Background
Clinical trials of systemic therapies for atopic dermatitis (AD) often exclude patients based on age and comorbidities.
Objectives
We conducted a scoping review of observational studies and survey of International Eczema Council (IEC) members on the treatment of AD in patients with liver disease, renal disease, viral hepatitis, HIV, or history of malignancy.
Methods
We searched MEDLINE via Ovid, Embase via Ovid, and Web of Science from inception to September 14, 2020. We mapped the available evidence on the use of cyclosporine, methotrexate, azathioprine, mycophenolate, systemic corticosteroids, and dupilumab for AD in older adults (≥65 years) and adults with the previously mentioned comorbidities. We surveyed IEC members on their preferred systemic medications for each patient population.
Results
We identified 25 studies on the use of systemic medications in special populations of adults with AD. Although IEC members preferred dupilumab as the first-line systemic agent across all special populations, many could not identify viable third-line systemic therapy options for some populations.
Conclusions
Data on systemic therapy for AD for older adults and adults with comorbidities are limited. Although IEC members' access to systemic therapies differs geographically, expert opinion suggests that dupilumab is preferred for those patients.
CAPSULE SUMMARY
-
-
Randomized clinical trials (RCTs) for atopic dermatitis often exclude older patients or patients with significant comorbidities, and safety data for these special patient populations are limited
-
-
Our survey of clinical practice patterns indicates that dupilumab is the preferred treatment across all special patient population
For patients with moderate-to-severe atopic dermatitis (AD) refractory to topical therapy, systemic treatment is often indicated.1 Currently available on-label or off-label, systemic medications include methotrexate, cyclosporine, azathioprine, mycophenolate, and dupilumab. Systemic corticosteroids are commonly used, although it is recommended that their long-term use should be avoided in patients with AD.2,3 The choice of which systemic therapy to choose is complex and must factor in effectiveness, safety, cost, availability and patient-specific factors, such as age, comorbidities, drug-drug interactions, and patient preference.1
Randomized clinical trials and network meta-analyses can be helpful for patients and clinicians to understand the relative efficacy and safety of treatments,4,5 but the populations included in randomized clinical trials (RCTs) for AD are often limited. In a systematic review, a third of AD systemic therapy trials had explicit upper age limits, and 70% had other exclusion criteria that would preferentially exclude older adults.6 The trials in that systematic review also commonly excluded people with liver disease, renal disease, viral hepatitis, HIV, or a history of malignancy.6 Observational data can help fill some of those gaps, but a systematic review of observational studies found only 2 small studies on the safety of systemic therapy for older adults with AD.7
To help guide clinical decision making for adults with AD in special populations, we conducted a scoping review of the literature and a survey of International Eczema Council (IEC) members. The aim of the scoping review was to identify literature on the use of systemic therapy for adult AD with comorbid liver disease, renal disease, viral hepatitises B and C, HIV, and a history of malignancy. The aim of the survey was to describe practice patterns of clinicians with expertise in AD.
MATERIALS AND METHODS
Scoping Review
We conducted the scoping review according to the methodological framework of Arksey and O'Malley8 and reported results in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis extension for Scoping Review (Supplemental Table S1, http://links.lww.com/DER/A97).9 We registered a protocol on Open Science Framework (https://osf.io/j96s4).
We searched MEDLINE via Ovid, Embase via Ovid, and Web of Science from inception to September 14, 2020, using a search strategy developed with the assistance of a research librarian (Supplemental Tables S2–S4, http://links.lww.com/DER/A97, respectively). We also manually searched citations of potentially relevant review articles for additional studies not included in our electronic searches.
Two investigators (pairs of M.L., R.M., J.Y., M.C.) screened titles, abstracts, and full texts independently and in duplicate. When necessary, discrepancies were resolved by consulting a senior author (A.M.D.). We included observational studies and case reports reporting on the use of cyclosporine, methotrexate, azathioprine, mycophenolate, systemic corticosteroids, and dupilumab for AD in individuals with HIV, viral hepatitis B or C, liver disease, renal disease, or a history of malignancy.
The following elements were abstracted in duplicate from each full-text article meeting our inclusion criteria using a standardized form: study characteristics (author, year, study design, country, participant source, funding), participant characteristics (total sample size, mean age, number and proportion of participants with HIV, liver disease and type, renal disease and type, history of malignancy and type), treatment, adverse, and efficacy outcomes (if reported).
We performed a qualitative content analysis of included articles, and study characteristics were synthesized in a descriptive summary. We produced an evidence map categorizing patients based on treatment and condition.
The IEC Survey
The IEC is a global nonprofit organization consisting of councilors and associates from 24 countries with research and clinical expertise in AD (http://www.eczemacouncil.org/). An electronic questionnaire was developed by IEC members (A.M.D., C.F., J.T., K.K., R.B., A.N.) and sent using SurveyMonkey on September 1, 2020, to all 103 IEC councilors and associates, of which 66 responded.
As anchoring questions, participants were asked about their approach to systemic treatment of a 30-year-old patient with AD for whom childbearing is not an important consideration. Participants were then asked about their approach to systemic treatment of AD among adults in special patient populations, including those with significant liver disease, kidney impairment, history of malignancy, HIV infection, or chronic hepatitis B or C infection. The questionnaire consisted of a 2-part question for each patient population: (1) which systemic agent would you consider prescribing for the patient population, out of azathioprine, corticosteroids, cyclosporine, dupilumab, methotrexate, mycophenolate, or none of these, and (ii) rank the systemic treatments you would consider for the patient population by your preferred first-, second-, and third-line systemic treatments (Supplemental Table S5 for full questionnaire, http://links.lww.com/DER/A97).
Reminders were sent at 2, 3, and 4 weeks, and responses were accepted for 5 weeks following the date that the questionnaire was sent. We calculated the proportion of respondents selecting each medication for each question across all survey participants and then separately in subgroups of IEC members practicing in Europe and North America.
RESULTS
Scoping Review
A total of 9688 records were retrieved from our literature search, and after the removal of 1992 duplicates, 7696 records were assessed based on titles and abstracts (Fig. 1). Full texts of 571 records were assessed, of which 25 met our eligibility criteria.10–34 Eleven studies were conducted in Europe, 10 in the United States, 2 studies in Asia, 1 in Australia, and 1 study in Mexico. Figure 2 summarizes the number of participants reported by included studies within each special population group, coded by treatment type. Most studies were published in 2019 (6 studies) or 2020 (11 studies). Two studies were published in 2017, and the remaining 6 studies were published in 2009 or prior.
Figure 1.
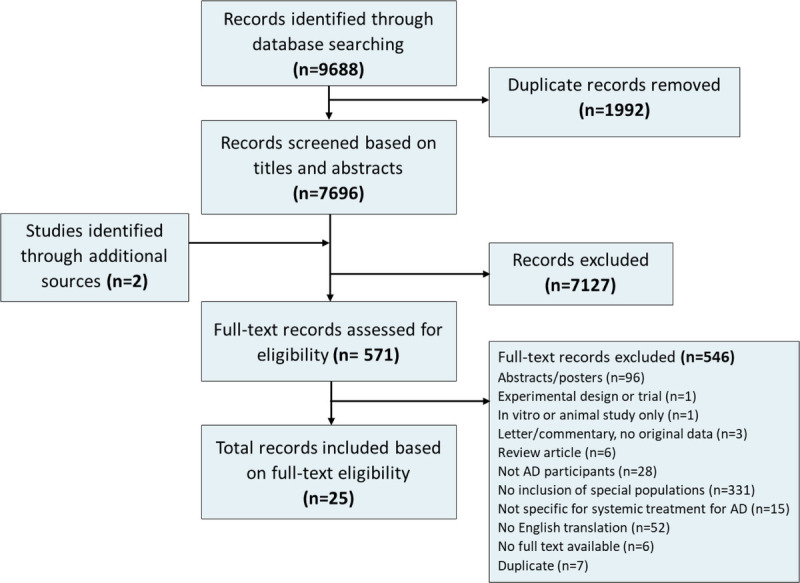
Study selection for the scoping review on the treatment of AD in people with HIV, viral hepatitis B or C, liver disease, renal disease, or a history of malignancy.
Figure 2.
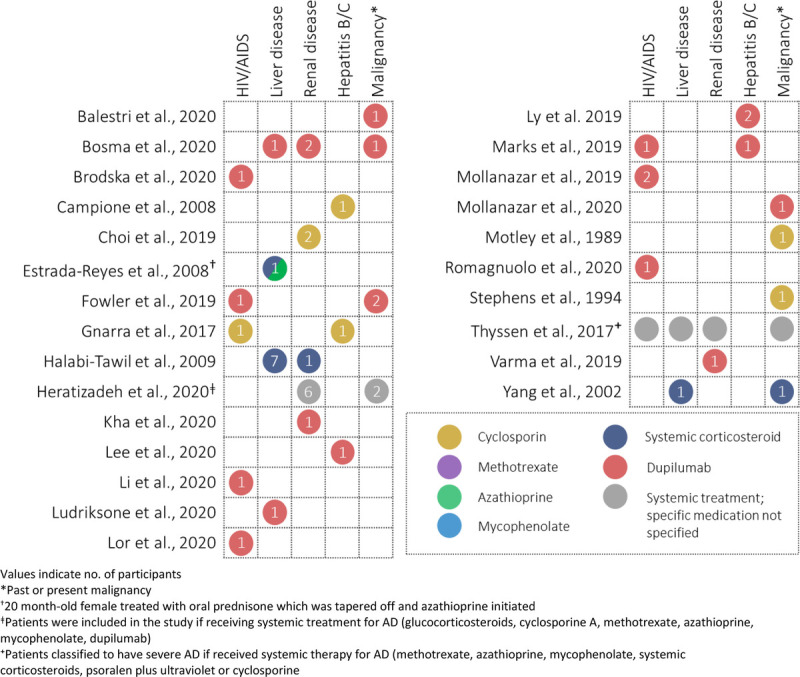
Evidence map of the studies on systemic therapy of AD in people with HIV, viral hepatitis B or C, liver disease, renal disease, or a history of malignancy.
Eight studies (6 case reports, 2 case series) included 9 AD participants with HIV/acquired immunodeficiency syndrome.11–15,17,33,34 Seven of these studies examined dupilumab treatment; 6 included adult patients with well-controlled HIV infections or under antiretroviral therapy, who experienced improvement under dupilumab treatment.11,13–15 Two of these studies reported no adverse effects on the patients' HIV disease after 15 months15 and 4 months14 of dupilumab treatment, and both reported undetectable viral loads (CD4 counts of 860 cells/μL14 and 668 cells/μL15). One study examining dupilumab included a patient with poorly controlled HIV infection (viral load, 276 copies/mL; CD4 count, 77 cells/μL) who reported improvements in self-reported itch intensity.17 One study examined cyclosporine treatment in an adolescent with perinatal transmission of HIV, resulting in complete clearance of skin lesions.12
Three studies included 3 cases with hepatitis C,12,14,21 and 2 studies included 3 cases with hepatitis B.18,22 Two patients treated with dupilumab were adults with a history of hepatitis C,14,21 and 1 adolescent patient with perinatal transmission of hepatitis C was treated with 12 months of cyclosporine and observed a reduction of hepatitis C virus RNA and alanine aminotransferase levels during the first phase of cyclosporine treatment.12 Ly et al22 examined 2 adult patients with chronic, well-controlled hepatitis B infections showing improvement with 20 months of dupilumab treatment, and Campione et al18 examined an adult patient with hepatitis B treated with 6 months of cyclosporine treatment and reported that liver function and hepatitis markers did not change with treatment.
Other liver diseases, including hepatosplenomegaly and acute liver failure due to Wilson disease, were reported in 11 participants across 5 studies (3 case reports, 2 cohorts).19,25,27,29,32 Three studies examined treatment with oral corticosteroids,19,27,32 with one of these studies also treating with 11 months of azathioprine after 1 month of oral prednisone.27 Two studies examined treatment with dupilumab,25,29 with one of these studies (Bosma et al25) reporting a patient with renal insufficiency and liver function abnormalities, who was treated with dupilumab.25
In addition to the study by Bosma et al,25 5 additional studies (2 case reports, 3 cohorts) reported a total of 13 participants with renal disease, including hydronephrosis, renal insufficiency, and end-stage renal disease.19,20,25,26,28,31 Three studies examined dupilumab treatment,20,25,31 1 study used cyclosporine treatment,26 1 study examined systemic corticosteroid treatment,19 and 1 study did not specify the systemic treatment used.28 Choi et al26 examined treatment with ciclosporin and reported that 1 patient with chronic kidney disease and 1 patient with end-stage renal failure on dialysis at the start of study, out of 92 patients in total. Varma et al31 presented a case report of a 22-month-old patient with a history of hydronephrosis who received 4 weeks of dupilumab. Kha et al20 presented a case report of a man with end-stage renal disease after kidney transplantation treated with 8 months of dupilumab. Halabi-Tawil et al19 reported 1 participant with membranous glomerulonephritis treated with systemic corticosteroids. Heratizadeh et al28 reported 6 patients (of 612) with comorbid renal insufficiency receiving systemic treatment (specific treatments received not specified).
Eight studies (4 case reports, 2 case series, 2 cohorts)10,11,16,24,25,28,30,32 included 3 participants with active malignancy, 1 participant who passed away because of complications of Hodgkin lymphoma, and 6 participants with past malignancy, including a history of breast cancer,10 skin cancer,28 and adenocarcinoma of the prostate.24 Three case reports presented 3 patients with lymphoma, examining treatment with dupilumab,16 intermittent oral corticosteroids,32 and cyclosporine.30 After 3 months of dupilumab treatment, Mollanazar et al16 noted a slight improvement in cutaneous T-cell lymphoma–specific laboratory values. Motley et al30 reported a stable condition in their T-cell lymphoma patient after 8 months of cyclosporine treatment. Bosma et al25 included 1 patient with active low-grade bladder cancer receiving dupilumab.
The IEC Survey
Sixty six of the 103 IEC councilors and associates (participation rate, 64.1%) responded to the survey. Respondents were from institutions in Africa (n = 1), Asia (n = 9), Australia (n = 1), Europe (n = 31), North America (n = 23), and South America (n = 1).
Across all special populations, dupilumab was the most common systemic treatment that respondents would consider prescribing (Supplemental Fig. S1 and Supplemental Table S6, http://links.lww.com/DER/A97) and was the systemic treatment most frequently selected as the preferred first-line agent for all patient populations (Table 1; Supplemental Table S7, http://links.lww.com/DER/A97). This was consistent in subgroup analyses limited to IEC members practicing in Europe and North America, respectively (Supplemental Figs. S2–S3 and Supplemental Tables S8, S9, http://links.lww.com/DER/A97).
TABLE 1.
Summary of the IEC Members' Ranked Preferred Systemic Treatments for the Treatment of Adults With AD in Special Patient Populations
| Most Commonly Preferred First-, Second-, and Third-Line Systemic Treatments for Patients Who Are Candidates for Systemic Therapy and Who: | First-LINE | Second-Line | Third-Line |
|---|---|---|---|
| Are 30 y old for whom childbearing is not an important consideration | Dupilumab (46.2%) | Cyclosporine (32.8%) Dupilumab (32.8%) |
Methotrexate (33.3%) |
| Are older (≥65 y) | Dupilumab (53.2%) | Dupilumab (25.8%) Methotrexate (25.8%) |
Mycophenolate (23.3%) |
| Have significant liver disease (excluding viral hepatitises B and C) | Dupilumab (76.7%) | Cyclosporine (48.1%) | None of these (27.9%) |
| Have significant kidney impairment | Dupilumab (76.3%) | Mycophenolate (25.5%) | None of these (33.3%) |
| Have a history of malignancy (other than KC/NMSC) presumed cured for <5 y | Dupilumab (73.7%) | Methotrexate (39.1%) | None of these (65.2%) |
| Have a history of malignancy (other than KC/NMSC) presumed cured for ≥5 y | Dupilumab (65.0%) | Methotrexate (28.1%) | None of these (24.4%) |
| Have an HIV infection | Dupilumab (67.3%) | Methotrexate (25.6%) | None of these (41.2%) |
| Have a chronic hepatitis B and/or C viral infection | Dupilumab (75.9%) | Corticosteroids (37.1%) | None of these (57.1%) |
Complete results for each special population and medication are given in Table E6.
KC/NMSC, keratinocyte carcinoma or non-melanoma skin cancer.
For older adults with AD, most respondents considered treatment with dupilumab (86.3%) and methotrexate (65.2%; Supplemental Fig. S1B, http://links.lww.com/DER/A97), which were also the most common preferred first- and second-line agents for older adults. Mycophenolate was the most common preferred third-line agent among older adults (23.3%).
For patients with significant liver disease, dupilumab (84.8%) was the treatment most frequently considered, followed by cyclosporine (37.9%; Supplemental Fig. S1B, http://links.lww.com/DER/A97). Dupilumab (76.7%) and cyclosporine (48.1%) received the highest number of responses for preferred first- and second-line therapy, respectively. Notably, the most frequently selected third-line treatment was none of the listed systemic treatments.
Respondents most frequently considered prescribing dupilumab (87.9%), methotrexate (34.8%), and mycophenolate (31.8%) for patients with significant kidney impairment (Supplemental Fig. S1D, http://links.lww.com/DER/A97). Dupilumab (76.3%) and mycophenolate (25.5%) were most commonly selected as preferred first- and second-line agents, respectively, for patients with significant kidney impairment.
For patients with a history of malignancy cured for less than 5 years and cured for 5 or more years, dupilumab (85.8%, 86.4%) and methotrexate (40.9%, 53.0%) were most frequently considered (Supplemental Figs. S1E, F, http://links.lww.com/DER/A97) and were the most frequent preferred first- and second-line treatments, respectively. None of the listed systemic treatments were most commonly selected for preferred third-line treatment. Results were similar for patients with HIV (Supplemental Fig. S1G, http://links.lww.com/DER/A97).
Most respondents would consider treatment with dupilumab for patients with chronic hepatitis B and/or C viral infection (Supplemental Fig. S1H, http://links.lww.com/DER/A97). However, 10 respondents (15.2%) indicated that none of the listed systemic treatments would be considered for this patient group. Dupilumab (75.9%) and cyclosporine (37.1%) were most commonly selected as the preferred first- and second-line treatment, but more than half of the respondents (57.1%) indicated that none of the listed systemic treatments were preferred third-line treatment.
DISCUSSION
In our scoping review of observational studies and case reports, we found limited evidence to guide systemic treatment decisions for older adults with AD and comorbid liver disease, renal disease, viral hepatitises B and C, HIV, or a history of malignancy. Our previous systematic reviews of RCTs and observational studies also found limited evidence for the treatment of older adults with systemic therapy.6,7
Ideally, all treatment decisions should be made on robust evidence applicable to the person with AD being treated. In the absence of such evidence, though, understanding expert practice patterns can be helpful. The results of our survey of IEC councilors and associates indicate that for all special populations under study, dupilumab would be the first-line systemic agent. Although cyclosporine was the most common second-line agent recommended for a hypothetical younger adult without comorbidities and for patients with liver disease, respondents tended to avoid it for patients with renal disease, viral hepatitises B and C, HIV, or a history of malignancy. This is similar to expert recommendations made in a recent review on treating psoriasis in special populations, which recommended against both cyclosporine and methotrexate for patients with chronic viral infections (hepatitis B, hepatitis C, and HIV).35 For patients with a history of malignancy, respondents favored methotrexate as a second-line agent.
Mycophenolate was the most commonly recommended third-line treatment for older adults, but the most common response for third-line treatment in the other special populations was “none of these.” This points to the current paucity of safe and effective treatments for severe AD, with dupilumab as the only targeted agent approved in most jurisdictions. Methotrexate, cyclosporine, azathioprine, and mycophenolate are all effective options, but their use is limited in AD patients with comorbidities due to: (1) their broad-spectrum immunomodulatory activity; (2) other potential toxicities, such as hepatotoxicity for methotrexate, azathioprine, and mycophenolate, and renal toxicity for cyclosporine; and (3) the lack of approval in moderate-to-severe AD for methotrexate, azathioprine, and mycophenolate.
Conclusions from our scoping review are limited by the low level of evidence from case reports and the limited number of observational studies included. Several reports also lacked sufficient detail on duration and safety of treatment. Our scoping review and survey are limited by their inclusion of only medications currently in widespread use. Many new systemic medications are being investigated for AD, but “real-world” data from observational studies are not yet available for them, and most IEC members do not have experience with these agents outside of clinical trial settings. Future work could replicate our studies, updated to include new medications, such as abrocitinib, baricitinib, upadacitinib, and tralokinumab. Access to different systemic medications differs geographically, so some IEC members' responses are likely influenced by local access.
As more biologic and other targeted agents are approved, there will be more options to use for older patients and those with comorbidities. Ideally, clinical trial inclusion criteria can be broadened to include such patients, but in the absence of that, high-quality observational data are needed. Ongoing AD registries will be instrumental in providing data on the safety of both new and older systemic agents for special populations of adults with AD, and observational studies should specifically aim to include older patients and those with comorbidities.
ACKNOWLEDGMENT
The authors thank Margaret Jung and Josephine Fazio for their help with survey development and dissemination.
Footnotes
A.M.D. has received compensation from the British Journal of Dermatology (reviewer and section editor), American Academy of Dermatology (guidelines writer), and National Eczema Association (grant reviewer). He has been a paid consultant for Canadian Agency for Drugs and Technology in Health. C.F. has received compensation from the British Journal of Dermatology (section editor). He is a chief investigator of the UK National Institute for Health Research–funded TREatment of Severe Atopic Eczema Trial and Softened Water for Eczema prevention trials as well as the UK-Irish Atopic eczema Systemic Therapy Register (A-STAR). He is also a principle investigator in the European Union Horizon 2020–funded BIOMAP Consortium (http://www.biomap-imi.eu/). His department has received funding from Sanofi-Genzyme for skin microbiome work. J.P.T. has been an advisor, investigator, or speaker for Regeneron, Sanofi-Genzyme, AbbVie, Pfizer, LEO Pharma, and Eli Lilly & Co. K.K. has received grants from Japan Tobacco, Inc; Kyowa Kirin; LEO Pharma; Maruho; Mitsubishi Tanabe Pharma; Ono Pharmaceutical; Pola Pharma; Procter & Gamble Company; Taiho Pharma; and Torii Pharmaceutical. R.B. is an advisory board member, consultant, speaker, and/or investigator for and receives honoraria and/or grant from AbbVie, Arcutis, Arena Pharma, Asana BioSciences, Bellus Health, Bluefin Biomedicine, Boehringer-Ingelheim, CARA, Eli Lilly, Evidera, Galderma, Incyte, Janssen, Kyowa Kirin, LEO Pharma, Pfizer, RAPT, Respivant, Sanofi-Genzyme, and Target RWE. R.B. is also an employee and shareholder of Innovaderm Research. V.A. is a principal investigator in the project 2018/23211-0 (Atopic Dermatitis) for FAPESP-Fundação de Amparo à Pesquisa do Estado de Sao Paulo and is a consultant and/or investigator in clinical trials for AbbVie, Sanofi, and Ely-Lilly. A.N. an advisory board member, consultant, speaker, and/or investigator for and receives honoraria and/or grant from AbbVie, Celgene, Sanofi-Genzyme, Pierre Fabre, Janssen, Eli Lilly, Leo Pharma, Galderma, Incyte, IsisPharma, Leo Pharma, L'Oréal, Novartis, Medac SAS, and Pfizer. The other authors have no funding or conflicts of interest to declare.
The International Eczema Council has received funding from AbbVie, Arena Pharmaceuticals, Asana BioSciences, Dermavant Sciences, Dermira, Eli Lilly and Company, Galderma, Incyte, Leo Pharma, Kyowa Kirin, Novartis, Pfizer, Sanofi-Genzyme and Regeneron Pharmaceuticals, and Sienna Biopharmaceuticals.
A.M.D. and M.L. contributed equally to this study.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.dermatitisjournal.com).
Contributor Information
Megan Lam, Email: Megan.lam@medportal.ca.
Carsten Flohr, Email: carsten.flohr@kcl.ac.uk.
Jacob P. Thyssen, Email: jacob.pontoppidan.thyssen@regionh.dk.
Kenji Kabashima, Email: kenjikabashima@gmail.com.
Robert Bissonnette, Email: rbissonnette@innovaderm.com.
Ncoza C. Dlova, Email: Dlovan@ukzn.ac.za.
Valeria Aoki, Email: valeria.aoki@gmail.com.
Max Chen, Email: max.chen@medportal.ca.
Joshua Yu, Email: josh.yu@medportal.ca.
Jie Wei Zhu, Email: jenny.zhu@medportal.ca.
Robert Micieli, Email: robertmicieli@gmail.com.
Audrey Nosbaum, Email: nosbaumaudrey@yahoo.fr.
REFERENCES
- 1.Simpson EL Bruin-Weller M Flohr C, et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J Am Acad Dermatol 2017;77(4):623–633. doi: 10.1016/j.jaad.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 2.Drucker AM Eyerich K de Bruin-Weller MS, et al. Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement. Br J Dermatol 2018;178(3):768–775. doi: 10.1111/bjd.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson EL Bieber T Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol 2016;74(3):491–498. doi: 10.1016/j.jaad.2015.10.043. [DOI] [PubMed] [Google Scholar]
- 4.Drucker AM Ellis AG Bohdanowicz M, et al. Systemic immunomodulatory treatments for patients with atopic dermatitis: a systematic review and network meta-analysis. JAMA Dermatol 2020;156(6):659–667. doi: 10.1001/jamadermatol.2020.0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawangjit R Dilokthornsakul P Lloyd-Lavery A, et al. Systemic treatments for eczema: a network meta-analysis. Cochrane Database Syst Rev 2020;9(9):CD013206. doi: 10.1002/14651858.cd013206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam M Zhu JW Maqbool T, et al. Inclusion of older adults in randomized clinical trials for systemic medications for atopic dermatitis: a systematic review. JAMA Dermatol 2020;156(11):1240–1245. doi: 10.1001/jamadermatol.2020.2940. [DOI] [PubMed] [Google Scholar]
- 7.Tang E Maqbool T Lam M, et al. Safety of systemic medications among older adults with psoriasis and atopic dermatitis: a systematic review of observational studies. J Cutan Med Surg 2021, 25, 397, 408. doi: 10.1177/1203475421993770. [DOI] [PubMed] [Google Scholar]
- 8.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol Theory Pract 2005;8(1):19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 9.Tricco AC Lillie E Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 10.Stephens RB, Lee ML, Cooper A. Cyclosporin treatment of atopic dermatitis: five case studies and literature review. Australas J Dermatol 1994;35(2):55–59. doi: 10.1111/j.1440-0960.1994.tb00895.x. [DOI] [PubMed] [Google Scholar]
- 11.Fowler E, Rosen J, Lev-Tov H, Yosipovitch G. Two cancer patients receiving dupilumab for treatment of atopic dermatitis. Acta Derm Venereol 2019;99(10):899–900. doi: 10.2340/00015555-3201. [DOI] [PubMed] [Google Scholar]
- 12.Gnarra M, De Simone C, Garcovich M, Garcovich S. Low-dose cyclosporine A in the treatment of severe atopic dermatitis complicated by chronic hepatitis C virus infection. Pediatr Dermatol 2017;34(3):374–376. doi: 10.1111/pde.13115. [DOI] [PubMed] [Google Scholar]
- 13.Li G, Berkenstock M, Soiberman U. Corneal ulceration associated with dupilumab use in a patient with atopic dermatitis. Am J Ophthalmol Case Rep. 2020;19:100848. doi: 10.1016/j.ajoc.2020.100848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marks DH, Piantadosi AL, Patil SU, Raff A, Yu J. Successful use of dupilumab for recalcitrant atopic dermatitis in an HIV patient. Dermatitis. 2019;30(4):276–277. doi: 10.1097/DER.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 15.Romagnuolo M, Angileri L, Tavecchio S, Marzano A V, Ferrucci S. Safety and efficacy of dupilumab in a patient with severe atopic dermatitis and HIV infection, with 15 months of follow-up. Clin Exp Dermatol 2020;45(6):762–763. doi: 10.1111/ced.14278. [DOI] [PubMed] [Google Scholar]
- 16.Mollanazar NK Savage KT Pousti BT, et al. Cutaneous T-cell lymphoma and concomitant atopic dermatitis responding to dupilumab. Cutis. 2020;106(3):131–132. doi: 10.12788/cutis.0066. [DOI] [PubMed] [Google Scholar]
- 17.Mollanazar NK Qiu CC Aldrich JL, et al. Use of dupilumab in patients who are HIV-positive: report of four cases. Br J Dermatol 2019;181(6):1311–1312. doi: 10.1111/bjd.18222. [DOI] [PubMed] [Google Scholar]
- 18.Campione E, Paternò EJ, Diluvio L, Bianchi L, Giorgetti G, Chimenti S. Combination of low-dosage cyclosporine and topical pimecrolimus in severe atopic dermatitis with chronic hepatitis B. Acta Derm Venereol 2008;88(1):74–75. doi: 10.2340/00015555-0327. [DOI] [PubMed] [Google Scholar]
- 19.Halabi-Tawil M Ruemmele FM Fraitag S, et al. Cutaneous manifestations of immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. Br J Dermatol 2009;160(3):645–651. doi: 10.1111/j.1365-2133.2008.08835.x. [DOI] [PubMed] [Google Scholar]
- 20.Kha C, Raji K, Chisolm S. Treatment of atopic dermatitis with dupilumab in a renal transplant patient. Dermatitis 2020;31(2):E17–E18. doi: 10.1097/DER.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 21.Lee DH, Cohen LM, Yoon MK, Tao JP. Punctal stenosis associated with dupilumab therapy for atopic dermatitis. J Dermatolog Treat 2021;32(7):737–40. doi: 10.1080/09546634.2019.1711010. [DOI] [PubMed] [Google Scholar]
- 22.Ly K, Smith MP, Thibodeaux Q, Beck K, Bhutani T, Liao W. Dupilumab in patients with chronic hepatitis B on concomitant entecavir. JAAD Case Rep 2019;5(7):624–626. doi: 10.1016/j.jdcr.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thyssen JP, Skov L, Hamann CR, Gislason GH, Egeberg A. Assessment of major comorbidities in adults with atopic dermatitis using the Charlson Comorbidity Index. J Am Acad Dermatol. 2017;76(6):1088–1092.e1. doi: 10.1016/j.jaad.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 24.Balestri R, Magnano M, Girardelli CR, Bortolotti R, Rech G. Long-term safety of combined biological therapy in a patient affected by arthropathic psoriasis and atopic dermatitis. Dermatol Ther 2020;33(4):e13498. doi: 10.1111/dth.13498. [DOI] [PubMed] [Google Scholar]
- 25.Bosma AL de Wijs LEM Hof MH, et al. Long-term effectiveness and safety of treatment with dupilumab in patients with atopic dermatitis: results of the TREAT NL (TREatment of ATopic eczema, the Netherlands) registry. J Am Acad Dermatol 2020;83(5):1375–1384. doi: 10.1016/j.jaad.2020.05.128. [DOI] [PubMed] [Google Scholar]
- 26.Choi E Cook A Phuan C, et al. Outcomes of prolonged and low-dose ciclosporin in an Asian population. J Dermatolog Treat 2021;32(4):432–437. doi: 10.1080/09546634.2019.1662881. [DOI] [PubMed] [Google Scholar]
- 27.Estrada-Reyes E Hernnández-Román MP Gamboa-Marrufo JD, et al. Hypereosinophilia, hyper-IgE syndrome, and atopic dermatitis in a toddler with food hypersensitivity. J Investig Allergol Clin Immunol 2008;18(2):131–135. [PubMed] [Google Scholar]
- 28.Heratizadeh A Haufe E Stölzl D, et al. Baseline characteristics, disease severity and treatment history of patients with atopic dermatitis included in the German AD Registry TREATgermany. J Eur Acad Dermatol Venereol 2020;34(6):1263–1272. doi: 10.1111/jdv.16078. [DOI] [PubMed] [Google Scholar]
- 29.Ludriksone L, Elsner P, Malessa C, Settmacher U, Schliemann S. Effectiveness and safety of dupilumab for atopic dermatitis in a liver transplant recipient: a case report. J Dtsch Dermatol Ges 2020;18(7):740–742. doi: 10.1111/ddg.14074. [DOI] [PubMed] [Google Scholar]
- 30.Motley RJ, Whittaker JA, Holt PJ. Resolution of atopic dermatitis in a patient treated with cyclosporin. Clin Exp Dermatol 1989;14(3):243–244. doi: 10.1111/j.1365-2230.1989.tb00943.x. [DOI] [PubMed] [Google Scholar]
- 31.Varma A, Tassavor M, Levitt J. The utility of dupilumab for use in the pediatric population. JAAD Case Reports. 2019;5(11):943–944. doi: 10.1016/j.jdcr.2019.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang CM Yang YH Lin YT, et al. Natural killer cell deficiency associated with Hodgkin's lymphoma: a case report. J Formos Med Assoc 2002;101(1):73–75. [PubMed] [Google Scholar]
- 33.Brodska P, Panzner P, Sedlacek D, Terl M, Cetkovska P. Use of dupilumab in a patient with atopic dermatitis, severe asthma, and HIV infection. Dermatol Ther. 2020;33(6):e14159. doi: 10.1111/dth.14159. [DOI] [PubMed] [Google Scholar]
- 34.Lor M, Villa N, Holland V. Safe and effective treatment of atopic dermatitis using dupilumab over 23 months in a patient with HIV. Dermatol Ther. 2020;33(6):e14271. doi: 10.1111/dth.14271. [DOI] [PubMed] [Google Scholar]
- 35.Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol 2019;80(1):43–53. doi: 10.1016/j.jaad.2018.06.056. [DOI] [PubMed] [Google Scholar]


