Abstract
Background
Odor identification (OI) dysfunction is an early marker of Alzheimer’s disease (AD), but it remains unclear how olfactory-related regions change from stages of subjective cognitive decline (SCD) and mild cognitive impairment (MCI) to AD dementia.
Methods
Two hundred and sixty-nine individuals were recruited in the present study. The olfactory-related regions were defined as the regions of interest, and the grey matter volume (GMV), low-frequency fluctuation, regional homogeneity (ReHo), and functional connectivity (FC) were compared for exploring the changing pattern of structural and functional abnormalities across AD, MCI, SCD, and normal controls.
Results
From the SCD, MCI to AD groups, the reduced GMV, increased low-frequency fluctuation, increased ReHo, and reduced FC of olfactory-related regions became increasingly severe, and only the degree of reduced GMV of hippocampus and caudate nucleus clearly distinguished the 3 groups. SCD participants exhibited reduced GMV (hippocampus, etc.), increased ReHo (caudate nucleus), and reduced FC (hippocampus-hippocampus and hippocampus-parahippocampus) in olfactory-related regions compared with normal controls. Additionally, reduced GMV of the bilateral hippocampus and increased ReHo of the right caudate nucleus were associated with OI dysfunction and global cognitive impairment, and they exhibited partially mediated effects on the relationships between OI and global cognition across all participants.
Conclusion
Structural and functional abnormalities of olfactory-related regions present early with SCD and deepen with disease severity in the AD spectrum. The hippocampus and caudate nucleus may be the hub joining OI and cognitive function in the AD spectrum.
Keywords: Alzheimer’s disease; caudate nucleus, hippocampus; mild cognitive impairment; MRI; odor identification; subjective cognitive decline
Significance Statement.
The olfactory system is the earliest region to be affected by AD pathology, and OI dysfunction is an early marker for predicting AD. However, it remains unclear how olfactory-related regions change from stages of SCD and MCI to AD dementia. Therefore, the present study first explored the changing patterns of structural and functional abnormalities of olfactory-related regions in participants with SCD, MCI, and AD. The results suggested that structural and functional abnormalities of olfactory-related regions present early with SCD and deepen with disease severity in the AD spectrum, and the hippocampus and caudate nucleus play the most important roles in joining OI dysfunction and cognitive impairment. The present study provides important novel insights into the pathophysiological substrate of OI dysfunction in SCD, MCI, and AD, and provides a potential target for the neuromodulation of AD spectrum diseases.
Introduction
Odor identification (OI) dysfunction is an early marker for indicating Alzheimer’s disease (AD) pathology and predicting development to dementia, with the advantage of non-invasiveness, cost-effectiveness, and high patient compliance (Casa and Ramirez 2020; Ubeda-Banon et al., 2020). In AD pathological progression, the emergence of OI dysfunction may be parallel to tau-mediated neuronal injury and earlier than memory impairment and clinical symptoms (Bathini et al., 2019; Murphy 2019). Additionally, OI dysfunction was associated with worse cognitive performance (Wilson et al., 2007), reduced hippocampal and entorhinal volume (Growdon et al., 2015), increased cortical amyloid burden (Bahar-Fuchs et al., 2010), lower ratios of CSF t-tau and P181-tau to Aβ 1-42 (Lafaille-Magnan et al., 2017), faster cognitive decline, and a higher rate of conversion to dementia (Devanand et al., 2008; Roberts et al., 2016) among community elderly individuals and patients with mild cognitive impairment (MCI).
Recent studies have suggested that OI dysfunction may also contribute to predicting dementia risk in individuals with subjective cognitive decline (SCD), which is regarded as the earliest stage of the AD spectrum (Jessen et al., 2014). Our previous results suggested that SCD patients exhibited worse OI compared with an increase with the progression of disease in the AD spectrum (Wang et al., 2020a). Furthermore, other studies demonstrated that OI dysfunction was associated with more subjective memory complaints (Sohrabi et al., 2009), increased temporal and parietal tau burden, temporal lobe atrophy (Risacher et al., 2017), and a higher risk of conversion to AD in SCD individuals (Tahmasebi et al., 2019). However, the underlying brain abnormalities related to OI dysfunction in SCD have not been fully elucidated.
According to the Braak stages, the olfactory system (especially the entorhinal and transentorhinal areas) is the first region to be affected by AD pathology (Braak and Braak 1997), and structural and functional abnormalities of olfactory regions have been repeatedly reported in patients with AD and MCI (Vasavada et al., 2017; Zhang et al., 2019; Kashibayashi et al., 2020). For SCD, studies also exhibited abnormalities of olfactory regions at the structural and functional levels (such as decreased hippocampal volume and thinner entorhinal cortex and decreased connectivity between the default mode network and hippocampus) (Wang et al., 2020b), but their direct relationships with OI have not yet been explored, and it remains unclear whether the abnormalities of other olfactory regions may also contribute to OI dysfunction in SCD. Moreover, how these structural and functional abnormalities of olfactory regions develop from SCD and MCI to AD needs to be further elucidated, because most of the previous studies explored the structural and functional abnormalities in AD, MCI, and SCD by using whole-brain analyses (Li et al., 2015; Wang et al., 2015). Additionally, some of the olfactory-related regions are relatively small, and their effect size may not reach a significant level compared with the other regions.
Therefore, the present study aimed to explore the changing pattern of structural and functional abnormalities of olfactory-related regions from SCD, MCI, and AD and explore their relationships with OI dysfunction and cognitive impairment. Combining what has been mentioned above, we hypothesized that structural and functional abnormalities of olfactory-related regions present in SCD patients would deepen in patients with MCI and AD, and these abnormalities mediated the relationship between OI and cognitive function. The present results provide a deeper understanding of the pathophysiological substrate of OI dysfunction in SCD, MCI, and AD, and provide potential targets for early neuromodulation.
MATERIALS AND METHODS
Participants
A total 269 patients were continuously recruited from the Affiliated Brain Hospital of Guangzhou Medical University and the community in Guangzhou. Participants were assigned to 1 of 4 groups: 70 with SCD, 118 with MCI, 31 with AD, and 50 normal controls (NCs). All patients or their legal guardians provided signed informed consent to participate in the study. This study was approved by the ethics committees of the Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital) (Ethical number: 2014, 078).
The diagnostic criteria of AD were based on the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association for probable AD (Dubois et al., 2007). Considering the compliance of different assessments, all AD patients included in the present study had mild or moderate AD, with a score ranging from 1 to 2 on the Clinical Dementia Rating. The diagnostic criteria of MCI were based on the Peterson criteria (with 1.5 SD below their agemates in cognitive scores and full score in activities of daily living) (Petersen, 2004). The SCD criteria included 2 major features (Jessen et al., 2014). The first was a self-experienced persistent decline in cognitive capacity relative to a previously normal cognitive status unrelated to an acute event. All respondents were asked the following questions: (1) Do you have complaints about your memory? Participants were asked to answer “yes” or “no”; (2) How long do you think your memory has been declining? The participants were asked to respond with the duration of memory decline; (3) Are you worried about your memory problems? The participants were asked to answer “yes” or “no”. If the answer was “yes”, then the following questions were asked: (3.1) Are you worried about remembering something? (3.2) Are you worried about where things are placed? (3.3) Are you worried about forgetting what you said? (3.4) Are you worried about forgetting a meeting or party? The participants were asked to answer “mildly,” “moderately,” or “severely.” Those who answered “yes” to the first question responded to the second question with a duration of memory decline of more than 0.5 years and indicated that the decline was unrelated to an acute event satisfied the first criterion. The second criterion was normal performance on standardized cognitive tests used to classify MCI, adjusted for age, sex, and education. NC individuals were age-matched, cognitively and physically healthy individuals without a complaint of memory decline. The exclusion criteria were as follows: (1) patients with a history of any neurodegenerative disease other than AD; (2) other conditions that significantly affect cognitive function, such as metabolic vitamin B12 deficiency, stroke, and neurosyphilis; (3) patients with psychosis or other psychiatric conditions, such as depression, anxiety, and suicidal behavior; (4) other situations that significantly influence olfaction, including active upper respiratory/sinus infection or respiratory distress at the time of testing, congenital or traumatic anosmia, known nasal polyps or tumors, current or recent (past 6 months) smoking, and alcohol or substance dependence. Participants underwent a structured interview followed by a standardized olfactory test, cognitive assessments, and neuroimaging scanning on the same day.
Assessment of Olfactory Function
For OI assessment, the Sniffin’ Sticks Screen 16 test (Hummel et al., 1997) was applied, which involves the presentation of odorants from felt-tip pens. To measure OI performance, odorized pens were used. The pen’s cap was opened by the experimenter for approximately 3 seconds, and the pen’s tip was placed approximately 2 cm in front of both nostrils. Participants were asked to smell 16 common odorants from the felt-tip pens and to name the odors using a multiple-choice format with 4 choices, only 1 of which was correct. The participants’ scores ranged from 0 to 16.
Assessment of Cognitive Function
Cognitive function in different cognitive domains was evaluated for differentiating NC and SCD from MCI (Jessen et al., 2014). The neuropsychological tests included global cognition (Mini-Mental State Examination [MMSE]), memory (long-term recall of Auditory Verbal Learning Task), executive function (Trail-Making Test B), attention (Symbol-Digit Modality Test), language (Boston Naming Test), and visuospatial skill (Rey-Osterrieth Complex Figure test). AD patients undergo only the MMSE test because of compliance.
Magnetic Resonance Imaging (MRI) Data Acquisition
Participants underwent MRI scans after neuropsychological assessments and olfactory tests. The Philips 3.0 T MR system in The Affiliated Brain Hospital of Guangzhou Medical University (Philips, Achieva, Amsterdam, Netherlands) was used to acquire the imaging data. For each participant, an anatomical image was obtained with a sagittal 3-dimensional gradient-echo T1-weighted sequence (TR = 8.2 ms, TED = 3.8 ms, TI = 1100 ms, flip angle = 8°, 188 slices, slice thickness = 1 mm, Gap = 0 mm, matrix = 256 × 256, inversion time = 0). Sagittal resting-state fMRI datasets of the whole brain were obtained in 6 minutes with a single-shot gradient echo-planar imaging pulse sequence. The resting-state fMRI scanning parameters were as follows: TE = 30 ms, TR =2 000 ms, flip angle = 90 degrees, numbers of slices = 33, slice thickness = 4 mm, matrix size = 64 × 64, and field of view = 220 × 220 mm.
Image Processing
The T1 images were preprocessed using the toolboxes Computational Anatomy Toolbox 12 in Statistical Parametric Mapping 12 (SPM 12, University College, London, UK) (Ashburner et al., 2004). Briefly, each T1 image was segmented into cerebrospinal fluid, white matter, and grey matter and then normalized to the Montreal Neurological Institute template. A Gaussian kernel filter of 8 × 8 × 8 mm3 was used to smooth the modulated image. Resting-state fMRI data preprocessing was carried out using the Data Processing Assistant for Resting-State 5.0 (Yan et al., 2016). The first 10 volumes were removed to preserve steady-state data only. The remaining images were corrected for timing differences and for head motion. Participants who had images with more than 2 mm translational movement or more than 2 degrees rotational movement were excluded from further analysis. The individual structural image (T1-weighted images) was coregistered to the mean functional image after motion correction. The transformed structural images were segmented into grey matter, white matter, and CSF. Nuisance signals, such as 6 head motion parameters, global signal, CSF signal, and white matter signal, were regressed out from each time series. Following this, the motion-corrected functional images were spatially normalized into the Montreal Neurological Institute space and resampled to 3 × 3 × 3 mm2 using the normalization parameters estimated during unified segmentation. Subsequently, the functional images were smoothed with a 6 × 6 × 6 mm3 full width at half maximum Gaussian kernel, and detrending was carried out. Finally, a bandpass filter (0.01 Hz <f < 0.1 Hz) was applied to reduce the effect of low-frequency drifts and high-frequency noise (Soares et al., 2016).
Analyses of Grey Matter Volume (GMV), Fractional Amplitude of Low-Frequency Fluctuation (ALFF), Regional Homogeneity, and Functional Connectivity
The voxelwise GMV was calculated by using Computational Anatomy Toolbox 12 in SPM 12. The ALFF (Zou et al., 2008), regional homogeneity (ReHo) (Zang et al., 2004), and functional connectivity (FC) (Fox and Raichle, 2007) were calculated by DPASF 4.5. For the images not processed with a filter, the sum of the absolute amplitudes of low frequency (ranging from 0.01 Hz to 0.08 Hz) was extracted from the time series of each voxel, which is defined as ALFF and reflects spontaneous neuronal activities (Zou et al., 2008). For the images not processed with smoothed, ReHo were calculated, and it reflects the degree of local regional neural activity coherence. Briefly, it was calculated as Kendall’s coefficient of concordance (or Kendall’s W) of the time course of a given voxel with those of its nearest neighbors (26 voxels). For the purpose of standardization, the ReHo value of each voxel was divided by the global mean ReHo value. Finally, the resulting ReHo images were spatially smoothed with a 6-mm FWHM Gaussian kernel (Zang et al., 2004). FC is defined as the temporal correlation (measured as Pearson’s r) in the high-amplitude, low-frequency, spontaneously generated blood oxygen level dependent (BOLD) signal between voxels (cubic “pixel” in a 3-dimensional brain image) or brain regions.
Definition of Olfactory-Related Regions
The olfactory-related regions included the piriform cortex, amygdala, entorhinal cortex, orbitofrontal cortex, hippocampus, parahippocampus, thalamus, insula, caudate nucleus, putamen, fusiform gyrus, temporal pole, and gyrus rectus (Fjaeldstad et al., 2017; Han et al., 2019). These olfactory-related regions were defined with WFU PickAtlas software (ANSIR, Wake Forest University, Winston-Salem, NC, USA) (Maldjian et al., 2003). Regions shown to be significant in ANOVA of ALFF and ReHo were selected as the seeds when calculating FC to the other olfactory-related regions.
Statistics
Statistical Package for Social Sciences version 25.0 (IBM SPSS 25.0, Chicago, IL, USA) was used for the statistical analyses. Across the 4 groups (NC, SCD, MCI, and AD), Analysis of Covariance (ANCOVA) was used to compare the scores of the OI and neuropsychological tests controlling for age, sex, and years of education. Post-hoc least significant difference tests were used for multiple comparisons. Additionally, ANCOVA was used to compare the GMV, ALFF, and ReHo among the 4 groups, and control variables included age, sex, and years of education. For the comparisons of GMV, the total intracranial volume was also included as a control variable. Least significant difference post hoc analysis was used for multiple comparisons. Multiple comparisons correction was performed using a cluster false discovery rate at P < .05. Regions that showed significant differences in the ANCOVA of ALFF and ReHo were selected as the seeds, and their FC to other olfactory-related regions were calculated respectively. Across all participants, partial correlations were used to explore the associations between OI, MMSE, and neuroimaging indicators, which had been shown to be significantly different post hoc of ANCOVA. Control variables included age, gender, and years of education. Furthermore, multiple linear regression analyses were used to explore which neuroimaging indicators were most associated with OI and MMSE. Mediation analyses were performed for potential variables screened in multiple linear regression analyses. The mediation model is established when the following conditions are met: (1) the independent variable (IV) has a significant effect on the dependent variable (DV); (2) the IV significantly predicts the mediator; (3) the mediator significantly affects the DV; and (4) when the mediator is excluded in the model, the effect of the IV on the DV decreases. In our analysis, OI scores were regarded as IV, MMSE scores as DVs, and neuroimaging indicators as mediators. PROCESS was used to investigate the mediation model among variables (Umeh 2019). Indirect effects were estimated with 1000 bootstrapped samples. Moreover, the Sobel test was performed to verify whether the mediating effect was significant (Sobel 1982).
RESULTS
Demographic, Olfactory, and Cognitive Information
The demographic, olfactory, and neuropsychological information of different participants is listed in Table 1. No significant difference was found in cognitive scores between the SCD and NC groups (P > .05). The MCI group exhibited lower cognitive scores than the NC and SCD groups, and AD exhibited lower scores of global cognition than the other 3 groups. The SCD and MCI groups exhibited lower OI scores than the NC group and higher OI scores than the AD group (P < .05).
Table 1.
Demographic, Olfactory, and Neuropsychological Information of the NC, SCD, MCI, and AD Groups
| NC (n = 50) | SCD (n = 70) | MCI (n = 118) | AD (n = 31) | F/χ 2 /Z | P | Post-hoc | |
|---|---|---|---|---|---|---|---|
| Male (%) | 18 (36.0%) | 23 (34.3%) | 34 (28.8%) | 12 (38.7%) | 1.647 | .649 | — |
| Age | 64.5 ± 4.4 | 67.3 ± 5.7 | 67.9 ± 7.7 | 69.9 ± 11.0 | 4.061 | .008 | A<B,C,D |
| Education, y | 10.5 ± 2.7 | 11.5 ± 2.9 | 9.1 ± 3.7 | 8.2 ± 4.6 | 10.208 | <.001 | A,B>C,D |
| Odor identification | 12.8 ± 2.0 | 10.8 ± 2.3 | 10.6 ± 2.3 | 6.6 ± 2.7 | 32.631 | <.001 | A>B,C>D |
| Global cognition | 27.2 ± 1.8 | 27.3 ± 2.0 | 25.4 ± 2.6 | 12.0 ± 4.5 | 271.592 | <.001 | A,B>C>D |
| Memory | 7.1 ± 2.0 | 6.9 ± 2.3 | 4.6 ± 2.6 | — | 38.861 | <.001 | A,B>C |
| Language | 23.7 ± 1.8 | 23.3 ± 2.7 | 19.9 ± 3.3 | — | 47.964 | <.001 | A,B>C |
| Executive function (sec) | 61.6 ± 21.9 | 60.0 ± 19.9 | 79.4 ± 32.4 | — | 18.541 | <.001 | A,B>C |
| Visuospatial skill | 27.6 ± 4.2 | 27.9 ± 3.8 | 25.1 ± 5.9 | — | 19.226 | <.001 | A,B>C |
| Attention | 36.7 ± 9.9 | 36.6 ± 9.7 | 30.0 ± 10.1 | — | 18.071 | <.001 | A,B>C |
Abbreviations: AD, Alzheimer’s disease; MCI, mild cognitive impairment; NC, normal control; SCD, subjective cognitive decline.
In the post-hoc comparison, A represents the NC group, B represents the SCD group, C represents the MCI group, and D represents the AD group.
Comparisons of Olfactory-Related Regions Among the AD, MCI, SCD, and NC Groups
Comparison of GMV in Olfactory-Related Regions
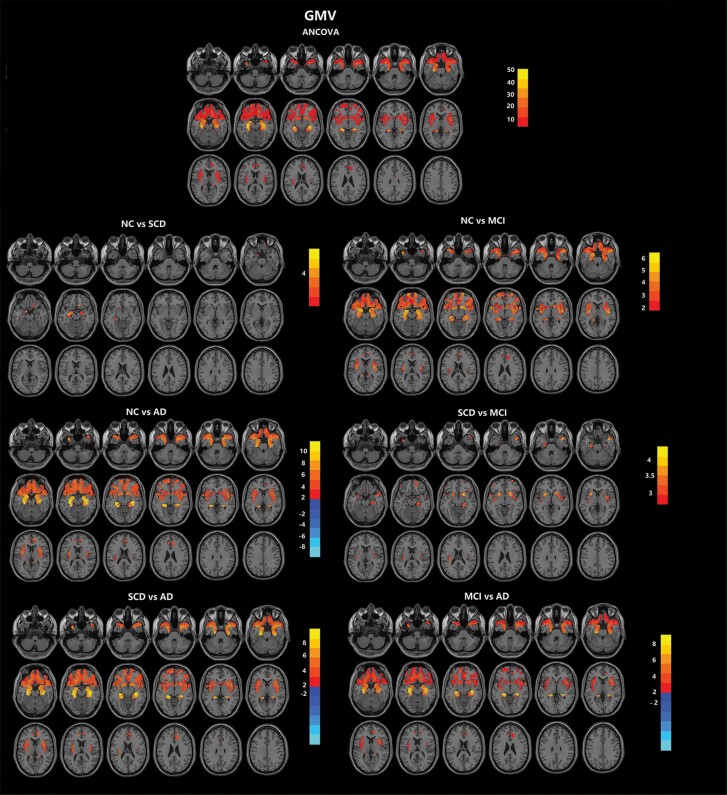
In the comparison of GMV, significant differences were found in the bilateral hippocampus, parahippocampus, temporal pole, insula, orbital frontal cortex, amygdala, fusiform gyrus, gyrus rectus, entorhinal cortex, caudate nucleus, putamen, left superior temporal gyrus, right putamen, and left insula across the 4 groups. In the post-hoc multiple comparisons, the GMV exhibited a tendency of AD < MCI < SCD < NC. Notably, the AD group exhibited increased GMV in the anterior caudate nucleus compared with the other 3 groups, although other portions of the caudate nucleus showed reduced GMV (Figure 1; Table 2).
Figure 1.
Comparison of GMV in olfactory-related regions among the AD, MCI, SCD, and NC groups. The olfactory-related regions were defined as the ROI, including the piriform cortex, amygdala, entorhinal cortex, orbitofrontal cortex, hippocampus, parahippocampus, thalamus, insula, caudate nucleus, putamen, fusiform gyrus, temporal pole, and gyrus rectus. Across the 4 groups, significant differences in GMV were found in the bilateral orbital frontal cortex, temporal pole, hippocampus, parahippocampus, insula, fusiform gyrus, amygdala, caudate nucleus, putamen, rectus, entorhinal cortex, thalamus, and piriform cortex. More details of the comparison are listed in Table 2. Multiple comparison correction was performed using a false discovery rate at P < .05. The color scale bar shows the logarithmic scale of P values (-log10). The closer to yellow or light blue, the more significant the difference between groups. Abbreviations: AD, Alzheimer’s disease; ANCOVA, analysis of covariance; GMV, grey matter volume, MCI, mild cognitive impairment; NC, normal control; SCD, subjective cognitive decline.
Table 2.
Comparison of Neuroimaging Indicators in Olfactory-Related Regions in the NC, SCD, MCI, and AD Groups
| Peak MNI | ||||||
|---|---|---|---|---|---|---|
| Comparison | Brain regions | x | y | z | Cluster size | F |
| GMV | ||||||
| F test | Bilateral orbital frontal cortex, temporal pole, hippocampus, parahippocampus, insula, fusiform gyrus, amygdala, caudate nucleus, putamen, rectus, entorhinal cortex, thalamus, piriform cortex | −25.5 | −28.5 | −6 | 48 687 | 50.10 |
| Right caudate | 18 | 24 | 12 | 35 | 7.12 | |
| NC vs SCD | Left hippocampus, parahippocampal, amygdala | −12 | 1.5 | −21 | 959 | 4.62 |
| Right hippocampus, parahippocampal, amygdala | 27 | −10.5 | −13.5 | 252 | 4.02 | |
| Right rectus, entorhinal cortex | 12 | 25.5 | −15 | 199 | 4.03 | |
| Right rectus, entorhinal cortex | 10.5 | 37.5 | −24 | 43 | 3.56 | |
| Left caudate nucleus, putamen | −10.5 | 6 | −12 | 30 | 3.69 | |
| Left hippocampus | −12 | −34.5 | 7.5 | 31 | 3.83 | |
| NC vs MCI | Bilateral orbital frontal cortex, temporal pole, hippocampus, parahippocampus, insula, fusiform gyrus, amygdala, caudate nucleus, putamen, rectus, entorhinal cortex | −12 | 1.5 | −21 | 46 601 | 6.37 |
| NC vs AD | Bilateral orbital frontal cortex, temporal pole, hippocampus, parahippocampus, insula, fusiform gyrus, amygdala, caudate nucleus, putamen, rectus, entorhinal cortex, thalamus | −30 | −13.5 | −13.5 | 48 667 | 11.80 |
| Right caudate nucleus | 21 | 25.5 | 10.5 | 35 | −4.01 | |
| SCD vs MCI | Right putamen, insula, caudate, amygdala | 19.5 | 6 | −9 | 2332 | 4.34 |
| Left putamen, parahippocampal, insula, fusiform gyrus, hippocampus | −24 | 16.5 | 4.5 | 1676 | 4.33 | |
| Right temporal pole | 45 | 6 | −24 | 1236 | 4.18 | |
| Left insula | −36 | −27 | 22.5 | 865 | 3.46 | |
| Right hippocampus, parahippocampal, fusiform gyrus | 22.5 | −24 | −7.5 | 832 | 3.94 | |
| Right orbital frontal cortex | 28.5 | 66 | −6 | 442 | 3.81 | |
| Left temporal pole | −55.5 | 6 | −4.5 | 197 | 3.38 | |
| Right orbital frontal cortex, insula | 34.5 | 30 | −7.5 | 167 | 3.08 | |
| Left orbital frontal cortex | −45 | 43.5 | −7.5 | 146 | 3.17 | |
| Left temporal pole | −34.5 | 0 | −43.5 | 83 | 3.68 | |
| Left orbital frontal cortex | −3 | 63 | −1.5 | 30 | 2.82 | |
| SCD vs AD | Bilateral orbital frontal cortex, temporal pole, hippocampus, parahippocampus, insula, fusiform gyrus, amygdala, caudate nucleus, putamen, rectus, entorhinal cortex | −27 | −21 | −24 | 48 553 | 9.62 |
| Right caudate nucleus | 21 | 25.5 | 10.5 | 35 | −3.21 | |
| MCI vs AD | Bilateral orbital frontal cortex, temporal pole, hippocampus, parahippocampus, insula, fusiform gyrus, amygdala, caudate nucleus, putamen, rectus | −25.5 | −30 | −4.5 | 40 180 | 8.90 |
| Right orbital frontal cortex | 28.8 | 55.5 | −6 | 188 | 3.01 | |
| Right caudate nucleus | 18 | 24 | 12 | 35 | −3.99 | |
| Left temporal pole | −55.5 | 3 | 0 | 14 | 2.34 | |
| ALFF | ||||||
| F test | Right hippocampus, parahippocampal, orbital frontal cortex, insula, putamen, amygdala, bilateral rectus | 36 | −33 | −9 | 1488 | 14.47 |
| Left hippocampus, parahippocampal, fusiform gyrus, amygdala | −36 | −24 | −15 | 299 | 12.26 | |
| Left caudate nucleus, insula, putamen | −39 | −3 | 15 | 231 | 7.54 | |
| NC vs AD | Right hippocampus, parahippocampal, fusiform gyrus | 15 | −9 | −15 | 277 | −4.20 |
| Bilateral rectus | 3 | 21 | −18 | 58 | −3.86 | |
| Right caudate nucleus | 12 | 12 | −3 | 26 | −3.37 | |
| SCD vs AD | Right hippocampus, parahippocampal, caudate nucleus, insula, putamen, orbital frontal cortex, rectus, amygdala | 27 | −30 | −12 | 1318 | −5.21 |
| MCI vs AD | Right hippocampus, parahippocampal, amygdala | 21 | 3 | −27 | 512 | −5.18 |
| Right caudate nucleus, rectus | 12 | 12 | −3 | 283 | −4.31 | |
| Right insula | 42 | 0 | 9 | 48 | −3.95 | |
| ReHo | ||||||
| F test | Right caudate nucleus | 15 | 18 | 15 | 69 | 8.82 |
| Left caudate nucleus | −15 | 3 | 24 | 28 | 9.28 | |
| NC vs SCD | Right caudate nucleus | 15 | 18 | 15 | 14 | −2.75 |
| Left caudate nucleus | −6 | 9 | 12 | 28 | −5.15 | |
| NC vs MCI | Right caudate nucleus | 12 | 15 | 12 | 10 | −2.50 |
| NC vs AD | Right caudate nucleus | 9 | 9 | 15 | 69 | −5.14 |
| Left caudate nucleus | −15 | 3 | 24 | 26 | 9.14 | |
| SCD vs AD | Right caudate nucleus | 15 | 21 | 6 | 65 | −4.15 |
| Left caudate nucleus | −15 | 3 | 24 | 27 | −4.12 | |
| MCI vs AD | Right caudate nucleus | 15 | 21 | 6 | 68 | −4.11 |
| Left caudate nucleus | −18 | 0 | 18 | 27 | −4.07 | |
| FC, left hippocampus | ||||||
| F test | Left parahippocampal | −27 | −36 | −12 | 74 | 11.59 |
| Right hippocampus, parahippocampal | 24 | −33 | −15 | 57 | 12.10 | |
| NC vs SCD | Left parahippocampal | −21 | −39 | −9 | 15 | 2.96 |
| Right hippocampus, parahippocampal | 27 | −33 | −9 | 11 | 2.65 | |
| NC vs AD | Left parahippocampal | −24 | −39 | −12 | 73 | 5.19 |
| Right hippocampus, parahippocampal | 27 | −33 | −15 | 57 | 5.24 | |
| SCD vs AD | Left parahippocampal | −27 | −36 | 0 | 66 | 4.41 |
| Right hippocampus, parahippocampal | 24 | −33 | −15 | 52 | 4.31 | |
| MCI vs AD | Left parahippocampal | −27 | −33 | −12 | 74 | 5.37 |
| Right hippocampus, parahippocampal | 24 | −33 | −15 | 57 | 5.58 | |
| FC, right hippocampus | ||||||
| F test | Right parahippocampal | 24 | −33 | −15 | 80 | 13.99 |
| Right caudate nucleus | 18 | 6 | 18 | 63 | 10.93 | |
| NC vs MCI | Right caudate nucleus | 18 | 3 | 21 | 25 | 3.34 |
| NC vs AD | Right parahippocampal | 27 | −33 | −15 | 76 | 5.21 |
| Right caudate nucleus | 18 | 6 | 18 | 63 | 5.29 | |
| SCD vs AD | Right parahippocampal | 21 | −33 | −12 | 70 | 4.91 |
| Right caudate nucleus | 18 | 6 | 18 | 57 | −4.40 | |
| MCI vs AD | Right parahippocampal | 24 | −33 | −15 | 80 | 5.72 |
| Right caudate nucleus | 18 | 6 | 15 | 51 | 4.17 | |
Abbreviations: AD, Alzheimer’s disease; ALFF, amplitude of low-frequency fluctuation; FC, functional connectivity; GMV, grey matter volume; MCI, mild cognitive impairment; MNI, Montreal Neurological Institute; NC, normal control; ReHo, regional homogeneity; SCD, subjective cognitive decline.
The olfactory-related regions were defined as the regions of interest, including the piriform cortex, amygdala, entorhinal cortex, orbito-frontal cortex, hippocampus, parahippocampus, thalamus, insula, caudate nucleus, putamen, fusiform gyrus, temporal pole, gyrus rectus.
Comparison of ALFF in Olfactory-Related Regions
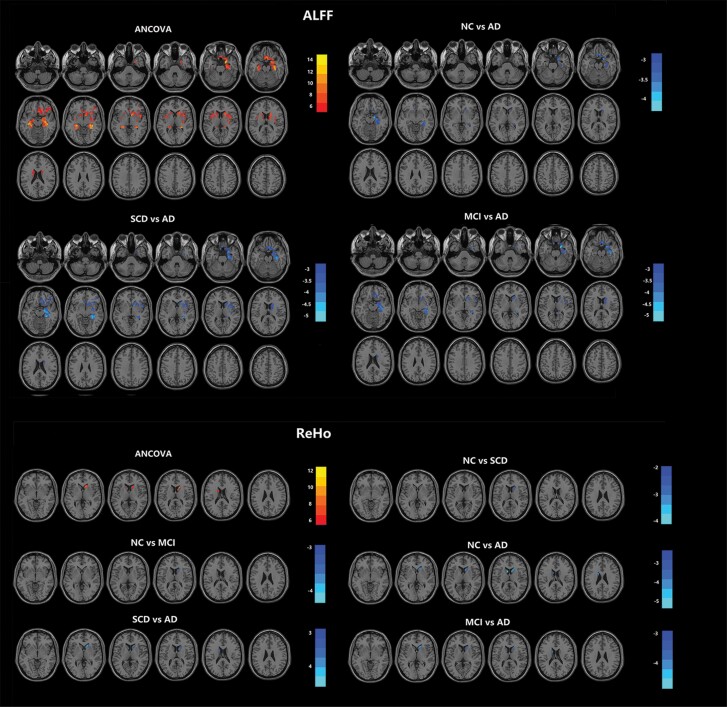
In the comparison of ALFF, significant differences were found in the bilateral hippocampus, bilateral parahippocampal gyrus, bilateral insula, bilateral putamen, bilateral gyrus rectus, left caudate nucleus, right amygdala, and right orbital-frontal cortex across the 4 groups. In the post-hoc multiple comparisons, the AD group exhibited increased ALFF compared with the other 3 groups (Figure 2; Table 2), and there was no significant difference between the other groups.
Figure 2.
Comparison of ALFF and ReHo in olfactory-related regions among the AD, MCI, SCD, and NC groups. The olfactory-related regions were defined as the ROI, including the piriform cortex, amygdala, entorhinal cortex, orbitofrontal cortex, hippocampus, parahippocampus, thalamus, insula, caudate nucleus, putamen, fusiform gyrus, temporal pole, and gyrus rectus. Across the 4 groups, significant differences in ALFF were found in the right hippocampus, parahippocampal cortex, orbital frontal cortex, insula, putamen, amygdala, bilateral rectus, left hippocampus, parahippocampal cortex, fusiform gyrus, amygdala, left caudate nucleus, insula, and putamen; significant differences in ReHo were found in the bilateral caudate nucleus. More details of the comparison are listed in Table 2. Multiple comparison correction was performed using a false discovery rate at P < .05. The color scale bar shows the logarithmic scale of P values (-log10). The closer to yellow or light blue, the more significant the difference between groups. Abbreviations: AD, Alzheimer’s disease; ALFF, amplitude of low-frequency fluctuation ReHo, regional homogeneity; ANCOVA, analysis of covariance; MCI, mild cognitive impairment; NC, normal control; SCD, subjective cognitive decline.
Comparison of ReHo in Olfactory-Related Regions
In the comparison of ReHo, significant differences were found in the bilateral caudate nuclei across the 4 groups. In the post-hoc multiple comparisons, the AD group exhibited increased ReHo compared with the other 3 groups. In addition, the SCD group and MCI group showed increased ReHo compared with the NC group (Figure 2; Table 2). No significant difference among the 4 groups was found.
Comparison of FC in Olfactory-Related Regions
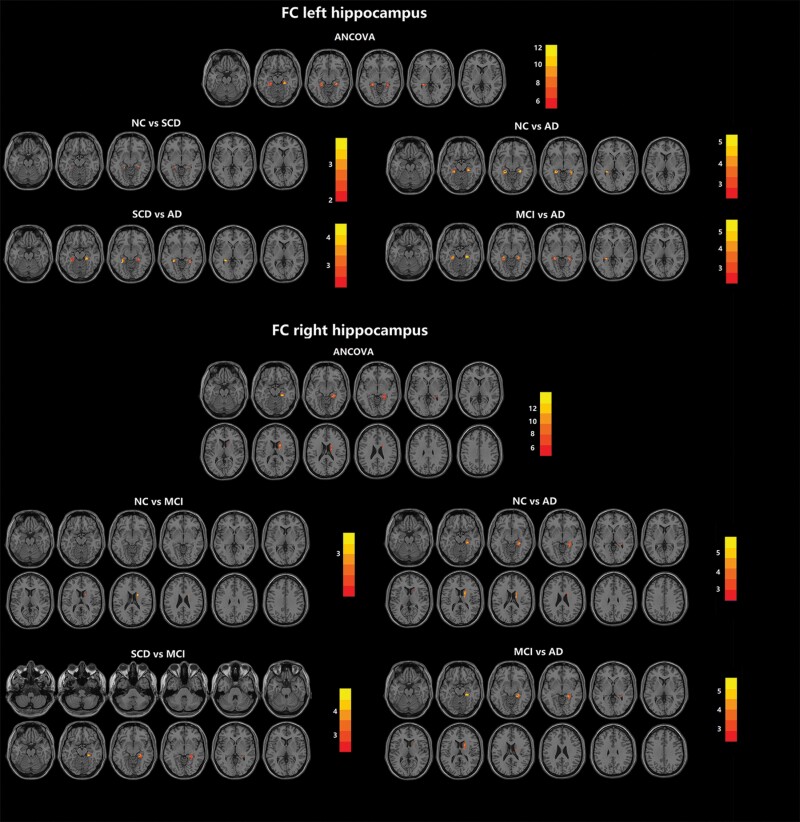
Regions shown to be significant in the ANCOVA of ALFF and ReHo were selected as seeds, including the bilateral hippocampus, bilateral parahippocampal gyrus, bilateral insula, bilateral putamen, bilateral gyrus rectus, caudate nucleus, right amygdala, and right orbital-frontal cortex. Across the 4 groups, significant differences were found in the FC of the right hippocampus and bilateral parahippocampus when the left hippocampus was chosen as the seed and in the FC of the right parahippocampus and right caudate nucleus when the right hippocampus was chosen as the seed. When the left hippocampus was chosen as the seed, the AD group exhibited decreased FC compared with the other 3 groups, and the SCD group exhibited decreased FC compared with the NC group. When the right hippocampus was chosen as the seed, the AD group exhibited decreased FC compared with the other 3 groups, and the MCI group exhibited decreased FC compared with the NC group (Figure 3; Table 2). No significant difference in FC was found when the other regions were chosen as the seeds. Taken together, the results of ANCOVA are summarized in Table 3.
Figure 3.
Comparison of FC in olfactory-related regions among the AD, MCI, SCD, and NC groups. The olfactory-related regions were defined as the ROI, including the piriform cortex, amygdala, entorhinal cortex, orbitofrontal cortex, hippocampus, parahippocampus, thalamus, insula, caudate nucleus, putamen, fusiform gyrus, temporal pole, and gyrus rectus. Across the 4 groups, significant differences were found in the FC of the right hippocampus and bilateral parahippocampus when the left hippocampus was chosen as the seed and in the FC of the right parahippocampus and right caudate nucleus when the right hippocampus was chosen as the seed. More details of the comparison are listed in Table 2. Multiple comparison correction was performed using a false discovery rate at P < .05. The color scale bar shows the logarithmic scale of P values (-log10). The closer to yellow, the more significant the difference between groups. Abbreviations: AD, Alzheimer’s disease; ANCOVA, analysis of covariance; FC, functional connectivity; MCI, mild cognitive impairment; NC, normal control; SCD, subjective cognitive decline.
Table 3.
Summary of Abnormal Olfactory-Related Regions Among the NC, SCD, MCI, and AD Groups
| Significant in ANOVA | Post-hoc | |
|---|---|---|
| Piriform cortex | GMV (bilateral) | GMV: AD was lower than other 3 groups (bilateral); MCI was lower than NC (bilateral) |
| Entorhinal cortex | GMV (bilateral) | GMV: AD was lower than other 3 groups (bilateral); MCI and SCD was lower than NC (bilateral) |
| Amygdala | GMV (bilateral) ALFF (bilateral) |
GMV: AD was lower than the other 3 groups (bilateral); MCI was lower than NC (bilateral) and SCD (right); SCD was lower than NC (bilateral) ALFF: AD was higher than MCI (right) |
| Hippocampus | GMV (bilateral) ALFF (bilateral) FC (left hippocampus to right hippocampus and bilateral parahippocampus; right hippocampus to right parahippocampus and right caudate) |
GMV: AD was lower than other 3 groups (bilateral); MCI was lower than NC and SCD (bilateral); SCD was lower than NC (bilateral) ALFF: AD was higher than other 3 groups (right) FC: AD was lower than other 3 groups (left hippocampus to right hippocampus and bilateral parahippocampus; right hippocampus to right parahippocampus and right caudate); MCI was lower than NC (right hippocampus to right caudate); SCD was lower than NC (left hippocampus to bilateral parahippocampus) |
| Parahippocampus | GMV (bilateral) ALFF (bilateral) FC (left hippocampus to bilateral parahippocampus; right hippocampus to right parahippocampus) |
GMV: AD was lower than other 3 groups (bilateral); MCI was lower than NC and SCD (bilateral); SCD was lower than NC (bilateral) ALFF: AD was higher than other 3 groups (right) FC: AD was lower than other 3 groups (left hippocampus to bilateral parahippocampus; right hippocampus to right parahippocampus); SCD was lower than NC (left hippocampus to bilateral parahippocampus) |
| Orbito-frontal cortex | GMV (bilateral) ALFF (right) |
GMV: AD was lower than the other 3 groups (bilateral), MCI was lower than NC and SCD (bilateral) ALFF: AD higher than the other 3 groups (right) |
| Thalamus | GMV (bilateral) | GMV: AD was lower than the other 3 groups (bilateral) |
| Insular | GMV (bilateral) ALFF (bilateral) |
GMV: AD was lower than the other 3 groups (bilateral); MCI was lower than NC and SCD (bilateral) ALFF: AD was higher than SCD and MCI (right) |
| Caudate nucleus | GMV (bilateral) ALFF (bilateral) ReHo(bilateral) FC (right hippocampus to right caudate) |
GMV: AD was lower than the other 3 groups (bilateral), and higher than the other 3 groups (right, anterior part); MCI was lower than NC (bilateral) and SCD (right); SCD was lower than NC (left) ALFF: AD was higher than the other 3 groups (right) ReHo: AD was higher than the other 3 groups (bilateral), MCI were higher than NC (right); SCD was higher than NC (bilateral) FC: AD was lower than the other 3 groups (right hippocampus to right caudate); MCI was lower than NC (right hippocampus to right caudate) |
| Putamen | GMV (bilateral) ALFF (bilateral) |
GMV: AD was lower than the other 3 groups (bilateral); MCI was lower than NC and SCD (bilateral); SCD was lower than NC (left) ALFF: AD was higher than SCD (right) |
| Fusiform gyrus | GMV (bilateral) ALFF (bilateral) |
GMV: AD was lower than the other 3 groups (bilateral); MCI was lower than NC and SCD (bilateral) ALFF: AD was higher than NC (right) |
| Temporal pole | GMV (bilateral) | GMV: AD was lower than the other 3 groups (bilateral); MCI was lower than NC and SCD (bilateral) |
| Gyrus rectus | GMV (bilateral) ALFF (bilateral) |
GMV: AD was lower than the other 3 groups (bilateral); MCI was lower than NC (bilateral); SCD was lower than NC (right) ALFF: AD was higher than NC (bilateral), MCI (right) and SCD (right) |
Abbreviations: AD, Alzheimer’s disease; ALFF, amplitude of low-frequency fluctuation; FC, functional connectivity; GMV, grey matter volume; MCI, mild cognitive impairment; MNI, Montreal Neurological Institute; NC, normal control; ReHo, regional homogeneity; SCD, subjective cognitive decline.
Associations Among OI Dysfunction, Cognitive Impairment, and Brain Abnormalities
Correlation Analyses
According to the ANCOVA, hippocampal and caudate abnormalities were included in the correlation analyses. Across all participants, OI was positively associated with MMSE scores (r = 0.582, P < .001), GMV (bilateral hippocampus), and FC (from right hippocampus to right caudate nucleus) and negatively associated with ReHo (right caudate nucleus) (Table 4). In addition, MMSE scores were positively associated with GMV (bilateral hippocampus and right caudate nucleus) and FC (left hippocampus to right hippocampus, right hippocampus to right caudate nucleus) and negatively associated with ALFF (ALFF of bilateral hippocampus) and ReHo (bilateral caudate nucleus) (P < .05) (Table 4). In the SCD group, OI was associated with GMV of the left hippocampus (r = 0.282, P = .034) and right hippocampus (r = 0.300, P = .023); MMSE was associated with GMV of the left hippocampus (r = 0.267, P = .029); OI was not associated with MMSE (r = 0.156, P = .248).
Table 4.
Correlations Between OI, MMSE, and Neuroimaging Indicators Across All Participants
| OI | Global cognition | |||
|---|---|---|---|---|
| r | P | r | P | |
| GMV of left hippocampus | 0.452 | <.001* | 0.510 | <.001* |
| GMV of right hippocampus | 0.439 | <.001* | 0.495 | <.001* |
| GMV of left caudate nucleus | −0.026 | .698 | -0.224 | .053 |
| GMV of right caudate nucleus | −0.065 | .336 | 0.224 | .009* |
| ALFF of right hippocampus | −0.055 | .422 | -0.152 | .016 |
| ALFF of right hippocampal | −0.072 | .295 | -0.186 | .003* |
| ReHo of left caudate nucleus | −0.043 | .528 | -0.144 | .035* |
| ReHo of right caudate nucleus | −0.238 | <.001* | -0.297 | <.001* |
| FC from left hippocampus to right hippocampus | −0.006 | .935 | 0.213 | .019* |
| FC from right hippocampus to right caudate nucleus | 0.180 | .009* | 0.124 | .050 |
Abbreviations: ALFF, amplitude of low-frequency fluctuation; FC, functional connectivity; GMV, grey matter volume; MMSE, Mini-Mental State Examination; OI, odor identification; ReHo, regional homogeneity.
*P < .05.
Regression Analyses
Among the different neuroimaging indicators associated with OI in the correlation analyses, GMV of the left hippocampus was the only variable associated with OI (R2 = 0.195, β = 0.442, t = 7.133, P < .001, 95% CI [14.334 to 25.283]) across all participants. Among the different neuroimaging indicators associated with MMSE in the correlation analyses, GMV of the left hippocampus (β = 0.486, t = 7.883, P < .001, 95% CI [29.483 to 49.124]) and ReHo of the right caudate nucleus (β = −0.155, t = −2.708, P = .007, 95% CI [−13.918 to −2.196]) were associated with MMSE (R2 = 0.271).
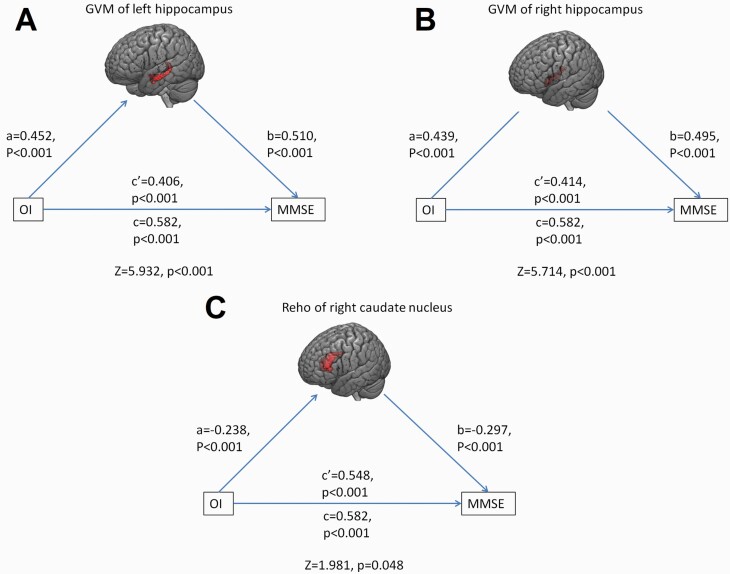
Mediation Analyses
Across all participants, the indirect effect of OI on MMSE through GMV of left hippocampus was 0.176 (Z = 5.932, P < .001), and the remaining direct effect of OI on MMSE was still significant, which manifested as OI positively affecting MMSE by upregulating GMV of left hippocampus (Figure 4A). The indirect effect of OI on MMSE through GMV of right hippocampus was 0.168 (Z = 5.714, P < .001), and the remaining direct effect of OI on MMSE was still significant, which manifested as OI positively affected MMSE by upregulating GMV of right hippocampus (Figure 4B). The indirect effect of OI on MMSE through ReHo of right caudate nucleus was 0.034 (Z = 1.981, P = .048), and the remaining direct effect of OI on MMSE was still significant, which manifested as OI positively affecting MMSE by downregulating ReHo of right caudate nucleus (Figure 4C). No significant mediating effect of other neuroimaging indicators on the relationships between OI and global cognition was found.
Figure 4.
Abnormalities in olfactory-related regions mediated the relationship between OI and global cognition. (A) The association between OI and MMSE was partially mediated by the GMV of the left hippocampus. (B) The association between OI and MMSE was partially mediated by GMV in the right hippocampus. (C) The association between OI and MMSE was partially mediated by ReHo of the right caudate nucleus. Abbreviations: GMV, grey matter volume; MMSE, Mini-Mental State Examination; OI, odor identification; ReHo, regional homogeneity.
Discussion
The present study first explored the changing pattern of structural and functional abnormalities of olfactory-related regions in participants with SCD, MCI, and AD, and the main findings were as follows: (1) from the SCD, MCI to AD groups, the reduced GMV, increased ALFF, increased ReHo, and reduced FC of olfactory-related regions became increasingly severe; (2) among various neuroimaging indicators, only the degrees of reduced GMV of the hippocampus and caudate nucleus clearly distinguish the AD, MCI, SCD, and NC groups; (3) SCD participants exhibited reduced GMV (bilateral hippocampus, bilateral parahippocampus, entorhinal cortex, right gyrus rectus, left caudate nucleus, and left putamen), increased ReHo (bilateral caudate nucleus), and reduced FC (between left and right hippocampus) compared with the NC group; and (4) across the 4 groups, reduced GMV of the bilateral hippocampus and increased ReHo of the right caudate nucleus were associated with OI dysfunction and global cognitive impairment, and they exhibited partially mediated effects on the relationships between OI and global cognition.
Most of the previous studies explored the brain structural and functional abnormalities in AD, MCI, and SCD patients by using whole-brain analyses (Li et al., 2015; Wang et al., 2015), but the abnormalities of their olfactory-related regions have not yet been fully clarified because some of the olfactory-related regions are relatively small, and their effect size may not reach a significant level compared with the other regions. By defining the olfactory-related regions as regions of interest, the present study demonstrated their changing pattern of olfactory-related regions in the AD spectrum, which can be summarized in Table 3. Generally, reduced GMV, increased ALFF and ReHo, and reduced FC in olfactory-related regions became increasingly obvious with the development of AD. This changing pattern can be explained by Murphy’s hypothesis: functional hyperactivation may be a compensation for increasingly serious OI dysfunction in the development of AD because patients need to make stronger efforts to maintain their normal olfactory perception when processing odor information; persistent functional hyperactivation may facilitate neurodegeneration, and the related accumulated neural damage may lead to structural abnormalities and cognitive impairment (Murphy 2019). Follow-up studies can further confirm this changing pattern of olfactory-related regions in the AD spectrum. In contrast, structural abnormalities were more widespread than resting-state functional abnormalities in olfactory-related regions in the present ANCOVA. On one hand, the ALFF, ReHo, and FC used in the present study may not be sufficient for mapping the pattern of resting-state functional abnormalities, and future studies, including network analyses, could provide a deeper understanding of the relationship between functional and structural abnormalities of olfactory-related regions in the AD spectrum. On the other hand, Murphy’s hypothesis is more strongly associated with the response to odors, since functional abnormalities of olfactory-related regions when processing olfactory information were reported in patients with MCI and AD (Vasavada et al., 2017). Future studies that include olfactory task-fMRI can further explore how the functional and structural abnormalities of olfactory-related regions interact with others.
The present study demonstrated structural and functional abnormalities of the hippocampus in SCD patients, which was consistent with previous studies (Perrotin et al., 2015; Dillen et al., 2017; Li et al., 2018; Scheef et al., 2019). Furthermore, hippocampal abnormalities were more obvious than abnormalities of other olfactory-related regions in SCD patients, and the GMV of the left hippocampus was the only neuroimaging variable associated with OI dysfunction and intact global cognition, suggesting the potential mediating role of the hippocampus in the relationship between olfaction and cognitive function. On one hand, the hippocampus is an important part of the secondary olfactory cortex and is responsible for encoding olfactory information and storing olfactory memory, which is essential for OI (Li et al., 2017). On the other hand, the hippocampus is among the first brain areas to be altered in AD, and the extent of the abnormalities may reflect the disease severity (Pini et al., 2016). The present results suggested that structural and functional abnormalities of olfactory-related regions are present early in the SCD stages of the AD spectrum, and the assessment of OI was more sensitive to reflecting hippocampal abnormalities in SCD patients than cognitive assessments.
Interestingly, not all the abnormalities of olfactory-related regions in the SCD group became more obvious in the MCI group (ALFF of the hippocampus, etc.), and the SCD group also exhibited more obvious abnormalities than the MCI group in ReHo of left caudate nucleus, etc. On one hand, the development of abnormalities of olfactory-related regions in AD spectrum may be nonlinear, and abnormalities in MCI are not necessary to be more severe than SCD. On the other hand, a portion of SCD and MCI patients may not progress to AD in follow-up, and their olfactory-related regions may remain intact, which reduces the difference between the 2 groups. Future studies including PET-CT or CSF markers can screen out Aβ-positive patients with SCD and MCI and provide a more precise damaging pattern of olfactory-related regions in the AD spectrum.
Apart from the hippocampus, the caudate nucleus was the only nucleus that exhibited different degrees of functional and structural abnormalities in the SCD, MCI, and AD groups. Furthermore, AD and MCI patients showed significantly reduced FC between the right hippocampus and right caudate. The caudate nucleus is not only a part of the olfactory pathway but is also involved in cognitive processes (attention, planning, and execution of behavior to achieve complex goals) and integrates major inputs from the dorsolateral and orbitofrontal cortices (Pini et al., 2016). Additionally, caudate abnormalities were found to be associated with MCI progression to AD and CSF tau levels (Wang et al., 2020b), and hippocampal–caudate connectivity was associated with dopamine D2 receptor availability (Nyberg et al., 2016), episodic memory, and contextually dependent navigation (Brown et al., 2012). Interestingly, AD patients exhibited a local increase in GMV in the anterior part of the caudate nucleus. Enlargement of the caudate nucleus in AD patients has also been reported in studies by Persson et al. and Tang et al. and could result from amyloid accumulation, neuroinflammation, and tissue reorganization caused by ventricular enlargement (Tang et al., 2014; Persson et al., 2018). Taken together, this evidence demonstrates that structural and functional changes in the caudate nucleus are important markers in the AD spectrum: they present early in the SCD stage and deepen with disease severity. The special role of the caudate nucleus in the AD spectrum (especially in the SCD stage) is worthy of further investigation.
Because the hippocampus and caudate nucleus exhibited different degrees of abnormalities in the AD, MCI, and SCD groups, we further explored their relationships with OI and cognition. Respectively, scores of OI and MMSE were associated with various hippocampal and caudate abnormalities, suggesting grey matter atrophy, functional hyperactivation, and impaired connectivity of olfactory-related regions involved in OI dysfunction and cognitive impairment. Furthermore, the mediation analyses suggest that GMV of the bilateral hippocampus and ReHo of the right caudate nucleus were partial mediators of the relationships between OI dysfunction and cognitive impairment, suggesting that OI may influence cognition not only directly but also through the mediation of the hippocampus and caudate nucleus. These results again confirm Murphy’s hypothesis: OI dysfunction may cause hyperactivation of overlapping regions related to olfaction and cognition, and persistent hyperactivation facilitates structural abnormalities, leading to cognitive impairment in AD spectrum diseases (Murphy 2019). Other overlapping regions (such as the orbital-frontal cortex, insula, and putamen) may also contribute to the relationship between OI and global cognition, but the present work indicates that the hippocampus and caudate nucleus may play the most crucial roles in this mediation. It should be noted that the present study, using resting-state fMRI to explore brain function in olfactory-related regions, can only provide information on correlations but not causality. Future studies using task-based fMRI and designing odor-related stimulus tasks can provide more information about how OI dysfunction, cognitive impairment, and brain abnormalities interact with each other.
There were limitations in the present study. First, the present study compared the difference in olfactory-related regions in the AD spectrum based on a cross-sessional cohort, which needs to be further confirmed by follow-up studies. Second, several other neurodegenerative diseases, such as Lewy body dementia and Parkinson’s disease, also exhibit OI dysfunction and cognitive impairment, which may be confounding factors of the present study. The lack of specificity of OI dysfunction needs to be improved by future studies, including assessments of Aβ and tau. Third, some of the AD patients were taking regular or irregular treatment with cholinesterase inhibitors, which may influence the results of functional analyses. Fourth, the current study only included OI, the strongest predictor of AD. Future studies including odor thresholds and discrimination could provide a deeper understanding of the relationship between olfaction and the AD spectrum. Finally, AAL 90 was used as the template to define the olfactory network in the present study, and further studies using templates with more detailed segments could provide a deeper understanding of how the olfactory network changes as the disease progresses in the AD spectrum.
Conclusions
In summary, the present study provided important novel insights into the pathophysiological substrate of OI dysfunction in SCD, MCI, and AD. We demonstrated that structural and functional abnormalities of olfactory-related regions are already present with SCD and deepen with disease severity in the AD spectrum. Additionally, the hippocampus and caudate nucleus may be the most important olfactory-related regions joining OI dysfunction and cognitive impairment, which provide potential targets for neuromodulation of AD spectrum diseases.
Acknowledgments
We thank Cong Ouyang, Weiru Zhang, Wanyuan Liang, and Chunying Dai for assistance in collecting the data. We are grateful for assistance from the Department of Neurology, the Department of Geriatric Psychiatry of the Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital).
This study was supported by a grant from the National Natural Science Foundation of China (nos. 81701341 and 82101508), the Guangzhou Municipal Psychiatric Diseases Clinical Transformation Laboratory (no. 201805010009), the Key Laboratory for Innovation Platform Plan, the Science and Technology Program of Guangzhou, China, the Science and Technology Plan Project of Guangdong Province (no. 2019B030316001), and the National Key Research and Development Program of China (no. 2016YFC0906300).
Contributor Information
Ben Chen, Memory Clinic, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, Guangdong Province, China.
Qiang Wang, Memory Clinic, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, Guangdong Province, China; Department of Geriatric Psychiatry, The Second People’s Hospital of Dali Bai Autonomous Prefecture, Dali, Yunnan Province, China.
Xiaomei Zhong, Memory Clinic, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, Guangdong Province, China.
Naikeng Mai, Memory Clinic, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, Guangdong Province, China.
Min Zhang, Memory Clinic, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, Guangdong Province, China.
Huarong Zhou, Memory Clinic, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, Guangdong Province, China.
Antje Haehner, Smell and Taste Clinic, Department of Otorhinolaryngology, Technische Universität Dresden, Dresden, Germany.
Xinru Chen, Memory Clinic, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, Guangdong Province, China.
Zhangying Wu, Memory Clinic, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, Guangdong Province, China.
Lavinia Alberi Auber, Department of Medicine, University of Fribourg, Fribourg, Switzerland; Swiss Integrative Center of Human Health, Fribourg, Switzerland.
Dongping Rao, Memory Clinic, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, Guangdong Province, China.
Wentao Liu, Memory Clinic, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, Guangdong Province, China.
Jinhong Zheng, Guangzhou Medical University, Guangzhou, Guangdong Province, China.
Lijing Lin, Guangzhou Medical University, Guangzhou, Guangdong Province, China.
Nanxi Li, Guangzhou Medical University, Guangzhou, Guangdong Province, China.
Sihao Chen, Guangzhou Medical University, Guangzhou, Guangdong Province, China.
Bingxin Chen, Guangzhou Medical University, Guangzhou, Guangdong Province, China.
Thomas Hummel, Smell and Taste Clinic, Department of Otorhinolaryngology, Technische Universität Dresden, Dresden, Germany.
Yuping Ning, Memory Clinic, Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, Guangdong Province, China; The First School of Clinical Medicine, Southern Medical University, Guangzhou, Guangdong Province, China; Guangdong Engineering Technology Research Center for Translational Medicine of Mental Disorders, Guangzhou, China.
Interest Statement
None.
References
- Ashburner J, Barnes G, Chen CC, Daunizeau J, Flandin G, Friston K, Glauche V, Henson R, Hutton C, Kiebel S (2004) Statistical parametric mapping. Pract Neurol 4:350–355. [Google Scholar]
- Bahar-Fuchs A, Chételat G, Villemagne VL, Moss S, Pike K, Masters CL, Rowe C, Savage G (2010) Olfactory deficits and amyloid-β burden in Alzheimer’s disease, mild cognitive impairment, and healthy aging: a PiB PET study. J Alzheimers Dis 22:1081–1087. [DOI] [PubMed] [Google Scholar]
- Bathini P, Brai E, Auber LA (2019) Olfactory dysfunction in the pathophysiological continuum of dementia. Ageing Res Rev 55:100956. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E (1997) Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 18:351–357. [DOI] [PubMed] [Google Scholar]
- Brown TI, Ross RS, Tobyne SM, Stern CE (2012) Cooperative interactions between hippocampal and striatal systems support flexible navigation. Neuroimage 60:1316–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casa MF, Ramirez A (2020) Minor neuropsychological deficits in patients with subjective cognitive decline. Neurology 95:e1134–e1143. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, de Leon MJ, Doty RL, Stern Y, Pelton GH (2008) Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biol Psychiatry 64:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillen KNH, Jacobs HIL, Kukolja J, Richter N, von Reutern B, Onur ÖA, Langen KJ, Fink GR (2017) Functional disintegration of the default mode network in prodromal Alzheimer’s disease. J Alzheimers Dis 59:169–187. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P (2007) Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 6:734–746. [DOI] [PubMed] [Google Scholar]
- Fjaeldstad A, Fernandes HM, Van Hartevelt TJ, Gleesborg C, Møller A, Ovesen T, Kringelbach ML (2017) Brain fingerprints of olfaction: a novel structural method for assessing olfactory cortical networks in health and disease. Sci Rep 7:42534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Growdon ME, Schultz AP, Dagley AS, Amariglio RE, Hedden T, Rentz DM, Johnson KA, Sperling RA, Albers MW, Marshall GA (2015) Odor identification and Alzheimer disease biomarkers in clinically normal elderly. Neurology 84:2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, Zang Y, Akshita J, Hummel T (2019) Magnetic resonance imaging of human olfactory dysfunction. Brain Topogr 32:987–997. [DOI] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G (1997) ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22:39–52. [DOI] [PubMed] [Google Scholar]
- Jessen F, et al. ; Subjective Cognitive Decline Initiative (SCD-I) Working Group . (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashibayashi T, Takahashi R, Fujita J, Kamimura N, Okutani F, Kazui H (2020) Correlation between regional brain volume and olfactory function in very mild amnestic patients. J Neurol Sci 411:116686. [DOI] [PubMed] [Google Scholar]
- Lafaille-Magnan ME, Poirier J, Etienne P, Tremblay-Mercier J, Frenette J, Rosa-Neto P, Breitner JCS; PREVENT-AD Research Group (2017) Odor identification as a biomarker of preclinical AD in older adults at risk. Neurology 89:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Hou XH, Liu HH, Yue CL, He Y, Zuo XN (2015) Toward systems neuroscience in mild cognitive impairment and Alzheimer’s disease: a meta-analysis of 75 fMRI studies. Hum Brain Mapp 36:1217–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Luo X, Zeng Q, Jiaerken Y, Xu X, Huang P, Shen Z, Xu J, Wang C, Zhou J, Zhang MM; Alzheimer’s Disease Neuroimaging Initiative (2018) Aberrant functional connectivity network in subjective memory complaint individuals relates to pathological biomarkers. Transl Neurodegener 7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu J, Liu Y, Zhu J, Liu N, Zeng W, Huang N, Rasch MJ, Jiang H, Gu X, Li X, Luo M, Li C, Teng J, Chen J, Zeng S, Lin L, Zhang × (2017) A distinct entorhinal cortex to hippocampal CA1 direct circuit for olfactory associative learning. Nat Neurosci 20:559–570. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Murphy C (2019) Olfactory and other sensory impairments in Alzheimer disease. Nat Rev Neurol 15:11–24. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Karalija N, Salami A, Andersson M, Wåhlin A, Kaboovand N, Köhncke Y, Axelsson J, Rieckmann A, Papenberg G, Garrett DD, Riklund K, Lövdén M, Lindenberger U, Bäckman L (2016) Dopamine D2 receptor availability is linked to hippocampal-caudate functional connectivity and episodic memory. Proc Natl Acad Sci U S A 113:7918–7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotin A, de Flores R, Lamberton F, Poisnel G, La Joie R, de la Sayette V, Mézenge F, Tomadesso C, Landeau B, Desgranges B, Chételat G (2015) Hippocampal subfield volumetry and 3D surface mapping in subjective cognitive decline. J Alzheimers Dis 48 Suppl 1:S141–S150. [DOI] [PubMed] [Google Scholar]
- Persson K, Bohbot VD, Bogdanovic N, Selbaek G, Braekhus A, Engedal K (2018) Finding of increased caudate nucleus in patients with Alzheimer’s disease. Acta Neurol Scand 137:224–232. [DOI] [PubMed] [Google Scholar]
- Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256:183–194. [DOI] [PubMed] [Google Scholar]
- Pini L, Pievani M, Bocchetta M, Altomare D, Bosco P, Cavedo E, Galluzzi S, Marizzoni M, Frisoni GB (2016) Brain atrophy in Alzheimer’s disease and aging. Ageing Res Rev 30:25–48. [DOI] [PubMed] [Google Scholar]
- Risacher SL, Tallman EF, West JD, Yoder KK, Hutchins GD, Fletcher JW, Gao S, Kareken DA, Farlow MR, Apostolova LG, Saykin AJ (2017) Olfactory identification in subjective cognitive decline and mild cognitive impairment: association with tau but not amyloid positron emission tomography. Alzheimers Dement 9:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RO, Christianson TJ, Kremers WK, Mielke MM, Machulda MM, Vassilaki M, Alhurani RE, Geda YE, Knopman DS, Petersen RC (2016) Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol 73:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheef L, Grothe MJ, Koppara A, Daamen M, Boecker H, Biersack H, Schild HH, Wagner M, Teipel S, Jessen F (2019) Subregional volume reduction of the cholinergic forebrain in subjective cognitive decline (SCD). Neuroimage Clin 21:101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares JM, Magalhães R, Moreira PS, Sousa A, Ganz E, Sampaio A, Alves V, Marques P, Sousa N (2016) A hitchhiker’s guide to functional magnetic resonance imaging. Front Neurosci 10:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel ME(1982) Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol 13:290–312. [Google Scholar]
- Sohrabi HR, Bates KA, Rodrigues M, Taddei K, Laws SM, Lautenschlager NT, Dhaliwal SS, Johnston AN, Mackay-Sim A, Gandy S, Foster JK, Martins RN (2009) Olfactory dysfunction is associated with subjective memory complaints in community-dwelling elderly individuals. J Alzheimers Dis 17:135–142. [DOI] [PubMed] [Google Scholar]
- Tahmasebi R, Zehetmayer S, Pusswald G, Kovacs G, Stögmann E, Lehrner J (2019) Identification of odors, faces, cities and naming of objects in patients with subjective cognitive decline, mild cognitive impairment and Alzheimer´s disease: a longitudinal study. Int Psychogeriatr 31:537–549. [DOI] [PubMed] [Google Scholar]
- Tang X, Holland D, Dale AM, Younes L, Miller MI; Alzheimer’s Disease Neuroimaging Initiative (2014) Shape abnormalities of subcortical and ventricular structures in mild cognitive impairment and Alzheimer’s disease: detecting, quantifying, and predicting. Hum Brain Mapp 35:3701–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda-Banon I, Saiz-Sanchez D, Flores-Cuadrado A, Rioja-Corroto E, Gonzalez-Rodriguez M, Villar-Conde S, Astillero-Lopez V, Cabello-de la Rosa JP, Gallardo-Alcañiz MJ, Vaamonde-Gamo J, Relea-Calatayud F, Gonzalez-Lopez L, Mohedano-Moriano A, Rabano A, Martinez-Marcos A (2020) The human olfactory system in two proteinopathies: Alzheimer’s and Parkinson’s diseases. Transl Neurodegener 9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeh KF (2019) Ethnic inequalities in doctor-patient communication regarding personal care plans: the mediating effects of positive mental wellbeing. Ethn Health 24:57–72. [DOI] [PubMed] [Google Scholar]
- Vasavada MM, Martinez B, Wang J, Eslinger PJ, Gill DJ, Sun X, Karunanayaka P, Yang QX (2017) Central olfactory dysfunction in Alzheimer’s disease and Mild cognitive impairment: a functional MRI study. J Alzheimers Dis 59:359–368. [DOI] [PubMed] [Google Scholar]
- Wang Q, Chen B, Zhong X, Zhou H, Zhang M, Mai N, Wu Z, Huang X, Haehner A, Chen X, Auber LA, Peng Q, Hummel T, Ning Y (2020a) Olfactory dysfunction is already present with subjective cognitive decline and deepens with disease severity in the Alzheimer’s disease spectrum. J Alzheimers Dis 79:585–595. [DOI] [PubMed] [Google Scholar]
- Wang WY, Yu JT, Liu Y, Yin RH, Wang HF, Wang J, Tan L, Radua J, Tan L (2015) Voxel-based meta-analysis of grey matter changes in Alzheimer’s disease. Transl Neurodegener 4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Huang W, Su L, Xing Y, Jessen F, Sun Y, Shu N, Han Y (2020b) Neuroimaging advances regarding subjective cognitive decline in preclinical Alzheimer’s disease. Mol Neurodegener 15:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA (2007) Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry 64:802–808. [DOI] [PubMed] [Google Scholar]
- Yan CG, Wang XD, Zuo XN, Zang YF (2016) DPABI: data processing and analysis for (resting-state) brain imaging. Neuroinformatics 14:339–351. [DOI] [PubMed] [Google Scholar]
- Zang Y, Jiang T, Lu Y, He Y, Tian L (2004) Regional homogeneity approach to fMRI data analysis. Neuroimage 22:394–400. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ji D, Yin J, Wang Z, Zhou Y, Ni H, Liu Y (2019) Olfactory fMRI activation pattern across different concentrations changes in Alzheimer’s disease. Front Neurosci 13:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF (2008) An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods 172:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]