Abstract
Sunlight inactivation rates of somatic coliphages, F-specific RNA bacteriophages (F-RNA phages), and fecal coliforms were compared in seven summer and three winter survival experiments. Experiments were conducted outdoors, using 300-liter 2% (vol/vol) sewage-seawater mixtures held in open-top chambers. Dark inactivation rates (kDs), measured from exponential survival curves in enclosed (control) chambers, were higher in summer (temperature range: 14 to 20°C) than in winter (temperature range: 8 to 10°C). Winter kDs were highest for fecal coliforms and lowest for F-RNA phages but were the same or similar for all three indicators in summer. Sunlight inactivation rates (kS), as a function of cumulative global solar radiation (insolation), were all higher than the kDs with a consistent kS ranking (from greatest to least) as follows: fecal coliforms, F-RNA phages, and somatic coliphages. Phage inactivation was exponential, but bacterial curves typically exhibited a shoulder. Phages from raw sewage exhibited kSs similar to those from waste stabilization pond effluent, but raw sewage fecal coliforms were inactivated faster than pond effluent fecal coliforms. In an experiment which included F-DNA phages and Bacteroides fragilis phages, the kS ranking (from greatest to least) was as follows: fecal coliforms, F-RNA phages, B. fragilis phages, F-DNA phages, and somatic coliphages. In a 2-day experiment which included enterococci, the initial concentration ranking (from greatest to least: fecal coliforms, enterococci, F-RNA phages, and somatic coliphages) was reversed during sunlight exposure, with only the phages remaining detectable by the end of day 2. Inactivation rates under different optical filters decreased with the increase in spectral cutoff wavelength (50% light transmission) and indicated that F-RNA phages and fecal coliforms are more susceptible than somatic coliphages to longer solar wavelengths, which predominate in seawater. The consistently superior survival of somatic coliphages in our experiments suggests that they warrant further consideration as fecal, and possibly viral, indicators in marine waters.
Enteric viruses are regarded as important pathogens in marine waters used for shellfish harvesting (35) and have also been implicated as agents of human gastroenteritis in both marine and fresh bathing waters (16). Although strong epidemiological evidence is lacking, the general assumption of the importance of enteric viruses in recreational waters led to the inclusion of criteria based on enteroviruses (enteric virus indicators) in the European Economic Community Bathing Water Directive (14).
Because enterovirus assays are costly and time-consuming, fecal bacteriophages—principally, somatic coliphages, F-specific RNA bacteriophages (F-RNA phages), F-DNA phages, and phages of Bacteroides fragilis—have been investigated as alternative enteric virus indicators (11, 20, 39). In marine waters, positive correlations between the presence of fecal phages, enteric viruses, and other pathogens have been recorded (4, 12). Mariño et al. (31) used a cumulative contamination index to assess microbial guidelines for European beaches and concluded that somatic coliphages best indicated fecal contamination levels.
Unfortunately, widespread adoption of fecal bacteriophages for marine water quality monitoring has been hampered by three interrelated problems. (i) Phages are difficult to extract from seawater, and there is little standardization of enumeration methods. This issue has still not been completely resolved, although several simple assays for 20- to 100-ml samples are now available (1, 38). (ii) Phages have rarely been included in studies of the disease risk associated with bathing, so epidemiological evidence to support their use is lacking. However, in two recent freshwater studies, both significant (29) and nonsignificant (32) relationships between F-RNA phage counts and disease risk were found. (iii) Compared to bacterial indicators, there is limited empirical information on the survival of fecal bacteriophages in sunlight-exposed seawater.
In an earlier paper (37), we outlined the interacting factors affecting the survival of fecal indicator bacteria in seawater—nutrient availability, salinity, temperature, pH, microbial predation, and solar radiation. The last factor appears to be the most important. The UV-B portion of the solar spectrum is the most bacteriocidal, causing direct (photobiological) DNA damage. At wavelengths above 329 nm, photochemical mechanisms become more important, usually acting through photosensitizers to damage cell membranes and tending to be more injurious in the presence of oxygen. Sunlight penetration in seawater decreases with decreasing wavelength, which tends to further increase the contribution of photochemical damage. Our earlier study (37) and that of Davies-Colley et al. (9) showed that greater sunlight exposure was required to inactivate enterococci in seawater, compared to fecal coliforms, and inactivation of both indicators decreased with increasing seawater depth. This depth pattern corresponded to attenuation of UV-A wavelengths (9) and was consistent with the results of optical filter experiments (37).
A range of interacting environmental factors have also been reported to affect the survival of bacteriophages (and other viruses) in seawater. These include salinity (2), temperature (7), attachment to colloids (17), and antiviral substances exuded by marine microbes (40). However, the effects of sunlight on viruses are not as well documented as those on bacteria. In fact, early investigators concluded that sunlight was of little or no importance in determining phage survival in seawater (3, 27). Subsequently, however, Kapuscinski and Mitchell (28) reported sunlight inactivation of laboratory phage strains. Although phage removal has been compared to that of bacteria at increasing distances from coastal outfalls (8, 30), removal rates were not empirically related to sunlight exposure. Other phage survival studies have either been conducted in the laboratory (7, 18, 26) or in field containers in which solar radiation was not measured (18, 26).
Two studies in which phage inactivation in relation to sunlight was measured produced conflicting results. Kapuscinski and Mitchell (28) reported that the F-RNA phage MS2 was more resistant to sunlight than the somatic coliphages φX174 and T7. In contrast, in studies at the United Kingdom Water Research Centre (WRc), when naturally occurring phages were exposed to sunlight in open beakers of seawater, somatic coliphages were found to be more resistant to inactivation than F-RNA phages (41). In both studies, the phages were more sunlight resistant than either Escherichia coli (28) or fecal (thermotolerant) coliforms (41).
In this work, we describe a 3-year study designed to quantify sunlight inactivation rates of fecal bacteriophages in seawater, using large chambers at an outdoor experimental area. This approach enabled the establishment of parallel experimental treatments, including the use of optical filters to measure phage inactivation by specific bands in the UV-visible spectrum. The study focused mainly on the survival of somatic coliphages, F-RNA phages, and fecal coliforms in the summer bathing season, but winter experiments were also conducted. One experiment included F-DNA and B. fragilis phages. Phage inactivation was compared to that of fecal coliforms (on media routinely used for fecal coliform monitoring), because the survival of the latter has been extensively studied in seawater (e.g., see references 9, 15, and 37). However, enterococci were included in one 2-day survival experiment, because they have now been adopted as marine recreational water quality indicators in some of the United States, Canada, Australia, and New Zealand.
MATERIALS AND METHODS
Experimental facilities.
The experimental setup was modified from that described by Sinton et al. (37). A new site was established in an unshaded area at Lincoln, 10 km south of Christchurch (latitude 43°S), New Zealand. Seawater-effluent mixtures were contained in white, plastic, open-top chambers (600 mm wide by 900 mm long by 680 mm deep) filled to a depth of 560 mm (volume, approximately 300 liters). The outsides of the tanks were lined with aluminum foil, to prevent light entry through the sidewalls.
Experimental procedures were designed to minimize between-chamber variability. To provide a thermal water jacket, the chambers were placed in a swimming pool filled with 13,000 liters of fresh water, to a level 100 mm below that of the seawater inside the chambers. In practice, there was a <0.5°C temperature difference between chambers, and temperatures were usually maintained to within 2°C of the target temperature (that prevailing in nearby coastal waters in that season). Heating was not required, but in summer the pool water was cooled to the target temperature with ice.
A submersible bilge pump on the bottom of each chamber was used to stir the seawater-effluent mixture. A timer switched on the pumps simultaneously for 3 min every half hour. A plastic sampling tube from each chamber was connected to a rubber seal on a sampling manifold beside the pool. During stirring, samples were collected by applying a vacuum to a sampling bottle attached to the appropriate seal.
Experimental procedures.
A total of 11 experiments—1 to assess variability and 10 survival experiments (Table 1)—were conducted, in summer and winter, over a period of 3 years. Each sunlight-exposed chamber was paired with a dark (control) chamber, and experiments generally contained the following common elements: one sunlight-exposed and one dark chamber, each containing 300 liters of a 2% (vol/vol) mixture of raw sewage in seawater, and each monitored hourly for somatic coliphages, F-RNA phages, and fecal coliforms, over a period of 7 or 8 daylight hours.
TABLE 1.
Summary of experimental program used in this study
| Expt no. | Season | Weather | Water temp (°C) | Indicators | Comment |
|---|---|---|---|---|---|
| 1 | Summer | Patchy clouds | 16.0 | Somatic coliphages, F-RNA phages, fecal coliforms | |
| 2 | Summer | Clear | 18.0 | Somatic coliphages, F-RNA phages, fecal coliforms | Comparison of raw sewage and waste stabilization pond effluent |
| 3 | Summer | Late clouds | 19.0 | Somatic coliphages, F-RNA phages, fecal coliforms | |
| 4 | Winter | Low clouds | 8.5 | Somatic coliphages, fecal coliforms | |
| 5 | Summer | Clear | 19.0 | Somatic coliphages, F-RNA phages, F-DNA phages, fecal coliforms, B. fragilis phages | |
| 6 | Summer | Low clouds | 19.0 | Somatic coliphages, F-RNA phages, F-DNA phages, fecal coliforms, enterococci, B. fragilis phages | Variability experiment |
| 7 | Summer | Clear | 19.0 | Somatic coliphages, F-RNA phages, fecal coliforms, enterococci | Two-day experiment |
| 8 | Winter | Patchy clouds | 9.0 | Somatic coliphages, fecal coliforms | |
| 9 | Summer | Clear | 17.0 | Somatic coliphages, fecal coliforms | |
| 10 | Summer | Clear | 19.5 | Somatic coliphages, F-RNA phages, fecal coliforms | Optical filter experiment (full sun, 318 nm, 342 nm, 396 nm, 556 nm, dark) |
| 11 | Winter | Clear | 9.0 | Somatic coliphages, F-RNA phages, fecal coliforms |
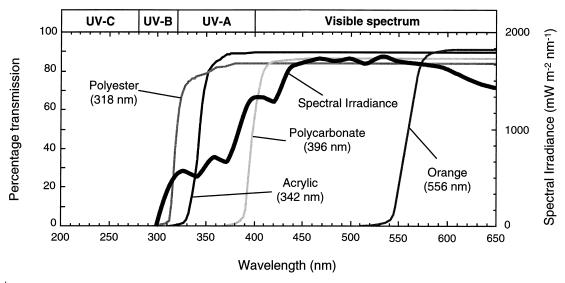
To exclude light, the dark chamber was covered with an aluminum lid and the sampling tube was wrapped in aluminum foil. In experiment 10, four additional chambers, each covered with a different long-pass optical filter, were used to gauge the contributions of different sunlight wavelengths to inactivation. Spectral transmission curves for these filters (polyester, acrylic, polycarbonate, and orange acrylic), together with an indicative solar spectrum, are presented in Fig. 1. The curves for the first three filters were compared with an action spectrum for E. coli and a solar irradiance spectrum by Sinton et al. (37). The maximum transmission of the filter materials was around 85 to 90%, because of reflection at the filter-air interfaces.
FIG. 1.
Spectral transmission curves for the optical filters used as chamber covers, including the spectral cutoff wavelength (λ50) for each filter (obtained by scanning the filter materials from 300 to 650 nm, against an air reference, with a model PU8800 spectrophotometer). Also shown is an indicative solar spectrum.
Two days before each experiment, up to 2,000 liters of unpolluted seawater was collected from nearby Lyttelton Harbour. The collection site, which was selected because of low fecal coliform counts (<5 CFU 100 ml−1), was a boat-launching ramp on the shoreline. The water was transported to the Lincoln site and was stirred (to ensure uniform clarity) before being pumped into the chambers.
Coarse-screened, raw sewage was used to seed the seawater in all experiments. However, one summer experiment (experiment 2 [Table 1]) included two further chambers (one sunlight exposed and one dark), each seeded with waste stabilization pond effluent. Each effluent seed was collected about 18 h before the experiment from the Christchurch sewage treatment plant and was stored overnight at about 5°C (tests showed no significant overnight change in bacterial or phage counts).
In each experiment, on the hour after the water surface was first exposed to direct sunlight, sewage was added to each chamber to give a concentration of 2% (vol/vol). In experiment 2, the concentration of waste stabilization effluent was 6% (vol/vol), to ensure an adequate initial microbial concentration. The first sample was collected immediately after stirring the seawater-effluent mixture with the bilge pumps for 3 min. Subsequent samples were collected on the hour. In experiment 7, sampling continued overnight (every 3 h) and hourly for 8 daylight hours on the following day. Two survival experiments included additional indicators—F-DNA and B. fragilis phages in experiment 5, and enterococci in experiment 7 (Table 1).
All six test organisms were included in the variability experiment (experiment 6), designed to gauge the overall variability associated with sample collection, transport, and assay. After 3 h of sunlight exposure, 10 samples were collected in quick succession (over a period of about 4 min) from one sunlight-exposed and one dark control chamber. The samples were transported to the laboratory and assayed as described below.
Although the sewage seed was the source of the test organisms in all experiments, because of low counts of naturally occurring B. fragilis phages in Christchurch sewage (unpublished data), laboratory-cultured B. fragilis phages were used to supplement the sewage seed in experiments 5 and 6 (see below [Laboratory analyses]).
All samples were collected in foil-wrapped, sterile glass bottles. The first 100 ml drawn into the evacuated sample bottle was discarded, and a 500-ml sample was collected. Because the manifold was about 1 m above the chambers, residual liquid in the tubes quickly flowed back into the chambers, thus minimizing cross-contamination between samples. In addition, after sampling, the manifold seals were rinsed with 70% ethanol and then sterile water.
Sample bottles were kept in the dark at 6 to 8°C for transfer to the laboratory. The holding time between collection and assay was typically ≤30 min.
Laboratory analyses.
Samples (1 to 2 ml) from chambers containing seawater-raw sewage mixtures were assayed for phages on overlay pour plates. In experiment 2, larger (100-ml) samples from the chambers containing seawater-waste stabilization pond effluent mixtures (where phage counts were lower) were assayed by the membrane filtration-swirling elution method of Sinton et al. (38). Because the same hosts were used and the data were normalized (as percentage survival curves), the different phage extraction methods were not considered likely to have influenced the results in experiment 2.
The bacterial host for the somatic coliphages was E. coli 13706/60. The host used for the recovery of F-RNA phages was Salmonella typhimurium WG49. E. coli RR (with the inclusion of RNase) was used for the recovery of F-DNA phages. The characteristics of these hosts and enumeration procedures are described by the International Organization for Standardization (23) and Sinton et al. (38).
The host for B. fragilis phages was B. fragilis HSP40 (ATCC 51477). This strain is resistant to kanamycin sulfate (100 mg liter−1) and vancomycin sulfate (7.5 mg liter−1) and was cultured anaerobically at 37°C for 4 to 5 h in Bacteroides phage recovery medium, to obtain a log-phase culture. A fresh culture was prepared from a slope culture for each assay. The base agar was modified blood agar base, and the overlay was brucella broth (prepared as an agar), both of which contained kanamycin sulfate (100 mg liter−1) and vancomycin sulfate (7.5 mg liter−1), as described by Tartera and Jofre (39). Samples were assayed by overlay pour plating. Host culture (0.5 ml) plus 1 ml of sample was added to a tube containing 2.5 ml of overlay agar held at 45°C. The tube was stirred on a vortex mixer, and the contents were poured onto the base layer and allowed to set. Plates were inverted and incubated anaerobically (in anaerobic jars with Oxoid gas-generating kits) at 37°C for 18 h.
The supplementary seed of B. fragilis phages (experiments 5 and 6) was prepared by first pour plating raw sewage using host HSP40 and confirming 10 plaques as B. fragilis phages by stabbing them onto host lawns of B. fragilis HSP40. Phages were harvested from these plates (by aseptic removal of agar plugs and resuspension in 5 ml of sterile water) and were added to the sewage to raise the count in the initial seawater-raw sewage mixture to >104 PFU per ml.
Samples were analyzed for fecal coliforms and enterococci by membrane filtration (Sartorius CN filter; 0.45 μm pore size), with dilutions prepared in phosphate buffer as required. Enumeration of fecal coliforms was by incubation on mFC agar (Gibco) at 44.5 ± 0.2°C for 24 ± 2 h (1). Enterococci were incubated on mE agar (Difco) at 41 ± 0.5°C for 48 h, followed by transfer to esculin iron agar for a further 20 min at 41 ± 0.5°C (1).
Bacterial counts were expressed as CFU per 100 ml; phage counts were expressed as PFU per 100 ml.
Solar radiation and temperature measurements.
Global (i.e., diffuse plus direct) solar radiation (GSR) was measured on site by using a LI-COR LI-200SA pyranometer connected to a LI-COR LI-1000 data logger. To maintain parity with local sea temperatures, the target temperature for each experiment was set to the mean Christchurch sea surface temperature for the relevant month. Chamber temperature was monitored hourly with a digital thermometer, with the probe suspended 200 mm below water level in one chamber.
Calculation of inactivation parameters.
A linear regression line was fitted to the (loge-transformed) counts from the dark chambers in each experiment to derive the dark inactivation rate (kD), in loge units per hour.
In tanks exposed to sunlight (including optically filtered sunlight in experiment 10), the percentage survival (p) at exposure time t was defined as p = 100N/N0, where N is the CFU or PFU count and N0 is the initial count. Each p value was corrected for dark inactivation by using the data from the dark control chamber for the particular experiment and the equation ps = p e−kDt, where pS is the corrected sunlight value.
A linear regression line was fitted to each set of (loge-transformed) bacteriophage sunlight inactivation ps values. Sunlight inactivation parameters for the phages were obtained from plots loge ps versus insolation (GSR, integrated from time 0 to t, i.e., cumulative GSR), in megajoules per square meter.
The bacterial sunlight inactivation curves usually displayed a recognizable shoulder, so the approach described by Sinton et al. (37) was adopted. A two-parameter, multitarget, kinetic expression (19) was fitted to the data:
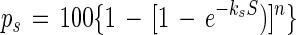 |
where S is insolation, kS is the sunlight inactivation coefficient (in meters squared per megajoule), and exponent n is a dimensionless parameter quantifying the size of the shoulder. The bacterial inactivation coefficient was obtained from the final slope of the inactivation curve (ks = −Δlogep/ΔS), by using the linear regression of logeps versus S, omitting points in the shoulder region, where present. The parameter n (the shoulder constant) was evaluated as n = p0/100, where logep0 is the y-axis intercept of the regression line.
To allow comparison with other studies, the insolation and time taken to achieve a 90% reduction in CFU or PFU count (the S90 and T90 values, respectively) were also calculated. The dark T90 was derived directly from the mean kD, as 2.303/kD (similarly for the sunlight T90), and S90 was derived directly from the mean kS, as 2.303/kS.
Because experiment 2 (Table 1) involved a comparison of two different effluent types and concentrations mixed in seawater, sunlight penetration into the mixtures was estimated based on the procedures described by Davies-Colley et al. (10). Spectral light absorption and scattering was measured on a Pye-Unicam PU8800 spectrophotometer, a spectral irradiance attenuation coefficient was calculated, and the average irradiance over the 560-mm depth of the chamber mixtures was expressed as a fraction of the incident spectral irradiance. These calculations showed that light attenuation by the seawater itself dominated in our experimental mixtures, and thus the average light exposure of the 2% sewage mixture was very similar to that of the 6% pond effluent mixture over most of the UV-visible spectrum. Accordingly, the survival curves in experiment 2 are presented as a function of incident insolation, uncorrected for attenuation in the different mixtures.
RESULTS
Data variability.
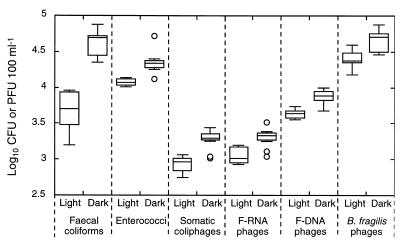
The results of the variability experiment (experiment 6) are presented as box plots in Fig. 2. Overall, fecal coliforms exhibited the highest degree of variability. The four phage indicators were broadly similar, with enterococcal counts in the sunlight tanks being the least variable.
FIG. 2.
Variability associated with sample collection, transport, and assay. Shown are box plots of the counts obtained from 10 samples collected over a period of 4 min from each tank. The crosspieces of each box plot represent (from top to bottom), maximum, upper-quartile, median, lower-quartile, and minimum values. An outlier (open circle) is defined as a point whose value is either above the upper quartile by 1.5 times the interquartile distance or below the lower quartile by 1.5 times the interquartile distance. Laboratory-cultured B. fragilis phages were added to the sewage inoculum.
Dark inactivation.
The mean kD and dark T90 values for each indicator are presented in Table 2, with the results broadly subdivided into winter (temperature range: 8 to 10°C) and summer (temperature range: 14 to 20°C) values. The somatic coliphage, F-RNA phage, and fecal coliform dark inactivation rates were higher in summer than in winter. In winter, kDs were highest for fecal coliforms and lowest for F-RNA phages, but in summer, somatic coliphages, F-RNA phages, and fecal coliforms had similar kDs. F-DNA phages were the most and B. fragilis phages and enterococci were the least rapidly inactivated in the dark (each based on a single summer experiment).
TABLE 2.
Dark inactivation parametersa
| Indicator | Mean dark inactivation parameter for:
|
|||
|---|---|---|---|---|
| Summerb
|
Winterc
|
|||
| kD (h−1) | T90 (h) | kD (h−1) | T90 (h) | |
| Somatic coliphage | 0.044 (n = 7; 56%) | 52 | 0.023 (n = 3; 96%) | 102 |
| F-RNA phage | 0.044 (n = 6; 59%) | 52 | 0.015 (n = 1) | 156 |
| Fecal coliform | 0.047 (n = 7; 39%) | 49 | 0.036 (n = 3; 27%) | 63 |
| F-DNA phage | 0.115 (n = 1) | 20 | ||
| B. fragilis phage | 0.009 (n = 1) | 257 | ||
| Enterococcus | 0.005 (n = 1) | 446 | ||
Mean kDs and T90s for summer and winter temperature ranges (14 to 20°C, and 8 to 10°C, respectively). For each kD, the number of experiments (n) and, where applicable, coefficient of variation are given in parentheses.
Experiments 1 to 3, 5, 7, 9, and 10.
Experiments 4 and 8.
Sunlight inactivation.
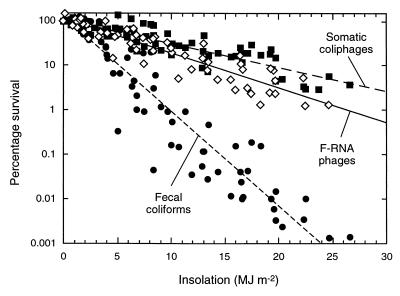
Percentage survival data for somatic coliphages, F-RNA phages, and fecal coliforms from all 10 survival experiments are presented in Fig. 3 and Table 3. Comparison of the regression line slopes in Fig. 3, according to the method of Zar (43), showed that both phages were inactivated significantly more slowly than fecal coliforms (P < 0.001) and the somatic coliphages were inactivated significantly more slowly than the F-RNA phages (P < 0.05).
FIG. 3.
Inactivation in seawater, as a function of insolation, of somatic coliphages (■), F-RNA phages (◊), and fecal coliform bacteria (●) from untreated sewage (data from all survival experiments). The fecal coliform data are the linear portions of the inactivation curves (i.e., shoulder points, where present, have been removed). The R2 values are as follows: for somatic coliphages, 0.82; for F-RNA phages, 0.86; and for fecal coliforms, 0.89.
TABLE 3.
Mean sunlight inactivation parametersa
| Indicator |
kS (m2 MJ−1)
|
nS
|
S90 (MJ m−2)
|
T90 (h)
|
||||
|---|---|---|---|---|---|---|---|---|
| Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | |
| Somatic coliphage | 0.11 (n = 7; 26%) | 0.06 (n = 3; 57%) | NAb | NA | 20.9 | 36.8 | 7.0 | 51.3 |
| F-RNA phage | 0.17 (n = 6; 21%) | 0.10 (n = 1) | NA | NA | 13.7 | 22.8 | 4.8 | 20.3 |
| Fecal coliform | 0.47 (n = 7; 11%) | 0.37 (n = 3; 58%) | 1.23 | 2.09 | 4.7 | 7.2 | 1.7 | 7.7 |
| F-DNA phage | 0.14 (n = 1) | NA | 16.0 | 4.9 | ||||
| B. fragilis phage | 0.16 (n = 1) | NA | 14.0 | 4.3 | ||||
| Enterococcus | 0.27 (n = 1) | 9.47 | 15.8 | 6.9 | ||||
For each kS, the number of experiments (n) and, where applicable, the coefficient of variation are given in parentheses. NA, not applicable.
Table 3 also shows that, in summer, the S90 of the somatic coliphages and F-RNA phages was, respectively, 4.5 and 3 times that required for fecal coliforms. The equivalent winter figures were similar (5 and 3 times that required for fecal coliforms). The winter T90s for somatic coliphages, F-RNA phages, and fecal coliforms were, respectively, 7, 4, and 4.5 times longer than the summer T90s (although the winter F-RNA phage T90 was based on a single experiment).
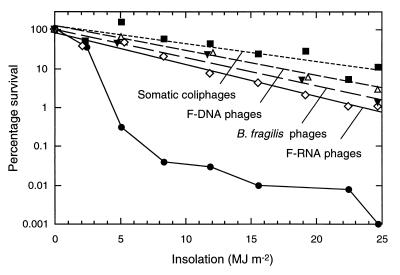
Figure 4 (experiment 5) shows that kSs of somatic coliphages, F-RNA phages, F-DNA phages, and B. fragilis phages were all markedly less than those for fecal coliforms. The overall inactivation rate ranking was (from least to greatest) somatic coliphages, F-DNA phages, B. fragilis phages, F-RNA phages, and fecal coliforms.
FIG. 4.
Inactivation in seawater, as a function of insolation, of somatic coliphages (■), F-RNA phages (◊), F-DNA phages (▵), phages of B. fragilis HSP40 (▾), and fecal coliform bacteria (●) from raw sewage. For clarity, some data points are slightly offset, and the phage data are presented as regression lines (the R2 values are as follows: for somatic coliphages, 0.84; for F-DNA phages, 0.97; for B. fragilis phages, 0.96; and for F-RNA phages, 0.98).
The actual counts (CFU or PFU 100 ml−1) of the indicators in the first sample of the survival experiments (as a mean of up to 10 samples, where appropriate) were as follows: for fecal coliforms, 1.2 × 105; for somatic coliphages, 3.4 × 103; for F-RNA phages, 8.5 × 103; for F-DNA phages, 8.6 × 103; for B. fragilis phages (supplemented), 9.6 × 104; and for enterococci, 7.9 × 103.
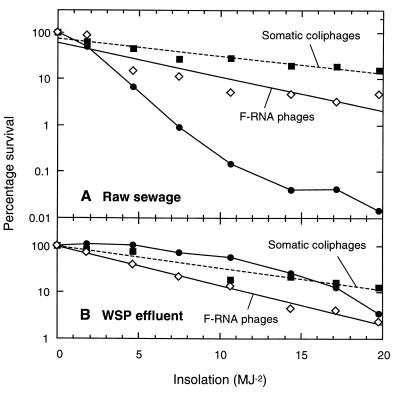
Effect of effluent type.
Figure 5 shows that the kSs in seawater were similar for somatic coliphages and F-RNA phages from raw sewage and waste stabilization pond effluent but that fecal coliforms from raw sewage (kS = 0.46 m2 MJ−1) were inactivated more rapidly than fecal coliforms from pond effluent (kS = 0.16 m2 MJ−1).
FIG. 5.
Inactivation in seawater, as a function of insolation, of somatic coliphages (■), F-RNA phages (◊), and fecal coliform bacteria (●) from raw sewage (A) and waste stabilization pond (WSP) effluent (B). For clarity, the phage data are presented as regression lines (the raw sewage R2 values are as follows: for somatic coliphages, 0.89; and for F-RNA phages, 0.90; the WSP effluent R2 values are as follows: for somatic coliphages, 0.96; and for F-RNA phages, 0.99).
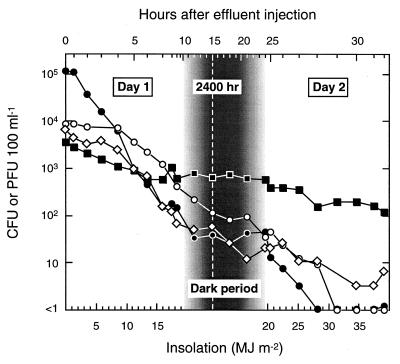
Two-day experiment.
The results of the 2-day experiment are presented in Fig. 6. Both days were fine with no clouds. The survival curves are plotted against insolation (lower x axis) during daylight periods and against time during the overnight period (upper x axis). The y axis gives actual (log10) concentrations rather than percentage survival. The order of the counts at the start of the experiment (from greatest to least: fecal coliforms, enterococci, F-RNA phages, and somatic coliphages) was reversed towards the end of day 2 (fecal coliform counts fell below 1 CFU 100 ml−1 1 h before that of enterococci).
FIG. 6.
Inactivation in seawater of somatic coliphages (■), F-RNA phages (◊), enterococci (○), and fecal coliform bacteria (●) from untreated sewage, as a function of insolation and time. The insolation scale is linear during daylight hours; the time scale is linear during the overnight period. Samplings in which no CFU were detected in 100 ml are presented as <1 on the (log10) y axis.
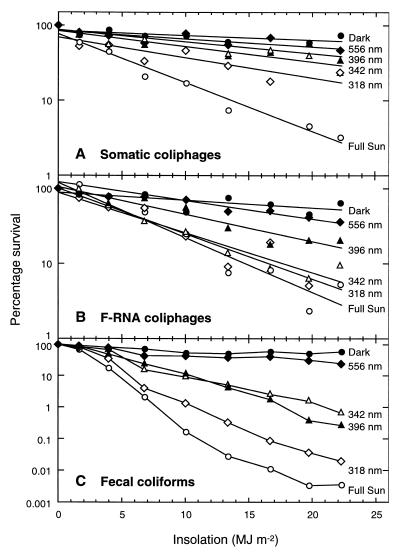
Contributions of different spectral regions to inactivation.
In the optical filter experiment (experiment 10), the order of the inactivation curves (Fig. 7) matched the order of the 50% light transmission wavelengths (λ50s) for both the somatic coliphages and the F-RNA phages. The fecal coliform curves broadly matched the λ50s, although there was an anomalous juxtaposition of the curves associated with the 342- and 396-nm filters. Figure 7 shows that the fecal coliforms (plotted on a more-compressed x-axis scale) were inactivated more rapidly than the phages at all wavelengths. Fecal coliforms and F-RNA phages were inactivated by a wide range of wavelengths, whereas somatic coliphages were more sensitive to wavelengths below 318 nm (UV-B).
FIG. 7.
Inactivation in seawater of somatic (A), and F-RNA phages (B), and fecal coliforms (C) from untreated sewage, in the dark (●), under full sun (○), and under 556-nm (orange) (⧫), 396-nm (polycarbonate) (▴), 342-nm (acrylic) (▵), and 318-nm (polyester) (◊) optical filters. For clarity, the phage data are presented as regression lines. Note the difference in scale of the fecal coliform y axis.
DISCUSSION
Data variability.
The results of the variability experiment (Fig. 2) suggest that the phage inactivation parameters were more robust than those for fecal coliforms. However, the F-RNA phage plots in Fig. 2, which were derived from assays using a single S. typhimurium WG49 host culture, probably underestimate the variability in the survival experiments, where fresh cultures were required every 2 h. In spite of careful quality control (23), F-RNA phage survival data were more variable than those for somatic coliphages, possibly due to different levels of F pilus production between sequential WG49 cultures. Similar problems with this host have been reported by the WRc (41).
Dark inactivation.
Dark inactivation rates were low for all the indicators. The overall kD ranking of the phages in Table 2 (from greatest to least: somatic coliphages, F-RNA phages, F-DNA phages, and B. fragilis phages) is consistent with the findings of Chung and Sobsey (7) that B. fragilis phages are longer-lived than F-RNA phages in laboratory-stored seawater. Although there was no difference between the summer kD values for somatic coliphages and F-RNA phages, dark inactivation at winter temperatures was higher for somatic coliphages (Table 2). In contrast, the WRc (41) reported lower inactivation rates for somatic coliphages than for F-RNA phages in dark seawater microcosms at both 10 and 20°C. However, faster dark inactivation is likely in many parts of the world, where both summer and winter seawater temperatures are higher than those used in our study and in the WRc experiments.
The faster dark inactivation of fecal coliforms in seawater in summer compared to winter (Table 2) is in agreement with the findings of Gameson (15) and our earlier investigation (37). Increased dark inactivation of sewage bacteria at higher temperatures has been attributed to the detrimental effects of increased metabolism in a low-nutrient environment and increases in the activity of predatory and lytic marine microflora (15). The faster dark inactivation of both somatic coliphages and F-RNA phages at summer temperatures (Table 2) is consistent with the results reported by the WRc (41) and Chung and Sobsey (7). Marine microbes appear to produce extracellular, filter-passing, antiviral compounds (40), so increases in antiviral activity may be expected at higher temperatures.
Sunlight inactivation.
All test organisms were more rapidly inactivated in sunlight than in the dark. A comparison of the summer T90 values (Tables 2 and 3) shows that for the three principal organisms (measured in six or more experiments), this difference was most marked for fecal coliforms, followed by F-RNA phages and somatic coliphages (inactivation 29, 11, and 7 times more rapid, respectively). This ranking reflects the relative resistance of these indicators to sunlight, because their dark T90 values (Table 2) were the same or similar. For the enterococci, B. fragilis phages, and F-DNA phages, the rates of inactivation were 65, 60, and 20 times more rapid, respectively.
The greater sunlight resistance of the phages compared to fecal coliforms was observed in all experiments (Fig. 3). These findings are similar to those reported by the WRc (41) for an experiment in which beakers containing seawater seeded with somatic coliphages, F-RNA phages, and fecal (thermotolerant) coliforms were exposed to sunlight (measured with a solarimeter). However, the fecal coliform, somatic coliphage, and F-RNA phage S90 values in Table 3 were above the ranges reported by the WRc (41)—respectively, 1.4 to 1.5 MJ m−2, 9.4 to 19.7 MJ m−2, and 3.3 to 4.3 MJ m−2. This may reflect the fact that the microbes in the sewage seed used in our study were hardier than the laboratory-cultured organisms (albeit isolated from natural sources) used in the WRc experiment or were particle associated (34). The mean summer S90 for fecal coliforms in Table 3 (4.7 MJ m−2) is broadly similar to the 6.3 MJ m−2 reported in our earlier investigation (37), suggesting that S90 values are reasonably robust parameters for comparing different studies.
The results for the F-DNA and B. fragilis phages (Fig. 5; Table 3) suggest that they are inactivated at rates intermediate between those of somatic coliphages and F-RNA phages. There appear to be no reported studies on sunlight inactivation of F-DNA phages in natural waters, although Davies-Colley et al. (10) recorded their inactivation by sunlight in waste stabilization pond effluent. Similarly, although B. fragilis phages have been reported to be more susceptible to UV inactivation than F-RNA phages (6), the effects of solar radiation on B. fragilis phages in natural waters do not appear to have been reported previously.
Most of the fecal coliform curves exhibited a shoulder. This is generally interpreted as representing the number of targets that need to be hit before a CFU is inactivated (19). As in our earlier study (37), shoulders for enterococci were larger than those for fecal coliforms (Fig. 6; Table 3), and the fecal coliform curves tended to flatten towards the end of the day. Dissolved oxygen levels did not change in the sunlight-exposed chamber throughout experiment 6 (data not presented), suggesting no decrease in the photooxidative impact of sunlight. Possible explanations for this curve flattening include photoreactivation resulting from longer solar wavelengths in the late afternoon (19, 24) and the presence of a sunlight-resistant subset of fecal coliforms, possibly as a result of association with particles (34).
All the bacteriophage curves were exponential, with no shoulder. This curve type has been described by Harm (19) as a “one-hit, one-target” curve. Although minor shoulders have been noted in pure-culture studies of double-stranded DNA phages, the equivalent curves reported for single-stranded DNA and RNA phages have been strictly exponential (19, 24).
Effect of effluent type.
It was estimated that the fecal coliforms from the waste stabilization pond effluent had already been exposed to 42 h of sunlight (∼80 MJ m−2) at the time of collection. Thus, their superior survival rate suggests that they were sunlight-resistant survivors of the raw sewage fecal coliforms. The similar rates of inactivation of phages from the sewage and pond effluent suggest that the phage genome offers a relatively limited scope for the development of sunlight resistance or that there were marked differences in particle associations between the bacteria and phages in the two effluents. Overall, these results suggest that enteric phage inactivation rates in seawater are likely to be far less dependent on the effluent source than those of fecal coliforms.
Two-day experiment.
Four distinct inactivation patterns were discernible in the 2-day experiment (Fig. 6). On day 1, the fecal coliform curve exhibited a small shoulder followed by a phase of rapid, exponential inactivation, with the curve flattening in the late afternoon. The overnight counts rose slightly, suggesting some dark repair (24). After sunrise on day 2, the curve resumed an exponential reduction phase, at a rate similar to that on day 1. Fecal coliform counts were first to fall below 1 CFU 100 ml−1, at 1300 h on day 2.
The shoulder for enterococci on day 1 was much larger than that for fecal coliforms, the log-linear phase was shallower, there was little evidence of photoreactivation in the late afternoon, and the overnight curve was flatter. Enterococci were slower to recommence exponential inactivation on day 2 but still fell below 1 CFU 100 ml−1 1 h after the fecal coliforms. Thus, in spite of larger shoulders and slower exponential inactivation, the combination of a lower initial count and a comparative lack of repair meant there was little difference over 2 days between the times to extinction (<1 CFU 100 ml−1) of fecal coliforms and enterococci. A similar result was recorded in our earlier study (37). These relative 2-day inactivation patterns may explain the often small differences between the disease risk prediction abilities of fecal coliforms and enterococci in some epidemiological studies (e.g., see references 13 and 22).
The F-RNA phage inactivation curve was steeper than that for somatic coliphages. There was no evidence of photoreactivation or dark repair, but F-RNA phages were still detectable at the end of day 2 (6 PFU 100 ml−1). The somatic coliphage slopes were similar on both days, and there was little overnight inactivation. Despite having the lowest initial count, the final somatic coliphage count was the highest (120 PFU 100 ml−1). These results suggest that, for marine outfall plume travel times of 1 to 2 days, somatic coliphages may be more useful as fecal indicators than fecal coliforms, enterococci, or F-RNA phages.
Contribution of spectral regions to inactivation.
The elucidation of sunlight inactivation mechanisms for enteric phages is practical only if it is assumed that little, if any, replication occurs in sewage-polluted marine waters (in contrast, sunlight inactivation of natural marine phage populations is likely to involve complex interactions between sunlight, phages, and their hosts). It is generally assumed that enteric F-RNA phages do not replicate in marine environments, because F-specific pilus synthesis occurs only above 30°C. Although somatic coliphage replication has been observed in laboratory microcosms (5, 33), the evidence for replication under natural conditions is equivocal. To multiply, phages appear to require concentrations of at least 104 CFU of susceptible host cells 100 ml−1 (42)—conditions which were met in the above studies only by seeding the microcosms with host cells. In addition, replication in natural waters appears to be unlikely for somatic coliphages able to grow at the temperatures (35 to 37°C) commonly used in laboratory assays (36). Because there was no evidence of phage replication in our study, even in the dark tanks, we have assumed that sunlight inactivation mechanisms, and in particular the optical filter experiments, can be interpreted in terms of their effects on the phage virions themselves.
Figure 7 shows that somatic coliphages were highly susceptible to the UV-B component of sunlight (58% of inactivation was attributable to wavelengths below 318 nm). In contrast, F-RNA phages were susceptible to all the components of the solar spectrum below 556 nm but were not particularly susceptible to damage by UV-B wavelengths (only 17% of inactivation was attributable to wavelengths below 318 nm). Fecal coliforms were inactivated by a wide range of solar wavelengths. The results are broadly similar to those reported by Sinton et al. (37), where half the fecal coliform inactivation was attributed to wavelengths above (and half below) 360 nm, and to those reported by Davies-Colley et al. (9), who found that the depth dependence of inactivation matched the depth profile of radiation at 360 nm. Overall, the results in Fig. 7 indicate that the increase in sunlight attenuation in seawater that occurs with decreasing wavelength (25), removes the shorter wavelengths, to which somatic coliphages are more susceptible, while allowing penetration of longer wavelengths, to which F-RNA phages and fecal coliforms are more susceptible.
Phage inactivation in sunlight-exposed seawater occurs when solar radiation results in damage to the capsid and/or nucleic acid genome. The spectral irradiance curve in Fig. 1 suggests that little photobiological (direct) damage to the capsid proteins is likely. This is because light absorption in proteins peaks at around 280 nm but falls sharply above this wavelength and is minimal above 300 nm (19). Thus, capsid damage is more likely to result from photochemical mechanisms. F-RNA phage susceptibility to longer wavelengths (Fig. 7) is consistent with a photochemical mechanism of inactivation. Davies-Colley et al. (10) suggested that F-RNA phage inactivation at longer wavelengths in waste stabilization pond effluent is due to photooxidative damage to host-binding proteins.
Greater capsid damage at longer wavelengths may be one reason why F-RNA phages were inactivated faster than somatic coliphages. Photobiological damage to the nucleic acids, which absorb light at wavelengths of >230 nm more strongly than do proteins (19), may be another reason, although the extent to which this occurs in seawater is unclear. Studies on UV (254-nm) sterilization of effluents have shown that F-RNA phages are actually more resistant than somatic coliphages to photobiological damage (21). Thus, if photobiological mechanisms contributed to the observed phage inactivation differences in our study, the implication is that RNA is more readily damaged than DNA at longer wavelengths.
Different repair mechanisms may also contribute to differences in phage inactivation rates. Phage repair of nucleic acid damage is expressed only after host infection (19, 24). The mechanism (bacterial excision resynthesis repair) involves enzymatic excision of damaged oligonucleotides from one DNA strand, followed by nucleotide resynthesis using the complementary strand. Thus, most somatic coliphages (double-stranded DNA) have this repair capability, whereas single-stranded DNA and RNA phages do not (19). Thus, excision repair is consistent with the lower inactivation of somatic coliphages compared to F-RNA phages and (single-stranded) F-DNA phages in our study. However, it does not explain why fecal coliforms were inactivated more rapidly than somatic coliphages, because excision repair is even more effective in repairing damage to the bacterial DNA itself (19). The difference is probably due to the greater overall susceptibility of fecal coliforms to a wide range of inactivating wavelengths (Fig. 7). Although standard indicator bacterial media were selected to maximize the relevance of our results to commonly used monitoring procedures, it should be noted that the shape of survival curves of sunlight-damaged cells may be influenced by interactions with inhibitory media and temperatures.
General conclusions.
The principal finding of our study was that somatic coliphages exhibited consistently superior survival in sunlight-exposed seawater compared to fecal coliforms and F-RNA phages. They were also more sunlight resistant than enterococci, F-DNA phages, and B. fragilis phages. Somatic coliphages have the advantage of a relatively straightforward assay procedure compared to F-RNA phages, but two arguments are often advanced against their use. First, they are a heterogeneous group, so the selected host will determine the subset enumerated and thus determine the count. This objection has been largely negated by the increasing adoption of E. coli ATCC 13706 derivatives as standard hosts (1, 38). Second, it has been suggested that somatic coliphages may replicate in the environment, although, as noted above, it is doubtful whether this occurs in marine waters.
Although our results (based on limited data) also showed that F-DNA and B. fragilis phages survived almost as well as somatic coliphages, there are problems associated with these indicators. Assaying for these phages is more complex than assaying for somatic coliphages, there is little information available on the sanitary significance of F-DNA phages, and low counts of B. fragilis phages in sewage-polluted marine waters (8) may limit their use as indicators.
In conclusion, our findings that somatic coliphages persist in sunlight-exposed seawater longer than fecal coliforms, enterococci, and F-RNA phages suggest that they warrant further consideration as fecal, and possibly viral, indicators in marine waters. Work is continuing in our laboratory on somatic coliphage and F-RNA phage inactivation rates following effluent discharges to fresh and saline waters and on determining whether somatic coliphages from sewage can replicate under natural conditions.
ACKNOWLEDGMENTS
We gratefully acknowledge the assistance of R. J. Davies-Colley, National Institute of Water and Atmospheric Research, for his advice throughout this study and for his critical comment on the manuscript. M. J. Noonan, Lincoln University, A. M. Donnison, Meat Industry Research Institute of New Zealand, and the journal’s referees also provided valuable comments. We also thank E. Gerard for technical assistance in the field and in the laboratory and the staff of the Christchurch City Council for providing access to the sewage treatment plant.
This research was funded by the New Zealand Public Good Science Fund, administered by the Foundation for Research, Science, and Technology.
REFERENCES
- 1.American Public Health Association. Standard methods for the examination of water and wastewater. 19th ed. Washington, D.C: American Public Health Association; 1995. [Google Scholar]
- 2.Ayres P A. Coliphages in sewage and the marine environment. Soc Appl Bacteriol Symp Ser. 1977;6:275–298. [Google Scholar]
- 3.Berry S M, Noton B G. Survival of bacteriophages in seawater. Water Res. 1976;10:323–327. [Google Scholar]
- 4.Borrego J J, Moriñigo M A, de Vicente A, Cornax R, Romero P. Coliphages as an indicator of faecal pollution in water. Its relationship with indicator and pathogenic micro-organisms. Water Res. 1987;21:1473–1480. [Google Scholar]
- 5.Borrego J J, Cornax R, Moriñigo M A, Martinee-Manzanares E, Romero P. Coliphages as an indicator of faecal pollution in water. Their survival and productive infectivity in natural aquatic environments. Water Res. 1990;24:111–116. [Google Scholar]
- 6.Bosch A, Tartera C, Gajardo R, Diez J M, Jofre J. Comparative resistance of bacteriophages active against Bacteroides fragilis to inactivation by chlorination or ultraviolet radiation. Water Sci Technol. 1989;21:221–226. [Google Scholar]
- 7.Chung H, Sobsey M D. Comparative survival of indicator viruses and enteric viruses in seawater and sediment. Water Sci Technol. 1993;27:425–428. [Google Scholar]
- 8.Cornax R, Moriñigo M A, Balebona M C, Castro D, Borrego J J. Significance of several bacteriophage groups as indicators of sewage pollution in marine waters. Water Res. 1991;25:673–678. [Google Scholar]
- 9.Davies-Colley R J, Bell R G, Donnison A M. Sunlight inactivation of enterococci and fecal coliforms within sewage effluent diluted in seawater. Appl Environ Microbiol. 1994;6:2049–2058. doi: 10.1128/aem.60.6.2049-2058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies-Colley R J, Donnison A M, Speed D J, Ross C M, Nagels J W. Inactivation of faecal indicator microorganisms in waste stabilization ponds: interaction of environmental factors with sunlight. Water Res. 1999;33:1220–1230. [Google Scholar]
- 11.Debartolomeis J, Cabelli V J. Evaluation of an Escherichia coli host strain for enumeration of F male-specific bacteriophages. Appl Environ Microbiol. 1991;57:1301–1305. doi: 10.1128/aem.57.5.1301-1305.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutka B J, El Shaarawi A, Martins M T. North and South American studies on the potential of coliphage as a water quality indicator. Water Res. 1987;21:1127–1135. [Google Scholar]
- 13.El-Sharkawi F, Hassan M N E R. The relation between the state of pollution in Alexandria swimming beaches and the occurrence of typhoid among bathers. Bull High Inst Public Health Alexandria. 1982;12:337–351. [Google Scholar]
- 14.European Economic Community. 1975. Directive du conseil concernant la qualité des eaux de gaignade du 8 decembre 1975. Journal Official de Communantes Europennes L31/1.
- 15.Gameson A L H. Investigations of sewage discharges to some British coastal waters, part 1. Bacterial mortality, p. 1–34. Water Research Centre Technical Report. TR 201. Medmenham, United Kingdom: Water Research Centre Environment; 1984. [Google Scholar]
- 16.Gammie A J, Wyn-Jones A M. Does hepatitis A pose a significant health risk to recreational water users? Water Sci Technol. 1997;35:171–177. [Google Scholar]
- 17.Gerba C P, Schailberger G E. The effect of particulates on virus survival in seawater. J Water Pollut Control Fed. 1975;47:93–103. [PubMed] [Google Scholar]
- 18.Girones R, Jofre J, Bosch A. Natural inactivation of enteric viruses in seawater. J Environ Qual. 1989;18:34–39. [Google Scholar]
- 19.Harm W. Biological effects of ultraviolet radiation. London, United Kingdom: Cambridge University Press; 1980. [Google Scholar]
- 20.Havelaar A H, van Olphen M, Drost Y. F-specific RNA bacteriophages are adequate model organisms for enteric viruses in fresh water. Appl Environ Microbiol. 1993;59:2956–2962. doi: 10.1128/aem.59.9.2956-2962.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Havelaar A H, Pot-Hogeboom W M, Koot W, Pot R. F-specific bacteriophages as indicators of the disinfection efficiency of secondary effluent with ultraviolet radiation. Ozone Sci Eng. 1987;9:353–368. [Google Scholar]
- 22.Holmes P R. Research in health risks at bathing beaches in Hong Kong. J Inst Water Environ Manag. 1989;3:488–495. [Google Scholar]
- 23.International Organization for Standardization. Water quality-detection and enumeration of bacteriophages, part I. Enumeration of F-specific RNA bacteriophages. ISO 10705-1. Geneva, Switzerland: International Organization for Standardization; 1995. [Google Scholar]
- 24.Jagger J. Solar-UV actions on living cells. New York, N.Y: Praeger; 1985. [Google Scholar]
- 25.Jerlov N G. Elsevier Oceanography Series. 14. Marine optics. Amsterdam, The Netherlands: Elsevier Scientific Publishing Co.; 1976. [Google Scholar]
- 26.Jofre J, Bosch A, Lucena F, Girones R, Tartera C. Evaluation of Bacteroides fragilis bacteriophages as indicators of the virological quality of water. Water Sci Technol. 1986;18:167–173. [Google Scholar]
- 27.Kapuscinski R B, Mitchell R. Processes controlling virus inactivation in coastal water. Water Res. 1980;14:363–371. [Google Scholar]
- 28.Kapuscinski R B, Mitchell R. Sunlight-induced mortality of viruses and Escherichia coli in coastal seawater. Environ Sci Technol. 1983;17:1–6. doi: 10.1021/es00107a003. [DOI] [PubMed] [Google Scholar]
- 29.Lee J V, Dawson S R, Ward S, Surman S B, Neal K R. Bacteriophages are a better indicator of illness rates than bacteria amongst users of a white water course fed by a lowland river. Water Sci Technol. 1997;35:165–170. [Google Scholar]
- 30.Lucena F, Lasobras J, McIntosh D, Forcadell M, Jofre J. Effect of distance from the polluting focus on relative concentrations of Bacteroides fragilis phages and coliphages in mussels. Appl Environ Microbiol. 1994;60:2272–2277. doi: 10.1128/aem.60.7.2272-2277.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mariño F J, Martinez-Manzanares E, Moriñigo M A, Borrego J J. Applicability of the recreational water quality standard guidelines. Water Sci Technol. 1995;31:27–31. [Google Scholar]
- 32.Medema G J, van Asperen I A, Havelaar A H. Assessment of the exposure of swimmers to microbiological contaminants in fresh waters. Water Sci Technol. 1997;35:157–163. [Google Scholar]
- 33.Parry O T, Whitehead J A, Dowling L T. Temperature sensitive coliphage in the environment. In: Goddard M, Butler M, editors. Viruses and wastewater treatment. Oxford, United Kingdom: Pergamon Press; 1981. pp. 277–279. [Google Scholar]
- 34.Qualls R G, Flynn M P, Johnson J D. The role of suspended particles in ultraviolet disinfection. J Water Pollut Control Fed. 1983;55:1280–1285. [Google Scholar]
- 35.Schwartzbrod L, Boher S. Viruses and shellfish. Water Sci Technol. 1993;27:313–319. [Google Scholar]
- 36.Seely N D, Primrose S B. The effect of temperature on the ecology of aquatic bacteriophages. J Gen Virol. 1980;46:87–95. [Google Scholar]
- 37.Sinton L W, Davies-Colley R J, Bell R G. Inactivation of enterococci and fecal coliforms from sewage and meatworks effluents in seawater chambers. Appl Environ Microbiol. 1994;60:2040–2048. doi: 10.1128/aem.60.6.2040-2048.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinton L W, Finlay R K, Reid A J. A simple membrane filtration-elution method for the enumeration of F-RNA, F-DNA and somatic coliphages in 100 ml water samples. J Microbiol Methods. 1996;25:257–269. [Google Scholar]
- 39.Tartera C, Jofre J. Bacteriophages active against Bacteroides fragilis in sewage-polluted water. Appl Environ Microbiol. 1987;53:1632–1637. doi: 10.1128/aem.53.7.1632-1637.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toranzo A E, Barja J L, Hetrick F M. Antiviral activity of antibiotic-producing marine bacteria. Can J Microbiol. 1982;28:231–238. doi: 10.1139/m82-031. [DOI] [PubMed] [Google Scholar]
- 41.Water Research Centre. Surrogate viral indicators. Prepared for the Department of the Environment by the Foundation for Water Research. Medmenham, United Kingdom: Water Research Centre; 1991. [Google Scholar]
- 42.Wiggins B A, Alexander M. Minimum bacterial density for bacteriophage replication: implications for significance of bacteriophages in natural ecosystems. Appl Environ Microbiol. 1985;49:19–23. doi: 10.1128/aem.49.1.19-23.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zar J. Biostatistical analysis. 3rd ed. Upper Saddle River, N.J: Prentice-Hall Inc.; 1996. [Google Scholar]