Abstract
Background
Low functional capacity, malnutrition, and anaemia are associated with an increased risk of complications after surgery. These high-risk indicators can be improved through preoperative interventions. The aim of the study was to examine the effect of screening for modifiable high-risk factors combined with targeted interventions on postoperative complications in patients undergoing colorectal cancer surgery.
Methods
A controlled before-and-after study was conducted including patients with colorectal cancer undergoing elective curative surgery between August 2015 and October 2018, in two institutions (intervention and control hospital). The intervention consisted of a screening for anaemia, low functional capacity, and nutritional status and their implementation (iron supplementation, prehabilitation, nutritional supplements, and consultation with a dietician), for a minimum of 4 weeks before surgery. The primary outcome was a composite measure consisting of unplanned admission to the intensive care unit, complications with Clavien–Dindo score of 3a or above, length of hospital stay less than 10 days, readmission, or death within 30 days during the postoperative course.
Results
A total of 1591 patients were included for analysis with 839 at the intervention hospital and 752 at the control hospital. In a difference-in-difference analysis, adjusted for age, sex, smoking, stage of disease, ASA score, surgical approach, and surgical procedure, the intervention was associated with a 10.9 per cent (95 per cent c.i. 2.1 to 19.7 per cent) absolute risk reduction of a complicated postoperative course, primarily due to a reduction in severe complications.
Conclusion
The combined intervention of screening and prehabilitation was associated with a decreased risk of a complicated course, primarily in a reduction of severe complications.
Prehabilitation of high-risk surgical patients is a promising addition to the patient care pathway to improve postoperative outcomes. However, evidence of the impact of high-risk screening and prehabilitation in a clinical setting is scarce. This controlled before-and-after study including 1591 patients with colorectal cancer found that the combined intervention with preoperative screening for anaemia, low functional capacity, and malnutrition with concomitant targeted prehabilitation was associated with an absolute risk reduction of 10.9 per cent (95 per cent c.i. 2.1 to 19.7 per cent) of a complicated course after curative intended surgery.
Introduction
Complications after major surgery are associated with a long-term reduction in quality of life1, functional capacity2, survival3, and increased health-related costs4. Thus, preoperative screening for comorbidity and risk stratification are important, but are generally limited to organ-specific risks and without means of preoperative risk reduction5. In patients with cancer, the risk of disease progression may limit the timeframe for preoperative optimization, and the modification of risk factors. Low functional capacity6, anaemia7, and poor nutritional status8 are all associated with adverse outcomes in colorectal cancer and are all potentially modifiable risk factors through preoperative interventions9,10, or ‘prehabilitation’ and several randomized clinical trials have been conducted in this field11,12. Trials with prehabilitation in high-risk patients investigating short-term interventions have been shown to reduce postoperative complications13,14. Thus, preoperative screening for modifiable high-risk indicators in the domains of functional capacity, anaemia, and nutritional status, could provide helpful information to decision-making, including whether to initiate prehabilitation15. This study aimed to examine a composite outcome for a complicated postoperative course before and after implementing a screening tool for high-risk patients with modifiable indicators, along with specific interventions aimed toward these indicators before elective colorectal cancer surgery.
Methods
Study design and setting
The study was a controlled before-and-after study, a quasi-experimental observational study design, conducted at an intervention hospital and a control hospital, both providing data before (1 August 2015 to 31 January 2017) and after (1 March 2017 to 4 October 2018) implementation of the intervention, which consisted of high-risk indicator screening and prehabilitation, see following section. The intervention hospital was the Department of Surgery, Zealand University Hospital, Roskilde, and the control hospital was the Department of Surgery, Slagelse Hospital.
Both departments are geographically placed in the same region of Zealand in Denmark, have similar size and volume of surgeries, and adhere to the national guidelines for the treatment of colorectal cancer. Minimally invasive surgery and enhanced recovery after surgery (ERAS)16,17 have been the standard of care at both centres. A comparison of the two centres is provided in Table S1.
Intervention
On 1 March 2017, a screening tool for modifiable high-risk indicators was introduced at the Department of Surgery, Zealand University Hospital, Roskilde for all patients referred with colorectal cancer. The screening tool assessed patients in three domains: anaemia, physical capacity, and nutritional status. The screening tool consisted of a one-page document (Supplementary material), in which haemoglobin, timed up and go (TUG), weight loss, BMI, albumin, ASA classification, and WHO performance status18 had to be registered by the surgeon at the initiation visit. Patients were considered as having modifiable high risk if one of the following was present: haemoglobin above 11.28 g/dl (above 7 mmol/l) for both men and women19, TUG greater or equal to 15 s20,21, weight loss of 10–15 per cent during the last 6 months, BMI below 18.5 kg/m2, or albumin below 30 g/l. The TUG test was performed at the visit and registered as the time spent rising from a chair, walking 2 × 3 m and sitting down again22. If the screening identified a modifiable high-risk indicator, the surgeon could choose to refer the patient for one or more prehabilitation interventions.
For patients in need of anaemia correction, intravenously administered Iron(III)isomaltoside was given on the day of the initiation visit, or as soon as possible (see Supplementary material for full description). Training was performed by referral to a physiotherapist in the patient’s respective municipality for a minimum of four weeks. Nutritional counselling was performed by a dietician with a specialty in colorectal cancer in one session approximately 1 h in length. The current intake was estimated by a general diet history and a 24-h recall. Total energy requirements were estimated by the Harris–Benedict equation23 with an added factor of 1.3–1.5, depending on daily activities. Total protein consumption was aimed at greater than or equal to 1.5 g protein/kg bodyweight. Further, the surgeon prescribed three to four protein drinks (Fresubin, Fresenius Kabi®) daily. Adherence to and compliance with the training, dietary counselling, and nutritional supplements was not recorded.
Inclusion and exclusion criteria
At both institutions, all patients aged 18 or above, referred with colorectal cancer were eligible for inclusion. Patients were excluded if they did not undergo curative intended surgery, emergency resections, or other surgical procedures that did not require skin incision. Patients who were referred to neoadjuvant treatment were assessed by the screening tool after the oncological treatment.
Data sources
Data from the patients treated before the screening implementation were retrospectively extracted from all colorectal procedures in the predefined period recorded in the hospital’s electronic registry. Data were then retrieved by screening electronic health records by two authors and validated by a senior author. A full description of the data extraction is provided in Supplementary material. The data from patients treated after the implementation were prospectively collected from hard-copy screening charts filled out by the surgeon for the intervention hospital and for the control group by registration at the outpatient clinic. Patient and treatment characteristics were subsequently collected from electronic health records.
Modifiable high-risk factors and patients
A ‘modifiable high-risk indicator’ was defined as a factor associated with increased risk of postoperative morbidity, but changeable through preoperative interventions. On this basis, a ‘modifiable high-risk patient’ was defined as a patient who tested positive at least for one indicator.
Outcomes of interest and subgroups comparison
The primary outcome was ‘a complicated postoperative course’, a predefined composite endpoint defined by a hospital stay more than 10 days, unplanned admission to intensive care, readmission within 30 days, a complication with a Clavien–Dindo24 score of 3a or higher within 30 days, or death within 30 days after surgery. Further, each component of a complicated course was analysed separately.
Secondary outcomes were the change in the comprehensive complication index (CCI)25, and 30-days alive and out of hospital index26.
After selection, patients were categorized in each hospital as ‘before implementation’ and ‘after implementation’ according to the timeframe of surgical treatment. The ‘before implementation’ groups were considered as retrospective historical cohorts, whereas the ‘after implementation’ groups were considered as prospective. All groups provided data for the subsequent analyses of the change in outcomes associated with the implementation of the intervention.
Subgroups analyses were conducted at the intervention hospital for patients with modifiable high-risk indicators and patients without and for patients with modifiable high-risk indicators referred for prehabilitation and those who were not.
Variables
Clinical variables included sex and patients’ age (see following section for categories) and smoking status (divided into non-smoker, previous smoker, current smoker, or unknown). Polypharmacy was defined as the prescription of five or more drugs. The surgical procedures were divided into right-sided, which included extended right-sided hemicolectomies and transverse colectomies; left-sided hemicolectomies/sigmoid resections, which included extended left-sided hemicolectomies; rectal resections; and total colectomy. The surgical approach was defined as either open/converted or minimally invasive surgery. The stage of disease was defined by the postoperative pathology description according to UICC staging and computed tomography for distant metastasis. Patients who received neoadjuvant oncological treatment and showed a complete response were defined as stage 0. Patients who were suspected of having (and treated for) malignant disease with oncological resections, but later showed benign pathology were also defined as stage 0. Readmissions were counted as any admission to a hospital with a length of more than 24 h. Postoperative complications were graded by the Clavien–Dindo classification24 and the CCI25. Planned collection of data on alcohol consumption, BMI, weight loss, and WHO performance status was not available for the retrospective before groups due to poor registration in medical records.
Ethics
The study was approved by The Danish Data Protection Agency (REG-044-2019) and representatives of both hospitals’ boards of directors. The intervention was introduced uniformly as a standard of care, and the study was performed as quality assurance. Patients gave informed consent for the surgical treatment, including all perioperative care according to Danish legislation. All reporting was conducted in line with STROBE guidelines27 (Supplementary material).
Statistics
Continuous variables were presented as means and standard deviations or median and interquartile ranges; categorical variables were presented using frequencies and percentages. The patients’ age was first tested for the linearity assumption of the logistic regression and if this was non-significant, this was divided into preplanned groups of under 65, 65–69, 70–74, and 75 years or older.
Differences in patient and treatment characteristics between groups were compared using a Student’s t test on continuous data with acceptable normal distribution, Wilcoxon signed rank test on data without a normal distribution, and Fisher’s exact test and chi-squared test for dichotomous and categorical data. In comparisons between groups before and after implementation of the intervention, a multiple logistic regression was used. Directed acyclic graphs were used to identify potential confounders and the logistic regression models were subsequently adjusted for age, sex, smoking status, ASA score, UICC stage, surgical procedure, and surgical approach (Supplementary material). A possible interaction between age and modifiable high risk was checked. For the primary outcome, a difference-in-differences analysis28,29 was conducted using general estimated equations with a binomial distribution and identity link to estimate absolute risk differences. The difference-in-difference model included variables that specified whether the patient was treated at the intervention or control hospital, whether the surgery occurred before or after implementation of the intervention, and the interaction between these two variables. The coefficient of the interaction can be interpreted as the independent association between the implementation of the intervention and the outcome accounting for changes in secular trends and regression towards the mean30. The difference-in-difference model was in addition, adjusted for the potential confounders of age, sex, smoking status, ASA score, UICC stage, surgical procedure, and surgical approach identified through the directed acyclic graphs. For the explorative secondary outcome of CCI for patients who developed a complication after surgery, an ANOVA was performed comparing the two groups before and after implementation of the intervention. All above-mentioned analyses were preplanned. Subsequently two unplanned analyses were performed in the manuscript revision process: a test of the inter-rater reliability of the extraction process using Cohen’s κ statistics, and an analysis of the change in days alive and out of hospital before and after implementation of the intervention using ANOVA. The inter-rater analysis was conducted to test the agreement of data entries between the junior authors and the subsequent validated data collected by the senior author, thus, showing the consistency of the data extraction process (Table S2 and Supplementary material). A full description of the missing data can be found in Table S2, which were handled by multiple imputations. All analyses were performed in SAS 9.4 (SAS Institute 2013).
Results
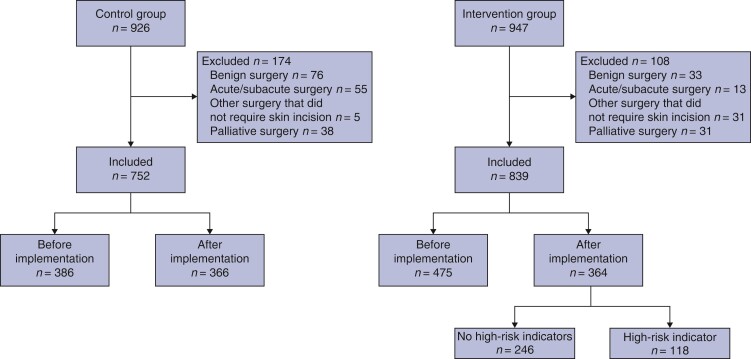
A total of 1873 patients were included in the study with 947 patients included in the intervention group and 926 in the control group (Fig. 1). Subsequently, 282 patients were excluded, leaving 1591 eligible patients for analysis, with 418 patients (26 per cent) having a complicated a hospital course. The patient and clinical characteristics of the two groups are presented in Table 1. The two groups differed in ASA score (P < 0.001), frequency of minimally invasive surgery (93 per cent versus 89 per cent, P < 0.001), and surgical procedure (P = 0.01) with the control hospital having a higher frequency of rectal resections (33 per cent versus 26 per cent). A full comparison of the groups and results of the inter-rater reliability analysis is provided in Tables S2–S4). In the intervention hospital, 364 patients were included after implementation of the intervention, and of those 118 (32 per cent) were identified with at least one modifiable high-risk indicator (Table S5). Patients included in the intervention hospital after implementation had a significantly higher ASA score (P = 0.004), but a lower frequency of polypharmacy (20 per cent versus 28 per cent, P < 0.001) and a higher frequency of minimally invasive surgery (96 per cent versus 91 per cent, P = 0.03) (Table 2).
Fig. 1.
Flowchart of inclusion of patients
Table 1.
Patient and treatment characteristics of included patients at the intervention and control hospital
| Control hospital (n = 752) | Intervention hospital (n = 839) | P | |
|---|---|---|---|
| Age (years), mean (s.d.) | 69.1 (9.98) | 69.5 (9.75) | 0.42 |
| Male sex | 401 (53) | 466 (56) | 0.40 |
| ASA score | <0.001 | ||
| I | 153 (20) | 208 (25) | |
| II | 446 (59) | 521 (62) | |
| III | 151 (20) | 97 (12) | |
| Missing | 2 (0.3) | 13 (1.5) | |
| Polypharmacy (5 or more drugs/day) | 214 (28) | 203 (24) | 0.08 |
| Smoking status | 0.91 | ||
| Non-smoker | 318 (42) | 343 (41) | |
| Former smoker | 301 (40) | 341 (41) | |
| Current smoker | 122 (16) | 136 (16) | |
| Missing | 11 (1.5) | 19 (2) | |
| Stage of disease | 0.24 | ||
| Not malignant or complete remission by neoadjuvant therapy | 48 (6) | 35 (4) | |
| I | 182 (24) | 211 (25) | |
| II | 231 (31) | 281 (33) | |
| III | 257 (34) | 275 (33) | |
| IV | 34 (5) | 38 (5) | |
| Surgical approach | <0.001 | ||
| Primary open | 15 (2) | 20 (2) | |
| Minimally invasive surgery | 670 (89) | 782 (93) | |
| Converted minimally invasive surgery | 67 (9) | 37 (4) | |
| Surgical procedure | 0.01 | ||
| Right-sided hemicolectomy* | 251 (33) | 332 (40) | |
| Left-sided hemicolectomy/sigmoid resection | 239 (32) | 276 (33) | |
| Rectal resection | 251 (33) | 219 (26) | |
| Total colectomy | 11 (1.5) | 12 (1.4) |
Right-sided hemicolectomy includes extended right-sided colectomies and transverse resections. Values are n (%) unless stated otherwise.
Table 2.
Baseline patients’ characteristics and outcomes in the intervention hospital before and after implementation
| Before implementation (n = 475) | After implementation (n = 364) | P | |
|---|---|---|---|
| Age (years), mean (s.d.) | 69 (10.0) | 70 (9.4) | 0.20 |
| Male sex | 270 (57) | 196 (54) | 0.40 |
| ASA score | 0.004 | ||
| I | 126 (27) | 82 (23) | |
| II | 302 (64) | 219 (60) | |
| III | 40 (8) | 57 (16) | |
| Missing | 7 (1.5) | 6 (1.6) | |
| Polypharmacy (5 or more drugs/day) | 131 (28) | 72 (20) | <0.001 |
| Smoking status | 0.40 | ||
| Non-smoker | 193 (41) | 150 (41) | |
| Former smoker | 201 (42) | 140 (38) | |
| Current smoker | 71 (15) | 65 (18) | |
| Missing | 10 (2) | 9 (2) | |
| Stage of disease | 0.06 | ||
| Not malignant or complete remission by neoadjuvant therapy | 20 (4) | 14 (4) | |
| 1 | 111 (23) | 100 (27) | |
| 2 | 151 (32) | 130 (36) | |
| 3 | 175 (37) | 100 (27) | |
| 4 | 18 (4) | 20 (5) | |
| Surgical approach | 0.03 | ||
| Primary open | 13 (3) | 7 (2) | |
| Minimally invasive surgery | 434 (91) | 348 (96) | |
| Converted minimally invasive surgery | 28 (6) | 9 (2) | |
| Type of operation | 0.01 | ||
| Right-sided hemicolectomy* | 173 (36) | 159 (44) | |
| Left-sided hemicolectomy/ sigmoid resection | 152 (32) | 124 (34) | |
| Rectal resection | 140 (29) | 79 (22) | |
| Total colectomy | 10 (2) | 2 (0.5) | |
| Length of stay (days) median (i.q.r.) | 4 (3–6) | 4 (2–5) | 0.03 |
| Unplanned stay at ICU | 34 (7) | 16 (4) | 0.11 |
| 30-day mortality | 4 (0.84) | 2 (0.55) | 0.70 |
| Complication with Clavien–Dindo score 3a or above | 104 (22) | 29 (8) | <0.001 |
| No. (%) readmission | 69 (15) | 35 (10) | 0.04 |
| 30-days alive and out of hospital median (i.q.r.) | 26 (23–27) | 26 (24–27) | 0.001 |
Right-sided hemicolectomy includes extended right-side colectomies and transverse resections. Values are n (%) unless stated otherwise.
Primary outcome
In the multivariable analyses comparing after versus before implementation, adjusted for age, sex, smoking, stage of disease, ASA, surgical procedure, and surgical approach found an odds ratio (OR) of a complicated hospital course of 0.54 (95 per cent c.i. 0.38 to 0.77) at the intervention hospital, whereas it remained unchanged at the control hospital (OR 1.05 (95 per cent c.i. 0.75 to 1.47)) (Table 3). In the difference-in-difference analysis, implementation of the intervention was associated with an absolute risk reduction of 10.9 per cent (95 per cent c.i. 2.1 to 19.7 per cent) of a complicated course and 11.6 per cent (95 per cent c.i. 3.3 to 19.8 per cent) of developing a complication Clavien–Dindo score of 3a or higher.
Table 3.
Association between before and after implementation of the intervention. Results of multivariable logistic regression analyses and the difference-in-difference analysis, both adjusted for age, sex, smoking status, ASA score, stage of disease, surgical procedure, and surgical approach
| After versus before implementation | Before versus after implementation and intervention versus control absolute risk reduction (95% c.i.) | ||
|---|---|---|---|
| Intervention hospital (n = 839) Odds ratio (95% c.i.) | Control hospital (n = 752) Odds ratio (95% c.i.) | ||
| Complicated course | 0.54 (0.38 to 0.77) | 1.05 (0.75 to 1.47) | 10.9% (2.1 to 19.7), P = 0.02 |
| Complication Clavien–Dindo score 3a or above | 0.30 (0.19 to 0.48) | 0.92 (0.60 to 1.40) | 11.6% (3.3 to 19.8), P = 0.006 |
| Duration of hospital stay 10 days or more | 0.61 (0.38 to 1.00) | 1.53 (0.99 to 2.37) | 7.5% (−2.2 to 17.2), P = 0.13 |
| Unplanned stay at ICU | 0.60 (0.31 to 1.15) | 1.17 (0.57 to 2.42) | 2.2% (−6.9 to 11.4), P = 0.63 |
| Readmission within 30 days | 0.65 (0.41 to 1.02) | 0.81 (0.53 to 1.25) | 2.3% (−4.3 to 8.9), P = 0.50 |
| 30-day mortality | 0.59 (0.10 to 3.58) | 0.59 (0.12 to 2.89) | 0.5% (−11.7 to 12.8), P = 0.93 |
Secondary outcomes
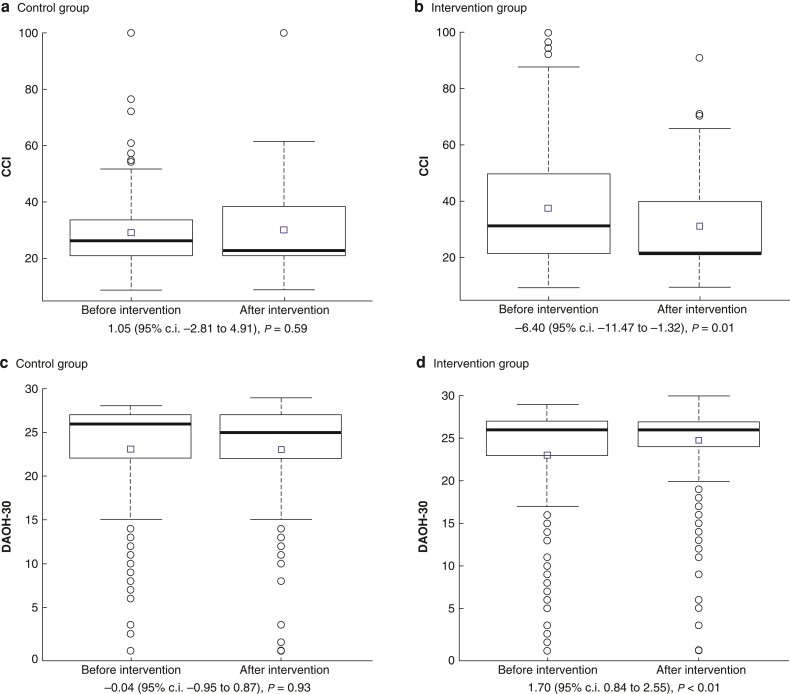
In the analysis of the CCI of patients who developed a complication, a significant decrease in the intervention group after implementation (P = 0.01) was documented, whereas no significant change was found in the control group (P = 0.59) (Fig. 2). When comparing 30-days alive and out of hospital before and after implementation, a mean increase of 1.70 days (95 per cent c.i. 0.84 to 2.55) was found at the intervention centre after implementation, whereas it remained unchanged at the control centre (−0.04 days (95 per cent c.i. −0.95 to 0.87)). Of the 118 patients identified with at least one modifiable high-risk indicator with 93 (79 per cent) had a haemoglobin level above 11.28 g/dl, 19 (16 per cent) had a TUG of 15 s or higher, and 35 (30 per cent) had decreased nutritional status. Seventy-four patients (63 per cent) were referred to a prehabilitation effort of a median length of 29 days (i.q.r. 27–35 (range 6–70) days). A full description can be found in Tables S6 and S7. No difference in a complicated course was found between the modifiable high-risk group that underwent an intervention compared with the group without modifiable high-risk indicators (OR 1.52 (95 per cent c.i. 0.71 to 3.23)). Furthermore, no difference was found between the modifiable high-risk group compared with the group without modifiable high-risk indicators (OR 1.26 (95 per cent c.i. 0.66 to 2.40)).
Fig. 2.
Boxplots and results of the ANOVA
Boxplots of the severity of complications measured by the comprehensive complication index and 30-days alive and out of hospital, before and after implementation in the intervention and control group. Results of the ANOVA are shown in the boxes in the top right corner. The square represents the mean and extreme observations by circles CCI, comprehensive complication index; DAOH-30, 30-days alive and out of hospital.
Discussion
In this study, a screening tool with an evaluation of three modifiable high-risk indicators, along with prehabilitation initiatives aimed at these indicators, was introduced in a clinical setting. Of the 364 consecutive screened patients with colorectal cancer, 32 per cent had a minimum of one high-risk indicator. In the difference-in-differences analysis, the intervention was associated with an absolute risk reduction of a complicated course by 10.9 per cent (95 per cent c.i. 2.1 to 19.7 per cent), primarily from a reduction in the frequency of severe complications.
Low functional capacity, anaemia, and poor nutritional status are all known risk factors for adverse outcomes following colorectal cancer surgery7,22,31. In three randomized controlled trials, preoperative exercise has reduced the risk of postoperative complications after major surgery13,14,32. Two of the studies were conducted in patients with colorectal cancer and both selected high-risk patients with low functional capacity in a setting with preoperative nutritional counselling and one of them with concomitant anaemia correction13,14. Other prehabilitation studies in patients with colorectal cancer without the high-risk patient focus have not been able to show the same effect33,34 and a recent study including frail patients was not able to show a reduction in complications12. The primary benefit of preoperative nutritional support is found when intervening preoperatively in malnourished patients35. Anaemia is associated with postoperative complications and perioperative blood transfusions36 but whether anaemia correction alone reduces risks of complications remains unanswered. This suggests that interventions towards patients with modifiable high-risk indicators utilizing the potential synergy of a multimodal intervention could provide an additional effect. However, clinical randomized studies that involve demanding interventions and high patient participation, such as multimodal prehabilitation, have innate volunteer and selection bias37.
A primary strength of this study is introducing the screening tool and prehabilitation interventions as an addition to a clinical setting, which included minimal invasive surgery and patient care within ERAS standards16,17. Adherence to ERAS principles reduces postoperative complications after colorectal cancer surgery38, thus the results show the effect of the intervention in an optimized clinical setting. Further, by introducing screening and prehabilitation into a clinical setting, the external validity is high without the risk of volunteer bias.
However, using a quasi-experimental study design has several limitations39, and causality between the screening or prehabilitation interventions and the reduced risk of a complicated course needs to be questioned when interpretating the data. The risk assessment tool could introduce a selection bias where the surgeons would be less inclined to offer surgery for patients with high-risk indicators. A possible indication of this can be seen in the significant reduction in patients with polypharmacy after the implementation. Thus, causality between prehabilitation and risk reduction cannot be inferred, which is a limitation. Another limitation was that data regarding compliance and adherence to the dietary and physical training were not collected. Further, even though the analyses were adjusted for secular trends, open surgery, co-morbidities, and surgical procedure a chronological bias could still remain. In colorectal cancer surgery, the risk of complications, mortality, and length of stay has been reduced in recent years through the introduction of several interventions, technological advances, and policies. However, the pace at which these are implemented at the departments may differ and potentially affect the parallel trends assumption of the difference-in-differences analyses. An indication of chronological bias can be seen in the significantly higher frequency of minimally invasive surgery after implementation at the intervention hospital (96 per cent versus 91 per cent). Another difference between the groups was the higher frequency of right-sided hemicolectomies. This procedure is associated with a lower risk of complications and generally shorter length of stay compared to sigmoid and rectal cancer surgery but to meet these potential confounders, both the surgical approach and surgical procedure were introduced in the analyses. Interestingly, no difference was documented in the subgroup analyses, between patients with high-risk indicators, and those without, and between patients with high-risk indicators who underwent prehabilitation efforts and those who did not. Interpretation of these results is difficult as they could indicate that the primary effect lies in the screening and subsequent selection of patients for surgery, that prehabilitation negated the risk associated with the high-risk indicators, and/or that the surgeons selected only patients severely affected by the high-risk indicators. However, the lack of statistical significance of these results may well lie in the limited sample sizes in these subgroups, which is indicated by the wide confidence intervals. The data abstractors were not blinded for the study objective, which despite the good inter-rater reliability, could lead to bias. Last, there was a difference in available information from the medical records. Performance status, BMI, weight loss, and alcohol consumption were missing in the majority of cases in both before groups, which shows the lacking interest in these risk factors before the intervention. By increasing the focus on high-risk patients and setting a high-risk screening as standard of care combined with interventions aimed directly at these risk factors, the surgeons may have altered their perception, decisions, and care40. Though, speculative, this could explain why after implementation, the intervention group had a significantly higher frequency of ASA III patients (16 per cent versus 8 per cent), despite the lower frequency of polypharmacy.
Recent systematic reviews concerning prehabilitation find significant heterogeneity between studies with varying evidence and lack of clinical outcomes, concluding the need for more studies to identify the optimal screening and prehabilitation program before implementation41,42. Numerous screening tools have been suggested but only a few parameters seem to be valid and reliable to predict complications43. Predictive measures that have the potential to be altered before surgery are therefore especially of clinical interest. Further studies are needed to identify more potential modifiable risk factors and randomized trials with prehabilitation focusing on modifiable high-risk patients to estimate accurate risk reduction.
Supplementary Material
Acknowledgements
R.D.B. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. R.D.B. and C.G. conceived the idea for the study. R.D.B., C.G., J.R.E., and I.G. planned the study. R.D.B., C.G., F.B., R.E.G.M., and J.R.E. collected the data. R.D.B. performed the statistical analysis. All authors interpreted the results. R.D.B. prepared the manuscript, and all others critically revised the manuscript for important content. All authors approved the final version of the manuscript for publication.
Disclosure. I.G. reports educational grants from Johnson & Johnson, Intuitive Surgical, Olympus, and an unrestricted research grant from Pharmacosmos, outside the submitted work. The authors declare no other conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The datasets used in the study are available from the corresponding author upon reasonable request.
References
- 1. Brown SR, Mathew R, Keding A, Marshall HC, Brown JM, Jayne DG. The impact of postoperative complications on long-term quality of life after curative colorectal cancer surgery. Ann Surg 2014;259:916–923 [DOI] [PubMed] [Google Scholar]
- 2. Couwenberg AM, de Beer FSA, Intven MPW, Burbach JPM, Smits AB, Consten ECJet al. . The impact of postoperative complications on health-related quality of life in older patients with rectal cancer: a prospective cohort study. J Geriatr Oncol 2018;9:102–109 [DOI] [PubMed] [Google Scholar]
- 3. Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg 2005;242:326–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel AS, Bergman A, Moore BW, Haglund U. The economic burden of complications occurring in major surgical procedures: a systematic review. Appl Health Econ Health Policy 2013;11:577–592 [DOI] [PubMed] [Google Scholar]
- 5. Bose S, Talmor D. Who is a high-risk surgical patient? Curr Opin Crit Care 2018;24:547–553 [DOI] [PubMed] [Google Scholar]
- 6. Minnella EM, Liberman AS, Charlebois P, Stein B, Scheede-Bergdahl C, Awasthi Ret al. . The impact of improved functional capacity before surgery on postoperative complications: a study in colorectal cancer. Acta Oncol 2019;58:573–578 [DOI] [PubMed] [Google Scholar]
- 7. Bruns ERJ, Borstlap WA, van Duijvendijk P, van der Zaag-Loonen HJ, Buskens CJ, van Munster BCet al. . The association of preoperative anemia and the postoperative course and oncological outcome in patients undergoing rectal cancer surgery. Dis Colon Rectum 2019;62:823–831 [DOI] [PubMed] [Google Scholar]
- 8. Hu W-H, Cajas-Monson LC, Eisenstein S, Parry L, Cosman B, Ramamoorthy S. Preoperative malnutrition assessments as predictors of postoperative mortality and morbidity in colorectal cancer: an analysis of ACS-NSQIP. Nutr J 2015;14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gillis C, Buhler K, Bresee L, Carli F, Gramlich L, Culos-Reed Net al. . Effects of nutritional prehabilitation, with and without exercise, on outcomes of patients who undergo colorectal surgery: a systematic review and meta-analysis. Gastroenterology 2018;155:391–410 [DOI] [PubMed] [Google Scholar]
- 10. Moran J, Guinan E, McCormick P, Larkin J, Mockler D, Hussey Jet al. . The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery 2016;160:1189–1201 [DOI] [PubMed] [Google Scholar]
- 11. Lau CSM, Chamberlain RS. Prehabilitation programs improve exercise capacity before and after surgery in gastrointestinal cancer surgery patients: a meta-analysis. J Gastrointest Surg 2020;24:2829–2837 [DOI] [PubMed] [Google Scholar]
- 12. Carli F, Bousquet-Dion G, Awasthi R, Elsherbini N, Liberman S, Boutros Met al. . Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial. JAMA Surg 2020;155:233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barberan-Garcia A, Ubré M, Roca J, Lacy AM, Burgos F, Risco Ret al. . Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg 2018;267:50–56 [DOI] [PubMed] [Google Scholar]
- 14. Berkel AEM, Bongers BC, Kotte H, Weltevreden P, de Jongh FHC, Eijsvogel MMMet al. . Effects of community-based exercise prehabilitation for patients scheduled for colorectal surgery with high risk for postoperative complications. Ann Surg 2021;275:e299–e306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Milder DA, Pillinger NL, Kam PCA. The role of prehabilitation in frail surgical patients: a systematic review. Acta Anaesthesiol Scand 2018;62:1356–1366 [DOI] [PubMed] [Google Scholar]
- 16. Scott MJ, Baldini G, Fearon KCH, Feldheiser A, Feldman LS, Gan TJet al. . Enhanced recovery after surgery (ERAS) for gastrointestinal surgery, part 1: pathophysiological considerations. Acta Anaesthesiol Scand 2015;59:1212–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feldheiser A, Aziz O, Baldini G, Cox BPBW, Fearon KCH, Feldman LSet al. . Enhanced recovery after surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand 2016;60:289–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Young J, Badgery-Parker T, Dobbins T, Jorgensen M, Gibbs P, Faragher Iet al. . Comparison of ECOG/WHO performance status and ASA score as a measure of functional status. J Pain Symptom Manage 2015;49:258–264 [DOI] [PubMed] [Google Scholar]
- 19. van Rooijen S, Carli F, Dalton SO, Johansen C, Dieleman J, Roumen Ret al. . Preoperative modifiable risk factors in colorectal surgery: an observational cohort study identifying the possible value of prehabilitation. Acta Oncol 2017;56:329–334 [DOI] [PubMed] [Google Scholar]
- 20. Robinson TN, Wu DS, Sauaia A, Dunn CL, Stevens-Lapsley JE, Moss Met al. . Slower walking speed forecasts increased postoperative morbidity and 1-year mortality across surgical specialties. Ann Surg 2013;258:582–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–148 [DOI] [PubMed] [Google Scholar]
- 22. Huisman MG, van Leeuwen BL, Ugolini G, Montroni I, Spiliotis J, Stabilini Cet al. . ‘Timed Up & Go’: a screening tool for predicting 30-day morbidity in onco-geriatric surgical patients? A multicenter cohort study. PLoS ONE 2014;9:e0086863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frankenfield DC, Muth ER, Rowe WA. The Harris–Benedict studies of human basal metabolism. J Am Diet Assoc 1998;98:439–445 [DOI] [PubMed] [Google Scholar]
- 24. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Slankamenac K, Nederlof N, Pessaux P, de Jonge J, Wijnhoven BPL, Breitenstein Set al. . The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg 2014;260:757–763 [DOI] [PubMed] [Google Scholar]
- 26. Jerath A, Austin PC, Wijeysundera DN. Days alive and out of hospital. Anesthesiology 2019;131:84–93 [DOI] [PubMed] [Google Scholar]
- 27. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JPet al. . The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–1457 [DOI] [PubMed] [Google Scholar]
- 28. Dimick JB, Ryan AM. Methods for evaluating changes in health care policy. JAMA 2014;312:2401.– [DOI] [PubMed] [Google Scholar]
- 29. Wing C, Simon K, Bello-Gomez RA. Designing difference in difference studies: best practices for public health policy research. Annu Rev Public Health 2018;39:453–469 [DOI] [PubMed] [Google Scholar]
- 30. Lechner M. The estimation of causal effects by difference-in-difference methods. Found Trends Econom 2010;4:165–224 [Google Scholar]
- 31. Ugolini G, Ghignone F, Zattoni D, Veronese G, Montroni I. Personalized surgical management of colorectal cancer in elderly population. World J Gastroenterol 2014;20:3762–3777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair. Ann Surg 2016;264:47–53 [DOI] [PubMed] [Google Scholar]
- 33. Bousquet-Dion G, Awasthi R, Loiselle S-È, Minnella EM, Agnihotram RV, Bergdahl Aet al. . Evaluation of supervised multimodal prehabilitation programme in cancer patients undergoing colorectal resection: a randomized control trial. Acta Oncol 2018;57:849–859 [DOI] [PubMed] [Google Scholar]
- 34. Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa Aet al. . Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology 2014;121:937–947 [DOI] [PubMed] [Google Scholar]
- 35. Jie B, Jiang Z-M, Nolan MT, Zhu S-N, Yu K, Kondrup J. Impact of preoperative nutritional support on clinical outcome in abdominal surgical patients at nutritional risk. Nutrition 2012;28:1022–1027 [DOI] [PubMed] [Google Scholar]
- 36. Pang Q-Y, An R, Liu H-L. Perioperative transfusion and the prognosis of colorectal cancer surgery: a systematic review and meta-analysis. World J Surg Oncol 2019;17:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barreto P de S, Ferrandez A-M, Saliba-Serre B. Are older adults who volunteer to participate in an exercise study fitter and healthier than nonvolunteers? The participation bias of the study population. J Phys Act Heal 2013;10:359–367 [PubMed] [Google Scholar]
- 38. Adamina M, Kehlet H, Tomlinson GA, Senagore AJ, Delaney CP. Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery 2011;149:830–840 [DOI] [PubMed] [Google Scholar]
- 39. Goodacre S. Uncontrolled before-after studies: discouraged by Cochrane and the EMJ. Emerg Med J 2015;32:507–508 [DOI] [PubMed] [Google Scholar]
- 40. Fry DE. The hawthorne effect revisited. Dis Colon Rectum 2018;61:6–7 [DOI] [PubMed] [Google Scholar]
- 41. Ismail H, Cormie P, Burbury K, Waterland J, Denehy L, Riedel B. Prehabilitation prior to major cancer surgery: Training for surgery to optimize physiologic reserve to reduce postoperative complications. Curr Anesthesiol Rep 2018;8:375–385 [Google Scholar]
- 42. Luther A, Gabriel J, Watson RP, Francis NK. The impact of total body prehabilitation on post-operative outcomes after major abdominal surgery: a systematic review. World J Surg 2018;42:2781–2791 [DOI] [PubMed] [Google Scholar]
- 43. Apóstolo J, Cooke R, Bobrowicz-Campos E, Santana S, Marcucci M, Cano Aet al. . Predicting risk and outcomes for frail older adults: an umbrella review of frailty screening tools. JBI Database Syst Rev Implement Rep 2017;15:1154–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used in the study are available from the corresponding author upon reasonable request.