Abstract
The change in vegetative cover of a Hawaiian soil from forest to pasture led to significant changes in the composition of the soil bacterial community. DNAs were extracted from both soil habitats and compared for the abundance of guanine-plus-cytosine (G+C) content, by analysis of abundance of phylotypes of small-subunit ribosomal DNA (SSU rDNA) amplified from fractions with 63 and 35% G+C contents, and by phylogenetic analysis of the dominant rDNA clones in the 63% G+C content fraction. All three methods showed differences between the forest and pasture habitats, providing evidence that vegetation had a strong influence on microbial community composition at three levels of taxon resolution. The forest soil DNA had a peak in G+C content of 61%, while the DNA of the pasture soil had a peak in G+C content of 67%. None of the dominant phylotypes found in the forest soil were detected in the pasture soil. For the 63% G+C fraction SSU rDNA sequence analysis of the three most dominant members revealed that their phyla changed from Fibrobacter and Syntrophomonas assemblages in the forest soil to Burkholderia and Rhizobium–Agrobacterium assemblages in the pasture soil.
Dispersal of inoculum and selection for growth provided by the environment are the two main factors that determine which organisms dominate a habitat. Vegetation is one environmental factor thought to be a major determinant of the composition of the soil microbial community since it provides the primary resource for heterotrophic growth. Since different plant species are comprised of different carbon compounds, different microorganisms might be expected to grow in soil under different plant communities. Furthermore, the plant community may alter other physical and chemical features of the soil and hence favor the growth of different microbial species. Little data exists, however, on the influence of vegetation on the composition of soil microbial communities.
We investigated the influence of vegetative cover on the structure and composition of a microbial community developed in a volcanic ash soil on the Big Island of Hawaii. Because of its young age and geographic isolation, Hawaii is depauperate in macrobiological species, providing a simpler ecosystem for study. Two adjacent soils from the Kohala region, one which has been continuously covered by a native tropical forest and one covered by a grass pasture, which replaced the native rain forest approximately 80 years ago, were compared. DNA-based methods were used rather than culturing methods to compare the soil communities since they better recover the dominant prokaryotic populations. Furthermore, community DNA allows analysis at different levels of taxon resolution and provides more quantitative comparisons than culture-based methods. The three levels of resolution used in order of increasing taxon resolution and decreasing comprehensiveness are as follows. First, community DNA was fractionated by its guanine and cytosine (G+C) content and the amount of DNA having each G+C content was quantified. Second, DNA fractions of two different G+C contents, one from a fraction of high biomass (63% G+C content) and the other from a fraction of low biomass (35% G+C content), were then selected for analysis of small subunit (SSU-) rRNA genes amplified by PCR. The ribosomal DNA (rDNA) clone libraries were screened by amplified rDNA restriction analysis (ARDRA) to determine pattern abundance profiles. Third, the dominant clones in the 63% biomass fraction from each soil were sequenced and analyzed phylogenetically. Differences in the bacterial community due to vegetation were seen by all three methods.
MATERIALS AND METHODS
Soil origin and sampling.
The forest site is located within the Kohala Forest Reserve on the Big Island of Hawaii (20°03′N, 155°41′E) and has never been cleared by humans. The forest site is the same as the Kohala site in the study of a chronosequence by Crews et al. (1). The pasture site was cleared in the 1920’s for ranchland and lies immediately adjacent to the forest. The pasture was seeded with the African grass Pennisetum clandestinum, which now dominates this species-poor site. Cattle graze the pasture.
The parent material of this Typic Placandept is a volcanic ash deposit, tephra, which was deposited 150,000 years ago. The sampling sites are at 1,122-m elevation, which corresponds to a mean annual air temperature of 16°C (2). The mean annual rainfall is near 2,500 mm (3). The pristine closed canopy rain forest is dominated (83% of the cover) by a single species, the native tree Metrosideros polymorpha, the tree most widespread on the Hawaiian islands (8). The well-developed understory vegetation is dominated by native tree ferns of Cibotium spp. (25% of the cover). Other trees and shrubs at this site are those of the genus Cheirodendron (18% of the cover), understory trees of the genus Coprosoma (4% of the cover), and shrubs of the genus Vaccinium (2% of the cover), accounting for most of the remaining cover (1). The exotic plant species Psidium cattleianum and Hedychium garnerianum are also present in low abundance (15). The total number of vascular plant species per 0.2 ha is 53, while the number of native plant species is 45 (1). In temperate forests there are about 140 vascular plant species per 0.2 ha (7).
Soil samples were collected in 1992, 1994, and 1999. Forest samples were from sites located 30 to 50 m apart, and pasture samples were from sites that spanned 5 km. Samples were taken from the upper 8- to 10-cm layer of the umbric A horizon after removal of the overlaying organic root and litter layer and placed immediately in sterile plastic bags on wet ice. After 48 h samples were shipped on dry ice to our laboratory in Michigan and stored at −20°C. The soil moisture content was determined by drying soil overnight at 100°C. Soil mechanical and chemical analyses were done by the Soil Analysis Laboratory, Michigan State University, using the methods described in Peck et al. (13).
DNA extraction, purification, and G+C fractionation.
DNA was extracted from 10 g of soil by using the direct lysis method of Holben (4) with the following modifications: a lower shaker speed, an extended shaking time, a 100-fold-increased phosphate concentration (due to soil acidity), and no addition of EDTA to the lysis buffer (12). Extraction efficiency was determined by comparing the amount of DNA extracted with the expected amount of DNA calculated from the difference in direct microscopic counts of bacterial cells before and after lysis. DNA was quantified by fluorometry (12). The amount of extracellular DNA was determined following extraction with sodium phosphate before lysis (data not shown).
DNA fragments were separated according to G+C content following the procedure of Holben and Harris (5). Briefly, DNA was mixed with the A- and T-specific dye bis-benzimidazole, and the buoyant density of the resulting DNA–bisbenzimide complex was decreased in proportion to the amount of dye bound. A gradient of percent G+C concentration was then established by equilibrium density gradient ultracentrifugation. The amount of DNA in each fraction was determined by absorption spectroscopy. To allow PCR amplification DNA fractions were purified from the CsCl and the dye by repeated extractions in CsCl-saturated isopropanol followed by spin column chromatography (Wizard PCR MiniPrep; Promega, Madison, Wis.) as previously described (12).
rRNA analysis.
G+C fractions (with 63 and 35% G+C contents) derived from two subsamples of soil from composite samples representing a 20-cm2 area each of forest and pasture were used to produce replicate SSU rRNA clone libraries. SSU rRNA genes were amplified by PCR from the DNA fractions by using the eubacterium-specific primers fD1 and rP2 of Weisburg et al. (18). PCRs were performed with Taq DNA polymerase (Boehringer Mannheim, Indianapolis, Ind.) according to the manufacturer’s protocol. To ensure that only soil bacterial rRNA genes were amplified the following quality control steps were implemented: determination of primer purity by using high-performance liquid chromatography, preparation of fresh oligonucleotide primers for each use, a preamplification heating step to maximize PCR sensitivity and specificity, and the use of stock solutions for negative controls (12). Amplified products were separated in agarose gels, and the bands were visualized by UV excitation after ethidium bromide staining. Prior to cloning, the amplified SSU rDNA fragments were purified by spin column chromatography (Wizard PCR Miniprep; Promega) and an equimolar amount of amplified PCR products was ligated to the vector pCR II (Invitrogen Corp., Carlsbad, Calif.) and transformed into Escherichia coli Top-10F′ competent cells. A primer pair specifically designed to complement the polylinker of the vector pCR II was used to amplify plasmid inserts directly from the transformant cells for SSU rDNA gene screening (19). To screen for SSU rDNA diversity, the amplified inserts were digested with two sets of tetrameric restriction endonucleases, HaeIII with HhaI and MspI with RsaI (12). The resulting fragments were electrophoretically resolved, and the similarities between the electrophoretic patterns were analyzed by using GelCompar (Applied Mathematics, Kortrijk, Belgium) cluster analyses by comparative numerical analysis with unweighted pair-group method using arithmetic averages (UPGMA). Individual clones were grouped by using a cutoff of 97% similarity and a 5% error rate for the band position. Each different SSU rRNA pattern is termed a phylotype.
Determination of nucleotide sequences and phylogenetic analysis.
Purified SSU rDNA clone inserts were sequenced in both directions by the fluorescent DiDeoxy termination method by using automated fluorescent Taq cycle sequencing on the ABI Catalyst 800 and ABI 373A sequencing system (Applied Biosystems, Foster City, Calif.). Partial sequences were done for the first 500 bp of the 5′ region (E. coli; numbering positions 11 through 529), and the dominant and novel types were fully sequenced. All sequences were aligned, and phylogenetic relationships were inferred as described previously (12). Only unambiguously aligned nucleotide positions were used for the sequence analysis. Potential chimeric artifacts were evaluated by CHECK_CHIMERA (11) and mglobalCHI (9) and by comparing the phylogenetic affiliations of the 5′ end of a 16S rRNA gene with those of the 3′ end. All phylogenetic nomenclature was based on the RDP-II version 7.0 (11).
Nucleotide sequence accession numbers.
The sequence data for the Hawaiian rain forest soil clones HRS-46, HRS-47, HRS-50, HRS-55, HRS-56, and HRS-60 and the Hawaiian pasture soil clones HPS-45, HPS-49, HPS-52, HPS-54, HPS-61, and HPS-64 have been deposited in the GenBank database under accession nos. AF165267 to AF165280.
RESULTS
The shift in vegetative cover from forest to pasture caused some changes in soil properties, including a decrease in soil acidity and organic carbon and an increase in bulk density (Table 1). The higher cation-exchange capacity in the forest soil than in the pasture soil is likely due to its higher soil organic matter content.
TABLE 1.
Differences in soil properties between the Kohala forest soil and pasture soil
| Soil | Soil classification | pH | Total carbon (%) | Total nitrogen (%) | NO3−-N (μg/g) | NH4+-N (μg/g) | Cation exchange capacity (meq/100 g) | Soil bulk density (g/cm3) | Dominating plant |
|---|---|---|---|---|---|---|---|---|---|
| Forest | Typic Placandept | 4.2 | 13.5 | 1.5 | 2.2 | 83.3 | 89.3 | 0.498b | M. polymorphac |
| Pasture | Typic Placandept | 5.2 | 8.1 | NDa | 5.6 | 22.4 | 51.9 | 0.587 | P. clandestinumd |
ND, not determined.
Data from Crews et al. (1).
The overstory is dominated by M. polymorpha, a native C-3 tree.
P. clanestinum is an African C-4 grass.
The G+C content profile of soil DNA was shifted to significantly higher G+C content of DNA with the change to pasture (Fig. 1). These profiles are highly habitat specific since both small- and large-scale replicate samples showed the same shift. Furthermore, three additional samples from other, similar Big Island forest soils gave the same forest profile, and one additional pasture sample gave the pasture profile (data not shown). The standard deviations (SDs) of the mean curve differences for the small-scale replicates of forest and pasture soil communities were small (SDs were 0.14 and 0.27, respectively) (Fig. 1).
FIG. 1.
Comparison of G+C content profiles of DNA extracted from three forest soils and three pasture soils in the Kohala area. One soil supports a rain forest, and the adjacent soil was converted to pasture 80 years ago. Samples collected in the same year were from the same area, while samples collected in different years were from sites distant from the previous sampling location in the habitat type.
The majority of soil DNA had G+C content in the 55 to 70% range for both soils, but the major peaks were 61% G+C content for the forest soil DNA and 67% G+C content for the pasture soil DNA. The pasture soil community exhibited an additional peak in the 42 to 45% G+C content range, which was especially prominent for the samples collected in 1994, and displayed a shoulder peak of 71% G+C content.
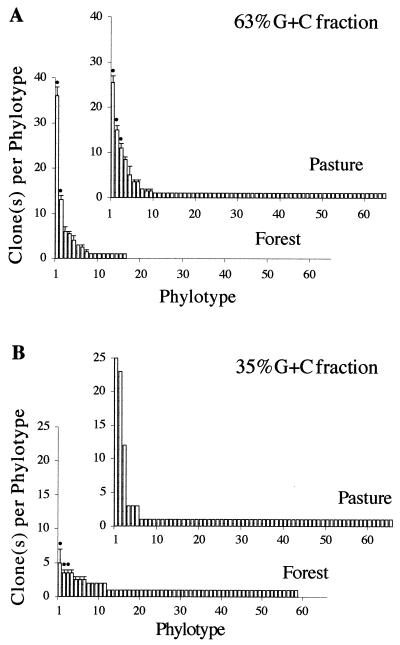
The same phylotypes were observed for replicate soil samples for the three dominant phylotypes in eight of the nine cases for the forest and the pasture soils (Fig. 2). Examples of ARDRA data documenting the presence of the same phylotypes in replicates of the pasture soil are shown in Fig. 3. The dominant phylotypes, however, were different between forest and pasture samples, suggesting that the populations were different at the two sites. Nondominant phylotypes were very different, even between replicates of the same vegetation type, as would be expected if the clone library were generated from a random process. Twenty-two percent of the phylotypes of rare clones (n = 1) were found in replicates of the pasture community with 63% G+C content, while 12% of the rare clones were found in replicates of the forest community. Only seven of the rare phylotypes were found under both vegetation types. The two most dominant phylotypes of the fraction of the forest soil with 35% G+C content had frequencies ± SDs of only 6.5% ± 2.5% and 4.5% ± 0.5%, but were found in both replicates.
FIG. 2.
Frequency distribution of the total number of SSU rDNA gene phylotypes from the 63% G+C content fractions (A) and the 35% G+C content fractions (B) from the rain forest and pasture soils. Each graph shows the median and range (bars) for data for two replicates. A dot is placed above each phylotype for which clones were identical in pattern and rank for the two replicates. A replicate of the pasture sample with 35% G+C content was not available.
FIG. 3.
An ARDRA profile following double digestion with HaeIII and HhaI of clones from the 63% fraction of the pasture soil. Similar patterns can be seen for three groups of clones, each found in both replicates, lanes, 2, 5, 15; 6, 7, 8, 10, 14, 17 and 1, 9, 11, 12. Lanes 1 to 8 contain clones from replicate A, and lanes 9 to 18 contain clones from replicate B. Plasmid pBR322 digested with HaeIII was used as a size marker (lanes M).
The phylotype abundance curves (Fig. 2) of both the forest soil and pasture soil replicated well with very low SDs. The two profiles of the 63% fraction of the forest soil were similar, i.e., 94% of all data fell within a single SD range (SD, ± 1.17) of the mean curve difference. The corresponding number for the profiles of the 63% fraction of the pasture profile was 93% (SD, ± 0.77).
Microbial rDNA diversity in the 63% G+C content fraction of the pasture soil differed from that of the forest soil. The rank abundance profiles of the 63% G+C content fractions are separated by the stronger trend for dominance in the forest soil but the much greater phylotype richness in the pasture soil (Fig. 2A). The four most dominant members contributed 77% ±6% (mean ± SD) of the forest soil community clones while they represented about half, i.e., 47% ± 3%, of the pasture soil community clones. With 62 ± 4 phylotypes among ca. 120 clones selected, the pasture had a phylotype richness which was almost 2.2 ± 0.4 times greater than that for the forest soil. The difference in abundance within the four most dominant phylotypes was more prominent in the forest soil community. Clones of the first phylotype were almost three times as abundant as those of the next group, while the corresponding difference in the pasture clones was under 60% (Fig. 2A).
The pasture soil displayed a prominent rRNA dominance structure of the first three phylotypes from the 35% G+C content fraction (Fig. 2B), while both replicates of the forest soil showed no significant dominance pattern or phylotype frequency over 10%. The three most dominant members represent 47% of the pasture clone community but only about one third of that in the forest soil community, where they constitute only 15% ± 3% (mean ± SD) of the clone community. The phylotype richness of the 35% G+C content fraction was about 43% ± 4% greater than that for the forest soil.
One clone of each of the three dominant phylotypes was sequenced. Most clones sequenced were moderately to distantly related to sequences of known taxa (Table 2). The dominant clones from the two vegetation types were very different; they represented two very different phyla, Fibrobacter and Proteobacteria. Many of the clones within the Fibrobacter group were dissimilar to each other (Table 3), while those within the Proteobacteria were affiliated with Burkholderia and more similar to each other (Tables 2 and 3).
TABLE 2.
Phylogenetic affiliations based on SSU rDNA genes of the three most dominant phylotypes of the two replicates from the 63% G+C content fractions of the rainforest soil and the pasture soil
| Phylotype | Replicate | Relative abundanceb (%) | Phylogenetic affiliationc | Most closely related organism in RDP database | % similarity |
|---|---|---|---|---|---|
| Forest soil | |||||
| HRS-46a | A | 43 | Fibrobacter phylum | Env. str. MC 9 | 94.3 |
| HRS-55a | A | 15 | Fibrobacter phylum | Acidobacterium capsulatum | 96.5 |
| HRS-60 | A | 9 | Thermoanaerobacter and Rel. | Desulfotomaculum geothermicum | 80.8 |
| HRS-47a | B | 48 | Fibrobacter phylum | Env. str. MC26 | 93.0 |
| HRS-56a | B | 18 | Fibrobacter phylum | A. capsulatum | 96.0 |
| HRS-50a | B | 6 | Fibrobacter phylum | Env. str. MC 27 | 96.5 |
| Pasture soil | |||||
| HPS-54a | A | 21 | Beta-proteobacteria | Burkholderia pseudomallei | 95.5 |
| HPS-61 | A | 11 | Beta-proteobacteria | B. pseudomallei | 89.7 |
| HPS-49 | A | 8 | Alpha-proteobacteria | Afipia clevelandensis | 99.0 |
| HPS-52 | B | 19 | Beta-proteobacteria | B. pseudomallei | 91.2 |
| HPS-45 | B | 13 | Beta-proteobacteria | Burkholderia gladioli | 93.4 |
| HPS-64a | B | 9 | Alpha-proteobacteria | A. clevelandensis | 93.2 |
Data based on a nearly complete sequence
Relative abundance of the clones belonging to a phylotype, calculated by dividing the number of clones belonging to the phylotype by the total number of clones analyzed.
Only unambiguously aligned regions were used for the analysis.
TABLE 3.
Sequence similarities among SSU rDNA clones from dominant members of the 63% G+C content fractions of the rain forest community and pasture soil community
| Clone no. | Percent SSU rDNA nucleotide sequence similarity for clone no.:
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Fibrobacter–Acidobacterium group | |||||
| 1, HRS-46 | |||||
| 2, HRS-47 | 98.7 | ||||
| 3, HRS-50 | 97.0 | 82.3 | |||
| 4, HRS-55 | 86.9 | 91.0 | 88.2 | ||
| 5, HRS-56 | 87.8 | 92.1 | 87.2 | 98.7 | |
| Burkholderia group | |||||
| 1, HPS-45 | |||||
| 2, HPS-52 | 99.6 | ||||
| 3, HPS-54 | 98.0 | 99.0 | |||
| 4, HPS-61 | 97.0 | 97.0 | 97.2 | ||
DISCUSSION
High soil microbial diversity, even in this young, isolated Hawaiian site, makes the detection and analysis of community components challenging. The methods used here, however, were able to distinguish clear differences in microbial communities between the forest and pasture soils. Because of the high microbial diversity, distinguishing statistically between communities at the same site and communities at different sites is often very difficult. In this study large- and small-scale replicate samples analyzed by G+C content profile showed less variability among replicates than between habitats. Furthermore, the ARDRA abundance patterns and rDNA sequence analysis also showed differences between habitats. It is important that these methods analyze a community at different levels of resolution and different levels of comprehensiveness. The method of analyzing the G+C content provides an analysis at a coarser level of taxon distinction but is comprehensive for all DNA, while the rDNA analysis is effective to nearly the species level but can only be done for amplifiable and selected dominant members. Hence, together they form a complementary suite with which to better analyze community differences.
A radical change in vegetation like the replacement of a rain forest with a pasture certainly constitutes a major shift in the soil environment, especially in carbon sources for the microbes, and accompanying changes in soil properties, e.g., a decrease in soil acidity and an increase in bulk density (Table 1). These soil changes for the forest to pasture shift are similar to those found in other ecological studies (14, 16). Furthermore, cattle have grazed in the pasture site while the rooting of wild pigs mixes the forest soil surface. Hence there are both primary and secondary factors that could be important determinants of the current microbial communities associated with the vegetation shift.
The change from forest to pasture correlated with a change of dominant phyla that were sampled in the 63% G+C content fraction, from the Fibrobacter phylum in the forest soil to the Proteobacteria in the pasture soil. The dominant clones from the pasture soil are closely related to members of the Burkholderia cepacia subgroup, which are known to be nutritionally versatile and fast growing, and many are dominant grass rhizosphere bacteria (17). The pasture habitat is consistent with this ecotype.
If DNA content is proportional to biomass, which is likely, the shift in vegetative cover to pasture resulted in a 49% change in the microbial composition (Fig. 1; compare areas 1 through 4). This suggests that at least half of the total soil microbial biomass was replaced by the vegetation change. There is also an obvious shift to genera of higher G+C content in the pasture soil. The peak in the 41 to 45% G+C content range in the pasture soil is also noteworthy since this has not been seen before in other soils in our experience. These differences between pasture and forest vegetation are not merely seasonal, as indicated by the high degree of similarity of total community profiles within a vegetation type (Fig. 1) collected over a 2- to 5-year period.
Two of the dominant phylotypes of the forest soil were found to be closely related (Tables 2 and 3) and to be members of the newly recognized Acidobacterium division. Because of their abundance in many soil environments representatives of this division are of potential environmental importance. Although only three cultured members are known, organisms of this group are well-represented by environmental clone sequences from independent studies (6, 10, 12), especially soil community studies of vastly different soil types ranging from dry sandy soil in the Australian desert to Typic Placandept under tropical, montane rain forest (10, 12). We were, however, unable to detect this group among the dominant strains in the DNA with 63% G+C content from the pasture soil.
With pasture abandonment and succession to secondary forest changes in carbon substrates and soil properties should occur, though at different rates. It would be interesting to know whether the microbial community returns to its original state, and if so, in concert with which of the soil environmental changes.
ACKNOWLEDGMENTS
This work was supported by a grant (DEB 9120006) from the National Science Foundation to the Center for Microbial Ecology.
We thank P. Vitousek and T. Crews for providing site access and information and assisting in sampling.
REFERENCES
- 1.Crews T E, Kitayama K, Fownes J H, Riley R H, Herbert D A, Mueller-Dombois D, Vitousek P M. Changes in soil phosphorous fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology. 1995;76:1407–1424. [Google Scholar]
- 2.Department of Geography, University of Hawaii. Atlas of Hawaii. Honolulu, Hawaii: University of Hawaii Press; 1983. [Google Scholar]
- 3.Giambelluca T W, Nullet M A, Schroeder T A. Rainfall atlas of Hawaii. Honolulu, Hawaii: Department of Land and Natural Resources; 1986. [Google Scholar]
- 4.Holben W E. Isolation and purification of bacterial DNA from soil. In: Weaver R, et al., editors. Methods of soil analysis, part 2. Microbiological and biochemical properties. Soil Science Society of America book series 5. Madison, Wis: Soil Science Society of America; 1994. pp. 727–751. [Google Scholar]
- 5.Holben W E, Harris D. DNA-based monitoring of total bacterial community structure in environmental samples. Mol Ecol. 1995;4:627–631. doi: 10.1111/j.1365-294x.1995.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 6.Hugenholtz P, Goebel B M, Pace N R. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol. 1998;180:4765–4774. doi: 10.1128/jb.180.18.4765-4774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huston M A. Biological diversity. Cambridge, England: Cambridge University Press; 1994. [Google Scholar]
- 8.Kitayama K, Mueller-Dombois D. Vegetation changes along gradients of long-term soil development in the Hawaiian montane rainforest zone. Vegetatio. 1995;120:1–20. [Google Scholar]
- 9.Komatsoulis G A, Waterman M S. A new computational method for detection of chimeric 16S rRNA artifacts generated by PCR amplification from mixed bacterial populations. Appl Environ Microbiol. 1997;63:2338–2346. doi: 10.1128/aem.63.6.2338-2346.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuske C R, Barns S M, Busch J D. Diverse uncultivated bacterial groups from soils of the arid southwestern United States that are present in many geographic regions. Appl Environ Microbiol. 1997;63:3614–3621. doi: 10.1128/aem.63.9.3614-3621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maidak B L, Cole J R, Parker C T, Jr, Garrity G M, Larsen N, Li B, Lilburn T G, McCaughey M J, Olsen G J, Overbeek R, Pramanik S, Schmidt T M, Tiedje J M, Woese C R. A new version of the RDP (Ribosomal Database Project) Nucleic Acids Res. 1999;27:171–173. doi: 10.1093/nar/27.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nüsslein K, Tiedje J M. Characterization of the dominant and rare members of a young Hawaiian soil bacterial community with small-subunit ribosomal DNA amplified from DNA fractionated on the basis of its guanine and cytosine composition. Appl Environ Microbiol. 1998;64:1283–1289. doi: 10.1128/aem.64.4.1283-1289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peck T R, Megel D, Eik K, Whitney D A, Warncke D, Munter R C, Brown J R, Knudsen D, Dahnke W C, Eckert D J, Beegle D, Fixen P E, Schulte E E. Recommended chemical soil test procedure for the north central region. Fargo, N. Dak.: North Dakota Agricultural Experiment Station, North Dakota State University; 1988. [Google Scholar]
- 14.Reiners W A, Bouwman A F, Parsons W F J, Keller M. Tropical rainforest conversion to pasture: changes in vegetation and soil properties. Ecol Appl. 1994;4:363–377. [Google Scholar]
- 15.Riley R H, Vitousek P M. Nutrient dynamics and nitrogen trace gas flux during ecosystem development in montane rain forest. Ecology. 1995;76:292–304. [Google Scholar]
- 16.Spaans E J A, Baltissen G A M, Bouma J, Miedema R, Lansu A L E, Schoonderbeek D, Wielemaker W G. Changes in physical properties of young and old volcanic surface soils in Costa Rica after clearing of tropical rain forest. Hydrogeological Processes. 1989;3:383–392. [Google Scholar]
- 17.Viallard V, Poirier I, Cournoyer B, Haurat J, Wiebkin S, Ophel-Keller K, Balandreau J. Burkholderia graminis sp. nov., a rhizospheric Burkholderia species, and reassessment of [Pseudomonas] phenazinium, [Pseudomonas] pyrrocina and [Pseudomonas] glathei as Burkholderia. Int J Syst Bacteriol. 1998;48:549–563. doi: 10.1099/00207713-48-2-549. [DOI] [PubMed] [Google Scholar]
- 18.Weisburg W W, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J, Davey M E, Figueras J B, Rivkina E, Gilichinsky D, Tiedje J M. Phylogenetic diversity of a bacterial community determined from Siberian tundra soil DNA. Microbiology. 1997;143:3913–3919. doi: 10.1099/00221287-143-12-3913. [DOI] [PubMed] [Google Scholar]