Abstract
Recently, the first 7-T MR system was approved for clinical use in the United States. Unfortunately, relatively few metallic implants have undergone testing to determine if they are acceptable or pose hazards to research subjects and patients at this ultra-high-field strength. Therefore, in lieu of not performing a research or clinical MRI exam at 7-T, the supervising physician may make a decision to scan the individual with an untested metallic implant based on an analysis of the risks vs. the benefits. We present a case report of a research subject with bilateral, total knee replacement implants that safely underwent MRI of the brain at 7-T and provide guidelines for healthcare professionals to follow in order to ensure safety in research subjects or patients with metallic implants referred for 7-T scans.
Keywords: Magnetic resonance imaging (MRI), 7-Tesla, Brain, MRI implants, MRI safety
1. Introduction
In 2017, the Food and Drug Administration (FDA) approved the first 7-Tesla magnetic resonance (MR) system, more than doubling the static magnetic field strength that was previously available for clinical use in the United States [1]. The use of 7-T MRI (commonly referred to as ultra-high-field or UHF MRI) in the brain is believed to result in improved clinical outcomes because of the ability to detect subtle anatomical, functional, and metabolic abnormalities that may be un-appreciated using lower field strength MR systems [2–4].
As indicated by Kraff and Quick [5], a precondition for the clinical application of 7-T MRI is to conduct safety testing of implants. While investigators have initiated evaluations of passive (i.e., an implant that does not contain electronic components and/or an external source of power) metallic implants in association with 7-T scanners, to date, relatively few implants have undergone proper testing [6–9]. Because of this lack of information, both research and clinical uses of 7-T MRI may be limited because it excludes volunteer subjects and patients with metallic implants.
In this report, we present a case of a research subject with bilateral, total knee replacement (TKR) implants (TKR is one of the most commonly performed orthopedic procedures [10]), who underwent MRI of the brain at 7-T and explain our strategy that allowed the safe use of UHF MRI in this individual despite the presence of untested implants. Guidelines are provided to ensure safety in similar research subjects or patients referred for 7-T scans.
2. Materials & methods
The research subject involved in this Case Report was a 56-year-old woman with bilateral, TKR implants. Descriptive information for the orthopedic implants is presented in Table 1. Besides the implants, this individual denied a prior history of relevant surgical procedures or health conditions. Under an Institutional Review Board-approved research protocol and following written and verbal informed consent, the research subject underwent research that involved testing specific sequences and protocols. MRI was performed at 7-T (MAGNETOM, Terra, Siemens, Siemens Medical Solutions) using a single channel-transmit/32 channel-receive head radiofrequency (RF) coil (note, this FDA approved, 7-Tesla scanner currently has transmit/receive RF head and knee coils, only). For this scanner, the highest “patient accessible” spatial gradient magnetic field (meaning the greatest spatial gradient magnetic field that a patient with a metallic implant passes through while being positioned at isocenter of the MR system) is 820-gauss/cm which is located 98-cm from the isocenter. Because of the fact that the materials (i.e., polyethylene, cobalt-chromium-molybdenum alloy, titanium-aluminum-vanadium alloy, and tantalum) used to make the TKR implants were nonmetallic or known to have a low magnetic susceptibility and the implants would not be located in the immediate area of the transmitted RF energy (note that RF power density rapidly decreases outside the transmit RF coil), the supervising neuroradiologist made the decision to proceed with the MRI exam after a careful analysis of the risks versus the benefits (i.e., there were no concerns related to magnetic field interactions or MRI-related heating for this individual’s untested implants).
Table 1.
Information for the research subject’s TKR implants (Zimmer Biomet, Warsaw, IN).
| Description | Material | Knee |
|---|---|---|
| Zimmer Persona Posterior Stabilized Femoral Standard, Cemented Core Set, Size 6 | Cobalt-Chromium-Molybdenum (Co-Cr-Mo) Alloy | Right |
| Zimmer Nexgen Trabecular Metal, Tibial Component, Fixed Bearing, Size 3 | Titanium-Aluminum-Vanadium Alloy with a coating of porous Tantalum | Right |
| Zimmer Nexgen Legacy Posterior Stabilized (LPS)-Flex Articular Insert, Size 12 mm | Polyethylene | Right |
| Zimmer Persona Posterior Stabilized Femoral Standard, Cemented Core Set, Size 6 | Cobalt-Chromium-Molybdenum (Co-Cr-Mo) Alloy | Left |
| Zimmer Nexgen Trabecular Metal, Tibial Component, Fixed Bearing, Size 3 | Titanium-Aluminum-Vanadium Alloy with a coating of porous Tantalum | Left |
| Zimmer Nexgen Legacy Posterior Stabilized (LPS)-Flex Articular Insert, Size 10 mm | Polyethylene | Left |
For the 7-T MRI scan of the brain, the following imaging parameters were used: 3D T1-weighted magnetization prepared, two rapid-acquisition gradient-echo (MP2RAGE) sequence (TR = 4500 ms, TE = 3.4 ms, TI1 = 1000 ms, T12 = 3200 ms, α1/α2 = 4°/5°, 224 slices, effective voxel resolution = isotropic 0.7 mm3), T2-weighted turbo spin echo sequence (TR = 3000 ms, TE = 73 ms, α = 140°, 24 slices, effective voxel resolution = 0.15 × 0.15 × 2.0 mm3), time-of-flight sequence (TR = 12 ms, TE = 4.67 ms, α = 17°, 72 slices, effective voxel resolution = isotropic 0.3 mm3); 3D multi echo susceptibility-weighted imaging with six equidistant echoes ranging from 4 to 23 ms (TR = 27 ms, α = 13°, 160 slices), optimized multi-shell diffusion-weighted with b-values of 700 s/mm2, 1000 s/mm2, 2000 s/mm2, 45 diffusion-encoding direction per shell, 19 non-diffusion-weighted images, acquired at AP and PA phase encoding directions (TR = 5900 ms, TE = 69 ms, 90 slices, effective voxel resolution = isotropic 1.3 mm3).
3. Results
The 7-T MR images of the brain demonstrated no significant findings (Figs. 1 and 2). The research subject did not complain of discomfort, pain, or other sensations during or after the MRI exam. A six month follow up of the research subject revealed no new symptoms and there were no abnormalities revealed on physical examination at that time.
Fig. 1.
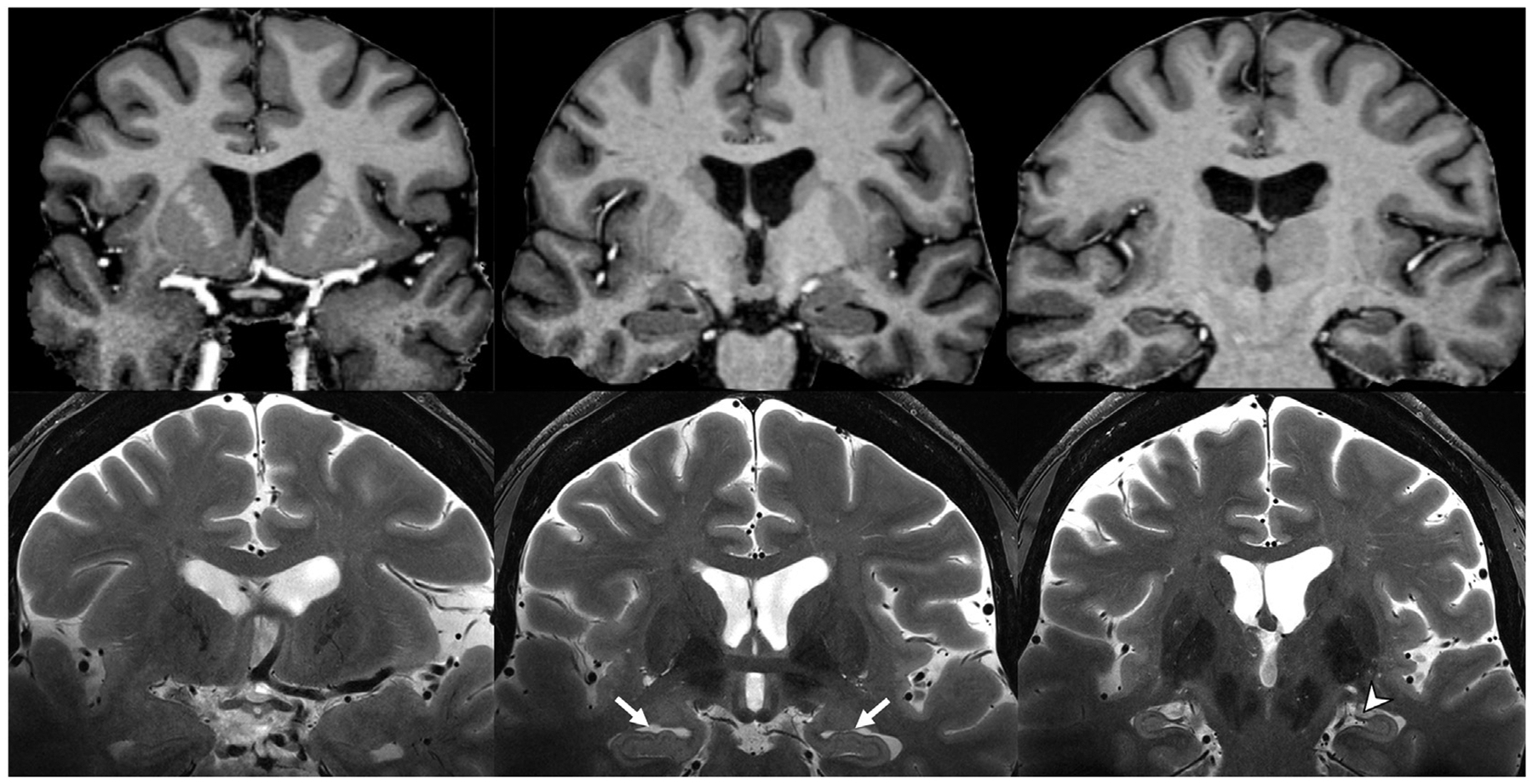
Skull-stripped coronal plane T1 MP2RAGE (top row) and unprocessed T2-weighted (bottom row) MR images. Note the contrast between grey and white matter on the T1 MP2RAGE, and the hippocampal digitations (white arrows), fimbria (arrowhead), and stratum layers of the hippocampus resolved on the T2-weighted images at 7-T.
(MP2RAGE, magnetization prepared, two rapid-acquisition gradient-echo).
Fig. 2.
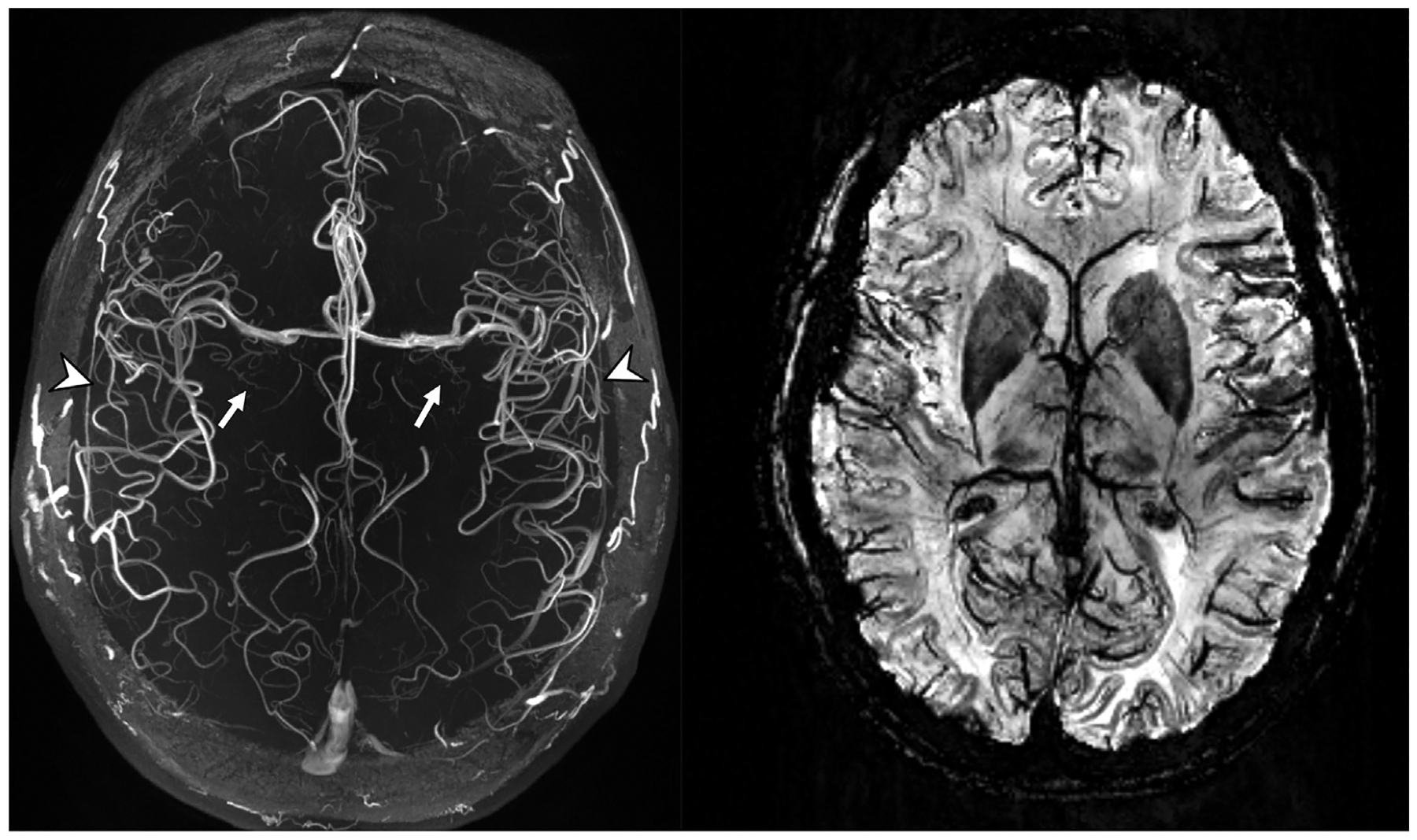
Axial plane TOF-MRA MIP (left) and SWI MinIP (right) MR images. Note the conspicuity of the lenticulostriate arteries (white arrows) arising from the MCAs, the high contrast seen in the insular branches (arrowheads) of the MCAs, and the high susceptibility and spatial resolution allowing better definition of deoxyhemoglobin in the smaller veins on the SWI image.
(MCA, middle cerebral artery; MinIP, minimum intensity projection; MIP, maximum intensity projection; SWI, susceptibility-weighted imaging; TOF-MRA, time-of-flight, magnetic resonance angio-graphy).
4. Discussion
Various studies have reported the clinical superiority of 7-T MRI for neurological and musculoskeletal applications [2–4], while whole-body applications are currently limited primarily because of the lack of suitable RF coils [4,5]. A limiting factor that impacts the research or clinical utilization of 7-T relates to the performance of exams in individuals with metallic implants. In general, most 7-T research facilities conservatively exclude all subjects with metallic implants regardless of the type or location. Presently, only a small number of biomedical products have undergone proper testing and subsequently labeled with MRI-specific information (i.e., labeled “MR Conditional”) [9,13]. If 7-T MRI is to reach its full potential as an imaging modality, scanning must become available to research subjects and patients with metallic implants [5].
The main concerns for passive metallic implants are magnetic field interactions (i.e., translational attraction and torque) and RF-induced heating [5–9,11,13–15]. From a magnetic field interaction consideration, translational attraction and/or torque may cause movement or dislodgment of a ferromagnetic implant resulting in an uncomfortable sensation, a minor-to-severe injury, or a fatality [9]. Translational attraction is dependent on the strength of the static magnetic field, the spatial gradient magnetic field, the mass of the implant, the shape of the implant and its magnetic susceptibility. Torque or rotational force, which predominates in the area of the MR system where the static field is most homogenous, influences metallic implants that have an elongated shape. From a practical standpoint, in addition to giving attention to translational attraction and torque, the “intended in vivo use” must be contemplated for metallic implants, particularly with respect to the various mechanisms that may provide retention or counterforce for the object in situ (e.g., the implant may be held in place by granulation or ingrowth of tissue; by sutures, fasteners or clips; by cement, etc.).
With respect to RF-induced implant heating, ex vivo testing using worst-case conditions has demonstrated that only minor temperature changes occur in association with objects that are relatively small passive implants (e.g., hemostatic clips, heart valve prostheses, ossicular implants, vascular access ports, ocular implants, etc.) [9,14–16]. By comparison, excessive heating and burns can occur with implants that have elongated configurations or that form conducting loops of certain diameters [9,14–16]. In general, for excessive implant heating to occur in association with MRI, all or part of the metallic object must be exposed to the RF energy deposited by the transmit RF coil of the scanner [9,14–16].
In this case example, we describe the use of 7-T MRI of the brain in a research subject with TKR implants (Fig. 3). To the best of our knowledge, this is the first report of an individual with untested metallic implants that safely underwent a scan using a UHF scanner. Because the TKR implants were made of materials that were nonmetallic or had a low magnetic susceptibility and were implanted in an anatomic area far removed from the transmitted RF energy, there were no deleterious effects anticipated for the scan. By following a similar strategy, a research subject or patient can safely undergo a 7-T MRI procedure and potentially benefit from the enhanced capabilities associated with UHF imaging.
Fig. 3.
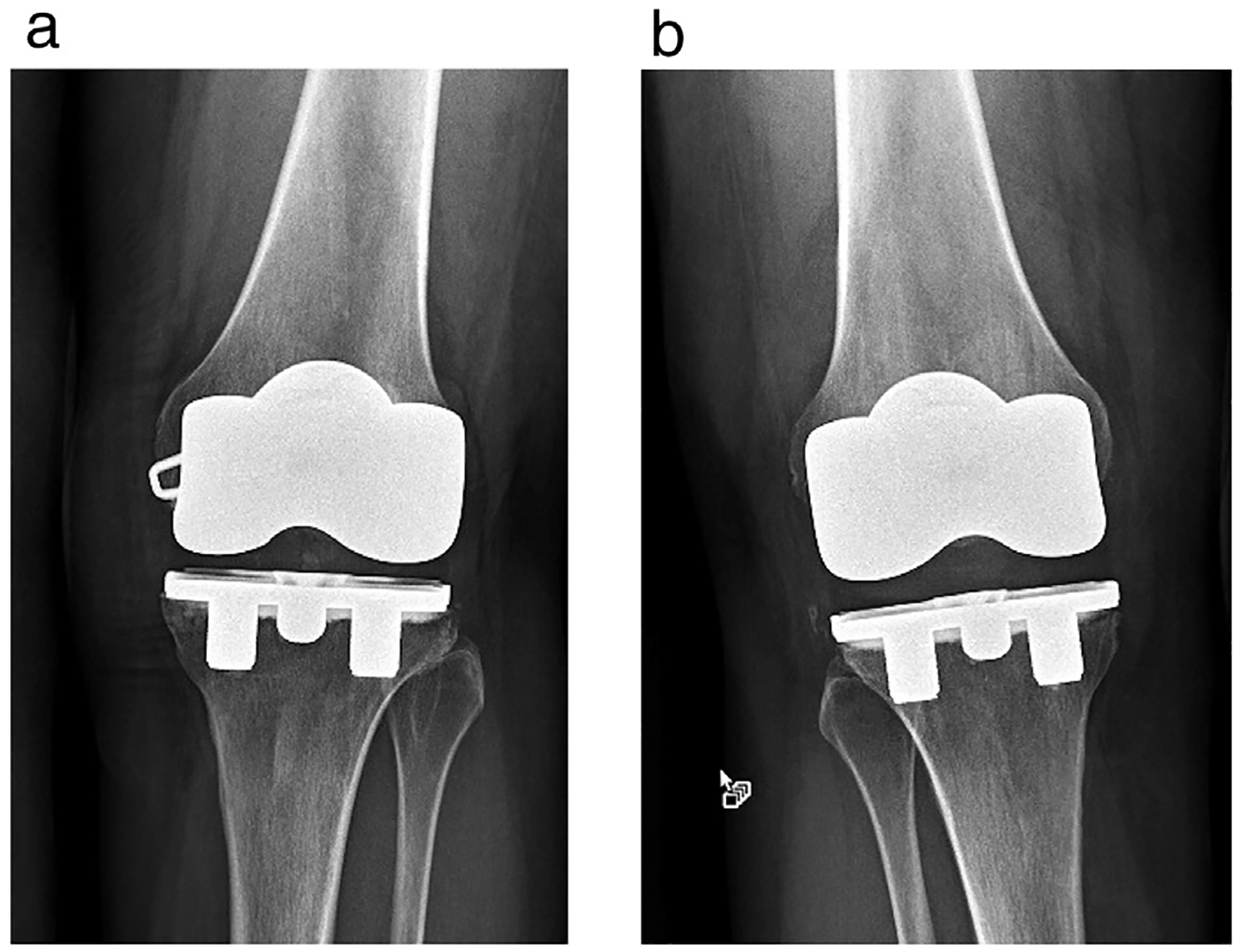
X-rays showing the TKR implants for the research subject that underwent MRI of the brain at 7-T. (a) Left knee, (b) Right knee.
5. Conclusions and recommendations
In conclusion, it is feasible to safely perform MRI of the brain at 7-T in an individual with an untested metallic implant by conducting an analysis of the possible risks. We propose the following conservative guidelines that can be utilized to manage a research subject or patient with an untested implant referred for a 7-T scan:
The supervising physician (e.g., the MRI-trained radiologist) must perform an assessment of the risks associated with the implant versus the benefits of the MRI exam.
Because of the lack of MRI-related labeling for the implant, a physician must provide written and verbal informed consent to the individual and include a discussion of the potential benefits, risks, and alternative imaging procedure that may be appropriate.
The type of implant and its anatomical location relative to the body part that will be imaged must be determined and considered during the risk versus benefit analysis.
The material used to make the implant must be identified in order to establish if there may be issues with respect to magnetic field interactions and heating. Typically, material information can be obtained by contacting the manufacturer. Some common low magnetic susceptibility, metallic materials that are used for implants include cobalt-chromium-molybdenum alloy, nitinol, austenitic stainless steel (e.g., 316L, 316LVM, etc.), platinum, and platinum-iridium, titanium-aluminum-vanadium alloy, tantalum, commercially pure titanium, and titanium alloy [9,12].
Since all metals are conductors, the position of the implant relative to the transmitted RF energy must be considered with respect to the potential for excessive heating [9,11]. Thus, to ensure the least amount of risk for the individual, the entire implant must be located outside and a suitable distance from the area of the transmitted RF energy. (Note, as previously indicated, 7-Tesla MR system approved by the FDA currently has transmit/receive RF head and knee coils, only. Accordingly, a relatively straightforward decision may be made with respect to hazards related to excessive implant heating for this scanner configuration. Once a transmit body or other transmit, body-part-specific RF coils become available, the prediction of implant heating will become a greater challenge.)
Continuously monitor the individual visually and verbally throughout the MRI exam. If the individual reports any unusual sensation, the scan must be immediately discontinued.
References
- [1].U.S. Department of Health and Human Services, U.S. Food and Drug Administration. FDA clears first 7 T magnetic resonance imaging device. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm580154.htm, Accessed date: 12 October 2017.
- [2].Trattnig S, Springer E, Bogner W, et al. Key clinical benefits of neuroimaging at 7 T. NeuroImage 2018;168:477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Balchandani P, Naidich TP. Ultra-high-field MR neuroimaging. AJNR Am J Neuroradiol 2015;36:1204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barisano G, Sepehrband F, Ma S, et al. Clinical 7T MRI: Are we there yet? A review about magnetic resonance imaging at ultra-high field. Br J Radiol. 2018;91:20180492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kraff O, Quick HH. 7 T: physics, safety, and potential clinical applications. J Magn Reson Imaging 2017;46:1573–89. 10.1002/jmri.25723. [DOI] [PubMed] [Google Scholar]
- [6].Sammet CL, Yang X, Wassenaar PA, et al. RF-related heating assessment of extra-cranial neurosurgical implants at 7 T. Magn Reson Imaging 2013;31:1029–34. [DOI] [PubMed] [Google Scholar]
- [7].Feng DX, McCauley JP, Morgan-Curtis FK, et al. Evaluation of 39 medical implants at 7.0 T. Br J Radiol 2015;88:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dula AN, Virostko J, Shellock FG. Assessment of MRI issues at 7 T for 28 implants and other objects. Am J Roentgenol 2014;202:401–5. [DOI] [PubMed] [Google Scholar]
- [9].Shellock FG. Shellock FG, editor. Reference manual for magnetic resonance safety, implants, and devices. 2018 ed. California: Biomedical Research Publishing Group; 2018. [Google Scholar]
- [10].Steiner C, Andrews R, Barrett M, Weiss A. HCUP projections: mobility/orthopedic procedures 2003 to 2012. US Agency Healthc Res Qual (Internet). 2012; (#2012-3):110 Available from: http://www.hcup-us.ahrq.gov/reports/projections/2012-03.pdf; 2012. [Google Scholar]
- [11].Woods TO. Standards for medical devices in MRI: present and future. J Magn Reson Imaging 2007;26:1186–9. [DOI] [PubMed] [Google Scholar]
- [12].Schenck JF. The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med Phys 1996;23:815–50. [DOI] [PubMed] [Google Scholar]
- [13].U.S. Department of Health and Human Services, U.S. Food and Drug Administration. Establishing safety and compatibility of passive implants in the magnetic resonance (MR) environment guidance for industry and Food and Drug Administration staff, document issued on December 11. 2014.
- [14].Bottomley PA. Turning up the heat on MRI. J Am Coll Radiol 2008;5:853–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dempsey MF, Condon B, Hadley DM. Investigation of the factors responsible for burns during MRI. J Magn Reson Imaging 2001;13:627–31. [DOI] [PubMed] [Google Scholar]
- [16].Smith CD, et al. Interactions of MRI magnetic fields with elongated medical implants. J Appl Phys 2000;87:6188–90. [Google Scholar]


