Abstract
The 2019 United States outbreak of E-cigarette (e-cig), or Vaping, Associated Acute Lung Injury (EVALI) has been linked to presence of vitamin E acetate (VEA) in Δ8tetrahydrocannabinol (Δ8THC)-containing e-liquids, as supported by VEA detection in patient biological samples. However, the pathogenesis of EVALI and the complex physicochemical properties of e-cig emissions remain unclear, raising concerns on health risks of vaping. This study investigates the effect of Δ8THC/VEA e-liquids and e-cig operational voltage on in vitro toxicity of e-cig aerosols. A novel E-cigExposure Generation System platform was used to generate and characterize e-cig aerosols from a panel of Δ8THC/VEA or nicotine-based e-liquids at 3.7 or 5 V. Human lung Calu-3 cells and THP-1 monocytes were exposed to cell culture media conditioned with collected e-cig aerosol condensate at doses of 85 and 257 puffs/m2 lung surface for 24 h, whereafter specific toxicological endpoints were assessed (including cytotoxicity, metabolic activity, reactive oxygen species generation, apoptosis, and inflammatory cytokines). Higher concentrations of gaseous volatile organic compounds were emitted from Δ8THC/VEA compared with nicotine-based e-liquids, especially at 5 V. Emitted PM2.5 concentrations in aerosol were higher for Δ8THC/VEA at 5 V and averagely for nicotine-based e-liquids at 3.7 V. Overall, aerosols from nicotine-based e-liquids showed higher bioactivity than Δ8THC/VEA aerosols in THP-1 cells, with no apparent differences in Calu-3 cells. Importantly, presence of VEA in Δ8THC and menthol flavoring in nicotine-based e-liquids increased cytotoxicity of aerosols across both cell lines, especially at 5 V. This study systematically investigates the physicochemical and toxicological properties of a model of Δ8THC/VEA and nicotine e-cigarette condensate exposure demonstrating that pyrolysis of these mixtures can generate hazardous toxicants whose synergistic actions potentially drive acute lung injury upon inhalation.
Keywords: EVALI e-cigs vaping acute lung injury, e-cigs aerosols nanoparticles, e-cigs aerosols ultrafine particles, e-cigs aerosol lung toxicity, e-cigs aerosols respiratory effects
The “E-cigarette (e-cig), or Vaping, Associated Acute Lung Injury” or EVALI outbreak started in the United States in July 2019 and lasted until February 18, 2020, when the Centers for Disease Control and Prevention (CDC) ended case reporting as the SARS-COV-2 pandemic arose in the United States. The outbreak led to 2807 hospitalizations, including 68 deaths. The EVALI outbreak raised public health concerns on the health risks associated with vaping, especially among the younger population (CDC, 2020). Due to the heterogeneity of clinical signs and symptoms that accompany the alveolar damage and hypoxemia, the diagnosis of EVALI is made by excluding any other possible cause of acute lung injury, such as infection or alternative diagnosis, in patients that used e-cigs in the 90 days before the onset of the symptoms, and presented with pulmonary infiltrates on chest radiograph or ground-glass opacities on chest computed tomography (CDC, 2019). The analysis performed on biological samples of EVALI patients, such as bronchoalveolar lavage (BAL) (Blount et al., 2019; Taylor et al., 2019), and e-cig products, including vaping devices, e-liquids, packaging, or other documentation (FDA, 2020), showed that the primary e-liquid constituents were Δ9tetrahydrocannabinol (Δ9THC) and vitamin E acetate (VEA) (Blount et al., 2020; Duffy et al., 2020; FDA, 2020; Muthumalage et al., 2020a; Taylor et al., 2019), whereas nicotine vaping alone was found only in a small percentage of cases (Blount et al., 2020; FDA, 2020; Taylor et al., 2019).
From the limited investigations on the e-cigs provided by EVALI patients, there was clearly a substantial difference between the marijuana medical products (MMP) and the EVALI cartridges. Although the conventional MMP contained Δ9THC 80%–90% + Terpenes 10%–20%, the EVALI cartridges contained <50% Δ9THC, due to increased Δ8THC, leading to an unusual ratio of Δ9THC/Δ8THC isomers. Additional numerous other chemicals, including VEA in a concentration range of 16%–88% (most commonly 16%–58%) or medium-chain triglyceride (MCT, concentration range 3%–24%) (Duffy et al., 2020; FDA, 2020; Muthumalage et al., 2020a), pesticide residues, terpenes, manufacturing/pesticides/automotive chemicals (eg, n-butane, benzene, xylene, pentane, etc.), solvents (eg, ethanol, acetone, ethylbenzene, toluene, etc.), PCP-polycaprolactone/household chemicals (eg, methacrolein, acetaldehyde, etc.), other toxic compounds (eg, acrolein, pentadiene compounds, hexane compounds, methyl vinyl ketone, etc.), and elements included Si, Cu, Ni, and Pb (Muthumalage et al., 2020a), were unexpectedly present.
To date, efforts have focused on characterizing vaping emissions from nicotine-based e-liquids either physicochemically or toxicologically (Zhao et al., 2016, 2018a), whereas the literature regarding Δ8THC/VEA-based e-liquids is very scarce. Hence, the causative agent or mechanism underlying EVALI is still unknown. Indeed, the majority of studies focused only on the physicochemical characterization (Jiang et al., 2020; Lanzarotta et al., 2020; Meehan-Atrash et al., 2019; Mikheev et al., 2020) or biological in vivo/in vitro assessment (Bhat et al., 2020; Jiang et al., 2020; Matsumoto et al., 2020; Muthumalage et al., 2020b; Muthumalage and Rahman, 2019) of e-liquids or aerosols generated from a single e-liquid compound (ie, THC or VEA or other additives), which is not representative of e-liquids used by e-cig consumers.
The physical, chemical, and toxicological properties of e-cig emissions depend on many variables, consisting of e-liquid composition, device type (coil material, power supply, voltage, temperature), and puffing topography (CDC U.S. Department of Health and Human Services 2016; Zhao et al., 2016, 2018a,b). However, due to the lack of regulations on the manufacturing and commercialization of e-cigs, the e-liquids and devices are widely customizable, causing the generation of a variety of chemical mixtures in the e-cig aerosols, in which the toxic components can potentially exert synergistic effects in the development of the EVALI syndrome.
The novel E-cig-Exposure Generation System (E-cig-EGS) platform was used to perform a systematic investigation of aerosols generated from combinations of Δ8THC/VEA/terpenes at various real-world concentrations, compared with nicotine-based e-liquids, linking the generation parameters to physicochemical properties of aerosols, using state-of-the-art analytical methods. Consequently, the biological and toxicological assessment of each sampled aerosol was performed using 2 physiologically relevant cell lines, Calu-3 epithelial cells, and THP-1 monocyte, which represent the first line of defense in the airways against particle and toxicants released in the e-cig aerosols, and thus are more involved in the pathogenesis of acute lung injury. Several important endpoints were analyzed for understanding mechanisms of toxicity (eg, cell viability, reactive oxygen species (ROS) generation, mitochondrial dysfunction, activation of caspase-3, and release of pro- and anti-inflammatory biomarkers).
Although the most psychoactive cannabis derivate is Δ9THC, due to federal restrictions on research-use of cannabis products, this study tested Δ8THC as a surrogate of Δ9THC. Although chemically the 2 are close, their toxicological profiles may differ. Moreover, the fact that Δ8THC escapes the FDA and other potential state regulations, by being manufactured from legal hemp-derived cannabidiol (CBD), increases its popularity and use especially in e-liquids, even though they have not been evaluated or approved by the FDA for safe use in any circumstance (NIDA, 2021, September 20).
This study is among the first to systematically investigate the physicochemical properties and cellular toxicity of Δ8THC/VEA and nicotine aerosols, with the goal of understanding and quantifying the risks of exposure to e-cig emissions, and more importantly, elucidating the mechanistic aspects driving the development of EVALI.
MATERIALS AND METHODS
E-cig Cartridges and E-liquids
The Δ8THC/VEA e-cigs were prepared using the following materials: CCell cartridge 510 thread (1 ml) (Hamilton Devices, Sacramento, California), Δ8THC, (+/−)-A-tocopherol acetate (VEA) (cat. no. T3376-100G, Sigma-Aldrich), lab-prepared mixture of terpenes (Terp) containing an equal concentration of myrcene (cat. no. W276212-100G-K, Sigma-Aldrich), caryophyllene (cat. no. 22075-5ML-F, Sigma-Aldrich), limonene (cat. no. 183164-100ML, Sigma-Aldrich), and pinene (cat. no. CRM40339, Sigma-Aldrich).
The nicotine-based e-cigs were prepared using the following materials: KangerTech refillable cartridges T3S series clear cartomizer 1.8 ohm (Rock Bottom Vapes, LLC, Longwood, Florida), nicotine (cat. no. 470301-868, VWR), propylene glycol (PG) high purity (cat. no. 97061-956, VWR), glycerol (vegetable glycerin, VG), lab reagent (cat. no. BDH1172-1LP, VWR), and menthol (cat. no. 470301-734, VWR).
The refillable cartridges were filled with 0.8 ml of e-liquid composed of a combination of Δ8THC/VEA/Terp (4 Δ8THC/VEA e-cigs) or nicotine/PG/VG/menthol (3 nicotine-based e-cigs) in varying proportions and were operated at e-cig operating voltage 3.7 or 5 V, as listed in Supplementary Table 1. All the Δ8THC/VEA and nicotine-based e-liquid constituents and cartridges were prepared and kindly provided by Jeff Rawson PhD, Postdoctoral Fellow from the Whitesides Laboratory, Department of Chemistry and Chemical Biology, Harvard University.
E-cig Exposure Generation System
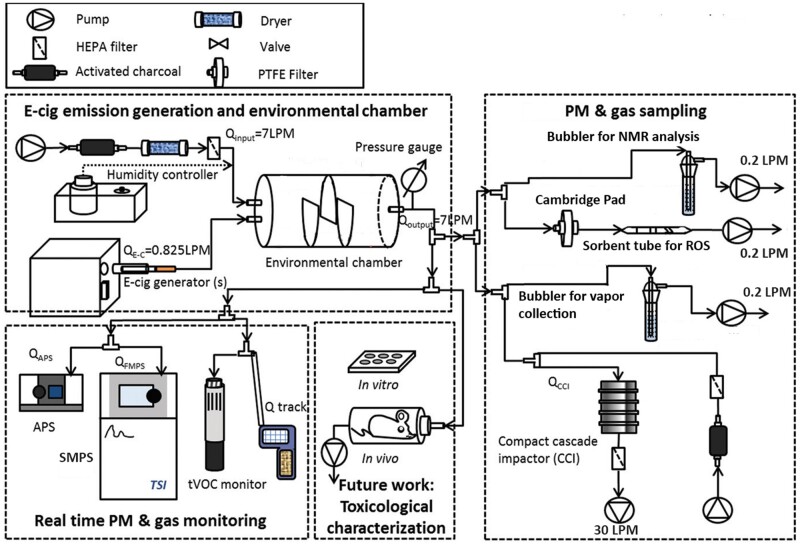
The recent E-cig-EGS platform developed by the authors was used to generate real-world e-cig aerosol exposures for real-time monitoring and detailed physicochemical and toxicological characterization, as shown in Figure 1 (Zhao et al., 2016). Briefly, a fully programmable single-port e-cig generator (ECAG; e_Aerosols LLC, Central Valley, New York) was connected to the desired e-cig cartridge to generate exposures under precisely controlled puffing patterns and operational voltage. The generated e-cig aerosol was mixed and diluted with HEPA-filtered and activated charcoal-filtered ambient air in a 7-l cylindrical environmental chamber. The residence time of the aerosol in the chamber was set to 60 s to mimic lung “washout time” during active vaping in e-cig users (Invernizzi et al., 2007). The temperature and relative humidity of the chamber was controlled at 24°C and 90% respectively. The E-cig-EGS was connected with real-time and time-integrated monitoring and sampling instrumentation for physicochemical and toxicological characterization (Demokritou et al., 2004; Pal et al., 2015).
Figure 1.
Schematic of the E-cig-Exposure Generation System (E-cig-EGS). E-cig-EGS (adapted from Zhao et al., 2016) for the generation, monitoring, sampling, and detailed physicochemical and toxicological characterization of real-world e-cig emissions.
The E-cig-EGS was connected to the prefilled cartridge as described previously. A modified puffing protocol was applied, with a puff volume of 55 ml, puff duration of 4 s, and an interval of 30 s between consecutive puffs, reflecting real-world e-cig consumer behaviors (Farsalinos et al., 2013a; Zhao et al., 2016; 2018a).
Real-Time Monitoring of E-Cig Emissions
After aerosol mixing in the environmental chamber, the released particulate matter (PM) number concentration in the e-cig aerosol and its number-size distribution were simultaneously monitored. A scanning mobility particle sizer spectrometer (NanoScan SMPS, Model 3910; TSI Inc., Shoreview, Minnesota) was used to measure nano-PNC as a function of mobility particle diameter (MPD) in the range of 10–420 nm. An aerodynamic particle sizer spectrometer (APS, Model 3321; TSI Inc.) was used to measure PNC as a function of APD in the range of 0.5–20 µm. For both SMPS and APS, the intersample duration was set to 60 s, the same as the aerosol residence time in the environmental chamber.
In addition, the gaseous byproducts in the e-cig aerosol (CO and CO2) and the environmental conditions (temperature and relative humidity) in the chamber were monitored using Q-Trak Indoor Air Quality (IAQ) Monitor (Model 8551, TSI Inc.) every 1 min. The total volatile organic compound (tVOC) levels were also measured using a photoionization detector sensor (TG-502 probe, GrayWolf Sensing Solutions, Shelton, Connecticut), equipped with a sensitive ppb-level probe, with a sample collected every 10 s. Background measurements of each parameter were collected for at least 2 min before the beginning of each aerosol generation/sampling experiment to ensure a stable, clean background. Real-time monitoring of e-cig emissions was assessed for a total of 10 min after the start of the puffing protocol.
Sampling of e-cig emissions
After 10 min of real-time monitoring following the initiation of the puffing protocol, the e-cig aerosol from the environmental chamber was bubbled through a fritted-head impinger (porosity A [145–174 µm] tip: Ace Glass Inc., Vineland, New Jersey) containing 20 ml of cell culture medium, either Eagle’s minimum essential medium (EMEM, American Type Culture Collection, ATCC, Rockville, Maryland) or Roswell Park Memorial Institute (RPMI) 1640 Medium (Gibco BRL, Rockville, Maryland) for subsequent in vitro toxicological characterization. The impinger sampling flow rate was set to 0.2 l/min and the sampling duration was 30 min. Immediately following sampling, the culture media were aliquoted in 15 ml falcon tubes and stored at 4°C until cell exposure.
In addition, the generated PM in the e-cig aerosol was size fractionated and sampled using the 30 l/min Harvard Compact Cascade Impactor (CCI) (Demokritou et al., 2004) and characterized for mass concentration as a function of size by gravimetric analysis. Three substrates (Teflon filter for PM0.1 and polyurethane foams [PUF] for PM0.1–2.5 and PM>2.5 size fractions) were used to sample PM in the CCI. Before and after sampling, the PUFs and Teflon filters were weighed using a Mettler Toledo XPE analytical microbalance (Mettler-Toledo LLC, Columbus, Ohio, USA). The weight difference was calculated and normalized to determine the average PM mass concentration (mg/m3) corresponding to each size fraction in the e-cig aerosol.
Cell Culture and Exposure
Human lung epithelial cell line Calu-3 and human peripheral blood monocytes THP-1 cells, (ATCC), were cultured in Eagle's EMEM medium (ATCC) and Roswell Park Memorial Institute (RPMI) 1640 Medium (Gibco BRL), respectively, supplemented with 10% fetal bovine serum, 100 U/ml of penicillin G and 100 mg/ml streptomycin and grown at 37°C in 5% CO2.
Cells (passage number between 2 and 8) were seeded in 96-well plates at a density of 0.5 × 106 cells/ml in 100 µl growth medium for a single well and exposed for 24 h. Both cell lines were exposed to 2 doses of the conditioned cell culture media generated with 85 and 257 puffs/m2 of e-cig aerosol for the basic cytotoxicity assays, whereas the higher dose was administered for the acute inflammatory biomarker assessment (see below on dose justification). Untreated cells were used as a negative control. Conventional cigarette smoke sampled in the appropriate cell culture medium was used as a comparator in all cellular toxicological experiments. The smoke generated from an entire cigarette was bubbled directly into the impinger containing 20 ml of EMEM or RPMI medium, connected to a pump with a flow rate of 0.2 l/min (Supplementary Figure 1). The schematic for cigarette smoke sampling methodology is shown in Supplementary Figure 1. All cellular exposures were done using 3 independent biological samples and 3 technical replicates.
Cellular dose justification
The exposures in this study were large bolus doses of conditioned media generated with 85 and 257 puffs/m2 of e-cig aerosol for 24 h. These doses were selected to mimic 1 and 3 months of vaping and were calculated based on the approximate number of puffs reported by e-cig users: 13 puffs/vaping session (Farsalinos et al., 2013b) or 120–180 puffs/day (Etter and Bullen, 2011; Kosmider et al., 2018; Tackett et al., 2015). The average of 200 puffs/day was normalized on the total exposure area of lung epithelial cells in an adult, which is 70 m2. The resulting 2.85 puffs/m2/day represents the number of puffs that reaches each square meter area of lung surface each day, in an adult that vapes 200 puffs/day. Then we calculated the number of puffs vaped over a period of 30 and 90 days (1 and 3 months), which resulted in 6000 and 18 000 puffs, respectively. Thus, the target cellular exposure concentrations corresponding to 1 month and 3 months of vaping were calculated to be 85 and 257 puffs/m2, respectively (eg 2.85 puffs/m2 × 30 days = 85.5 puffs/m2 which is equal to 6000 puffs in 30 days/70 m2 = 85.7 puffs/m2). Subsequently, the volume of stock solution to be administered in bolus for 24 h was determined based on the e-cig aerosol concentration (units of puffs/ml) in the stock solution and the cell surface area. For more details on the cellular exposure dose calculations, please refer to the Supplementary Information.
Cell Viability Assay
Lactate dehydrogenase (LDH) released in the supernatant of Calu-3 and THP-1 cells treated for 24 h in a 96-well plate was used to assess the cell viability and measured using the Pierce LDH assay kit (cat. no. 88953, ThermoFisher Scientific, Waltham, Massachusetts) according to manufacturer’s instructions. The absorbance was measured spectrophotometrically at a wavelength of 490 and 680 nm. Percent cytotoxicity was calculated by normalizing LDH activity of the treatments based on the maximum activity induced by the positive control, as described previously (Singh et al., 2022).
Metabolic Activity Assay
PrestoBlue HS cell viability reagent (cat. no. P50201, ThermoFisher Scientific) was used according to the manufacturer’s instructions to assess the cellular metabolic activity. Fluorescence was measured at 560 nm (excitation)/590 nm (emission). Percent metabolic activity was normalized to the response from the negative control (untreated cells) assigned as 100%, as described previously (Singh et al., 2022).
Oxidative Stress/Reactive Oxygen Species Assay
ROS analysis was performed after 6 h treatment in a 96-well plate, using CellROX green reagent (cat. no. C10444, ThermoFisher-Scientific) according to the manufacturer’s instructions. Fluorescence was measured at 480 nm (excitation)/520 nm (emission). Percent ROS generation was normalized to the maximum ROS generation induced by the positive control assigned as 100%, as published previously (Singh et al., 2022).
Mitochondrial Membrane Potential Assay
Mitochondrial membrane potential (ΔΨm) was measured after 24 h treatment of Calu-3, using JC-1 Mitochondrial Membrane Potential Detection Kit (cat. no. 30001, Biotium Inc., Fremont, California), according to the manufacturer’s instructions. Calu-3 cells were exposed in a 96-well plate containing 100 µl/well of cell culture medium. Cells treated with carbonyl cyanide 3-chlorophenylhydrazone 0.1 mM for 30 min at 37°C were used as a positive control. Briefly, after 24 h exposure, the cell culture medium was removed, replaced with 100 μl of JC-1 reagent working solution, and plates were incubated in a 37°C cell culture incubator for 15 min. The supernatant was removed, and cells were then washed once with 200 μl PBS. Red and green fluorescence were measured at 550 nm (excitation)/660 nm (emission), and 485 nm (excitation)/535 nm (emission), respectively, using a fluorescence microplate reader. To calculate the ΔΨm, the ratio of red/green fluorescence was measured: the ratio is decreased in dead and apoptotic cells compared with healthy cells. Percent ΔΨm was normalized to the response from the negative control (untreated cells) assigned as 100%.
Caspase-3 Activation Assay
Caspase-3 DEVD-R110 Fluorometric HTS Assay Kit (Biotium Inc., Fremont, California) was used to detect the activation of Caspase-3 in treated Calu-3 cells, according to the manufacturer’s instructions. Calu-3 cells were exposed in a 96-well plate containing 100 µl/well of cell culture medium. Cells treated with staurosporine 2 µM for 2 h at 37°C were used as a positive control. Briefly, after 24 h exposure, 100 µl/well of assay buffer, containing enzyme-substrate (acDEVD)2 R110 (2 mM) and cell lysis/assay buffer at a ratio of 50 µl substrate per 1 ml buffer, was added to each well, the plate was incubated for 30 min and fluorescence was measured at 470 nm (excitation)/520 nm (emission). Percent caspase-3 activation was normalized to the response from the negative control (untreated cells) assigned as 100%.
Inflammatory Response Assessment
The supernatants from Calu-3 cells exposed to the higher dose representative of 257 puffs/m2 (3 months) were collected after 24 h exposure and shipped in dry ice for cytokine and chemokine assessment using the Human Cytokine Array/Chemokine Array 48-Plex (HD48) assay (Eve Technologies, Calgary, Alberta). The samples were prepared according to Eve Technologies protocol, as previously described (Singh et al., 2022). The total concentration of cytokines/chemokines released was normalized to the number of cells and the negative control.
Statistical Analysis
The real-time aerosol characteristics (PNC and size distributions) were averaged across the consecutive measurements collected after reaching a steady state. Aerosol data analysis was performed in the TSI Aerosol Instrument Manager Software (AIM, v9.0) and average PNC and relevant aerosol size statistics (median, mode, geometric mean, and geometric standard deviation) were reported for each vaping atmosphere. All the cellular experiments were performed using 3 independent biological samples and each test was conducted in 3 technical triplicates.
Statistical analyses for all real-time and toxicological data were performed on GraphPad Prism using 2-way ANOVA tests and corrected for multiple comparisons: Dunnett’s test was used to compare e-cig liquid composition versus baseline (Δ8THC 80%/VEA 20% for real-time data or negative/positive control for toxicological data); Sidak’s test was used to compare 3.7 versus 5 V for real-time data and 85 versus 257 puffs/m2 (1 vs 3 months) for toxicological data.
RESULTS
Real-Time Characterization of Released Aerosol PM
Figure 2 and Supplementary Figure 2 show the aerosol PNC as a function of size (in the nano to micron regime) in the e-cig aerosol generated from Δ8THC/VEA- and nicotine-based e-cigs, respectively, after reaching steady-state condition (approximately 1 min after initiation of the puffing protocol, irrespective of e-liquid type or operational voltage). Supplementary Table 2 summarizes the tPNC and the mean and modal aerosol sizes (along with standard deviations) at steady state during the e-cig aerosol generation from each e-liquid composition at the specified operational voltage, as measured using the APS (aerodynamic diameter range 0.5–20 µm) and SMPS (mobility diameter range 10–420 nm) instrumentation. In more detail in the following sections.
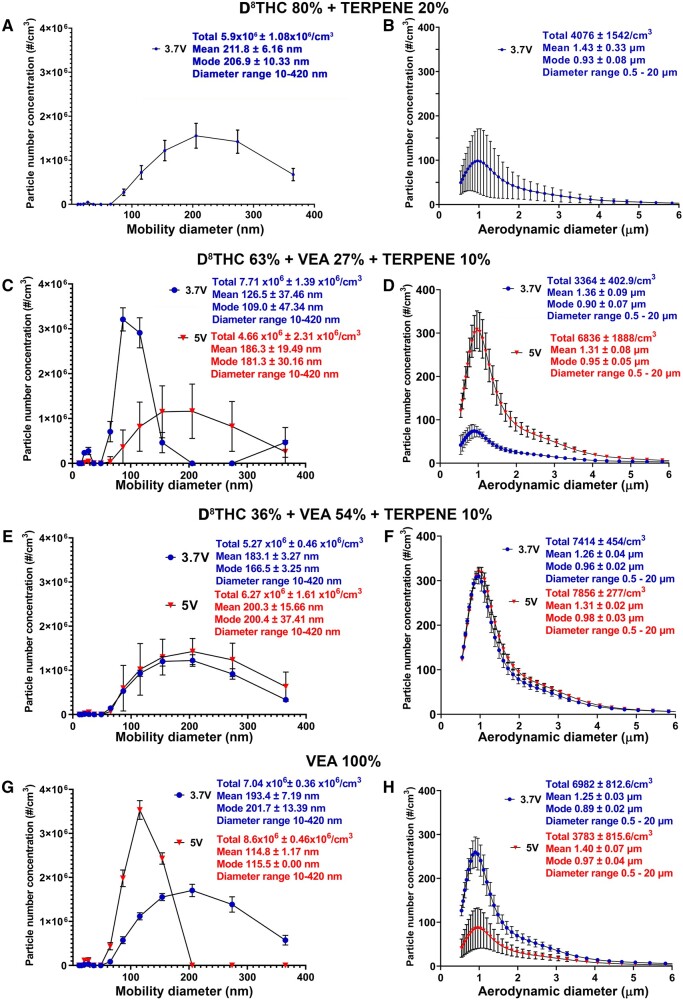
Figure 2.
The total article number concentration and size distribution depend on both e-liquid composition and operational voltage. Comparison of number concentration, mean, and mode of particles generated from the 4 different THC-based e-liquid combinations, generated at 3.7 versus 5 V. (A, C, E, G) mobility diameter range of 10–420 nm; (B, D, F, H) aerodynamic diameter range of 0.5–20 µm. Aerosol measurements were taken every 60 s for a period of 10 min for both APS and SMPS, and then the average of the consecutive readings of particle number concentration and mean/modal aerosol size was calculated. Thus the N was at least 10 for both measurements.
Δ8THC/VEA-Based E-Liquids
For the 4 different Δ8THC/VEA e-liquid compositions (corresponding to VEA 0%—27%—54%—100%), the nano-PNC in the e-cig aerosol varied (between 4.7 × 106 and 8.6 × 106 particles/cm3) across the 2 operational voltage, and the mean aerosol MPD ranged between 115 and 212 nm (Figure 2 and Supplementary Table 2). The micro-PNC was between 3364 and 7865 particles/cm3, whereas the mean APD remained between 1.25 and 1.43 µm.
Effect of e-liquid composition
The tPNC for both size ranges was sensitive to e-liquid composition (ie, VEA concentration), without any apparent specific trend (Figure 2 and Supplementary Figure 2). For instance, at operational voltage 3.7 V, increasing the e-liquid VEA concentration from 0% to 27% raised the nano-PNC (from 5.9 × 106 to 7.7 × 106 particles/cm3), followed by decrease to 5.3 × 106 particles/cm3 at VEA 54% and subsequent increase to 7.0 × 106 particles/cm3 at VEA 100%. Similarly, the micro-PNC initially decreased from 4076 particles/cm3 at VEA 0% to 3364 particles/cm3 at VEA 27%, subsequently increased to 7414 particles/cm3 at VEA 54% and marginally decreased to 6982 particles/cm3 at VEA 100%. Similar fluctuations in PNC with e-liquid composition were also observed at the higher operational voltage 5 V: the nano-PNC steadily increased with increasing VEA concentration, starting from 4.7 × 106 particles/cm3 at VEA 27%, to 6.3 × 106 particles/cm3 at VEA 54%, and finally 8.6 × 106 particles/cm3 at VEA 100%. At the same voltage, the micro-PNC initially climbed from 6836 particles/cm3 at VEA 27% to 7856 particles/cm3 at VEA 54%, but then declined significantly to 3783 particles/cm3 at VEA 100%. The VEA concentration’s effect on the average nanoparticle size was also apparent for both operational voltages, whereas the micron-sized particle number-size distributions remained relatively similar across the different compositions (Supplementary Figure 3). For instance, at 3.7 V, the e-liquid with VEA 27% had a significantly different nanoparticle size distribution (mean MPD: 126 nm) compared with the other compositions (mean MPD: 183–212 nm). At 5 V, the e-liquid with VEA 100% differed significantly (mean MPD: 115 nm) from the remaining compositions (mean MPD: 186–200 nm).
Effect of operating voltage
As is evident from Figure 2, the PNC for both size ranges and the nanoparticle size distribution (10–420 nm) were also dependent on the operational voltage utilized. Increasing operational voltage from 3.7 to 5 V increased the nano-PNC for e-liquids with VEA 54% (from 5.3 × 106 to 6.3 × 106 particles/cm3) and VEA 100% (from 7.0 × 106 to 8.6 × 106 particles/cm3) but decreased for VEA 27% (from 7.7 × 106 to 4.7 × 106 particles/cm3). Similarly, the voltage increase raised micro-PNC for VEA 27% (from 3364 to 6836 particles/cm3) and VEA 54% (from 7414 to 7856 particles/cm3), but significantly reduced them for VEA 100% (from 6982 to 3783 particles/cm3). The nanoparticle number-size distribution remained relatively the same across the 2 operational voltages for VEA 54% composition (mean MPD: 183–200 nm) but significantly shifted for the VEA 27% and VEA 100% compositions with operational voltage change. For VEA 27%, the nanoparticle size distribution shifted toward larger sizes at the higher voltage (mean MPD: from 126 nm at 3.7 V to 186 nm at 5 V), whereas for VEA 100%, it shifted toward smaller sizes with the increase in voltage (mean MPD: from 193 nm at 3.7 V to 115 nm at 5 V).
Nicotine-Based E-Liquids
For the 3 different nicotine-based e-liquid compositions (corresponding to Nic 2%–5%, Nic 5% + Menthol 2%), the released nano-PNC was in the range of (5.2–9.2) × 106 particles/cm3 and the mean MPD remained between 110 and 125 nm, across the 2 operational voltages (Supplementary Figure 2 and Table 2). The micro-PNC varied between 4052 and 7048 particles/cm3, and the mean APD remained relatively constant in the range of 1.25–1.31 µm.
Effect of e-liquid composition
Both the nano- and micro-tPNC were affected by the nicotine/menthol content in the e-liquid for a given operational voltage, whereas the aerosol size distribution and the mean aerosol size in both size regimes remained relatively the same across the different compositions (Supplementary Figs. 2 and 4). At operational voltage 3.7 V, the nano-PNC marginally decreased with nicotine content increase, from 9.2 × 106 particles/cm3 at Nic 2% to 8.6 × 106 particles/cm3 at Nic 5%, whereas the micro-PNC increased from 6178 to 6890 particles/cm3. The addition of menthol 2% to Nic 5% e-liquid (same voltage) further decreased both the nano-PNC to 6.4 × 106 particles/cm3 and the micro-PNC to 4052 particles/cm3.
Effect of operating voltage
The effect of operational voltage (3.7 vs 5 V) was assessed for one of the nicotine-based e-liquids (Nic 5% + menthol 2%). Both nano- and micron-PNC were sensitive to operational voltage utilized, but not the aerosol number-size distributions (Supplementary Figure 2). Nano-PNC decreased slightly with increasing operational voltage, from 6.4 × 106 particles/cm3 at 3.7 V to 5.2 × 106 particles/cm3 at 5 V, whereas micro-PNC rose steeply from 4052 particles/cm3 at 3.7 V to 7048 particles/cm3 at 5 V.
Real-Time Monitoring of Released Gaseous VOCs
Figures 3A–C show the real-time evolution of the total gaseous VOCs’ concentration (tVOCs, ppm levels) released in the e-cig aerosol for all the different vaping atmospheres generated, for the first 10 min after the start of the puffing protocol. The concentration of tVOCs increased linearly or exponentially (sigmoidal or S-shaped growth curve) with time, depending on the e-liquid composition and the operational voltage. In more detail in the following sections.
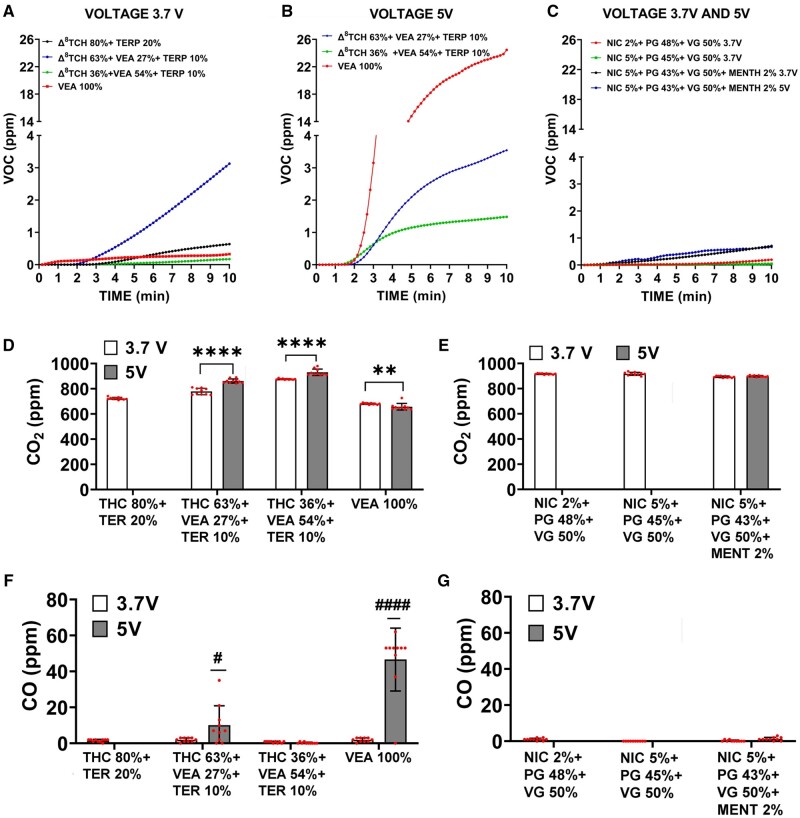
Figure 3.
The emission of gaseous pollutants (VOC, CO2, and CO) depends on both e-liquid composition and operational voltage. E-cig gaseous pollutants generation from different e-liquid combinations and under different operating voltages. A–C, Total VOC monitored for 10 min by tVOC monitor. D, E, CO2 generation monitored for 30 min by Qtrak. F, G, CO generation monitored for 30 min by Qtrak. The measurement of VOC, CO2, and CO were recorded only for the conditions listed in Supplementary Table 1, therefore only 4 e-liquid compositions were examined at both voltages 3.7 and 5 V. All values are represented as the mean ± SE. * p < .05, values statistically different between 2 voltages. Measurements were taken every 60 s for a period of 10 min, thus N = 10.
Δ8THC/VEA-Based E-Liquids
The maximum tVOC concentration observed at the end of 10 min varied widely between the various vaping atmospheres generated from Δ8THC/VEA-based e-cigs (range: 0.173–24.5 ppm), depending on both e-liquids VEA concentration and operational voltage (Figs. 3A and 3B).
Effect of e-liquid composition
VEA concentration significantly affected the peak tVOC concentration for a given operational voltage, but without a definite correlation. At 3.7 V, the maximum tVOC concentration was achieved by VEA 27% (3.1 ppm), followed by VEA 0% (0.638 ppm), VEA 100% (0.327 ppm), and VEA 54% (0.173 ppm). At 5 V, the e-liquid with the highest tVOC concentration was VEA 100% (24.5 ppm), followed by VEA 27% (3.5 ppm) and VEA 54% (1.5 ppm).
Effect of operating voltage
Increasing the operational voltage from 3.7 to 5 V seemed to affect the tVOC evolution curve over time and increase the peak released tVOC concentration for all e-liquid compositions. Whereas the tVOC growth curve mostly remained linear at 3.7 V, it was exponential with a sigmoidal growth at 5 V with concentrations plateauing close to the 10-min mark. The most dramatic rise in tVOC concentration with the voltage increase was observed for VEA 100% (from 0.327 to 24.5 ppm, 75-fold increase), followed by VEA 54% (from 0.173 to 1.5 ppm, 9-fold increase), and VEA 27% (from 3.1 to 3.5 ppm, 1.1-fold increase).
Nicotine-Based E-Liquids
The peak tVOC concentration released in nicotine-based vaping atmospheres ranged between 0.044 and 0.707 ppm (Figure 3C), overall lower than Δ8THC/VEA-based e-liquids. The composition of nicotine did not affect the peak tVOC concentration (Nic 2%: 0.196 ppm vs Nic 5%: 0.044 ppm), whereas the addition of menthol flavoring significantly raised the tVOC concentration (Nic 5% + menthol 2%: 0.707 ppm vs Nic 5%: 0.044 ppm, 16-fold increase). Increasing the operational voltage from 3.7 to 5 V did not significantly alter the tVOC concentration released from the Nic 5% + menthol 2% e-cig (0.707–0.674 ppm, respectively).
Real-Time Monitoring of Released CO2 and CO
Figures 3D–G show the bar graph concentration (in ppm levels) of CO2 (D–E) and CO (F–G) released in the e-cig aerosol across the different vaping atmospheres, averaged for the first 10 min after the start of the puffing protocol. In more detail in the following sections.
Δ8THC/VEA-Based E-Liquids
The average released CO2 level varied between 650 and 925 ppm (Figure 3D), whereas the average CO level ranged from negligible levels to 46 ppm (Figure 3F). The release of both CO2 and CO was significantly affected by the e-liquid composition as well as the operational voltage, with more dramatic effects observed for CO levels with changes in composition and voltage.
Effect of e-liquid composition
The CO2 level significantly increased initially in a dose-dependent manner with the increase in VEA concentration (VEA 0%: 723 ppm, VEA 27%: 779 ppm, VEA 54%: 876 ppm, p < .0001 vs VEA 0%), but then significantly decreased to 681 ppm (p < .0001 vs VEA 0%) at VEA 100%, when the e-cig was operated at 3.7 V (Figure 3D). Similarly, at the operating voltage of 5 V, the CO2 level augmented from VEA 27% (862 ppm) to VEA 54% (925 ppm) (p < .0001 vs VEA 0%) and then declined to 650 ppm at VEA 100% (p < .0001 vs VEA 0%). The effect of VEA concentration on CO levels was quite pronounced at operational voltage 5 V (Figure 3F). Although the e-liquids VEA 0% and VEA 54% released negligible levels of CO, VEA 27% exhibited an average CO level of 10 ppm (peak: 35 ppm) (p < .05 vs VEA 0%) and VEA 100% showed the highest average CO level at 46 ppm (peak: 62 ppm) (p < .0001 vs VEA 0%).
Effect of operational voltage
Increasing the voltage from 3.7 to 5 V significantly increased the released CO2 levels for VEA 27% (from 779 to 862 ppm, p < .0001) and VEA 54% (from 876 to 925 ppm p < .0001) e-liquids but suppressed the CO2 levels for VEA 100% (from 681 to 650 ppm p < .0001) (Figure 3D). The CO levels increased tremendously with the operational voltage increase for VEA 27% (from negligible level to 10 ppm average, p < .05) and VEA 100% (from negligible level to 46 ppm average, p < .0001) but were unaffected for the VEA 0% and VEA 54% compositions where they remained negligible at both voltages (Figure 3F).
Nicotine-Based E-Liquids
The average CO2 levels released from nicotine-based e-cigs remained relatively similar (approximately 900 ppm) across the different nicotine/menthol concentrations and voltage conditions, whereas the CO concentrations did not reach detectable levels for any of the nicotine atmospheres.
Time-Integrated Mass-Size Distribution of Sampled Aerosol PM
Supplementary Table 3 and Figure 5 present the average PM mass concentration data (in units of mg/m3) over the sampling period of the size-fractionated and collected aerosol particles in 3 size fractions (PM0.1, PM0.1–2.5, PM>2.5) generated from the various vaping atmospheres. In more detail in the following sections.
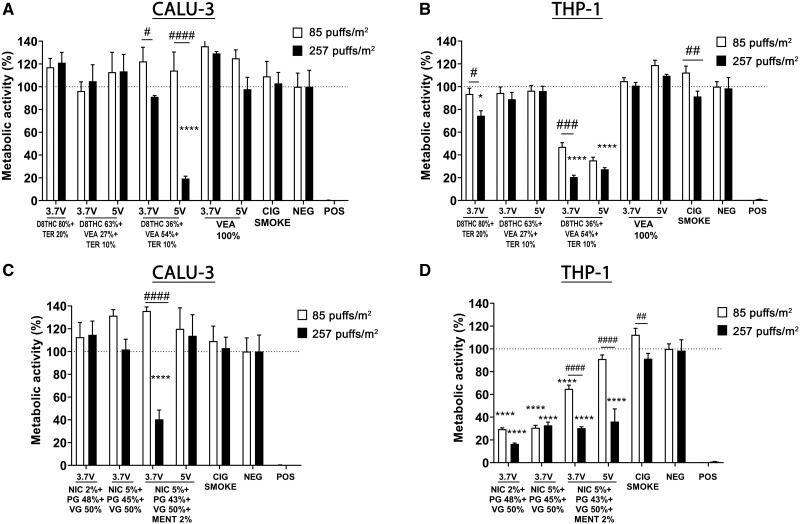
Figure 5.
Alterations in metabolic activity in Calu-3 and THP-1 cell lines are dependent on e-cigs e-liquid composition and operational voltage. Acute in vitro cytotoxicity assessment through cells metabolic activity, using Presto blue assay, following exposure to e-cigs aerosols generated from different concentrations of THC/VEA- or nicotine-based e-liquids, under 2 operational voltages (3.7 or 5 V), or cigarette smoke, at 2 doses of 85 and 257 puffs/m2 (1 and 3 months), on human lung epithelial cells (Calu-3) (A, C) and peripheral monocytes (THP-1) (B, D). All values are represented as the mean± SE. * p < .05, ** p < .01, *** p < .001, **** p < .0001 compared with negative control. # p < .05, ## p < .01, ### p < .001, #### p < .0001 compare between 2 doses. Statistical analysis was performed on an average of 3 independent biological samples; fold-changes >20% were deemed to be statistically significant (p < .05).
Δ8THC/VEA-Based E-Liquids
The total PM mass concentration (across the 3 size fractions) released from the different Δ8THC/VEA e-liquids ranged widely between 67 and 910 mg/m3, depending on e-liquid VEA concentration and operational voltage. The bulk of the PM mass (94–99 wt%) was distributed between the PM0.1 (11–60 wt%) and PM0.1–2.5 (38–88 wt%) size fractions for all Δ8THC/VEA atmospheres, whereas the PM>2.5 size fraction constituted 1–6 wt% of the total PM mass.
Effect of e-liquid composition and operating voltage
The released total PM mass concentration significantly increased with increasing e-liquid VEA concentration at both operational voltages. In addition, increasing the voltage from 3.7 to 5 V dramatically raised the PM mass concentration for all Δ8THC/VEA e-liquids. At 3.7 V, VEA 100% released the highest PM mass concentration (311 mg/m3) followed by VEA 54% (306 mg/m3), VEA 27% (114 mg/m3), and VEA 0% (67 mg/m3). A similar trend was observed at 5 V along with tremendously increased PM mass concentrations for each e-liquid compared with 3.7 V (VEA 100%: 910 mg/m3, VEA 54%: 447 mg/m3, VEA 27%: 245 mg/m3).
Nicotine-Based E-Liquids
The total PM mass concentration released in the different nicotine-based vaping atmospheres ranged from 240 to 668 mg/m3, with the majority of the PM mass lying in the PM0.1–2.5 size fraction (82–87 wt%), followed by PM0.1 (11–16 wt%) and PM>2.5 (1–2 wt%) size fractions. Increasing nicotine content in e-liquid significantly raised the PM mass concentration, from 300 mg/m3 (Nic 2%) to 417 mg/m3 (Nic 5%) at operational voltage 3.7 V. Furthermore, adding menthol 2% flavoring to the Nic 5% e-liquid further increased the PM concentration to 668 mg/m3, at the same voltage. However, at the higher operational voltage of 5 V, the PM concentration from the Nic 5% + menthol 2% e-liquid significantly declined to 240 mg/m3.
Cytotoxicity of E-Cig Emissions
In this section, we present the results for the acute in vitro cytotoxicity assessment of the generated and sampled e-cig aerosols from the different e-liquid compositions under the investigated voltage conditions for both Calu-3 and THP-1 cell lines.
Cell Viability (LDH and PrestoBlue Assays)
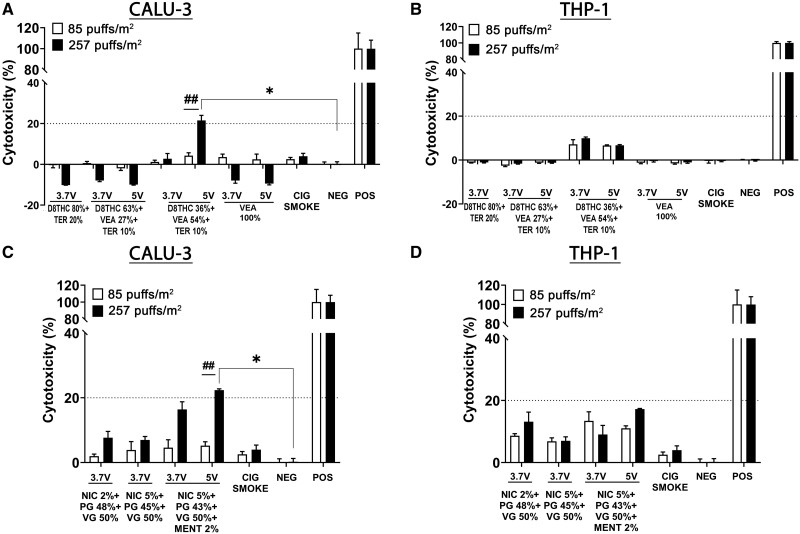
Figure 4 shows the results for the LDH release after 24 h from Calu-3 human lung epithelial cells (Figs. 4A and 4C for Δ8THC/VEA- and nicotine-based e-liquids, respectively) and THP-1 human monocyte cell line (Figs. 4B and 4D for Δ8THC/VEA- and nicotine-based e-liquids, respectively), exposed to 2 doses of e-cig aerosol corresponding to 85 and 257 puffs/m2 (1 and 3 months). Neither of the e-cig aerosol samples induced significant LDH release in both cell lines, except for the higher dose (257 puffs/m2, 3 months) of aerosol produced from VEA 54% at 5 V and Nic 5% + menthol 2% at 5 V that induced a slight but significant increase of LDH release after 24 h (>20%, p < .05) in the Calu-3 cells (Figs. 4A and 4C).
Figure 4.
Cell viability was affected by the presence of VEA in Δ8THC and menthol flavoring in nicotine-based e-liquids especially at 5 V. Acute in vitro cell viability assessment, using LDH assay, following exposure to e-cigs aerosols generated from different concentrations of THC/VEA or nicotine-based e-liquids, under 2 operational voltages (3.7 or 5 V), or cigarette smoke, at 2 doses of 85 and 257 puffs/m2 (1 and 3 months), on human lung epithelial cells (Calu-3) (A, C) and peripheral monocytes (THP-1) (B, D). All values are represented as the mean± SE. * p < .05, values statistically different from untreated cells. # p < .05, values statistically different between 2 doses. Statistical analysis was performed on an average of 3 independent biological samples; fold changes >20% were deemed to be statistically significant (p < .05).
An indirect method to measure cell viability is the cells’ metabolic activity. After 24 h exposure of Calu-3 to both concentrations of aerosols, the metabolic activity did not decrease significantly. However, similar to the LDH release, aerosols emitted from VEA 54% (5 V) and nicotine 5%+ menthol 2% (3.7 V) induced a significant and dose-dependent reduction of metabolic activity in Calu-3 epithelial cells (****p < .0001 compared with negative control, ####p < .0001 higher dose vs lower dose) (Figs. 5A and 5C).
Similar to Calu-3 epithelial cells, THP-1 monocyte cells experienced a statistically significant dose-dependent diminution in metabolic activity when exposed to both doses of VEA 54% aerosol generated at 3.7 or 5 V (****p < .0001) (Figure 5B). In addition, THP-1 cells exposed to both doses of nicotine-containing aerosols also showed a statistically significant decrease in metabolic activity (****p < .0001), except for the lower dose of Nic 5% + menthol 2% (3.7 V) (Figure 5D).
To better understand the cytotoxic effect of the aerosol generated from VEA 54% (5 V), both cell lines were exposed to higher concentrations of the same aerosol, 1020, 510, 255, and 85 puffs/m2, corresponding to 12-, 6-, 3-, and 1-month vaping periods (Supplementary Figure 6). Although Calu-3 epithelial cells did not exhibit any significant release of LDH or decrease in metabolic activity for any of the doses tested (Supplementary Figs. 6A and 6C), THP-1 monocytes showed a dose-dependent increase of LDH release compared with the negative control, and a decreasing metabolic activity only at 257 puffs/m2 (3 months) (Supplementary Figs. 6B and 6D).
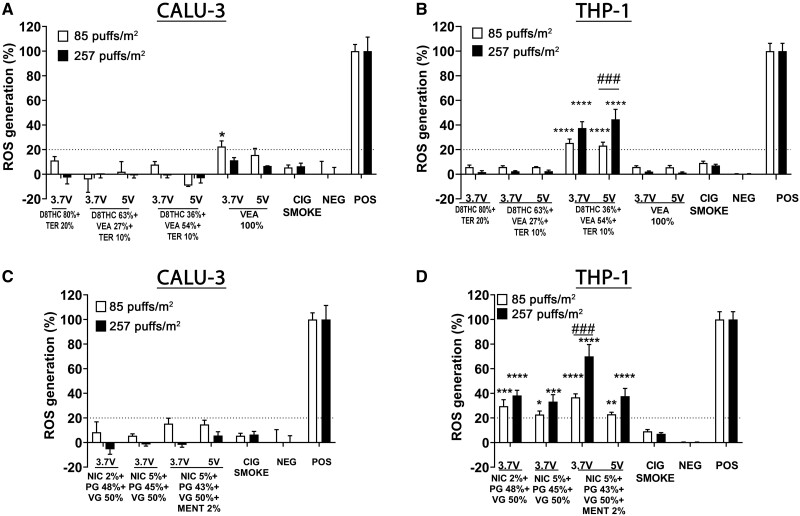
Cell ROS Production
Figure 6 shows the ROS generation from human lung epithelial cells (Calu-3) (Figs. 6A and 6C) and human monocyte cell line (THP-1) (Figs. 6B and 6D), exposed to 2 doses of aerosols, corresponding to 85 and 257 puffs/m2 (1 and 3 months). Aerosols generated from Δ8THC- or nicotine-containing e-liquids did not induce a significant generation of ROS in Calu-3 epithelial cells. Conversely, the THP-1 monocyte cells exposed to both doses of VEA 54% at 3.7 and 5 V generated a significant amount of ROS in a dose-dependent manner (****p < .0001) (Figure 6B). THP-1 monocyte cells exposed to both concentrations of aerosols from nicotine-based e-liquids also showed a dose-dependent generalized augmented production of ROS (****p < .0001) (Figure 6D).
Figure 6.
In THP-1 monocytes, but not in Calu-3 cells, VEA 54% (3.7 and 5 V) induced a significant and dose-dependent reactive oxygen species (ROS) generation. Acute in vitro assessment of ROS generation in human lung epithelial cells (Calu-3) (A, C) and peripheral monocytes (THP-1) (B, D) exposed for 24 h to aerosols generated from different concentrations of Δ8THC/VEA or nicotine-based e-liquids under 2 operational voltages (3.7 or 5 V), or cigarette smoke, at 2 doses of 85 and 257 puffs/m2 (1 and 3 months). All values are represented as the mean± SE. * p < .05, ** p < .01, *** p < .001, **** p < .0001 compared with negative control. # p < .05, ## p < .01, ### p < .001, #### p < .0001 compare between 2 doses. Statistical analysis was performed on an average of 3 independent biological samples; fold changes >20% were deemed to be statistically significant (p < .05).
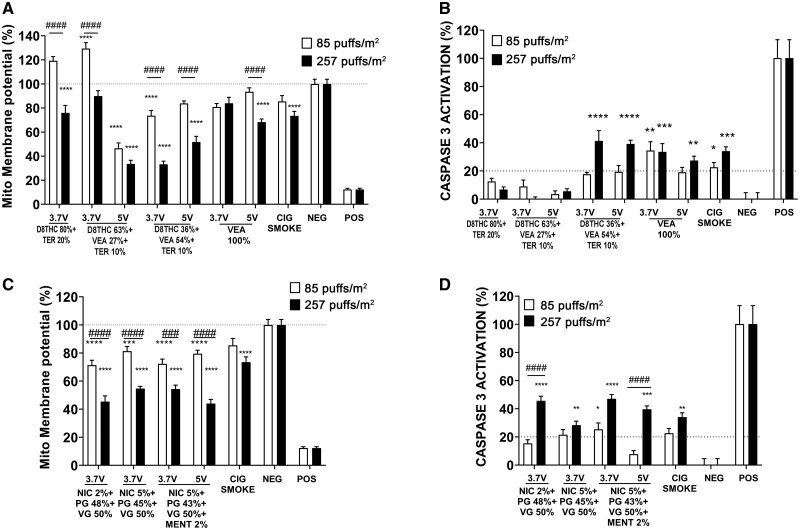
Apoptosis JC1: Caspase-3
To understand better the mechanism of cell damage, the alteration of mitochondrial membrane potential (ΔΨm) and the activation of caspase-3 were measured in Calu-3 epithelial cells. Overall, all the e-cig aerosols induced a dose-dependent loss in ΔΨm and activation of caspase-3 (Figure 7). Specifically, in the Δ8THC group, the most significant mitochondrial perturbation was caused by both doses of VEA 27% at 5 V (−50% and −60% for 85 and 257 puffs/m2, respectively, ****p < .0001) and the high dose of VEA 54% at 3.7 and 5 V (−60% and −50% respectively, ****p < .0001) (Figure 7A). Among the nicotine group, all the high doses induced a significant reduction of ΔΨm (−50%, ****p < .0001), whereas the lower doses only induced a slight decrease in ΔΨm (−20%, ****p < .0001) (Figure 7C).
Figure 7.
High doses of e-cigs aerosols induce the greater mitochondrial membrane potential (ΔΨm) alteration and caspase-3 activation in Calu-3 cells. Acute in vitro assessment of mitochondrial membrane depolarization and apoptosis, using JC-1 die (A, C) and caspase-3 activation (B, D) in human lung epithelial cells (Calu-3) exposed for 24 h to aerosols generated from different concentrations of Δ8THC/VEA or nicotine-based e-cigs at 2 different voltages (3.7 or 5 V) or cigarette smoke, at 2 doses of 85 and 257 puffs/m2 (1 and 3 months). All values are represented as the mean± SE. * p < .05, ** p < .01, *** p < .001, **** p < .0001 compared with negative control. # p < .05, ## p < .01, ### p < .001, #### p < .0001 compare between 2 doses. Statistical analysis was performed on an average of 3 independent biological samples; fold changes >20% were deemed to be statistically significant (p < .05).
The assessment of the caspase-3 activation showed that among the aerosols generated from Δ8THC-based e-liquids, high doses of VEA 54% generated at both operational voltages (3.7 and 5 V) led to an increased caspase-3 activation (approximately 40%, ****p < .0001 compared with negative control) (Figure 7B). Aerosol generated from both doses of VEA 100% at 3.7 V and the high dose of VEA 100% at 5 V also significantly stimulated the activation of caspase-3 (3.7 V: approximately 40%, ****p < .0001; 5 V: approximately 30%, **p < .01 compared with negative control), as well as cigarette smoke (approximately 30%, ***p < .001 compared with negative control) (Figure 7B). Among the cells treated with nicotine-based aerosols, the higher doses induced significant activation of caspase-3 (approximately 40%, ****p < .0001 compared with the negative control, except Nic 5% + PG 45% + VG 50% approximately 25% **p < .001), whereas the lower dose had a very mild effect (approximately 20%, compared with negative control) (Figure 7D).
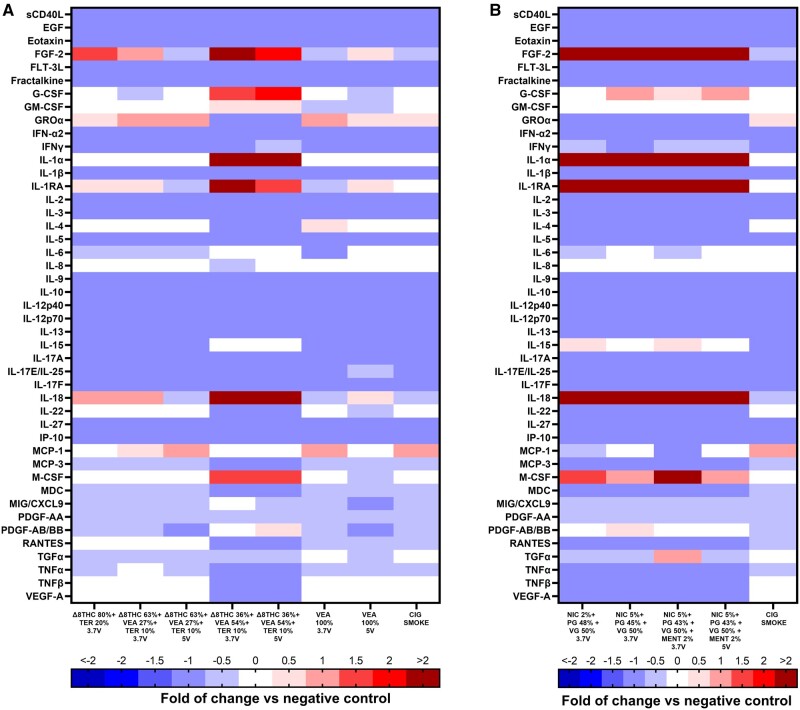
In Vitro Inflammatory Response
Figure 8 and Supplementary Table 4 show the analysis of the cytokines released in the supernatant of the lung epithelial cells exposed to the higher dose of e-cig emissions for 24 h. Δ8THC containing emissions induced a general suppression of pro- and anti-inflammatory markers, including sCDL40, EGF, Eotaxin, FTL-3L, Fractalkine, IFN-α2, IFN-γ, IL-1β, IL-2, -3, -5, -9, -10, -12p40, -12p70, -13, -17A, IL-17E/-25, -17F, -27, IP-10, MCP-3, PDGF-AA, TGF-α, and TNF-α.
Figure 8.
Aerosols generated from Δ8THC 36% + VEA 54% + Ter 10% and Nic 5% + menthol 2% are the most bioactive emission. Measured levels of cytokines and chemokines in the supernatant of Calu-3 exposed for 24 h to aerosols generated from different concentrations of Δ8THC/VEA or nicotine-based e-liquids at 2 different voltages (3.7 or 5 V) or cigarette smoke, at high dose of 257 puffs/m2 (3 months). Statistical analysis was performed on an average of 3 biological samples. Values are presented as fold of change versus negative control (0). For the statistical analysis, please refer to the text or Supplementary Table 4.
In contrast with the general trend, the exposure to VEA 54% at both 3.7 and 5 V did not affect the secretion of IL-6, IL-15, and MCP-1, whereas it induced upregulation of FGF-2 (p < .01 and p < .0001, respectively), G-CSF (both p < .001), IL-1α (both p < .0001), IL-1RA (p < .001 and p < .01, respectively), IL-18 (p < .05 and p < .001, respectively), M-CSF (both p < .001), and PDGF-AB/BB (p < .05 and p < .0001, respectively) and downregulation of GRO-α (both p < .0001), IL-4 (both p < .0001), IL-22 (both p < .0001), MDC (p < .001 and p < .01, respectively), RANTES (both p < .0001), TNF-β (both p < .0001), and VEGF-A (both p < .0001) (Figure 8A). Δ8THC 80% + TER 20% only upregulated FGF-2 (p < .01), GROα (p < .05); VEA 100% aerosol generate at 3.7 and 5 V upregulated FGF-2 (p < .0001, only 5 V), GRO-α (both p < .0001), IL-4 (only 3.7 V p < .05), whereas downregulated IL-6 (p < .0001 only 3.7 V), IL-15 (p < .0001), PDGF-AB/BB (both p < .0001) and RANTES (p < .001 and p < .0001, respectively). VEA 27% at 3.7 and 5 V also increased the release of FGF-2 (only at 3.7 V p < .05), GRO-α (both p < .0001), and MCP-1 (p < .05 at 5 V) (Figure 8A). The only cytokines that were not affected by any treatment were IL-8 and Gm-CSF (Figure 8A). IL-7, MIP-1, and MIP-3 were undetected (data not shown).
Similarly to aerosol generated by Δ8THC containing e-liquid, aerosols from the nicotine based e-liquids also suppressed the release of sCD40L, EGF, Eotaxin, FLT-3L, fractalkine, GRO- α, IFN-α2, IFN-γ, IL-1β, -2, -3, -4, -5, -9, -10, -12p40, -12p70, -13, -17A, 17E/IL-25, 17F, -22, -27, IP-10, MCP-3, MDC, MIG, MIP-1B, PDGF-AA, RANTES, TNF-α, TNF-β, VEGF-A, and stimulated the release of FGF-2, IL-1α, IL-1RA, IL-18, and M-CSF. G-CSF, GM-CSF, IL-8, MCP-1, and PDGF-AB/BB were not affected by any nicotine e-cig aerosol. IL-7 and MIP-1A were not detected (Figure 8B).
The most bioactive emission was Nic 5% + menthol 2% generated at 3.7 V, which in addition to the aforementioned biomarkers, increased the release of IL-15 (p < .001) and TGF-α (p < .0001), and inhibited the secretion of IL-6 (p < .01) (Figure 8B). The aerosols released from Nic 2% also suppressed the release of IL-6 (p < .05) and MCP-1 (p < .01), whereas increasing the secretion of IL-15 (p < .05) (Figure 8B).
DISCUSSION
The main goal of our study was to investigate the physicochemical properties and potential cytotoxicity of aerosols generated from various concentrations and operational voltages of Δ8THC/VEA-based e-liquids, as they have been presumably associated with EVALI, due to their detection in the majority of patients’ samples and vaping products (Blount et al., 2020; Duffy et al., 2020; FDA, 2020; Muthumalage et al., 2020a; Taylor et al., 2019), compared with conventional nicotine e-cigs.
Using a versatile E-cig-EGS, which enables the systematic generation of real-world e-cig exposures, and through the adjustment of operational parameters (such as e-cig brand/type, e-liquid composition, operating voltage, and puffing topography) (Zhao et al., 2016, 2018a,b), this study tested combinations of Δ8THC, VEA, and terpenes at various concentrations derived from EVALI patients’ products and compared them with the conventional MMP concentration (Δ8THC 80% + Terp 20%) (Supplementary Table 1). Because a small proportion of patients reported only nicotine vaping, 4 different combinations of nicotine, diluents (PG: VG), and flavors (menthol) were also analyzed (Supplementary Table 1) as comparators. In addition, because the inspected EVALI cartridges showed burns and marks of high temperature, hence high operational voltage, the e-cigs containing only selected e-liquids combination were operated at 2 different voltages, 3.7 (low) and 5 V (high) (Supplementary Table 1).
Effect of E-Liquid Composition and Voltage on Particles’ Physical Properties
We believe this study to be the first to provide a comprehensive and systematic physical characterization of particles and gases emitted by pyrolysis of Δ8THC + Terp with or without VEA in comparison with those of nicotine-based e-liquids.
The only previous study investigating the particle size distribution of aerosol generated from VEA e-liquid alone showed a variation of tPNC between 107 and 108 particles/cm3, with count median diameter (CMD) range 50–200 nm mostly depending on the puff flow rate and operational voltage: as the flow rate increased, the CMD decreased while the tPNC increased (Mikheev et al., 2020). Although no data are available on the characterization of Δ8THC-based aerosols, the nicotine-based ones showed tPNC variation between 106 and 109 particles/cm3 (Fuoco et al., 2014; Lampos et al., 2019; Zhao et al., 2016), with a CMD range of 24–450 nm (Ingebrethsen et al., 2012; Scungio et al., 2018), and a bimodal (Mikheev et al., 2016; Zhao et al., 2016) or unimodal (Fuoco et al., 2014; Zhang et al., 2013) size distribution, related to different puffing parameters, e-liquid compositions, and e-cigs devices. In agreement with our previous study (Zhao et al., 2016), real-time monitoring data revealed that tPNC emitted from both Δ8THC- and nicotine-based e-liquids was in the range of 106 particles/cm3, and the majority of particles were <500 nm in size, with a single peak approximately 153 nm (coefficient of variation, CV, 27%). Thousands of particles/cm3 were reported in the size range 0.5–20 µm with a single peak approximately 1.30 µm (CV 5%) (Supplementary Table 2 and Figs. 2–5). The subtle variations in the aerosol mean size were e-liquid composition and voltage dependent, however, no significant differences in terms of tPNC or size distribution were noticed between conventional nicotine and Δ8THC aerosols, even in the presence of VEA.
Additionally, the variability in particle mass concentration across all the Δ8THC-based aerosols was in the range 66.61–908.9 mg/m3, due to different e-liquid composition and voltage, where the lower and the higher amount were emitted by Δ8THC 80% + Terp 20% (3.7 V) and VEA 100% (5 V), respectively. Nicotine-based e-liquids generated PM with a mass concentration in the range 239.69–668.48 mg/m3, the minimum and maximum corresponding to Nic 5% + PG 43% + VG 50% + menthol 2% at 5 and 3.7 V, respectively.
Emissions from Δ8THC 36% + VEA 54% + Terp 10% or VEA 100% e-liquids show a PM mass-size distribution similar to the nicotine-based ones, whereas the PM mass concentrations were similar for the Δ8THC/VEA e-liquids (305.78–909.36 mg/m3) and nicotine-based ones (239.69–668.48 mg/m3). The larger mass concentration was reported for the PM0.1–2.5 size fraction regardless of the voltage. This is in agreement with our previous data published for nicotine-based e-liquids (Zhao et al., 2016, 2018a,b). In contrast, emissions from Δ8THC 80% + Terp 20% or Δ8THC 63% + VEA 27% + Terp 10% regardless of the voltage showed a PM concentration (66.6–163.15 mg/m3) and size distribution more evenly distributed between PM0.1 and PM0.1–2.5 size fractions (Supplementary Table 3 and Figure 5). Such variations in the PM mass size distribution and concentration can be attributed to the differences in e-liquid composition and operating voltage.
Recently, Meehan-Atrash et al. investigated the tVOC emission from pure THC or THC/Terpenes 90:10 v/v, generated through 2 different methods, dabbing or CCell cartridges vaporizer, operated at 3 voltages: 3.2, 4.0, or 4.8 V. They reported that tVOCs increased in a voltage-dependent manner, across the 3 voltage vaping conditions (Meehan-Atrash et al., 2019). In our study, tVOC, as well as CO2 and CO, were also voltage- and e-liquid composition dependent without any specific pattern (Figure 3).
The concentration of CO2 varied between 657 and 930 ppm within the Δ8THC group, whereas in the nicotine group varied between 893 and 918 ppm, in a voltage and e-liquid composition-dependent manner (Figure 3). In contrast, the CO concentration was negligible across all the Δ8THC and nicotine atmospheres, even though VEA 100% (5 V) and Δ8THC 63% + VEA 27% + Terp 10% (5 V) reached an average of 46 and 10 ppm, respectively (Figure 3). The World Health Organization (WHO) determined the time-weighted average exposure limits for some CO concentrations so that the carboxyhemoglobin level of 2.5% was not exceeded, consisting in 8 h for 9 ppm CO, 1 h for 26 ppm CO, and 30 min for 52 ppm CO. Hence, besides the fact that e-cigs containing only VEA are not available in the real-world, the combination Δ8THC 63% + VEA 27% + Terp 10% (5 V) releases the higher amount of CO only when the e-liquid is running out, and the temperature is consequently higher, therefore the average of 10 ppm would only apply to a few minutes of vaping and thus likely would not exceed the recommended limit for 8 h.
In summary, the tPNC, PM mass-size distribution, tVOC, and CO2 levels for Δ8THC/VEA e-liquids exhibited a different trend compared with conventional nicotine-based ones, thus suggesting that multiple chemical reactions can take place in the pyrolysis of combinations of e-liquids at various operational voltages, which leads to the release of a mixture of chemicals.
Most likely, the chemical composition will be different as well across the 2 classes of e-liquids. Pyrolysis of the e-liquids is known to generate a complex mixture of organic chemicals and heavy metals, depending on the e-liquid composition and the temperature inside the cartridge which is driven by the applied voltage (Jiang et al., 2020; Ko and Kim, 2022). It is well-known that combustion temperature can alter the size, number concentration, and chemical composition of emitted aerosols and the nature and concentration of gaseous byproducts (Geiss et al., 2016; Ko and Kim, 2022; Lechasseur et al., 2019; Zhao et al., 2018a). This is evident in the case of VEA 100% where the higher voltage (5 V) induces an extremely high concentration of CO, VOC, and particle number/mass concentration compared with the other atmospheres, which is likely driven by the accelerated combustion of pure VEA at the higher temperature produced inside the cartridge. We are currently conducting a detailed chemical analysis of the sampled e-cig aerosols, and it will be reported in a future manuscript.
Effect of E-Liquid Composition and Voltage on Cytotoxicity and Inflammation
In vitro acute toxicological assessment of the emission from Δ8THC and nicotine-based e-liquids was conducted using human lung epithelial cells Calu-3 and human peripheral monocytes THP-1. These cell lines were selected because the EVALI syndrome is characterized by a disruption of the alveolar epithelium, and because the alveolar macrophages represent the first line of defense against particles in the lungs. Both cell lines were exposed to 2 different concentrations of aerosols, 85 and 257 puffs/m2, representative of 1 and 3 months vaping, to assess potential dose-response relationships and mimic the exposure necessary for the development of EVALI (Christiani, 2020; Layden et al., 2020). Emissions from a conventional tobacco cigarette were used as a comparator.
The results showed that most of the emissions from Δ8THC/VEA and nicotine-based e-liquids did not affect viability, metabolic activity, and ROS generation of either Calu-3 epithelial cells or THP-1 monocytes (Figs. 4–6). The only exceptions were the higher dose of aerosol from Δ8THC 36% + VEA 54% + Terp 10% (5 V) and nicotine 5% + PG 43% + VG 50% + menthol 2% that induced a significant increase of LDH release (>20%, p < .05), and a reduction in metabolic activity (p < .0001) in Calu-3 epithelial cells (Figs. 4 and 5), and Δ8THC 36% + VEA 54% + Terp 10% (3.7 and 5 V) and nicotine-based aerosols, that induced a sharp and dose-dependent decrease in metabolic activity and increased ROS generation (p < .0001) in THP-1 monocytes, compared with cigarette smoke and control-treated cells (Figs. 5 and 6). Taken together, this data show that the most bioactive aerosol generated from the Δ8THC/VEA e-liquid group was Δ8THC 36% + VEA 54% + Terp 10% (5 V). However, the cytotoxicity did not increase in Calu-3 epithelial cells when they were challenged with increasing doses of the aerosol, up to 1020 puffs/m2 (12 months), whereas a statistically significant dose-dependent release of LDH was recorded in THP-1 monocytes, accompanied by a decreased metabolic activity only after 257 puff/m2 (3 months) dose exposure (Supplementary Figure 6). Previous investigations on the in vitro cytotoxic effect of aerosols generated from MCT, VEA, CBD/counterfeit vape cartridge, as well as nicotine and-based e-liquids, showed an overall dose- and time-dependent cell death and ROS generation, which were also correlated with e-liquid composition and aerosol generation parameters (puffing protocol, operational voltage) (Husari et al., 2016; Jiang et al., 2020; Lerner et al., 2015; Matsumoto et al., 2020; Muthumalage et al., 2020b; Muthumalage and Rahman, 2019; Rowell et al., 2017; Zhang et al., 2021).
Exposure of epithelial cells to aerosols generated from Δ8THC/VEA e-liquids leads to mitochondrial dysfunction, expressed as a dose-dependent alteration of mitochondrial membrane potential, which did not correspond necessarily to activation of caspase-3 and apoptosis. Among the Δ8THC/VEA-based e-liquids, Δ8THC 36% + VEA 54% + Terp 10% at 3.7 and 5 V (loss ΔΨm > 20%), and Δ8THC 63% + VEA 27% + Terp 10% at 5 V (loss ΔΨm > 20%) induced the higher mitochondrial effect (Figure 7), whereas the activation of caspase 3 was only observed when cells were exposed to high doses of Δ8THC 36% + VEA 54% + Terp 10% aerosol (3.7 and 5 V), and both doses of VEA 100% (3.7 and 5 V) (Figure 7). In comparison, all nicotine-based e-liquids, as well as cigarette smoke, induced a significant dose-dependent alteration of the mitochondrial membrane potential and activation of the apoptosis cascade. This is in agreement with the current literature (Clapp et al., 2019; Correia-Álvarez et al., 2020; Zahedi et al., 2019).
Finally, to better understand the link between the exposure of epithelial cells to aerosols generated from Δ8THC/VEA-based e-liquids and their inflammatory status, we measured the cytokines and chemokines released in the supernatant of epithelial cells exposed to the higher dose for 24 h. The results clarify that among the Δ8THC/VEA group the most bioactive aerosol was Δ8THC 36% + VEA 54% + Terp 10% (3.7 and 5 V), with effects similar to the nicotine group. Although most cytokines and chemokines were suppressed by all aerosols in both groups, the above-mentioned Δ8THC/VEA generated aerosols induced hypersecretion of FGF-2, G-CSF, GM-CSF, IL-1α, IL-1RA, IL-18, MDC, PDGF-AB/BB, and suppression of GRO-α, TNFβ, and VEGF-A (Figure 8).
Previous studies investigating the toxic effect of CBD, diluents, such as PG, VG, MCT, or VEA, or flavorings, such as menthol, cinnamaldehyde, either as e-liquid or aerosolized, showed a varying degree of cytotoxicity, barrier dysfunction, and inflammation both in vitro and in vivo depending on e-liquid composition, puffing protocol, operational voltage, and cell lines (Clapp et al., 2019; Crotty Alexander et al., 2018; Jiang et al., 2020; Leslie et al., 2017; Muthumalage et al., 2019; 2020b; Muthumalage and Rahman, 2019; Park et al., 2022; Phipps et al., 2010; Sundar et al., 2016). Although the most frequent biomarkers of e-cigs aerosol toxicity identified in biological samples are IL-1β, IL-6, IL-8, IL-13 IFN-γ, MCP-1, MCSF, and TNF-α (Ghosh et al., 2019; Park et al., 2022; Scott et al., 2018; Shields et al., 2017; Sundar et al., 2016), clinical studies on acute lung injury have highlighted the critical role in the disease’s development played by pro- and anti-inflammatory cytokines, such as IL-1β, TNF-α, IL-1RA, and MIF, IL-8, found in the human BAL (Goodman et al., 2003). Laboratory findings in EVALI patients also showed elevated inflammatory markers (such as erythrocyte sedimentation rate, c-reactive protein, etc.), but the BAL outcomes found were mostly nonspecific, suggestive of a nonspecific inflammatory process (Blagev et al., 2019; Butt et al., 2019; Layden et al., 2020; Matsumoto et al., 2020; Muthumalage et al., 2020b; Triantafyllou et al., 2019). In the present study, aerosols generated from Δ8THC 36% + VEA 54% + Terp 10% (3.7 and 5 V), but not from Δ8THC/Terp or pure VEA, are as potent as aerosols generated from nicotine-based e-liquids in increasing a limited number of proinflammatory cytokines and suppressing the majority of them, whereas inducing a higher level of cytotoxicity.
This is one of the first studies to assess the in vitro acute toxic and inflammatory effect of aerosols generated from the combination of Δ8THC/VEA e-liquids on human lung epithelial cells and human monocytes aiming to find a causative agent for the development of EVALI. Previous studies focused on the in vitro/in vivo toxicological characterization of either parent e-liquids or aerosols generated from conventional nicotine, CBD alone, or nicotine/THC diluents, such as PG, VG, MCT, or VEA (Bhat et al., 2020; Jiang et al., 2020; Matsumoto et al., 2020; Muthumalage et al., 2020b; Muthumalage and Rahman, 2019) rather than the real-world combinations of the above. Their findings revealed that there is a considerable biological difference between the parent e-liquid and their aerosols and between aerosols generated from individual components and a mixture of e-liquids (Bhat et al., 2020; Jiang et al., 2020; Matsumoto et al., 2020; Muthumalage et al., 2020b; Muthumalage and Rahman, 2019). In agreement with the aforementioned literature, the present study confirms the different toxicological profiles correlated with the e-liquid composition (ie, VEA 100% vs Δ8THC 36% + VEA 54% + Terp 10%), but also with the operational voltage (ie, 3.7 and 5 V). As evident from the real-time aerosol number-size distribution and time-integrated PM mass-size distribution, nearly all of the particles generated by vaping are in the fine particle size (<2.5 µm), which means they are deposited in the deeper bronchioalveolar regions of the respiratory tract, where they could affect a range of adverse pulmonary outcomes depending on their chemical composition (Lechasseur et al., 2019; Phipps et al., 2010; Xing et al., 2016; Zhai et al., 2022).
However, the in vitro cell models and exposure system applied in the present study have many intrinsic limitations: the single-cell monolayer does not account for the cell-to-cell interaction, presence of other stimuli, or underlying conditions, which are commonly present in the context of EVALI (Layden et al., 2020; Taylor et al., 2019); cells exposures in this study were large bolus doses of conditioned media generated with 85 and 257 puffs/m2 of e-cig aerosol for 24 h, this submerged culture model does not completely recapitulate in vivo aerosol exposures, this dose is larger than a typical e-cig user would vape in that same period, and does not take into consideration clearance/repair mechanisms. Air-liquid interface (ALI) exposure systems can also be used to directly expose human airway epithelial cells to aerosols. In such a system, intact aerosol, including gaseous, semivolatile, and particle phases, is directly and intermittently delivered to the apical surface of the lung epithelial cells, mimicking the physiological airways exposure (Lacroix et al., 2018; Li, 2016; Upadhyay and Palmberg, 2018; Zavala et al., 2020). Although the findings of this study are not physiologically relevant compared with ALI or in vivo models, the use of in vitro cellular models, especially in comparative studies, provides useful information on endpoints of interest for understanding the associations between e-liquid composition/operational parameters and potential toxicity mechanisms in the development of EVALI.
In addition, a critical issue recognized in the aerosol collection is the potential loss of the major volatile toxicant contained in the gaseous phase of e-cig aerosols during bubbling, and the physicochemical alteration of toxicants due to reaction with media during the sampling process and in the timeframe between sampling and cell exposure. Moreover, Calu-3 human epithelial cells, used in place of primary human airways epithelial cells, are derived from a nonsmall-cell lung cancer cell line, were chosen for the initial and preliminary toxicological characterization of Δ8THC/VEA-derived aerosols, due to the rapid polarization in a stable culture that resembles the human lung epithelium-derived cells. They have also been extensively used in the toxicological assessment of e-cigs aerosol (Leslie et al., 2017; Rowell et al., 2017; Zhang et al., 2021).
Although this preliminary study focuses on the analysis of aerosols generated from “real-world” concentrations of Δ8THC/VEA/terpenes, including more controls, like Δ8THC alone, terpene alone, menthol alone, and PG/VG alone will be beneficial in the future for a more detailed physical, chemical, and toxicological characterization.
Other limitations in our study include the lack of deeper investigation on the role of macrophages in the development, progression, and resolution of the aggressive inflammatory process in EVALI. The involvement of both alveolar and interstitial macrophages in the EVALI syndrome is particularly important because they play a key role in the recognition and elimination of pathogens in the early stage (Higgins et al., 2008), and in the resolution of inflammation, regulation of fibroproliferative response, and wound healing in the late stage of disease (Bellingan, 2002; Gordon, 2003; Huang et al., 2018). Moreover, lipid-laden macrophages in the alveolar space were a common feature of the affected individuals (Christiani, 2020; Jonas, 2020).
These limitations are due to the fact that the present study focuses more on the human lung epithelial cells, as they represent a physical barrier that is disrupted by e-cigs aerosols in the EVALI syndrome. In this regard, it is crucial to evaluate the integrity of epithelial cell junctions, hence the epithelial barrier dysfunction and permeability alteration, which in turn aggravates the inflammatory response, and facilitates the recruitment of the immune cells (Crotty Alexander et al., 2018; Muthumalage et al., 2019). Because our data show suppression of most cytokines following both Δ8THC and nicotine e-cig aerosols exposure, further studies using a more relevant cellular model are warranted to understand the mechanism of the immune modulation.
Furthermore, RNA-sequencing transcriptome analysis of the Calu-3 epithelial cells exposed to the e-cig aerosols is underway and we will report any mechanistic findings in a future manuscript.
CONCLUSION
This is one of the first studies to provide a comprehensive and systematic physicochemical and toxicological characterization of aerosols emitted from Δ8THC and VEA-based e-liquids, as they have been associated with the development of EVALI in the young healthy population in the United States in 2019–2020. The E-cig-EGS platform was used to generate real-world e-cig exposures for real-time monitoring and physicochemical and toxicological characterization of aerosols generated from combinations of different concentrations of Δ8THC + Terp with or without VEA and VEA 100% at 2 operational voltages (3.7 and 5 V). Conventional nicotine-based e-liquids were also used as comparators. The main finding of the study is that e-liquid composition and operational voltage influenced the physicochemical properties of each aerosol (tPNC and size distribution, particulate mass-size distribution, release of VOC, and gaseous pollutants), as well as their biological and inflammatory effects (cell death, mitochondrial dysfunction, apoptosis, cytokines). Overall, the comparison between Δ8THC and nicotine e-liquid groups did not show particular differences, such that it was not possible to find a strong correlation between exposure to a specific aerosol generated from the Δ8THC/VEA group and the development of EVALI. Further organic chemical speciation of the aerosols is needed to identify chemical differences between aerosols generated between Δ8THC/VEA and nicotine-based e-liquid groups. However, the present study supports the concept that there was a significant difference between the individual e-liquid component (ie, VEA 100%) and the e-liquid mixture (ie, Δ8THC + VEA + Terp), in terms of both physicochemical characteristics and bioactivity. Therefore, it is fundamental, in the future, to avoid focusing on the toxicity of parent e-liquids or aerosols generated from individual diluents, as they do not reflect the real physicochemical exposure. The combination of chemicals can generate a mixture of toxicants whose synergistic actions can drive bioactivity and EVALI. Further comprehensive in vitro/in vivo inhalation studies, based also on a proteomic, genomic, and metabolomic approach, are necessary to validate the in vitro toxicological effects and to address the specific cascade of events that leads to diffuse alveolar damage and respiratory failure in EVALI patients. Findings from this study will help risk assessors, and public health and governmental agencies, for the development of Δ8THC and nicotine e-cig regulations, to limit the epidemic of e-cigs in the young population and prevent new cases of EVALI due to the incorporation of unregulated chemicals in e-cigs.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
This investigation was made possible by the T32 grant no. HL007118 from NIH. The cytokine/chemokines analysis was made possible by grant no. P30ES000002 from the Integrated Health Sciences Facility (IHSFC) of the Harvard NIEHS Center for Environmental Health. The e-cigs cartridges and Δ8THC e-liquids were kindly provided by Jeff Rawson PhD, Postdoctoral Fellow from Prof. George M. Whitesides Laboratory, Department of Chemistry and Chemical Biology, Harvard University.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary Material
Contributor Information
Antonella Marrocco, Department of Environmental Health, Center for Nanotechnology and Nanotoxicology, Harvard T.H. Chan School of Public Health, Harvard University, Boston, Massachusetts 02115, USA.
Dilpreet Singh, Department of Environmental Health, Center for Nanotechnology and Nanotoxicology, Harvard T.H. Chan School of Public Health, Harvard University, Boston, Massachusetts 02115, USA.
David C Christiani, Department of Environmental Health, Center for Nanotechnology and Nanotoxicology, Harvard T.H. Chan School of Public Health, Harvard University, Boston, Massachusetts 02115, USA.
Philip Demokritou, Department of Environmental Health, Center for Nanotechnology and Nanotoxicology, Harvard T.H. Chan School of Public Health, Harvard University, Boston, Massachusetts 02115, USA; Department of Environmental and Population Health Bio-Sciences, Environmental Occupational Health Sciences Institute, School of Public Health, Rutgers University, Piscataway, New Jersey 08854, USA.
REFERENCES
- Bellingan G. J. (2002). The pulmonary physician in critical care 6: The pathogenesis of ALI/ARDS. Thorax 57, 540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat T. A., Kalathil S. G., Bogner P. N., Blount B. C., Goniewicz M. L., Thanavala Y. M. (2020). An animal model of inhaled vitamin E acetate and EVALI-like lung injury. N. Engl. J. Med. 382, 1175–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagev D. P., Harris D., Dunn A. C., Guidry D. W., Grissom C. K., Lanspa M. J. (2019). Clinical presentation, treatment, and short-term outcomes of lung injury associated with e-cigarettes or vaping: A prospective observational cohort study. Lancet 394, 2073–2083. [DOI] [PubMed] [Google Scholar]
- Blount B. C., Karwowski M. P., Morel-Espinosa M., Rees J., Sosnoff C., Cowan E., Gardner M., Wang L., Valentin-Blasini L., Silva L., et al. (2019). Evaluation of bronchoalveolar lavage fluid from patients in an outbreak of e-cigarette, or vaping, product use-associated lung injury - 10 states, August-October 2019. MMWR Morb. Mortal. Wkly. Rep. 68, 1040–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount B. C., Karwowski M. P., Shields P. G., Morel-Espinosa M., Valentin-Blasini L., Gardner M., Braselton M., Brosius C. R., Caron K. T., Chambers D., Lung Injury Response Laboratory Working Group, et al. (2020). Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N. Engl. J. Med. 382, 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt Y. M., Smith M. L., Tazelaar H. D., Vaszar L. T., Swanson K. L., Cecchini M. J., Boland J. M., Bois M. C., Boyum J. H., Froemming A. T., et al. (2019). Pathology of vaping-associated lung injury. N. Engl. J. Med. 381, 1780–1781. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2019). For state, local, territorial, and tribal health departments. Electronic cigarettes. Smoking & tobacco use. 2019 lung injury surveillance primary case definition (CDC), September 18, 2019. Available at: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease/health-departments/index.html.
- Centers for Disease Control and Prevention (CDC). (2020). Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Available at: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html. Accessed November 27, 2020.
- CDC U.S. Department of Health and Human Services. (2016). E-cigarette use among youth and young adults: A report of the surgeon general. Atlanta, GA.
- Christiani D. C. (2020). Vaping-induced lung injury. N. Eng. J. Med. 382, 960–962. [DOI] [PubMed] [Google Scholar]
- Clapp P. W., Lavrich K. S., van Heusden C. A., Lazarowski E. R., Carson J. L., Jaspers I. (2019). Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am. J. Physiol. Lung Cell. Mol. Physiol.237 316, L470–L486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia-Álvarez E., Keating J. E., Glish G., Tarran R., Sassano M. F. (2020). Reactive oxygen species, mitochondrial membrane potential, and cellular membrane potential are predictors of e-liquid induced cellular toxicity. Nicotine Tob. Res. 22, S4–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty Alexander L. E., Drummond C. A., Hepokoski M., Mathew D., Moshensky A., Willeford A., Das S., Singh P., Yong Z., Lee J. H., et al. (2018). Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 314, R834–R847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demokritou P., Lee S. J., Ferguson S. T., Koutrakis P. (2004). A compact multistage (cascade) impactor for the characterization of atmospheric aerosols. J. Aerosol Sci. 35, 281–299. [Google Scholar]
- Duffy B., Li L., Lu S., Durocher L., Dittmar M., Delaney-Baldwin E., Panawennage D., LeMaster D., Navarette K., Spink D. (2020). Analysis of cannabinoid-containing fluids in illicit vaping cartridges recovered from pulmonary injury patients: Identification of vitamin E acetate as a major diluent. Toxics 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter J. F., Bullen C. (2011). Electronic cigarette: Users profile, utilization, satisfaction and perceived efficacy. Addiction 106, 2017–2028. [DOI] [PubMed] [Google Scholar]
- Farsalinos K. E., Romagna G., Allifranchini E., Ripamonti E., Bocchietto E., Todeschi S., Tsiapras D., Kyrzopoulos S., Voudris V. (2013a). Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int. J. Environ. Res. Public Health 10, 5146–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K. E., Romagna G., Tsiapras D., Kyrzopoulos S., Voudris V. (2013b). Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: Implications for research protocol standards definition and for public health authorities' regulation. Int. J. Environ. Res. Public Health 10, 2500–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. (2020). Lung illnesses associated with use of vaping products. Available at: https://www.fda.gov/news-events/public-health-focus/lung-injuries-associated-use-vaping-products.
- Fuoco F. C., Buonanno G., Stabile L., Vigo P. (2014). Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ. Pollut. 184, 523–529. [DOI] [PubMed] [Google Scholar]
- Geiss O., Bianchi I., Barrero-Moreno J. (2016). Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int. J. Hyg. Environ. Health 219, 268–277. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Coakley R. D., Ghio A. J., Muhlebach M. S., Esther C. R. Jr, Alexis N. E., Tarran R. (2019). Chronic e-cigarette use increases neutrophil elastase and matrix metalloprotease levels in the lung. Am. J. Resp. Crit. Care Med. 200, 1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. B., Pugin J., Lee J. S., Matthay M. A. (2003). Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 14, 523–535. [DOI] [PubMed] [Google Scholar]
- Gordon S. (2003). Alternative activation of macrophages. Nat. Rev. Immunol. 3, 23–35. [DOI] [PubMed] [Google Scholar]
- Higgins D. M., Sanchez-Campillo J., Rosas-Taraco A. G., Higgins J. R., Lee E. J., Orme I. M., Gonzalez-Juarrero M. (2008). Relative levels of M-CSF and GM-CSF influence the specific generation of macrophage populations during infection with mycobacterium tuberculosis. J. Immunol. 180, 4892–4900. [DOI] [PubMed] [Google Scholar]
- Huang X., Xiu H., Zhang S., Zhang G. (2018). The role of macrophages in the pathogenesis of ALI/ARDS. Mediators Inflamm. 2018, 1264913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husari A., Shihadeh A., Talih S., Hashem Y., El Sabban M., Zaatari G. (2016). Acute exposure to electronic and combustible cigarette aerosols: Effects in an animal model and in human alveolar cells. Nicotine Tob Res. 18, 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebrethsen B. J., Cole S. K., Alderman S. L. (2012). Electronic cigarette aerosol particle size distribution measurements. Inhal. Toxicol. 24, 976–984. [DOI] [PubMed] [Google Scholar]
- Invernizzi G., Ruprecht A., De Marco C., Paredi P., Boffi R. (2007). Residual tobacco smoke: Measurement of its washout time in the lung and of its contribution to environmental tobacco smoke. Tob. Control. 16, 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Ahmed C. M. S., Martin T. J., Canchola A., Oswald I. W. H., Garcia J. A., Chen J. Y., Koby K. A., Buchanan A. J., Zhao Z., et al. (2020). Chemical and toxicological characterization of vaping emission products from commonly used vape juice diluents. Chem. Res. Toxicol. 33, 2157–2163. [DOI] [PubMed] [Google Scholar]
- Jonas A. (2020). Lipid-laden alveolar macrophages and vaping: Lessons from EVALI. EBioMedicine 60, 103010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko T. J., Kim S. A. (2022). Effect of heating on physicochemical property of aerosols during vaping. Int. J. Environ. Res. Public Health 19, 1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider L., Jackson A., Leigh N., O’Connor R., Goniewicz M. L. (2018). Circadian puffing behavior and topography among e-cigarette users. Tob. Regul. Sci. 4, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix G., Koch W., Ritter D., Gutleb A. C., Larsen S. T., Loret T., Zanetti F., Constant S., Chortarea S., Rothen-Rutishauser B., et al. (2018). Air–liquid interface in vitro models for respiratory toxicology research: Consensus workshop and recommendations. Appl. In Vitro Toxicol. 4, 91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampos S., Kostenidou E., Farsalinos K., Zagoriti Z., Ntoukas A., Dalamarinis K., Savranakis P., Lagoumintzis G., Poulas K. (2019). Real-time assessment of e-cigarettes and conventional cigarettes emissions: Aerosol size distributions, mass and number concentrations. Toxics 7, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzarotta A., Falconer T. M., Flurer R., Wilson R. A. (2020). Hydrogen bonding between tetrahydrocannabinol and vitamin E acetate in unvaped, aerosolized, and condensed aerosol e-liquids. Anal. Chem. 92, 2374–2378. [DOI] [PubMed] [Google Scholar]
- Layden J. E., Ghinai I., Pray I., Kimball A., Layer M., Tenforde M. W., Navon L., Hoots B., Salvatore P. P., Elderbrook M., et al. (2020). Pulmonary illness related to e-cigarette use in Illinois and Wisconsin - final report. N. Engl. J. Med. 382, 903–916. [DOI] [PubMed] [Google Scholar]
- Lechasseur A., Altmejd S., Turgeon N., Buonanno G., Morawska L., Brunet D., Duchaine C., Morissette M. C. (2019). Variations in coil temperature/power and e‐liquid constituents change size and lung deposition of particles emitted by an electronic cigarette. Physiol. Rep. 7, e14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner C. A., Sundar I. K., Yao H., Gerloff J., Ossip D. J., McIntosh S., Robinson R., Rahman I. (2015). Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 10, e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie L. J., Vasanthi Bathrinarayanan P., Jackson P., Mabiala Ma Muanda J. A., Pallett R., Stillman C. J., Marshall L. J. (2017). A comparative study of electronic cigarette vapor extracts on airway-related cell lines in vitro. Inhal. Toxicol. 29, 126–136. [DOI] [PubMed] [Google Scholar]
- Li X. (2016). In vitro toxicity testing of cigarette smoke based on the air-liquid interface exposure: A review. Toxicol. In Vitro 36, 105–113. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Fang X., Traber M. G., Jones K. D., Langelier C., Hayakawa Serpa P., Calfee C. S., Matthay M. A., Gotts J. E. (2020). Dose-dependent pulmonary toxicity of aerosolized vitamin E acetate. Am. J. Respir. Cell Mol. Biol. 63, 748–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan-Atrash J., Luo W., McWhirter K. J., Strongin R. M. (2019). Aerosol gas-phase components from cannabis e-cigarettes and dabbing: Mechanistic insight and quantitative risk analysis. ACS Omega 4, 16111–16120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheev V. B., Brinkman M. C., Granville C. A., Gordon S. M., Clark P. I. (2016). Real-time measurement of electronic cigarette aerosol size distribution and metals content analysis. Nicotine Tob. Res. 18, 1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheev V. B., Klupinski T. P., Ivanov A., Lucas E. A., Strozier E. D., Fix C. (2020). Particle size distribution and chemical composition of the aerosolized vitamin E acetate. Aerosol. Sci. Technol. 54, 993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumalage T., Friedman M. R., McGraw M. D., Ginsberg G., Friedman A. E., Rahman I. (2020a). Chemical constituents involved in e-cigarette, or vaping product use-associated lung injury (EVALI). Toxics 8, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumalage T., Lamb T., Friedman M. R., Rahman I. (2019). E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci. Rep. 9, 19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumalage T., Lucas J. H., Wang Q., Lamb T., McGraw M. D., Rahman I. (2020b). Pulmonary toxicity and inflammatory response of e-cigarette vape cartridges containing medium-chain triglycerides oil and vitamin E acetate: Implications in the pathogenesis of EVALI. Toxics 8, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumalage T., Rahman I. (2019). Cannabidiol differentially regulates basal and lps-induced inflammatory responses in macrophages, lung epithelial cells, and fibroblasts. Toxicol. Appl. Pharmacol. 382, 114713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIDA. (2021, September 20). CDC health advisory: Increases in availability of cannabis products containing delta-8 THC and reported cases of adverse events. Available at: https://nida.Nih.Gov/news-events/emerging-trend/cdc-health-advisory-increases-in-availability-cannabis-products-containing-delta-8-thc-reported. Accessed March 4, 2022.
- Pal A. K., Watson C. Y., Pirela S. V., Singh D., Chalbot M. C., Kavouras I., Demokritou P. (2015). Linking exposures of particles released from nano-enabled products to toxicology: An integrated methodology for particle sampling, extraction, dispersion, and dosing. Toxicol. Sci. 146, 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. A., Crotty Alexander L. E., Christiani D. C. (2022). Vaping and lung inflammation and injury. Annu. Rev. Physiol. 84, 611–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps J. C., Aronoff D. M., Curtis J. L., Goel D., O'Brien E., Mancuso P. (2010). Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of Streptococcus pneumoniae. Inf. Immun. 78, 1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell T. R., Reeber S. L., Lee S. L., Harris R. A., Nethery R. C., Herring A. H., Glish G. L., Tarran R. (2017). Flavored e-cigarette liquids reduce proliferation and viability in the Calu3 airway epithelial cell line. Am. J. Physiol. Lung Cell. Mol. Physiol. 313, L52–L66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A., Lugg S. T., Aldridge K., Lewis K. E., Bowden A., Mahida R. Y., Grudzinska F. S., Dosanjh D., Parekh D., Foronjy R., et al. (2018). Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax 73, 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scungio M., Stabile L., Buonanno G. (2018). Measurements of electronic cigarette-generated particles for the evaluation of lung cancer risk of active and passive users. J. Aerosol. Sci. 115, 1–11. [Google Scholar]
- Shields P. G., Berman M., Brasky T. M., Freudenheim J. L., Mathe E., McElroy J. P., Song M.-A., Wewers M. D. (2017). A review of pulmonary toxicity of electronic cigarettes in the context of smoking: A focus on inflammation. Cancer Epidemiol. Prevent. Biomark 26, 1175–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D., Marrocco A., Wohlleben W., Park H.-R., Diwadkar A. R., Himes B. E., Lu Q., Christiani D. C., Demokritou P. (2022). Release of particulate matter from nano-enabled building materials (NEBMS) across their lifecycle: Potential occupational health and safety implications. J. Haz. Mat. 422, 126771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar I. K., Javed F., Romanos G. E., Rahman I. (2016). E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget 7, 77196–77204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackett A. P., Lechner W. V., Meier E., Grant D. M., Driskill L. M., Tahirkheli N. N., Wagener T. L. (2015). Biochemically verified smoking cessation and vaping beliefs among vape store customers. Addiction 110, 868–874. [DOI] [PubMed] [Google Scholar]
- Taylor J., Wiens T., Peterson J., Saravia S., Lunda M., Hanson K., Wogen M., D'Heilly P., Margetta J., Bye M., et al. ; Lung Injury Response Task Force. (2019). Characteristics of e-cigarette, or vaping, products used by patients with associated lung injury and products seized by law enforcement - Minnesota, 2018 and 2019. MMWR Morb. Mortal. Wkly. Rep. 68, 1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafyllou G. A., Tiberio P. J., Zou R. H., Lamberty P. E., Lynch M. J., Kreit J. W., Gladwin M. T., Morris A., Chiarchiaro J. (2019). Vaping-associated acute lung injury: A case series. Am. J. Respir. Crit. Care Med. 200, 1430–1431. [DOI] [PubMed] [Google Scholar]
- Upadhyay S., Palmberg L. (2018). Air-liquid interface: Relevant in vitro models for investigating air pollutant-induced pulmonary toxicity. Toxicol. Sci. 164, 21–30. [DOI] [PubMed] [Google Scholar]
- Xing Y. F., Xu Y. H., Shi M. H., Lian Y. X. (2016). The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 8, E69–E74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi A., Phandthong R., Chaili A., Leung S., Omaiye E., Talbot P. (2019). Mitochondrial stress response in neural stem cells exposed to electronic cigarettes. iScience 16, 250–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala J., Freedman A. N., Szilagyi J. T., Jaspers I., Wambaugh J. F., Higuchi M., Rager J. E. (2020). New approach methods to evaluate health risks of air pollutants: Critical design considerations for in vitro exposure testing. Int. J. Environ. Res. Public Health 17, 2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai X., Wang J., Sun J., Xin L. (2022). PM(2.5) induces inflammatory responses via oxidative stress-mediated mitophagy in human bronchial epithelial cells. Toxicol. Res. 11, 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Jones M. M., Dornsife R. E., Wu T., Sivaraman V., Tarran R., Onyenwoke R. U. (2021). JUUL e-liquid exposure elicits cytoplasmic Ca2+ responses and leads to cytotoxicity in cultured airway epithelial cells. Toxicol. Lett. 337, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Sumner W., Chen D. R. (2013). In vitro particle size distributions in electronic and conventional cigarette aerosols suggest comparable deposition patterns. Nicotine Tob. Res. 15, 501–508. [DOI] [PubMed] [Google Scholar]
- Zhao J., Nelson J., Dada O., Pyrgiotakis G., Kavouras I. G., Demokritou P. (2018a). Assessing electronic cigarette emissions: Linking physico-chemical properties to product brand, e-liquid flavoring additives, operational voltage and user puffing patterns. Inhal. Toxicol. 30, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Pyrgiotakis G., Demokritou P. (2016). Development and characterization of electronic-cigarette exposure generation system (Ecig-EGS) for the physico-chemical and toxicological assessment of electronic cigarette emissions. Inhal. Toxicol. 28, 658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Zhang Y., Sisler J. D., Shaffer J., Leonard S. S., Morris A. M., Qian Y., Bello D., Demokritou P. (2018b). Assessment of reactive oxygen species generated by electronic cigarettes using acellular and cellular approaches. J. Hazard. Mater. 344, 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.