Abstract
To evaluate the microbial populations involved in the reduction of Fe(III) in an acidic, iron-rich sediment, the anaerobic flow of supplemental carbon and reductant was evaluated in sediment microcosms at the in situ temperature of 12°C. Supplemental glucose and cellobiose stimulated the formation of Fe(II); 42 and 21% of the reducing equivalents that were theoretically obtained from glucose and cellobiose, respectively, were recovered in Fe(II). Likewise, supplemental H2 was consumed by acidic sediments and yielded additional amounts of Fe(II) in a ratio of approximately 1:2. In contrast, supplemental lactate did not stimulate the formation of Fe(II). Supplemental acetate was not consumed and inhibited the formation of Fe(II). Most-probable-number estimates demonstrated that glucose-utilizing acidophilic Fe(III)-reducing bacteria approximated to 1% of the total direct counts of 4′,6-diamidino-2-phenylindole-stained bacteria. From the highest growth-positive dilution of the most-probable-number series at pH 2.3 supplemented with glucose, an isolate, JF-5, that could dissimilate Fe(III) was obtained. JF-5 was an acidophilic, gram-negative, facultative anaerobe that completely oxidized the following substrates via the dissimilation of Fe(III): glucose, fructose, xylose, ethanol, glycerol, malate, glutamate, fumarate, citrate, succinate, and H2. Growth and the reduction of Fe(III) did not occur in the presence of acetate. Cells of JF-5 grown under Fe(III)-reducing conditions formed blebs, i.e., protrusions that were still in contact with the cytoplasmic membrane. Analysis of the 16S rRNA gene sequence of JF-5 demonstrated that it was closely related to an Australian isolate of Acidiphilium cryptum (99.6% sequence similarity), an organism not previously shown to couple the complete oxidation of sugars to the reduction of Fe(III). These collective results indicate that the in situ reduction of Fe(III) in acidic sediments can be mediated by heterotrophic Acidiphilium species that are capable of coupling the reduction of Fe(III) to the complete oxidation of a large variety of substrates including glucose and H2.
Reclamation of surface mining of lignite in wide areas of Germany, Poland, and the Czech Republic has led to the formation of numerous acidic mine lakes (45). These lakes have high concentrations of sulfate and iron due to the oxidation of sulfide-containing minerals of the mine tailings (35). The oxidation of elemental sulfur and Fe(II) by chemolithotrophic acidophilic bacteria, such as species of Thiobacillus and Leptospirillum, has been extensively investigated (21, 40). However, reductive processes [e.g., the dissimilatory reduction of sulfate and Fe(III)] under acidic conditions have received relatively little attention (15, 20, 24, 49).
In pH-neutral aquifers and aquatic and marine sediments, the reduction of Fe(III) is regarded as an important process for the degradation of naturally occurring organic compounds and anthropogenic matter (10, 30). Although the capacity to reduce Fe(III) is widespread among neutrophilic bacteria, fermentative microorganisms do not effectively couple the reduction of Fe(III) to the oxidation of organic compounds (28). In contrast, bacteria that conserve energy via the reduction of Fe(III) utilize short-chain organic acids, H2, or aromatic compounds; such organisms include species of Shewanella, Pelobacter, Geobacter, or Desulfuromonas (27, 28, 37, 40). Acetate is a key intermediate in anaerobic habitats and has been used as a substrate for the isolation of Fe(III)-reducing organisms from sediments and aquifers (9, 12, 31).
High rates of Fe(III) reduction in iron-rich sediments of acidic coal mine lakes were recently reported (6, 16, 38). Under these low-pH conditions, the reduction of Fe(III) might be mediated by Thiobacillus ferrooxidans and Thiobacillus thiooxidans, which can use elemental sulfur as an electron donor (8, 21, 40, 41). Some heterotrophic acidophiles of the genus Acidiphilium also have the capacity to reduce Fe(III) under aerobic or anaerobic conditions (23, 24). To better understand the microbial populations potentially involved in the reduction of Fe(III) in these acidic sediments, (i) the effects of various electron donors on the reduction of Fe(III) were evaluated, (ii) the Fe(III)-reducing microflora was enumerated, and (iii) Fe(III)-reducing microorganisms of these sediments were isolated and characterized.
MATERIALS AND METHODS
Field site and sampling.
Sediments were obtained from an acidic coal mine lake situated in the Lusatian mining area in east central Germany. The pH of the lake water and the maximum summer temperature of the upper sediment approximated to 3 and 12°C, respectively. No oxygen was detected at the water-sediment interface. The upper sediment zone (0 to 4 cm) was enriched with amorphous iron and contained no reduced sulfur components (FeS, FeS2, and S0) (38). Replicate sediment cores were collected in August 1996, February 1997, and August 1998 with a gravity corer in Plexiglas tubes (inner diameter, 5.9 cm), transported to the laboratory, and sectioned under an N2 atmosphere within 24 h. Detailed descriptions of the site and the solid and the pore water phases of the sediment are given elsewhere (38).
Preparation of sediment microcosms.
Sediment from five replicate cores was pooled under anoxic conditions, and 40 g (fresh weight) of the upper iron-rich sediment was transferred to sterile 150-ml infusion bottles (Merck ABS, Dietikon, Switzerland) inside a Mecaplex anaerobic chamber (100% N2 gas phase). Bottles were closed with rubber stoppers and screw-cap seals, flushed with sterile argon for 15 min, and incubated in the dark at 12°C with an initial overpressure of 20 to 25 kPa of argon at room temperature. Substrates were added as sterile stock solutions or as sterile gas.
Enumeration of the sediment microflora.
Numbers of cultured cells were determined by the most-probable-number (MPN) technique with three replicates incubated at 15°C in various media as indicated; MPN values were calculated from standard MPN tables and were within 95% certainty (1). Total bacteria in sediments were enumerated by the 4′,6-diamidino-2-phenylindole (DAPI) procedure (39) as modified for sediment samples (7). The DAPI-stained bacteria were counted by using a Nikon Optiphot microscope equipped with an EF-D episcopic fluorescence attachment (Nippon Kogaku K.K., Tokyo, Japan).
Media and isolation.
For culturing acidophilic Fe(III)-reducing bacteria, an acidic Fe-tryptone soya broth (Fe-TSB) medium (23) was used. The medium contained 0.025% TSB-basal salts at a pH of 2.5 and was supplemented with 5 mM glucose. The medium was boiled, cooled, and dispensed under N2. Ferric sulfate was added from an anoxic 500 mM stock solution (pH 1.7, sterilized by membrane filtration [0.2-μm pore size]) to a concentration of 35 mM, leading to the partial formation of an orange precipitate that might be jarosite (4). The final pH approximated to 2.3. Reduction of Fe(III) was determined visually by the disappearance of the orange precipitate, the complete decolorization of the orange medium, and the formation of a white precipitate and also analytically by measuring the accumulation of Fe(II). For culturing pH-neutral Fe(III)-reducing bacteria, an FePPi medium (9) was used; the medium contained 2.5 g of NaHCO3, 1.5 g of NH4Cl, 0.6 g of KH2PO4, 0.1 g of KCl, and 0.5 g of yeast extract per liter; 10 ml of vitamins (13); and 10 ml of trace metals (13). Soluble ferric pyrophosphate [Fe4(P2O7)3, 3 g liter−1] was added as the electron acceptor, and glucose (5 mM) was added as the electron donor. Alternative supplemental electron donors were cellobiose (3 mM), acetate (5 mM), and H2 (15 mM). The gas phase was N2-CO2 (80:20); the final pH of the medium approximated to 6.7. Reduction of Fe(III) was determined visually by a change of the medium from yellow to colorless and the formation of a white precipitate and also analytically by measuring the accumulation of Fe(II). Enrichment cultures were streaked on solidified media supplemented with either 1% agarose for the acidic Fe-TSB medium or 1.5% agar for the pH-neutral FePPi medium. Isolated colonies were transferred to liquid medium and then sequentially restreaked three times on solidified medium. Cultures were considered to be pure based on uniform colony and cell morphologies. Unless otherwise indicated, isolates were cultivated at 30°C. Growth in media lacking Fe(III) was monitored as optical density at 660 nm with a Spectronic 501 spectrophotometer (Bausch and Lomb, Rochester, N.Y.). Growth of JF-5 in Fe-TSB medium was monitored by direct counting of cells in a Thoma chamber.
Analytical techniques.
The rate of Fe(III) reduction by sediments was estimated by determining the amount of Fe(II) formed (44). Aliquots (0.2 ml) of the sediment were taken with sterile syringes, transferred to 9.8 ml of 0.5 N HCl, and incubated for 1 h at room temperature (29). Fe(II) was measured after the addition of acetate by the phenanthroline method (47). Total iron was determined with an inductively coupled plasma optical emission spectrometer (GBC Scientific Equipment, Melbourne, Victoria, Australia); Fe(III) was calculated as the difference between Fe(II) and total iron.
Headspace gases (H2, O2, CO2, and CH4) were measured with Hewlett-Packard Co. (Palo Alto, Calif.) 5980 series II gas chromatographs (26). Gas values were estimated by Henry’s law and included the total amounts in both the liquid and the gas phases. To compare the amount of a specific gas (millimoles per bottle or tube filled with variable amounts of water depending on the experiment) with the concentration of a substrate consumed or of Fe(II) formed, the total amount of the gas for 1,000 ml of water to achieve a millimolar concentration was calculated. In this study, no distinction was made between CO2 and its carbonate forms. Concentrations were corrected for the changing liquid-to-gas-phase volume ratio due to liquid samplings (0.7 ml). Aliphatic acids, aromatic compounds, alcohols, and sugars were determined with Hewlett-Packard 1090 series II high-performance liquid chromatographs (26). Nitrate and sulfate were analyzed by ion chromatography (26). Sediment pH was measured with an Ingold U457-S7/110 combination pH electrode.
Electron microscopy.
Cells of JF-5 were cultivated at 30°C either anaerobically in Fe-TSB medium supplemented with glucose and various concentrations of Fe(III) (10, 35, and 65 mM) or aerobically in TSB medium lacking Fe(III) and supplemented with glucose. Cells were fixed by adding glutaraldehyde to a final concentration of 2% (vol/vol) and harvested by centrifugation. For negative staining (50), aqueous solutions of neutralized phosphotungstic acid (1% [wt/vol]) were used for visualizing the blebs on the cell periphery, whereas uranyl acetate (2% [wt/vol], pH 4.5) was adequate for visualizing the detached blebs. For thin-section preparations, pelleted cells were embedded in agar (2% [wt/vol]) and fixed in glutaraldehyde-OsO4 (48). After being stained for 7 min with 2% uranyl acetate and, subsequently, for 5 min with lead citrate (43), specimens were examined in a model CEM 902A microscope (Zeiss, Oberkochen, Germany).
DNA isolation and determination of G+C content of DNA.
The DNA was isolated by standard methods (11). The G+C content of the DNA was determined by high-performance liquid chromatography (34).
16S rRNA gene sequencing and phylogenetic analysis.
Genomic DNA was extracted, amplified by PCR, and purified (42). Purified PCR products were sequenced by using an ABI PRISM Ready Reaction dye terminator kit (Applied Biosystems, Foster City, Calif.). Sequence reaction mixtures were electrophoresed with an Applied Biosystems model 373A DNA sequencer. Alignments of the sequence were done manually, and determinations of similarity values were done by using the ae2 editor (33). Accession numbers for sequences used for comparison of JF-5 with the closest relatives are as follows: Acidiphilium cryptum ATCC 33463T, D30773 (25); A. cryptum B-Het4, X75265 (17); and Acidiphilium organovorum ATCC 443141T, D30775 (25). The accession number of the sequence of the closest relative of CH-1 (Clostridium butyricum MMP3 DSM 2478) is X68177.
Nucleotide sequence accession number.
The 16S rRNA gene sequence of strain JF-5 has been deposited in the EMBL database under accession no. Y18446. The accession numbers of A. cryptum DSM 2389T and DSM 9467 are Y18445 and Y18447, respectively.
RESULTS
Effects of supplemental electron donors on the formation of Fe(II) in acidic sediments.
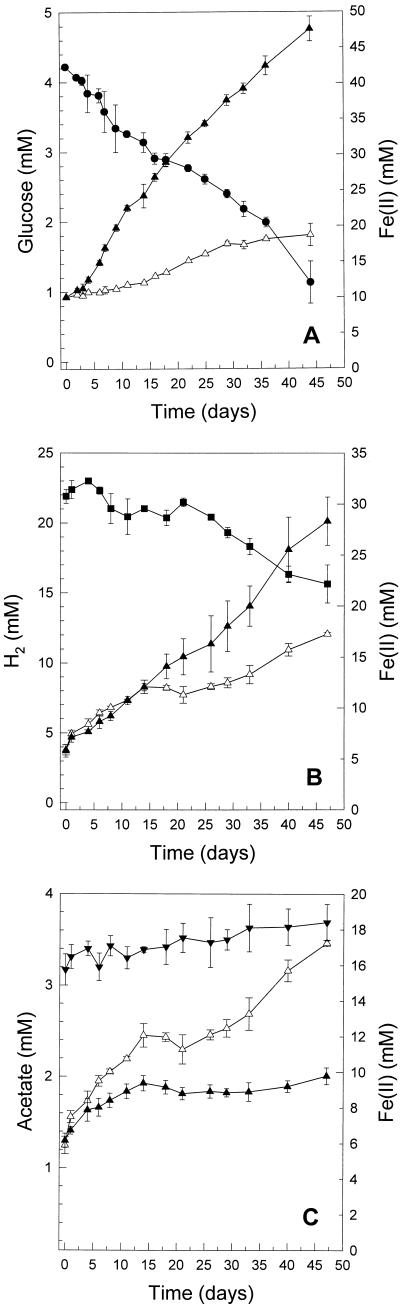
In unsupplemented sediment microcosms, the concentration of Fe(II) increased with time at a rate of 232 μmol liter−1 day−1. Since nitrate was negligible, and since sulfate (20 mM) was not consumed and methane was not produced, the reduction of Fe(III) appeared to be the main terminal electron-accepting process that was coupled to the oxidation of naturally occurring electron donors. In glucose-supplemented sediment microcosms, glucose was consumed without apparent delay and stimulated the formation of Fe(II) and CO2 (Fig. 1A and data not shown). No fermentation products (e.g., short-chain organic acids, alcohols, or H2) were detected during the consumption of glucose. Compared to controls lacking glucose, the production of CO2 was enhanced from 5.3 to 12.8 mM. Forty-two percent of the reducing equivalents that were theoretically obtained from glucose were recovered in Fe(II). The pH of the sediment increased from 3.2 to 5.8 during the time in which glucose was consumed; in contrast, the pH of the control increased only slightly, from 3.2 to 3.6. In microcosms supplemented with cellobiose (2.8 mM), 21% of the reducing equivalents theoretically obtained from cellobiose were recovered in Fe(II). Cellobiose was initially converted to glucose. During the subsequent consumption of glucose, lactate, acetate, and propionate were detected in low concentrations (0.5, 1.9, and 1.2 mM, respectively) and the pH increased to 5.7.
FIG. 1.
Effect of supplemental electron donors on the formation of Fe(II). Sediment was supplemented with glucose (A), H2 (B), or acetate (C) and incubated under an argon gas phase at 12°C. Presented are the averages (± standard deviations) of triplicate microcosms. Symbols: ●, glucose; ■, H2; ▾, acetate; ▴, Fe(II); ▵, Fe(II) control.
When H2 was added to sediment microcosms, H2 was consumed concomitantly with the formation of Fe(II) (Fig. 1B). Sulfate was not consumed, and neither acetate nor methane was detected (data not shown). Approximately 93% of the reducing equivalents theoretically obtained from H2 were recovered in Fe(II). The pH increased from 3.2 to 3.8. In microcosms supplemented with lactate (2 mM), small amounts of lactate were consumed (0.7 mM) and yielded acetate (0.4 mM) (data not shown). The formation of Fe(II) was not stimulated by lactate. In the presence of supplemental acetate, the formation of Fe(II) increased with a lower rate during the first 12 days of incubation than that of unsupplemented controls and then ceased completely (Fig. 1C). Acetate was not consumed over an incubation period of 140 days, and the final pH remained relatively stable at 3.3.
Enumeration of Fe(III) reducers.
The above findings indicated that these acidic sediments contained microorganisms capable of coupling the reduction of Fe(III) to the oxidation of glucose and H2. Since H2 is a known substrate for neutrophilic Fe(III)-reducing microorganisms (27), Fe(III) reducers were enumerated both under acidic and under pH-neutral conditions. The cultured number of microorganisms capable of Fe(III) reduction with glucose as the electron donor approximated 4 × 103 (g [wet weight] of sediment−1) in acidic Fe-TSB medium compared to 2.3 × 103 (g [wet weight] of sediment−1) in pH-neutral FePPi medium (Table 1). In acidic Fe-TSB medium, supplemental glucose was partially consumed (up to 2 mM) and no fermentation products were detected. Fe(III) was totally reduced to Fe(II). In pH-neutral FePPi medium, the complete consumption of supplemental glucose (5 mM) yielded formate, acetate, propionate, and butyrate (2.0, 6.8, 1.6, and 1.3 mM, respectively), and approximately 3 mM Fe(II) was formed. The numbers of cultured acetate- or H2-utilizing Fe(III) reducers in pH-neutral FePPi medium were negligible (Table 1). In FePPi medium, supplemental acetate was not consumed. Supplemental H2 was consumed in FePPi medium; however, its consumption was coincident with the production of acetate. The average H2-to-acetate ratio in all positive dilutions approximated to 4.3:1, a value indicative of H2-dependent acetogenesis (13). The total number of DAPI-stained microbes in the sediment approximated to 7.2 × 105 (g [wet weight] of sediment−1).
TABLE 1.
MPN values of Fe(III)-reducing microbes and total counts of DAPI-stained microbes obtained from acidic sedimentsa
| Metabolic type | Medium | pH | MPN (g [wet wt] of sediment−1)b |
|---|---|---|---|
| Glucose-utilizing Fe(III) reducer | Fe-TSB | 2.3 | 4 × 103 (8.6 × 102–1.9 × 104) |
| Glucose-utilizing Fe(III) reducer | FePPi | 6.7 | 2.3 × 103 (4.9 × 102–1.1 × 104) |
| H2-utilizing Fe(III) reducer | FePPi | 6.7 | <3 |
| Acetate-utilizing Fe(III) reducer | FePPi | 6.7 | <3 |
| Total DAPI-stained microbes | NAc | NA | 7.2 × 105 (5.8 × 105–8.6 × 105) |
MPN dilutions were incubated in three replicates at 15°C for 9 months.
Values in parentheses represent the ranges of the MPN values within 95% certainty.
NA, not applicable.
Isolation of JF-5.
Repeated transfers from the highest growth-positive dilution of the MPN series in acidic Fe-TSB medium supplemented with glucose yielded a stable, Fe(III)-reducing, glucose-utilizing enrichment culture. No fermentation products were detected. On solidified Fe-TSB medium incubated under an N2 gas phase, colonies appeared after 8 weeks simultaneously with a complete decolorization of the orange plates. Colonies were shiny, convex, and white with a slightly beige center and had a maximum diameter of 2 mm. One representative strain, JF-5, was selected for further characterization.
Morphological and physiological characteristics of JF-5.
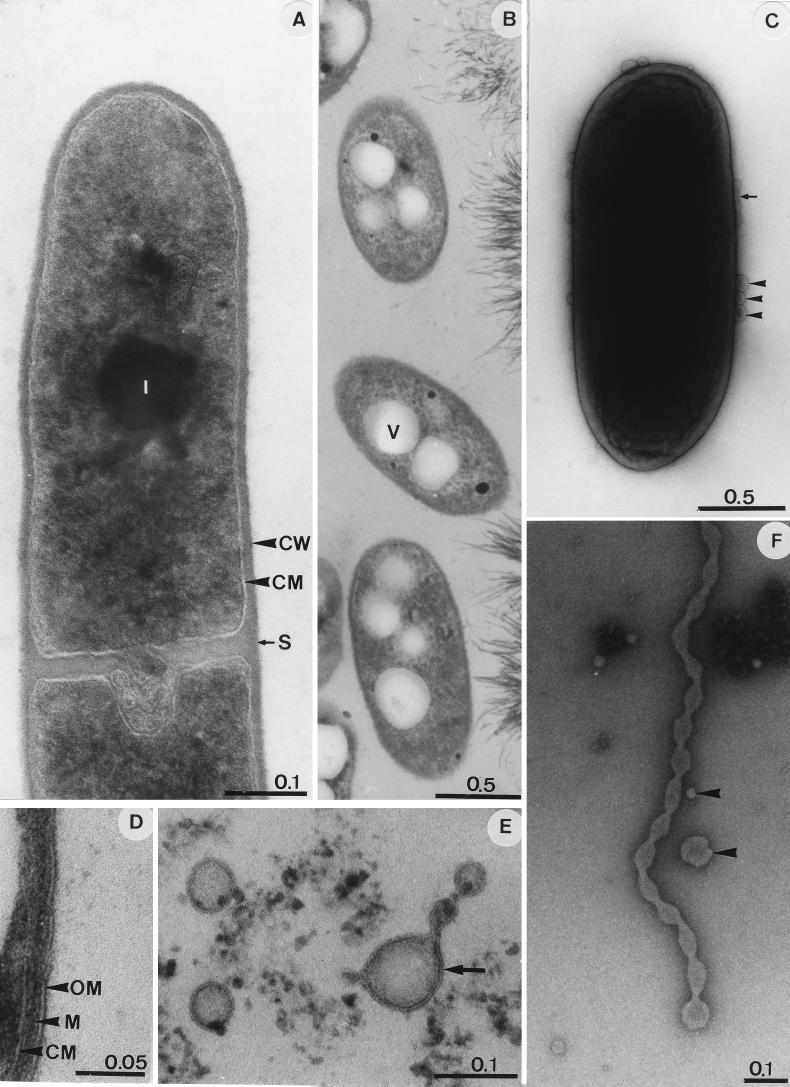
Cells of JF-5 were facultative anaerobic, motile, gram-negative (Fig. 2D), short rods (Fig. 2C). Electron microscopy revealed peritrichous inserted flagella. JF-5 did not grow aerobically on solidified glucose-supplemented TSB medium lacking Fe(III). In liquid glucose-supplemented aerobic TSB medium lacking Fe(III), growth appeared after a lag phase of 3 to 7 days. In general, the cultivation conditions in TSB medium appeared to favor growth under Fe(III)-reducing conditions. However, growth occurred rapidly and without a lag phase in an aerobic basal mineral salt solution supplemented with yeast extract (0.3 g liter−1) (19). Neither growth nor the utilization of glucose was observed in anaerobic glucose-supplemented TSB medium lacking Fe(III). JF-5 grew at temperatures ranging from 12 to 37°C; no growth was observed at 5, 10, or 40°C. The optimal temperature was 35°C. Although no spores could be detected, growth and Fe(III) reduction were observed after heating cultures for 15 min at 80°C and for 5 min at 95°C. Under aerobic conditions, growth was observed over a pH range of 2.1 to 5.8; the pH optimum was 3.2.
FIG. 2.
Electron micrographs of strain JF-5 grown in aerobic TSB medium lacking Fe(III) (A) or in Fe-TSB medium (B to F). (A, B, and D to F) Thin-section micrographs. (C) Whole cell, negatively stained with 2% phosphotungstate. Single blebs (arrowheads) and a short “chain” consisting of four blebs (small arrow) are visible on the cell periphery. (D) Cell envelope of JF-5 with features of a gram-negative cell. (E) Micrograph showing that blebs and extrusions are membrane surrounded (arrow). (F) The long extrusion which originates from the cytoplasmic membrane reveals constrictions. Arrowheads indicate single free blebs of different sizes. Bar lengths are shown in micrometers. Abbreviations: CM, cytoplasmic membrane; CW, cell wall; I, inclusion body; S, septum (division point); M, murein layer; OM, outer membrane; V, vesicle.
Cells grown in Fe-TSB medium contained large intracellular vesicles (Fig. 2B); in comparison, cells grown in aerobic TSB medium lacking Fe(III) had a dense cytoplasm (Fig. 2A). Cells grown in Fe-TSB medium had blebs (i.e., protrusions still in contact with the cytoplasmic membrane [46]) on the cell surface (Fig. 2C). Over 80% of the cells examined formed blebs. In contrast, cells grown in aerobic TSB medium lacking Fe(III) rarely formed blebs. The number of bleb-forming cells in Fe-TSB medium was independent of the amount of Fe(III) added (data not shown). In thin sections, projections and detached vesicles were also detected (Fig. 2F). Both the projections and detached vesicles were enclosed by a membrane (Fig. 2E). The morphological continuity between the cytoplasmic membrane, the blebs, and projections indicated that the membranes of the blebs and projections originated from the cytoplasmic membrane.
Oxidation of organic compounds coupled to the reduction of Fe(III) by JF-5.
Substrate utilization and the reduction of Fe(III) by JF-5 were observed with the following substrates (in Fe-TSB medium): glucose, fructose, xylose, ethanol, glycerol, malate, glutamate, fumarate, citrate, and H2. No fermentation products were detected. No Fe(III) reduction or substrate utilization was observed with lactose, cellobiose, lactate, formate, pyruvate, acetate, vanillate, or benzoate. JF-5 could not respire even low amounts of acetate (300 μM). In the presence of acetate (5 mM; 300 μM), growth and the reduction of Fe(III) were inhibited in glucose-, ethanol-, and H2-supplemented cultures. In aerobic TSB medium lacking Fe(III), growth and substrate utilization were observed with glucose, glycerol, citrate, succinate, and malate but not with lactose, glutamate, fumarate, ethanol, and H2. In aerobic TSB medium supplemented with nitrate (5 mM) and glucose, substrate utilization or growth was not observed.
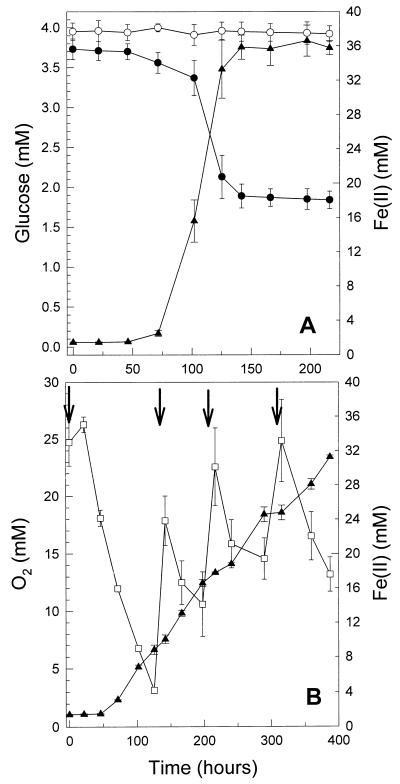
In Fe-TSB medium supplemented with glucose, the consumption of 1.8 mM glucose was concomitant with the reduction of approximately 35 mM Fe(III) (Fig. 3A) and yielded an increase in cell numbers from 1.2 × 107 to 2.6 × 108 cells ml−1, indicating that growth-supportive energy was conserved. Glucose oxidation, CO2 formation, and Fe(III) reduction approximated the following stoichiometry: C6H12O6 + 24Fe(III) + 24OH− → 6CO2 + 24Fe(II) + 18H2O (Table 2). In aerobic Fe-TSB medium supplemented with glucose, the formation of Fe(II) was concomitant with the consumption of oxygen (Fig. 3B), indicating that oxygen and Fe(III) were corespired.
FIG. 3.
Formation of Fe(II) and consumption of glucose by strain JF-5 incubated in Fe-TSB medium (A) and formation of Fe(II) and consumption of O2 by strain JF-5 incubated in Fe-TSB medium supplemented with O2 (B). O2 and glucose were added at the indicated time intervals (arrows). Presented are the averages (± standard deviations) of triplicate experiments. Symbols: ●, glucose; ○, glucose-supplemented TSB medium lacking Fe(III); ▴, Fe(II); □, O2.
TABLE 2.
Carbon and reductant balances obtained from glucose cultures of isolate JF-5a
| Culture no. | Fe(III) added (mM) | Fe(II) formedb (mM) | Glucose consumed (mM) | CO2 produced (mM) | Reductant obtained (mM) | Carbon recovered (%) | Reductant recovered (%) |
|---|---|---|---|---|---|---|---|
| 1 | 65.2 ± 2.8 | 66.0 ± 1.3 | 2.7 ± 0.1 | 16.9 ± 0.8 | 64.8 | 104.3 | 101.9 |
| 2 | 35.0 ± 1.5 | 34.0 ± 0.8 | 1.6 ± 0.3 | 10.3 ± 1.3 | 38.4 | 107.3 | 88.5 |
| 3 | 10.0 ± 1.7 | 12.0 ± 1.1 | 0.6 ± 0.2 | 4.2 ± 0.9 | 14.4 | 116.7 | 83.3 |
JF-5 was cultivated in triplicate in Fe-TSB medium supplemented with 4.0 mM glucose and various concentrations of Fe(III) (as indicated).
Concentrations of Fe(II) were corrected for the concentration of Fe(II) present at the beginning of the experiment and for the concentration of Fe(II) formed in controls lacking glucose.
Phylogenetic analysis of the 16S rRNA gene and G+C content of JF-5.
Phylogenetic analysis of the almost complete 16S rRNA gene sequence (93.3% of the Escherichia coli sequence) with the database of 16S rRNA gene sequences (33) indicated that JF-5 is a member of the alpha subclass of the Proteobacteria. The highest sequence similarity value (99.6%) was to an Australian isolate of A. cryptum (strain B-Het4 [17]). A sequence similarity value of 99.2% was obtained with the sequence of the type strain of A. organovorum, ATCC 43141T. The presence of almost identical 16S rRNA gene sequences in the type strains of A. cryptum and A. organovorum has been reported previously (25). The DNA base composition of strain JF-5 was 68.3 mol% G+C. The G+C content of the type strain of A. cryptum, ATCC 33463T, is 69.8 mol% (19).
Enrichment and isolation of CH-1.
Since sugar-fermenting microorganisms were present and possibly involved in the reduction of Fe(III) in acidic sediments, a stable cellobiose-fermenting, Fe(III)-reducing culture was enriched in pH-neutral FePPi medium. Isolate CH-1 was obtained on solidified FePPi medium. Colonies were surrounded by a decolorized zone and were white, smooth, irregular, and convex with a diameter of 3 to 4 mm. CH-1 was a strictly anaerobic, gram-labile, spore-forming rod (3 to 6 by 0.6 μm). Cellobiose (4.0 mM) was fermented to formate, acetate, butyrate, and H2 (7.3, 3.3, 6.0, and 4.1 mM, respectively). Concomitant with the consumption of cellobiose, 4.4 mM Fe(II) was formed, indicating that approximately 2.3% of the cellobiose-derived reducing equivalents were utilized in the reduction of Fe(III). In the absence of cellobiose, no Fe(II) was formed. The reduction of Fe(III) was also coupled to the fermentation of glucose. CH-1 was able to grow at pH 4.4 but not at pH 3.9. Phylogenetic analysis of the 16S rRNA gene sequence indicated that CH-1 was 100.0% identical to C. butyricum MMP3 DSM 2478.
DISCUSSION
The geochemistry of this acidic sediment differs from that of other sedimentary environments in which Fe(III) reduction is a main terminal electron-accepting process (29, 30, 44). In these acidic coal mine lakes, Fe(III) is sedimented permanently at high rates, yielding an orange, fluffy sediment zone of reactive amorphous Fe(III)-(hydr)oxide which consists mainly of schwertmannite (38). Schwertmannite is a poorly crystallized Fe(III)-oxyhydroxysulfate (5) that can be formed in iron- and sulfate-rich waters (3, 4). Since the reactivity of Fe(III)-(hydr)oxides as electron acceptors for microbial processes is linked to their degree of crystallinity (29, 36, 40), schwertmannite might be easily degraded due to its amorphous structure. Sediments that contain high amounts of crystalline Fe(III)-(hydr)oxides are readily depleted of microbially reducible Fe(III) (29, 44). The linear rate of Fe(II) formation by sediments during the 44-day incubation period (Fig. 1A) implies that the availability of reducible Fe(III) was not a limiting factor. However, under in situ conditions, electron donors might be limited, because only very low quantities of autochthonous organic matter reach the water-sediment interface (38).
In most pH-neutral sedimentary Fe(III)-reducing environments, H2 and acetate are expected to be major intermediates in the oxidation of fermentable organic substrates (12). Geobacter and Shewanella species which can metabolize these substrates and can be easily isolated from those sediments are thought to be important catalysts of Fe(III) reduction in sedimentary environments (12, 37). In sediment microcosms, supplemental H2 stimulated the formation of Fe(II) in a 1:1.9 ratio (Fig. 1B). This ratio approximates the following stoichiometry: H2 + 2Fe(III) → 2H+ + 2Fe(II), indicating that the oxidation of H2 was completely coupled to the reduction of Fe(III). Isolate JF-5 was obtained from the highest growth-positive dilution of the MPN series on the sediment and was capable of H2 utilization at in situ pH. The number of cultured neutrophilic H2-utilizing Fe(III) reducers was negligible. The capacity of mesophilic acidophiles to oxidize H2 under aerobic conditions is also known for T. ferrooxidans (14).
Supplemental acetate was not consumed in sediment microcosms; indeed, acetate appeared to inhibit the formation of Fe(II) (Fig. 1C). Even low concentrations of acetate also inhibited growth and the reduction of Fe(III) by JF-5. The inhibitory effect of acetic acid on growth of acidophilic bacteria has been previously described (19). Under pH-neutral conditions in FePPi medium, supplemental acetate also did not stimulate the reduction of Fe(III) in sediment MPN dilutions (Table 1). Thus, acetate does not appear to be an important electron donor for the reduction of Fe(III) in these acidic sediments. Lactate, a substrate for many Fe(III)-reducing organisms (27, 37), also did not stimulate the formation of Fe(II) in sediment microcosms and was not utilized by JF-5. These collective results indicate that the Fe(III)-reducing microorganisms present in this acidic sediment are distinct from most well-described neutrophilic nonfermentative Fe(III) reducers.
Fermentable substrates like glucose or cellobiose were consumed in sediment microcosms and stimulated the formation of Fe(II) (Fig. 1A and data not shown). Many sugar-fermenting microorganisms are capable of the reduction of Fe(III) (28). However, the amounts of reducing equivalents usually recovered in Fe(II) are in a range of 0.03 to 3%, demonstrating that the reduction of Fe(III) is only a minor pathway for these microorganisms (28). The fermentative isolate CH-1 transferred approximately 2.3% of the reducing equivalents obtained from cellobiose or glucose to Fe(III). The 16S rRNA gene sequence of CH-1 was identical to that of C. butyricum, which is known to have the capacity to reduce Fe(III) (18). In sediment microcosms, however, high amounts of reducing equivalents obtained from glucose were recovered in Fe(II), suggesting that nonfermentative processes were also involved in the reduction of Fe(III). This observation is contradictory to other studies on glucose metabolism in Fe(III)-reducing sediments that demonstrate that sugars are utilized in a microbial food chain by the combined activity of fermentative bacteria and fatty acid-oxidizing Fe(III)-reducing bacteria (28, 32). To date, the complete oxidation of sugars to CO2 with Fe(III) as the sole electron acceptor by a pure culture has not been clearly demonstrated (23, 28). The nonfermentative isolate JF-5 readily reduced Fe(III) via the oxidation of glucose, with 83 to 102% of the glucose-derived reducing equivalents being recovered in Fe(II) (Table 2).
On the basis of the 16S rRNA gene sequence similarity, JF-5 was closely related to an Australian isolate of A. cryptum that was isolated from an acidic leaching environment (17). In general, species of Acidiphilium are described as being obligate aerobes; however, some isolates of Acidiphilium, including A. cryptum, have the capacity to reduce Fe(III) under aerobic or microaerophilic conditions (21–23, 40). In the absence of oxygen, the growth of Acidiphilium strain SJH is coupled to the reduction of Fe(III) in glucose-TSB liquid medium; however, stoichiometries for glucose consumption and Fe(III) reduction for this strain have not been determined (23). Indeed, the general capacity of Acidiphilium species to completely oxidize organic substrates to CO2 via the reduction of Fe(III) has not been adequately resolved.
In contrast to A. cryptum and other species of acidophilic heterotrophs that are heat sensitive (19, 23), JF-5 grew after pasteurization without delay and could survive boiling. The formation of blebs by JF-5 (Fig. 2C) has not been described for A. cryptum (19). In general, blebs that originate from the cytoplasmic membrane have not been observed in other gram-negative bacteria (46). The formation of numerous blebs and the appearance of intracellular vesicles under Fe(III)-reducing conditions appear to be characteristic features of JF-5. The function of these blebs is not resolved. It can be speculated that an enlargement of a cytoplasmic membrane might enhance cellular contact in order for the cell to get into contact with nonsoluble Fe(III) hydroxides that are utilized as electron acceptors. The capacity of JF-5 and other Acidiphilium species to corespire O2 and Fe(III) (23) is dissimilar to that of other facultative Fe(III)-reducing organisms (e.g., Shewanella species) that do not significantly reduce Fe(III) until O2 is completely removed (2). Under acidic conditions, substantial amounts of dissolved Fe(III) are available in solution, and the redox potential for the reduction of soluble Fe(III) to soluble Fe(II) (+0.77 V) is close to the redox potential for the reduction of O2 to H2O (+0.82 V) (28, 37). Shewanella does not grow at low pH values, and the redox potential for the reduction of solid Fe(III)-(hydr)oxides is much lower than that of soluble Fe(III). Thus, the small difference in these redox potentials might facilitate the use of soluble Fe(III) as an alternative electron acceptor even in the presence of the energetically more favorable O2. The capacity of Acidiphilium species to utilize a variety of substrates and to reduce Fe(III) both in the presence and in the absence of oxygen indicates that they might be of ecological significance in the turnover of iron at oxic-anoxic interfaces in acidic sediments.
ACKNOWLEDGMENTS
We express sincere appreciation to Christine Nohlen for assistance in obtaining the sediments, to Bettina Popp from Central Analytics for the determination of the total iron content, to Rita Grotjahn and Rita Schineis for preparation of the electron micrographs, to Carola Matthies and Ariane Peine for helpful discussions, and to Harold L. Drake for his support and critical review of the manuscript.
This study was supported by the German Ministry for Education, Research, Science, and Technology (BMBF).
REFERENCES
- 1.Alef K. Methodenhandbuch Bodenmikrobiologie: Aktivitäten, Biomasse, Differenzierung. Landsberg/Lech, Germany: Ecomed; 1991. pp. 44–49. [Google Scholar]
- 2.Arnold R G, Hoffmann M R, DiChristina T J, Picardal F W. Regulation of dissimilatory Fe(III) reduction activity in Shewanella putrefaciens. Appl Environ Microbiol. 1990;56:2811–2817. doi: 10.1128/aem.56.9.2811-2817.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bigham J M, Carlson L, Murad E. Schwertmannite, a new iron oxyhydroxysulphate from Pyhäsalmi, Finland, and other localities. Mineralogical Magazine. 1994;58:641–648. [Google Scholar]
- 4.Bigham J M, Schwertmann U, Pfab G. Influence of pH on mineral speciation in a bioreactor simulating acid mine drainage. Appl Geochem. 1996;11:845–849. [Google Scholar]
- 5.Bigham J M, Schwertmann U, Carlson L, Murad E. A poorly crystallized oxyhydroxysulfate of iron formed by bacterial oxidation of Fe(II) in acid mine waters. Geochim Cosmochim Acta. 1990;54:2743–2758. [Google Scholar]
- 6.Blodau C, Hoffmann S, Peine A, Peiffer S. Iron and sulfate reduction in the sediments of acidic mine lake 116 (Brandenburg, Germany): rates and geochemical evaluation. Water Air Soil Pollut. 1998;108:249–270. [Google Scholar]
- 7.Bott T L, Kaplan L A. Bacterial biomass, metabolic state, and activity in stream sediments, in relation to environmental variables and multiple assay comparisons. Appl Environ Microbiol. 1985;50:508–522. doi: 10.1128/aem.50.2.508-522.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brock T D, Gustafson J. Ferric iron reduction by sulfur- and iron-oxidizing bacteria. Appl Environ Microbiol. 1976;32:567–571. doi: 10.1128/aem.32.4.567-571.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caccavo F, Jr, Lonergan D J, Lovley D R, Davis M, Stolz J F, McInerney M J. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microbiol. 1994;60:3752–3759. doi: 10.1128/aem.60.10.3752-3759.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canfield D E, Thamdrup B, Hansen J E. The anaerobic degradation of organic matter in Danish coastal sediments: iron reduction, manganese reduction, and sulfate reduction. Geochim Cosmochim Acta. 1993;57:3867–3883. doi: 10.1016/0016-7037(93)90340-3. [DOI] [PubMed] [Google Scholar]
- 11.Cashion P, Holder-Franklin M A, McCully J, Franklin M. A rapid method for the base ratio determination of bacterial DNA. Anal Biochem. 1977;81:461–466. doi: 10.1016/0003-2697(77)90720-5. [DOI] [PubMed] [Google Scholar]
- 12.Coates J D, Phillips E J P, Lonergan D J, Jenter H, Lovley D R. Isolation of Geobacter species from diverse sedimentary environments. Appl Environ Microbiol. 1996;62:1531–1536. doi: 10.1128/aem.62.5.1531-1536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drake H L. Acetogenesis, acetogenic bacteria, and the acetyl-CoA “Wood/Ljungdahl” pathway: past and current perspectives. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall, Inc.; 1994. pp. 3–60. [Google Scholar]
- 14.Drobner E, Huber H, Stetter K O. Thiobacillus ferrooxidans, a facultative hydrogen oxidizer. Appl Environ Microbiol. 1990;56:2922–2923. doi: 10.1128/aem.56.9.2922-2923.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortin D, Davis B, Beveridge T J. Role of Thiobacillus and sulfate-reducing bacteria in iron biocycling in oxic and acidic mine tailings. FEMS Microbiol Ecol. 1996;21:11–24. [Google Scholar]
- 16.Friese K, Wendt-Potthoff K, Zachmann D W, Fauville A, Mayer B, Veizer J. Biogeochemistry of iron and sulfur in sediments of an acidic mining lake in Lusatia, Germany. Water Air Soil Pollut. 1998;108:231–247. [Google Scholar]
- 17.Goebel B M, Stackebrandt E. Cultural and phylogenetic analysis of mixed microbial populations found in natural and commercial bioleaching environments. Appl Environ Microbiol. 1994;60:1614–1621. doi: 10.1128/aem.60.5.1614-1621.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammann R, Ottow J C G. Reductive dissolution of Fe2O3 by saccharolytic clostridia and Bacillus polymyxa under anaerobic conditions. Z Pflanzenernaehr Bodenkd. 1974;137:108–115. [Google Scholar]
- 19.Harrison A P., Jr Acidiphilium cryptum gen. nov., sp. nov., heterotrophic bacterium from acidic mineral environments. Int J Syst Bacteriol. 1981;31:327–332. [Google Scholar]
- 20.Herlihy A T, Mills A L. Sulfate reduction in freshwater sediments receiving acid mine drainage. Appl Environ Microbiol. 1985;49:179–186. doi: 10.1128/aem.49.1.179-186.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson D B. Mineral cycling by microorganisms: iron bacteria. In: Allsopp D, Colwell R R, Hawksworth D L, editors. Microbial diversity and ecosystem function. Cambridge, United Kingdom: CAB International, University Press; 1995. pp. 137–159. [Google Scholar]
- 22.Johnson D B. Biodiversity and ecology of acidophilic microorganisms. FEMS Microbiol Ecol. 1998;27:307–317. [Google Scholar]
- 23.Johnson D B, McGinness S. Fe(III) reduction by acidophilic heterotrophic bacteria. Appl Environ Microbiol. 1991;57:207–211. doi: 10.1128/aem.57.1.207-211.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson D B, Ghauri M A, McGinness S. Biogeochemical cycling of iron and sulphur in leaching environments. FEMS Microbiol Rev. 1993;11:63–70. [Google Scholar]
- 25.Kishimoto N, Kosako Y, Wakao N, Tano T, Hiraishi A. Transfer of Acidiphilium facilis and Acidiphilium aminolytica to the genus Acidocella gen. nov., and emendation of the genus Acidiphilium. Syst Appl Microbiol. 1995;18:85–91. [Google Scholar]
- 26.Küsel K, Drake H L. Effects of environmental parameters on the formation and turnover of acetate by forest soils. Appl Environ Microbiol. 1995;61:3667–3675. doi: 10.1128/aem.61.10.3667-3675.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovley D R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovley D R. Dissimilatory metal reduction. Annu Rev Microbiol. 1993;47:263–290. doi: 10.1146/annurev.mi.47.100193.001403. [DOI] [PubMed] [Google Scholar]
- 29.Lovley D R, Phillips E J P. Availability of Fe(III) for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl Environ Microbiol. 1986;52:751–757. doi: 10.1128/aem.52.4.751-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovley D R, Phillips E J P. Organic matter mineralization with reduction of Fe(III) in anaerobic sediments. Appl Environ Microbiol. 1986;51:683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovley D R, Phillips E J P. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988;54:1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovley D R, Phillips E J P. Requirement for a microbial consortium to completely oxidize glucose in Fe(III)-reducing sediments. Appl Environ Microbiol. 1989;55:3234–3236. doi: 10.1128/aem.55.12.3234-3236.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maidak B L, Olsen G J, Larsen N, Overbeek R, McCaughey M J, Woese C R. The Ribosomal Database Project. Nucleic Acids Res. 1996;24:82–85. doi: 10.1093/nar/24.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mesbah M, Premachandran U, Whitman B. Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography. Int J Syst Bacteriol. 1989;39:159–167. [Google Scholar]
- 35.Miller G C, Lyons W B, Davis A. Understanding the water quality of pit lakes. Environ Sci Technol. 1996;30:118A–123A. doi: 10.1021/es9621354. [DOI] [PubMed] [Google Scholar]
- 36.Munch J C, Ottow J C G. Preferential reduction of amorphous to crystalline iron oxides by bacterial activity. Soil Sci. 1980;129:15–21. [Google Scholar]
- 37.Nealson K H, Myers C R. Microbial reduction of manganese and iron: new approaches to carbon cycling. Appl Environ Microbiol. 1992;58:439–443. doi: 10.1128/aem.58.2.439-443.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peine A, Peiffer S. Neutralisierungsprozesse in Sedimenten saurer Restseen des Braunkohletagebaus. Wasserkalender. 1998;33:48–72. [Google Scholar]
- 39.Porter K G, Feig Y S. The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr. 1980;25:943–948. [Google Scholar]
- 40.Pronk J T, Johnson D B. Oxidation and reduction of iron by acidophilic bacteria. Geomicrobiol J. 1992;10:153–171. [Google Scholar]
- 41.Pronk J T, DeBruyn J C, Bos P, Kuenen J G. Anaerobic growth of Thiobacillus ferrooxidans. Appl Environ Microbiol. 1992;58:2227–2230. doi: 10.1128/aem.58.7.2227-2230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rainey F A, Ward-Rainey N, Kroppenstedt R M, Stackebrandt E. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage; proposal for Nocardiopsaceae fam. nov. Int J Syst Bacteriol. 1996;46:1088–1092. doi: 10.1099/00207713-46-4-1088. [DOI] [PubMed] [Google Scholar]
- 43.Reynolds E S. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol. 1963;7:208. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roden E R, Wetzel R G. Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnol Oceanogr. 1996;41:1733–1748. [Google Scholar]
- 45.Schultze M, Geller W. The acid lakes of lignite mining district of the former German Democratic Republic. In: Reuther R, editor. Geochemical approaches to environmental engineering of metals. Berlin, Germany: Springer-Verlag; 1996. pp. 89–105. [Google Scholar]
- 46.Specka U, Spreinat A, Antranikian G, Mayer F. Immunocytochemical identification and localization of active and inactive α-amylase and pullulanase in cells of Clostridium thermosulfurogenes EM1. Appl Environ Microbiol. 1991;57:1062–1069. doi: 10.1128/aem.57.4.1062-1069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamura H, Goto K, Yotsuyanagi T, Nagayama M. Spectrophotometric determination of iron(II) with 1,10-phenanthroline in the presence of large amounts of iron(III) Talanta. 1974;21:314–318. doi: 10.1016/0039-9140(74)80012-3. [DOI] [PubMed] [Google Scholar]
- 48.Traub W H, Acker G, Kleber I. Ultrastructural surface alterations of Serratia marcescens after exposure to polymyxin B and/or fresh human serum. Chemotherapy. 1976;22:104–113. doi: 10.1159/000221919. [DOI] [PubMed] [Google Scholar]
- 49.Tuttle J H, Dugan P R, Randles C I. Microbial dissimilatory sulfur cycle in acid mine water. J Bacteriol. 1969;97:594–602. doi: 10.1128/jb.97.2.594-602.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valentine R C, Shapiro B M, Stadtman E R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biogeochemistry. 1968;7:2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]