Abstract
As the popularity of injection lipolysis increases, several side effects of injection lipolysis have been reported. In this case, A 53-year-old woman visited our outpatient clinic with a new round-shaped protruding mass (size: 5.0 cm × 3.0 cm) in the submental area. The patient had received the injection lipolysis treatment before the visit. She had received injections in the submental area at 1-week intervals (i.e., 4, 5, and 6 weeks). We performed contrast-enhanced computed tomography of the neck for differential diagnosis and found a 5.0 cm × 3.7 cm × 2.1 cm rim-enhanced fluid-density lesion in the submental. Hence, surgical removal of the lesion was planned based on the diagnosis of unspecified complicated fluid collection. The removed mass was a 3.0 cm × 2.0 cm × 2.0 cm whitish fibrous tissue. Histological examination revealed mucormycosis infection. Although several side effects of lipolysis have been reported to date, mucormycosis infection in the submental area has not been reported before.
KEY WORDS: Complication, immunocompetent, lipolysis, mucormycosis, submandibular space infection
Introduction
Noninvasive alternatives to liposuction in common practice are radiofrequency ablation, cryolipolysis, high-intensity focused ultrasound, and injection lipolysis.[1] Among several noninvasive alternatives, injections intended to dissolve fat have attracted interest from many physicians. However, with the increase in the popularity of injection lipolysis, several side effects of injection lipolysis have been reported like hyperpigmentation, infection, dermatitis, skin necrosis, and skin contour deformity.[2]
Mucormycosis is an aggressive infection caused by a ubiquitous group of molds known as mucormycetes.[3] Incidents of mucormycosis in immunocompetent hosts are rare, with 80% of all cases occurring in immunocompromised patients.[4,5]
Through this case report, we would like to discuss the side effects of mucormycosis infection that occurred after injection lipolysis in the submental area in an immunocompetent individual.
Case Report
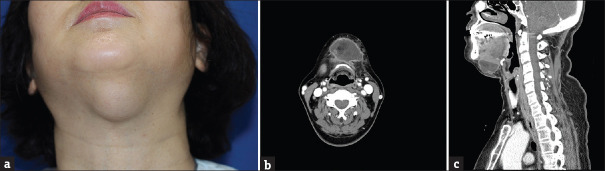
A 53-year-old woman visited our outpatient clinic with a new round-shaped protruding mass (size: 5.0 cm × 3.0 cm) in the submental area [Figure 1a]. The patient had no known drug allergies or a family history of major illnesses. The patient had received the injection lipolysis treatment before the visit. She had received injections in the submental area at 1-week intervals (i.e., 4, 5, and 6 weeks). The components of the injection were deoxycholic acid and phosphatidylcholine. After each procedure, no infections such as heating sensation, itching sensation, and erythematous skin lesion occurred; however, a mass-like lesion in the submental area gradually increased in size after each injection. We performed contrast-enhanced computed tomography of the neck for differential diagnosis and found a 5.0 × 3.7 × 2.1 cm rim-enhanced fluid-density lesion in the submental. In addition, soft-tissue edema, infiltration around the rim, and thickening of the left platysma muscle was observed [Figure 1b and c]. There were no specific abnormal findings in laboratory findings.
Figure 1.
Preoperative findings. (a) Protruding mass of 5 x 3 cm in the submental area, (b) Computed tomography findings (Axial view): rim-enhanced fluid-density lesion in the submental area, (c) Computed tomography findings (Sagittal view): soft -tissue edema, infiltration around the rim
Hence, surgical removal of the lesion was planned based on the diagnosis of unspecified complicated fluid collection. After examining the mass-like tissue located below the platysma muscle, total excision of the lesion was performed. The removed mass was a 3.0 cm × 2.0 cm × 2.0 cm whitish fibrous tissue with no evidence of grains or vasculature involvement [Figure 2].
Figure 2.
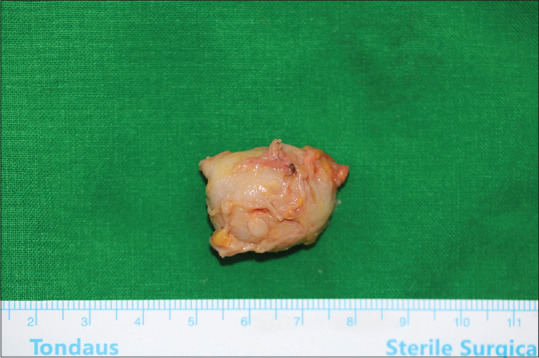
Removed mass (size: 3.0 × 2.0 × 2.0 cm, whitish, fibrous tissue with no evidence of grains or vasculature involvement)
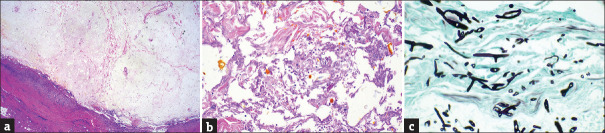
Histological examination revealed cystic changes with necrosis and chronic active inflammation [Figure 3a]. Fungal hyphae intermingled with necrotic tissue were observed [Figure 3b]. Gomori methenamine silver staining of the specimen highlighted the broad and noseptate hyphae with nearly 90° branching [Figure 3c].
Figure 3.
Pathologic findings. (a) H and E staining; magnification × 40, (b) H and E staining; magnification × 100, (c) Gomori methenamine silver staining; magnification × 400
The patient did not complain of any general symptoms both before and after surgery. However, laboratory tests after surgery confirmed the presence of 906.9 pg/mL 1-3-β-D-glucan. To prevent complications, the patient was administered 300 mg posaconazole every 12 hours on the first day, then 300 mg once daily for 3 months. Originally intended to be treated with intravenous amphotericin B, the patient refused intravenous amphotericin B treatment. Therefore, I had no choice but to start treatment with oral administration of posaconazole as a secondary choice. At 3 months after surgery, outpatient observation confirmed uneventful complete healing, without further recurrence or other complications. In addition, as a result of the laboratory test, it was confirmed that the level of 1-3-β-D-glucan was also reduced to 37.1 pg/mL.
Discussion
Complications following injection lipolysis are rare.[6] A clinical study investigating the stability of injection lipolysis indicated that the most common side effect after injection lipolysis is lesser esthetic improvement than that expected.[2] Further, other side effects of injection lipolysis are hyperpigmentation, late itching, persistent pain beyond 2 weeks, bacterial infection, atypical mycobacterial infection, chronic skin irritation or dermatitis, open wound, and permanent skin contour deformity requiring treatment.[2] The complication of mucormycosis infection after injection lipolysis has not been reported to date. A study applied the protocol used during breast augmentation surgery as a treatment before injection lipolysis and reported no infection after the protocol.[1,7] Development of a new pretreatment protocol to prevent side effects such as infection, which may occur after injection lipolysis, is required.
Risk factors for mucormycosis infection are poorly controlled diabetes, organ transplantation, long-term steroid use, malignancy, neutropenia, skin trauma, and burns.[3,4] Cutaneous mucormycosis results from the direct inoculation of fungal spores in the skin. Dissemination from internal organs to the skin is extremely rare.[8] In this case report, the patient was immunocompetent and did not have any other underlying diseases that could be risk factors. Hence, we presumed that the route of infection is injection lipolysis.
Cutaneous mucormycosis has several clinical manifestations. Symptoms such as facial swelling, skin or mucosal necrosis, pain, and fever may occur during invasive cutaneous mucormycosis infection. The progression of facial cutaneous mucormycosis is very rapid; mucormycosis has been reported to penetrate into the tissue surrounding the infection.[3,4] Based on the degree of invasiveness, cutaneous mucormycosis can be classified as localized, deep extension, or disseminated infection.[4] The typical symptom of cutaneous mucormycosis is a necrotic eschar accompanied by surrounding erythema and induration; however, in this case, the patient had no symptoms (e.g., pain, tenderness, skin problem) other than a protruding skin lesion.[8]
Considering the potential severity of mucormycosis infection, active treatment should be initiated immediately after mucormycosis infection is suspected.[9] In our case, immediately after mucormycosis was diagnosed by biopsy, active treatment was initiated. Surgical excision and antifungal treatment are the standard treatments for primary cutaneous mucormycosis.[5,10] Surgical excision should proceed as perfectly as possible until healthy margins are obtained. In our case, preoperative computed tomography confirmed the presence of a 5.0 cm × 3.7 cm × 2.1 cm rim-enhanced fluid-density lesion and successful surgical excision of the isolated mucormycosis prevented invasion into the muscle and tissue around mucormycosis infection. In addition to surgical excision, timely and adequate treatment with antifungal medication is important. To date, only two antifungal agents, namely amphotericin B and posaconazole, are known to have reliable activity against mucormycosis.[10] Currently, no clear standard exists for the duration of the use of antifungal agents. Antifungal agents are mainly taken up to the resolution of symptoms of infection and substantial radiographical improvement. The duration of administration varies from 1 week to 3 years (average duration, 6 months).[10] In our case, no special symptoms of infection were observed after the surgical removal of mucormycosis; however, antifungal treatment was administered for a total of 3 months to prevent complications like fungal infection. Also, the reason that antifungal treatment was administrated is that 1-3-β-D-glucan tested after surgery showed a high value of 906.9 pg/mL. This is supported by the fact that the 1-3 β-D-Glucan test can be used to monitor response to treatment during other fungal infection. In addition, surgical excision of mucormycosis effectively treated the infection. First, the contrast-enhanced computed tomography of the neck before surgical treatment allowed accurate preoperative evaluation of the location and size of the lesion. Second, the patient was immunocompetent, and therefore, wound healing occurred uneventfully.
The standard for diagnosis of the mucormycosis is histological biopsy and microbiological culture.[5] Unfortunately, in our case, the diagnosis for mucormycosis was only diagnosed by histologic method. When the submental mass was removed, the inflammatory findings such as heating sense and erythematous skin lesion were not identified, so we did not think of performing microbiological culture. We think this is the limitation of this study. However, according to previous study, culture growth is positive in only 1.5-2.5% of cases of mucormycosis.[5] Pathologically, the fungal forms of mucormycosis are characterized by wide, aseptate or sparsely septated hyphae. Also, the angle of branching is approximately 90°. The pathological difference from Aspergillus species is that the angle of branching of mucormycosis species is closer to 90° than that of Aspergillus species.[5] In our case, the pathological characteristics of mucormycosis were confirmed [Figure 3a-c].
Although several side effects of lipolysis have been reported to date, mucormycosis infection in the submental area has not been reported before. This case report indicates that the possibility of mucormycosis infection after injection lipolysis should be considered.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Thomas MK, D'Silva JA, Borole AJ. Injection lipolysis: A systematic review of literature and our experience with a combination of phosphatidylcholine and deoxycholate over a period of 14 years in 1269 patients of Indian and South East Asian origin. J Cutan Aesthet Surg. 2018;11:222–8. doi: 10.4103/JCAS.JCAS_117_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duncan DI, Chubaty R. Clinical safety data and standards of practice for injection lipolysis: A retrospective study. Aesthet Surg J. 2006;26:575–85. doi: 10.1016/j.asj.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Jundt JS, Wong MEK, Tatara AM, Demian NM. Invasive cutaneous facial mucormycosis in a trauma patient. J Oral Maxillofac Surg. 2018;76:1930.e1–5. doi: 10.1016/j.joms.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi (Basel) 2019;5:26. doi: 10.3390/jof5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paduraru M, Moreno-Sanz C, Gallardo JMO. Primary cutaneous mucormycosis in an immunocompetent patient. BMJ Case Rep. 2016;2016:bcr2016214982. doi: 10.1136/bcr-2016-214982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan DI, Palmer M. Fat reduction using phosphatidylcholine/sodium deoxycholate injections: Standard of practice. Aesthetic Plast Surg. 2008;32:858–72. doi: 10.1007/s00266-008-9188-9. [DOI] [PubMed] [Google Scholar]
- 7.Thomas M, D'Silva JA, Borole AJ, Chilgar RM. Periprosthetic atypical mycobacterial infection in breast implants: A new kid on the block! J Plast Reconstr Aesthet Surg. 2013;66:e16–9. doi: 10.1016/j.bjps.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S23–34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 9.Lass-Flörl C. Zygomycosis: Conventional laboratory diagnosis. Clin Microbiol Infect. 2009;15(Suppl 5):60–5. doi: 10.1111/j.1469-0691.2009.02999.x. [DOI] [PubMed] [Google Scholar]
- 10.Mtibaa L, Halwani C, Tbini M, Boufares S, Souid H, Sassi RB, et al. Successful treatment of rhino-facial mucormycosis in a diabetic patient. Med Mycol Case Rep. 2020;27:64–7. doi: 10.1016/j.mmcr.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]