Abstract
Background
Every year, over 4 million children are treated for severe acute malnutrition with varying program performance. This study sought to explore the predictors of time to recovery from and non-response to outpatient treatment of SAM.
Methods
Children with weight-for-height z-score (WHZ) <-3 and/or mid-upper arm circumference (MUAC) <115 mm, without medical complications were enrolled in a trial (called MANGO) from outpatient clinics in Burkina Faso. Treatment included a weekly ration of ready-to-use therapeutic foods. Recovery was declared with WHZ ≥-2 and/or MUAC ≥125 mm, for two weeks without illness. Children not recovered by 16 weeks were considered as non-response to treatment. Predictors studied included admission characteristics, morbidity and compliance during treatment and household characteristics. Cox proportional hazard models were fitted and restricted mean time to recovery calculated. Logistic regression was used to analyse non-response to treatment.
Results
Fifty-five percent of children recovered and mean time to recovery was eight weeks while 13% ended as non-response to treatment. Independent predictors of longer time to recovery or non-response included low age, being admitted with WHZ <-3, no illness nor anaemia at admission, illness episodes during treatment, skipped or missed visits, low maternal age and not practising open defecation. Eighty-four percent of children had at least one and 59% at least two illness episodes during treatment. This increased treatment duration by 1 to 4 weeks. Thirty-five percent of children missed at least one treatment visit. One missed visit predicted 3 weeks longer and two or more missed visits 5 weeks longer treatment duration.
Conclusions
Both longer time to recovery and higher non-response to treatment seem most strongly associated with illness episodes and missed visits during treatment. This indicates that prevention of illnesses would be key to shortening the treatment duration and that there is a need to seek ways to facilitate adherence.
Introduction
Severe acute malnutrition (SAM) is a condition that occurs when the food intake does not meet the nutritional requirements either as a consequence of poor intake or disease [1]. In children 6–59 months of age, SAM is diagnosed when a child presents with a weight-for-height z-score (WHZ) <-3, a mid-upper-arm circumference (MUAC) <125 mm or nutritional oedema [2]. While the overall prevalence of SAM is unknown, in 2020 it was estimated that 2% of all children below the age of 5 years presented a WHZ <-3 translating to more than 13.6 million children suffering from severe wasting at any time [3]. Children with SAM have a 11.6 increased risk of mortality compared to children with no nutritional deficits living in the same contexts [4].
Generally SAM arises in contexts with social, political and economic factors affecting food availability and where infections and inflammation are common [1]. This is also why no single intervention has been shown to reduce the incidence that requires a holistic package of interventions [5].
According to the World Health Organisation (WHO), children with SAM without medical complications are treated as outpatients with weekly check-up visits [6]. The treatment includes a systematic antibiotic regimen and ready-to-use therapeutic foods (RUTF), prescribed according to the weight of the child and continued until discharge [6]. RUTF are energy and nutrient dense pastes usually composed of peanut butter, milk powder, oil, sugar and a vitamin and mineral complex designed to fulfil the nutritional needs of children recovering from SAM [7]. Recovery is defined as having reached a WHZ ≥-2 for those admitted with a WHZ <-3 or a MUAC ≥125 mm for those admitted with a MUAC <115 mm for at least two weeks [6]. Children that never attain recovery within a maximum treatment time are called non-response to treatment [8].
In 2020, around 5 million children were treated for SAM globally [9]. With 13.6 million children suffering from severe wasting at any time [3] and applying of incidence correction factor of 3.5 [10] to account for all new cases arising in a year, this translates to 47.6 million episodes of severe wasting in a year. Thus, the current coverage of treatment is around 10%. This is when excluding the burden of MUAC cases which, if added, would translate to an even lower coverage of treatment. Such low coverage warrants reflection on how to improve it possibly by optimising and better targeting treatment in order to expand it to more cases.
Children treated for SAM in different contexts present with varying mean treatment time and proportion of non-response to treatment [11–15]. Longer time in treatment and high percent of non-response to treatment increase the cost of treatment and question the effectiveness of the current treatment protocol. Little is known about the factors influencing treatment duration and non-response to treatment. Understanding who requires longer treatment time and which children are at the greatest risk of non-response to treatment could help guide the optimisation of SAM treatment and explain differences in program performance observed between different contexts.
The current study seeks to explore the predictors of time to recovery from and non-response to treatment of SAM in a community based treatment setting. The study is based on data collected during a randomised controlled trial comparing the efficacy of a reduced dose of RUTF with a standard dose on the treatment outcomes of children with uncomplicated SAM managed in outpatient care. The trial showed no significant effect of the dose reduction on the weight gain velocity, length of stay in treatment, recovery percent or proportion of non-response to treatment [16].
Methods
Ethics
The MANGO study was performed in accordance with the principles in the Declaration of Helsinki. The research protocol was approved by the national ethics committee (Comité d’éthique pour la Recherche en Santé) and the clinical trials board (Direction Générale de la Pharmacie, du Médicament et des Laboratoires) of Burkina Faso and was registered at the IRSCTN registry http://www.isrctn.com/ISRCTN50039021. Caregivers of participating children gave their informed consent in a written form.
Study design and setting
This study is based on data collected prospectively as part of a randomized controlled trial (called MANGO), which compared the efficacy of a reduced RUTF dose to a standard RUTF dose in the management of SAM without medical complications in children 6–59 months of age in a non-inferiority design. The results from the randomized controlled trial have been published elsewhere [16–22]. The study recruited a total of 801 children which provides a good sample size for analysing predictors compared to previous studies looking at time to recovery with samples sizes starting from 200 and most around 400 children treated [23–34].
The trial was conducted in 10 health centres of the Fada N’Gourma health district in eastern Burkina Faso. Malaria was endemic with 69.3% of children in the region presenting a positive rapid test [35]. In 2016, the prevalence of severe wasting (WHZ <-3) and moderate wasting (WHZ between -3 and -2) was 2.4% and 8.6%, respectively [36]. Most households depend on small scale farming and livestock ownership [37] and 32% of the population lives more than 10 km away from nearest health post [38].
Study participants and treatment protocol
The participants included all children enrolled in the clinical trial for which the enrolment procedures have been described in detail elsewhere [16]. In short, participants were children presenting with WHZ <-3 and/or MUAC <115 mm but without medical complications, at the 10 participating health centres. Children with any grade of oedema or no appetite were referred to inpatient care.
Treatment followed the Burkina Faso national community-based management of acute malnutrition (CMAM) guidelines [39] in all aspects except the RUTF dose. Half of the children received a reduced dose from the third treatment week onwards: One sachet per day to children weighing <7 kg and two to children ≥7 kg, representing a reduction between 30 and 54% compared to standard dose depending on the weight category [16]. Co-morbidities diagnosed during SAM treatment were managed according to national protocol [39]. In case of medical complications, weight loss of over 5% at any point or stagnant weight defined as no more than 100g weight gained over 4 weeks, during treatment, children were referred to inpatient care, as per the Burkina national CMAM protocol [39]. Nutritional treatment was continued weekly until recovery.
Recovery was defined as having attained 1) a WHZ ≥-2 for those admitted with a WHZ <-3, or 2) a MUAC ≥125 mm for those admitted with a MUAC <115 mm, or 3) a WHZ ≥-2 and a MUAC ≥125 mm for those admitted with both WHZ<-3 and MUAC <115 mm, for 2 consecutive weeks and absence of illness. Children still not having reached the anthropometric recovery criteria by 16 weeks of treatment were declared as non-response to treatment. Other discharge categories included defaulting (defined as having missed 3 consecutive visits and confirmed alive), loss-to follow up (defined as having missed 3 consecutive visits and not confirmed alive), death, and false discharge (those discharged wrongly after verification of discharge anthropometrics).
Data collection
Upon admission, the child’s caregiver was interviewed regarding household socio-economic characteristics, care practices and recent morbidity of the child. Anthropometric measurements and a clinical examination were performed at each visit from admission to discharge. Weight was measured using an electronic scale (SECA 876) to the nearest 100 g, height using a wooden measuring board (locally made) to the nearest 1 mm, and MUAC using a non-stretchable colourless measuring tape to the nearest 1 mm. Z-scores were calculated using the WHO standards and STATA command zscore06 [40]. Haemoglobin (HemoCue) was measured at admission. All data were collected via tablets using the Open Data Kit (ODK1 software) and continuous data monitoring and cleaning was performed. Data monitoring included among other thing, checking duplicate entries and outliers, anthropometric decimal distributions and correct prescription of medication according to diagnosed conditions. Any potential data problem resulted in action. Data cleaning was based on double checks of electronic data against patient registries or therapeutic cards.
Outcomes
Two outcomes were studied: 1) time to recovery defined as days passed from admission to treatment until discharge as recovered, and 2) non-response to treatment defined as not reaching the anthropometric recovery criteria within 16 weeks. Recovery and non-response were dichotomised to recovered or non-recovered and response or non-response, respectively. For the study of time to recovery, non-recovered cases were right censored contributing to the analysis of time to recovery until exit from study. Patients referred to inpatient care were excluded from the analysis in order to limit potential bias that could be introduced due to their short length of stay in treatment.
Predictors
Predictors included in the analyses were variables describing 1) admission characteristics, 2) morbidity and compliance to treatment visits during treatment and 3) household characteristics. Admission characteristics included sex, age, WHZ, MUAC, height for-age z-score (HAZ), admission criteria (WHZ <-3, MUAC <115 mm or both), any illness, anaemia, breastfeeding status and low birth weight. Illness at admission was defined as any caregiver reported illness (including cough, diarrhoea, fever, vomiting, skin lesions) observed in the week prior to admission or diagnosed by study nurse upon admission. Anaemia was defined as a haemoglobin level <110 g/l [41]. Low birth weight (<2500 g) was confirmed from an official birth certificate or health card. Morbidity and compliance variables included an episode of malaria, acute respiratory illness (ARI) or diarrhoea during treatment and number of illness episodes as well as number of skipped and missed visits. Malaria episode during treatment was defined as an armpit temperature of >37.5°C, a positive malaria rapid diagnostic test (RDT) and a negative RDT at admission. ARI was defined as cough reported by caregiver in the past week or diagnosed by study nurse during visit. Diarrhoea included acute, persistent or dysenteric forms and was defined as 3 or more loose stools per day as reported by caregiver in the past week or diagnosed by study nurse. Illness episodes included any caregiver reported or nurse diagnosed illness in the past week. Skipped visits were those that were planned in advance and thus the caregiver was prescribed double dose of RUTF to cover 2 weeks of home treatment. Missed visits were unplanned and thus represent gaps in RUTF prescription. Household characteristics included caregiver’s age, education level, whether caregiver was the household head, number of children under 5 years of age in the household, water source, open defecation (the practice of defecating in nature instead of a toilet facility) or not, food security status, distance from health centre and urban or rural setting. Safe water source was defined as a protected well, pump or tap while unsafe water source included unprotected wells and rivers, lakes and ponds. Food security assessment was based on the Household Food Insecurity Access Scale (HFIAS) [42]. Distance from health centre was estimated by the caregivers as the time needed for a return trip with the available transportation means.
Data analysis
All children included in the MANGO trial regardless of their treatment arm (reduced RUTF or standard RUTF) were included in the analysis. Baseline characteristics of the study population are summarized as percent (n) or mean ±SD. Cox proportional hazard regression models were fitted to quantify effects of predictors on time to recovery with resulting hazard ratios describing the increased or decreased chance of recovery. Kaplan Meier plots were used to visualize results concerning age and admission categories. Restricted mean time to recovery was estimated for significant categorical predictors. Logistic regression was used to evaluate predictors of non-response to treatment. Both unadjusted and models adjusted for sex and age at admission were fitted. A p-value below 0.05 was used to declare statistical significance. All analyses were performed using STATA 15 (StataCorp, USA) and Kaplan Meier plots were produced in GraphPad Prism 8 (GraphPad Software, USA).
Results
In total, 801 children were enrolled in the RCT, of which 54% (n = 433) recovered with a median [IQR] time to recovery of 8 weeks [5–12]. Thirteen percent (n = 101) were considered non-response to treatment, 20% (n = 157) were referred to inpatient care due to stagnant weight, weight loss or medical complication, 10% (n = 83) defaulted, 3% (n = 24) were falsely discharged, 2 children died and 1 was lost to follow up. Excluding the 157 referrals, 644 children contributed to the analysis of time to recovery and all 801 to the analysis of non-response to treatment.
At admission, children were on average 13 months old, 86% were breastfed, 79% had an illness, and 80% had anaemia (Table 1). Most households (83%) had access to a safe drinking water source but 79% were practising open defecation. During treatment, 84% of children had at least one illness episode with 59% reporting at least two episodes. Up to 35% of children missed at least one treatment visit with 16% missing more than one visit.
Table 1. Characteristics of children included in the analysis of time to recovery.
| Characteristics | Values |
|---|---|
| 1. Admission characteristics | |
| Male, % (n) | 47 (305) |
| Age, months | 13.4 ± 8.4 |
| WHZ at admission | -3.0 ± 0.7 |
| MUAC at admission, mm | 113.1 ± 6.2 |
| HAZ at admission | -2.4 ± 1.3 |
| Admission criteria, % (n) | |
| WHZ only | 27 (171) |
| MUAC only | 39 (252) |
| both WHZ and MUAC | 34 (221) |
| Illness, % (n) | 79 (507) |
| Anaemia, % (n) | 80 (514) |
| Low birth weight, % (n)1 | 20 (84) |
| Breastfeeding, % (n) | 86 (552) |
| 2. Morbidity and compliance during treatment | |
| Malaria episode, % (n) | 16 (105) |
| ARI episode, % (n) | 47 (303) |
| Diarrhoea episode, % (n) | 27 (177) |
| Number of illness episodes, % (n) | |
| none | 16 (102) |
| one | 25 (163) |
| two | 18 (113) |
| three or more | 41 (266) |
| Number of skipped* visits, % (n) | |
| none | 48 (309) |
| one | 25 (163) |
| two or more | 27 (172) |
| Number of missed* visits, % (n) | |
| none | 65 (418) |
| one | 19 (120) |
| two or more | 16 (106) |
| 3. Household characteristics | |
| Maternal age, years | 27.9 ± 7.7 |
| Mother has some formal education, % (n) | 24 (154) |
| Caregiver is the household head, % (n) | 3 (21) |
| Number of children under 5 in the household, % (n) 1 | |
| only index child | 31 (201) |
| two | 34 (220) |
| three or more | 34 (218) |
| Using safe water source, % (n) | 83 (533) |
| Open defecation, % (n) | 77 (496) |
| Household is food secure, % (n) | 89 (575) |
| >30 min return distance from health centre, % (n) | 63 (407) |
| Urban setting, % (n) | 14 (89) |
Values are mean ± SD unless otherwise indicated
1 Birth weight was only available for 410 children and number of children in the household for 639 of all 644 children included in the time to recovery analysis
* skipped visits refer to those that were planned and thus benefitted from double RUTF prescription as opposed to missed visits that were unplanned
ARI, acute respiratory infection; HAZ, height-for-age z-score; MUAC, mid-upper arm circumference; WHZ, weight-for-height z-score.
Unadjusted and adjusted estimates of associations with time to recovery are presented in Table 2. When adjusted for sex and age at admission, independent predictors of longer time to recovery included low age, low WHZ at admission, being admitted with WHZ <-3 (compared to only MUAC <115mm), not having illness or anaemia at admission, having any co-morbidity episode during treatment, higher number of missed treatment visits, low maternal age and not practising open defecation. For example, for every 1 z-score increase in WHZ at admission, children have 26% higher chance of recovery.
Table 2. Predictors of time to recovery (days) among 644 children during outpatient treatment of severe acute malnutrition.
| Predictors | n | Unadjusted | Sex and age adjusted2 | |||||
|---|---|---|---|---|---|---|---|---|
| Event | Censored | HR1 | 95% CI | p-value | HR1 | 95% CI | p-value | |
| 1.Admission characteristics | ||||||||
| Sex | ||||||||
| Male | 209 | 96 | Ref | Ref | ||||
| Female | 224 | 115 | 0.86 | 0.71; 1.04 | 0.12 | 0.88 | 0.73; 1.07 | 0.20 |
| Age, months | 433 | 211 | 1.01 | 1.00; 1.03 | 0.004 | 1.01 | 1.00; 1.02 | 0.007 |
| WHZ | 433 | 211 | 1.12 | 0.98; 1.27 | 0.10 | 1.26 | 1.09; 1.46 | 0.002 |
| MUAC, mm | 433 | 211 | 1.01 | 1.00; 1.03 | 0.15 | 1.00 | 0.99; 1.02 | 0.85 |
| HAZ | 433 | 211 | 0.93 | 0.87; 1.00 | 0.055 | 0.95 | 0.88; 1.02 | 0.16 |
| Admission criteria | ||||||||
| WHZ only | 102 | 69 | Ref | Ref | ||||
| MUAC only | 185 | 67 | 1.31 | 1.03; 1.67 | 0.028 | 1.80 | 1.36; 2.38 | <0.001 |
| both WHZ & MUAC | 146 | 75 | 1.05 | 0.82; 1.36 | 0.68 | 1.26 | 0.97; 1.65 | 0.086 |
| Any illness | ||||||||
| No | 78 | 59 | Ref | Ref | ||||
| Yes | 355 | 152 | 1.40 | 1.09; 1.79 | 0.008 | 1.35 | 1.05; 1.73 | 0.017 |
| Anaemia | ||||||||
| No | 78 | 52 | Ref | Ref | ||||
| Yes | 355 | 159 | 1.50 | 1.18; 1.92 | 0.001 | 1.60 | 1.25; 2.06 | <0.001 |
| Low birth weight | ||||||||
| No | 222 | 104 | Ref | Ref | ||||
| Yes | 54 | 30 | 0.81 | 0.60; 1.09 | 0.16 | 0.80 | 0.60; 1.08 | 0.15 |
| Breastfeeding | ||||||||
| No | 66 | 26 | Ref | Ref | ||||
| Yes | 367 | 185 | 0.64 | 0.49; 0.84 | 0.001 | 0.70 | 0.48; 1.01 | 0.057 |
| 2.Morbidity and compliance during treatment | ||||||||
| Malaria episode | ||||||||
| No | 382 | 157 | Ref | Ref | ||||
| Yes | 51 | 54 | 0.42 | 0.32; 0.57 | <0.001 | 0.43 | 0.32; 0.58 | <0.001 |
| ARI episode | ||||||||
| No | 248 | 93 | Ref | Ref | ||||
| Yes | 185 | 118 | 0.44 | 0.37; 0.54 | <0.001 | 0.45 | 0.37; 0.55 | <0.001 |
| Diarrhoea episode | ||||||||
| No | 342 | 125 | Ref | Ref | ||||
| Yes | 91 | 86 | 0.43 | 0.44 | 0.35; 0.56 | <0.001 | ||
| Number of illness episodes | 433 | 211 | 0.57 | 0.53; 0.61 | <0.001 | 0.57 | 0.53; 0.61 | <0.001 |
| Number of skipped* visits | 433 | 211 | 0.67 | 0.61; 0.73 | <0.001 | 0.66 | 0.60; 0.73 | <0.001 |
| Number of missed* visits | 433 | 211 | 0.60 | 0.53; 0.67 | <0.001 | 0.59 | 0.52; 0.67 | <0.001 |
| 3.Household characteristics | ||||||||
| Maternal age, years | 433 | 211 | 1.02 | 1.00; 1.03 | 0.029 | 1.01 | 1.00; 1.03 | 0.048 |
| Maternal education | ||||||||
| No formal education | 333 | 157 | Ref | Ref | ||||
| Some formal education | 100 | 54 | 0.84 | 0.67; 1.05 | 0.13 | 0.84 | 0.67; 1.05 | 0.13 |
| Number of children under 5 in the household | 432 | 207 | 1.04 | 0.97; 1.11 | 0.31 | 1.05 | 0.98; 1.12 | 0.16 |
| Caregiver is the household head | ||||||||
| No | 416 | 207 | Ref | Ref | ||||
| Yes | 17 | 4 | 1.51 | 0.93; 2.45 | 0.098 | 1.36 | 0.83; 2.23 | 0.22 |
| Safe water source | ||||||||
| No | 62 | 49 | Ref | Ref | ||||
| Yes | 371 | 162 | 1.27 | 0.97; 1.66 | 0.082 | 1.24 | 0.95; 1.62 | 0.12 |
| Open defecation | ||||||||
| No | 90 | 58 | Ref | Ref | ||||
| Yes | 343 | 153 | 1.30 | 1.03; 1.65 | 0.025 | 1.32 | 1.04; 1.67 | 0.021 |
| Food insecure household | ||||||||
| No | 383 | 192 | Ref | Ref | ||||
| Yes | 50 | 19 | 0.92 | 0.69; 1.24 | 0.59 | 0.86 | 0.64; 1.16 | 0.33 |
| Return time from health centre | ||||||||
| ≤30 min | 156 | 81 | Ref | Ref | ||||
| >30 min | 277 | 130 | 0.97 | 0.80; 1.19 | 0.80 | 1.01 | 0.83; 1.23 | 0.93 |
| Setting | ||||||||
| Rural | 375 | 180 | Ref | Ref | ||||
| Urban | 58 | 31 | 0.92 | 0.70; 1.22 | 0.58 | 0.89 | 0.67; 1.17 | 0.40 |
1 HR>1 means faster recovery
2 when analysing sex as a predictor, only age was included as adjustment and when analysing age as a predictor only sex was included as adjustment
*skipped visits refer to those that were planned and thus benefitted from double RUTF prescription as opposed to missed visits that were unplanned
ARI, acute respiratory infection; HR, Hazard Ratio; MUAC, mid-upper arm circumference; WHZ, weight-for-height z-score.
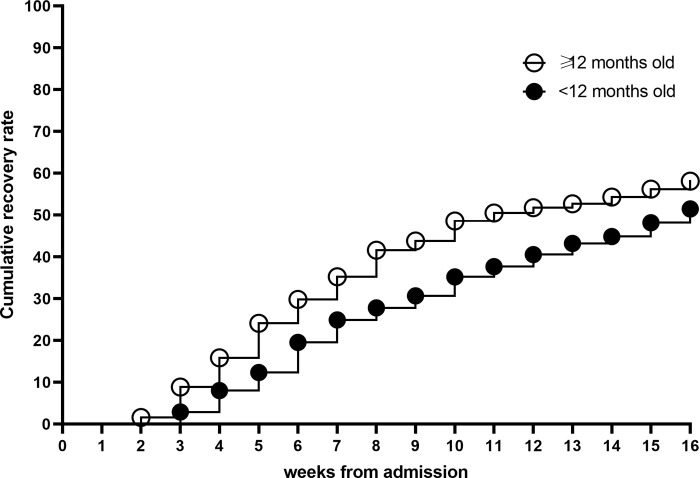
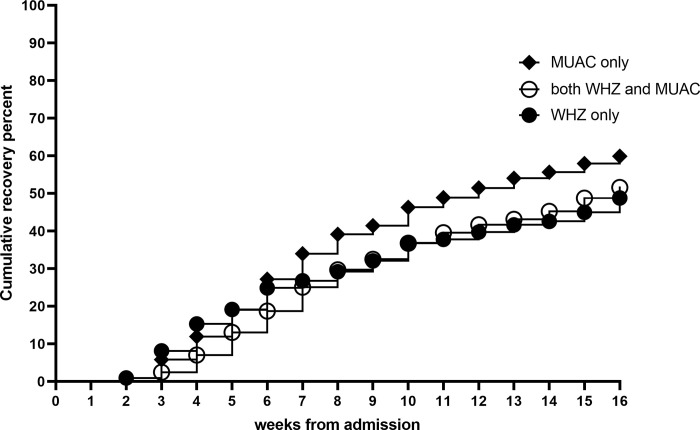
The mean time to recovery for significant categorical variables are presented in Table 3. Children <12 months of age required 13 more days to reach recovery compared to children ≥12 months of age (Fig 1). Being admitted with only MUAC criteria and thus a WHZ ≥-3 predicted faster recovery compared to those admitted with WHZ only (Fig 2) with an average of 15 days shorter time to recovery compared to WHZ only or 10 days shorter compared to those with both admission criteria (Table 3). Illness and malaria at admission were associated with 6 and 14 days faster recovery, respectively. On the contrary, having one, two or more than two illness episodes during treatment was associated with 1, 4 and 8 weeks longer time to recovery, respectively, compared to those with no illness episodes during treatment. One skipped visit increased time to recovery by 2 weeks and one missed visit by 3 weeks. Open defecation was associated with 1 week faster recovery. Including children referred to inpatient care in the analysis of different factors with time to recovery resulted in similar associations.
Table 3. Estimated restricted mean time (days) to recovery from SAM by significant predictor characteristics.
| Predictors | mean (days) | Unadjusted | Sex and age adjusted2 | ||||
|---|---|---|---|---|---|---|---|
| Difference | 95%CI | p-value | Difference | 95%CI | p-value | ||
| 1.Admission characteristics | |||||||
| Age | |||||||
| <12 months | 75.7 | ||||||
| ≥12 months | 62.3 | -13.5 | -18.9; -8.1 | <0.001 | -13.4 | -18.8; -8.0 | <0.001 |
| Admission criteria | |||||||
| WHZ only | 73.8 | ||||||
| MUAC only | 66.0 | -7.8 | -14.8; -0.9 | 0.027 | -15.1 | -22.6; -7.6 | <0.001 |
| WHZ & MUAC | 73.5 | -0.2 | -7.4; 6.9 | 0.95 | -4.8 | -12.1; 2.5 | 0.20 |
| Any illness | |||||||
| No | 76.5 | ||||||
| Yes | 68.9 | -7.6 | -14.4; -0.9 | 0.026 | -6.2 | -12.9; 0.6 | 0.075 |
| Anaemia | |||||||
| No | 81.4 | ||||||
| Yes | 67.7 | -13.7 | -20.1; -7.4 | <0.001 | -14.5 | -21.1; -7.9 | <0.001 |
| Breastfeeding | |||||||
| No | 57.8 | ||||||
| yes | 72.6 | 14.7 | 7.1; 22.4 | <0.001 | 8.9 | -1.3; 19.1 | 0.086 |
| 2.Morbidity and compliance during treatment | |||||||
| Malaria episode | |||||||
| no | 66.2 | ||||||
| yes | 91.5 | 25.3 | 19.2; 31.4 | <0.001 | 24.2 | 18.0; 30.3 | <0.001 |
| ARI episode | |||||||
| no | 58.0 | ||||||
| yes | 83.0 | 24.9 | 20.0; 29.9 | <0.001 | 24.0 | 19.0; 29.1 | <0.001 |
| Diarrhoea episode | |||||||
| no | 63.6 | ||||||
| yes | 87.9 | 24.4 | 19.0; 29.7 | <0.001 | 23.0 | 17.5; 28.4 | <0.001 |
| Number of illness episodes | |||||||
| none | 39.2 | ||||||
| one | 46.6 | 7.4 | 1.6; 13.1 | 0.012 | 6.9 | 1.2; 12.6 | 0.017 |
| two | 66.5 | 27.3 | 21.0; 33.7 | <0.001 | 26.9 | 20.6; 33.2 | <0.001 |
| more than two | 92.2 | 53.0 | 47.8; 58.2 | <0.001 | 52.7 | 47.4; 58.0 | <0.001 |
| Number of skipped1 visits | |||||||
| none | 56.1 | ||||||
| one | 71.4 | 15.3 | 9.1; 21.5 | <0.001 | 16.2 | 10.2; 22.2 | <0.001 |
| more than one | 91.1 | 35.0 | 29.6; 40.3 | <0.001 | 35.6 | 30.2; 41.0 | <0.001 |
| Number of missed1 visits | |||||||
| none | 59.1 | ||||||
| one | 83.0 | 23.9 | 17.8; 30.1 | <0.001 | 23.2 | 16.8; 29.6 | <0.001 |
| more than one | 98.7 | 39.6 | 34.5; 44.7 | <0.001 | 39.4 | 34.1; 44.6 | <0.001 |
| 3.Household characteristics | |||||||
| Open defecation | |||||||
| no | 75.7 | ||||||
| yes | 68.9 | -6.7 | -13.2; -0.2 | 0.042 | -7.0 | -13.5; -0.5 | 0.036 |
1 skipped visits refer to those that were planned and thus benefitted from double RUTF prescription as opposed to missed visits that were unplanned
2 when analysing age categories as a predictor only sex was included as adjustment
ARI, acute respiratory infection; MUAC, mid-upper arm circumference; OR, odds ratio; WHZ, weight for height z-score.
Fig 1. Kaplan Meier plot of cumulative recovery from SAM by age category during outpatient treatment.
Fig 2. Kaplan Meier plot of cumulative recovery from SAM by admission criteria during outpatient treatment.
Factors associated with non-response to treatment were largely similar to those associated with time to recovery in that factors predicting a slower recovery also predicted non-response to treatment and factors predicting faster recovery also predicted not ending up non-response (see Table 4).
Table 4. Predictors of non-response to outpatient treatment of SAM in 801 patients without medical complications at admission.
| Predictors | n | Unadjusted | Sex and age adjusted2 | |||||
|---|---|---|---|---|---|---|---|---|
| Non-response | Response | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| 1.Admission characteristics | ||||||||
| Sex | ||||||||
| Male | 41 | 355 | Ref | Ref | ||||
| Female | 60 | 345 | 1.51 | 0.99; 2.30 | 0.058 | 1.46 | 0.95; 2.24 | 0.082 |
| Age | 101 | 700 | 0.97 | 0.94; 1.00 | 0.039 | 0.97 | 0.94; 1.00 | 0.051 |
| WHZ | 101 | 700 | 0.96 | 0.72; 1.30 | 0.80 | 0.80 | 0.58; 1.09 | 0.16 |
| MUAC | 101 | 700 | 0.98 | 0.95; 1.01 | 0.30 | 1.00 | 0.96; 1.04 | 0.98 |
| HAZ | 101 | 700 | 1.11 | 0.94; 1.30 | 0.22 | 1.05 | 0.89; 1.24 | 0.58 |
| Admission criteria | ||||||||
| WHZ only | 30 | 179 | Ref | Ref | ||||
| MUAC only | 33 | 276 | 0.71 | 0.42; 1.21 | 0.21 | 0.41 | 0.22; 0.75 | 0.004 |
| WHZ & MUAC | 38 | 245 | 0.93 | 0.55; 1.55 | 0.77 | 0.66 | 0.38; 1.14 | 0.14 |
| Any illness | ||||||||
| no | 31 | 143 | Ref | Ref | ||||
| yes | 70 | 557 | 0.58 | 0.37; 0.92 | 0.020 | 0.61 | 0.38; 0.97 | 0.038 |
| Anaemia | ||||||||
| no | 29 | 144 | Ref | Ref | ||||
| yes | 72 | 556 | 0.64 | 0.40; 1.03 | 0.065 | 0.63 | 0.39; 1.01 | 0.057 |
| Low birth weight | ||||||||
| no | 54 | 338 | Ref | Ref | ||||
| yes | 19 | 91 | 1.31 | 0.74; 2.31 | 0.34 | 1.25 | 0.70; 2.23 | 0.44 |
| Breastfeeding | ||||||||
| no | 9 | 103 | Ref | Ref | ||||
| yes | 92 | 597 | 1.76 | 0.86; 3.61 | 0.12 | 1.19 | 0.48; 2.94 | 0.71 |
| 2.Morbidity and compliance during treatment | ||||||||
| Malaria episode | ||||||||
| no | 65 | 603 | Ref | Ref | ||||
| yes | 36 | 97 | 3.44 | 2.17; 5.46 | <0.001 | 3.41 | 2.14; 5.42 | <0.001 |
| ARI episode | ||||||||
| no | 23 | 380 | Ref | Ref | ||||
| yes | 78 | 320 | 4.03 | 2.47; 6.56 | <0.001 | 3.81 | 2.33; 6.23 | <0.001 |
| Diarrhoea episode | ||||||||
| no | 50 | 507 | Ref | Ref | ||||
| yes | 51 | 193 | 2.68 | 1.75; 4.09 | <0.001 | 2.57 | 1.68; 3.95 | <0.001 |
| Number of illness episodes | 101 | 700 | 1.95 | 1.72; 2.21 | <0.001 | 1.95 | 1.72; 2.21 | <0.001 |
| Number of skipped1 visits | 101 | 700 | 1.95 | 1.65; 2.29 | <0.001 | 1.94 | 1.65; 2.29 | <0.001 |
| Number of missed1 visits | 101 | 700 | 2.01 | 1.69; 2.38 | <0.001 | 2.04 | 1.71; 2.42 | <0.001 |
| 3.Household characteristics | ||||||||
| Maternal age | 101 | 700 | 1.00 | 0.97; 1.02 | 0.74 | 1.00 | 0.97; 1.03 | 0.92 |
| Mother has received some formal education | ||||||||
| no | 70 | 536 | Ref | Ref | ||||
| yes | 31 | 164 | 1.45 | 0.92; 2.29 | 0.113 | 1.45 | 0.91; 2.29 | 0.12 |
| Number of children under 5 in the household | 101 | 700 | 0.86 | 0.72; 1.03 | 0.103 | 0.83 | 0.69; 1.00 | 0.054 |
| Safe water source | ||||||||
| no | 18 | 122 | Ref | Ref | ||||
| yes | 83 | 578 | 0.97 | 0.56; 1.68 | 0.92 | 1.06 | 0.61; 1.84 | 0.83 |
| Open defecation | ||||||||
| no | 34 | 158 | Ref | Ref | ||||
| yes | 67 | 542 | 0.57 | 0.37; 0.90 | 0.016 | 0.58 | 0.37; 0.91 | 0.018 |
| Food insecure household | ||||||||
| no | 90 | 613 | Ref | Ref | ||||
| yes | 11 | 87 | 0.86 | 0.44; 1.67 | 0.66 | 0.97 | 0.50; 1.91 | 0.94 |
| Return time from health centre | ||||||||
| ≤30min | 41 | 263 | Ref | Ref | ||||
| >30min | 60 | 437 | 0.88 | 0.58; 1.35 | 0.56 | 0.82 | 0.53; 1.27 | 0.37 |
| Setting | ||||||||
| rural | 80 | 608 | Ref | Ref | ||||
| urban | 21 | 92 | 1.73 | 1.02; 2.94 | 0.041 | 1.93 | 1.13; 3.31 | 0.017 |
1skipped visits refer to those that were planned and thus benefitted from double RUTF prescription as opposed to missed visits that were unplanned
2 when analysing sex as a predictor, only age was included as adjustment and when analysing age as a predictor only sex was included as adjustment
ARI, acute respiratory infection; MUAC, mid-upper arm circumference; OR, odds ratio; WHZ, weight-for-height z-score.
Discussion
In this study, only 55% of children admitted to outpatient treatment of SAM recovered while 13% were discharged non-response to treatment. The mean time to recovery was 8 weeks and was most strongly associated with illness episodes and missed and skipped visits during treatment. Up to 59% of children had at least two illness episodes during treatment which increased the treatment duration by nearly 4 weeks with two episodes and over 7 weeks with more than two episodes. Up to 35% of children missed at least one visit which increased the time to recovery by 3 weeks with one and over 5 weeks with two or more missed visits. Similarly, non-response to treatment seemed most strongly associated with illness episodes and missed visits during treatment increasing the odds of non-response to 1.95 for each additional illness episode and 2.04 for each additional missed visit.
The mean time to recovery was longer than reported by most previous studies conducted in outpatient settings [14, 15, 27, 31, 32, 34, 43, 44]. Most of the variability in length of stay in treatment between different studies and contexts can probably be ascribed to differences in discharge criteria: in many studies recovery was declared when children reached a weight-for height >85% of WHO median growth reference regardless of being admitted with low WHZ or MUAC [14, 24–26, 30–32, 43, 45]. Additionally, often the recommendation [6] of presenting the anthropometric recovery criteria for at least 2 weeks is not followed [14, 24–26, 30, 44]. These deviations from the WHO issued protocol have consequences on the program performance indicators [46].
Illness episodes during treatment were the strongest predictor of recovery and non-response. Few outpatient studies have looked at co-morbidities occurring during treatment. Yebyo et al. (2013) found a significant association between co-morbidities and lower recovery rate but did not distinguish between illnesses diagnosed at admission or during treatment [14]. The relatively long treatment duration in the current study probably contributed to the observation that illnesses diagnosed during treatment were more predictive of recovery than those diagnosed at admission. Children were systematically treated for the diagnosed illnesses and the observation that these episodes were still associated with remarkably longer treatment time is noteworthy. It highlights the importance of preventing the conditions as it seems that once children catch diarrhoea, malaria or ARI during SAM treatment, the illnesses substantially slow their recovery trajectory.
One missed visit increased the time to recovery by more than 3 weeks. The reason for missing a visit was in most cases related to time or accessibility impediments to travel to the health centre to receive treatment. This calls for seeking more flexible and possibly more closely available services; flexible in the sense of providing services on several week days instead of just one and more closely available potentially in the form of community health worker delivered treatment [47]. Interestingly, even when children had benefitted from double RUTF prescription, one skipped visit still increased the time to recovery by more than 2 weeks. This suggests that increased visit spacing could result in longer treatment time. This said, part of the increased time (1 week) can be explained by missed and skipped visits occurring towards the end of treatment artificially lengthening treatment time when recovery can only be declared upon reaching discharge criteria during two consecutive visits.
We observed that higher age was associated with faster recovery. Previous studies in outpatient settings have found positive [27, 34], negative [32] and no [14, 31, 33, 43] associations between age and recovery. This could partly be due to the use of different cut-offs for defining age groups ranging from 12 months to 36 months. Worth noting though, in our study only 10% of children were ≥24 months old at admission, meaning that in a context with an older SAM population, the association could be different.
Being admitted based on MUAC only (ie. MUAC <115 mm and WHZ ≥-3) was associated with 2 weeks quicker recovery and 0.41 the odds of non-response than being admitted with WHZ only (ie. WHZ <-3 and MUAC ≥115 mm). This indicates either that children presenting with a low WHZ potentially have a different pathophysiological status that requires a longer treatment time or that reaching a WHZ ≥-2 takes longer than reaching a MUAC ≥125 mm. This observation is to be considered when observing success rates from programs implementing MUAC only admission criteria as based on the current study excluding children with WHZ <-3 but MUAC > 115mm would result in better recovery rate.
Illness at admission were associated with faster recovery, contrary to results from most previous studies showing slower recovery in inpatient [24, 25, 28–30, 45, 48] and outpatient settings [14] among those admitted with co-morbidity. Interestingly however, one study from Gambia showed that higher cortisol at admission (indicating acute illness) predicted higher WAZ gain during treatment [49]. The authors suspected that this was due to children with high cortisol being more sick and when treated for their condition responding faster by also gaining weight. In somewhat similar lines, another study found that children that failed the appetite test at admission, possibly indicating sub-clinical illness, had higher weight gains during treatment compared to children having passed the appetite test [50]. Our observation offers support to this hypothesis in that it would seem that children with co-morbidities respond fastest to the treatment. It could be that their malnutrition is a secondary condition related to a primary co-morbidity that when managed correctly allows a rapid return to a normal health and nutrition status. Consequently, children with no apparent co-morbidities at admission possibly have a different causal pathway to malnutrition and maybe a different pathophysiological state leading to slower response to treatment. It is also possible that they present with some underlying chronic hard-to-detect pathology such as environmental enteropathy [51–53] or congenital heart diseases [54] that increase the nutrient needs and could affect the time to recovery.
Anaemia at admission was associated with faster recovery. This is in contrast to previous studies in inpatient settings reporting slower recovery rates among children with anaemia at admission [24, 25, 30, 45]. It could be that children admitted to inpatient care because of medical complications and in addition presenting with anaemia have a different pathophysiologic profile to children in outpatient care with anaemia but who don’t present medical complications and that would explain a slower response to treatment. It could also be that the inpatient treatment with therapeutic feeds such as F75 and F100 that do not contain iron [55] would slow down the recovery of anaemic patients. Regardless, anaemia is a condition driven by multiple factors including infections and nutritional deficiencies [56]. Similarly to the possible explanation for the faster recovery among children with illnesses at admission, it could be that these children, when managed properly via medical and nutritional treatment, respond quickly to treatment.
Open defecation was associated with slightly faster recovery. This association was contrary to what we expected. It could be that due to poor hygiene conditions these children entered treatment after an enteric illness and weight loss and subsequently responded fast to treatment. However, controlling for diarrhoea at admission did not reduce the association.
Living in a more urban setting was associated with higher non-response rate compared to a more rural setting and we do not have a hypothesis for why this should be. Among previous studies looking into factors influencing time to recovery two have reported no association between residence and recovery [31, 34] and one reported a quicker recovery among those coming from a more urban setting [27].
We found no association between HAZ at admission and time to recovery or non-response to treatment. Categorising HAZ into <-2 and ≥-2 did not reveal an association either. This is worth noting as there has been some interest in looking at the treatment outcomes of concurrently stunted and wasted children. Previously there has even been concern that short wasted children would not respond adequately to treatment although this has been shown not to be the case [57]. Our findings are consistent with this observation.
This study has several strengths including that it was done prospectively as part of a clinical trial with few missing data. The data quality was high with nurse diagnosed morbidity and strict respect of recovery criteria to investigate time to recovery and non-response to treatment.
The study also has limitations. The population studied was very young (mostly <24 months old) so some of the associations could be different if the full age range of 6–59 months were present. Also, the use of strict referral criteria identifying children with stagnant weight gain and weight loss led to high referral rate (20%) and many children being excluded from the time to recovery analysis. However, including these children in the analysis did not change the associations. Additionally, the findings could be context specific as the causal pathways and the response to treatment may be different in other settings.
In conclusion, illness episodes during treatment were common and also the strongest predictor of non-recovery and non-response, in addition to missed visits. Over half of the children suffered at least 2 illness episodes during treatment increasing time to recovery by nearly 4 weeks with two episodes and over 7 weeks with more than two episodes. This indicates that while correct diagnosis and management of co-morbidities is crucial, prevention would be key to shortening the treatment duration. Missing a visit during treatment also increased treatment time considerably by 3 weeks which calls for reflecting the service delivery to better accommodate clients’ needs to rearrange appointments and potentially further decentralise health services. In general, we call for more data on children with SAM, both prior and during treatment, in order to better understand causal pathways and pathophysiology of children admitted to care and subsequently their specific needs for recovery.
Acknowledgments
We thank the study participants and caregivers for participating in the trial and the research teams for all their efforts in implementing the trial. Any views or opinions reflected here are those of the authors, expressed in a personal capacity, and do not necessarily reflect those of their employer.
Data Availability
The full dataset used to produce the current manuscript is available from the ZENODO platform: https://zenodo.org/record/4274090 (https://zenodo.org/record/4274090#.YlXrS8jMI2w).
Funding Statement
The data collection for this study was funded by Action Against Hunger France, European Commission's Civil Protection and Humanitarian aid Operations (grant number ECHO/-WF/BUD/2015/91065), Children's Investment Fund Foundation (grant: "CIFF03: Reinventing community based management of malnutrition"), European Commission's Civil Protection and Humanitarian aid Operations Enhanced Response Capacity (grant number ECHO/ERC/BUD/2016/91006) and Humanitarian Innovation Fund (HIF) a programme managed by ELRHA (Enhancing Learning and Research for Humanitarian Assistance). Funding provided by these organisations covered the study conception and field implementation of the study, including the salary of STK and VN for the full length of the study. Other authors received no specific funding for their work related to the current study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bhutta ZA, Berkley JA, Bandsma RHJ, Kerac M, Trehan I, Briend A. Severe childhood malnutrition. Nat Rev Dis Primer. 2017. Sep 21;3:17067. doi: 10.1038/nrdp.2017.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization, UNICEF. WHO child growth standards and the identification of severe acute malnutrition in infants and children: a joint statement by the World Health Organization and the United Nations Children’s Fund. [Internet]. 2009. [cited 2018 Mar 12]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK200775/ [PubMed] [Google Scholar]
- 3.United Nations Children’s Fund (UNICEF), World Health Organization, International, Bank for Reconstruction and Development/The World Bank. Levels and trends in child malnutrition: Key Findings of the 2020 Edition of the Joint Child Malnutrition Estimates. World Health Organization; 2020. [Google Scholar]
- 4.Olofin I, McDonald CM, Ezzati M, Flaxman S, Black RE, Fawzi WW, et al. Associations of Suboptimal Growth with All-Cause and Cause-Specific Mortality in Children under Five Years: A Pooled Analysis of Ten Prospective Studies. Wiley AS, editor. PLoS ONE. 2013. May 29;8(5):e64636. doi: 10.1371/journal.pone.0064636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet Lond Engl. 2013. Aug 3;382(9890):427–51. doi: 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Guideline: Updates on the Management of Severe Acute Malnutrition in Infants and Children [Internet]. 2013. [cited 2018 Apr 17]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK190328/ [PubMed] [Google Scholar]
- 7.WHO, WFP, UNSCN, UNICEF. Community-based management of severe acute malnutrition: a joint statement by the World Health Organization, the World Food Programme, the United Nations System Standing Committee on Nutrition and the United Nations Children’s Fund. Geneva: UNICEF; 2007. [Google Scholar]
- 8.Sphere Project, editor. The Sphere handbook: humanitarian charter and minimum standards in humanitarian response. Fourth edition. Geneva, Switzerland: Sphere Association; 2018. 406 p. [Google Scholar]
- 9.UNICEF. Responding to COVID-19: UNICEF Annual report 2020 [Internet]. 2020. Available from: https://www.unicef.org/media/100946/file/UNICEF%20Annual%20Report%202020.pdf
- 10.Isanaka S, Andersen CT, Cousens S, Myatt M, Briend A, Krasevec J, et al. Improving estimates of the burden of severe wasting: analysis of secondary prevalence and incidence data from 352 sites. BMJ Glob Health. 2021. Mar;6(3):e004342. doi: 10.1136/bmjgh-2020-004342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burza S, Mahajan R, Marino E, Sunyoto T, Shandilya C, Tabrez M, et al. Community-based management of severe acute malnutrition in India: new evidence from Bihar. Am J Clin Nutr. 2015. Apr 1;101(4):847–59. doi: 10.3945/ajcn.114.093294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linneman Z, Matilsky D, Ndekha M, Manary MJ, Maleta K, Manary MJ. A large-scale operational study of home-based therapy with ready-to-use therapeutic food in childhood malnutrition in Malawi. Matern Child Nutr. 2007. Jul;3(3):206–15. doi: 10.1111/j.1740-8709.2007.00095.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maust A, Koroma AS, Abla C, Molokwu N, Ryan KN, Singh L, et al. Severe and Moderate Acute Malnutrition Can Be Successfully Managed with an Integrated Protocol in Sierra Leone. J Nutr. 2015. Nov 1;145(11):2604–9. doi: 10.3945/jn.115.214957 [DOI] [PubMed] [Google Scholar]
- 14.Yebyo HG, Kendall C, Nigusse D, Lemma W. Outpatient therapeutic feeding program outcomes and determinants in treatment of severe acute malnutrition in tigray, northern ethiopia: a retrospective cohort study. PloS One. 2013;8(6):e65840. doi: 10.1371/journal.pone.0065840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James PT, Van den Briel N, Rozet A, Israël A-D, Fenn B, Navarro-Colorado C. Low-dose RUTF protocol and improved service delivery lead to good programme outcomes in the treatment of uncomplicated SAM: a programme report from Myanmar. Matern Child Nutr. 2015. Oct;11(4):859–69. doi: 10.1111/mcn.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kangas ST, Salpéteur C, Nikièma V, Talley L, Ritz C, Friis H, et al. Impact of reduced dose of ready-to-use therapeutic foods in children with uncomplicated severe acute malnutrition: A randomised non-inferiority trial in Burkina Faso. Persson LÅ, editor. PLOS Med. 2019. Aug 27;16(8):e1002887. doi: 10.1371/journal.pmed.1002887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kangas ST, Salpéteur C, Nikièma V, Talley L, Briend A, Ritz C, et al. Vitamin A and iron status of children before and after treatment of uncomplicated severe acute malnutrition. Clin Nutr. 2020. Nov;39(11):3512–9. doi: 10.1016/j.clnu.2020.03.016 [DOI] [PubMed] [Google Scholar]
- 18.Kangas ST, Kaestel P, Salpéteur C, Nikièma V, Talley L, Briend A, et al. Body composition during outpatient treatment of severe acute malnutrition: Results from a randomised trial testing different doses of ready-to-use therapeutic foods. Clin Nutr. 2020. Nov;39(11):3426–33. doi: 10.1016/j.clnu.2020.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.N’Diaye DS, Wassonguema B, Nikièma V, Kangas ST, Salpéteur C. Economic evaluation of a reduced dosage of ready‐to‐use therapeutic foods to treat uncomplicated severe acute malnourished children aged 6–59 months in Burkina Faso. Matern Child Nutr [Internet]. 2021. Feb 23 [cited 2021 Mar 26]; Available from: https://onlinelibrary.wiley.com/doi/10.1111/mcn.13118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikièma V, Kangas ST, Salpéteur C, Ouédraogo A, Lachat C, Bassolé NHI, et al. Adequacy of Nutrient Intakes of Severely and Acutely Malnourished Children Treated with Different Doses of Ready-To-Use Therapeutic Food in Burkina Faso. J Nutr. 2021. Feb 11;nxaa393. doi: 10.1093/jn/nxaa393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikièma V, Fogny NF, Kangas ST, Lachat C, Salpéteur C. Availability, use, and consumption practices of ready-to-use therapeutic foods prescribed to children with uncomplicated severe acute malnutrition aged 6–59 months during outpatient treatment in Burkina Faso. Appetite. 2022. Jan;168:105751. doi: 10.1016/j.appet.2021.105751 [DOI] [PubMed] [Google Scholar]
- 22.Nikièma V, Fogny NF, Salpéteur C, Lachat C, Kangas ST. Complementary feeding practices and associated factors of dietary diversity among uncomplicated severe acute malnourished children aged 6–23 months in Burkina Faso. Matern Child Nutr [Internet]. 2021. Oct [cited 2021 Dec 17];17(4). Available from: https://onlinelibrary.wiley.com/doi/10.1111/mcn.13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wondim A, Tigabu B, Kelkay MM. Time to Recovery from Severe Acute Malnutrition and Its Predictors among Admitted Children Aged 6–59 Months at the Therapeutic Feeding Center of Pawi General Hospital, Northwest Ethiopia: A Retrospective Follow-Up Study. Int J Pediatr. 2020. Mar 11;2020:1–9. doi: 10.1155/2020/8406597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kebede MA, Teshome GS, Fenta FA, Bantigegn KA. Recovery time from severe acute malnutrition and associated factors among under-5 children in Yekatit 12 hospital, Addis Ababa, Ethiopia- a retrospective cohort study. Epidemiol Health. 2020. Feb 2;e2020003. doi: 10.4178/epih.e2020003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagnew F, Dejenu G, Eshetie S, Alebel A, Worku W, Abajobir AA. Treatment cure rate and its predictors among children with severe acute malnutrition in northwest Ethiopia: A retrospective record review. Madiba S, editor. PLOS ONE. 2019. Feb 20;14(2):e0211628. doi: 10.1371/journal.pone.0211628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mekuria G, Derese T, Hailu G. Treatment outcome and associated factors of severe acute malnutrition among 6–59 months old children in Debre Markos and Finote Selam hospitals, Northwest Ethiopia: a retrospective cohort study. BMC Nutr [Internet]. 2017. Dec [cited 2020 Feb 11];3(1). Available from: http://bmcnutr.biomedcentral.com/articles/10.1186/s40795-017-0161-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atnafe B, Roba KT, Dingeta T. Time of recovery and associated factors of children with severe acute malnutrition treated at outpatient therapeutic feeding program in Dire Dawa, Eastern Ethiopia. Gopichandran V, editor. PLOS ONE. 2019. Jun 13;14(6):e0217344. doi: 10.1371/journal.pone.0217344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fikrie A, Alemayehu A, Gebremedhin S. Treatment outcomes and factors affecting time-to-recovery from severe acute malnutrition in 6–59 months old children admitted to a stabilization center in Southern Ethiopia: A retrospective cohort study. Ital J Pediatr. 2019. Apr 11;45(1):46. doi: 10.1186/s13052-019-0642-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassen SL, Astatkie A, Mekonnen TC, Bogale GG. Survival Status and Its Determinants among Under-Five Children with Severe Acute Malnutrition Admitted to Inpatient Therapeutic Feeding Centers in South Wollo Zone, Amhara Region, Ethiopia. J Nutr Metab. 2019;2019:2643531. doi: 10.1155/2019/2643531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asres DT, Prasad RPCJ, Ayele TA. Recovery time and associated factors of severe acute malnutrition among children in Bahir Dar city, Northwest Ethiopia: an institution based retrospective cohort study. BMC Nutr [Internet]. 2018. Dec [cited 2020 Apr 27];4(1). Available from: https://bmcnutr.biomedcentral.com/articles/10.1186/s40795-018-0224-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desalegn M, Kifle W, Birtukan T, Amanuel T. Treatment outcome of severe acute malnutrition and determinants of survival in Northern Ethiopia: A prospective cohort study. Int J Nutr Metab. 2016. Apr 30;8(3):12–23. [Google Scholar]
- 32.Mengesha MM, Deyessa N, Tegegne BS, Dessie Y. Treatment outcome and factors affecting time to recovery in children with severe acute malnutrition treated at outpatient therapeutic care program. Glob Health Action. 2016;9:30704. doi: 10.3402/gha.v9.30704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moramarco S, Amerio G, Kasengele Chipoma J, Nielsen-Saines K, Palombi L, Buonomo E. Filling the Gaps for Enhancing the Effectiveness of Community-Based Programs Combining Treatment and Prevention of Child Malnutrition: Results from the Rainbow Project 2015−17 in Zambia. Int J Environ Res Public Health. 2018. 22;15(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shanka N, Lemma S, Abyu D. Recovery Rate and Determinants in Treatment of Children with Severe Acute Malnutrition using Outpatient Therapeutic Feeding Program in Kamba District, South West Ethiopia. Journal of Nutritional Disorders and Therapy [Internet]. 5th ed. 2016; Available from: doi: 10.4172/2161-0509.1000155 [DOI] [Google Scholar]
- 35.Institut National de la Statistique et de la Démographie—INSD/Burkina Faso, ICF International. Burkina Faso Enquête Démographique et de Santé et à Indicateurs Multiples (EDSBF-MICS IV) 2010 [Internet]. Calverton, Maryland, USA: Institut National de la Statistique et de la Démographie—INSD/Burkina Faso and ICF International; 2012. Available from: http://dhsprogram.com/pubs/pdf/FR256/FR256.pdf [Google Scholar]
- 36.Ministère de la Santé Burkina Faso. SMART 2016. Enquête nutritionnelle nationale Burkina Faso. [Internet]. 2016. Available from: https://www.humanitarianresponse.info/sites/www.humanitarianresponse.info/files/documents/files/smart_2016.pdf
- 37.Reseau du système d’alerte précoce (FEWS NET). Zones et profils de moyens d’existence au Burkina Faso. [Internet]. Available from: https://fews.net/sites/default/files/documents/reports/bf_profile_fr.pdf
- 38.Ministère de la Santé. Annuaire statistique en santé. Burkina Faso; 2016. [Google Scholar]
- 39.Ministère de la santé BF. Protocole national prise en charge intégrée de la malnutrition aiguë (PCIMA) Burkina Faso. 2014. [Google Scholar]
- 40.Leroy JL. zscore06: Stata command for the calculation of anthropometric z-scores using the 2006 WHO child growth standards. 2011. [Google Scholar]
- 41.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization, 2011. (WHO/NMH/NHD/MNM/11.1); [Google Scholar]
- 42.Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: Indicator Guide. Version 3. [Internet]. 2007th ed. FANTA Project; [cited 2019 Jan 23]. Available from: https://www.fantaproject.org/sites/default/files/resources/HFIAS_ENG_v3_Aug07.pdf [Google Scholar]
- 43.Liben ML, Wuneh AG, Shamie R, G/Her K. Factors associated with child survival in children admitted to outpatient therapeutic program at public health institutions in Afar Regional State, Ethiopia: a prospective cohort study. J Health Popul Nutr. 2019. 27;38(1):35. doi: 10.1186/s41043-019-0193-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irena AH, Bahwere P, Owino VO, Diop EI, Bachmann MO, Mbwili-Muleya C, et al. Comparison of the effectiveness of a milk-free soy-maize-sorghum-based ready-to-use therapeutic food to standard ready-to-use therapeutic food with 25% milk in nutrition management of severely acutely malnourished Zambian children: an equivalence non-blinded cluster randomised controlled trial. Matern Child Nutr. 2015. Dec;11 Suppl 4:105–19. doi: 10.1111/mcn.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baraki AG, Akalu TY, Wolde HF, Takele WW, Mamo WN, Derseh B, et al. Time to recovery from severe acute malnutrition and its predictors: a multicentre retrospective follow-up study in Amhara region, north-west Ethiopia. BMJ Open. 2020. Feb;10(2):e034583. doi: 10.1136/bmjopen-2019-034583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guesdon B, Roberfroid D. Substandard discharge rules in current severe acute malnutrition management protocols: An overlooked source of ineffectiveness for programmes? Field Exchange. 2019. Jul;29. [Google Scholar]
- 47.Alvarez Morán JL, Alé GBF, Charle P, Sessions N, Doumbia S, Guerrero S. The effectiveness of treatment for Severe Acute Malnutrition (SAM) delivered by community health workers compared to a traditional facility based model. BMC Health Serv Res. 2018. Dec;18(1):207. doi: 10.1186/s12913-018-2987-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Desyibelew HD, Fekadu A, Woldie H. Recovery rate and associated factors of children age 6 to 59 months admitted with severe acute malnutrition at inpatient unit of Bahir Dar Felege Hiwot Referral hospital therapeutic feeding unite, northwest Ethiopia. PloS One. 2017;12(2):e0171020. doi: 10.1371/journal.pone.0171020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nabwera HM, Bernstein RM, Agbla SC, Moore SE, Darboe MK, Colley M, et al. Hormonal Correlates and Predictors of Nutritional Recovery in Malnourished African Children. J Trop Pediatr. 2018. 01;64(5):364–72. doi: 10.1093/tropej/fmx075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zangenberg M, Abdissa A, Johansen ØH, Tesfaw G, Friis H, Briend A, et al. Critical evaluation of the appetite test for children with severe acute malnutrition. Trop Med Int Health [Internet]. 2020. Jan 6 [cited 2020 Jan 22]; Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/tmi.13360 [DOI] [PubMed] [Google Scholar]
- 51.Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014. Jun;510(7505):417–21. doi: 10.1038/nature13421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owino V, Ahmed T, Freemark M, Kelly P, Loy A, Manary M, et al. Environmental Enteric Dysfunction and Growth Failure/Stunting in Global Child Health. PEDIATRICS. 2016. Dec 1;138(6):e20160641–e20160641. doi: 10.1542/peds.2016-0641 [DOI] [PubMed] [Google Scholar]
- 53.Prendergast AJ, Kelly P. Interactions between intestinal pathogens, enteropathy and malnutrition in developing countries: Curr Opin Infect Dis. 2016. Jun;29(3):229–36. doi: 10.1097/QCO.0000000000000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Argent AC, Balachandran R, Vaidyanathan B, Khan A, Kumar RK. Management of undernutrition and failure to thrive in children with congenital heart disease in low- and middle-income countries. Cardiol Young. 2017. Dec;27(S6):S22–30. doi: 10.1017/S104795111700258X [DOI] [PubMed] [Google Scholar]
- 55.WHO. Management of severe malnutrition: a manual for physicians and other senior health workers. 1999. [Google Scholar]
- 56.Calis JCJ, Phiri KS, Faragher EB, Brabin BJ, Bates I, Cuevas LE, et al. Severe Anemia in Malawian Children. N Engl J Med. 2008. Feb 28;358(9):888–99. doi: 10.1056/NEJMoa072727 [DOI] [PubMed] [Google Scholar]
- 57.Fabiansen C, Phelan KP, Cichon B, Ritz C, Briend A, Michaelsen KF, et al. Short children with a low midupper arm circumference respond to food supplementation: an observational study from Burkina Faso1,2. Am J Clin Nutr. 2016. Feb 6;103(2):415–21. doi: 10.3945/ajcn.115.124644 [DOI] [PubMed] [Google Scholar]