Abstract
Background
Ketamine has emerged as a fast-acting and powerful antidepressant, but no head to head trial has been performed, Here, ketamine is compared with electroconvulsive therapy (ECT), the most effective therapy for depression.
Methods
Hospitalized patients with unipolar depression were randomized (1:1) to thrice-weekly racemic ketamine (0.5 mg/kg) infusions or ECT in a parallel, open-label, non-inferiority study. The primary outcome was remission (Montgomery Åsberg Depression Rating Scale score ≤10). Secondary outcomes included adverse events (AEs), time to remission, and relapse. Treatment sessions (maximum of 12) were administered until remission or maximal effect was achieved. Remitters were followed for 12 months after the final treatment session.
Results
In total 186 inpatients were included and received treatment. Among patients receiving ECT, 63% remitted compared with 46% receiving ketamine infusions (P = .026; difference 95% CI 2%, 30%). Both ketamine and ECT required a median of 6 treatment sessions to induce remission. Distinct AEs were associated with each treatment. Serious and long-lasting AEs, including cases of persisting amnesia, were more common with ECT, while treatment-emergent AEs led to more dropouts in the ketamine group. Among remitters, 70% and 63%, with 57 and 61 median days in remission, relapsed within 12 months in the ketamine and ECT groups, respectively (P = .52).
Conclusion
Remission and cumulative symptom reduction following multiple racemic ketamine infusions in severely ill patients (age 18–85 years) in an authentic clinical setting suggest that ketamine, despite being inferior to ECT, can be a safe and valuable tool in treating unipolar depression.
Keywords: Electroconvulsive therapy, ketamine infusion, major depressive disorder, psychotic depression, racemic ketamine
Significance Statement.
The current trial is the first, to our knowledge, randomized clinical trial with an adequate sample size that compares ketamine with another active antidepressant treatment.
Electroconvulsive therapy (ECT) is superior to racemic ketamine infusions both in terms of higher remission rates and greater reduction of depressive symptoms in hospitalized patients suffering from depression.
Even in the absence of fast-onset antidepressant effects, remission can be attained if multiple ketamine infusions are administered.
A similar proportion of patients remitting following ketamine infusions or ECT relapsed during a 12-month follow-up period.
Racemic ketamine is safe and effective for treating depressions, including depressions with psychotic features.
Older patients seem to be less responsive to ketamine, whereas younger patients seem to respond as well to ketamine as to ECT.
Introduction
Depression is a debilitating disorder affecting millions globally (Kessler and Bromet 2013), causing severe suffering and reduced life expectancy due to associated somatic co-morbidities and elevated suicide risk (Laursen et al., 2016). The burden on society is immense, and depression is a leading contributor to disability-adjusted life years(DALYs et al., 2015). The depressive state involves many brain systems, but antidepressants in use mainly target monoaminergic systems. As many as 30% of patients respond unsatisfactorily to current antidepressant regimes(Rush et al., 2006). Ketamine is a safe and well-studied anaesthetic and analgesic drug antagonistic at the N-methyl-D-aspartate receptor. The reports of a glutamate-modulating drug that alleviated depressive symptoms, causing patients to remit within hours after 1 i.v. subanaesthetic dose, received enormous attention(Berman et al., 2000; Zarate et al., 2006). However, side-effects of ketamine treatment in depressed patients, especially long-term effects, are still only partially known, and concerns for the safety of ketamine as an antidepressant treatment have been raised. Acute, transient side-effects are common, including dizziness, dissociation, hypertension, anxiety, and cognitive changes. There are also reports of urological and hepatic toxicity in recreational users and patients treated for pain with repeated high doses(Short et al., 2018). More importantly, there are reasons to believe that ketamine has addictive properties with risk for tolerance and dependency(Short et al., 2018). Depression has traditionally not been viewed as a condition that can be remedied quickly, and the possibility of a rapid therapeutic onset was a paradigm shift. However, published clinical trials of racemic ketamine have been small, with short follow-up times; in addition, multiple ketamine infusions(Caddy et al., 2015) have rarely been used, and no trial with a sufficient sample size using an active comparator has been conducted. Electroconvulsive therapy (ECT) is the most effective antidepressant therapy in use for almost a century. It acts via a controlled epileptic seizure elicited through a current delivered via electrodes on the skull. ECT has a faster therapeutic onset than antidepressant drugs. Even so, a number of treatment sessions are required before remission is achieved(Ferrier 2019). Accessibility to ECT varies and can be restricted by lacking resources. Unbalanced depictions in popular culture have created a negative but largely erroneous public image of ECT. Some patients are reluctant to accept ECT over concerns about cognitive side effects, which are common and can be disturbing but are rarely persistent(Semkovska and McLoughlin 2010). Given the remarkable claims of the efficacy of ketamine, in particular its rapid onset, a head to head comparison with ECT is warranted. In the first (to our knowledge) trial using an active control, we compare the efficacy of multiple racemic ketamine infusions with standard ECT in hospitalized patients with unipolar depression.
METHODS
Study Design
The KetECT trial was a randomized, parallel, open-label multicenter (6 clinics) study testing the hypothesis that ketamine is non-inferior to ECT in antidepressant efficacy. A non-inferiority design was chosen because we found it improbable that ketamine would be more effective than ECT but wanted to compare it with the most effective treatment. Assuming fewer cognitive side effects and faster onset and considering that ketamine treatment can be administered without anaesthesia, ketamine could be regarded as a relevant treatment alternative with even lower remission rates than ECT. Given a remission rate of 60% for ECT(Kellner et al., 2010), the non-inferiority margin for ketamine was set at 40%. We planned for 97 patients per group, a significance level of 5%, and a power of 80%. If ECT actually resulted in remission in 60% of cases, a remission rate of 54% following ketamine would be necessary to ascertain with 95% confidence that the remission rate with ketamine was not <40%. The trial was approved by The Swedish Ethical Board in Lund (Dnr 2011/796), and the Swedish Medical Products Agency, Uppsala, Sweden, and registered at the registry of the European Medical Agency, EudraCT (2011-001520-37), and clinicaltrials.gov (NCT02659085). Each participant provided oral and written informed consent.
Patients and Randomization
We screened hospitalized patients scheduled for ECT at the participating university hospitals. Hospitalized patients, aged 18–85 years, diagnosed with unipolar depression according to the DSM-IV with a score of ≥20 on the Montgomery–Åsberg Depression Rating Scale (MADRS)(Montgomery and Asberg 1979) were informed about an ongoing study comparing ECT with a new experimental treatment. Patients received oral and written information and were encouraged to involve relatives or friends in the decision to take part in the study. No patients were recruited through referrals or advertisements.
After obtaining written consent, a sequentially numbered sealed envelope was opened to reveal treatment allocation. The randomization scheme (1:1, block size 6 per site) was developed by a statistician not otherwise involved in the study. Inclusion and exclusion criteria are listed in supplementary Table 1 in the Appendix.
Treatments
Ketamine was administered i.v. at a fixed dose of 0.5 mg/kg over 40 minutes. ECT patients were oxygenated and anesthetized and obtained muscle relaxants according to clinical routines and stimulated based on age and sex (for ECT parameters see supplementary Table 2, Appendix). All patients fasted overnight and received treatments thrice weekly in the morning. Concomitant medications were unrestricted. However, drug adjustments followed the same procedures as for ECT for all patients. Depression severity was evaluated with MADRS at baseline, 4–5 hours after the first treatment session, the day after each subsequent treatment session, and at follow-ups. Treatment sessions (maximum 12) were administered until remission or maximal antidepressant effect (as judged by the treating psychiatrist) was achieved. When the study was designed, little data was available on the effectiveness of multiple ketamine infusions. We wanted to avoid prolonged individual patient suffering by exposure to a potentially ineffective treatment (ketamine) but ensure that ECT recipients received an adequate number of treatment sessions. Symptom reduction after 6 ECT treatment sessions strongly predicts remission rates(Husain et al., 2004). Based on this, we set a checkpoint after 6 treatment sessions, where patients with <25% reduction of their MADRS baseline score were classified as non-responders and received no further treatment sessions as study participants.
Outcomes
The primary outcome was remission after completed treatment. Remission was defined as a MADRS score ≤10 persisting over at least 2 subsequent treatment sessions or a minimum of 5 days. Temporary MADRS scores ≤10 succeeded by higher scores was not classified as remission. Participants were classified as responders if MADRS scores decreased by at least 50% following a complete treatment series. A complete treatment series ranged from between 1 and 12 treatment sessions.
As this was an open label study, neither raters nor patients were blinded. Raters went through a training session with educational video material of a clinical interview followed by discussions to standardize rating procedures. Secondary outcomes included changes in MADRS, total number of sessions and number of sessions to remission, relapse rate, and adverse events. Patients were followed up 1 week and 3, 6, and 12 months after completed treatment. Relapse was defined as when a patient was considered to meet the criteria for depression. The occurrence of and time to relapse were determined based on patient information at follow-ups and confirmed, if needed, by medical records. Adverse events (AEs) were monitored throughout the treatment and at follow-ups and assessed based on the investigator’s clinical judgements. In conjunction with MADRS ratings, patients listed all experienced AEs irrespective of the perceived connection to the treatment. Causality to the received treatment was classified as very likely, probable, possible, unlikely, or not related by the investigator. AEs classified as very likely or probable were included in the analysis.
To quantify treatment-emergent psychotic or dissociative symptoms, we used a modified Brief Psychiatric Rating Scale (Overall JE, 1962), comprising items 7–11, 14–15, 20, 22–24, and the Clinician Administered Dissociative States Scale (Bremner et al., 1998). Ratings were done at baseline, 1 hour, and 4–5 hours, the day after the first treatment, and after 6 sessions.
After randomization, before receiving any treatment, patients expressed expectations and fears regarding treatment outcome using a Visual Analogue Scale.
Outcomes not specified in the study protocol are post-hoc analyses of remission rates in patients with psychotic depression and analyses of the interaction of age group, sex, and site with treatment. Changes in concomitant medications association with remission rates was also analyzed. A 2-member independent safety committee reviewed data from the first 33 patients to determine trial continuation or early termination. An external monitor reviewed data documentation and study protocol adherence at regular intervals.
Statistical Analysis
The main analyses were based on all patients who received at least 1 treatment. Additionally, an intention-to-treat analysis that included all randomized patients was performed regarding the primary outcome. Proportions were compared using chi-square tests. One-way ANOVA was used to compare Visual Analogue Scale scores for expectations and fears. T-tests were used to compare MADRS scores and number of treatment sessions. A Kaplan-Meier survival analysis was used to illustrate time to relapse. A binary logistic regression with treatment, age group (18–50 years, 51–85 years), sex, and site as cofactors, and the interaction of the latter 3 with treatment, evaluated their impact on the likelihood of remitting. Statistical analyses were conducted using SPSS 26.0 (IBM, Armonk, New York, USA, 2019). The 95% confidence interval of the difference between remission rates was calculated according to Newcombe(Newcombe, 1998). Effects sizes were calculated as Cohen d or Cohen dRM for comparisons between or within groups, respectively.
Role of the Funding Sources
The funding sources had no role in any aspect of the study.
RESULTS
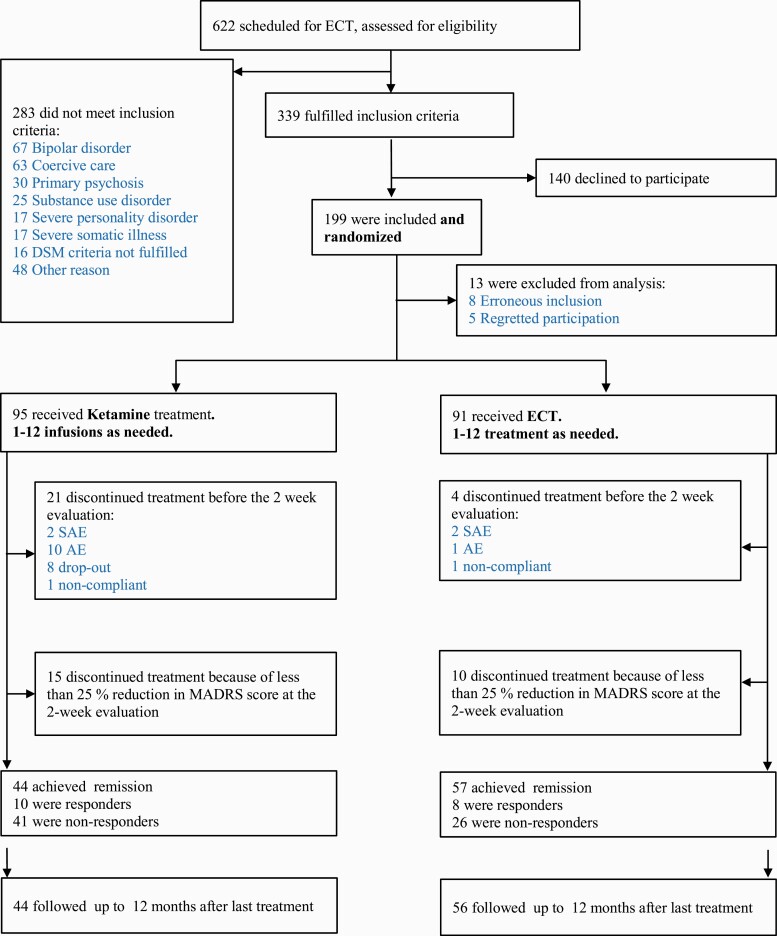
We screened 622 patients for eligibility and included 199 patients (Figure 1). A total 186 patients received at least 1 session and are included in the analyses. Remitters were followed-up for 12 months following the final treatment session. Twenty-seven patients missed at least 1 follow-up visit. Time to relapse or confirmation of continued remission at 12 months was collected for all but 1 remitter. Baseline demographics, site inclusion data, and current medications are presented in Table 1. Outcomes are listed in Table 2, and in supplemental Table 3 in the Appendix.
Figure 1.
Trial profile of a randomized controlled trial comparing electroconvulsive therapy (ECT) and multiple infusions with racemic ketamine for treatment of major depressive disorder in hospitalized patients. AE, adverse event; MADRS, Montgomery Åsberg Depression Rating Scale; SAE, serious adverse event.
Table 1.
Patient Demographics and Site Data
| Variable | ECT | Ketamine |
|---|---|---|
| (n = 91) | (n = 95) | |
| Study sites | ||
| Lund | 58 | 56 |
| Malmö | 11 | 14 |
| Örebro | 12 | 11 |
| Helsingborg | 9 | 9 |
| Linköping | 1 | 4 |
| Halmstad | 0 | 1 |
| Demographics | ||
| Mean age, y (range) | 50 ± 18 (20–85) | 55 ± 18 (18–84) |
| Female sex | 58/91 (64%) | 61/95 (64%) |
| Body mass index (kg/m2) | 27.6 ± 5.7 | 25.5 ± 4.2 |
| Major depressive disordera | ||
| Single episode, moderate (32.1) | 6 (7%) | 2 (2%) |
| Single episode, severe without psychotic features (32.2) | 25 (27%) | 26 (27%) |
| Single episode, severe with psychotic features (32.3) | 6 (7%) | 11 (12%) |
| Single episode, unspecified (32.9) | 1 (1%) | 4 (4%) |
| Recurrent, moderate (33.1) | 13 (14%) | 13 (14%) |
| Recurrent, severe, without psychotic features (33.2) | 31 (34%) | 31 (33%) |
| Recurrent, severe, with psychotic features (33.3) | 8 (9%) | 7 (7%) |
| Recurrent, unspecified (33.9) | 0 (0%) | 1 (1%) |
| Mixed anxiety-depressive disorder (41.2) | 1 (1%) | 0 (0%) |
| Psychotic symptoms present | 14/91 (15%) | 18/95 (19%) |
| Additional psychiatric diagnosis | 28 (31%) | 31 (33%) |
| History | ||
| Previous ECT | 34/91 (37%) | 40/95 (42%) |
| Good effect of previous ECT | 24/34 (71%) | 26/39 (67%) |
| Previous psychotherapy | 61/89 (69%) | 51/93 (55%) |
| Duration of current episode (wk)b | 14 (8–14) | 14 (8–28) |
| Number of previous episodes | 3 (1–6.5) | 3 (1–7.5) |
| First depressive episodeb | 12/91 (13%) | 12/88 (14%) |
| Previous suicide attempt | 46/90 (51%) | 38/95 (40%) |
| Previous serious suicide attempt | 40/44 (91%) | 29/38 (76%) |
| Number of previous suicide attemptsb | 2 (1–3) | 2 (1–2) |
| Self-harm | 21/89 (24%) | 12/92 (13%) |
| Family history (first-degree relative) | ||
| Alcoholism | 12/91 (13%) | 12/95 (13%) |
| Depression | 37/91 (41%) | 44/95 (46%) |
| Bipolarity | 7/91 (8%) | 6/95 (9%) |
| Schizophrenia | 3/91 (3%) | 1/95 (1%) |
| Suicide | 8/91 (9%) | 7/95 (7%) |
| Obsessive compulsive disorder | 3/91 (3%) | 1/95 (1%) |
| Drugs used at the time of inclusion | ||
| Mood stabilizers | 15 (16%) | 9 (9%) |
| Antidepressants | 77 (85%) | 75(79%) |
| Anxiolytics | 63 (69%) | 58 (61%) |
| Antipsychotics | 20 (24%) | 18 (19%) |
| Central stimulants | 1 (1%) | 1 (1%) |
| Hypnotics | 68 (75%) | 61 (64%) |
| None | 5 (6%) | 6 (6%) |
| Recently prescribed drugsc | ||
| Mood stabilizers | 6 (7%) | 4 (4%) |
| Antidepressants | 40 (44%) | 41 (43%) |
| Anxiolytics | 35 (38%) | 39 (41%) |
| Antipsychotics | 17 (19%) | 13 (14%) |
| Hypnotics | 33 (36%%) | 44 (46%) |
| None | 32 (35%) | 29 (31%) |
Abbreviations: ECT, electroconvulsive therapy. Shown are the number of patients included per site, the prevalence of different diagnoses, and demographic data, including family history and medical history. Values are mean ± SD or number of patients (percentages in parenthesis).
a Sub-diagnoses are according to the International Classification of Diseases and Related Health Problems–Tenth Revision (ICD-10).
b Indicates median value (with interquartile range in parenthesis). Outcome per site is presented in supplementary Table 4 in the Appendix.
c Drugs prescribed between the period 4 weeks prior to and 2 weeks after the date of inclusion.
Table 2.
Trial Outcomes in Patients With MDD Randomized to ECT or Racemic Ketamine Infusions
| Outcome | ECT | Ketamine | P | 95% CI | Odds ratio | NECT | NKet |
|---|---|---|---|---|---|---|---|
| Remission | 57/91 (63%) | 44/95 (46%) | .026 | (2.0%, 30%) | 0.5 [0.3, 0.9] | 91 | 95 |
| ITT | 57/94 (61%) | 44/97 (45%) | .034 | (1.1%, 29%) | 0.5 [0.3, 1.0] | 94 | 97 |
| Young (18–50 y)a | 26/52 (50%) | 22/36 (61%) | .39 | 1.6 [0.7, 3.7] | 52 | 36 | |
| Old (51–85 y)a | 31/39 (77%) | 22/59 (37%) | <.001 | 0.2 [0.06, 0.4] | 39 | 59 | |
| Psychotic depressionsa | 11/14 (79%) | 9/18 (50%) | .15 | 0.3 [0.06, 1.3] | 14 | 18 | |
| MADRS | Cohen´s dc | ||||||
| Baseline | 34.5 ± 5.7 | 33.1 ± 6.3 | .11 | (−0.34, 3.2) | 0.23 | 91 | 95 |
| Final | 12.2 ± 11.1 | 16.9 ± 13.1 | .009 | (1.2, 8.2) | 0.40 | 91 | 95 |
| Change in | 22.4 ± 11.4 | 16.1 ± 12.0 | <.001 | (2.9, 9.7) | 0.53 | 91 | 95 |
| Change inb | 22.4 ± 11.4 | <.001 | (20.0, 24.8) | 1.91 | 91 | ||
| Change inb | 16.0 ± 12.1 | <.001 | (13.6, 18.5) | 1.35 | 95 | ||
| MADRS baseline | |||||||
| Young (18–50) | 34.8 ± 5.5 | 32.3 ± 6.0 | .048 | (0.03, 5.0) | 0.43 | 52 | 36 |
| Old (51–85) | 34.0 ± 6.0 | 33.5 ± 6.5 | .49 | (−2.1, 3.1) | 0.08 | 39 | 59 |
| Psychotic depressions | 37.1 ± 4.6 | 37.6 ± 6.1 | .79 | (−4.5, 3.5) | 0.010 | 14 | 18 |
| MADRS final | |||||||
| Young (18–50 y) | 14.8 ± 11.3 | 12.9 ± 10.1 | .41 | (−2.7, 6.6) | 0.19 | 52 | 36 |
| Old (51–85 y) | 8.7 ± 9.8 | 19.4±14.2 | <.001 | (5.9, 15.9) | 0.91 | 39 | 59 |
| Psychotic depressions | 10.1 ± 11.1 | 19.4 ± 16.7 | .069 | (−19.4, 0.78) | 0.68 | 14 | 18 |
| Change in MADRS | |||||||
| Young (18–50)b | 20.0 ± 11.3 | <.001 | (16.8, 23.2) | 1.8 | 52 | ||
| Young (18–50)b | 19.4 ± 9.7 | <.001 | (16.2, 22.7) | 2.0 | 36 | ||
| Old (51–85)b | 25.3 ± 11.1 | <.001 | 2.3 | 39 | |||
| Old (51–85)b | 14.1 ± 12.1 | <.001 | 1.1 | 59 | |||
| Psychotic depressions | 27.0 ± 11.8 | 18.2 ± 13.0 | .058 | (−0.31, 17.9) | 0.71 | 14 | 18 |
| Psychotic depressionsb | 27.0 ± 11.8 | <.001 | (20.2, 33.8) | 2.3 | 14 | ||
| Psychotic depressionsb | 18.2 ± 13.0 | <.001 | (11.8, 24.7) | 1.4 | 18 | ||
| Binary regression analysis | ExpB | 95% CI ExpB | |||||
| Treatment * site 1 | 1.29 | .58 | 0.56, 2.97 | ||||
| Treatment * site 2 | 0.80 | .71 | 0.24, 2.65 | ||||
| Treatment * site 3 | 0.76 | .62 | 0.25, 2.32 | ||||
| Treatment * site 4 | N/A | N/A | N/A | ||||
| Treatment * site 5 | 0.15 | .10 | 0.02, 1.39 | ||||
| Treatment * agegroup | 12.6 | <.001 | (3.1, 51.2) | ||||
| Treatment * sex | 0.4 | .19 | (0.10, 1.6) | ||||
| Relapse | Hazard ratio | ||||||
| Relapse frequency | 36/56 (64%) | 31/44 (70%) | .44 | HR 0.83 [0.51, 1.34] | 56 | 44 | |
| Survival analysis | .30 | ||||||
| No. of treatments | |||||||
| Mean, in total | 7.8 ± 2.4 | 6.8 ± 3.3 | .02 | (0.2, 1.8) | 91 | 95 | |
| Median (IQR) | 8 (6–10) | 6 (5–9) | |||||
| Mean, to remission | 6.0 ± 2.3 | 6.0 ± 2.7 | .84 | (−1.1, 0.9) | 57 | 44 | |
| Median (IQR) | 6 (4–8) | 6 (5–8) | |||||
| Hope of improvement (VAS) | 6.3 ± 2.7 | 5.8 ± 3.1 | .32 | (−0.4, 1.3) | 90 | 90 | |
| Fear of negative outcomes (VAS) | 4.2 ± 3.0 | 4.6 ± 2.9 | .37 | (−1.3, 0.5) | 90 | 89 |
Abbreviations: ECT, electroconvulsive therapy; IQR, interquartile range; ITT, intention to treat analysis; MADRS, Montgomery Åberg Depression Rating Scale; MDD, major depressive disorder; VAS, Visual Analogue Scale. Data are number and percentage of participants (remission and relapse frequency), mean (± SD), and median (IQR) values. Numbers per group indicate the number of participants contributing to each comparison. All presented P values are uncorrected.
a Analyses of patients younger and older than 50 years and of the sub-group of patients diagnosed with psychotic depression were post-hoc analyses that were not explicitly pre-specified in the study protocol.
b Within-group MADRS changes from baseline to post-treatment were analyzed with paired t tests.
c Effects sizes were calculated as Cohen d (mean difference / (SD12+SD22)/2) for between-group comparisons or Cohen dRM (mean difference / √(SD12+SD22 −(2r SD1+SD2)), where r is the correlation between measured pairs for within-group comparisons. Additional data are presented in supplementary Table 3 in Appendix.
Primary Outcome: Remission Rates
In the ECT group, 63% of the patients (57/91) remitted compared with 46% of patients (44/95) treated with ketamine. The remission rate was significantly higher in the ECT group (chi2 = 5.0, P = .026; OR = 0.51 [0.29, 0.92]). The 95% confidence interval of the difference in remission rates was estimated between 2.0% and 30%. In an intention-to-treat analysis that included 5 patients who were randomized but regretted participation and received no treatment, of 94 ECT recipients 57 (61%) remitted compared with 44 of 97 (45%) of ketamine-treated patients (chi2 = 4.5, P = .034, 95% CI [1.1%, 29%]).
Secondary Outcomes
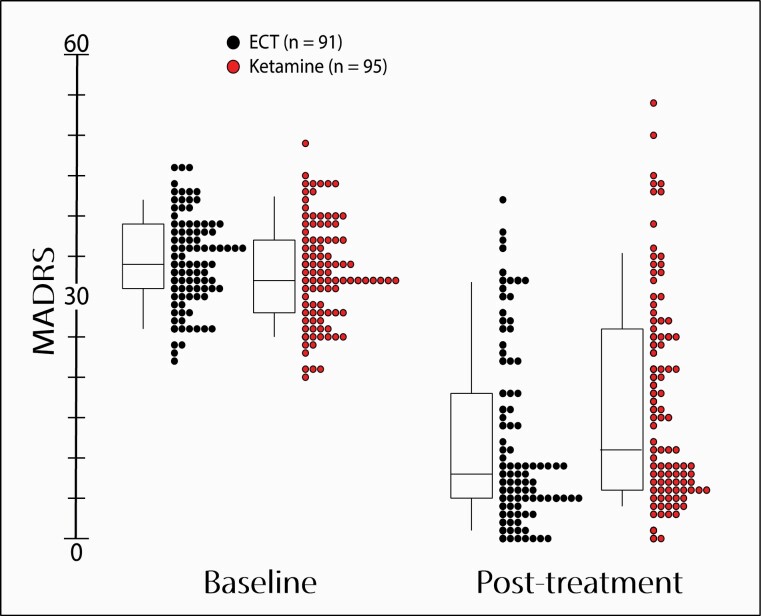
Prior to the 2-week evaluation, 21 and 4 patients dropped out mainly due to AEs from the Ketamine and the ECT group, respectively. At the 2-week evaluation, 15 and 10 patients discontinued treatment because of <25 % reduction in MADRS score in the ketamine and ECT groups, respectively. MADRS scores, indicating depression severity, were 34.5 ± 5.7 (ECT) and 33.1 ± 6.3 (Ket) at baseline (P = .11, 95% CI [-0.34, 3.4]). Final MADRS scores were significantly lower in the ECT group (ECT: 12.2 ± 11.1, Ket: 16.9 ± 13.1; P = .009, 95% CI [1.2, 8.2]) (Figures 2 and 3). We noted all incidences of dose modulation, switching to or addition of a novel antidepressant drugs, between 4 weeks prior to and 2 weeks after the inclusion date to control for a potential influence on remission probability. Such events did not differ between treatment groups (P = .91) or between remitters, responders, and non-responders (P = .62).
Figure 2.
Montgomery Åsberg Depression Rating Scale (MADRS) scores at baseline and after completed treatment in hospitalized patient with major depressive disorder (MDD) randomized to electroconvulsive therapy or multiple infusions with racemic ketamine. Patients were administered between 1 and 12 treatment sessions on a per-need basis. Boxes enclose mean scores ± 1 SD. Midlines indicate median values. Vertical lines indicate 10th to 90th percentiles. Circles indicate individual MADRS scores for participants in the electroconvulsive therapy (ECT) (black) and ketamine (red) treatment groups, respectively. AE, adverse event.
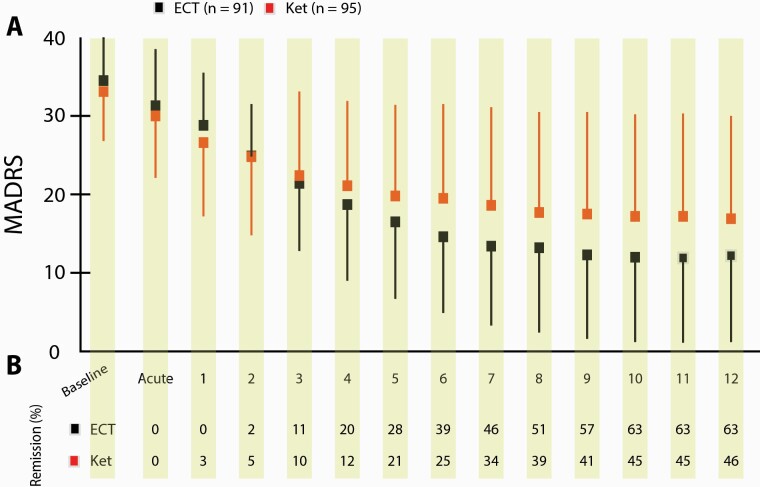
Figure 3.
Mean Montgomery Åsberg Depression Rating Scale (MADRS) score and remission rate over successive treatment sessions in hospitalized patient with major depressive disorder (MDD) randomized to electroconvulsive therapy (ECT) or multiple infusions with racemic ketamine. (A) Mean MADRS scores over the 4-week treatment (thrice weekly) period. Boxes and lines indicate mean scores and 1-sided SD for ECT (black) and ketamine (red). Numbers on the x-axis denote the treatment session that preceded the rating. Baseline and acute indicate ratings done prior to receiving any treatment and 4–5 hours after receiving the first treatment, respectively. (B) Numbers indicate the accumulated percentage of remitters in the treatment groups over time. AE, adverse event; Ket, racemic ketamine.
The binary logistic regression analysis revealed a significant interaction between treatment and age group (Wald chi2 = 12.6, P < .001) but not between treatment and psychotic symptoms (P = .41). In the ECT group, remission was significantly more likely in patients older than 50 years, of whom 77% remitted, compared with younger patients, of whom 50% achieved remission (chi2 = 8.3, P = .004). The opposite relationship was found in the ketamine group, where the remission rate was 61% in the younger age group compared with only 37% in older patients (chi2 = 5.1, P = .034).
Correspondingly, patients older than 50 years receiving ECT had both significantly higher remission rates (ECT: 31/39, 77%, Ket: 22/59, 37 %, chi2 = 16.8, P < .001, OR: 0.15 [0.060, 0.39]) and lower final MADRS scores (ECT: 8.7 ± 9.8, Ket: 19.4 ± 14.2, P < .001, Cohen d = 0.91 [0.68. 1.13]) relative to ketamine patients in the same age category. In the younger age group, neither remission rate (ECT: 26/52, 50%, Ket: 22/36, 61%, chi2 = 1.1, P = .39; OR: 1.6 [0.66, 3.7]) nor final MADRS scores (ECT: 14.8 ± 11.3, Ket: 12.9 ± 10.1, P = .41, Cohen d = 0.18 [0.036, 0.40]) differed significantly between treatment groups. Similar proportions of patients in both treatment groups were diagnosed with depressive disorder with psychotic features (ECT: 14/91, 15%, Ket: 18/95, 18%, chi2 = 0.41, P = .56). A post-hoc analysis assessing the antidepressant effect specifically in this sub-group found no significant difference in remission rates (ECT: 79%, Ket: 50%, chi2 = 2.7, P = .15, OR: 0.27 [0.056, 1.3]) or final MADRS scores (ECT: 10.1 ± 11.1, Ket: 19.4 ± 16.7, P = .069, Cohen d = 0.68). Among these patients, MADRS scores decreased with 27.8 ± 11.8 in the ECT group (Cohen dRM = 2.3) and 18.2 ± 13.0 in the ketamine group (Cohen dRM = 1.4).
Patients in the ECT group received significantly more treatment sessions than patients in the ketamine group (ECT: 7.8 ± 2.4, Ket: 6.8 ± 3.3, P = .02). Comparing the number of treatment sessions by treatment response (remitters, responders, non-responders) revealed that the number of treatment sessions was significantly larger in the ECT group only among non-responders (ECT: 7.2 ± 2.9, Ket: 5.2 ± 2.8, P = .002), whereas remitters and responders received an almost identical number of treatment sessions. There was no difference in the numbers of treatment sessions among remitters between treatment groups (ECT: 6.0 ± 2.3, Ket: 6.0 ± 2.7, P = .84).
Expectations and Fears
Expectations of improvement (ECT: 6.3 ± 2.7, Ket: 5.3 ± 3.1, P = .32) and fear of negative outcomes (ECT: 4.2 ± 3.0, Ket: 4.6 ± 2.9, P = .37) did not differ between treatment groups.
Adverse Events
There was a distinct pattern of AEs associated with each treatment (Table 3). ECT patients reported significantly more headaches, muscle pain, and amnesia and twice as often suffered side effects lasting 24 hours or longer (ECT: 48/90, Ket: 20/91, chi2 = 18.5, P < .001). A large proportion of ECT recipients (n = 21) reported prolonged amnesia, which in some cases resolved only after between 3 and 6 months (n = 3) or persevered during the whole 12-month follow-up period (n = 3). Long-lasting muscle pain was also more common in the ECT group (ECT: 28/90, Ket: 4/91, chi2 = 21.5, P < .001). Ketamine infusions significantly more often led to reports of dissociative side effects, anxiety, blurred vision, euphoria, vertigo, and diplopia (P = .001 for all). Aggregated Clinician Administered Dissociative States Scale and Brief Psychiatric Rating Scale scores were significantly higher in the ketamine group 1 hour after the first treatment (P = .01), but not at other time points. More patients in the ECT group reported a severe AE (SAE) (ECT: 23/90, Ket: 14/91, chi2 = 2.6, P = .09). Eleven and 2 SAEs in the ECT and ketamine group, respectively, were classified as very likely or probably caused by the treatment. In the ECT group, SAEs consisted of hypertonia, amnesia (n = 4), deep-vein thrombosis, hypoxia, post-treatment catatonia, and seizures (the latter 2 in the same patient). Two SAEs and 1 AE led to termination of ECT. Both SAEs in the ketamine group were panic attacks during the first infusion. Both patients declined additional ketamine treatments. Twenty-one ketamine patients dropped out before receiving 6 treatment sessions. Ten AEs emerging during ketamine infusions led to recipients terminating participation. Eight patients dropped out due to a combination of lack of clinical improvement and discomfort from the infusions. One patient in each treatment group left study participation by checking out from the ward. One patient died from suicide 3 months after remitting following a series of 12 ECT sessions. Nine other patients attempted suicide during follow-up (ECT: 5, Ket: 4).
Table 3.
Adverse Events
| No. of patients reporting AE | P | No. of patients reporting long-lasting (>24 h) AE | Median duration, d (range) | ||||
|---|---|---|---|---|---|---|---|
| Type of AE | ECT (n = 90) | Ketamine (n = 91) | ECT (n = 90) | Ketamine (n = 91) | ECT (n = 90) | Ketamine (n = 91) | |
| Euphoria | 0 | 19 | <.001 | 0 | 1 | ||
| Dissociative symptoms | 14 | 55 | <.001 | 3 | 7 | 1 (1–5) | 2 (1–11) |
| Anxiety | 16 | 41 | <.001 | 2 | 2 | 1 (1) | 1 (1) |
| Affect lability | 3 | 11 | .048 | 0 | 2 | 1 (1) | |
| Fatigue | 19 | 20 | n.s. | 6 | 4 | 7 (1–28) | 17 (1–27) |
| Sleep disturbance | 0 | 1 | n.s. | 0 | 1 | 6 (6) | |
| Confusion | 22 | 23 | n.s. | 5 | 1 | 1 (1–40) | 1 (1) |
| Paranoid delusions | 2 | 1 | n.s. | 0 | 0 | ||
| Amnesia | 26 | 8 | <.001 | 19 | 0 | 28 (1–365)a | |
| Vertigo | 22 | 63 | <.001 | 5 | 6 | 1 (1–5) | 2 (1–6) |
| Paresthesia | 2 | 3 | n.s. | 0 | 0 | ||
| Seizures | 2 | 0 | n.s. | 0 | 0 | ||
| Myoclonus | 1 | 0 | n.s. | 0 | 0 | ||
| Blurred vision | 0 | 18 | <.001 | 0 | 3 | 1 (1) | |
| Diplopia | 2 | 28 | <.001 | 0 | 0 | ||
| Headache | 72 | 20 | <.001 | 15 | 6 | 1 (1–3) | 1 (1–3) |
| Tinnitus | 2 | 1 | n.s. | 1 | 0 | 1 (1) | |
| Hypertonia | 4 | 1 | n.s. | 0 | 0 | ||
| Tachy/bradychardia | 6 | 0 | .029 | 0 | 0 | ||
| Hypotension | 1 | 0 | n.s. | 0 | 0 | ||
| Thrombosis/swelling | 1 | 1 | n.s. | 1 | 0 | 67 (67) | |
| Abdominal pain | 2 | 0 | n.s. | 1 | 0 | 3 (3) | |
| Emesis | 6 | 2 | n.s. | 0 | 0 | ||
| Constipation/diarrhea | 1 | 3 | n.s. | 0 | 2 | 4 (3–5) | |
| Nausea | 23 | 25 | n.s. | 3 | 4 | 1 (1–4) | 1 (1–2) |
| Salivation/dry mouth | 1 | 22 | <.001 | 0 | 2 | 2 (1–3) | |
| Sore throat | 3 | 1 | n.s. | 2 | 0 | 3 (2–4) | |
| Laryngo/bronchospasm | 5 | 0 | n.s. | 0 | 0 | ||
| Desaturation | 12 | 4 | .039 | 0 | 0 | ||
| Infection | 0 | 1 | n.s. | 0 | 0 | ||
| Skin irritation | 1 | 3 | n.s. | 0 | 0 | ||
| Muscle pain | 48 | 13 | <.001 | 26 | 4 | 2 (1–211) | 5 (1–11) |
| Urine retention | 0 | 1 | n.s. | 0 | 0 | ||
| ECT (n = 90) | Ketamine (n = 91) | P | |||||
| No. of patients reporting AE | 85 | 85 | n.s. | ||||
| No. of patients reporting long-lasting AEs | 49 | 21 | <.001 | ||||
| No. of AEs per participant | 7.8 ± 5.4 | 12.0 ± 10.9 | <.001 | ||||
| No. of patients reporting SAEs | 23 | 14 | .09 | ||||
| No. of suicide attempts | 6 | 4 | |||||
| No. of patients attempting suicide | 6 | 4 | |||||
| No. of suicides | 1 | 0 | |||||
Abbreviations: AE, adverse event; ECT, electroconvulsive therapy; n.s., non significant; SAE, serious adverse event. All AEs reported were classified as “very likely” or “probably” to be related to the treatment are included.
a A maximum duration of 365 days indicates the AE had not resolved at the 12-month follow-up.
Relapse
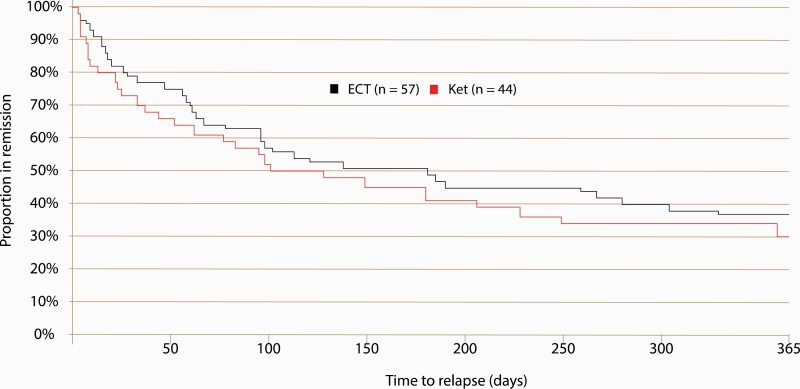
During the 12-month follow-up period, 64% of the remitters in the ECT group relapsed compared with 70% in the ketamine group (ECT: 36/56, Ket: 31/44 (log rank P = .44, HR [95% CI] = 0.83, [0.51–1.34]). A survival analysis revealed no significant difference between the treatment groups in the time to relapse (chi2 = 0.061, P = .30) (Figure 4).
Figure 4.
Kaplan-Meier curves of time to relapse during a 12-month follow-up period in patients with major depressive disorder (MDD) remitting following randomization to electroconvulsive therapy (ECT) or multiple infusions with racemic ketamine. Hospitalized patients with major depressive disorder were randomized to ECT (black line) or multiple infusions with racemic ketamine (red line). Remitters were followed-up at 1 week and at 3, 6, and 12 months after their last treatment session. Ket, racemic ketamine.
Discussion
To our knowledge, this is the first randomized controlled trial comparing ketamine with an active control with an adequate sample size. Ketamine was administered to hospitalized, severely ill patients and compared with ECT, the most effective antidepressant treatment available. Remission rates were significantly higher, and decreases in MADRS scores were significantly larger in patients after ECT treatment than after ketamine infusions.
Despite inferiority of ketamine, remission in almost one-half (46%) of patients is itself clinically meaningful. Surprisingly, we found little evidence of a fast onset antidepressant effect of ketamine. Unlike previous studies that reported remission levels between 30% and 50% following 1 (Berman et al., 2000; Zarate et al., 2006; Vande Voort et al., 2016; Feifel et al., 2017; Perez-Esparza et al., 2018; Vidal et al., 2018) or 2 (Rasmussen et al., 2013) infusions, we found that acute remission was very rare and that, on average, patients needed 6 treatment sessions to achieve remission. Continued administration should therefore not be discouraged in patients who do not exhibit symptom relief after the first sessions.
Reported AEs were typical for ECT(Ferrier I 2019) and ketamine(aan het Rot et al., 2010). Headaches and muscle pain were common after ECT, but also physiological reactions, such as tachycardia and desaturation, were reported, and a handful of patients reported amnesia also present at the end of the 12-month follow-up.
AEs in the ketamine group generally terminated during or shortly after infusions, often involving perceptual changes, with reactions from feelings of euphoria to severe anxiety. The higher dropout rate in the ketamine group was a direct consequence of the difficulty some patients had to cope with the side effects. NMDA-receptor antagonists possess psychotomimetic(Noghuchi 2002) properties, and patients with psychotic depressions have therefore been excluded from previous studies. One-half of patients with psychotic depression remitted after ketamine, with no indications of adverse reactions particular for these patients. A corresponding 79% remission level was observed in response to ECT. The lack of statistical significance is explained by the limited number of patients with psychotic symptoms (14 and 18 in the ECT and ketamine group, respectively) and contrasted by a robust effect size (Cohen d = 0.68) when comparing the final MADRS scores. Being older than 50 years increased the likelihood of remitting following ECT but decreased the likelihood to remit with ketamine. Patients over 50 remitted twice as often after ECT compared with ketamine, but we found no difference in remission rates or final MADRS in younger patients. Age could thus impact treatment outcome, in accordance with ECT being more effective in older patients(Geduldig and Kellner 2016) and indices of failure of ketamine in geriatric patients(Szymkowicz et al., 2014).
The study has limitations. There was no placebo group, but the superiority of racemic ketamine over placebo infusions has been demonstrated in several trials(Berman et al., 2000; Zarate et al., 2006; Sos et al., 2013; Fava et al., 2020). In our opinion, it was not ethical to withhold active treatment for patients in need of immediate relief. The trial was open label. Blinding ketamine patients would have required redundant anaesthesia, which might have interfered with the treatment effect. Patients were hospitalized and interacted with staff on a 24-hour basis. To blind assessors would have interfered with daily clinical routines. MADRS ratings were done simultaneously with recording AEs as an integrated part of the physician–patient interaction.
We rejected the idea of independent raters as many patients required delicate handling. By minimizing changes in clinical routine, we believe patients who otherwise would have declined participation have been able to take part. Some potential participants declined because of previous positive experiences of ECT. This loss of patients expected to respond well to ECT might have introduced a small potential bias in favor of ketamine.
Participating sites all had long-time experience with ECT but no experience administering ketamine. Staffs, and some patients, were familiar with side effects common to ECT but were less prepared for the adverse psychological effects of ketamine. This, and knowing ECT was available after the study, probably contributed to the higher dropout rate in the ketamine group. Some of these patients possibly could have benefitted from additional infusions. For representativity, suicidal patients and patients suffering from co-morbid psychiatric conditions were not excluded. There was no washout period so as not to study treatment effects in drug-free patients who recently discontinued long- term medication. No patients were recruited through advertisements or referrals. Instead, we recruited severely ill, hospitalized patients about to receive ECT. We believe this better resembles the real-world clinical situation and increases the generalizability of our findings. The value of a flexible number of infusions is self-evident. If we had limited to 3 or 6 infusions, drastically fewer patients in the ketamine group would have remitted. Finally, we followed remitters, losing only 1 participant during the follow-up, for 12 months. This is longer than previous studies and fundamentally important to evaluate the clinical value of ketamine. We found that a substantial proportion of patients, 30% and 36% in the ketamine and ECT group, respectively, remained relapse free during the whole 12-month follow-up period, falsifying assumptions that the antidepressant effect of ECT or of ketamine is necessarily short lasting. Future studies should investigate if the larger group of less severely depressed patients who gain little benefit from available antidepressants responds as well to ketamine infusions as the current cohort did.
Upwards of one-half of patients who complete a successful ECT series relapse after 2–4 months(Tew et al., 2007) and many require hospitalization within a year(Nordenskjold et al., 2011). Similar concerns have been raised about ketamine as patients were reported to relapse within 1 (Murrough et al., 2013; Ghasemi et al., 2014; Shiroma et al., 2014; Singh et al., 2016); Vande Voort et al., 2016); Cusin et al., 2017; Feifel et al., 2017; Perez-Esparza et al., 2018; Vidal et al., 2018) or a few months(Loo et al., 2016; Wilkinson et al., 2017). Increasing evidence suggests continuation ECT may reduce relapse rates, and it is possible that continuing ketamine infusions after remission would also decrease relapse rates. Further studies need to investigate the relative benefits of different continuation regimens.
The role of age in predicting response to ketamine and ECT should be clarified. Improving tolerability is important, given the high dropout rate in the ketamine group, and reducing the time to remission by increasing the frequency of infusions should be tested systematically.
CONCLUSION
Non-inferiority of ketamine was not demonstrated. ECT was superior to ketamine infusions both in terms of higher remission rates and greater reduction of depressive symptoms and remains the most effective treatment for severe depression. In particular, older patients and patients with psychotic symptoms have high remission rates with ECT, which was confirmed in this study. The onset of action of ketamine was not faster than ECT and the antidepressant effect not necessarily transient. Even so, with almost one-half of severely depressed recipients remitting, ketamine could be a viable option when ECT is unavailable or contraindicated or in cases when patients decline ECT. We believe that ketamine infusion can be offered as an alternative treatment option perhaps for younger patients in some cases or patients who have experienced severe side effects from ECT. It is important to realize that remission can be achieved after multiple infusions even in the absence of rapidly appearing antidepressant effects and to not stop after only a single or a few infusions. Preparations prior to and psychological support during infusions might encourage patients not to discontinue prematurely because of side-effects such as anxiety and dissociation. Individual patients may prefer ketamine. Nevertheless, given the high remission rate, in particular for patients who are older or present with psychotic symptoms, the superior efficacy of ECT must be emphasized.
Supplementary Material
Acknowledgments
We dedicate this work to late professor Peter Höglund, who contributed immensely with his expertise, but sadly passed away before its completion. We thank professors Olle Lindvall and Åsa Petersén for their work with the safety committee, and the clinical staff at the participating clinics and all patients who contributed by accepting to take part. The paper was edited by www.elevatescientific.com.
The study was funded by the Swedish Research Council (Grant number: 2015-00799), The Crafoord Foundation, Skåne Regional Council, The Königska Foundation, Lions forsknings foundation Skåne, and OM Perssons donation foundation.
Contributor Information
Joakim Ekstrand, Department of Clinical Sciences, Faculty of Medicine, Lund University, Lund, Sweden.
Christian Fattah, Department of Clinical Sciences, Faculty of Medicine, Lund University, Lund, Sweden.
Marcus Persson, Department of Clinical Sciences, Faculty of Medicine, Lund University, Helsingborg, Sweden.
Tony Cheng, Department of Clinical Sciences, Faculty of Medicine, Lund University, Malmö, Sweden.
Pia Nordanskog, Department of Clinical and Experimental Medicine, Linköping University, Linköping, Sweden.
Jonas Åkeson, Department of Clinical Sciences, Faculty of Medicine, Lund University, Malmö, Sweden.
Anders Tingström, Department of Clinical Sciences, Faculty of Medicine, Lund University, Lund, Sweden.
Mats B Lindström, Department of Clinical Sciences, Faculty of Medicine, Lund University, Malmö, Sweden.
Axel Nordenskjöld, Faculty of Medicine and Health, Örebro University, Örebro, Sweden.
Pouya Movahed Rad, Department of Clinical Sciences, Faculty of Medicine, Lund University, Lund, Sweden.
Interest Statement
A.N., J.E., and P.M.R. has received lecturer honoraria from Lundbeck.
References
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139–145. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM (1998) Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 11:125–136. [DOI] [PubMed] [Google Scholar]
- Caddy C, Amit BH, McCloud TL, Rendell JM, Furukawa TA, McShane R, Hawton K, Cipriani A (2015) Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev 9:CD011612. [DOI] [PubMed] [Google Scholar]
- Cusin C, Ionescu DF, Pavone KJ, Akeju O, Cassano P, Taylor N, Eikermann M, Durham K, Swee MB, Chang T, Dording C, Soskin D, Kelley J, Mischoulon D, Brown EN, Fava M (2017) Ketamine augmentation for outpatients with treatment-resistant depression: preliminary evidence for two-step intravenous dose escalation. Aust N Z J Psychiatry 51:55–64. [DOI] [PubMed] [Google Scholar]
- Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, Ionescu DF, Mathew SJ, Chang LC, Iosifescu DV, Murrough J, Debattista C, Schatzberg AF, Trivedi MH, Jha MK, Sanacora G, Wilkinson ST, Papakostas GI (2020) Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry 25:1592–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Malcolm B, Boggie D, Lee K (2017) Low-dose ketamine for treatment resistant depression in an academic clinical practice setting. J Affect Disord 221:283–288. [DOI] [PubMed] [Google Scholar]
- Ferrier IWJ (2019) The ECT handbook. 4th ed. Cambridge, UK: Cambridge University Press. [Google Scholar]
- GBD 2013 DALYs and HALE Collaborators (2015) Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet 386:2145–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geduldig ET, Kellner CH (2016) Electroconvulsive therapy in the elderly: new findings in geriatric depression. Curr Psychiatry Rep 18:40. [DOI] [PubMed] [Google Scholar]
- Ghasemi M, Kazemi MH, Yoosefi A, Ghasemi A, Paragomi P, Amini H, Afzali MH (2014) Rapid antidepressant effects of repeated doses of ketamine compared with electroconvulsive therapy in hospitalized patients with major depressive disorder. Psychiatry Res 215:355–361. [DOI] [PubMed] [Google Scholar]
- Husain MM, Rush AJ, Fink M, Knapp R, Petrides G, Rummans T, Biggs MM, O’Connor K, Rasmussen K, Litle M, Zhao W, Bernstein HJ, Smith G, Mueller M, McClintock SM, Bailine SH, Kellner CH (2004) Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry 65:485–491. [DOI] [PubMed] [Google Scholar]
- Kellner CH, Knapp R, Husain MM, Rasmussen K, Sampson S, Cullum M, McClintock SM, Tobias KG, Martino C, Mueller M, Bailine SH, Fink M, Petrides G (2010) Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br J Psychiatry 196:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Bromet EJ (2013) The epidemiology of depression across cultures. Annu Rev Public Health 34:119–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen TM, Musliner KL, Benros ME, Vestergaard M, Munk-Olsen T (2016) Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord 193:203–207. [DOI] [PubMed] [Google Scholar]
- Loo CK, Gálvez V, O’Keefe E, Mitchell PB, Hadzi-Pavlović D, Leyden J, Harper S, Somogyi AA, Lai R, Weickert CS, Glue P (2016) Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr Scand 134:48–56. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV (2013) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe RG (1998) Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med 17:873–890. [DOI] [PubMed] [Google Scholar]
- Noghuchi K (2002) NMDA antagonist-induced neurotoxicity and psychosis. In: Handbook of neurotoxicology (Massaro EJ, ed), pp 199–205. Totowa,NJ: Humana Press. [Google Scholar]
- Nordenskjold A, von Knorring L, Engström I (2011) Predictors of time to relapse/recurrence after electroconvulsive therapy in patients with major depressive disorder: a population-based cohort study. Depress Res Treat 2011:470985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR (1962) The brief psychiatric rating scale. Psychol Rep 10:799–812. [Google Scholar]
- Perez-Esparza R, Corona T, Ruiz-García RG, Oñate-Cadena N, de la Fuente-Sandoval C, Ramírez-Bermúdez J (2018) Time until relapse after augmentation with single-dose ketamine in treatment-resistant depression. Psychiatry Clin Neurosci 72:623. [DOI] [PubMed] [Google Scholar]
- Rasmussen KG, Lineberry TW, Galardy CW, Kung S, Lapid MI, Palmer BA, Ritter MJ, Schak KM, Sola CL, Hanson AJ, Frye MA (2013) Serial infusions of low-dose ketamine for major depression. J Psychopharmacol 27:444–450. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917. [DOI] [PubMed] [Google Scholar]
- Semkovska M, McLoughlin DM (2010) Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry 68:568–577. [DOI] [PubMed] [Google Scholar]
- Shiroma PR, Albott CS, Johns B, Thuras P, Wels J, Lim KO (2014) Neurocognitive performance and serial intravenous subanesthetic ketamine in treatment-resistant depression. Int J Neuropsychopharmacol 17:1805–1813. [DOI] [PubMed] [Google Scholar]
- Short B, Fong J, Galvez V, Shelker W, Loo CK (2018) Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry 5:65–78. [DOI] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, Pinter C, Murrough JW, Sanacora G, Shelton RC, Kurian B, Winokur A, Fava M, Manji H, Drevets WC, Van Nueten L (2016) A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry 173:816–826. [DOI] [PubMed] [Google Scholar]
- Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T (2013) Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuro Endocrinol Lett 34:287–293. [PubMed] [Google Scholar]
- Szymkowicz SM, Finnegan N, Dale RM (2014) Failed response to repeat intravenous ketamine infusions in geriatric patients with major depressive disorder. J Clin Psychopharmacol 34:285–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew JD Jr, Mulsant BH, Haskett RF, Joan P, Begley AE, Sackeim HA (2007) Relapse during continuation pharmacotherapy after acute response to ECT: a comparison of usual care versus protocolized treatment. Ann Clin Psychiatry 19:1–4. [DOI] [PubMed] [Google Scholar]
- Vande Voort JL, Morgan RJ, Kung S, Rasmussen KG, Rico J, Palmer BA, Schak KM, Tye SJ, Ritter MJ, Frye MA, Bobo WV (2016) Continuation phase intravenous ketamine in adults with treatment-resistant depression. J Affect Disord 206:300–304. [DOI] [PubMed] [Google Scholar]
- Vidal S, Gex-Fabry M, Bancila V, Michalopoulos G, Warrot D, Jermann F, Dayer A, Sterpenich V, Schwartz S, Vutskits L, Khan N, Aubry JM, Kosel M (2018) Efficacy and safety of a rapid intravenous injection of ketamine 0.5 mg/kg in treatment-resistant major depression: an open 4-week longitudinal study. J Clin Psychopharmacol 38:590–597. [DOI] [PubMed] [Google Scholar]
- Wilkinson ST, Wright D, Fasula MK, Fenton L, Griepp M, Ostroff RB, Sanacora G (2017) Cognitive behavior therapy may sustain antidepressant effects of intravenous ketamine in treatment-resistant depression. Psychother Psychosom 86:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.