Abstract
Atopic dermatitis is a chronic inflammatory skin condition that affects approximately 18 million people in the United States. Assessing the extent and severity of atopic dermatitis is critical for determining baseline disease burden and treatment effectiveness for both investigators and clinicians. Considerable efforts over the past several decades have been made in developing a highly validated instrument called the Eczema Area and Severity Index (EASI). Although several guides exist for the EASI, questions continue to arise regarding its use and interpretation. This review was developed to serve as the definitive guide for the EASI and to address commonly asked questions.
The Eczema Area and Severity Index (EASI) was developed in 1998 and later validated to meet the demands of investigators in need of a standardized evaluation tool for severity of atopic dermatitis (AD) signs in clinical studies.1,2 It was designed by modifying the Psoriasis Area and Severity Index scoring system, an assessment developed for patients with psoriasis.2 The EASI assessment integrates body surface and the intensity of lesional skin into one composite score. After an extensive review of more than 16 instruments, the Harmonizing Outcome Measures in Eczema (HOME) initiative identified the EASI as the recommended core instrument for measuring signs in all AD clinical trials.3 This selection was based on consensus voting after several studies revealed that the EASI demonstrated adequate psychometric properties, including validity, responsiveness, internal consistency, and intraobserver and interobserver reliability.4 Because of the performance of the scale and endorsement by the HOME group, the EASI has become the most widely utilized signs/severity scale in AD trials.5 Although the HOME Web site provides a training video and manual to help guide the proper use of the EASI, questions continue to emerge among clinicians and investigators regarding the scale. The purpose of this review is to provide additional guidance on the current use of the scale and to serve as the definitive reference for its use.
THE EASI BASICS
The EASI requires approximately 6 minutes when performed by a trained investigator.6 Reference tables to aid in the steps and calculation of the EASI score are presented in Figure 1 for both adult and pediatric patient sets. The EASI is assessed following these steps:
Figure 1.
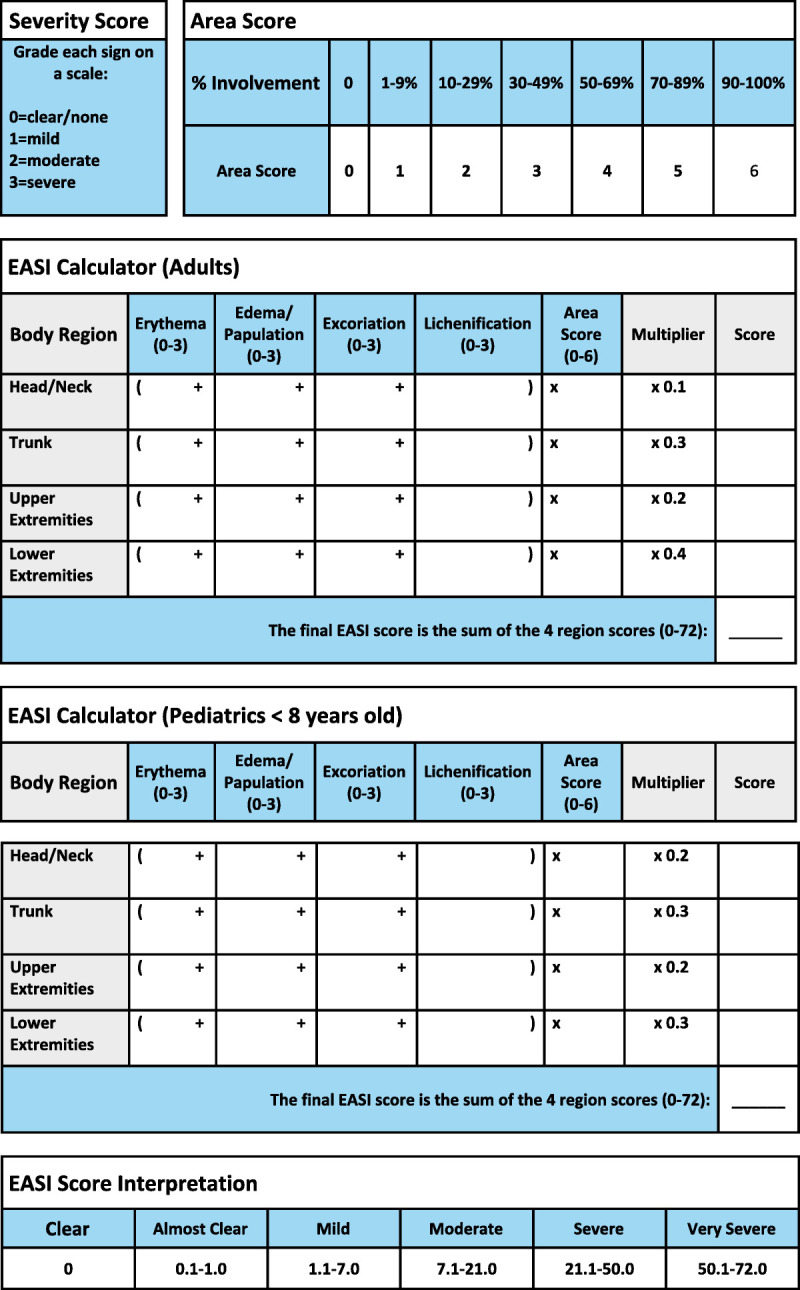
Area of Involvement
First, the area of involvement must be visually estimated in each of the 4 body regions separately (head and neck, upper extremities, trunk, and lower extremities) and assigned an area score: 1 (1%–9%), 2 (10%–29%), 3 (30%–49%), 4 (50%–69%), 5 (70%–89%), and 6 (90%–100%). The feet and buttocks are included as part of the lower extremities, whereas the axilla and groin are counted as part of the trunk (Fig. 2).
Figure 2.
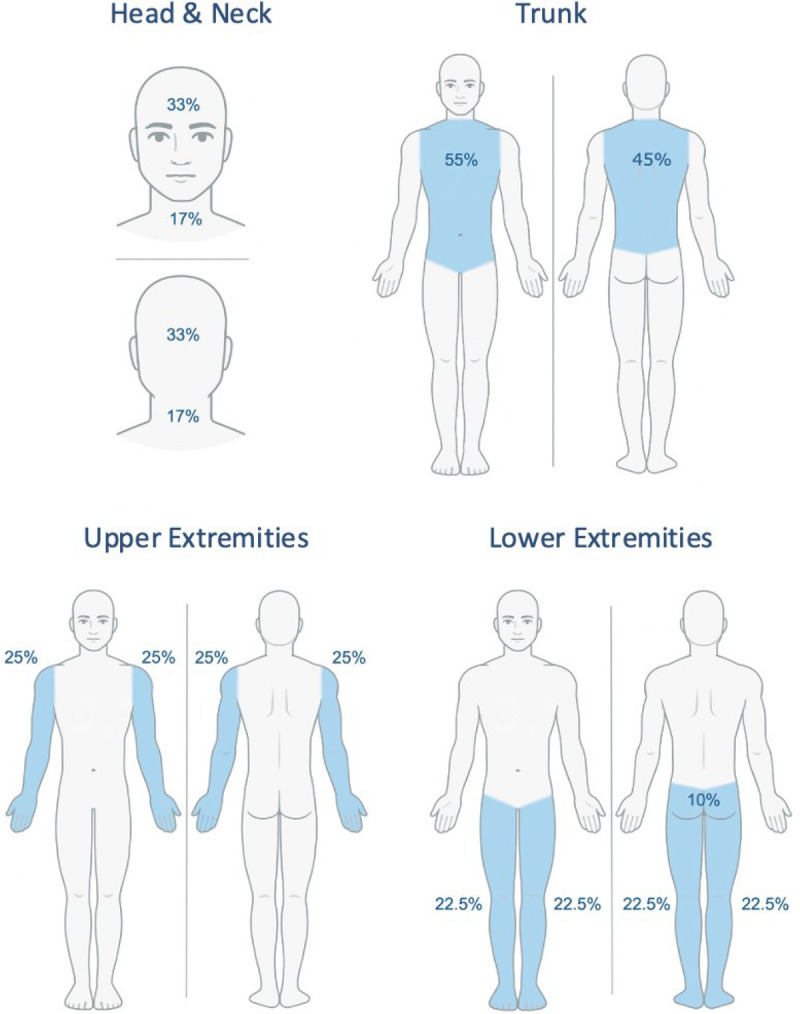
Area of involvement.
2. Intensity of Lesions
Next, each region is assessed separately for 4 signs: erythema, edema/papulation, excoriation, and lichenification. Each sign is assigned an intensity score from 0 to 3, with 0 being absent; 1, mild; 2, moderate; and 3, severe. Half points may be used between points 1 and 3 (eg, 1.5 and 2.5 but not 0.5) as any sign present should be treated as at least mild.2 It is important to note that only inflamed areas should be included in the assessment. Xerosis, ichthyosis, keratosis pilaris, urticaria, and postinflammatory pigment changes should not be included unless underlying eczema is present. Regions that present with varying severity of a particular sign should be roughly averaged across involved areas only; half units may be useful in this scenario.
3. Region Score
Each region is assigned an adult (>8 years old) or pediatric multiplier that reflects the relative contribution of that region to the total body surface area (BSA). The region score is calculated separately for each region by multiplying the sum of the regional intensity score by the regional area score and the region-specific multiplier.
4. Final EASI Score
The final EASI score is the summation of the 4 regional scores, ranging from 0 to 72.
A score of 0 indicates clear or no eczema, 0.1 to 1.0 indicates almost clear, 1.1 to 7 indicates mild disease, 7.1 to 21 indicates moderate disease, 21.1 to 50 indicates severe disease, and greater than 51 indicates very severe disease.6
The EASI should be completed independently of previous EASI assessments—it is a static tool. As with any clinician assessment, there is some subjectivity to the measurement. Although the interrater reliability has been shown to be adequate per rigorous definitions, the most accurate results emerge when the same investigator is used for assessments in a participant over the course of a study.4,7 Scoring patients together is an effective method for ensuring 2 investigators are aligned on measurements. Training through Web sites like the homeforeczema.org and trifectaclinical.com also ensures more accurate and consistent assessments.8
TIPS FOR ASSESSING AREA OF INVOLVEMENT
The EASI was designed to make estimating BSA a simple and straightforward process. The EASI utilizes area assessments that rate the 4 involved regions on a 0% to 100% scale for each region. It is important to note that this assessment is different from how the BSA is estimated by other instruments, such as the rule of 9's in The SCORing Atopic Dermatitis (SCORAD) and BSA calculations using the handprint method. Because the EASI, total BSA, and SCORAD are often included simultaneously in studies and performed at the same visit, confusion may arise, and errors may result. To address this problem, some studies have utilized 1 BSA measurement method and applied it to all 3 calculations. Although this approach reduces discrepancies in BSA calculation and streamline assessments, investigators must recognize that utilizing a different method for BSA calculation may be at odds with the original validated scale. Further validation work is needed to confirm the acceptability of other BSA assessments for the EASI calculation. Studies reporting the EASI that do not utilize regional percentages according to the original validation should identify the modification in the methods section in their articles.
Some investigators find visually subdividing a region useful for creating more accurate and reproducible estimates (Fig. 2). For example, in the head and neck region, estimating regional percentages is easily performed by dividing the head and neck into thirds, including face, scalp, and neck both anteriorly and posteriorly.
TIPS FOR ASSESSING ERYTHEMA
Erythema is defined as skin redness caused by increased blood flow to superficial capillaries. Mild erythema (score of 1) should be considered barely perceptible and appears light pink on lightly pigmented skin. Moderate erythema is a dull red that is clearly distinguishable on lightly pigmented skin. Severe erythema is a deep, dark, or fiery bright red. A common pitfall when evaluating erythema is underestimating the severity of inflammation in patients with darker skin. Clinicians must consider the underlying skin pigment when grading erythema, and often, investigators will increase the score by 1 grade or more in heavily pigmented skin. Examples of the various grades of erythema in skin of different pigmentation levels are shown in Figure 3. Grading erythema in more pigmented skin improves with experience and with an understanding that the shades of erythema may be more violaceous than pink in darker skin tones. Increasing scores for erythema on darker skin tones prevents underestimation of erythema and thus disease severity in patients with skin of color.
Figure 3.
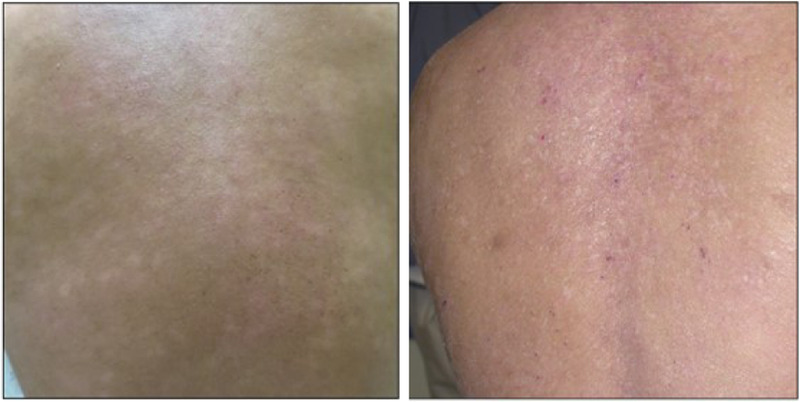
Examples of erythema; both images show an erythema severity score of 2.
TIPS FOR ASSESSING EDEMA/PAPULATION
Edema and papulation represent clinical signs of acute spongiosis and inflammation. These features are typically the most challenging of the 4 AD signs to evaluate by images and are generally best assessed by palpating the skin. Mild edema reflects a barely perceptible elevation of skin. Lesions that are visually perceptible should be scored as moderate, and eczematous plaques with distinct step off borders or very raised papules qualify as severe. Examples of edema/papulation can be seen in Figure 4.
Figure 4.
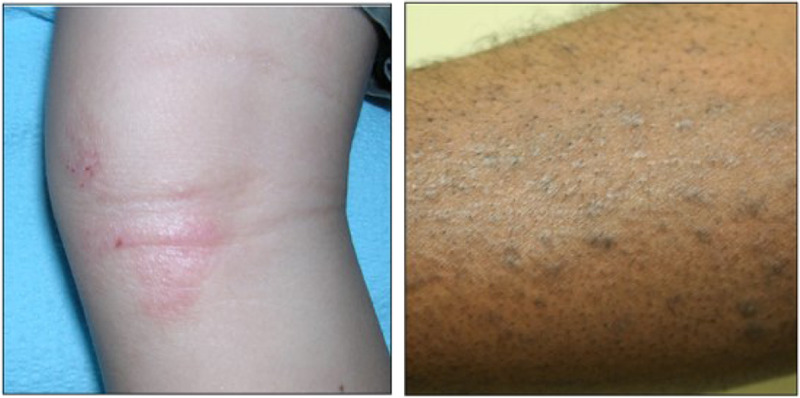
Edema/papulation; both images show an edema/papulation severity score of 2.
TIPS FOR ASSESSING EXCORIATION
Excoriations are physical evidence of pruritus from scratching or rubbing, and they denote an area of broken skin surface. Two dimensions of excoriations, density and depth, may be utilized and integrated for the final assessment. Mild excoriations are characterized by scant, superficial lesions lacking density. Moderate excoriations are either diffuse, superficial lesions or scant, deeper excoriations. Severe excoriations reflect very dense gouging of the skin with some areas being deep; linear scratch areas may also be present in moderate or severe lesions. Examples of excoriation severity are shown in Figure 5.
Figure 5.
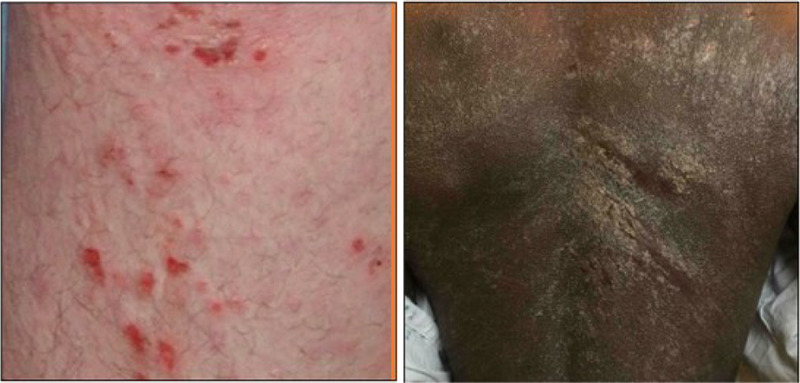
Excoriation, both images show an excoriation severity score of 3.
TIPS FOR ASSESSING LICHENIFICATION
Lichenification is a leathery thickening of the epidermis and is defined by accentuation of skin markings due to prolonged scratching or rubbing in chronic disease. Mild lichenification is defined as slight thickening of the epidermis, with markings only minimally exaggerated. It is often barely perceptible and determined by touch. Moderate lichenification is clearly thickened epidermis with exaggerated skin markings. Thickened skin markings that create deep furrows should be graded as severe. It is important to note that lichenification is classically the last sign to disappear after treatments and can delay the overall response by weeks.
In darker skin types, lichenification may present with firm, flat-topped, discrete papules (“follicular lichenification”) and should be graded as chronic lesions of lichenification. Prurigo nodules are larger, firm, and more protuberant papules or nodules that form in some patients as a response to scratching and should also be graded as areas of lichenification. Examples of lichenification can be seen in Figure 6.
Figure 6.
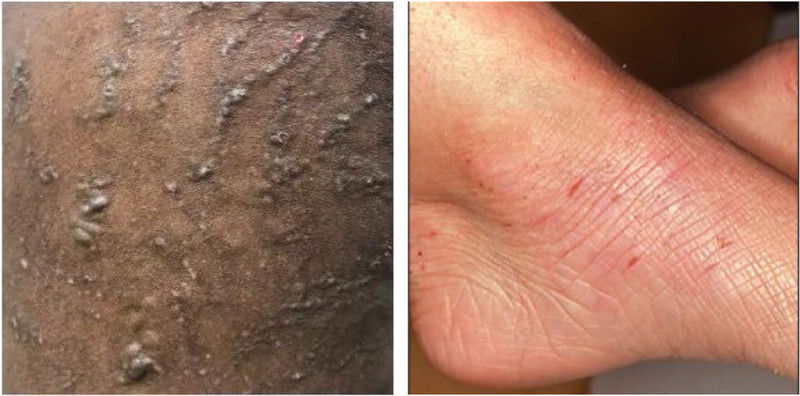
Lichenification; both images show a lichenification severity score of 3.
LIMITATIONS
The EASI is an extensively validated instrument for use in AD clinical trials, but researchers should be aware of its limitations. One limitation is that it has shown only moderate interrater reliability, and we suggest that the same investigator perform the EASI throughout the trial when possible.3,4 Investigators should also be aware that the EASI weighs extent and severity equally, and thus, there may be a heterogeneous patient population with the same EASI score.9 Although there have been some recent concerns about the suitability of the EASI for mild AD, these theoretical concerns have not born out in phase 3 clinical trials of patients with milder disease, where the EASI readily distinguished between active drug and placebo.8,10 Assessment of patients with pigmented skin has presented challenges to evaluators, especially when measuring erythema.11 A recent comparison between the EASI, SCORAD, and IGA using trained investigators showed excellent intrarater reliability for all scores and participants, but only the EASI had excellent interrater reliability; this study also showed that erythema did not significantly contribute to the variability of the measure.12 Although we recommend additional work on this subject, current knowledge supports the EASI for patients with skin of color. Last, all aspects of the scale are subjective to some degree, ranging from estimation of BSA to the intensity of lesions. With proper training and guides, such as this article, the EASI represents a reliable and accurate measure of the intensity of signs.
SUMMARY
As recommended by the HOME group, we advocate using the EASI scale in all AD clinical trials, along with other instruments included in the HOME core outcome set.3 Before initiating a study, it is recommended to train investigators on performing the EASI scale, using this article and other EASI training platforms as a guide. Performing the EASI as it was designed and validated is instrumental in maintaining robust validity of study data. This manual can also provide guidance on common areas of debate and facilitate an efficient application of the scale. See Table 1 for frequently asked questions and answers.
TABLE 1.
Frequently Asked Questions
| Frequently Asked Questions | Answers |
|---|---|
| Can half points be used when grading intensity of lesions (eg, erythema or lichenification)? | Half points may be used between points 1 and 3 (eg, 1.5 and 2.5 but not 0.5) as any sign present should be treated as at least mild |
| How do we grade erythema in darkly pigmented skin? | Clinicians must consider the underlying skin pigment when grading erythema, and this often means increasing the erythema grade by 1 |
| Should prurigo nodules be included in grading? | Prurigo nodules are included in the grading as areas of lichenification |
| Can I use other methods to calculate the BSA for the EASI score? | Although not recommended, clinicians using other BSA methods to calculate the EASI should report the modification in their article |
| How do we calculate the EASI when a child turns 8 y old in a study? | If a child turns 8 during the study, there is no clearly defined protocol, and the decision to switch scales should be determined on a case-by-case basis |
| Is estimating the BSA different among age groups? | No, the estimation methods remain the same. However, the multiplier in the EASI score changes between adults and children <8 y old (Fig. 1) |
| How do I grade xerosis (dryness), ichthyosis, and hyperlinear palms? | Unless there is active eczema in these areas, they are not included in the EASI assessment |
Footnotes
E.L.S. reports personal fees from AbbVie, Amgen, Arena Pharmaceuticals, Aslan Pharma, Boston Consulting Group, Collective Acumen, LLC (CA), Dermira, Eli Lilly, Evidera, ExcerptaMedica, Forte Bio RX, Galderma, GlaxoSmithKline, Incyte, Janssen, Kyowa Kirin Pharmaceutical Development, Leo Pharm, Medscape LLC, Pfizer, Physicians World LLC, Regeneron, Sanofi-Genzyme, Trevi Therapeutics, and WebMD. He also reports grants (or principal investigator role) from AbbVie, Amgen, Arcutis, Aslan, Corevita, Dermira, Eli Lilly, Incyte, Kyowa Hakko Kirin, Leo Pharmaceuticals, Merck, Novartis, Pfizer, Regeneron, Sanofi, and TARGET-DERM. These potential conflicts of interest have been reviewed and managed by OHSU. The other authors have no funding or conflicts of interest to declare.
Contributor Information
Jon M. Hanifin, Email: hanifinj@ohsu.edu.
Wenelia Baghoomian, Email: baghoomi@ohsu.edu.
Erin Grinich, Email: grinich@ohsu.edu.
Yael A. Leshem, Email: yael.leshem@gmail.com.
Michael Jacobson, Email: jacobsmi@ohsu.edu.
Eric Lawrence Simpson, Email: simponse@ohsu.edu.
REFERENCES
- 1.Tofte S Graeber M Cherill R, et al. Eczema Area and Severity Index (EASI): a new tool to evaluate atopic dermatitis. J Eur Acad Dermatol Venereol 1998;11. [DOI] [PubMed] [Google Scholar]
- 2.Hanifin JM Thurston M Omoto M, et al. The Eczema Area and Severity Index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 2001;10(1):11–18. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- 3.Schmitt J Spuls PI Thomas KS, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol 2014;134(4):800–807. doi: 10.1016/j.jaci.2014.07.043. [DOI] [PubMed] [Google Scholar]
- 4.Schmitt J Langan S Deckert S, et al. Assessment of clinical signs of atopic dermatitis: a systematic review and recommendation. J Allergy Clin Immunol 2013;132(6):1337–1347. doi: 10.1016/j.jaci.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Rehal B, Armstrong AW. Health outcome measures in atopic dermatitis: a systematic review of trends in disease severity and quality-of-life instruments 1985–2010. PLoS One 2011;6(4):e17520. doi: 10.1371/journal.pone.0017520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leshem YA Hajar T Hanifin JM, et al. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol 2015;172(5):1353–1357. doi: 10.1111/bjd.13662. [DOI] [PubMed] [Google Scholar]
- 7.Bożek A, Reich A. Assessment of intra- and inter-rater reliability of three methods for measuring atopic dermatitis severity: EASI, objective SCORAD, and IGA. Dermatology 2017;233(1):16–22. doi: 10.1159/000472711. [DOI] [PubMed] [Google Scholar]
- 8.Silverberg JI Lei D Yousaf M, et al. What are the best endpoints for Eczema Area and Severity Index and Scoring Atopic Dermatitis in clinical practice? A prospective observational study. Br J Dermatol 2021;184(5):888–895. doi: 10.1111/bjd.19457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chopra R Vakharia PP Sacotte R, et al. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br J Dermatol 2017;177(5):1316–1321. doi: 10.1111/bjd.15641. [DOI] [PubMed] [Google Scholar]
- 10.Papp K Szepietowski JC Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol 2021;85(4):863–872. doi: 10.1016/j.jaad.2021.04.085. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol 2002;147(5):920–925. doi: 10.1046/j.1365-2133.2002.04965.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhao CY Hao EY Oh DD, et al. A comparison study of clinician-rated atopic dermatitis outcome measures for intermediate- to dark-skinned patients. Br J Dermatol 2017;176(4):985–992. doi: 10.1111/bjd.15271. [DOI] [PubMed] [Google Scholar]


