Abstract
Microbial populations, their distributions, and their aquatic environments were studied over a year (1997) at an acid mine drainage (AMD) site at Iron Mountain, Calif. Populations were quantified by fluorescence in situ hybridizations with group-specific probes. Probes were used for the domains Eucarya, Bacteria, and Archaea and the two species most widely studied and implicated for their role in AMD production, Thiobacillus ferrooxidans and Leptospirillum ferrooxidans. Results show that microbial populations, in relative proportions and absolute numbers, vary spatially and seasonally and correlate with geochemical and physical conditions (pH, temperature, conductivity, and rainfall). Bacterial populations were in the highest proportion (>95%) in January. Conversely, archaeal populations were in the highest proportion in July and September (∼50%) and were virtually absent in the winter. Bacterial and archaeal populations correlated with conductivity and rainfall. High concentrations of dissolved solids, as reflected by high conductivity values (up to 125 mS/cm), occurred in the summer and correlated with high archaeal populations and proportionally lower bacterial populations. Eukaryotes were not detected in January, when total microbial cell numbers were lowest (<105 cells/ml), but eukaryotes increased at low-pH sites (∼0.5) during the remainder of the year. This correlated with decreasing water temperatures (50 to 30°C; January to November) and increasing numbers of prokaryotes (108 to 109 cells/ml). T. ferrooxidans was in highest abundance (>30%) at moderate pHs and temperatures (∼2.5 and 20°C) in sites that were peripheral to primary acid-generating sites and lowest (0 to 5%) at low-pH sites (pH ∼0.5) that were in contact with the ore body. L. ferrooxidans was more widely distributed with respect to geochemical conditions (pH = 0 to 3; 20 to 50°C) but was more abundant at higher temperatures and lower pHs (∼40°C; pH ∼0.5) than T. ferrooxidans.
Acid mine drainage (AMD) has long been recognized to be greatly impacted by microbial activity. Iron-oxidizing chemolithotrophs increase the rate of pyrite oxidation by accelerating the rate-limiting step, the oxidation of Fe2+ to Fe3+ (16). Ferric iron oxidizes pyrite by the following reaction: FeS2(s) + 14Fe3+(aq) + 8H2O(l) → 15Fe2+(aq) + 2SO42−(aq) + 16 H+. In low-pH environments, the ferric iron necessary to drive this reaction is generated primarily by microorganisms because the rate of ferrous iron oxidation is slow (17). Hence, the microbial communities involved in sulfide dissolution and acid generation have received considerable attention for several decades, due to both the environmental pollution that often results and the economic prospects of bioleaching.
To date, laboratory studies of sulfide mineral dissolution and AMD production have largely concentrated on the role of two iron-oxidizing species, Thiobacillus ferrooxidans and, more recently, Leptospirillum ferrooxidans. These organisms are the most readily cultured from acidic drainage waters and have been found to greatly accelerate the rate of pyrite oxidation. However, it is well established that culturing studies, while necessary to assess microbial physiology, are poor indicators of microbial diversity in situ (1, 11). While molecular studies that circumvent culturing biases in AMD environments are not numerous, those that have been conducted thus far largely support the findings of culturing studies: AMD environments support limited microbial diversity, and most species are readily obtained via culturing (5, 6, 12). Most molecular studies of the microbial diversity of AMD environments have been indirect, relying on DNA extraction, PCR amplification, and cloning techniques. While these methods are superior to culturing studies as a means of assessing microbial diversity, they are nonquantitative due to biases associated primarily with DNA extraction and PCR amplification (4, 13, 14, 18). In situ molecular studies of AMD environments with oligonucleotide probes are few (3, 15) and thus far suggest that our understanding of microbial diversity at low pHs is incomplete. Hence, an understanding of the process of pyrite dissolution and AMD formation is limited by our understanding of naturally occurring populations of microorganisms. In particular, the abundance and distribution of the two species (T. ferrooxidans and L. ferrooxidans) most commonly isolated from AMD environments and used for laboratory studies are not well established.
Probing techniques that target whole cells provide quantitative assessments of environmental microbial populations (for examples, see references 3, 7, and 15). We used fluorescence in situ hybridization to evaluate the abundance and distribution of T. ferrooxidans, L. ferrooxidans, Archaea, Bacteria, and Eucarya in an AMD environment as functions of geochemical and environmental conditions. Schrenk et al. have reported probing results for the month of January (15). Here, we report results for samples collected over a 1-year period to determine how populations at this site vary with conditions.
MATERIALS AND METHODS
Study site.
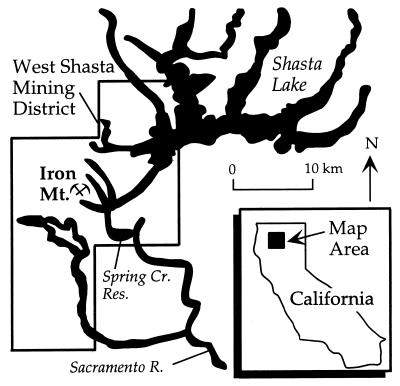
Iron Mountain is located in the West Shasta Mining district near Redding, Calif. (Fig. 1). The site is a massive sulfide deposit in rhyolitic host rock that has been mined for the extraction of gold, zinc, copper, and silver. Mining operations were conducted both underground and in open pits. Underground mining has resulted in over 10 miles of interconnected tunnels. Mining has also resulted in fracturing and overall increased permeability of the solid, unprocessed portions of the deposit. These activities have increased the sulfide mineral surface area and the total reactive ore that is exposed to oxygen and surface water.
FIG. 1.
Location map of Iron Mountain.
Conditions at Iron Mountain such as temperature and pH vary considerably, both spatially and seasonally. Spatially, pH levels as low as −3.5 and temperatures in excess of 60°C in some of the subsurface environments have been reported (8, 9). Drainage streams typically have pH values of 2 to 4 and temperatures of 15 to 20°C. Seasonal variations in geochemical conditions result from alternating wet and dry seasons, during the winter and summer months, respectively. January rainfalls in excess of 35 in. are common, contrasting with the little or no rainfall observed from June through September.
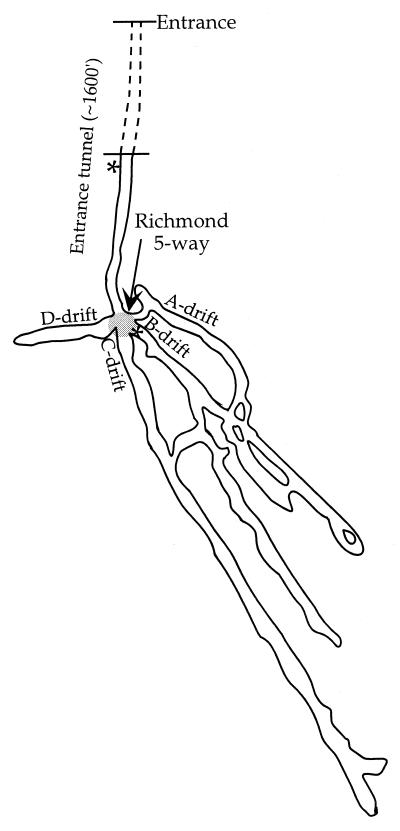
We compared two environments sampled in January, July, September, and November: a high-temperature, low-pH environment and a lower-temperature, higher-pH environment. At lower pH and higher temperatures, we examined a disused mine that is in contact with the pyrite ore body at a subsurface site known as the Richmond five-way. The five-way is located approximately 1,300 ft. into the mountain and is a junction between the entrance tunnel and four tunnels that extend further into the mountain (Fig. 2). Temperatures and pH values vary at the five-way but generally range from 40 to 50°C and 0 to 1, respectively. The representative sampling site for the mine environment is an area of the five-way referred to as “B-drift” (Fig. 2). The second location is characterized by higher pH values and lower temperatures (pH 2 to 4; ∼20°C). For this environment, we focused on streams peripheral to the ore body and drainage in the entrance tunnel to the Richmond five-way mine. For the drainage environment, the designation “tunnel” is used.
FIG. 2.
Schematic map of the Richmond five-way and entrance tunnel. Sampling sites are marked with asterisks.
Environmental measurements and sample collection.
Environments within Iron Mountain mine were sampled in January, July, September, and November 1997. Conductivity and pH measurements were made with an Orion model 126 conductivity meter and an Orion model 290A pH meter with an Orion model 9107 temperature-correcting electrode. Conductivity calibration was performed with a 100-mS/cm reference solution. Reference solutions of pHs 1, 2, and 4 were used for pH meter calibration.
Water, sediment, and slime samples were collected from each environment (see above) in sterile 15-ml Falcon tubes and fixed on-site with 3% paraformaldehyde in phosphate-buffered saline (PBS) solution (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4 [pH 7.4 at 25°C]). Samples were collected from the same vicinity within the Richmond mine during each sampling trip.
Probe design and specificity.
Oligonucleotide probe design, sequences, and stringency specifications have been reported previously (15). Interference of pyrite with the fluorescence in situ hybridization protocol was tested by hybridization to fixed control cultures that had been either spotted to (Escherichia coli DH5α, L. ferrooxidans ATCC 29047, and Aureobasidium pullulans were obtained courtesy of R. Spear, Department of Plant Pathology, University of Wisconsin) or allowed to colonize the mineral surface (T. ferrooxidans ATCC 19859 and Sulfolobus solfataricus were obtained courtesy of A. Tsang, Department of Bacteriology, University of Wisconsin).
Hybridization procedure.
Fixed cells in suspension and pyritic sediment particles were spotted separately at optimal concentrations of 107 to 109 cells/ml (by dilution in 70% ethanol) on gelatin-coated [0.25% gelatin and 0.01% KCr(SO4)2] multiwelled glass slides (10 wells/slide; 10 μl of sample/well) and allowed to dry in a sterile hood. Pyritic sediments were rinsed once in 70% ethanol prior to being spotted onto slides to remove unattached cells and debris. The hybridization procedure followed the protocol of Li et al. (7).
Samples were examined by epifluorescence and light microscopy with a Leica DMRX epifluorescence microscope equipped with an HBO 100-W mercury arc lamp and red, green, and violet dichroic filter cubes. Cells containing DNA in all fixed samples were nonspecifically stained with 4′,6-diamidino-2-phenylindole (1.5 μg/ml; DAPI; Sigma Chemical Co.). Vectashield (Vector Laboratories) was used to prevent photobleaching. Images were captured with a charge-coupled-device camera and NIH Image 1.61 software for the Power Macintosh.
Cell counts were made directly (image analysis software was not used for counting purposes) by averaging the cell numbers obtained in a minimum of 10 fields of view per well from three to six wells. The total number of hybridized cells was estimated by counting the cells that were hybridized with the probes Bac 338, Arch 915, and Euk 502. All data are shown as the proportion of cells within a group (domain or species) relative to the sum of cells hybridized in all domains. Counts were made for both cells in suspension (solutions surrounding sediments) and cells attached to pyritic sediment particles. Calibrations to determine the volume of material represented in a field of view and determinations of the approximate percentage of cells lost during the washing protocols were performed experimentally with control cultures.
RESULTS
Environmental conditions.
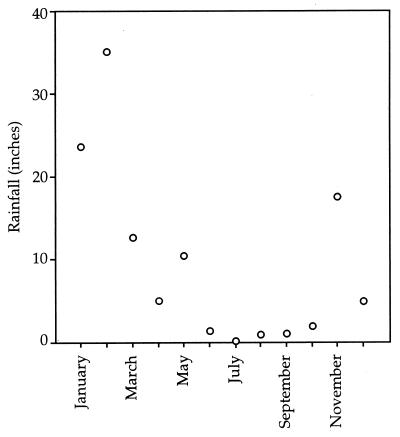
Rainfall records for 1997 were provided by the treatment plant engineers (Stauffer Management Co.) at Iron Mountain and have been plotted in Fig. 3. Rainfall in the winter was seasonally high: the site received more than 20 in. of rain in January and more than 30 in. in February. From February through March, the amount of rainfall was considerably lower, and rainfall was virtually absent from June through September. Rain picked up again in November, and the site received nearly 20 in. of rainfall during that month.
FIG. 3.
Monthly rainfall totals during 1997 at Iron Mountain. Data was provided by Stauffer Management Co. and reflects readings taken at the treatment facility.
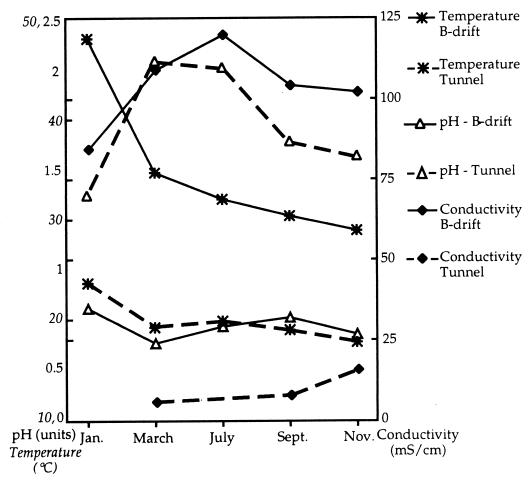
Temperature, conductivity, and pH measurements made in the tunnel and B-drift are shown in Fig. 4. Temperatures decreased in both environments throughout the year, while conductivity rose during the dry summer months and then decreased in the fall. This trend was more pronounced in B-drift than in the tunnel. The pH in B-drift varied by only 0.22 pH unit over the year. In the tunnel, the pH rose almost a unit over the summer and then decreased in the fall.
FIG. 4.
Environmental conditions measured at B-drift and the tunnel during 1997.
Microbial population distribution over time.
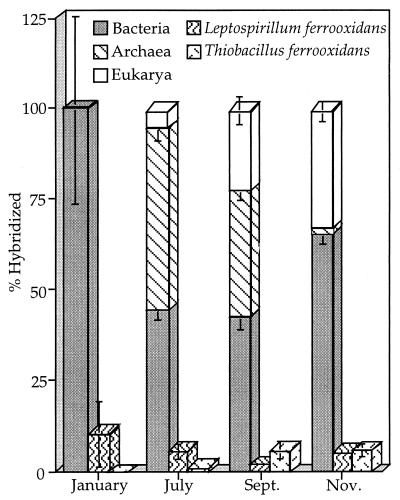
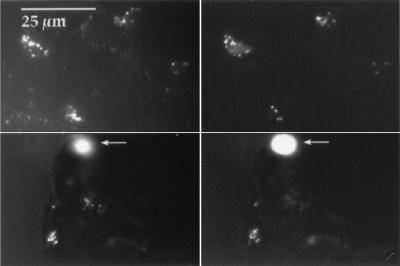
The relative distributions of the three domains, T. ferrooxidans, and L. ferrooxidans over the course of 1997 in B-drift sediments are shown in Fig. 5. Most notable is the initial falling proportion of bacteria followed by a rebound, which is inversely correlated with the rising proportion of archaea followed by a decline, over the course of the year. Examples of hybridized and DAPI-stained archaeal cells from B-drift are shown in Fig. 6.
FIG. 5.
Microbial populations at B-drift for January, July, September, and November 1997. The numbers used to plot the relative proportions of cells represent the sum of cells on surfaces and in suspension associated with the sediments. Cell numbers are normalized to the sum of the domain counts and thus reflect viable cell proportions. Error bars reflect standard errors for counting procedures (see text). Only the lower error bars are shown for stacked data.
FIG. 6.
Image of probing of archaeal cells in B-drift sediments. For both the upper and lower sets of images, the cells on the left are stained with DAPI and viewed under UV fluorescence and the cells on the right are hybridized with the CY 3 archaeal probe. The upper two images show cells in suspension, while the lower two show cells adhering to pyrite sediment surfaces (separated from solution and rinsed with ethanol prior to probing). Arrows point to clusters of cells, stained (left) or hybridized (right), that are out of the plane of focus due to the irregular shape of natural pyrite sediments.
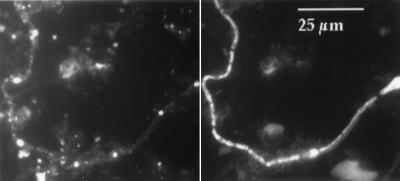
L. ferrooxidans numbers also decreased over the year, while the number of T. ferrooxidans organisms rose from undetectable to very low levels (Fig. 5). Eucarya were absent in January and rose during the dry season. Eukaryotic filaments comprised the matrix of slime streamers that developed after the rainy season in the Richmond five-way. Prokaryotic cells occurred in high numbers within the slime streamers as well. Figure 7 shows hybridized and DAPI-stained eukaryotic filaments from the five-way.
FIG. 7.
Example of eukaryotic filaments at the Richmond five-way. (Left) DAPI-stained cells (viewed under UV); (right) filaments hybridized with the CY 3 eukaryote probe.
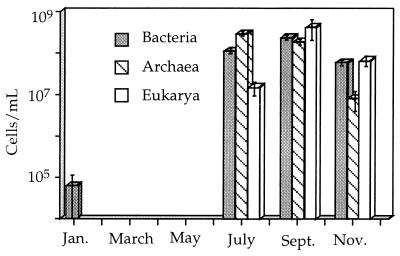
Total cell numbers also varied spatially and seasonally. Cell numbers in free-flowing water were generally low (<105 cells/ml). In slime streamers and within sediments, they were considerably higher (up to 108 to 109 cells/ml). The number of cells per milliliter for bacteria, archaea, and eucarya in B-drift sediments over the course of 1997 are shown in Fig. 8. These numbers reflect the total number of cells that were hybridized in suspension and attached to sediment surfaces.
FIG. 8.
Cells per milliliter for each domain in B-drift sediments over the course of 1997. Error bars reflect standard errors for counting procedures (see text).
Microbial population distribution: mine environment versus drainage.
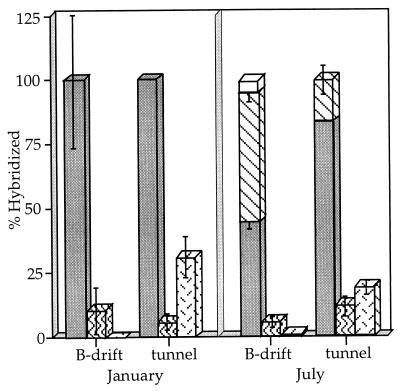
A previous assessment of the microbial distributions associated with different sample types in the tunnel and mine environments (slimes, solutions, and sediments) for January 1997 has been reported (15). In Fig. 9, a comparison between the microbial populations in B-drift and the tunnel, with sample types that dominate the respective environments and contain the highest cell densities, is shown. A comparison between completely analogous sample types was not possible, because pyritic sediments are not present in the tunnel outside of the ore body and slime streamers are completely absent from the five-way for part of the year. Data for B-drift hybridizations are for cells associated with pyritic sediments, while data for tunnel hybridizations are for slime streamers. The microbial populations shown in Fig. 9 are those for January and July 1997, which represent the end members’ environmental conditions (Fig. 4).
FIG. 9.
Comparison of microbial populations at the tunnel and B-drift at the two end-member extremes under environmental conditions represented in Fig. 4. Patterns are the same as were used for Fig. 5. Error bars reflect standard errors for counting procedures (see text). Only the lower error bars are shown for stacked data.
Bacteria dominated both B-drift and the tunnel in January. In the tunnel, T. ferrooxidans was abundant and L. ferrooxidans was present, though in lower numbers. In contrast, B-drift sediments lacked T. ferrooxidans entirely in January and had low numbers of L. ferrooxidans. These general trends were also seen in July. While certain microenvironments, such as slime streamers within the five-way, have been found to contain high numbers of L. ferrooxidans (15), no sites sampled thus far within the lower-pH, higher-temperature environments have been found to contain significant numbers of T. ferrooxidans organisms (2).
Archaea were not detected in January in either environment; their numbers rose to high proportions in July, though more significantly in B-drift than the tunnel. While eucarya increased in B-drift during the summer, they remained undetectable in the tunnel.
DISCUSSION
The abundances of eucarya, bacteria, and archaea show seasonal correlations with geochemical conditions at the Richmond five-way. Spatially, pH influences the prevailing microbial community, as exemplified by the low levels of T. ferrooxidans at sites where the pH is less than 1 (Fig. 9). However, for B-drift and the tunnel, these data do not suggest that the more minor, seasonal changes in pH (Fig. 4) have significant effects on the aspects of the microbial community that we examined. Of the environmental parameters measured in this study, temperature and conductivity have the strongest impacts on the prevailing microbial community. Temperature and conductivity vary as a function of rainfall over the course of the year (Fig. 4), and these trends mirror many of those seen in microbial distributions. Most notably, the occurrence of archaea correlates with the rise in conductivity during the dry summer months, suggesting that the archaeal species at this site have a competitive advantage under high-ionic-strength conditions. While the conditions at the Richmond five-way are extreme relative to those in better-studied AMD environments, more-acidic, higher-temperature, and higher-metal-load sites do exist at Iron Mountain deeper in the tunnels (9). As archaea dominate at the five-way under high-ionic-strength conditions, it is possible that they may dominate year-round at more extreme (and less accessible) sites.
The increase in the eukaryote population in the fall correlates with decreasing temperatures in the mine and with increasing prokaryote populations. While eukaryotes proportionally increase throughout the year, their numbers actually decrease in November (Fig. 8) as prokaryote populations diminish. This is expected, as the decreasing prokaryote population cannot support as much eukaryotic (heterotrophic) biomass.
The low abundance of T. ferrooxidans at the Richmond five-way over the year indicates that this species is not a significant member of the microbial community at acid-generating sites (those in contact with the pyrite ore body). T. ferrooxidans is abundant only in more moderate-pH (1.5 to 2.3), low-temperature environments peripheral to the ore body at Iron Mountain. This is expected, because the pH and temperature conditions at B-drift are outside of this species’ normal growth range (10). In fact, T. ferrooxidans occurs at detectable levels only when the temperature drops to within its growth range; its continued low abundance is likely due to the low-pH conditions that persist. The role that T. ferrooxidans plays in iron oxidation at this site occurs after pyrite dissolution and the subsequent acidification of surface waters. In fact, T. ferrooxidans may play a beneficial role at this site through the oxidation of ferrous to ferric iron in drainage streams, as ferric iron precipitates more readily at higher pHs than ferrous iron. The precipitation of iron plays an important role in the treatment of contaminated drainage, as toxic metals, such as arsenic and cadmium, are adsorbed onto the surfaces of precipitates.
L. ferrooxidans is more abundant in extremely low-pH environments than T. ferrooxidans. However, our sampling has shown that this species is spatially restricted within the five-way. In the samples examined, it was found in highest abundance in association with slime streamers and in suspension within the water column. L. ferrooxidans was found to be in lower abundance within the pyrite sediments and thus may not be the most important acid-generating species at Iron Mountain.
At all sites examined in this study, T. ferrooxidans and L. ferrooxidans comprised less than 50% of the viable microbial population (Fig. 8 and 9). At sites in contact with the ore body, these species comprised less than 25% of the microbial population. Numerous studies have explored the rates and mechanisms of pyrite oxidation by the acidophilic chemolithotrophs T. ferrooxidans and L. ferrooxidans. However, their applicability to natural systems, in which fluctuating communities of microorganisms that may or may not include them exist, is unknown. In this study, we addressed the following questions: what individual microorganisms and groups of microorganisms are active at sites of acid generation, and how do they fluctuate in response to environmental conditions? To our knowledge, this is the first study to quantitatively track microbial population shifts at an AMD site by molecular techniques, to show that these shifts are functions of geochemical and physical parameters. In order to provide the context critical to understanding the community dynamics involved in acid generation, further studies are needed to determine the dominant species in AMD environments as a function of environmental conditions.
ACKNOWLEDGMENTS
We thank Matthew Schrenk for his input to this study and Brett Goebel and Norman Pace for assistance with sample collection. We also thank Iron Mountain Mine Inc. for site access and Stauffer Management Co. for providing assistance and access to records.
Funding was provided by NSF grant CHE-9521731.
REFERENCES
- 1.Amann R I, Ludwig W, Schleifer K-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards, K. J., T. M. Gihring, and J. F. Banfield. Unpublished data.
- 3.Edwards K J, Goebel B M, Rodgers T M, Schrenk M O, Gihring T M, Cardona M M, Hu B, McGuire M M, Hamers R J, Pace N R, Banfield J F. Geomicrobiology of pyrite (FeS2) dissolution: a case study at Iron Mountain, California. Geomicrobiol J. 1999;16:165–179. [Google Scholar]
- 4.Farrelly V, Rainey F A, Stackebrandt E. Effect of genome size and rrn gene copy number on PCR amplification of 16S rRNA genes from a mixture of bacterial species. Appl Environ Microbiol. 1995;61:2798–2801. doi: 10.1128/aem.61.7.2798-2801.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goebel B M, Stackebrandt E. The biotechnological importance of molecular biodiversity studies for metal bioleaching. In: Priest F G, Ramos-Cormenzana A, Tindall B J, editors. Bacterial diversity and systematics. New York, N.Y: Plenum Press; 1994. pp. 259–273. [Google Scholar]
- 6.Goebel B M, Stackebrandt E. Molecular analysis of the microbial biodiversity in a natural acidic environment. In: Jerez C A, Vargas T, Toledo H, Wiertz J V, editors. Biohydrometallurgical processing. Santiago, Chile: University of Chile; 1995. pp. 43–52. [Google Scholar]
- 7.Li S, Spear R N, Andrews J H. Quantitative fluorescence in situ hybridization of Aureobasidium pullulans on microscope slides and leaf surfaces. Appl Environ Microbiol. 1997;63:3261–3267. doi: 10.1128/aem.63.8.3261-3267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordstrom D K. Chemical modeling of acid mine waters in the Western United States. In: Mallard G E, Aronson D E, editors. U.S. Geological Survey Toxic Substances Hydrology Program: proceedings of the technical meeting, 1991. U.S. Reston, Va: Geological Survey; 1994. p. 83. [Google Scholar]
- 9.Nordstrom, D. K., C. N. Alpers, C. J. Ptacek, and D. W. Blowes. Submitted for publication.
- 10.Norris P R. Acidophilic bacteria and their activity in mineral sulfide oxidation. In: Erlich H L, Brierley C, editors. Microbial mineral recovery. New York, N.Y: McGraw-Hill; 1990. pp. 3–27. [Google Scholar]
- 11.Pace R N, Stahl D A, Lane D J, Olsen G J. The analysis of natural microbial population by ribosomal RNA sequences. Adv Microb Ecol. 1986;9:1–55. [Google Scholar]
- 12.Pizarro J, Jedlicki E, Orellana O, Romero J, Espejo R T. Bacterial populations in samples of bioleached copper ore as revealed by analysis of DNA obtained before and after cultivation. Appl Environ Microbiol. 1996;62:1323–1328. doi: 10.1128/aem.62.4.1323-1328.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polz M F, Cavanaugh C M. Bias in template-to-product ratios in multitemplate PCR. Appl Environ Microbiol. 1998;64:3724–3730. doi: 10.1128/aem.64.10.3724-3730.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reysenbach A-L, Giver L J, Wickham G S, Pace N R. Differential amplification of rRNA genes by polymerase chain reaction. Appl Environ Microbiol. 1992;58:3417–3418. doi: 10.1128/aem.58.10.3417-3418.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrenk M O, Edwards K J, Goodman R M, Hamers R J, Banfield J F. Distribution of Thiobacillus ferrooxidans and Leptospirillum ferrooxidans: implications for generation of acid mine drainage. Science. 1998;279:1519–1522. doi: 10.1126/science.279.5356.1519. [DOI] [PubMed] [Google Scholar]
- 16.Singer P C, Stumm W. Acidic mine drainage: the rate-determining step. Science. 1970;167:1121–1123. doi: 10.1126/science.167.3921.1121. [DOI] [PubMed] [Google Scholar]
- 17.Singer P C, Stumm W. Proceedings of the 2nd Symposium on Coal Mine Drainage Research. Pittsburgh, Pa: National Coal Association; 1968. Kinetics of the oxidation of ferrous iron; pp. 12–34. [Google Scholar]
- 18.Suzuki M T, Giovannoni S J. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl Environ Microbiol. 1996;62:625–630. doi: 10.1128/aem.62.2.625-630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]