Abstract
Background
With increased use of antibiotics in high-risk patients, the investigation of new antibiotics to cover potentially resistant pathogens is warranted. In this prospective randomized trial, we compared ceftolozane/tazobactam (C/T), a new cephalosporin/β-lactamase inhibitor, to the standard of care (SOC) for the empiric treatment of neutropenia and fever in patients with hematological malignancies.
Methods
We enrolled 100 patients to receive intravenous (IV) C/T or SOC antibiotics (cefepime, piperacillin/tazobactam, or meropenem) in combination with gram-positive antibacterial agents. We evaluated responses at the end of IV therapy (EOIV), test of cure (TOC; days 21–28), and late follow-up (LFU; days 35–42).
Results
We analyzed 47 C/T patients and 50 SOC patients. C/T patients had a higher rate of favorable clinical response at EOIV (87% vs 72%). A 1-sided noninferiority analysis indicated that C/T was at least not inferior to the SOC for favorable clinical response at EOIV (P = .002), TOC (P = .004), and LFU (P = .002). Superiority tests showed that C/T led to significantly lower rates of clinical failure at TOC (6% vs 30%; P = .003) and LFU (9% vs 30%; P = .008). C/T and SOC patients with documented infections had similar rates of favorable microbiological response. Serious adverse events leading to drug discontinuation (2% vs 0%; P = .48) and overall mortality (6% vs 4%; P = .67) were similar in both groups.
Conclusions
The empiric use of C/T in high-risk patients with hematological malignancies and febrile neutropenia is safe and associated with better clinical outcomes than SOC antimicrobial agents.
Clinical Trials Registration
Keywords: cancer patients, febrile neutropenia, fever, immunocompromised, leukemia, neutropenia, neutropenic fever
Ceftolozane/tazobactam can be used as empiric treatment in cancer patients with neutropenia and fever. It is safe and is associated with better clinical outcomes compared to other standard-of-care antimicrobial agents.
Patients with hematologic malignancy (HM) and recipients of hematopoietic stem cell transplantation receive intensive chemotherapy that often induces prolonged neutropenia, which puts these patients at particularly high risk for potentially life-threatening infections, particularly if not recognized and treated promptly [1, 2]. Delaying appropriate antibiotic therapy in patients with Pseudomonas aeruginosa bloodstream infections (BSIs) or other resistant gram-negatives pathogens has been associated with poor outcomes and increased mortality [3–8].
The careful evaluation of these patients’ signs and symptoms, antimicrobial prophylaxis, prior infections, previous antimicrobial use, and potential antimicrobial resistance is crucial to guide the appropriate selection of antimicrobial therapy. Although only 20%–30% of patients with neutropenia and fever have a clinically or microbiologically documented infection [2], the rate of infections caused by gram-negative pathogens is increasing with the emergence of antimicrobial-resistant strains [9–13]. Hence, standard empirical therapy with cefepime, piperacillin-tazobactam, or carbapenems could be inappropriate for cancer patients with febrile neutropenia (FN), particularly those with a history of infection or colonization with an antibiotic-resistant organism (such as extended-spectrum β-lactamase [ESBL]–producing Enterobacteriaceae or resistant Pseudomonas) [14]. Any initial empirical antibiotic therapy given for FN should include an antipseudomonal β-lactam agent to cover the most virulent and resistant gram-negative pathogens. Antibiotics against gram-positive pathogens should be added for suspected catheter-related infection, skin or soft tissue infection, pneumonia, or hemodynamic instability [2]. If antimicrobial resistance is suspected, the addition of other antimicrobials should be considered, particularly if the patient is hemodynamically unstable.
Despite successful antibiotic stewardship programs, antibiotic resistance continues to emerge, particularly in patients with gram-negative bacterial infections. Ceftolozane/tazobactam (C/T), a combination of a novel antipseudomonal cephalosporin antibiotic and an established β-lactamase inhibitor, was initially developed to address antimicrobial resistance in cases of serious infections caused by gram-negative pathogens [15]. The addition of tazobactam extends the spectrum of C/T coverage to include ESBL-producing Enterobacteriaceae. C/T is now approved in the United States for the treatment of complicated intra-abdominal infections in combination with metronidazole; complicated urinary tract infections, including pyelonephritis; and ventilated nosocomial pneumonia [16–19]. Few case reports have reported promising results with favorable outcomes in HM and HCST patients with FN and P aeruginosa infections or other resistant gram-negative organisms [20–22]. However, the empiric use of C/T for the treatment of FN has not been evaluated in a prospective randomized trial. In this study, we compared C/T with standard-of-care (SOC) antibiotics (cefepime, meropenem, or piperacillin/tazobactam) given with anti–gram-positive antibiotics for the empiric treatment of FN in patients with HM.
METHODS
Study Design
This single-center, prospective, randomized, open-label comparative study was approved by our Institutional Review Board and is registered at ClinicalTrials.gov (NCT03485950). Written informed consent was obtained from all patients or their authorized representatives.
Patient Population
Eligible patients were aged ≥18 years, had HM, presented to our emergency center with FN, and required hospitalization for intravenous (IV) empiric antibiotic therapy. Patients were excluded if they were allergic to any cephalosporin antibiotic; previously received IV antibiotics for >24 hours; had a confirmed viral or fungal infection; had an alanine aminotransferase level >5 times the upper limit of normal (ULN), total bilirubin level >3 times the ULN, or creatinine clearance ≤30 mL minute; or had a history of seizure disorder.
Randomization, Treatment, and Monitoring
Between May 2018 and October 2020, 100 patients were enrolled and randomized using our institutional Clinical Trial Conduct website to receive either C/T (1.5 g every 8 hours) or SOC antibiotics (cefepime [2 g every 8 hours], meropenem [1 g every 8 hours], or piperacillin/tazobactam [4.5 g every 6 hours], at the discretion of the treating physician) for at least 72 hours and up to 14 days.
Patients in either group could receive antibacterial agents (vancomycin, linezolid, or daptomycin) for gram-positive infections if indicated for suspected line infection, skin and soft tissue infection, pneumonia, hemodynamic instability, history of methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci colonization, or severe mucositis, according to the Infectious Diseases Society of America clinical practice guidelines for the use of antimicrobial agents in neutropenic patients with cancer [2]. The choice and duration of gram-positive coverage was determined by the treating physician.
Early de-escalation after 72 hours was encouraged and implemented if appropriate after assessing the patient and reviewing the culture results as an antimicrobial stewardship practice and as predetermined in the protocol. A switch to oral or narrower spectrum or a once-daily IV agent against gram-negative pathogens for the purposes of outpatient treatment was allowed after 72 hours. The original IV randomized treatment could have been discontinued either as (1) de-escalation (in patients who improved and became afebrile) or (2) due to insufficient therapeutic effect, including incomplete clinical resolution or persistence of fever, that requires alternative antimicrobial therapy. The investigator or treating physician was encouraged to continue study therapy[ies] for at least 72 hours before considering such patients as a clinical failure and prematurely discontinuing study therapy(ies). Patients who were switched prior to 72 hours for reasons other than study drug–related adverse events or resistant organisms were considered as indeterminate. The decision to de-escalate was made by either the investigator or the treating physician, who were not blinded to treatment arm. Empirical treatment of Clostridioides difficile with oral vancomycin or with IV or oral metronidazole could be added at any time for patients with symptoms of abdominal cramping and diarrhea or if C difficile infection was strongly suspected clinically.
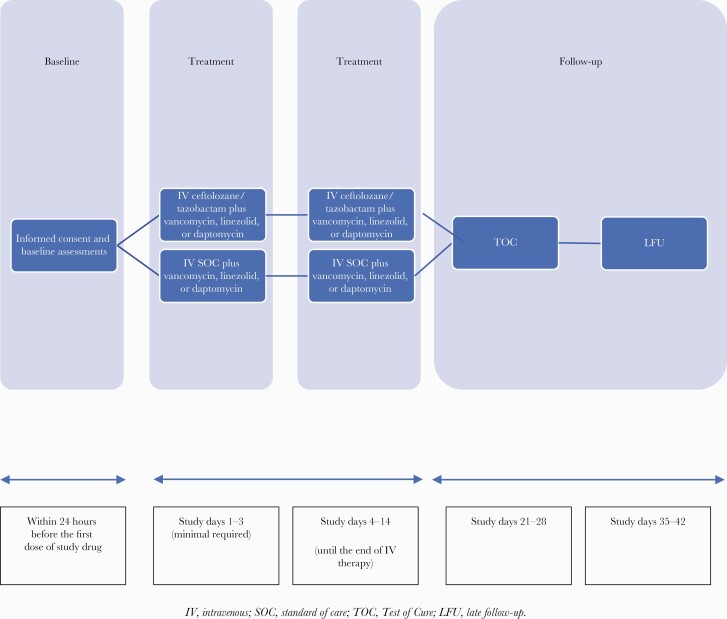
Patients were followed through the end of IV therapy (EOIV). Patients were evaluated 21–28 days after starting IV antibiotic therapy for test of cure (TOC) and 35–42 days after starting therapy as a late follow-up (LFU) (Figure 1). Clinical and microbiological responses were assessed at EOIV, TOC, and LFU.
Figure 1.
Study design. Abbreviations: IV, intravenous; LFU, late follow-up; SOC, standard of care; TOC, test of cure.
All patients were monitored throughout the study period, including the 30 days after the last study drug dose, for the development of adverse events (AEs), serious AEs (SAEs), and study drug–related AEs.
Analysis Populations and Outcomes
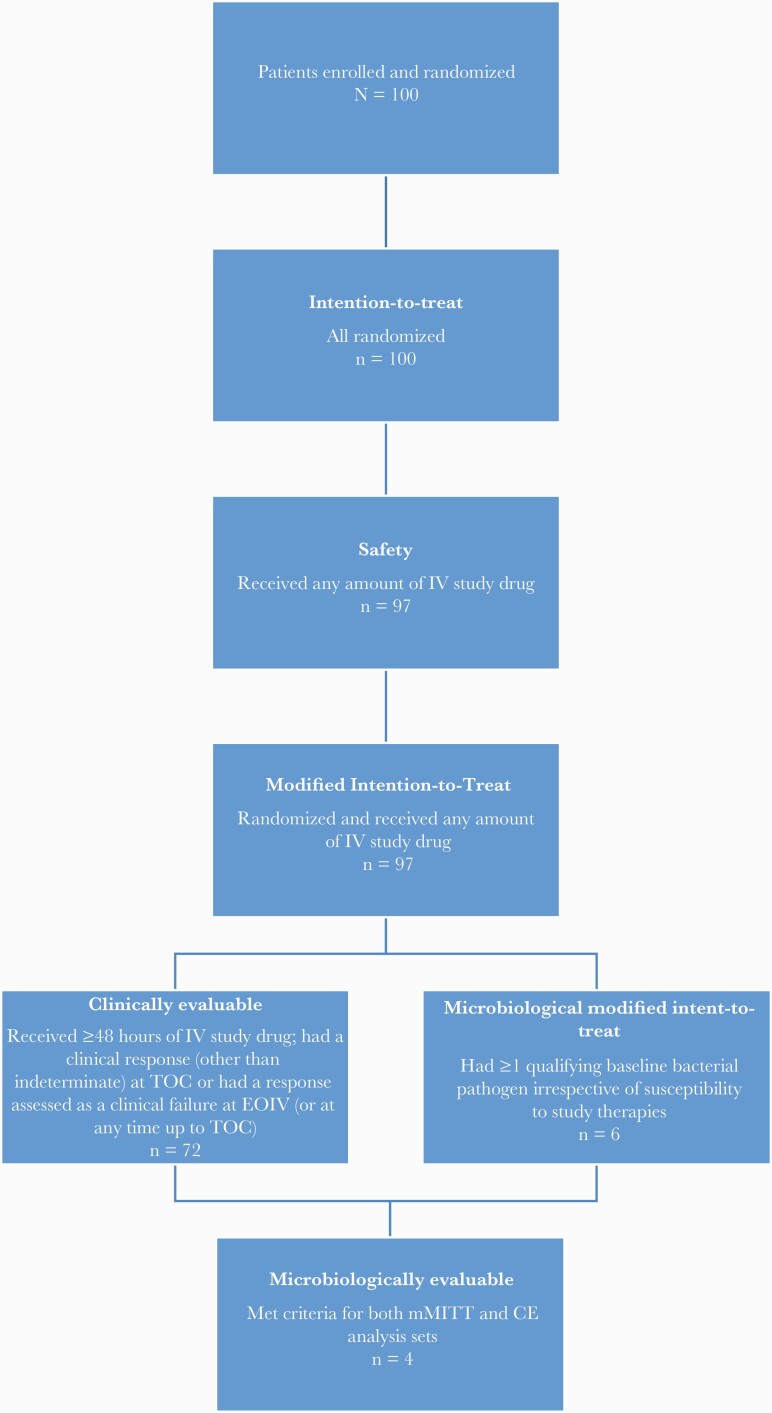
The analysis sets included efficacy and safety analyses and are shown in Figure 2.
Figure 2.
Patient population and analysis sets. Abbreviations: CE, clinically evaluable; EOIV, end of intravenous therapy; IV, intravenous; mMITT, microbiological modified intention-to-treat; TOC, test of cure.
The efficacy analysis included clinical and microbiological responses and was based on the modified intent-to-treat (MITT) and microbiological MITT (mMITT) analysis sets.
The primary objective of the study was to show that the efficacy of C/T plus vancomycin, daptomycin, or linezolid is noninferior to the SOC plus vancomycin, daptomycin, or linezolid as empiric therapy in cancer patients with FN with respect to favorable clinical response. The primary efficacy parameter was the proportion of patients in the MITT analysis set with favorable clinical response at EOIV.
The safety analysis set included all randomized patients who received any amount of IV inpatient study drug. Safety was assessed by assessing AEs and SAEs that were attributed to the study drug throughout the study as well as 30-day mortality.
The secondary efficacy parameters included the proportion of patients in the mMITT and clinically evaluable (CE) analysis sets with favorable clinical response at EOIV; the proportion in the MITT analysis set with favorable clinical response at TOC and LFU; the proportion in the mMITT and CE analysis sets with favorable clinical response by baseline gram-negative pathogen at EOIV, TOC, and LFU; the proportion in the mMITT and microbiologically evaluable (ME) analysis sets with a favorable microbiological response (defined as eradication or presumed eradication of the infecting pathogen) by baseline gram-negative pathogen at EOIV, TOC, and LFU; the proportion in the MITT and mMITT analysis sets with infection-related mortality at TOC and LFU; and 30-day all-cause mortality.
Definitions
FN was defined as either a single oral temperature measurement of 38.3°C (≥101°F) or a temperature of 38.0°C (≥100.4°F) sustained over a 1-hour period, with an absolute neutrophil count of <500 cells/mL.
A favorable clinical response was defined as the resolution of all acute signs and symptoms (mainly fever resolution as fever may represent the only sign of infection in patients with neutropenia who may have decreased inflammatory response) of the primary infection at EOIV, TOC, and LFU.
A clinical failure was defined as a fever persisting 96 hours after the initiation of the study drug; discontinuation of study drug after at least 72 hours due to insufficient therapeutic effect including persistence, incomplete clinical resolution, or worsening in signs and symptoms (mainly fever) that requires alternative antimicrobial therapy; a documented breakthrough gram-negative BSI; (ie, a recurrent BSI or a new BSI with a gram-negative pathogen not present at baseline but occurring while on study drug); a documented study drug–resistant gram-negative pathogen that required alternative antimicrobial therapy; the discontinuation of study drug therapy owing to an AE and the requirement for alternative antimicrobial; death resulting from the primary infection; or >1 therapy switch after the discontinuation of the original IV treatment. Another episode of FN during the follow-up period was not considered a clinical failure unless the patient presented with a relapse of the same documented infection that was present at baseline.
The clinical outcome was classified as indeterminate in any of the following: patient developed a documented invasive fungal or viral infection at any time during the study; patient has a documented infection with gram-positive organisms; study data were not available for evaluation of efficacy for any reason including death in which FN was clearly noncontributory or patient was lost to follow-up; or patient withdrew from the study for reasons other than clinical failure.
In patients with microbiologically documented infections, microbiological response was defined as either eradication of the original baseline pathogen; presumed eradication (no available culture and the clinical response assessed as a cure); persistence; presumed persistence (no available culture and the clinical response assessed as a failure); or indeterminate (no culture and the clinical response assessed as indeterminate).
Statistical Analysis
Continuous and categorical variables were summarized by treatment group using descriptive statistics (medians and ranges) and frequency distributions (counts and percentages), respectively. Summaries were provided for all randomized patients who received any amount of inpatient IV study drug (MITT analysis set), and efficacy summaries were provided for the subgroup of patients with a gram-negative pathogen identified (mMITT analysis set) and other subgroups including patients in the CE analysis set and ME analysis set defined by the protocol (Figure 2). The χ2 or Fisher exact test was used to compare categorical variables, and Wilcoxon rank-sum test was used to compare continuous variables. One-sided χ2 test for noninferiority was performed for the primary endpoint (favorable clinical response) with a noninferiority margin of 10%. If a noninferiority was found, then a 2-sided χ2 test for superiority was performed for a further comparison. All the tests were at the significance level of .05 and most of them except the noninferiority tests were 2-sided. The statistical analyses were performed using SAS version 9.3 software (SAS Institute, Cary, North Carolina).
RESULTS
Patient Characteristics
Among the 100 patients enrolled and randomized during the study period, 97 patients (47 in the C/T group and 50 in the SOC group) received at least 1 dose of the study drug. Three C/T patients withdrew consent before receiving any dose of the study drug and were excluded from the analysis.
The patients’ characteristics are shown in Table 1. The C/T and SOC groups did not differ significantly in terms of age, gender, race, underlying disease, hospital stay duration, intensive care unit (ICU) admission, or requirement for mechanical ventilation. Antibiotic prophylaxis received before the onset of FN was similar in both arms (81% in C/T arm vs 90% in SOC arm; P = .2). The main antibiotics given as prophylaxis consisted of fluoroquinolones (82%), followed by cefpodoxime (12%), then others such as amoxicillin, trimethoprim/sulfamethoxazole, cefdinir, etc. The C/T and SOC groups had similar rates of microbiologically documented infections at baseline, most commonly BSI (28% and 24%, respectively; P = .68). The groups also had similar rates of isolated gram-negative and -positive pathogens. The 6 patients with documented infections caused by gram-negative pathogens at baseline included 3 C/T patients with BSIs, 1 C/T patient with a urinary tract infection, and 2 SOC patients with BSIs. Isolated pathogens included Escherichia coli (1 C/T patient and 2 SOC patients), P aeruginosa (2 C/T patients), and Klebsiella pneumoniae (1 C/T patient). Two of the isolated gram-negative pathogens—1 E coli in the SOC group and 1 K pneumoniae in the C/T group, neither of which was carbapenem-resistant—were classified as ESBL-producing multidrug-resistant organisms (MDROs). Compared to SOC, patients in C/T had at baseline a higher rate of clinically documented infection (34% vs 6%; P = .018) and sepsis (62% vs 30%; P = .002), but a lower rate of unexplained fever (38% vs 70%; P = .002) (Supplementary Table 1). Only 1 patient in C/T, who had a clinically documented infection (pneumonia), had an ICU admission related to his FN episode. The median study drug duration of the C/T group (3 days [range, 1–6 days]) was shorter than that of the SOC group (4 days [range, 1–14 days]; P = .11). The SOC group had a significantly higher rate of patients who received study drugs for >5 days (20% vs 2%; P = .006).
Table 1.
Characteristics of Patients Who Received Ceftolozane/Tazobactam and Those Who Received the Standard of Care
| Characteristic | Ceftolozane/Tazobactam (n = 47) |
Standard of Care (n = 50) |
P Value |
|---|---|---|---|
| Age, y, median (range) | 60 (25–84) | 55 (18–79) | .12 |
| Sex | .65 | ||
| Male | 28 (60) | 32 (64) | |
| Female | 19 (40) | 18 (36) | |
| Race/ethnicity | .85 | ||
| White | 32 (68) | 35 (70) | |
| Black | 4 (9) | 5 (10) | |
| Hispanic | 6 (13) | 7 (14) | |
| Asian | 0 (0) | 1 (2) | |
| Middle Eastern | 3 (6) | 1 (2) | |
| Other | 2 (4) | 1 (2) | |
| Hematological malignancy | .32 | ||
| ALL | 9 (19) | 12 (24) | |
| AML | 19 (40) | 22 (44) | |
| CML | 3 (6) | 0 | |
| Lymphoma | 9 (19) | 6 (12) | |
| Other | 7 (15) | 10 (20) | |
| BMT within 1 y prior to fever | 6 (13) | 9 (18) | .48 |
| Autologous | 2/6 (33) | 4/9 (44) | |
| Allogeneic | 4/6 (67) | 5/9 (56) | |
| Type of allogeneic transplant | |||
| Matched unrelated donor | 0 | 1/5 (20) | |
| HLA matched related donor | 4/4 (100) | 4/5 (80) | |
| GVHD | 1/6 (17) | 1/8 (13) | >.99 |
| Temperature at baseline, °C, median (IQR) | 37.3 (36.9–38.2) | 37.5 (37.0–38.3) | .31 |
| Temperature at initial presentation, °C | .32 | ||
| <36 | 0 | 0 | |
| 36–38 | 3 (6) | 7 (14) | |
| >38 | 44 (94) | 43 (86) | |
| Microbiological documentation (positivity) | 13 (28) | 12 (24) | .68 |
| Site of microorganism(s)a | |||
| Genitourinary tract | 2 | 1 | |
| Blood | 11 | 12 | |
| Gram-negative bacterial pathogen | 4 (9) | 2 (4) | .43 |
| Gram-negative alone | 2 | 2 | |
| Gram-negative and -positive (mixed infection) | 2 | 0 | |
| Organisms recovered in positive culturesb | |||
| Escherichia coli | 0 | 2 | |
| Klebsiella pneumoniae | 1 | 0 | |
| Pseudomonas aeruginosa | 1 | 0 | |
| MRSA | 2 | 1 | |
| Rothia mucilaginosa | 1 | 0 | |
| Streptococcus viridans | 5 | 5 | |
| Staphylococcus epidermidis | 1 | 2 | |
| Enterococcus faecalis | 0 | 2 | |
| E faecalis + E coli | 1 | 0 | |
| E faecalis + P aeruginosa | 1 | 0 | |
| CVC the source of BSI isolation | 7/11 (64) | 7/12 (58) | >.99 |
| Hospital stay duration, d, median (IQR) | 6 (4–9) | 7 (4–11) | .84 |
| ICU admission | 2 (4) | 3 (6) | >.99 |
| Mechanical ventilation | 2 (4) | 1 (2) | .61 |
Data are presented as No. of patients (%) unless otherwise indicated.
Abbreviations: ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; BMT, bone marrow transplantation; BSI, bloodstream infection; CML, chronic myeloid leukemia; CVC, central venous catheter; GVHD, graft-vs-host disease; HLA, human leukocyte antigen; ICU, intensive care unit; IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus.
One patient had 2 sites of organisms.
Four patients had 2 or 3 organisms.
The group’s treatment regimen characteristics are shown in Supplementary Table 2. In the SOC group, the most commonly used antibiotics were cefepime (76%) followed by piperacillin/tazobactam (20%) and meropenem (4%). In both groups, >90% of the patients received gram-positive coverage, with linezolid being the most commonly used agent. De-escalation at end of IV study drug occurred similarly in both groups (94% in C/T and 84% in SOC; P = .14), although patients on C/T were more likely to de-escalate to IV study drug compared to SOC (55% vs 21%) (Supplementary Table 3).
Clinical Response
Detailed clinical outcomes are presented in Table 2 and Supplementary Tables 1, 4, and 5. At EOIV, for the MITT population, C/T patients had a higher rate of favorable clinical response than SOC patients did (87% vs 72%). The 1-sided noninferiority analysis indicated that C/T was not inferior to the SOC (P = .002), which was consistent with the lower limit of the 90% confidence interval (.013) of their rate difference being greater than the noninferiority limit (–0.10). Moreover, the lower limit of the 95% CI of the difference in the favorable clinical response rates (–.014) was also greater than the noninferiority limit (–0.10).
Table 2.
Clinical Outcome of Patients Who Received Ceftolozane/Tazobactam and Those Who Received the Standard of Care
| Clinical Outcome | Ceftolozane/Tazobactam (n = 47) |
Standard of Care (n = 50) |
P Value |
|---|---|---|---|
| Clinical outcome at EOIV | .10 | ||
| Favorable clinical response | 41 (87) | 36 (72) | |
| Clinical failure | 2 (4) | 9 (18) | |
| ndeterminate | 4 (9) | 5 (10) | |
| Clinical outcome at TOC | .01 | ||
| Clinical cure | 34 (72) | 28 (56) | |
| Clinical failure | 3 (6) | 15 (30) | |
| Indeterminate | 10 (21) | 7 (14) | |
| Clinical outcome at LFU | .028 | ||
| Clinical cure | 33 (70) | 26 (52) | |
| Clinical failure | 4 (9) | 15 (30) | |
| Indeterminate | 10 (21) | 9 (18) | |
| Mortality during the study | 3 (6) | 2 (4) | .67 |
| Duration between last dose of study drug and death, d, median (range) | 17 (15–34) | 29 (28–29) | .77 |
| Infection-related mortality | 0 (0) | 0 (0) | |
| 30-d all-cause mortality | 2 (4) | 2 (4) | >.99 |
Data are presented as No. of patients (%) unless otherwise specified.
Abbreviations: EOIV, end of intravenous therapy; LFU, late follow-up; TOC, test of cure.
At EOIV, C/T patients had lower rates of clinical failure (4% vs 18%) and indeterminate clinical response (9% vs 10%). In both groups, the most common reason for clinical failure at EOIV was persistent fever. One SOC patient required alternative therapy owing to a BSI with E coli that was resistant to the selected study drug (cefepime). It is to note that 76% of patients in the SOC arm received cefepime and 20% received piperacillin/tazobactam, whereas only 4% received meropenem) (Supplementary Table 1).
At TOC, C/T was also found to be noninferior to the SOC with regard to favorable clinical response (P = .004). Furthermore, superiority tests showed a significant difference between the groups’ distributions of clinical outcomes (P = .01), with the C/T group having a significantly lower rate of clinical failure (6% vs 30%; P = .003). Analyses of LFU data also yielded similar results, with the C/T group again having a significantly lower rate of clinical failure (9% vs 30%; P = .008).
The clinical outcomes at EOIV, TOC, and LFU for the mMITT and CE populations are presented in Supplementary Tables 5B and 5C, respectively.
Microbiological Response
The C/T and SOC groups’ rates of microbiological response at EOIV, TOC, and LFU among patients who had microbiologically documented infections at baseline did not differ significantly (Table 3 and Supplementary Tables 1, 4, and 5).
Table 3.
Microbiological Outcome of Patients Who Received Ceftolozane/Tazobactam and Those Who Received the Standard of Care
| Outcome | Ceftolozane/Tazobactam (n = 47) |
Standard of Care (n = 50) |
P Value |
|---|---|---|---|
| Microbiologically documented infection | 13 (28) | 12 (24) | .68 |
| Microbiological response at EOIV | .86 | ||
| Persistence | 1/13 (8) | 1/12 (8) | |
| Eradication | 11/13 (85) | 9/12 (75) | |
| Presumed eradication | 0/13 (0) | 1/12 (8) | |
| Indeterminate | 1/13 (8) | 1/12 (8) | |
| Microbiological response at TOC | .64 | ||
| Persistence | 0/13 (0) | 1/12 (8) | |
| Eradication | 3/13 (23) | 2/12 (17) | |
| Presumed eradication | 8/13 (62) | 9/12 (75) | |
| Indeterminate | 2/13 (15) | 0/12 (0) | |
| Microbiological response at LFU | .33 | ||
| Persistence | 0/13 (0) | 1/12 (8) | |
| Eradication | 2/13 (15) | 3/12 (25) | |
| Presumed eradication | 81/3 (62) | 8/12 (67) | |
| Indeterminate | 3/13 (23) | 0/12 (0) | |
| Relapse | 0/12 (0) | 0/11 (0) |
Data are presented as No. of patients (%) unless otherwise indicated.
Abbreviations: EOIV, end of intravenous therapy; LFU, late follow-up; TOC, test of cure.
The microbiologic responses at EOIV, TOC, and LFU in the mMITT and ME populations are presented in Supplementary Table 5D and 5E, respectively. In the mMITT population, of the 4 C/T patients with documented infections caused by gram-negative pathogens, 1 had an indeterminate clinical outcome at TOC. This patient had a BSI caused by an ESBL/MDRO K pneumoniae that was susceptible to C/T; however, the patient was switched to meropenem <72 hours after initiating therapy. Of the 2 SOC patients with documented infections caused by gram-negative pathogens, 1 had clinical failure owing to a BSI caused by an ESBL/MDRO E coli that was resistant to cefepime and required a switch to meropenem <72 hours after initiating therapy with cefepime.
In patients with documented gram-negative infections at baseline, there was no relapse or emergence of gram-negative resistant organisms during the follow-up period. Five patients developed a new bacteremia with a different gram-negative organism that was not present at baseline and that occurred after the end of IV therapy: 4 in the CT arm and 1 in the SOC arm. In the CT arm, 4 patients developed 5 episodes of bacteremia after EOIV and during the follow-up period. The recovered isolates consisted of E coli (2 patients), P aeruginosa (1 patient), and K pneumoniae and Stenotrophomonas (1 patient). In the SOC arm, 1 patient developed E coli bacteremia. Except for the Stenotrophomonas, none of the isolates were resistant to the randomized study drugs that they received. The microbiological outcomes of these patients were not considered as failures as the bacteremia occurred after end of IV study drug and not while on study drug.
Safety
The C/T and SOC groups’ overall rates of AEs and SAEs did not differ significantly (Table 4). Although the rate of drug-related AEs tended to be higher in the C/T group (17% vs 6%; P = .09), the rate of drug-related SAEs were similar in both groups (2% vs 0%; P = .48) (Table 4). In addition, the groups’ rates of study drug–related AEs and SAEs that led to drug discontinuation did not differ significantly. The most common study drug–related AEs in the C/T group were increased liver function tests (aminotransferase and bilirubin levels), rash, increased alkaline phosphatase level, and headache. Both groups had a 30-day mortality rate of 4%, and their rates of mortality during the study did not differ significantly (6% vs 4%; P = .67). There was no study drug–related death in either group (Table 2 and Supplementary Table 5F–I). All SAEs and non-SAEs, regardless of their attribution, are presented in Supplementary Tables 6 and 7, respectively. Non-SAEs reported with a frequency of at least 5% are presented in Table 5.
Table 4.
Adverse Events and Serious Adverse Events of Patients Who Received Ceftolozane/Tazobactam and Those Who Received the Standard of Care
| Event | Ceftolozane/Tazobactam (n = 47) |
Standard of Care (n = 50) |
P Value |
|---|---|---|---|
| Total AEs | 38 (81) | 37 (74) | .42 |
| Total SAEs | 33 (70) | 33 (66) | .66 |
| Study drug–related AE | 8 (17) | 3 (6) | .09 |
| ALT increased (>ULN) | 2 (4) | 1 (2) | |
| Bilirubin increased (>1.5 ULN) | 1 (2) | … | |
| Rash | 5 (11) | 2 (4) | |
| Alkaline phosphatase increased (>ULN) | 1 (2) | … | |
| Headache | 1 (2) | … | |
| Study drug–related SAE | 1 (2) | 0 (0) | .48 |
| Bilirubin increased (>1.5 ULN) | 1 (2) | … | |
| Study drug–related AE resulting in drug discontinuation | 2 (4) | 2 (4) | >.99 |
| ALT increased (>ULN) | … | 1 (2) | |
| Bilirubin increased (>1.5 ULN) | 1 (2) | … | |
| Rash | 1 (2) | 1 (2) | |
| Study drug–related SAE resulting in drug discontinuation | 1 (2) | 0 (0) | .48 |
| Bilirubin increased (>1.5 ULN) | 1 (2) | … | |
| Mortality | 3 (6) | 2 (4) | .67 |
| Study drug–related mortality | 0 | 0 | |
| 30-d all-cause mortality | 2 (4) | 2 (4) | >.99 |
Data are presented as No. of patients (%) unless otherwise indicated.
Abbreviation: AE, adverse event; ALT, alanine aminotransferase; SAE, serious adverse event; ULN, upper limit of normal.
Table 5.
Nonserious Adverse Events With a Frequency Threshold >5% Among Patients Who Received Ceftolozane-Tazobactam and Those Who Received the Standard of Care
| Event | Ceftolozane/Tazobactam (n = 47) |
Standard of Care (n = 50) |
|---|---|---|
| Total | 9 (19) | 6 (12) |
| ALT elevation (>ULN) | 3 (6) | 3 (6) |
| Urinary tract infection | 3 (6) | 0 (0) |
| Rash | 5 (11) | 3 (6) |
Data are presented as No. (%) of patients. Events were collected by systematic assessment.
Abbreviation: ALT, alanine aminotransferase; ULN, upper limit of normal.
DISCUSSION
Our findings show that C/T is noninferior and may be superior to the SOC in patients with HM and FN. In the MITT population, compared with SOC patients, C/T patients not only had a noninferior rate of favorable clinical response at EOIV, TOC, and LFU but also had a significantly lower rate of clinical failure at TOC and LFU.
In our study, the most commonly used antibiotic in the SOC arm was cefepime (76%) followed by piperacillin/tazobactam (20%) and meropenem (4%). Microbiologically documented infections with either gram-negative or -positive pathogens were identified at baseline in 25% of our patients and were primarily BSIs (92%), with similar distributions in both groups. Our documented infection rate is similar to those reported previously [23]. Although the rate of gram-negative infections is increasing, gram-negative pathogens were isolated in only 6% of our patients, mostly from blood (83%). Of the 6 patients with documented gram-negative infections, 2 (33%) had an ESBL/MDRO pathogen that required an early switch of the antibiotic <72 hours after initiating therapy.
Given the increasing rate of ESBL-producing gram-negative bacteria [10], the empiric use of fourth-generation β-lactams such as cefepime or extended-spectrum penicillin/β-lactamase inhibitors such as piperacillin/tazobactam could be suboptimal, especially in high-risk patients with underlying malignancy and FN. C/T, a combination of a novel cephalosporin and an established β-lactamase inhibitor, could be used as an alternative to carbapenems. C/T has shown in vitro activity against a wide range of gram-negative pathogens, including multidrug-resistant P aeruginosa and ESBL-producing Enterobacteriaceae [24–30]. The lower rates of clinical failure observed with C/T at TOC and LFU remain unclear given the very low number of documented gram-negative MDROs.
In this study we used the C/T limited dose of 1.5 g every 8 hours given that the 3-g dose was still considered investigational at the time of registration of this study. The 3-g dose has since been approved by the Food and Drug Administration for the treatment of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia and should be considered as the preferred dose in patients with FN to empirically cover multidrug-resistant P aeruginosa.
In the present study, the median study drug duration of the C/T group (3 days) was shorter than that of the SOC group (4 days; P = .08). Only 1 C/T patient (2%) but 10 SOC patients (20%) received study drugs for > 5 days (P = .006). This difference could be due to the early de-escalation procedures that were implemented after the patients’ clinical improvement, which occurred earlier and more frequently in the C/T arm.
Both the C/T and SOC groups had high rates of AEs and SAEs that were equally distributed, which reflects the complexity of this severely immunocompromised patient population. Although C/T patients tended to have a higher rate of study drug–related AEs, both groups had relatively few study drug–related AEs and SAEs that led to drug discontinuation.
This is the first randomized controlled trial that evaluated the safety and efficacy of C/T in high-risk patients with HM and FN. Our study had several limitations. The study enrolled eligible patients, including those without a history of an ESBL-producing organism or an MDRO. However, such patients are severely immunosuppressed and are frequently hospitalized for complications resulting from their intensive chemotherapy. In addition, the study included very few patients with documented gram-negative pathogens. Another limitation is the open-label design of the study, which could have introduced a potential source of bias in the assessment of the patient. Although most of the endpoints were objective based on measurable endpoints such as temperature for the clinical outcome or cultures for microbiologic documentation, the decision to de-escalation or to discontinue study drug and the attribution of the AE to study drug may have been biased by the assessor who was not blinded to the treatment agent.
CONCLUSIONS
C/T is efficacious and safe, and its prudent and judicious use should be considered, in the context of local patterns of antibiotic resistance, for the empiric treatment of high-risk patients with HM and FN, particularly those with a history of infection or colonization with resistant gram-negative organisms and/or prior hospitalization and antibiotic overuse. Closely monitoring patients, optimizing antibiotic selection, and reassessing and de-escalating antibiotic treatment using culture results and susceptibility patterns should be implemented to attain the shortest effective duration of therapy and limit the emergence of resistant pathogens.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. A.-M. C., R. H., and I. R. were responsible for study conception and protocol design. A. M. C. wrote the first draft of the manuscript. A.-M. C., R. H., A. E. M., A. D. S., V. E. M., S. A., P. C., and I. R. were responsible for patient screening, enrollment, and follow-up. S. A. and R. D. were responsible for data collection. A.-M. C., R. H., S. A., and R. D. have verified the underlying data. Y. J. and Y. Y. designed the statistical analysis plan, analyzed the data, and developed the figures and the tables. A.-M. C., R. H., and I. R. were responsible for overall project and data management. All authors had full access to the study data and were responsible for the final decision to submit the manuscript for publication. All authors were responsible for critical review of drafts and approval of the final manuscript.
Acknowledgments. We thank Salli Saxton in the MD Anderson Department of Infectious Diseases, Infection Control and Employee Health for helping with the submission of the manuscript, and Joe Munch in the MD Anderson Research Medical Library for editing the manuscript. This assistance was funded by The University of Texas MD Anderson Cancer Center.
Patient consent. Written informed consent was obtained from all patients or their authorized representatives. This single-center, prospective, randomized, open-label comparative study was approved by our Institutional Review Board and is registered at ClinicalTrials.gov (NCT03485950).
Data sharing. The study protocol, statistical analysis plan, lists of de-identified individual data, and generated tables and figures will be made available upon request by qualified scientific and medical researchers for legitimate research purposes. Requests should be sent to achaftari@mdanderson.org and yijiang@mdanderson.org. Data will be available on request for 6 months from the date of publication. Investigators are invited to submit study proposal requests detailing research questions and hypotheses in order to receive access to these data.
Financial support. This research was supported by Merck & Co and by the National Institutes of Health/National Cancer Institute through an The University of Texas MD Anderson Cancer Center Support Grant (number P30CA016672).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Anne-Marie Chaftari, Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Ray Hachem, Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Alexandre E Malek, Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Victor E Mulanovich, Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Ariel D Szvalb, Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Ying Jiang, Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Ying Yuan, Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Shahnoor Ali, Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Rita Deeba, Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Patrick Chaftari, Department of Emergency Medicine, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Issam Raad, Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
REFERENCES
- 1. Zuckermann J, Moreira LB, Stoll P, Moreira LM, Kuchenbecker RS, Polanczyk CA.. Compliance with a critical pathway for the management of febrile neutropenia and impact on clinical outcomes. Ann Hematol 2008; 87:139–45. [DOI] [PubMed] [Google Scholar]
- 2. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52:e56–93. [DOI] [PubMed] [Google Scholar]
- 3. Lodise TP Jr, Patel N, Kwa A, et al. Predictors of 30-day mortality among patients with Pseudomonas aeruginosa bloodstream infections: impact of delayed appropriate antibiotic selection. Antimicrob Agents Chemother 2007; 51:3510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH.. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother 2005; 49:1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rottier WC, Ammerlaan HS, Bonten MJ.. Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae and patient outcome: a meta-analysis. J Antimicrob Chemother 2012; 67:1311–20. [DOI] [PubMed] [Google Scholar]
- 6. Martinez-Nadal G, Puerta-Alcalde P, Gudiol C, et al. Inappropriate empirical antibiotic treatment in high-risk neutropenic patients with bacteremia in the era of multidrug resistance. Clin Infect Dis 2020; 70:1068–74. [DOI] [PubMed] [Google Scholar]
- 7. Gudiol C, Albasanz-Puig A, Laporte-Amargos J, et al. Clinical predictive model of multidrug resistance in neutropenic cancer patients with bloodstream infection due to Pseudomonas aeruginosa. Antimicrob Agents Chemother 2020; 64:e02494-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Averbuch D, Tridello G, Hoek J, et al. Intercontinental study on pre-engraftment and post-engraftment gram-negative rods bacteremia in hematopoietic stem cell transplantation patients: risk factors and association with mortality. J Infect 2020; 81:882–94. [DOI] [PubMed] [Google Scholar]
- 9. Trecarichi EM, Tumbarello M.. Antimicrobial-resistant gram-negative bacteria in febrile neutropenic patients with cancer: current epidemiology and clinical impact. Curr Opin Infect Dis 2014; 27:200–10. [DOI] [PubMed] [Google Scholar]
- 10. Irfan S, Idrees F, Mehraj V, Habib F, Adil S, Hasan R.. Emergence of carbapenem resistant gram negative and vancomycin resistant gram positive organisms in bacteremic isolates of febrile neutropenic patients: a descriptive study. BMC Infect Dis 2008; 8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Montassier E, Batard E, Gastinne T, Potel G, de La Cochetiere MF.. Recent changes in bacteremia in patients with cancer: a systematic review of epidemiology and antibiotic resistance. Eur J Clin Microbiol Infect Dis 2013; 32:841–50. [DOI] [PubMed] [Google Scholar]
- 12. Pagano L, Caira M, Rossi G, et al. A prospective survey of febrile events in hematological malignancies. Ann Hematol 2012; 91:767–74. [DOI] [PubMed] [Google Scholar]
- 13. Hansen BA, Wendelbo O, Bruserud O, Hemsing AL, Mosevoll KA, Reikvam H.. Febrile neutropenia in acute leukemia. epidemiology, etiology, pathophysiology and treatment. Mediterr J Hematol Infect Dis 2020; 12:e2020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gustinetti G, Mikulska M.. Bloodstream infections in neutropenic cancer patients: a practical update. Virulence 2016; 7:280–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cluck D, Lewis P, Stayer B, Spivey J, Moorman J.. Ceftolozane-tazobactam: a new-generation cephalosporin. Am J Health Syst Pharm 2015; 72:2135–46. [DOI] [PubMed] [Google Scholar]
- 16. Solomkin J, Hershberger E, Miller B, et al. Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI). Clin Infect Dis 2015; 60:1462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wagenlehner FM, Umeh O, Steenbergen J, Yuan G, Darouiche RO.. Ceftolozane-tazobactam compared with levofloxacin in the treatment of complicated urinary-tract infections, including pyelonephritis: a randomised, double-blind, phase 3 trial (ASPECT-cUTI). Lancet 2015; 385:1949–56. [DOI] [PubMed] [Google Scholar]
- 18. Lucasti C, Hershberger E, Miller B, et al. Multicenter, double-blind, randomized, phase II trial to assess the safety and efficacy of ceftolozane-tazobactam plus metronidazole compared with meropenem in adult patients with complicated intra-abdominal infections. Antimicrob Agents Chemother 2014; 58:5350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kollef MH, Novacek M, Kivistik U, et al. Ceftolozane-tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): a randomised, controlled, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 2019; 19:1299–311. [DOI] [PubMed] [Google Scholar]
- 20. Fernandez-Cruz A, Alba N, Semiglia-Chong MA, et al. A case-control study of real-life experience with ceftolozane-tazobactam in patients with hematologic malignancy and Pseudomonas aeruginosa infection. Antimicrob Agents Chemother 2019; 63:e02340-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hakki M, Lewis JS 2nd. Ceftolozane-tazobactam therapy for multidrug-resistant Pseudomonas aeruginosa infections in patients with hematologic malignancies and hematopoietic-cell transplant recipients. Infection 2018; 46:431–4. [DOI] [PubMed] [Google Scholar]
- 22. Clerici D, Oltolini C, Greco R, et al. The place of ceftazidime/avibactam and ceftolozane/tazobactam for therapy of haematological patients with febrile neutropenia. Int J Antimicrob Agents 2021; 57:106335. [DOI] [PubMed] [Google Scholar]
- 23. Klastersky J, Ameye L, Maertens J, et al. Bacteraemia in febrile neutropenic cancer patients. Int J Antimicrob Agents 2007; 30:S51–59. [DOI] [PubMed] [Google Scholar]
- 24. Farrell DJ, Flamm RK, Sader HS, Jones RN.. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa with various resistance patterns isolated in U.S. hospitals (2011-2012). Antimicrob Agents Chemother 2013; 57:6305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sader HS, Farrell DJ, Castanheira M, Flamm RK, Jones RN.. Antimicrobial activity of ceftolozane/tazobactam tested against Pseudomonas aeruginosa and Enterobacteriaceae with various resistance patterns isolated in European hospitals (2011–12). J Antimicrob Chemother 2014; 69:2713–22. [DOI] [PubMed] [Google Scholar]
- 26. Sader HS, Farrell DJ, Flamm RK, Jones RN.. Ceftolozane/tazobactam activity tested against aerobic gram-negative organisms isolated from intra-abdominal and urinary tract infections in European and United States hospitals (2012). J Infect 2014; 69:266–77. [DOI] [PubMed] [Google Scholar]
- 27. Walkty A, Karlowsky JA, Adam H, et al. In vitro activity of ceftolozane-tazobactam against Pseudomonas aeruginosa isolates obtained from patients in Canadian hospitals in the CANWARD study, 2007 to 2012. Antimicrob Agents Chemother 2013; 57:5707–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sutherland CA, Nicolau DP.. Susceptibility profile of ceftolozane/tazobactam and other parenteral antimicrobials against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa from US hospitals. Clin Ther 2015; 37:1564–71. [DOI] [PubMed] [Google Scholar]
- 29. Tato M, Garcia-Castillo M, Bofarull AM, Canton R.. In vitro activity of ceftolozane/tazobactam against clinical isolates of Pseudomonas aeruginosa and Enterobacteriaceae recovered in Spanish medical centres: results of the CENIT study. Int J Antimicrob Agents 2015; 46:502–10. [DOI] [PubMed] [Google Scholar]
- 30. Estabrook M, Bussell B, Clugston SL, Bush K.. In vitro activity of ceftolozane-tazobactam as determined by broth dilution and agar diffusion assays against recent U.S. Escherichia coli isolates from 2010 to 2011 carrying CTX-M-type extended-spectrum beta-lactamases. J Clin Microbiol 2014; 52:4049–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.