Abstract
Locomotor speed is a basic input used to calculate one’s position, but where this signal comes from is unclear. We identified neurons in the supramammillary nucleus (SuM) of the rodent hypothalamus that were highly correlated to future locomotor speed and reliably drove locomotion when activated. Robust locomotion control was specifically identified in Tac1 (substance P)-expressing neurons (SuMTac1+), whose activation selectively controlled the activity of speed-modulated hippocampal neurons. In contrast, SuMTac1− cells weakly regulated locomotion, but potently controlled the spike-timing of hippocampal neurons and were sufficient to entrain local network oscillations. These findings highlight that the SuM not only regulates basic locomotor activity but also selectively shapes hippocampal neural activity in a manner that may support spatial navigation.
One-Sentence Summary:
Dissociated hypothalamic cell-types differentially drive locomotion and hippocampal cellular and network-level activity patterns.
Constructing and accessing a mental map of the environment during locomotion is an important adaptation facilitating survival and is supported by tracking self-motion (1). Mammalian locomotion is intimately tied to the occurrence of 6–12Hz hippocampal theta oscillations, such that theta begins prior to the onset of self-generated motion and increases in amplitude with respect to speed (2–6). Hippocampal theta temporally organizes the activity of place-coding neuronal assemblies into trajectories across past, present, and future locations, which is thought to subserve cognitive operations during spatial navigation (5–10). Tight coupling of theta to speed could be the result of shared neural circuitry between self-generated locomotion and theta control, providing a potential speed signal (1). Alternatively, speed could be derived from optic flow, vestibular input, or an efference copy from locomotor areas (11–14). Since the identification of speed-encoding neurons in brain areas like the hippocampus and entorhinal cortex that are thought to utilize a speed signal to calculate position (3,15–18), there is growing interest in understanding potential sources of speed input.
The medial septum is critical for hippocampal theta and is functionally coupled to locomotion (3, 19–21), but other brain areas have been proposed to contribute to this oscillation. One such area is the supramammillary nucleus (SuM) of the posterior hypothalamus (22–24), which also has recently identified roles in arousal (25), spike-timing coordination (26), and identification of novelty (27). As a proposed theta controller, neural activity in the SuM is likely also related to locomotion, but this has not been systematically investigated. In addition to innervating the medial septum, the SuM has highly divergent outputs targeting midbrain, where locomotor commands are integrated and brain regions involved in spatial navigation, including the hippocampus, entorhinal cortex, medial prefrontal cortex, nucleus reuniens, and claustrum (28). Thus, the SuM projects to theta, locomotor, and spatial navigation circuitry, but the functional relevance of this positioning remains poorly understood.
Using electrophysiological data from rats navigating a continuous alternation task for reward (26), we first investigated how the spiking activity of SuM neurons relates to locomotor speed and hippocampal theta oscillations (Fig. 1A). Similar to previous observations in the midbrain locomotor region (MLR) (29), we found a large proportion of SuM units with firing rates that were significantly coupled to locomotor speed with the majority displaying a positive correlation (Fig. 1B,C; fig. S1A,C,D). Interestingly, SuM “speed cells” were more correlated with future speed, with an average offset of 1.2 seconds, than real-time speed (Fig. 1D, fig S1A,D). After adjusting for temporal offsets, the firing rate of 99% of SuM units were modulated by speed (fig. S1B). Most speed cells retained their correlation with immobility data withheld (fig. S1E,F) and were more weakly coupled to acceleration than speed (fig. S2). SuM unit activity was also correlated to hippocampal theta amplitude in real-time, but this was less accurately modeled than speed (fig. S3). Thus, SuM activity and locomotion are strongly coupled.
Fig. 1. SuM representation of speed and hippocampal theta.
A, Recording paradigm; data from using unrestrained rats (26), reanalyzed here for speed- and theta-related investigations. B, Example SuM unit (recorded by tetrodes) that positively correlates to speed (i.e. a speed cell). Inset represents these data as a scatter plot. C, Distribution of speed vs. firing rate Pearson r values. Pie chart shows percentage of units for positive (r=0.36±0.023, mean±sem), negative (r=−0.24±0.024), and non-significant cells (r=0.018±0.012). D, Distribution of temporal offsets for positive speed cells. sem=0.19. E, Two example SuM unit spiking activities. Orange cell shows phase-locked firing with respect to hippocampal theta whereas the grey cell does not. F, Quantification of theta-related firing for two example units from E. The top panel shows spike-field coherence and the bottom shows theta-rhythmic spiking. G, Units were clustered into theta cells based on quantification from F. H, Distribution of speed scores among clustered “theta cells” from G.
We then addressed whether speed-related neuronal activity is propagated to projection targets, because not all locally recorded SuM units are necessarily projection neurons. In vivo 2-photon calcium imaging of SuM axon terminals innervating the dentate gyrus (DG) and CA2 of hippocampus was performed on head-fixed mice (fig. S4A). Indeed, speed-correlated activity in SuM axons was observed in both regions (fig. S4B,C). Extensive collateralization of SuM axons was also determined (fig. S5) and may contribute to the similar proportion of positively and negatively correlated cells in both regions (fig. S4B).
Next, we examined the coupling between SuM action potentials and hippocampal theta waves (Fig. 1E). 30.7% of units displayed high coherence with hippocampal theta and theta-rhythmic spiking (Fig. 1F,G, see methods). SuM “theta cells” typically fired near the trough of CA1 theta with a slight prospective bias (fig. S6). Much like the overall SuM population, most SuM theta cells were positively correlated with locomotor speed (Fig. 1H).
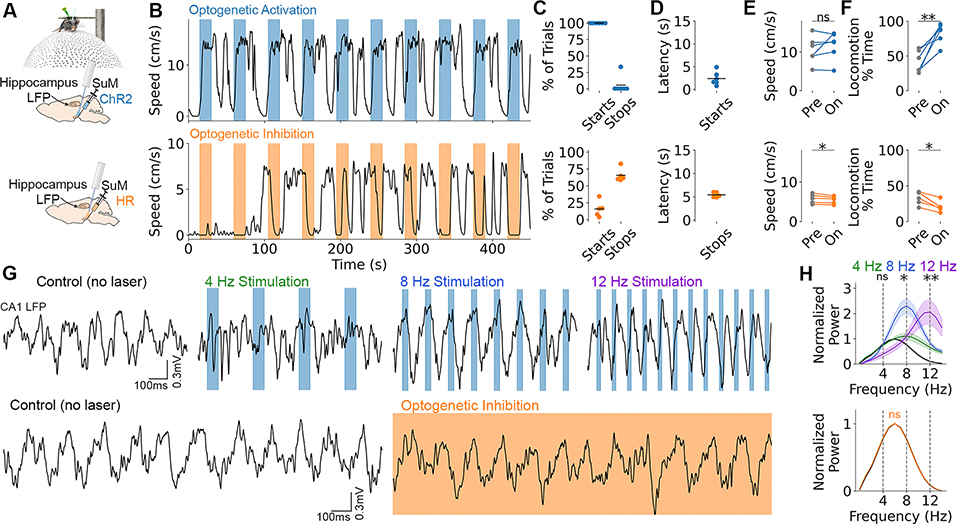
To test the functional involvement of the SuM in locomotion and theta, we injected the SuM with recombinant adeno-associated virus (rAAV) to express the optogenetic proteins, channelrhodopsin-2 (ChR2, excitatory) or halorhodopsin (HR, inhibitory), under control of a pan-neuronal promoter (Fig. 2A,G). Light activation of ChR2 drove locomotion on 100% of trials with an average latency of 2.4±0.6 seconds in head-fixed mice (Fig. 2B–F). Locomotion lagged behind an increase in theta amplitude (fig. S7), which is consistent with prospective encoding of speed but real-time encoding of theta amplitude (Fig. 1D vs. fig. S3A). The frequency of laser pulses reliably entrained hippocampal local field potential (LFP) at 8 and 12 Hz (Fig. 2G,H), similar to previous optogenetic manipulations in the medial septum (3,4,20,21). 94% of CA1 neurons were entrained by laser pulses, however, the preferred firing phase shifted considerably from spontaneous theta (fig. S8). Optogenetic inhibition with HR halted locomotion on 65.6±0.6 % of trials with a latency of 5.4±0.2 seconds (Fig. 2B–F). Consistent with previous studies (26,30), SuM inhibition did not alter hippocampal theta during locomotion (Fig. 2G,H). Thus, optogenetic manipulation of the SuM robustly and bidirectionally controls locomotion but does not inhibit spontaneous hippocampal theta, despite robust spike and LFP entrainment with ChR2.
Fig. 2. Optogenetic SuM modulation controls locomotion and hippocampal LFP.
A, Pan-neuronal SuM activation with ChR2 (blue) or inhibition with HR (orange) in head-fixed mice on a floating ball. B, Bidirectional locomotor effect with SuM activation (top) and inhibition (bottom). Colored bars denote laser on. C, Percent of trials where locomotion was initiated or halted for ChR2 (top) and HR (bottom). D, Latency of start vs. stop response. E, Speed during locomotor epochs before (pre) and during (on) light delivery. ChR2, t5=−1.14, p=0.31; HR, t4=3.07, p=0.037. F, Percent of time spent locomoting before (pre) and during (on) light delivery. ChR2, t5=−4.84, p=0.0047; HR, t4=4.60, p=0.010. G, Top: optogenetic activation at 4, 8, or 12Hz compared to no laser control. Blue bars denote laser on. Bottom: optogenetic inhibition (orange shading) vs. no laser control. Scalebar applies across rows. H, Quantification of power spectrum changes normalized to no laser. Top (ChR2): 4Hz power at 4Hz stimulation, t5=0.86, p=0.43; 8Hz power at 8Hz stimulation, t5=5.18, p=0.0035; 12Hz power at 12Hz stimulation, t5=3.99, p=0.010. Bottom (HR): t4=0.043, p=0.97. Data are mean±sem.
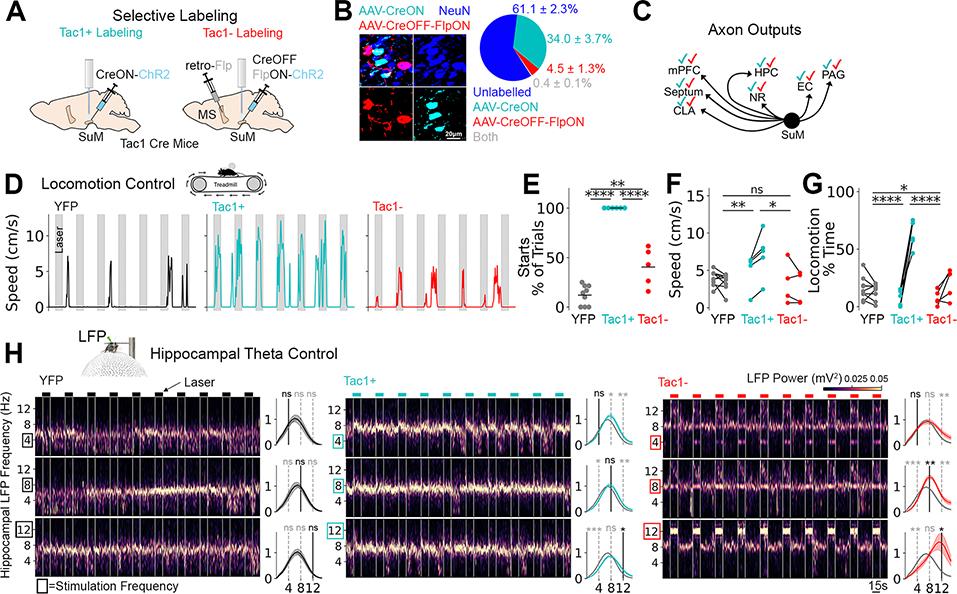
Considering the heterogeneity of SuM cell types (31), we then determined if locomotion control and spike/LFP entrainment were cell-type dependent. A subset of SuM neurons express Substance P, encoded by the Tac1 gene, and project to the hippocampus and other regions (32). Using the Tac1-Cre mouse line, we targeted two mutually exclusive populations. SuMTac1+ neurons were labelled with a CreON rAAV, whereas a smaller population of SuMTac1− projection neurons were labelled with an intersectional approach using a CreOFF-FlpON rAAV (33), facilitated by a retrograde rAAV carrying Flp in the medial septum (Fig. 3A,B). The axon outputs of both cell populations were similar, innervating the known and expected SuM target regions (Fig. 3C; fig. S9). However, SuMTac1+ and SuMTac1− cells had different intrinsic properties (fig. S10). Cell-types were further differentiated by the non-uniform axon innervation pattern of the dentate gyrus granule cell layer (fig. S11C) and increased vesicular GABA transporter content of SuMTac1− cells (fig. S11A,B), consistent with previously identified GABA/glutamate co-releasing SuM cells (34). Functionally, SuMTac1+ cell stimulation robustly drove locomotion on 100% of trials (Fig. 3D–G) but did not entrain hippocampal LFP (Fig. 3H). This locomotion was relatively slow and steady with an alternating stepping pattern (movie S1), consistent with exploratory locomotion and distinct from fight or flight responses seen with stimulation of other hypothalamic areas (35). In contrast, activation of SuMTac1− cells weakly controlled locomotion (Fig. 3D–G), but precisely controlled the frequency of hippocampal LFP at 8 and 12 Hz (Fig. 3H).
Fig. 3. Cell-type-dependence of locomotion initiation and LFP entrainment.
A, Labeling strategy to target mutually exclusive populations based on Tac1. B, Investigation of labelling specificity. Left: Image showing AAV-labelled Tac1+ (CreON) and Tac1− cells (CreOFF-FlpON) among other NeuN+ cells in the SuM. Right: quantification. C, Schematic showing checkmarks of each color (cyan: SuMTac1+, red: SuMTac1−) if axons were found in SuM target regions (see fig. S9). D, Representative locomotor activity during optogenetic activation at 8Hz. E, Percent of trials with locomotion initiation. ANOVA F2,17=103.6, p<0.0001, Tukey post-test. F, Locomotor speed before (left) vs. laser on (right). ANOVA was performed to determine group differences on changes in speed (pre vs. on). F2,15=7.2, p=0.0064, Tukey post-test. G, Percent of time locomoting before (left) vs. laser on (right). Differences in response change was assessed by ANOVA F2,17=57.8, p<0.0001, Tukey post-test. H, Optogenetic stimulation while head-fixed on floating ball at 4, 8, or 12 Hz. Spectrogram (left) and power spectral density changes (right, off vs. on) for each condition (columns) at each frequency (rows). Paired t-tests performed on light off vs. light on. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Given the robust control of movement initiation by SuMTac1+ neuron stimulation, we further examined the role of this cell-type in locomotion. Using 2-photon calcium imaging in head-fixed behaving mice, we observed a high proportion of SuMTac1+ cells whose activity were positively correlated with speed and active prior to locomotion onset (fig. S12). Similar results were obtained from Tac1+ cells in the ventral hypothalamus of zebrafish during swimming behavior, supporting evolutionary conservation of function (fig. S13). As with broad SuM inhibition, selective optogenetic inhibition of SuMTac1+ neurons also suppressed locomotion in head-fixed mice (fig. S14). Finally, we examined potential SuMTac1+ output pathways that reach the MLR, where the coordination of locomotor input and gait selection takes place (36). Using anterograde trans-synaptic tracing to label post-synaptic neurons (37), we found that midbrain periaqueductal grey neurons that specifically receive SuMTac1+ input, in turn, project to the MLR (fig. S15).
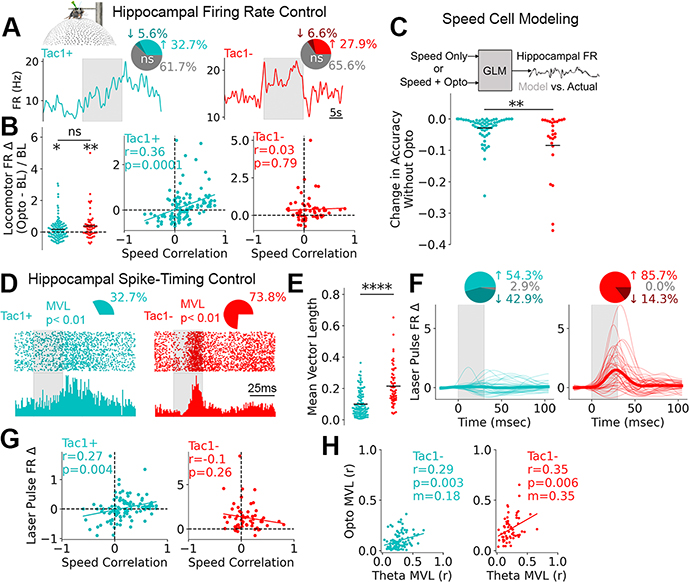
Finally, we determined how SuMTac1+ and SuMTac1− neurons alter firing rate and control the spike-timing of hippocampal neurons. Given the tight coupling of SuMTac1+ cells to locomotion, we hypothesized that spontaneously speed-correlated hippocampal neurons would be particularly sensitive to SuMTac1+ activation. On average, activation of both SuM cell-types increased the firing rate of the hippocampal units during locomotion (Fig. 4B) and resulted in similar proportions of units with significant firing rate alterations (Fig. 4A). The effect of SuMTac1+, but not SuMTac1− stimulation, was indeed correlated to the magnitude of spontaneous speed-modulation, such that the firing rates of positively correlated speed units increased, and negatively correlated cells decreased with optogenetic stimulation (Fig. 4B). We observed the opposite relationship with optogenetic inhibition of SuMTac1+ cells (fig. S14E). Hippocampal speed cell firing rates were then modelled from speed and laser timing as inputs (Fig. 4C). Modeling firing rates during SuMTac1+ stimulation produced considerably less error than SuMTac1− stimulation when laser timing was withheld (Fig. 4C), supporting that the effect of SuMTac1+ stimulation on locomotion and hippocampal speed cell firing rates are coupled.
Fig. 4. Hippocampal populations are differentially regulated by SuM cell-types.
A, Data were obtained from head-fixed mice on floating ball. Mean firing rate changes from two example hippocampal cells during SuMTac1+ or SuMTac1− optogenetic activation (grey bar). Pie charts display proportion of units with significantly altered locomotor firing rates. B, Locomotor firing rate change for light on vs. off (one-sample t-test, SuMTac1+ t105=2.56, p=0.012; SuMTac1− t57=3.28, p=0.0017; between sample t-test, t163=1.69, p=0.09) and as a function of speed correlation (calculated while laser is off). Y-label applies to all panels. C, Generalized linear model of hippocampal speed cell FR, with two sets of input (speed only vs. speed + opto). Change in modelling accuracy when optogenetic information was withheld (t89=3.28, p=0.0015). D, Hippocampal spike raster plots with histogram aligned to laser pulses (grey bar) during 8Hz stimulation. Pie charts show the proportion of units with significantly laser-modulated spike distributions. E, Quantification of non-uniform spike distributions from D. t-test t167=7.39, p=6.8×1012. F, Smoothed spike histograms of significantly laser-modulated cells. Thin lines are individual cells. Thick line is mean. Pie chart shows the directionality of modulation. G, Laser pulse-triggered firing rate change plotted against cells’ speed correlations (calculated while laser is off). H, Firing rate modulation by spontaneous theta vs. optogenetic laser pulses. Lines in B, G and H represent linear fits.
At a sub-second timescale, we also determined if hippocampal spike timing was altered relative to each laser pulse. Because SuMTac1− activation overrode spontaneous hippocampal theta and entrained LFP, we hypothesized that spike-timing would be more affected by activation of this cell-type. Indeed, SuMTac1− activation entrained spike-timing in more units with a greater average effect (Fig. 4D,E). Moreover, SuMTac1− activation increased the firing rate of most units in close proximity to the termination of each light pulse, whereas SuMTac1+ activation had mixed responses (Fig. 4F, fig. S16). Unlike SuMTac1− stimulation, the effect of SuMTac1+ stimulation depended on that unit’s speed correlation, such that positively correlated hippocampal speed cells were more likely to fire shortly after laser pulse onset and negatively correlated cells were suppressed (Fig. 4G). A significant correlation between the magnitude of optogenetic entrainment and the magnitude of spontaneous theta entrainment was observed for activation of both cell-types. In addition to the greater overall optogenetic entrainment (Fig. 4E), SuMTac1− activation resulted in a two-times greater slope of the optogenetic vs. spontaneous theta entrainment linear fit (Fig. 4H). Lastly, the preferred firing phase relative to hippocampal theta was more perturbed by SuMTac1− cell activation (fig. S17).
The finding that SuMTac1− cells potently regulate hippocampal spike-timing and are sufficient to entrain LFP is interesting in light of the lack of effect of SuM inhibition on spontaneous hippocampal theta. However, others have reported no change in hippocampal theta with SuM inhibition and lesions (26,30), despite remarkable effects with SuM activation (31). Recent work demonstrated that a sub-population of SuM units increase their activity under novel conditions (27). SuM cells from this previous study have several characteristics that overlap with SuMTac1− cells, including the non-uniform axonal innervation pattern and mixed neurotransmitter phenotype in the dentate gyrus (34). In another study, optogenetic inhibition of the SuM affected theta-range spike-timing in SuM-connected structures, but only at the decision point of the maze (26). Thus, SuMTac1− cells may be most influential to hippocampal network patterns in particularly salient situations, with little contribution to mechanisms underlying spontaneous theta. Consistent with this model, SuMTac1− activation caused a considerable shift in firing phase preferences from spontaneously generated theta, highlighting potentially different underlying mechanisms for SuMTac1−-evoked vs. spontaneous theta.
These data also advance our understanding of the SuM’s in role in locomotion and identifies a cell-type with functional properties relevant to spatial navigation. In addition to the tight coupling of SuMTac1+ activity to speed, we found that SuMTac1+ activation robustly drove locomotion while selectively regulating the activity of speed-sensitive hippocampal neurons. These data raise the intriguing potential role for SuMTac1+ neurons in distributing a speed signal throughout its many axon-termination sites and complements recent work outlining a pathway from the MLR to entorhinal cortex via the septum that relays speed (38). Given that SuMTac1+ cells encode future speed, the SuM may provide its synaptic partners with intended speed whereas executed speed is propagated from the MLR. Thus, the SuM may support a role in planning and error correction during locomotion by broadcasting a future speed signal.
Supplementary Material
Acknowledgments:
The authors thank Hiroshi Ito, Edvard Moser, and May-Britt Moser for generously sharing a previously published dataset for reanalysis for the specific purposes of this study and providing comments on the manuscript. The authors also thank Rika Kumar and Sylwia Felong for technical assistance.
Funding:
Canadian Institutes of Health Research postdoctoral fellowship (JSF)
National Institute of Mental Health (NIMH) K99MH11284002 (MLB)
American Epilepsy Society (AES) Junior Investigator Award (FTS)
Stanford Epilepsy Training Grant funded by the National Institute of Neurological Disorders and Stroke (NINDS) 5T32NS007280 (PMK, EH)
Swiss National Science Foundation Postdoctoral Fellowship (TG)
National Science Foundation Fellowship DGE-114747 (BA)
HHMI Gilliam Fellowship for Advanced Study (BA)
Gates Millennium Scholarship (BA)
Japan Society for the Promotion of Science (JSPS) Overseas (ST) Fellowship.
AES Postdoctoral Fellowship (BD)
NINDS K99NS117795 (BD)
National Institute of Health (NIH) 1U19NS104590 (IS, AL, MS).
NIMH 1R01MH124047 and 1R01MH124867 (AL)
Kavli Foundation (AL)
NIH, NSF, Gatsby, Fresenius, and NOMIS Foundations (KD).
Footnotes
Competing interests: M.J.S. is a scientific co-founder of Inscopix. GRIN lenses used in this studied were purchased from Inscopix
Data and materials availability: Code used in this manuscript came from publicly available resources referenced in the Material and Methods. Parts of the raw datasets and additional custom code are openly available at solteszlab.com/datasets and will continue to be formatted and uploaded.
References and Notes
- 1.O’Keefe J, Nadel L, The hippocampus as a cognitive map (Oxford: Clarendon Press, 1978). [Google Scholar]
- 2.Vanderwolf CH, Electroencephalogr. Clin. Neurophysiol. 26, 407–418 (1969). [DOI] [PubMed] [Google Scholar]
- 3.Foster TC, Castro CA, McNaughton BL, Science 244, 1580–1582 (1989). [DOI] [PubMed] [Google Scholar]
- 4.Fuhrmann F, et al. Neuron, 86, 1253–1264 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Colgin LL, Mechanisms and functions of theta rhythms. Annu. Rev. Neurosci. 36, 295–312 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Buzsáki G. Rhythms of the Brain (Oxford University Press, 2006). [Google Scholar]
- 7.O’Keefe J, Recce ML, Hippocampus 3, 317–330 (1993). [DOI] [PubMed] [Google Scholar]
- 8.Skaggs WE, McNaughton BL, Wilson MA, Barnes CA, Hippocampus 6, 149–172 (1996). [DOI] [PubMed] [Google Scholar]
- 9.Pastalkova E, Itskov V, Amarasingham. A, Buzsáki G, Science 321, 1322–1327 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta MR, Lee AK, Wilson MA, Nature 417, 741–746 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Sheeran WM, Ahmed OJ, Neurosci. Biobehav. Rev. 108, 821–833 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munn RG, Mallory CS, Hardcastle K, Chetkovich DM, Giocomo LM, Nat. Neurosci. 23, 239–251 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Escobar JA, Kornienko O, Latuske P, Kohler L, Allen K, K. eLife 23, e16937 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell MG et al. Nat. Neurosci. 21, 1096–1106 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwase M, Kitanishi T, Mizuseki K, Sci. Rep. 10, 1–23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kropff E, Carmichael JE, Moser MB, Moser EI, Nature 523, 419–424 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Justus D, et al. Nat. Neurosci. 20, 16–19 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Hinman JR, Brandon MP, Climer JR, Chapman GW, Hasselmo ME, Neuron 91, 666–679 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green JD, Arduini AA, J. Neurophysiol 17, 533–557 (1954). [DOI] [PubMed] [Google Scholar]
- 20.Dannenberg H, et al. J. Neurosci. 35, 8394–8410 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zutshi I, et al. Current Biology 28, 1179–1188 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirk IJ, McNaughton N, Neuroreport 2, 723–725 (1991). [DOI] [PubMed] [Google Scholar]
- 23.Kirk IJ, Oddie SD, Konopacki J, Bland BH, J. Neurosci 16, 5547–5554 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kocsis B, Vertes RP, J. Neurosci 14, 7040–7052 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen NP, et al. Nat. Comm. 8, 1–16 (2017). [Google Scholar]
- 26.Ito HT, Moser EI, Moser MB, Neuron 99, 576–587 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Chen S, et al. Nature 586, 270–274 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Vertes RP, J. Comp. Neurol. 326, 595–622 (1992). [DOI] [PubMed] [Google Scholar]
- 29.Lee AM, et al. Neuron 83, 455–466 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thinschmidt JS, Kinney GG, Kocsis B, Neuroscience 67, 301–312 (1995). [DOI] [PubMed] [Google Scholar]
- 31.Pan WX, McNaughton N, Prog. Neurobiol. 74:127–66 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Ino T, et al. Neurosci. Lett. 90, 259–264 (1988). [DOI] [PubMed] [Google Scholar]
- 33.Fenno LE, et al. Nat. Methods 11, 763–772 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Billwiller F, et al. Brain Struct. Funct 225, 1–26 (2020).31792694 [Google Scholar]
- 35.Li Y, et al. Neuron 97, 911–924 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Caggiano V, et al. Nature 553, 455–460 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zingg B, et al. Neuron 93, 33–47 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carvalho MM, et al. Cell Rep. 32, 108123 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris JA et al. Front. Neural Circuits 8, 76 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kropff E, Carmichael JE, Moser MB, Moser EI, Neuron 109, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fries P, Reynolds JH, Rorie AE, Desimone R, Science 291, 1560–1563 (2001). [DOI] [PubMed] [Google Scholar]
- 42.Rutishauser U, Ross IB, Mamelak AN, Schuman EM, Nature 464, 903–907 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Siapas AG, Lubenov EV, Wilson MA, Neuron 46,141–51 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Dana H, Nature Methods 16, 649–657 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Chen TW, Nature 499, 295–300 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahn M, et al. Nat. Comm. 9, 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madisen L et al. Neuron 85, 942–958 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaifosh P, Lovett-Barron M, Turi GF, Reardon TR, Losonczy A, Nat. Neurosci. 16, 1182–1184 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Danielson NB, et al. Neuron 90, 101–112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sparks FT, Liao A, Li W, Grosmark A, Soltesz I, Losonczy A, A. Nat. Comm. 11, 1–13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung JE, Neuron 95, 1381–1394 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ting JT, Daigle TL, Chen Q, Feng G, G. “Acute brain slice methods for adult and aging animals: application oftargeted patch clamp analysis and optogenetics” in Patch-Clamp Methods and Protocols, Martina M, Taverna S, Eds (Springer; New York, ed. 2, 2014). [Google Scholar]
- 53.Kaifosh P, Zaremba JD, Danielson NB, Losonczy A, Front. Neuroinform. 8, 80 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pachitariu M, et al. 10.1101/061507v2 (2017). [DOI]
- 55.Freeman J, et al. Nat. Methods 11, 941–950 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Štih V, Petrucco L, Kist AM, Portugues R, PLoS Comput. Biol. 15, e1006699 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lovett-Barron M, et al. Nat. Neurosci. 23, 959–967 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lovett-Barron M et al. Cell, 171: 1411–1423 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.H. M. Choi, et al. Development 145, dev165753 (2018).29945988 [Google Scholar]
- 60.Harris CR, et al. Nature 585, 357–362 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hunter JD, Comput. Sci. Eng. 9, 90–95 (2007). [Google Scholar]
- 62.Virtanen P, et al. Nat. Methods 17, 261–272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pedregosa F, J. Mach. Learn. Res. 12, 2825–2830 (2011). [Google Scholar]
- 64.Robitaille TP et al. Astron. Astrophys. 558, A33 (2013). [Google Scholar]
- 65.Berens P, J. Stat. Softw. 31, 1–21 (2009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.