Abstract
Background
Patients with glioblastoma (GBM) have a poor prognosis and limited effective treatment options. Bevacizumab has been approved for treatment of recurrent GBM, but there is questionable survival benefit. Based on preclinical and early clinical data indicating that CD105 upregulation may represent a mechanism of resistance to bevacizumab, we hypothesized that combining bevacizumab with the anti-CD105 antibody TRC105 may improve efficacy in recurrent GBM.
Methods
Phase I dose-escalation/comparative randomized phase II trial in patients with GBM. During phase I, the maximum tolerated dose (MTD) of TRC105 in combination with bevacizumab was determined. In phase II, patients were randomized 1:1 to TRC105 and bevacizumab or bevacizumab monotherapy. Patients received TRC105 (10 mg/kg) weekly and bevacizumab (10 mg/kg) every 2 weeks. Efficacy, as assessed by progression-free survival (PFS), was the primary endpoint; safety, quality of life, and correlative outcomes were also evaluated.
Results
In total, 15 patients were enrolled in phase I and 101 in phase II; 52 patients were randomized to TRC105 with bevacizumab and 49 to bevacizumab monotherapy. The MTD was determined to be 10 mg/kg TRC105 weekly plus bevacizumab 10 mg/kg every 2 weeks. An increased occurrence of grade ≥3 adverse events was seen in the combination arm, including higher incidences of anemia. Median PFS was similar in both treatment arms: 2.9 months for combination versus 3.2 months for bevacizumab monotherapy (HR = 1.16, 95% CI = 0.75–1.78, P = .51). Quality of life scores were similar for both treatment arms.
Conclusions
TRC105 in combination with bevacizumab was well tolerated in patients with recurrent GBM, but no difference in efficacy was observed compared to bevacizumab monotherapy.
Keywords: bevacizumab, CD105, glioblastoma, TRC105, angiogenesis
Key Points.
TRC105 given in combination with bevacizumab was well tolerated in patients with GBM.
TRC105 + bevacizumab did not improve outcomes vs bevacizumab alone in patients with recurrent GBM.
Importance of the Study.
Based on preclinical and early clinical data with the combination of TRC105 and bevacizumab in patients with other solid tumors, we hypothesized that blocking CD105 with the anti-CD105 antibody TRC105 could prevent the development of resistance to bevacizumab. There is an urgent need for novel treatments for patients with glioblastoma (GBM) as bevacizumab monotherapy has only modest efficacy. We investigated the combination of TRC105 and bevacizumab in patients with GBM. While this combination was well tolerated, no increased efficacy with the addition of TRC105 was demonstrated. Correlative analyses indicated that baseline presence of circulating endothelial cells may impact progression-free survival after bevacizumab therapy, which could be of interest for future research, including the development of combinatorial strategies that can increase efficacy or prevent bevacizumab resistance.
Glioblastoma (GBM), the most lethal primary malignant brain tumor, has a median survival of 16–18 months despite multimodality treatment that includes surgery, radiation therapy, and chemotherapy. There is a pressing need to develop innovative approaches in the treatment of this disease.1
GBM is a highly vascularized tumor relying heavily on angiogenesis.2 A key mediator in cancer angiogenesis is the angiogenic cytokine vascular endothelial growth factor (VEGF).3 Inhibition of VEGF signaling has been shown to inhibit glioma tumor growth in several models.4 Bevacizumab is a recombinant humanized monoclonal antibody (mAb) that binds and neutralizes VEGF activity. Bevacizumab was granted accelerated approval by the United States Food and Drug Administration (FDA) for treatment of recurrent GBM,5–7 which was subsequently converted to full approval in 2017. While bevacizumab has an acceptable safety profile and antiglioma activity as single agent, the survival benefit is still questionable. Development of rationally designed bevacizumab-based combinatorial strategies is needed in order to improve efficacy outcomes.
CD105 (endoglin) is a transforming growth factor β (TGFβ) binding protein that can be found on the surface of vascular endothelial cells.8 High expression of CD105 has been detected in tissues undergoing active vascularization, such as tumors.9 Microvessel density (MVD), as determined by the level of anti-CD105 mAb binding (CD105-MVD), has been shown to correlate with VEGF expression in GBM; patients with higher CD105-MVD tumors were reported to have a shorter survival time than patients with low CD105-MVD tumors.8 This was especially true when increased CD105-MVD was observed in the area situated 1–3.5 cm from the tumor.10 These outcomes suggest that CD105 expression has a possible prognostic value in patients with GBM. Moreover, increased levels of CD105 have been observed following VEGF inhibition11 and this may represent an escape mechanism for the tumor.
TRC105 is a chimeric anti-CD105 IgG1 antibody, formed of human Cκ and Cγ1 constant regions and murine Vκ and VH regions,12 that inhibits angiogenesis and has the potential to complement other anti-angiogenic therapies. Treatment with TRC105 monotherapy in patients with advanced tumors was shown to be well tolerated, with possibly early antitumor activity; 47% of patients achieved stable disease (SD) or better.13 Additionally, the adverse events (AEs) associated with TRC105 were distinct from those previously observed with VEGF inhibitors, indicating that these treatments may be safely combined.13 A phase Ib trial evaluating the combination of TRC105 plus bevacizumab in patients with advanced solid tumors has shown a tolerable safety profile, with some preliminary clinical activity.14 Hence, we hypothesized that combining these two agents in patients with GBM may improve efficacy outcomes and prevent development of bevacizumab treatment resistance.
Herein, we present data from North Central Cancer Treatment Group (NCCTG) N1174, a phase I/II study investigating treatment with TRC105 plus bevacizumab in patients with recurrent GBM. The aim of the trial was to determine the maximum tolerated dose (MTD) and evaluate the safety and efficacy of the combination. NCCTG is now part of the Alliance for Clinical Trials in Oncology.
Materials and Methods
Patients
Eligible patients were ≥18 years old, had evidence of tumor progression following radiation or other antitumor therapy, and had measurable or evaluable disease by gadolinium magnetic resonance imaging (MRI) or contrast computerized tomography (CT) scan. Additionally, all patients were required to have an Eastern Cooperative Oncology Group (ECOG) status of 0–2, a life expectancy ≥12 weeks, and adequate hematologic, hepatic, and renal function. The phase I component of the study enrolled patients with grade 3 or 4 gliomas, including astrocytoma, oligodendroglioma, and mixed gliomas, as determined by preregistration central pathology review. Patients were allowed to have received any number of prior chemotherapy regimens and a last dose of bevacizumab ≥2 weeks prior to registration. Patients were not eligible for the phase I part if they had experienced any prior hypersensitivity to bevacizumab. For the phase II part, patients with histological confirmation of GBM, gliosarcoma, or other grade 4 astrocytoma variants were eligible. Histologic diagnosis was confirmed by central pathology review. Patients were allowed to have had ≤1 chemotherapy or other nonantiangiogenic regimen at recurrence and have no prior exposure to bevacizumab. Patients were not eligible for phase II if they had had any prior exposure to a VEGF inhibitor.
Patients were ineligible for both study phases if they had prior hypersensitivity to recombinant antibodies or triptans, had other active malignancies, or uncontrolled infection. Additional exclusion criteria included a history of hypertensive crisis or hypertensive encephalopathy, history of bleeding diathesis, clinically significant cardiovascular or vascular disease, receipt of any other investigational agents, or prior treatment with TRC105. The study protocol was approved by the Mayo Clinic Institutional Review Board and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each individual participating in the study.
Study Design
This was a phase I dose-escalation/comparative randomized phase II trial in patients with GBM (NCT01648348). The primary objective of the phase I part of the study was to determine the maximum tolerated dose (MTD) of TRC105 when combined with bevacizumab. In addition, pharmacokinetics (PK) of TRC105 were determined as correlative research. For the phase II part of the study, primary objectives were to assess overall safety and occurrence of AEs, and to evaluate the efficacy of the combination versus bevacizumab monotherapy; progression-free survival (PFS) was the primary endpoint. Secondary objectives included the evaluation of 6-month PFS, overall survival (OS, defined as length of time from registration until death due to any cause), time to treatment failure (TTF, defined as the time from study registration until documentation of progression, unacceptable toxicity or refusal to continue study participation), and quality of life in both treatment arms. Correlative research during the phase II trial included the investigation of the relationship between circulating biomarkers of vascular response and efficacy.
For phase I, a standard 3 + 3 dose-escalation schedule was applied for TRC105. The following premedications were administered 0.5–2 hours prior to administration of TRC105: acetaminophen (650 mg), dexamethasone (20 mg), famotidine (20 mg), and cetirizine (10 mg). Dexamethasone doses were tapered over the treatment cycles if previous TRC105 infusions were well tolerated. TRC105 was administered intravenously (IV) using an infusion pump over 1–4 hours, with starting dose level set at 6 mg/kg weekly. Subsequent dose levels were 8 mg/kg and 10 mg/kg weekly. For all patients, the initial dose in cycle 1 was split over days 8 (3 mg/kg IV) and 11 (the remainder of the dose), and subsequent doses given fully on days 1 and 8 of each 2 week cycle. Bevacizumab was administered at the approved dose of 10 mg/kg on day 1 of each 2 week cycle. Three patients were included for each dose level and if 1 of 3 patients exhibited dose-limiting toxicity (DLT), an additional 3 patients were included at the same dose level. The MTD was defined as the highest dose when no more than 1 out of 6 patients had a DLT.
During phase II, patients were randomized 1:1 to TRC105 and bevacizumab or bevacizumab monotherapy. The TRC105 dose was 10 mg/kg weekly as determined in phase I. During cycle 1 the dose was split between days 8 (3 mg/kg) and 11 (7 mg/kg), and in subsequent cycles, the full dose was administered on days 1 and 8 of each 2 week cycle. Maximum administered doses of TRC105 were 850 mg for women and 1000 mg for men. Bevacizumab was given every 2 weeks, as a 10 mg/kg IV infusion on day 1 of each 2 week cycle. On the days when both bevacizumab and TRC105 were administered, bevacizumab was given first, followed by TRC105 premedications and TRC105.
Dose levels of TRC105 could be reduced when patients experienced AEs; in cycle 1 the day 11 dose could be reduced from 7 mg/kg to 3.5 mg/kg and in subsequent cycles doses could be reduced to 8, 6, or 3 mg/kg weekly. When dose-related toxicities were observed at the lowest of these doses, TRC105 was discontinued. Bevacizumab could be omitted for the cycle, delayed, or discontinued based on bevacizumab-related AEs experienced by patients.
Safety
All AEs were evaluated per National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) v4.0 at baseline and each evaluation. AEs were assessed for likelihood of being related to the study drug and categorized as definite, probable, possible, unlikely to be related, and unrelated.
Response Assessment
Tumor response was assessed using the Response Assessment Neuro-Oncology (RANO) criteria.15 Measurable disease was defined as bi-dimensionally contrast-enhancing lesions with clearly defined margins that had two perpendicular diameters ≥10 mm and were visible on ≥2 axial slices. Tumors around a cyst or surgical cavity were considered nonmeasurable unless a nodular component existed with a diameter ≥10 mm. Disease was considered nonmeasurable when there were uni-dimensionally lesions, masses without clearly defined margins, or lesions with a maximal perpendicular diameter <10 mm. For target lesions, a maximum of 5 measurable lesions were selected (based on the longest diameters), recorded, and measured at baseline. Target lesions were evaluated, and response was defined as complete response (CR) when there was a complete disappearance of all enhancing measurable and nonmeasurable disease that was sustained for ≥4 weeks and no new lesions had appeared. Partial response (PR) was defined as a ≥50% decrease compared with baseline in perpendicular diameters of all measurable enhancing lesions, which was sustained for ≥4 weeks, without new lesions or progression of nonmeasurable disease. Progressive disease (PD) was defined as a ≥25% increase in perpendicular diameters of enhancing lesions, compared with the smallest tumor measurement at baseline or at best response, occurrence of any new lesion, clear clinical deterioration, or failure to return for evaluation due to death or deterioration, or clear progression of nonmeasurable disease. Patients not qualifying for CR, PR, or PD who were clinically stable were deemed to have SD. All patients continued treatment until disease progression unless unacceptable toxicity occurred.
Pharmacokinetics
For patients enrolled in the phase I part of the study, serum samples were collected for PK analysis at pre- and postdose in cycle 1 days 8 and 11, cycle 2 days 1 and 8, at the end of the study or upon withdrawal, and 28 days after end of the study. Pre-dose samples were taken immediately prior to the start of TRC105 infusion, and post-dose samples were collected within 10 minutes of completion of the infusion.
Circulating Endothelial Cells
Planned correlative analyses included evaluation of cellular biomarkers of vascular response during the phase II part of the study. Total circulating endothelial cells (CEC) and CECs with expression of CD105, the putative target of TRC105, were determined at various time points. Whole blood samples were collected in EDTA tubes at baseline, prior to treatment in cycle 2, on cycle 2 day 3 (± 1 day), prior to treatment in cycle 3, and prior to treatment every 4 weeks thereafter for up to 5 times. In addition, samples for CEC analysis were acquired at disease progression, withdrawal, or study discontinuation. Samples had to be analyzed within 48 hours of collection for results to be considered valid. CECs were evaluated as previously described.16,17 Briefly, whole blood samples were lysed to remove red blood cells and then stained in BD Pharmingen Trucount© tubes for the absolute count calculation of endothelial cells. Endothelial cells were identified by their characteristic low forward/side scatter and CD146+CD3-CD31+ phenotype. Cells were additionally stained using CD105 to evaluate activated endothelial cells. Isotype controls were used to exclude non-specific staining, and 7-amino-actinomycin D staining to exclude dead cells.
Quality of Life
Quality of life was assessed with the EORTC Quality of Life QLQ-C15-PAL and QLQ-BN20 patient questionnaires. All patients were requested to complete these questionnaires at baseline and every four weeks thereafter. In addition, patient satisfaction with participation in the trial was assessed using the 4-item Was It Worth It (WIWI) questionnaire. The WIWI questionnaire was completed at 4 weeks and at disease progression, at time of patient withdrawal or study discontinuation, or 12 months from randomization, whichever occurred first. This questionnaire includes a few key questions to determine patient perception of a treatment being worth the trouble.
Statistical Analyses
All AEs and their severity were tabulated and summarized. For the safety analyses, all patients who received at least one dose of study drug were included; patients were analyzed according to the treatment that they received. Overall AE rates for grade ≥3 events were compared between the treatment groups using Chi-Square or Fisher’s Exact tests.18,19
For phase II, the primary endpoint was to evaluate the difference in efficacy between bevacizumab alone and bevacizumab in combination with TRC105 as determined by PFS. A sample size of 86 (43 per group) resulted in 90% power to detect at least a 3-month increase in median PFS. PFS was defined as time from randomization to disease progression, with death being documented as tumor progression. Patients who did not die or progress were censored at time of last tumor assessment. PFS was estimated using the Kaplan-Meier method.20 The patient groups were compared using log-rank tests. For efficacy analyses the intent-to-treat (ITT) population was used, defined as all eligible patients belonging to the treatment group to which they were randomized and regardless of receiving study treatment. A sensitivity analysis was included that used a modified ITT population, including only patients who had been registered and randomized, and who had received at ≥1 study treatment cycle without a major violation.
Secondary endpoints included 6-month PFS, overall survival (OS, defined as time from start of therapy to death by any cause with the distribution estimated using Kaplan-Meier), time to treatment failure (TTF, defined as the time from study registration until documentation of progression, unacceptable toxicity or refusal to continue study participation), and quality of life in both treatment arms. The modified ITT population was also used for the CEC analyses.
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Management Center. Data quality was ensured by review of data by the Alliance Statistics and Data Management Center and by the study chairperson following Alliance policies. The study was monitored at least twice annually by the Alliance Data and Safety Monitoring Board.
Results
Patients and Baseline Demographics
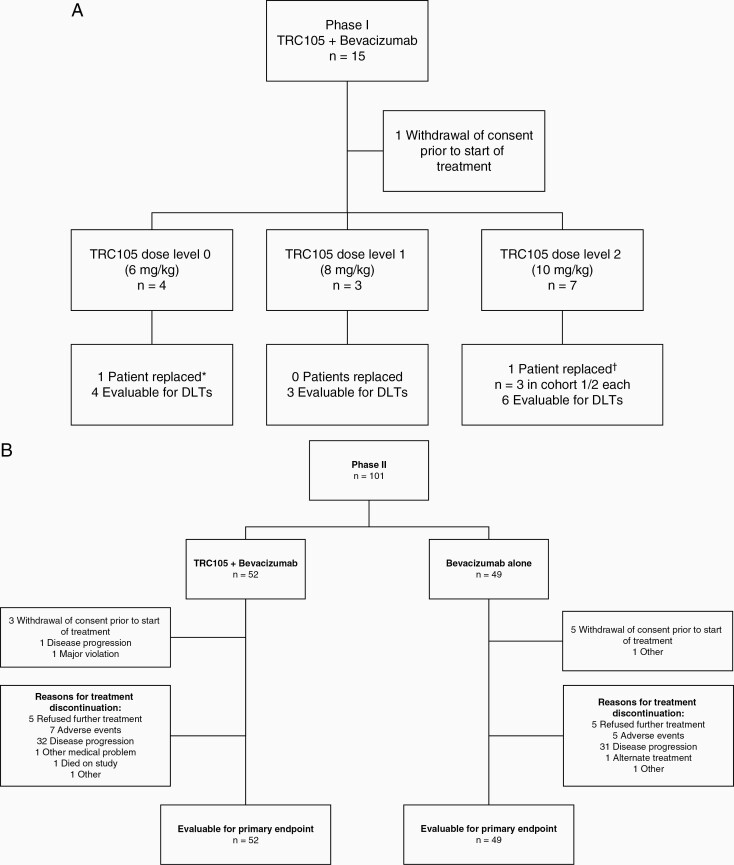
In total, 15 patients were enrolled for the phase I part of the trial (Figure 1A). One patient withdrew from the trial prior to receiving study treatment. Four patients started on dose level 0 (6 mg/kg weekly), 3 on dose level 1 (8 mg/kg), and 7 on dose level 2 (10 mg/kg) with, respectively, 4, 3 and 6 patients evaluable for DLTs. All patients in the phase I part of the study discontinued treatment. Reasons for treatment discontinuation were disease progression (n = 11), refusal of further treatment (n = 2), and AEs (n = 1). For the phase II part of the study, 101 patients were included and 52 were randomized to TRC105 with bevacizumab and 49 to bevacizumab alone (Figure 1B). Eight patients withdrew consent prior to beginning study treatment and were excluded from the primary analysis per ITT. At time of data analysis, all patients had discontinued treatment. The most common reasons for treatment discontinuation were disease progression (n = 63), AEs (n = 12), and refusal of further treatment (n = 10). Reasons for treatment discontinuation were not significantly different between the treatment arms (P = .75).
Figure 1.
CONSORT diagrams for the A) phase I and B) phase II parts of the study. *Replaced for maximum tolerated dose analysis due to disease progression prior to receiving TRC105. †Replaced for maximum tolerated dose analysis, the patient refused further treatment from cycle 1 day 11 onwards and withdrew consent.
Patient demographics and baseline characteristics for both phase I and II of the study are summarized in Table 1. When comparing the phase II arms, there was a difference in the extent of resection at recurrence at any time, with more patients having gross total resection in the TRC105 plus bevacizumab arm and more patients having subtotal resection in bevacizumab alone arm (P = .02).
Table 1.
Patient Demographics and Baseline Characteristics
| Characteristic | TRC105 + bev | TRC105 + bev | Bev alone |
|---|---|---|---|
| Phase I | Phase II | Phase II | |
| n = 15 | n = 52 | n = 49 | |
| Age, years, median (range) | 53.0 (44.0–65.0) | 58.5 (31.0–86.0) | 56.0 (32.0–75.0) |
| Gender, n (%) | |||
| Female | 4 (26.7) | 17 (32.7) | 12 (24.5) |
| Male | 11 (73.3) | 35 (67.3) | 37 (75.5) |
| Years since end RT, median (range) | 0.67 (0.08–2.17) | 0.67 (0.17–10.17) | 0.75 (0.17–3.50) |
| ECOG Performance Status | |||
| 0 | 4 (26.7) | 11 (21.2) | 10 (20.8) |
| 1 | 7 (46.7) | 29 (55.8) | 28 (58.3) |
| 2 | 4 (26.7) | 12 (23.1) | 10 (20.8) |
| Missing | 0 | 0 | 1 |
| Numberof priorchemo regimens, n (%) | |||
| 0 | 1 (6.7) | 8 (15.4) | 6 (12.5) |
| 1 | 5 (33.3) | 35 (67.3) | 34 (70.8) |
| 2 | 9 (60.0) | 9 (17.3) | 8 (16.7) |
| Missing | 0 | 0 | 1 |
| Corticosteroid use at entry, n (%) | |||
| Yes | 6 (40.0) | 27 (51.9) | 27 (56.2) |
| No | 9 (60.0) | 25 (48.1) | 21 (43.8) |
| Missing | 0 | 0 | 1 |
| Extent of resection (primary), n (%) | |||
| Biopsy | 3 (20.0) | 8 (15.4) | 4 (8.3) |
| Subtotal resection | 4 (26.7) | 18 (34.6) | 16 (33.3) |
| Gross total resection | 8 (53.3) | 26 (50.0) | 28 (58.3) |
| Missing | 0 | 0 | 1 |
| Extent of resection (recurrence), n (%) | |||
| None | 5 (33.3) | 30 (58.8) | 28 (59.6) |
| Biopsy | 1 (6.7) | 4 (7.8) | 0 |
| Subtotal resection | 3 (20.0) | 4 (7.8) | 12 (25.5) |
| Gross total resection | 6 (40.0) | 13 (25.5) | 7 (14.9) |
| Missing | 0 | 1 | 2 |
Bev, bevacizumab; ECOG, Eastern Cooperative Oncology Group; RT, radiation therapy
MTD Determination
In phase I, the most frequently occurring AEs regardless of relation to treatment were headache (n = 12), anemia (n = 7), epistaxis (n = 7), hypertension (n = 6), thromboembolic event (n = 3), and hyperglycemia (n = 2). One DLT occurred in the dose level 2 cohort: during cycle 1 one patient experienced grade 3 headache and hypertension. At cycle 2 the headache was resolved to grade 0 and the hypertension to grade 2. All other AEs in the trial were grade 1 or 2. The MTD was determined to be TRC105 10 mg/kg in combination with bevacizumab 10 mg/kg every 2 weeks.
Phase II Safety Data
AE data were available for 92 evaluable patients from phase II: 49 patients treated with TRC105 plus bevacizumab and 43 with bevacizumab monotherapy. The most common treatment-related AEs (TRAEs) that occurred in phase II are summarized in Table 2. The incidence of AEs regardless of attribution was compared between the two treatment arms. Significantly higher occurrences of anemia (P < .001), fatigue (P = .045), infusion-related reaction (P = .028), headache (P = .037), and epistaxis (P < .001) were observed in the combination arm compared with the bevacizumab monotherapy arm. There was also a general increase in the occurrence of any grade ≥3 events in the combination arm (P < .001); this difference was mainly driven by the incidence of grade ≥3 hematologic AEs (P < .001). There was no significant difference between the treatment groups for grade 4 AEs.
Table 2.
All Grade Treatment-Related AEs Occurring in ≥10 of Patients and Grade ≥3 Treatment-Related AEs
| All grade | Grade ≥3 | |||
|---|---|---|---|---|
| n = 92 | n = 92 | |||
| Adverse event, n (%) | Bev + TRC105 | Bev | Bev + TRC105 | Bev |
| (N = 49) | (N = 43) | (N = 49) | (N = 43) | |
| Anemia | 31 (63.3%) | 6 (14.0%) | 14 (28.6%) | 0 |
| Fatigue | 24 (49.0%) | 11 (25.6%) | 2 (4.1%) | 1 (2.3%) |
| Hypertension | 9 (18.4%) | 16 (37.2%) | 3 (6.1%) | 3 (7.0%) |
| Headache | 17 (34.7%) | 2 (4.7%) | 4 (8.2%) | 0 |
| Platelet count decreased | 9 (18.4%) | 9 (20.9%) | 1 (2.0%) | 1 (2.3%) |
| Epistaxis | 15 (30.6%) | 2 (4.7%) | 0 | 0 |
| Proteinuria | 5 (10.2%) | 8 (18.6%) | 0 | 1 (2.3%) |
| Infusion-related reaction | 10 (20.4%) | 1 (2.3%) | 1 (2.0%) | 0 |
| White blood cell decreased | 7 (8.9%) | 4 (9.3%) | 0 | 0 |
Efficacy
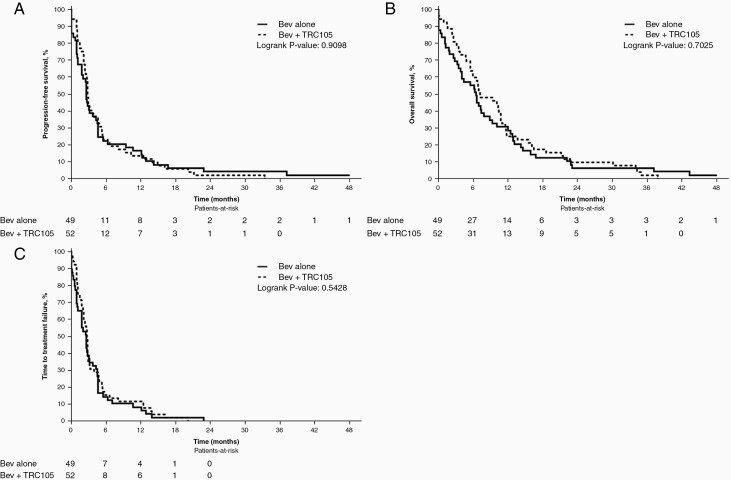
At the time of analysis, 13 patients were alive and remained progression-free; the median time of follow-up for these patients was 37.2 months (95% CI: 22.9–NA). The 6-month PFS rate was 0.25 (95% CI: 0.15–0.41) and 0.3 (95% CI: 0.19–0.48) for the TRC105 plus bevacizumab and bevacizumab arms, respectively. The median PFS for TRC105 plus bevacizumab was 2.9 (95% CI: 2.76–4.86) months, and for bevacizumab alone was 3.2 (95% CI: 2.60–4.63) months (Figure 2A). Median OS was 9.7 (95% CI: 6.74–11.53) months for TRC105 plus bevacizumab and 7.4 (95% CI: 6.54–12.71) months for bevacizumab alone (Figure 2B). The median TTF was 2.8 (95% CI: 2.14–3.22) and 2.6 (95% CI: 1.81–4.3) months for TRC105 plus bevacizumab and bevacizumab alone, respectively (Figure 2C). There was no significant difference between the treatment arms in terms of PFS (P = .51), overall survival (P = .81) or TTF (P = .57).
Figure 2.
Phase II efficacy endpoints. Kaplan-Meier curves for A) progression-free survival (PFS), B) overall survival (OS), and C) time to treatment failure (TTF) in patients with GBM receiving either TRC105 with bevacizumab (bev) or bevacizumab alone.
A sensitivity analysis of the survival data was undertaken, which included those patients who had received ≥1 cycle of treatment without a major treatment violation; 5 patients in the combination arm and 6 patients in the bevacizumab monotherapy arm were excluded from this analysis. Median PFS was 2.9 (95% CI: 2.76–4.86) months for TRC105 plus bevacizumab, and 3.2 (95% CI: 2.60–4.63) months for bevacizumab alone. Median overall survival for the combination arm was 10.2 (95% CI: 6.80–11.66) and 7.4 (95% CI: 6.54–12.71) months for the bevacizumab arm, while the median TTF was 2.8 (95% CI: 2.30–4.60) and 2.7 (95% CI: 2.04–4.53), respectively. In the sensitivity analysis, the differences between the treatment arms remained not significant for PFS (P = .52), overall survival (P = .91), or TTF (P = .66).
The best response to treatment is summarized in Table 3. For each of the treatment arms, 1 CR and 5 PRs were observed. SD was the best response for 33 patients treated with TRC105 plus bevacizumab and 26 patients treated with bevacizumab alone. Eight patients had PD after being treated with TRC105 plus bevacizumab and 7 patients had PD after treatment with bevacizumab alone. Overall, best response was not significantly different between the treatment arms (13% versus 16%, P = .97).
Table 3.
Best Response Rates in the Phase II
| Best response rate (95%CI) | Phase II | Phase II |
|---|---|---|
| TRC105 + bev | Bev alone | |
| n = 52 | n = 49 | |
| Missing/not evaluable | 5 | 10 |
| CR | 1/47 | 1/39 |
| 0.02 (0.00–0.11) | 0.03 (0.00–0.13) | |
| PR | 5/47 | 5/39 |
| 0.11 (0.04–0.23) | 0.13 (0.04–0.27) | |
| SD | 33/47 | 26/39 |
| 0.70 (0.55–0.83) | 0.67 (0.50–0.81) | |
| PD | 8/47 | 7/39 |
| 0.17 (0.08–0.31) | 0.18 (0.08–0.34) |
Bev, bevacizumab; CI, confidence interval; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Pharmacokinetics
TRC105 concentrations were determined during cycle 2, before and after treatment. Before treatment on cycle 2 day 1, the mean corrected concentration of TRC105 was 46.6 (± standard deviation of 37.4) µg/mL, which increased to 208.8 (± 25.3) µg/mL post-treatment. At day 8 of cycle 2, the TRC105 concentrations were 51.5 (± 30.6) µg/mL and 259.2 (± 94.6) µg/mL pre- and post-treatment, respectively, which fall within active therapeutic windows exceeding the target TRC105 concentration of 20 µg/mL both at peak and trough.
Circulating Endothelial Cells
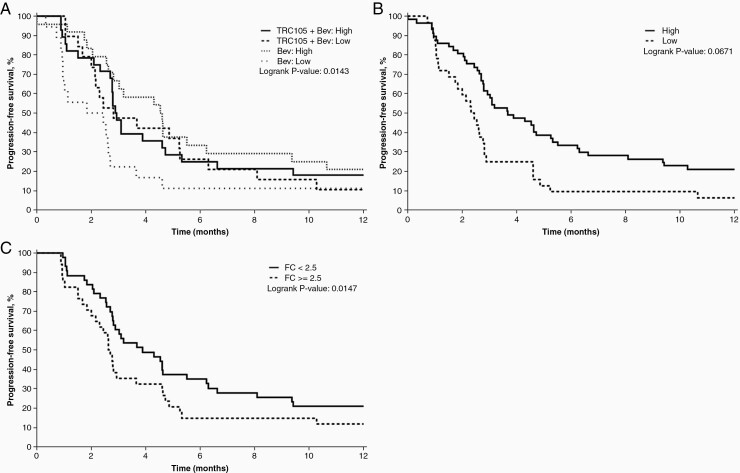
Baseline data for CEC analysis was available from 89 patients; 47 patients in the TRC105 plus bevacizumab arm and 42 patients in the bevacizumab alone arm. At baseline, no significant differences were observed between the treatment groups in numbers of total or CD105+ CECs or percentages of CD31+CD105+ CECs (Supplementary Table 1). Low numbers of CECs (≤27.86) were seen in 42% of patients and 39% of patients had no CD105+ CECs at baseline. The percentage of patients without CD105+ CECs at baseline was slightly lower in the TRC105 plus bevacizumab arm (30%) than in the bevacizumab alone arm (50%; P = .083). A lower baseline CEC number was associated with a worse PFS (hazard ratio [HR] = 1.75, P = .017). The treatment arm was an effect modifier on this relationship. The numbers of CECs at baseline were not significantly influential on PFS in patients treated with TRC105 plus bevacizumab (HR = 1.27, P = .45), but a significant influence of lower baseline CECs numbers on PFS was seen for patients receiving bevacizumab alone (HR = 2.55, p=0.007). When comparing treatment arms with low or high baseline CECs, patients with low CECs receiving bevacizumab alone had the shortest PFS (Figure 3A). A lower percentage of CD31+CD105+ CECs at baseline was also significantly associated with shorter PFS (HR = 1.80, P = .017; Figure 3B). Increases in the numbers of CECs with a fold change ≥2.5 (HR = 1.83, P = .014; Figure 3C) during treatment were associated with worse PFS.
Figure 3.
Circulating endothelial cells. Kaplan-Meier curves for progression-free survival (PFS) by A) treatment arm and low or high numbers of circulating endothelial cells (CECs) at baseline, B) low or high percentages of CD31+CD105+ CECs at baseline, C) fold change in CEC numbers from baseline to cycle 2 (approximately 1 month).
Quality of Life
In total, 45 patients in the TRC105 plus bevacizumab arm and 41 in the bevacizumab alone arm completed the EORTC-QLQ-BN20 questionnaire at baseline. Of these, 33 patients in the TRC105 plus bevacizumab arm and 32 in the bevacizumab alone arm had also completed questionnaires at cycle 2. Supplementary Table 2 shows an overview of the changes from baseline to cycle 2. A decrease in “future uncertainty” scores was reported by patients in both treatment arms (median –8.33 for both groups). Median scores for other topics did not change from baseline to cycle 2, and no significant difference was observed between the treatment arms. The overall quality of life median scores from the EORTC-QLQ-C15-PAL also did not significantly change from baseline to cycle 2 (Supplementary Table 3) for either arm, and results did not significantly differ between treatment arms. From available WIWI questionnaires, 26 patients (72.2%) receiving TRC105 plus bevacizumab and 23 patients (71.9%) receiving bevacizumab indicated it was worth being a part of the study.
Discussion
TRC105 in combination with bevacizumab was generally well tolerated in patients with GBM. Only one DLT occurred in the phase I part of the study, which was grade ≥3 headache and hypertension. As a result, the highest TRC105 dose tested in phase I, 10 mg/kg weekly in combination with bevacizumab 10 mg/kg on day 1 of each 2 week cycle, was established as the MTD and used for phase II of the study. Interestingly, results from the phase I portion of this study suggested it is feasible to enroll patients refractory to bevacizumab, a challenging population that is often excluded from participation in clinical trials.
In the phase II portion of the study, several AEs were more prominent in the TRC105 plus bevacizumab arm compared with bevacizumab alone, specifically anemia, fatigue, infusion-related reaction, headache, and epistaxis. These AEs have previously been associated with TRC105 in trials with patients with prostate cancer21 and hepatocellular carcinoma.22 The data presented within also indicate that treating patients with both TRC105 and bevacizumab appears to increase the severity of AEs, mainly the proportion of patients suffering with grade 3 AEs, compared with bevacizumab alone. This is in contrast to published data in patients with renal cell cancer where the combination of TRC105 plus bevacizumab did not increase the overall frequency of grade ≥3 AE compared with bevacizumab monotherapy.23 Similarly, a phase I trial in patients with solid tumors found no difference in the frequency or severity of AEs in patients who received TRC105 plus bevacizumab, compared with the safety profiles for each individual therapy (except from headache).14 The differences observed in this trial may be a result of the indication in which the combination is being studied and the possible increased susceptibility of this specific patient population to certain side effects such as headaches. It is of note that despite the increased frequency of AEs in the combination arm, this did not translate in differences in quality of life as assessed by the EORTC-QLQ-BN20 and EORTC-QLQ-C15-PAL questionnaires or the WIWI questionnaire. Furthermore, >70% of patients in both arms deemed that the participation in this trial was worth it.
The combination of TRC105 plus bevacizumab did not improve efficacy compared with bevacizumab alone. The median PFS, OS and TTF were similar in both treatment groups. The combination of TRC105 plus bevacizumab has previously shown activity in patients with solid tumors. In one study, 45% (n=14) of patients had a decrease in overall tumor burden; of these, 10 patients had previously progressed after a VEGF-targeted therapy.14 Similar to our data, a trial in patients with metastatic renal cell cancer also demonstrated that the combination of TRC105 plus bevacizumab did not improve patient outcomes; a lower PFS (2.8 months) with the combination therapy versus bevacizumab alone (4.6 months) was observed. The patients in that study were highly refractory however and could have progressed after several other VEGF-targeted agents prior to inclusion in the trial.23 While CD105 was previously seen to be upregulated after VEGF inhibition in mouse models,11 a study evaluating bevacizumab with TRC105 in patients with renal cell cancer found contrary results and observed a decrease in serum levels of CD105 after treatment with bevacizumab.23 It is possible that angiogenic factors other than CD105 are more prominent in patients with GBM after VEGF-targeted therapy, which could explain the lack of response seen in our trial.
Preliminary correlative analyses indicated that the duration of PFS may be influenced by numbers of CECs and percentages of CD31+CD105+ CECs at baseline. Lower levels of baseline CECs were associated with a worse PFS in patients treated with bevacizumab alone. In addition, patients without CECs at baseline who developed these cells during treatment with bevacizumab also had a lower PFS. However, these results need to be considered with caution as the number of patients who could be included in these analyses was limited and the results are considered exploratory and hypothesis-generating in nature. Nevertheless, this potential relationship between CECs and PFS in patients with GBM treated with bevacizumab is intriguing and warrants additional investigation.
GBM is a fatal malignancy, commonly refractory to all treatment options. Although our trial failed to demonstrate efficacy of the TRC105/bevacizumab combination in GBM, trials examining the combination of TRC105 with other VEGF targeting strategies did demonstrate promising results in other solid tumors. When TRC105 was administered in combination with sorafenib in patients with hepatocellular carcinoma, a response rate of 25% was observed with a duration of response ranging from 4.4–27.6 months. The majority of the confirmed PRs (4/5) were seen with a dose of 15 mg/kg TRC105 every 2 weeks and no responses were seen at doses <10 mg/kg [19]. Axitinib in combination with TRC105 also demonstrated preliminary activity in patients with metastatic renal cell carcinoma with 29% of patients having PR, and a median PFS of 11.3 months. This was a higher response rate and longer PFS than expected with axitinib alone. All 5 responders in this study had previously been unresponsive to sunitinib or pazopanib.24
In conclusion, TRC105 in combination with bevacizumab did not improve PFS compared to bevacizumab alone in patients with recurrent GBM. The combination of these 2 treatments has shown promising results in at least one trial for a different tumor type, as has TRC105 combined with other VEGF inhibitors. Although the development of TRC105 may be valuable for the development of future anti-cancer therapies, GBM does not appear to be an optimal indication for this strategy. Further studies are necessary to determine optimal bevacizumab combinations and alternative anti-angiogenic strategies for GBM treatment.
Supplementary Material
Contributor Information
Evanthia Galanis, Department of Oncology, Mayo Clinic, Rochester, Minnesota, USA; Department of Pathology, Mayo Clinic, Rochester, Minnesota, USA; Mayo Clinic, Rochester, Minnesota, USA.
S Keith Anderson, Alliance Statistics and Data Management Center, Mayo Clinic, Rochester, Minnesota, USA.
Erin Twohy, Alliance Statistics and Data Management Center, Mayo Clinic, Rochester, Minnesota, USA.
Nicholas A Butowski, Department of Neurological Surgery, University of California San Francisco, San Francisco, California, USA.
Adilia Hormigo, Department of Neurology, Icahn School of Medicine at Mount Sinai, The Tisch Cancer Institute, New York, New York, USA.
David Schiff, Department of Neurology, University of Virginia Health System, Charlottesville, Virginia, USA.
Antonio Omuro, Department of Neurology, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Kurt A Jaeckle, Department of Neurology, Mayo Clinic Florida, Jacksonville, Florida, USA.
Shaji Kumar, Department of Hematology, Mayo Clinic, Rochester, Minnesota, USA; Mayo Clinic, Rochester, Minnesota, USA.
Timothy J Kaufmann, Department of Neuroradiology, Mayo Clinic, Rochester, Minnesota, USA; Mayo Clinic, Rochester, Minnesota, USA.
Susan Geyer, Alliance Statistics and Data Management Center, Mayo Clinic, Rochester, Minnesota, USA.
Priya U Kumthekar, Northwestern Medicine, Lou and Jean Malnati Brain Tumor Institute, Chicago Illinois, USA.
Jian Campian, Washington University School of Medicine, Siteman Cancer Center, St. Louis, Missouri, USA.
Caterina Giannini, Mayo Clinic, Rochester, Minnesota, USA.
Jan C Buckner, Department of Oncology, Mayo Clinic, Rochester, Minnesota, USA; Mayo Clinic, Rochester, Minnesota, USA.
Patrick Y Wen, Center for Neuro-Oncology, Dana-Farber/Partners Cancer Care, Boston, Massachusetts, USA.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under awards UG1CA189823 (Alliance for Clinical Trials in Oncology National Cancer Institute Community Oncology Research Program Grant), U10CA180821, U10CA180882, UG1CA233180, UG1CA233290, and UG1CA233339. https://acknowledgments.alliancefound.org. Correlative analysis was supported with funding from TRACON Pharmaceuticals, San Diego, CA, USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest statement. E.G. Consulting or Advisory Role—Gradalis, Inc. (personal); Kiyatec, Inc. (personal); Agios Pharmaceuticals (Inst); Karyopharm Therapeutics, Inc. (Inst).Research/Grant/Clinical Trial Funding—Agios Pharmaceuticals (Inst); Celgene (Inst); MedImmune, Inc. (Inst); Tracon Pharmaceuticals (Inst). S.K.A. No relationships to disclose. N.A.B. Consulting or Advisory Role—Abbvie; Genentech/Roche; Medicenna; Omniox, Speakers’ Bureau—Genentech/Roche, Research Funding—Abbvie; Bristol-Myers Squibb; Five Prime Therapeutics; Lilly; Medicenna; Merck; Merrimack; Tocagen. A.H. No relationships to disclose. D.S. No relationships to disclose. D.D.T. No relationships to disclose. A.O. Consulting or Advisory Role—Alexion Pharmaceuticals; AstraZeneca; Bristol-Myers Squibb; Inovio Pharmaceuticals; Juno Therapeutics; Merck; Oxigene; Stemline Therapeutics. K.A.J. No relationships to disclose. S.K. Honoraria—Kesios Therapeutics; Noxxon Pharma; Skyline Diagnostics, Consulting or Advisory Role—Abbvie (Inst); Amgen (Inst); Bristol-Myers Squibb (Inst); Janssen Oncology (Inst); Takeda (Inst), Research Funding—Abbvie (Inst); Celgene (Inst); Janssen Oncology (Inst); Merck (Inst); Novartis (Inst); Sanofi (Inst); Takeda (Inst). T.J.K. No relationships to disclose. J.C.B. Travel, Accommodations, Expenses—Genentech/Roche. E.T. No relationships to disclose. C.G. No relationships to disclose. P.Y.W. No relationships to disclose.
Authorship statement. All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Evanthia Galanis, S. Keith Anderson, Erin Twohy and Susan Geyer. The first draft of the manuscript was written by Evanthia Galanis and S. Keith Anderson and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Clinicaltrials.gov Identifier: NCT01648348
References
- 1. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl 5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kargiotis O, Rao JS, Kyritsis AP. Mechanisms of angiogenesis in gliomas. J Neurooncol. 2006;78(3):281–293. [DOI] [PubMed] [Google Scholar]
- 3. Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005;69(Suppl 3):4–10. [DOI] [PubMed] [Google Scholar]
- 4. Stefanik DF, Fellows WK, Rizkalla LR, et al. Monoclonal antibodies to vascular endothelial growth factor (VEGF) and the VEGF receptor, FLT-1, inhibit the growth of C6 glioma in a mouse xenograft. J Neurooncol. 2001;55(2):91–100. [DOI] [PubMed] [Google Scholar]
- 5. Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist 2009;14(11):1131–1138. [DOI] [PubMed] [Google Scholar]
- 6. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 7. Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yao Y, Kubota T, Takeuchi H, Sato K. Prognostic significance of microvessel density determined by an anti-CD105/endoglin monoclonal antibody in astrocytic tumors: comparison with an anti-CD31 monoclonal antibody. Neuropathology 2005;25(3):201–206. [DOI] [PubMed] [Google Scholar]
- 9. Fonsatti E, Maio M. Highlights on endoglin (CD105): from basic findings towards clinical applications in human cancer. J Transl Med. 2004;2(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sica G, Lama G, Anile C, et al. Assessment of angiogenesis by CD105 and nestin expression in peritumor tissue of glioblastoma. Int J Oncol. 2011;38(1):41–49. [PubMed] [Google Scholar]
- 11. Bockhorn M, Tsuzuki Y, Xu L, et al. Differential vascular and transcriptional responses to anti-vascular endothelial growth factor antibody in orthotopic human pancreatic cancer xenografts. Clin Cancer Res. 2003;9(11):4221–4226. [PubMed] [Google Scholar]
- 12. Seon BK, Haba A, Matsuno F, et al. Endoglin-targeted cancer therapy. Curr Drug Deliv. 2011;8(1):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosen LS, Hurwitz HI, Wong MK, et al. A phase I first-in-human study of TRC105 (Anti-Endoglin Antibody) in patients with advanced cancer. Clin Cancer Res. 2012;18(17):4820–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon MS, Robert F, Matei D, et al. An open-label phase Ib dose-escalation study of TRC105 (anti-endoglin antibody) with bevacizumab in patients with advanced cancer. Clin Cancer Res. 2014;20(23):5918–5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 16. Khan SS, Solomon MA, McCoyJP, Jr. Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytometry B Clin Cytom. 2005;64(1):1–8. [DOI] [PubMed] [Google Scholar]
- 17. Mancuso P, Burlini A, Pruneri G, et al. Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood 2001;97(11):3658–3661. [DOI] [PubMed] [Google Scholar]
- 18. Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. J Royal Statistical Soc. 1922;85(1):87–94. [Google Scholar]
- 19. Pearson K. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling (PDF). Phil Mag. 1900;50(302):157–175. [Google Scholar]
- 20. Kaplan E, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 21. Karzai FH, Apolo AB, Cao L, et al. A phase I study of TRC105 anti-endoglin (CD105) antibody in metastatic castration-resistant prostate cancer. BJU Int 2015;116(4):546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duffy AG, Ma C, Ulahannan SV, et al. Phase I and preliminary phase II study of TRC105 in combination with sorafenib in hepatocellular carcinoma. Clin Cancer Res. 2017;23(16):4633–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dorff TB, Longmate JA, Pal SK, et al. Bevacizumab alone or in combination with TRC105 for patients with refractory metastatic renal cell cancer. Cancer 2017;123(23):4566–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choueiri TK, Michaelson MD, Posadas EM, et al. An open label phase Ib dose escalation study of TRC105 (anti-endoglin antibody) with axitinib in patients with metastatic renal cell carcinoma. Oncologist 2019;24(2):202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.