuous variables were compared using the Mann-Whitney U test. Kaplan-Meier analysis was performed to calculate the probability of overall survival (OS) and the log-rank test was used to compare OS between cohorts. Statistical analyses were performed in SPSS© (version 26). Of the 50 patients, 30 received frontline chemotherapy without a FLT3i (no FLT3i cohort), and 20 received induction with a FLT3i (FLT3i cohort). Baseline characteristics, treatment regimens, and outcomes of patients treated with or without a FLT3i-based therapy are summarized in Table 1. Patients in the FLT3i cohort were younger than the no FLT3i cohort (median age 58 vs. 67 years old, p=0.03), and more often were treated with an intensive induction (80% vs 43%, p=0.01).
Table 1.
Baseline clinical characteristics of patients treated with or without a FLT3 inhibitor-based therapy
| Characteristics | No FLT3i Cohort N= 30 N (%) [range] |
FLT3i Cohort N= 20 N (%) [range] |
p |
|---|---|---|---|
| Median age, years | 67 [23–84] | 58 [28–83] | 0.03 |
| Age >60 years old | 24 (80) | 8 (40) | 0.04 |
| Male gender | 17 (57) | 10 (50) | 0.64 |
| Type of AML | |||
| De novo | 25 (83) | 20 (100) | 0.16 |
| Secondary AML | 1 (3) | 0 (0) | |
| Therapy related | 4 (14) | 0 (0) | |
| WBC, x109/L | 5.2 [1.1–34.2] | 5.8 [0.9–378.4] | 0.44 |
| Hemoglobin, g/dl | 8.9 [7.7–11.6] | 8.7 [5.1–11.4] | 0.37 |
| Platelets, x109/L | 52 [9–222] | 31 [10–96] | 0.33 |
| Creatinine | 0.8 [0.5–1.4] | 0.7 [0.5–4.6] | 0.69 |
| Total Bilirubin | 0.7 [0.2–1. | 0.5 [0.3–1.3] | 0.08 |
| Peripheral blood blasts, % | 24 [0–91] | 19 [0–97] | 0.86 |
| Bone marrow blasts, % | 64 [15–96] | 72 [30–94] | 0.43 |
| Cytogenetics | |||
| Diploid karyotype | 15 (50) | 12 (60) | 0.77 |
| Adverse | 7 (23) | 4 (20) | |
| others | 8 (27) | 4 (20) | |
| FLT3 mutations* | |||
| ITD Allelic Frequencies | 0.03 [0.01–0.09] | 0.05 [0.01–0.09] | 0.45 |
| Other Mutations | |||
| NPM1 | 9/28 (32) | 12/20 (60) | 0.06 |
| RUNX1 | 7/21 (33) | 3/19 (16) | 0.20 |
| RAS | 5/27 (18) | 6/20 (30) | 0.36 |
| IDH2 | 8/29 (28) | 5/20 (25) | 0.84 |
| ASXL1 | 5/21 (24) | 2/19 (11) | 0.27 |
| IDH1 | 6/29 (21) | 1/19 (5) | 0.12 |
| DNMT3A | 2/27 (7) | 1/20 (5) | 0.74 |
| CEBPA | 1/26 (4) | 3/19 (16) | 0.16 |
| TP53 | 1/24 (4) | 1/19 (5) | 0.87 |
| Treatment | |||
| Low Intensity | 17 (57) | 4 (20) | 0.01 |
| High Intensity | 13 (43) | 16 (80) | |
| FLT3 inhibitors | |||
| Sorafenib | 0 (0) | 19 (96) | n/a |
| Quizartinib | 0 (0) | 1 (5) | |
| no FLT3i | 30 (100) | 0 (0) | |
| Treatment outcome | |||
| CR + CRi | 20 (67) | 17 (85) | 0.23 |
| No response | 7 (23) | 3 (15) | |
| Early death | 3 (10) | 0 (0) | |
| ASCT in CR1 | 8 (27) | 6 (20) | 0.80 |
FLT2 inhibitor; N, number; n/a, not applicable; WBC, white blood cell; CR/CRi, complete remission/Complete remission with incomplete count recovery; ASCT, allogeneic stem cell transplant; CR1, first remission
Only 1 patient had both ITD and D835 at baseline, others were isolated FLT-ITD
Response definitions were per international working group (IWG) criteria. Relapse is defined by the detection of blasts (>5%) in a bone marrow aspirate or by the detection of biopsy-proven extramedullary myeloid sarcoma
Overall, a total of 37 patients achieved a CR/CRi, and 12 (32%) relapsed with a median follow-up of 42 months (range 30–53 months) (CONSORT diagram: Supplemental (S.) Figure 1). Of the 12 relapses, 8 (8/30, 27%) and 4 (4/20, 20%) were among patients treated without and with FLT3i-based regimens, respectively. The overall incidence of FLT3-ITD positivity at relapse was 42% (5 of 12 relapsed patients). However, all of the five FLT3-ITD positive relapses were observed in the no FLT3i cohort (5 of 8), and no FLT3 positive relapses were seen in the FLT3i cohort (0 of 4), 63% versus 0%, respectively (p=0.038) (S. Figure 2A - D).
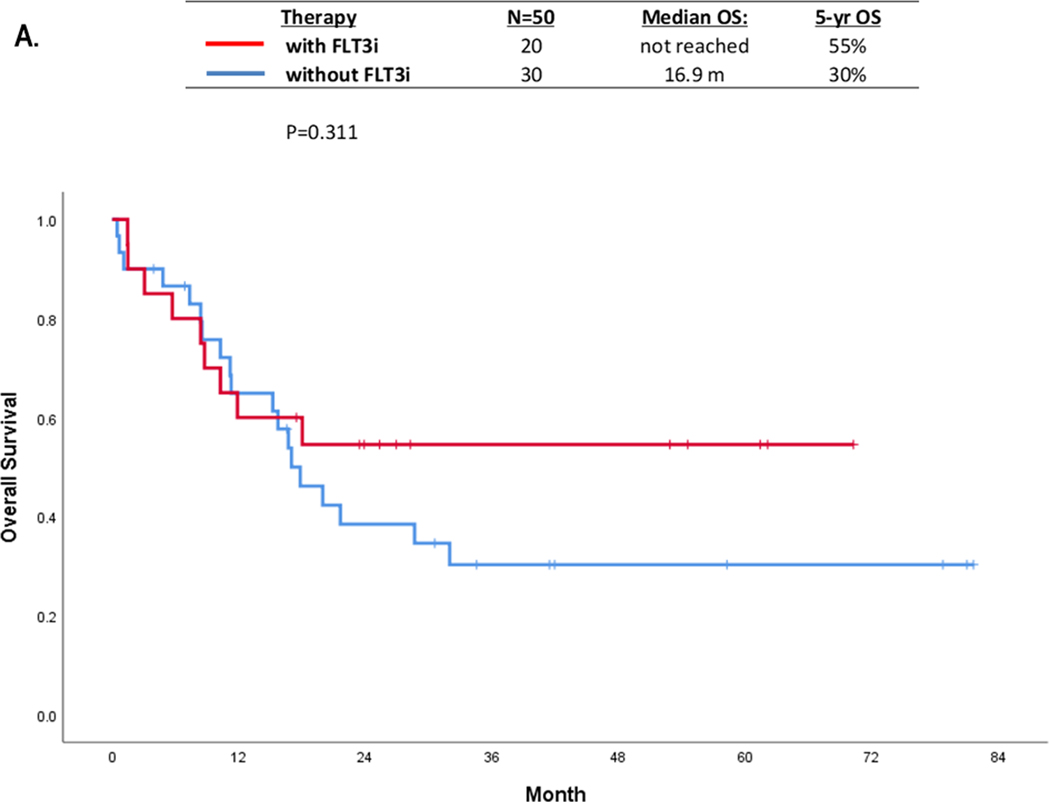
The median OS was not statistically different in the FLT3i cohort compared with no FLT3i cohort; not reached (N=20) versus 16.9 months (N=30), (p=0.31) (Figure 1A). We also investigated the impact of NPM1 co-mutations on OS. Although statistically not significant, NPM1 co-mutated patients had median OS of 32 months compared with 17 months in NPM1 negative patients(p=0.18) (S. Figure 3A). The 5-year OS, not censored for ASCT, in patients with NPM1 treated with FLT3i (n=12), NPM1 without FLT3i (n=9), no NPM1 with FLT3i (n=8), no NPM1 without FLT3i (n=19) were 58%, 40%, 50%, and 22% months, (p=0.38) respectively (S. Figure 3B).
FIGURE 1:
OS in patients withFLT3-ITD with AF≤0.1 by: (A) Treated with or without FLT3i containing regimen; (B) with or without ASCT inCR1, and (C) impact of NPM1 co-mutation and ASCT in CR1
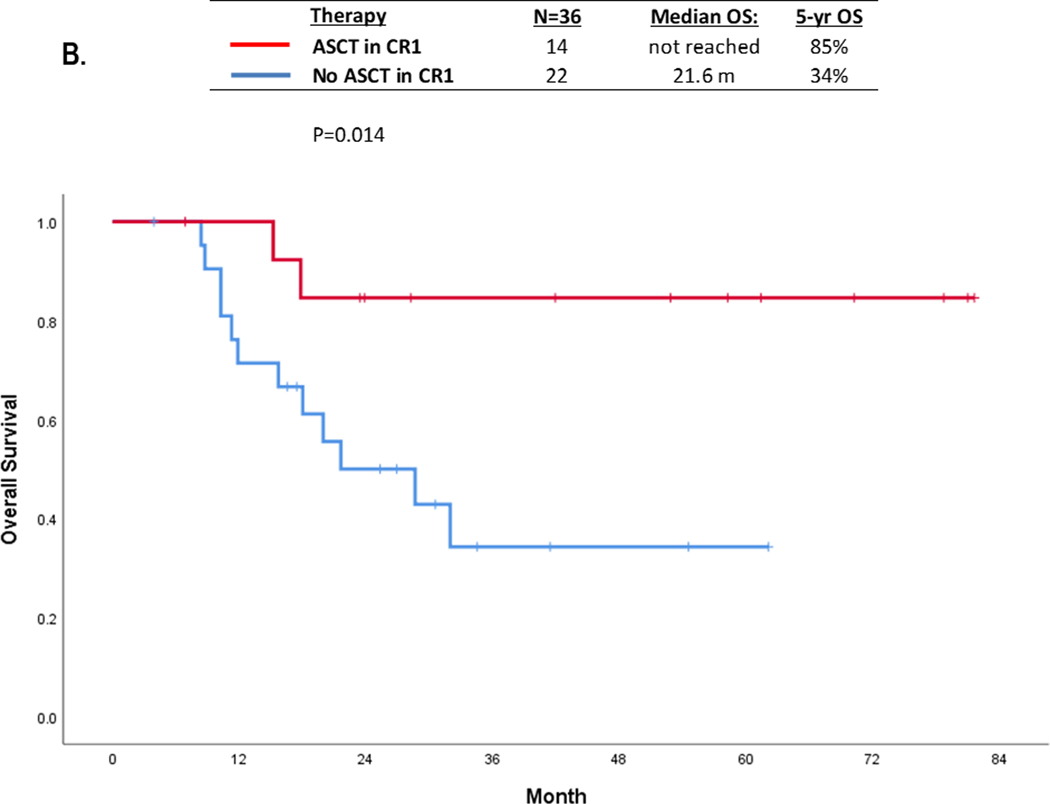
Overall, 14 patients underwent ASCT (6 FLT3i and 8 no FLT3i cohort) in the first remission with a median time to ASCT of 114 days (range 79–230 days). A landmark analysis (at 3-month post ASCT) showed that 5-year OS was significantly superior in patients who underwent ASCT (N=14) compared with those who did not (N=22), 85% vs. 34%, respectively (p=0.01) (Figure 1B). Given the heterogeneity of induction therapies used (intensive versus low-intensity chemotherapies), we performed landmark analysis in patients treated with intensive (N=25) or low-intensity regimens (n=11). When analyzed separately, the statistical significance of ASCT in CR1 was lost. However, there still was a trend for superior OS in transplant recipients regardless of chemotherapy intensity (S. Figure 4A-B).
Although the numbers were small, patients who received FLT3i-based induction and underwent ASCT achieved the longest OS in this very low FLT3 burden population. The 5-year OS rates in patients who received a FLT3i with induction followed by ASCT in CR1 (n=6), did not receive a FLT3i with induction followed by ASCT in CR1 (n=8), received a FLT3i with induction but did not undergo ASCT (n=10), and did not receive a FLT3i with induction and did not undergo ASCT (n=12) were 100%, 71%, 48%, and 27%, respectively (p=0.08) (S. Figure 5). The 5-year OS rates only in patients who received FLT3i and ASCT in CR1 (n=6) versus those who received neither FLT3i nor ASCT in CR1 were 100% vs 27% (n=12) (p=0.02). Among 14 ASCT recipients, 2 died; 1 due to relapse (FLT3 negative) and 1 death in remission.
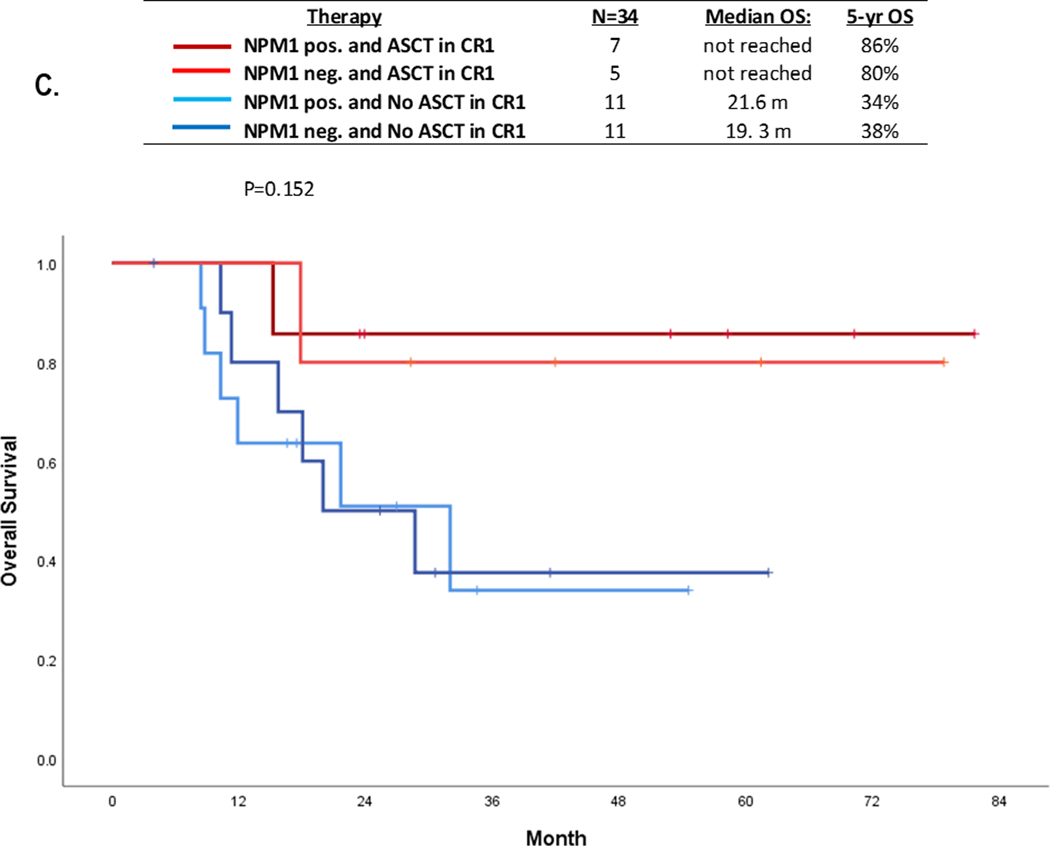
Interestingly, even in this very low FLT3 burden population ASCT was associated with favorable OS irrespective of the baseline NPM1 co-mutation status. Among the 34 patients (2 with no baseline NPM1 testing excluded), the 5-year OS in patients with NPM1 with ASCT (n=7), no NPM1 with ASCT (n=5), NPM1 without ASCT (n=11), and no NPM1 without ASCT (n=11) were 86%, 80%, 34%, and 38%, (p=0.15) respectively (Figure 1C).
Midostaurin was approved by the Food and Drug Administration and European Medicines Agency for patients with newly diagnosed FLT3-mutated AML based on improved survival in a randomized phase III study of induction chemotherapy with or without midostaurin (RATIFY) [4]. In a subsequent analysis, the addition of midostaurin was demonstrated to consistently improve survival in patients with low and high FLT3-ITD ARs supporting the use of midostaurin irrespective of the ITD AR [6]. However, this pivotal clinical trial excluded patients with FLT3-ITD AR < 0.05, and there remained a debate regarding the biological and clinical impact of using FLT3i’s and/or ASCT in very low FLT3 burden. In our study, the median FLT3-ITD AF was 0.03 for the entire group, and we demonstrated that the use of a FLT3i (sorafenib in 95% of the cases) non-significantly improved CR/CRi rates (85% versus 67%), reduced the incidence of FLT3 positive relapses (0% vs 63%) with a trend to improved OS. Given the high rate of FLT3 positive relapses even among patients with very low FLT3-ITD burden, we believe that future clinical trials investigating FLT3 inhibitors in AML should not exclude these patients.
Our analysis supports the use of FLT3i and ASCT in CR1 in patients with very low FLT3-ITD AF (≤ 0.1), irrespective of NPM1 co-occurrence. Patients who received a FLT3i with induction and underwent ASCT in CR1 had a 5-year OS of 100%, compared with 71% in patients who did not receive a FLT3i with induction but underwent ASCT in CR1, and only 27% in patients who neither received a FLT3i nor underwent ASCT in CR1. In addition, ASCT appeared to improve outcomes regardless of NPM1 co-mutation status among this low FLT3 burden population; the 5-year OS rates were 86% and 80% in patients with NPM1 positive or negative, respectively, who went to ASCT compared with 30–40% in NPM1 positive or negative patients who did not go to ASCT in CR1 (Figure 1C). We do acknowledge that the decision for ASCT needs to be further individualized for patients in this group based on co-existing factors including patient fitness, donor availability, MRD positivity, and co-occurring mutations.
Small cohort sizes and differences among baseline clinical charactheristics in FLT3i and no FLT3i cohorts limit our ability to make definitive conclusions. Our findings require confirmation by studies with a larger patient size. However, in our study,, we demonstrated that the use of FLT3i reduced the incidence of FLT3 positive relapses in AML patients with very low FLT3-ITD AFs (AF ≤0.1). Our data suggests that eligible patients should be considered to receive a FLT3i based therapy and be considered for ASCT in CR1 irrespective of their baseline FLT3 AF and NPM1 co-mutation status, after weighing all patient and disease specific factors for individual patients. ASCT non-eligible patients should be encouraged to enroll in novel maintenance clinical trials. To better understand the impact of very low FLT3 burden, future clinical trials investigating FLT3i based therapies should include all allelic burden FLT3-mutated patients.
Supplementary Material
Acknowledgements:
Funding:
This work was supported in part by the MD Anderson Cancer Centre Support Grant (CCSG) CA016672
Conflict of interest:
MY reports research funding from Pfizer and Daiichi Sankyo.
TK reports personal fees from Novartis, grants and personal fees from Pfizer, grants from BMS, grants and personal fees from Abbvie, grants and personal fees from Genentech, grants and personal fees from JAZZ, grants from Amgen, grants from AstraZeneca; Celgene: research grant; Incyte: research grant; Ascentage: research grant.
CD reports grants and personal fees from Abbvie, grants and personal fees from Agios, grants and personal fees from Novartis, grants and personal fees from ImmuneOnc, grants and personal fees from Daiichi Sankyo, grants and personal fees from Celgene, personal fees from Jazz, personal fees from Notable Labs, personal fees from MedImmune, grants from Calithera, personal fees from Bayer.
EJ reports research grants and consultancy from Abbvie, Adaptive biotechnology, Amgen, BMS, Pfizer, Takeda.
NS reports personal fees from Amgen, personal fees from AstraZeneca, grants and personal fees from Takeda, grants from Astellas.
GI reports grants and personal fees from Novartis, grants from Syndax, grants from Celgene.
FR reports honoraria and member of advisory board from Astellas, honoraria from Daichii, honoraria and member of advisory board from Novartis.
ND reports research funding from Daiichi Sankyo, Bristol-Myers Squibb, Pfizer, Karyopharm, Sevier, Genentech, Astellas, Abbvie, Genentech, Novimmune, Amgen, Trovagene, Gilead and ImmunoGen and has served in a consulting or advisory role for Daiichi Sankyo, Bristol-Myers Squibb, Pfizer, Novartis, Celgene, AbbVie, Genentech, Servier, Trillium, Syndax, Trovagene, Astellas, Gilead and Agios.
Other authors reported no relevant COIs.
Data Availability Statement:
Research Data is not shared
References
- 1.Kottaridis PD, et al. , The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood, 2001. 98(6): p. 1752–9. [DOI] [PubMed] [Google Scholar]
- 2.Dohner H, et al. , Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood, 2017. 129(4): p. 424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levis MJ, et al. , Effect of gilteritinib on survival in patients with FLT3-mutated (FLT3mut+) relapsed/refractory (R/R) AML who have common AML co-mutations or a high FLT3-ITD allelic ratio. Journal of Clinical Oncology, 2019. 37(15_suppl): p. 7000–7000. [Google Scholar]
- 4.Stone RM, et al. , Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med, 2017. 377(5): p. 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perl AE, et al. , Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N Engl J Med, 2019. 381(18): p. 1728–1740. [DOI] [PubMed] [Google Scholar]
- 6.Dohner K, et al. , Impact of NPM1/FLT3-ITD genotypes defined by the 2017 European LeukemiaNet in patients with acute myeloid leukemia. Blood, 2020. 135(5): p. 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research Data is not shared