Abstract
Burkholderia sp. strain LB400 is one of the most potent aerobic polychlorobiphenyl (PCB)-degrading microorganisms that have been characterized. Its PCB-dioxygenating activity originates predominantly or exclusively from the biphenyl dioxygenase encoded by its bph gene cluster. Analysis of the dioxygenation products of several di- to pentachlorinated biphenyls formed by this enzyme revealed a complex dependence of the regiospecificity and the yield of dioxygenation on the substitution patterns of both the oxidized and the nonoxidized rings. No dioxygenolytic attack involving chlorinated meta or para carbons was observed. Therefore, the ability of the enzyme to hydroxylate chlorinated carbons appears to be limited to the ortho position. However, it is not limited to monochlorinated rings, as evidenced by dioxygenation of the 2,4-disubstituted ring at carbons 2 and 3. This site of attack is strikingly different from that of the 2,5-dichlorinated ring, which has been shown to be dihydroxylated at positions 3 and 4 (J. D. Haddock, J. R. Horton, and D. T. Gibson, J. Bacteriol. 177:20–26, 1995). These results demonstrate that a second substituent of ortho-chlorinated rings crucially influences the site of dioxygenation at this ring and thereby determines whether or not the initial chlorobiphenyl oxidation product is further metabolized through the bph-encoded pathway. The 2,4-dichlorinated ring can alternatively be attacked at carbons 5 and 6. The preferred site crucially depends on the substitution pattern of the other ring. The formation of more than a single dioxygenation product was found predominantly with congeners that contain two chlorinated rings, both of which are similarly prone to dioxygenation or one is substituted only at carbon 3.
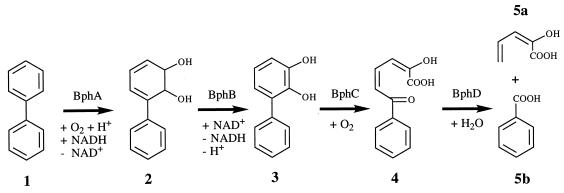
Microbes and enzymes capable of attacking halogenated aromatics are of increasing interest due to their potential use in the treatment of environmental contaminants. Industrial mixtures of polychlorobiphenyls (PCBs) constitute an important class of persistent and potentially carcinogenic pollutants. Certain aerobic bacteria are able to oxidize some PCB congeners by upper pathways that are basically identical in the different organisms (Fig. 1) (1, 4, 7, 10, 20). Genes encoding such pathways have been cloned and characterized (2, 11, 15, 18, 22), and the elucidation of the three-dimensional structure of pathway enzymes is in progress (14, 17, 25, 30).
FIG. 1.
Upper pathway for catabolism of biphenyls encoded by the bph locus of Burkholderia sp. strain LB400. Compounds shown are biphenyl (1), BDHD (2), DHB (3), HOPDA (4), 2-hydroxypenta-2,4-dienoic acid (5a), and benzoic acid (5b).
One microorganism with a superior ability to oxidize a wide range of chlorobiphenyl (CB) congeners is Burkholderia sp. strain LB400. A number of investigations focussed on the depletion of CBs by this organism (4–6, 12, 24). Fewer data are available on the metabolites that arise from the attack on CBs. The conversion of 2,5,2′,5′-CB into dead-end dihydrodiols has been described (6, 24). Furthermore, the chlorinated benzoates (CBAs) and acetophenones formed from a number of CBs have been identified (3, 4, 24). Genes, termed bph, encoding all enzymes of the pathway shown in Fig. 1 have been identified, cloned, and characterized (8, 9, 15, 16, 22). Transposon mutagenesis of strain LB400 indicated that the bph locus codes for a major pathway but did not rule out the existence of additional routes involved in the breakdown of CBs (23, 24). The construction of bph-harboring recombinant Escherichia coli strains and the isolation of the bph-encoded biphenyl 2,3-dioxygenase (BphA) permitted investigations of the CB catabolic activities directly ascribable to Bph enzymes. These investigations provided data on the substrate range of BphA (22) as well as on CB metabolites formed by this enzyme (13, 27), by biphenyl-2,3-dihydrodiol 2,3-dehydrogenase (BphB) (27), by 2,3-dihydroxybiphenyl 1,2-dioxygenase (BphC) (28), and by 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate hydrolase (BphD) (28). The results obtained are consistent with the view that the bph locus encodes a major CB catabolic route of strain LB400.
The regiospecificity of dioxygenation by the initial enzyme of the pathway is of particular importance, because the site (or sites) of dioxygenation of a CB determines the (potential) site(s) of attack by the subsequent enzymes of the pathway (Fig. 1). This predetermines whether and how further enzymatic degradation of the compound may take place. The products formed from a number of CBs by attack by BphA of strain LB400 show that the enzyme is able to hydroxylate meta and para carbons, that the site specificity of dioxygenation is influenced by the substituent pattern, and that an ortho-monochlorinated ring is readily and almost exclusively attacked at positions 2 and 3 (13, 27). However, these data do not permit predictions of dioxygenation patterns for other CBs and of whether their primary products are further metabolized. We have therefore characterized the oxidation of a larger range of congeners and investigated their further catabolism by the bph-encoded upper pathway.
MATERIALS AND METHODS
Chemicals.
PCB congeners (99% purity) were obtained from Lancaster Synthesis (White Lund, Morecambe, England), Promochem (Wesel, Germany), or Restek (Sulzbach, Germany). CBAs (98% purity) were purchased from Fluka AG (Buchs, Switzerland) or Lancaster Synthesis.
Bacterial strains, plasmids, and culture conditions.
The E. coli strain used in this study was BL21(DE3)/pLysS (31) harboring either pAIA111, pAIA13, pAIA15, pAIA50, or pAIA74. These plasmids are based on the phage T7 expression vector pT7-6 (32). pAIA111 carries bphA1A2A3A4, pAIA13 contains bphA1A2A3A4B, pAIA15 harbors bphC, pAIA50 carries bphA1A2A3A4BC, and pAIA74 contains bphA1A2A3A4BCD of Burkholderia sp. strain LB400. The constructions of pAIA111 (21), pAIA13 (27), pAIA50, and pAIA74 (28) have been described previously. pAIA15 was obtained from pDD372 (16) by cleavage with HindIII and StuI, filling in of the HindIII end, and recircularization with standard procedures (26). Bacteria were grown in Luria-Bertani medium (26) at 37°C unless otherwise indicated. Where appropriate, chloramphenicol and/or ampicillin at a concentration of 20 or 50 μg/ml, respectively, was used for selection.
Preparation of resting cells.
The preparation of resting cells was carried out as previously described (28).
Degradation of CBs by BphA or by BphA and BphB and analysis of products.
Resting cell suspensions (1 ml) (optical density at 600 nm [OD600] = 15) of E. coli BL21(DE3)/pLysS harboring either pAIA111 or pAIA13 were incubated on a rotary shaker with a 0.5, 1, or 2 mM nominal concentration of CB for 6 or 24 h at 30°C. The reaction mixtures were extracted with an equal volume of ethyl acetate. The organic layer was reextracted with one volume of 50 mM sodium phosphate buffer, pH 7.5, and dried over magnesium sulfate. To obtain butylboronate derivatives, the solvent was removed from 200 μl of extract, the residue was redissolved in 80 μl of acetone, 20 μl of a 2-mg/ml solution of n-butylboronic acid in acetone was added, and the solution was incubated at 50°C for 10 min (19). After derivatization, mixtures were evaporated to dryness under a stream of nitrogen and dissolved in 10 μl of n-octane. Samples (1 μl) were injected in the splitless mode (250°C injector temperature) into a Hewlett-Packard series II gas chromatograph equipped with a Hewlett-Packard Ultra 2 capillary column (5% diphenyl-, 95% dimethylpolysiloxane; length, 50 m; inside diameter, 0.2 mm; film thickness, 0.11 mm). Helium served as the carrier gas, and the following temperature program was used: 80°C (3 min), increases of 10°C/min to 288°C, and 20 min at 288°C. The gas chromatograph was coupled to a Hewlett-Packard model 5989A quadrupole mass spectrometer, which was operated in the electron impact mode.
Degradation of CBs by BphA, BphB, and BphC or by BphA and BphC and analysis of products.
Resting cell suspensions (4 ml) (OD600 = 2) of E. coli BL21(DE3)/pLysS harboring pAIA50 or a mixture of equal amounts of the same host harboring pAIA111 or pAIA15 were incubated as described above with a 30 μM nominal concentration of CB. The formation of 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoates (HOPDAs) was monitored at intervals between 1 and 24 h by visible spectral scanning of the assay mixtures with a Beckman model DU-70 spectrophotometer.
Degradation of CBs by BphA, BphB, BphC, and BphD and analysis of products.
Resting cell suspensions (1 ml) (OD600 = 10) of E. coli BL21(DE3)/pLysS harboring pAIA74 were incubated as above with a 125 μM nominal concentration of CB for 24 h. Cell-free supernatants were analyzed by high-performance liquid chromatography (HPLC) as described previously (28). CBAs were identified and quantitated by comparison with authentic standards.
RESULTS AND DISCUSSION
Conversion of CBs into dihydroxy compounds.
The attack upon several di- to penta-CBs by the bphA-encoded dioxygenase of Burkholderia sp. strain LB400 was investigated by incubation of individual congeners with a recombinant E. coli strain synthesizing this enzyme. The dioxygenation products formed were characterized by gas chromatography-mass spectrometry (GC-MS). The results are shown in Table 1. Half of the congeners yielded more than a single reaction product, indicating a relaxed regiospecificity of attack by the enzyme. Each of the metabolites detected was the product of a single dioxygenation of a CB molecule. Thus, “dioxygenation of a congener at different sites” or similar phrases throughout this work refer to the dioxygenation of different molecules of a congener. On the basis of apparent relative yields (deduced from total ion chromatogram [TIC] peak areas and thus based on the assumption that similar amounts of the different metabolites yield similar areas), the formation of one of the dioxygenation products appeared to be strongly preferred in most cases. Biphenyldihydrodiols (BDHDs) are formed by the dioxygenation of unchlorinated carbons, while dihydroxybiphenyls (DHBs) are formed by an attack at a semichlorinated pair of carbons and the subsequent elimination of hydrochloric acid (13, 27). As hydroxylation of a chlorosubstituted ring can lead to artifactual dechlorination during derivatization or GC-MS analysis (27), we confirmed the formation of DHBs by an entirely different experimental procedure that obviates derivatization. This procedure involved incubation of the CBs with recombinant bacteria synthesizing BphA and the third pathway enzyme, BphC, but not BphB. BphC does not attack BDHDs but converts 2,3-DHBs into HOPDAs (Fig. 1), which display characteristic absorption maxima between approximately 390 and 440 nm (28). Biphenyl (not shown) and 3,4- and 3,5-CB, for which dehalogenation had not been detected (29), served as negative controls. Positive controls were carried out with cells producing all three enzymes. The results are shown in Table 2. Whereas biphenyl and 3,4- and 3,5-CB were not converted to HOPDAs by cells synthesizing BphA and BphC, the other CBs were transformed to HOPDAs, which confirms that attack by BphA leads directly to the formation of DHBs.
TABLE 1.
Characterization of CB metabolites formed by dioxygenation catalyzed by the bph-encoded biphenyl dioxygenase of Burkholderia sp. strain LB400
| Substrate CB | No. of products
|
tr (min) of derivative (GC) | Apparent relative yield (%)c | Mass of derivative | No. of chlorines | Type of compound | |
|---|---|---|---|---|---|---|---|
| Possiblea | Observedb | ||||||
| 3,3′ | 2 | 2 | 24.82 | 85–95 | 322 | 2 | BDHD |
| 25.25 | 5–15 | 322 | 2 | BDHD | |||
| 3,4′ | 3 | 3 | 25.03 | 50–70 | 322 | 2 | BDHD |
| 25.18 | 20–40 | 322 | 2 | BDHD | |||
| 25.45 | 5–15 | 322 | 2 | BDHD | |||
| 4,4′ | 1 | 1 | 25.37 | 100 | 322 | 2 | BDHD |
| 2,3,2′ | 6 | 1 | 24.29 | 100 | 320 | 2 | DHB |
| 2,4,2′ | 6 | 2–3 | 23.92 | 90–95 | 320 | 2 | DHB |
| 24.22 | 3–10 | 320 | 2 | DHB | |||
| [25.41] | [1–2] | [356] | [3] | [BDHD] | |||
| 3,4,2′ | 5 | 1 | 25.61 | 100 | 320 | 2 | DHB |
| 3,5,2′ | 4 | 1 | 24.98 | 100 | 320 | 2 | DHB |
| 2,3,3′ | 4 | 2 | 25.64 | 90–95 | 356 | 3 | BDHD |
| 26.42 | 5–10 | 356 | 3 | BDHD | |||
| 2,4,3′ | 4 | 2–3 | 25.29 | 15–20 | 356 | 3 | BDHD |
| [25.50] | [2–3] | [356] | [3] | [BDHD] | |||
| 26.19 | 75–85 | 356 | 3 | BDHD | |||
| 2,3,4′ | 3 | 2 | 26.03 | 85–95 | 356 | 3 | BDHD |
| 26.39 | 5–15 | 356 | 3 | BDHD | |||
| 2,4,4′ | 3 | 2 | 25.48 | 20–40 | 320 | 2 | DHB |
| 25.65 | 60–80 | 356 | 3 | BDHD | |||
| 2,3,4,5 | 2 | 1 | 27.65 | 100 | 390 | 4 | BDHD |
| 2,4,2′,4′ | 2 | 1 | 25.69 | 100 | 354 | 3 | DHB |
| 2,3,4,5,2′ | 4 | 1 | 27.54 | 100 | 388 | 4 | DHB |
Based on the assumption that the enzyme cannot attack C-1 and chlorinated meta or para carbons. There are no indications of these types of attack, either in previous studies or in the present work.
A range is given when one or more products were observed in only marginal amounts. Data for such products are given in square brackets, as it cannot be ruled out that they may originate from contaminants.
Deduced from TIC peak areas.
TABLE 2.
Determination of dechlorinating dioxygenation of CBs by measurement of HOPDA formation in the presence and absence of BphBa
| CB | HOPDA formed by:
|
|||
|---|---|---|---|---|
| BphA and BphC
|
BphA, BphB, and BphC
|
|||
| λmax (nm) | A (5 h)b | λmax (nm) | A (5 h)b | |
| 3,4 | NF | NF | 440 ± 2c | ND |
| 3,5 | NF | NF | 439 ± 2c | ND |
| 2,4,2′ | 392 ± 2 | 0.72 | 392 ± 2 | 2.1 |
| 2,4,4′ | 436 ± 3 | 0.21 | 434 ± 2 | 0.33 |
| 2,4,2′,4′ | 396 ± 2 | 0.14 | 396 ± 2 | 0.05 |
ND, not determined; NF, not found.
Absorption at λmax after 5 h of incubation.
Taken from reference 28.
Conversion of CBs to CBAs.
Table 3 shows the CBAs detected and identified by HPLC-UV after transformation of 15 congeners by E. coli cells synthesizing the four enzymes of the bph-encoded upper pathway. All of these CBs yielded CBAs, indicating that all four enzymes of this pathway are able to metabolize congeners or their degradation products with a large variety of different chlorine substitution patterns, including compounds with up to four substituents on the nonoxidized ring. Bedard and Haberl (4) have shown that strain LB400 itself catabolizes 2,4,4′-CB to 4-CBA (13% yield) and 2,4-CBA (2%) and 3,4,2′-CB to 3,4-CBA (48 to 64%). Thus, the metabolism of these congeners appears to be similar in this strain and in E. coli harboring its bph genes. Strain LB400 converts 2,3,3′-CB into the dead-end product 2,3-CBA and the transient metabolite 2,3-dichloroacetophenone (4). Our recombinant E. coli strain also yielded 2,3-CBA and no 3-CBA. The formation of chloroacetophenone is currently being investigated.
TABLE 3.
Formation of CBAs from CBs by the enzymes of the bph-encoded degradative pathway of Burkholderia sp. strain LB400a
| CB | No. of CB dioxygenation productsb | CBA
|
||
|---|---|---|---|---|
| tr (min) (HPLC) | Position(s) of chlorine(s) | Yield (%) | ||
| 3,4′ | 3 | 5.05 | 4 | 10–20 |
| 4.83 | 3c | 5–10 | ||
| 2,3,4 | ND | 7.27 | 2,3,4 | 70–100 |
| 2,4,5 | ND | 8.61 | 2,4,5 | 70–100 |
| 3,4,5 | ND | 15.18 | 3,4,5d | 40–70d |
| 2,3,2′ | 1 | 4.52 | 2,3 | 70–100 |
| 2,4,2′ | 2–3 | 5.34 | 2,4 | 70–100 |
| 3.42 | 2e,f | <1 | ||
| 3,4,2′ | 1 | 7.59 | 3,4 | 40–70 |
| 3,5,2′ | 1 | 8.92 | 3,5 | 40–70 |
| 2,3,3′ | 2 | 4.52 | 2,3c | 20–40 |
| 2,4,3′ | 2–3 | 4.83 | 3 | 10–20 |
| 2,3,4′ | 2 | 4.52 | 2,3 | 3–6 |
| 2,4,4′ | 2 | 5.05 | 4c | 20–40 |
| 5.34 | 2,4c,f | 1–2 | ||
| 2,3,4,5 | 1 | 12.34 | 2,3,4,5 | 70–100 |
| 2,4,2′,4′ | 1 | 5.34 | 2,4 | 10–20 |
| 2,3,4,5,2′ | 1 | 12.34 | 2,3,4,5 | 40–70 |
ND, not determined.
A range is given when one or more products were observed only in marginal amounts (Table 1).
In previous experiments (28), this CBA was not detected, probably due to a lower activity of the HOPDA hydrolase.
Tentative data, as an authentic reference was not available.
Assignment based only on tr value.
As this product was observed only in marginal amounts, it cannot be ruled out that it may originate from a contaminant.
Assignment of sites of initial dioxygenation.
An assignment of the positions of initial dioxygenation from GC-MS data alone can be made only in exceptional cases, such as 4,4′-CB, where an attack at only a single site will yield a BDHD. However, the combination of different types of data frequently permitted assignment of the site of dioxygenation. For example, congeners that were dechlorinated and converted into DHBs by BphA must have been attacked at a chlorinated carbon. If the CB is monochlorinated or symmetrically dichlorinated and the substituents are at ortho or para positions, the identification of its metabolite as a DHB is sufficient for assignment of the site of dioxygenation, if we assume that position 1 or 1′, respectively, cannot be attacked. In some instances, we additionally transformed an otherwise analogous CB, which did not contain a presumptively eliminated chlorine, into a DHB and compared the chromatographic retention times (trs) of the two DHBs. For example, 3,4,2′-CB, transformed by BphA, and 3,4-CB, transformed by BphA and BphB, yielded coeluting DHBs (data not shown). As the DHB formed from 3,4-CB had been previously shown to be dioxygenated at carbons 2′ and 3′ (28), this was taken as an indication that 3,4,2′-CB had been attacked at the same positions. Our investigation of the CB metabolites formed by BphA of the gram-positive PCB degrader Rhodococcus globerulus P6 (21) was also helpful in identifying dioxygenation sites in the present work. This enzyme frequently formed only a single metabolite which could be identified. Thus, the comparison of tr values and mass spectra of the products formed by the two BphA’s revealed their identity or nonidentity. The conversion of CBs into CBAs or chlorinated HOPDAs was also used for site assignments. Only dioxygenation at ortho and meta carbons will lead to formation of these metabolites (Fig. 1). While CBAs in most cases can be identified by comparison with authentic standards, the absorption maxima of the electronic spectra of HOPDAs can be useful in identifying the attacked ring, since they are different for ortho-substituted and non-ortho-substituted compounds (28). The particular type of evidence used for dioxygenation site assignments is indicated in Table 4. For completeness, this table also contains data from earlier reports (4, 13, 27).
TABLE 4.
Regiospecificity of dioxygenation of various biphenyls chlorinated in both rings by the bph-encoded biphenyl dioxygenase of Burkholderia sp. strain LB400
| Congener | Site(s) of dioxygenationa | Evidence for site of dioxygenationb,c |
|---|---|---|
| 2,2′ | 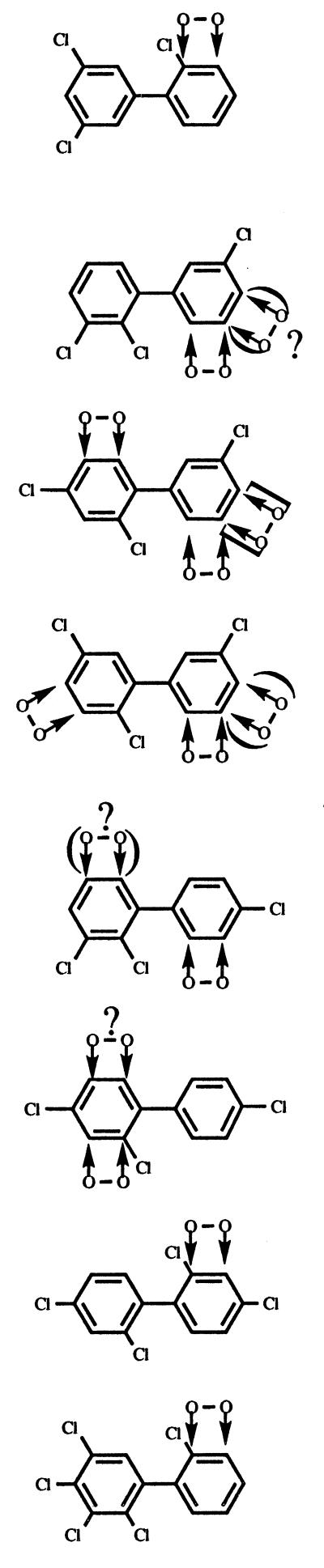 |
Elimination of HCl (13, 27). Essentially quantitative conversion of 2,2′-CB into 2-CBA (28). |
| Tentative assignment (13). | ||
| 2,3′ | 5′,6′, tentative assignment, consistent with no elimination of HCl (13) and formation of trace amounts of 2-CBA by strain LB400 (4). | |
| 2,3, elimination of HCl (13); almost quantitative conversion of 2,3′-CB into 3-CBA (28). | ||
| No elimination of HCl (13); almost quantitative conversion of 2,3′-CB into 3-CBA (28). | ||
| 2,4′ | Elimination of HCl; identical tr of monochloro-DHB derived from 4-CB (27); essentially quantitative conversion of 2,4′-CB into 4-CBA (28). | |
| 3,3′ | No elimination of HCl (13); no other possibility. | |
| No elimination of HCl (13); identical tr of P6 BphA product, identified as 5,6-dioxygenated (29); formation of 20–25% of 3-CBA (28). | ||
| 3,4′ | No elimination of HCl; formation of 3-CBA. | |
| No elimination of HCl; no other possibility. | ||
| No elimination of HCl; identical tr of P6 BphA product, identified as 5,6-dioxygenated (21); formation of 4-CBA (28). | ||
| 4,4′ | No elimination of HCl; formation of HOPDA (28). | |
| 2,3,2′ | Elimination of HCl; formation of 2,3-CBA. | |
| 2,4,2′ | Elimination of HCl; identical tr of dichloro-DHB derived from 2,4-CB. | |
| Tentative assignment, consistent with no elimination of HCl. | ||
| Elimination of HCl; probable formation of 2-CBA. | ||
| NMR (13). | ||
| 2,5,2′ | NMR (13). | |
| Tentative assignment, consistent with no elimination of HCl (13). | ||
| 3,4,2′ | Elimination of HCl; identical tr of dichloro-DHB derived from 3,4-CB; formation of 3,4-CBA. | |
| 3,5,2′ | Elimination of HCl; identical tr of dichloro-DHB derived from 3,5-CB; formation of 3,5-CBA. | |
| 2,3,3′ | Tentative assignment, consistent with no elimination of HCl. | |
| No elimination of HCl; formation of 20–40% of 2,3-CBA. | ||
| 2,4,3′ | No elimination of HCl; formation of 10–20% of 3-CBA. | |
| For the 3-chlorinated ring, no other possibilities in the absence of HCl elimination. Tentative assignment of marginal attack at carbons 4′ and 5′. | ||
| 2,5,3′ | 3,4 and 3′,4′, no other possibilities in the absence of HCl elimination (13). Tentative assignment of minor attack to carbons 4′ and 5′. | |
| Formation of trace amounts of 2,5-CBA and -CA by strain LB400 (4). | ||
| 2,3,4′ | Tentative assignment, consistent with no elimination of HCl. | |
| No elimination of HCl; formation of 2,3-CBA. | ||
| 2,4,4′ | Tentative assignment, consistent with no elimination of HCl.d | |
| Elimination of HCl; incubation with only BphA and BphC yields HOPDA (λmax = 436 nm); formation of 4-CBA. | ||
| 2,4,2′,4′ | Elimination of HCl; incubation with only BphA and BphC yields HOPDA (λmax = 396 nm); formation of 2,4-CBA. | |
| 2,3,4,5,2′ | Elimination of HCl; formation of 2,3,4,5-CBA. | |
Minor sites of dioxygenation (apparent yield, ≤15%) are shown in parentheses. Marginal sites of dioxygenation (Table 1) are shown in square brackets. Tentatively assigned sites are indicated by question marks.
CA, chloroacetophenone. NMR, nuclear magnetic resonance.
If no reference is given, evidence is provided by this work.
A trace amount of 2,4-CBA (Table 3) suggests an alternative or additional attack at carbons 2′ and 3′. However, it cannot be ruled out that this small quantity originates from a contaminant in the CB.
Characteristics of CB oxidation by BphA from strain LB400.
The present and previous studies of CB oxidation provide no indication of hydroxylation of chlorinated meta or para carbons. Specifically, 3-, 4-, 2,3-, and 2,5-substituted rings were not dechlorinated. The 2,4-dichlorinated ring of 2,4,2′-, 2,4,4′-, and 2,4,2′,4′-CB was dechlorinated at one of the carbons, but available evidence indicates that this reaction involved position 2. For example, the dioxygenation of 2,4,4′-CB yielded a dichloro-DHB which was converted into a HOPDA by BphC. This indicates that the hydroxy groups of the DHB were attached to ortho and meta carbons and thus rules out dioxygenation at positions 3,4, 4,5, or 3′,4′. The HOPDA showed a λmax of 433 nm, which indicates dioxygenation of the ortho-substituted ring (28) and is consistent with attack at carbons 2 and 3. Thus, all available evidence suggests that the attack of chlorinated carbons by this BphA is limited to the ortho position.
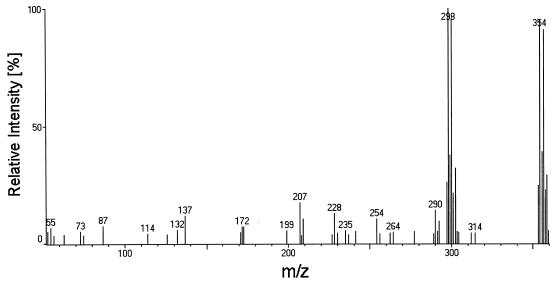
Although the hydroxylation of chlorinated carbons by BphA from strain LB400 appears to occur only at the ortho position, our results show (Tables 1 and 2) that the attack of a chlorinated ortho carbon is not limited to monochlorinated rings. The 2,4-disubstituted rings of 2,4,2′-, 2,4,4′-, and 2,4,2′,4′-CB were dioxygenated at positions 2 and 3. The mass spectrum of the sole product obtained from 2,4,2′,4′-CB is shown in Fig. 2. Its isotopically most abundant (10B:11B ratio = 1:4; 35Cl:37Cl ratio = 3:1) molecular ion (m/z = 354) and the fragment [M − 56]+, which is the most prominent ion typically formed from catechols due to the elimination of butene (19), indicate the formation of a trichlorinated DHB. The conversion of this congener into 2,4-CBA (Table 3) demonstrates dioxygenation at ortho and meta carbons. Taken together, both results indicate dioxygenation at positions 2 and 3. Evidence for 2,3-dioxygenation of 2,4,2′- and 2,4,4′-CB is provided in Table 4. This site of attack, which permits degradation of these congeners by all subsequent enzymes of the upper pathway (Table 3), is strikingly different from that of the 2,5-dichlorinated ring, which also possesses a chlorinated ortho carbon next to an unsubstituted meta carbon. Haddock et al. (13) reported that the dichlorinated ring of 2,5,2′-, 2,5,3′-, and 2,5,2′,5′-CB is dihydroxylated exclusively at carbons 3 and 4. This yields metabolites that are not further transformed by the next pathway enzyme (13). These results demonstrate that a second substituent on an ortho-chlorinated ring can crucially influence the site of dioxygenation and, as a result, the further breakdown of the compound.
FIG. 2.
Mass spectrum of the butylboronate derivative of the product obtained from 2,4,2′,4′-tetrachlorobiphenyl by dioxygenation catalyzed by the bph-encoded biphenyl dioxygenase of Burkholderia sp. strain LB400.
As shown above, the dichlorinated ring of 2,4,2′,4′-CB was dioxygenated at positions 2 and 3. In contrast, 2,4,3′-CB yielded no DHB but three BDHDs, one in marginal amounts (Table 1). The formation of BDHDs is inconsistent with 2,3-dioxygenation of this congener. The formation of 3-CBA from this CB (Table 3) indicates that one of the BDHDs is dihydroxylated at positions 5 and 6. Dioxygenation of 2,4,4′-CB gives rise to a DHB by attack at carbons 2 and 3 (Table 4). Additionally, this congener yields a BDHD as a major metabolite (Table 1). This may be formed by dioxygenation at positions 5 and 6. This assignment is consistent with the formation of a HOPDA with an absorption maximum at 434 nm (Table 2) and with the formation of 4-CBA (Table 3). Thus, the dichlorinated ring of 2,4,4′-CB is possibly dioxygenated at both pairs of vicinal ortho and meta carbons. These results indicate that the position of dioxygenation can be strongly influenced by the substitution pattern of the nonoxidized ring. Another example for this influence is 2,3′-CB. The ortho-chlorinated ring of this congener is 5,6-dioxygenated with a relative yield of 38% (13). In contrast, 2,2′- and 2,4′-CB are not or are only marginally attacked at this site (13, 27). These results suggest that a meta substituent on the nonoxidized ring promotes 5,6-dioxygenation of the 2-chlorinated ring. However, of five a,b,2′-CBs, including four with a and/or b in the meta position(s), only 2,4,2′-CB was 5′,6′-dioxygenated, though only in marginal amounts (Tables 1 and 4). This emphasizes the difficulty of deducing the site(s) of dioxygenation from the behavior of similarly substituted CBs.
Irrespective of the site of dioxygenation, a 2,4-disubstituted ring yielded more dioxygenation product (as deduced from absolute TIC peak areas) than a 4-monochlorinated ring, indicating that a higher chlorosubstitution of a ring may increase its susceptibility to enzymatic oxidation. A similar observation has been made by Nadim et al. (24), who found that strain LB400 depletes 2,4,2′,4′-CB much more rapidly than 4,4′-CB.
Relative and absolute yields of dioxygenation can also be influenced by the substitution pattern of the second ring. Thus, the para-chlorinated ring of 3,4′-CB yielded more dioxygenation product than that of 4,4′-CB, even though oxidation of the para-chlorinated ring of 3,4′-CB unfavorably competed with oxidation of the meta-chlorinated ring.
Several CBs yielded more than a single dioxygenation product, indicating that the productive binding of a given substrate to the BphA active site occurs in more than one orientation. The data shown in Table 4 reveal a complex dependence of the regiospecificity of dioxygenation on the substitution pattern of the biphenyl core.
As stated above, the yield of dioxygenation of one ring is dependent on the substitution pattern of the other. This is due not only to steric and electronic influences of the second ring on the binding to the active site and the reactivity of the attacked ring but also to the competition of the two rings for the active site and for dioxygenation. In spite of this complication, available data suggest the following approximate order of preferential dioxygenation for a number of different ring types (“ring preference”): unsubstituted > 2-chloro > 2,5-dichloro > 2,4-dichloro > < 3-chloro > 4-chloro (“> <” indicates that both preferences were observed). The dioxygenation of both rings of an asymmetrically substituted biphenyl is expected when rings are similarly prone to attack. This was frequently observed; examples are 3,4′-, 2,4,2′-, 2,5,2′-, 2,4,3′-, and 2,5,3′-CB. A similar ring preference has been deduced for strain LB400 on the basis of CBA and chloroacetophenone yields (4).
The only rings which were found to be meta- and para-dioxygenated are meta-monochlorinated rings and ortho- and meta-substituted 2,5-dichlorinated rings (Table 4). The latter may be regarded as a more highly substituted analogue of the former. While the 2,5-dichlorinated ring was attacked only at meta and para carbons (13), the 3-chlorinated ring was ortho- and meta- as well as meta- and para-dioxygenated. The apparent inability of the enzyme to 2,3-dioxygenate the 2,5-dichlorinated ring is surprising. The comparison of the dioxygenation of the 2,5-dichlorinated ring of 2,5,2′-, 2,5,3′-, and 2,5,2′,5′-CB with the dioxygenation of the 2-monochlorinated ring of 2,2′-, 2,3′-, and 2,5,2′-CB reveals that the 2-monochlorinated ring of the latter congeners can be positioned in the enzyme active site to be 2,3-dioxygenated, whereas the 2,5-dichlorinated ring of the former CBs presumably cannot. It seems less likely that this is due to the steric interference of the substituent at position 5 when carbons 2 and 3 of the 2,5-dichlorinated ring are facing the iron-bound activated oxygen species, because with 3,3′- and 2,5,3′-CB, the 3-monochlorinated ring becomes 5,6-dioxygenated, indicating no such steric hindrance in these cases. It is interesting to note that minor amounts of 2,5-dichloroacetophenone were detected as metabolites of 2,5,2′,5′-CB when degraded by strain LB400 (4, 24). This may suggest an additional attack by BphA of this dichlorinated ring, although formation of this metabolite via another pathway present in strain LB400 cannot strictly be ruled out (24).
A ring that can in principle be dioxygenated at two alternative sites was not attacked at both sites in any congener. Thus, the 3-chlorinated ring was dioxygenated at different sites in four of six congeners, and the 2-chlorinated ring was attacked at alternative sites essentially in only one of nine CBs. This again illustrates the influence of the substitution pattern of the nonoxidized ring.
Our data suggest that a congener is expected to yield more than a single dioxygenation product particularly when both rings are chlorinated, of which one carries a substituent at carbon 3, and/or when the congener contains two rings that are similarly prone to dioxygenation. However, it is not possible to reliably predict the exact sites of dioxygenation of as yet uninvestigated CBs.
Finally, our results show that most of the examined primary dioxygenation products that are dihydroxylated at ortho and meta carbons, including catabolites with up to four chlorines on the nonoxidized ring (2,3,4,5- and 2,3,4,5,2′-CB), are further degraded by all subsequent enzymes of the upper pathway. However, the 2,3-dihydrodiol of 4,4′-CB (28) and the 5′,6′-dihydrodiols derived from 2,5,3′-CB (28) and 2,4,3′-CB were not converted into CBAs. This indicates that in some cases catabolic bottlenecks exist after the first step of this LB400 pathway.
ACKNOWLEDGMENTS
We thank Silke Backhaus for excellent technical assistance and Annette Walter for carrying out some pilot experiments in this project.
This work was supported by BMBF WTZ grant CHL K0A 1B and by a grant from Fundacion Andes and the Consejo Nacional de Ciencia y Tecnología (CONICYT). K.N.T. gratefully acknowledges generous support by the Fonds der Chemischen Industrie.
REFERENCES
- 1.Ahmed M, Focht D D. Degradation of polychlorinated biphenyls by two species of Achromobacter. Can J Microbiol. 1973;19:47–52. doi: 10.1139/m73-007. [DOI] [PubMed] [Google Scholar]
- 2.Asturias J A, Timmis K N. Three different 2,3-dihydroxybiphenyl-1,2-dioxygenase genes in the gram-positive polychlorobiphenyl-degrading bacterium Rhodococcus globerulus P6. J Bacteriol. 1993;175:4631–4640. doi: 10.1128/jb.175.15.4631-4640.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedard D L. Bacterial transformation of polychlorinated biphenyls. In: Kamely D, Chakrabarty A, Omenn G S, editors. Biotechnology and biodegradation. Houston, Tex: Gulf Publishing Company; 1990. pp. 369–388. [Google Scholar]
- 4.Bedard D L, Haberl M L. Influence of chlorine substitution pattern on the degradation of polychlorinated biphenyls by eight bacterial strains. Microb Ecol. 1990;20:87–102. doi: 10.1007/BF02543870. [DOI] [PubMed] [Google Scholar]
- 5.Bedard D L, Unterman R, Bopp L H, Brennan M J, Haberl M L, Johnson C. Rapid assay for screening and characterizing microorganisms for the ability to degrade polychlorinated biphenyls. Appl Environ Microbiol. 1986;51:761–768. doi: 10.1128/aem.51.4.761-768.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bopp L H. Degradation of highly chlorinated PCBs by Pseudomonas strain LB400. J Ind Microbiol. 1986;1:23–29. [Google Scholar]
- 7.Catelani D, Colombi A, Sorlini C, Treccani V. 2-Hydroxy-6-oxo-6-phenylhexa-2,4-dienoate: the meta-cleavage product from 2,3-dihydroxybiphenyl by Pseudomonas putida. Biochem J. 1973;134:1063–1066. doi: 10.1042/bj1341063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowling D N, Pipke R, Dwyer D F. A DNA module encoding bph genes for the degradation of polychlorinated biphenyls (PCBs) FEMS Microbiol Lett. 1993;113:149–154. doi: 10.1111/j.1574-6968.1993.tb06506.x. [DOI] [PubMed] [Google Scholar]
- 9.Erickson B D, Mondello F J. Nucleotide sequencing and transcriptional mapping of the genes encoding biphenyl dioxygenase, a multicomponent polychlorinated-biphenyl-degrading enzyme in Pseudomonas strain LB400. J Bacteriol. 1992;174:2903–2912. doi: 10.1128/jb.174.9.2903-2912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furukawa K, Matsumura F, Tonomura K. Alcaligenes and Acinetobacter capable of degrading polychlorinated biphenyls. Agric Biol Chem. 1978;42:543–548. [Google Scholar]
- 11.Furukawa K, Miyazaki T. Cloning of a gene cluster encoding biphenyl and chlorobiphenyl degradation in Pseudomonas pseudoalcaligenes. J Bacteriol. 1986;166:392–398. doi: 10.1128/jb.166.2.392-398.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson D T, Cruden D L, Haddock J D, Zylstra G J, Brand J M. Oxidation of polychlorinated biphenyls by Pseudomonas sp. strain LB400 and Pseudomonas pseudoalcaligenes KF707. J Bacteriol. 1993;175:4561–4564. doi: 10.1128/jb.175.14.4561-4564.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddock J D, Horton J R, Gibson D T. Dihydroxylation and dechlorination of chlorinated biphenyls by purified biphenyl 2,3-dioxygenase from Pseudomonas sp. strain LB400. J Bacteriol. 1995;177:20–26. doi: 10.1128/jb.177.1.20-26.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han S, Eltis L D, Timmis K N, Muchmore S W, Bolin J T. Crystal structure of the biphenyl-cleaving extradiol dioxygenase from a PCB-degrading pseudomonad. Science. 1995;270:976–980. doi: 10.1126/science.270.5238.976. [DOI] [PubMed] [Google Scholar]
- 15.Hofer B, Backhaus S, Timmis K N. The biphenyl/polychlorinated biphenyl-degradation locus (bph) of Pseudomonas sp. LB400 encodes four additional metabolic enzymes. Gene. 1994;144:9–16. doi: 10.1016/0378-1119(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 16.Hofer B, Eltis L D, Dowling D N, Timmis K N. Genetic analysis of a Pseudomonas locus encoding a pathway for biphenyl/polychlorinated biphenyl degradation. Gene. 1993;130:47–55. doi: 10.1016/0378-1119(93)90345-4. [DOI] [PubMed] [Google Scholar]
- 17.Hülsmeyer M, Hecht H J, Niefind K, Hofer B, Eltis L D, Timmis K N, Schomburg D. Crystal structure of cis-biphenyl-2,3-dihydrodiol-2,3-dehydrogenase from a PCB degrader at 2.0 Å resolution. Protein Sci. 1998;7:1286–1293. doi: 10.1002/pro.5560070603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimbara K, Hashimoto T, Fukuda M, Koana T, Takagi M, Oishi M, Yano K. Cloning and sequencing of two tandem genes involved in degradation of 2,3-dihydroxybiphenyl to benzoic acid in the polychlorinated biphenyl-degrading soil bacterium Pseudomonas sp. strain KKS102. J Bacteriol. 1989;171:2740–2747. doi: 10.1128/jb.171.5.2740-2747.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirsch N H, Stan H-J. Gas chromatographic-mass spectrometric determination of chlorinated cis-1,2-dihydroxycyclohexadienes and chlorocatechols as their boronates. J Chromatogr A. 1994;684:277–287. [Google Scholar]
- 20.Massé R, Messier F, Péloquin L, Ayotte C, Sylvestre M. Microbial biodegradation of 4-chlorobiphenyl, a model compound of chlorinated biphenyls. Appl Environ Microbiol. 1984;47:947–951. doi: 10.1128/aem.47.5.947-951.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKay D B, Seeger M, Zielinski M, Hofer B, Timmis K N. Heterologous expression of biphenyl dioxygenase-encoding genes from a gram-positive broad-spectrum polychlorinated biphenyl degrader and characterization of chlorobiphenyl oxidation by the gene products. J Bacteriol. 1997;179:1924–1930. doi: 10.1128/jb.179.6.1924-1930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mondello F J. Cloning and expression in Escherichia coli of Pseudomonas strain LB400 genes encoding polychlorinated biphenyl degradation. J Bacteriol. 1989;171:1725–1732. doi: 10.1128/jb.171.3.1725-1732.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mondello F J, Bopp L H. Proceedings of Biotech USA 1987. London, England: Online International Ltd.; 1987. Genetic and cell-free studies of PCB biodegradation in Pseudomonas putida LB400; pp. 171–181. [Google Scholar]
- 24.Nadim L, Schocken M J, Higson F J, Gibson D T, Bedard D L, Bopp L H, Mondello F J. Proceedings of the 13th Annual Research Symposium on Land Disposal, Remedial Action, Incineration, and Treatment of Hazardous Waste. EPA/600/9-87/015. U.S. Cincinnati, Ohio: Environmental Protection Agency; 1987. Bacterial oxidation of polychlorinated biphenyls; pp. 395–402. [Google Scholar]
- 25.Nandhagopal N, Senda T, Hatta T, Yamada A, Masai E, Fukuda M, Mitsui Y. Three-dimensional structure of microbial 2-hydroxyl-6-oxo-6-phenylhexa-2,4-dienoic acid (HPDA) hydrolase (BphD enzyme) from Rhodococcus sp. strain RHA1, in the PCB degradation pathway. Proc Jpn Acad Ser B. 1997;73:154–157. [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 27.Seeger M, Timmis K N, Hofer B. Degradation of chlorobiphenyls catalyzed by the bph-encoded biphenyl-2,3-dioxygenase and biphenyl-2,3-dihydrodiol-2,3-dehydrogenase of Pseudomonas sp. LB400. FEMS Microbiol Lett. 1995;133:259–264. doi: 10.1111/j.1574-6968.1995.tb07894.x. [DOI] [PubMed] [Google Scholar]
- 28.Seeger M, Timmis K N, Hofer B. Conversion of chlorobiphenyls into phenylhexadienoates and benzoates by the enzymes of the upper pathway for polychlorobiphenyl degradation encoded by the bph locus of Pseudomonas sp. strain LB400. Appl Environ Microbiol. 1995;61:2654–2658. doi: 10.1128/aem.61.7.2654-2658.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeger, M., M. Zielinski, K. N. Timmis, and B. Hofer. Unpublished results.
- 30.Senda T, Sugiyama K, Narita H, Yamamoto T, Kimbara K, Fukuda M, Sato M, Yano K, Mitsui Y. Three-dimensional structures of free form and two substrate complexes of an extradiol ring-cleavage type dioxygenase, the BphC enzyme from Pseudomonas sp. strain KKS102. J Mol Biol. 1996;255:735–752. doi: 10.1006/jmbi.1996.0060. [DOI] [PubMed] [Google Scholar]
- 31.Studier F W. Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. J Mol Biol. 1991;219:37–44. doi: 10.1016/0022-2836(91)90855-z. [DOI] [PubMed] [Google Scholar]
- 32.Tabor S, Richardson C C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]