Abstract
Background
Sodium thiosulfate (STS) can be used to treat patients diagnosed with calciphylaxis, which is a rare life-threatening syndrome. However, our patients treated with the recommended STS regimen presented with serious adverse events, resulting in treatment withdrawal. Then an optimized STS regimen was used to increase the tolerance of patients to STS and improve treatment continuation. The curative effect of the new regimen is not yet definite. Therefore, this study aimed to evaluate the response to the use of the optimized STS regimen for the treatment of calciphylaxis in Chinese patients during the first three courses of treatment.
Methods
Demographic, clinical, and laboratory data were retrospectively collected on 31 calciphylaxis patients with chronic kidney disease (CKD) or end-stage kidney disease (ESKD) treated with the optimized STS regimen. The primary outcome was a clinical improvement. The secondary outcomes included survival rate and adverse events.
Results
Twenty-five patients (over 80%) achieved clinical improvement considering improvement or nonspecific changes of skin lesions (80.65%) and pain relief (100%). Furthermore, 54.84% of patients did not experience any adverse events and none died from complications. During a median follow-up of 9 months (interquartile range 4‒19), 27 patients (87.10%) survived; additionally, 13 patients (41.94%) survived after a one-year follow-up period.
Conclusion
The optimized STS regimen is relatively safe, associated with satisfactory outcomes, and well tolerated by patients for short to medium treatment duration. Hence, it is a promising approach for the treatment of patients diagnosed with calciphylaxis.
Keywords: Sodium thiosulfate, calciphylaxis, optimized STS regimen, clinical improvement
Introduction
Calciphylaxis, also known as calcific uremic arteriolopathy (CUA), is a rare life-threatening syndrome observed mostly in patients with CKD [1], particularly in those on dialysis with ESKD [2]. Initial manifestations involve painful skin lesions [3] including erythema, violaceous plaques, nodules, livedo, or purpura, progressing to necrosis or ulcers [4,5]. The prevalence of CUA is low and has an estimated annualized incidence of 3.49‰ in the United States [6], 0.4‰ in Germany [2], and less than 0.1‰ in Japan [7]. However, the prognosis is extremely poor, with 45–80% mortality being reported within one year [8–11].
Characteristic histologic features of CUA include vessel calcification, thromboses, endovascular fibrosis, fat necrosis, and dermal angioplasia [12–14]. Treatment with STS a potent antioxidant and chelator of calcium, can prevent the progression of calciphylaxis [15–17]. Although randomized controlled trials have not been performed, the benefits of STS for CUA patients had been highlighted in case reports [18–24] and observational studies [25–27].
The generally recommended STS regimen is 25 g (100 mL of a 25% solution) administered intravenously thrice a week during the last 30–60 min of each hemodialysis session [28]. Nevertheless, some patients treated with the above protocol presented with serious adverse events, resulting in treatment withdrawal [25,29,30]. At our center, 5 patients had a history of STS administration at the recommended dose. One patient died from septicemia within 6 months, one patient was lost to follow-up, and three patients discontinued the recommended STS treatment because of hypotension (one patient) and severe nausea and vomiting (two patients). The variations in response to treatment are agnogenic, and it may be attributed to factors such as different medication specification, dosage form, ethnicity, genotype, and susceptibility [31]. To increase the tolerance of patients to STS, we used an optimized STS regimen to improve treatment continuation. The curative effect of the new regimen is not yet definite. Therefore, aim of this study was to retrospectively evaluate the response to the optimized STS regimen for the treatment of calciphylaxis in Chinese patients during the first three courses of treatment.
Materials and methods
Optimized STS regimen
The optimized STS regimen called ‘Zhong Da STS Approach’ was named after Zhong Da Hospital, affiliated to Southeast University. This regimen includes four features: small initial dose, daily administration, increasing light-to-moderate dose, and repeated courses of treatment. A therapeutic schedule was designed with successive courses of treatment at a maintenance dose until complete resolution of symptoms. In detail, the protocol is as follows. An initial STS dose of 5 g (250 mL of 2% solution) is administered intravenously once a day. Next, the dose is increased by 1 g per day to the moderate dose as a maintenance dose (or to a maximum dose: 10 g, 250 mL of 4% solution). The one treatment course involves treatment for 3 weeks, followed by a treatment-free period of 2 weeks. Thereafter, the patients continue treatment with the next session. In addition, we used the following assistive treatment measures for these patients as appropriate: dialysis administration of optimization, analgesia and wound management, elimination of risk factors (such as correcting hypercalcemia and hyperphosphatemia), and hyperbaric oxygen therapy.
The off-label drug use for the indication and the STS treatment approach were approved by the Pharmaceutical and Drug Administration Committee of Southeast University School of Medicine, Nanjing. (Figure S1). This study complied with the principles of the Declaration of Helsinki and was approved by the institutional ethical review board of Zhongda Hospital in 2020 (approval identifier: 2020ZDSYLL037-P01) (Figure S2).
Study design and patient population
In this study, demographic, clinical, and laboratory information was recorded and analyzed for patients with CKD or ESKD who were diagnosed with calciphylaxis at Zhong Da Hospital affiliated to Southeast University between October 2017 and October 2019. Patients who did not receive the optimized STS treatment were excluded.
The case information was obtained from digital hospitalized patient records and did not involve revealing the identity of patients. Additionally, some patients died or were lost to follow-up when the project was initiated. Therefore, the requirement for informed consent from the patients was waived by the institutional review board in the approval records of the Ethics Committee (Figure S2). The study was registered in the Chinese Clinical Trial Registry (registration number: ChiCTR2000039084).
Study data collection
The evaluated biochemical parameters included the levels of hemoglobin (Hb), white blood cell (WBC), C-reactive protein (CRP), procalcitonin (PCT), serum albumin (ALB), alkaline phosphatase (ALP), calcium (Ca), phosphate (P), and parathyroid hormone (PTH), at baseline and after each course of treatment. All biochemical parameters were based on plasma or serum from fasting morning venous blood samples. In case of dialysis patients, the data were based on samples collected before the dialysis treatment.
Clinical data were collected from our regularly updated digital and follow-up records. Demographic and clinical data included age at diagnosis of calciphylaxis, age at onset of hemodialysis, gender, vascular access type, body mass index (BMI), primary kidney disease, comorbidities, medication history, numerical pain rating scale (NPRS), and information on skin lesions.
The NPRS is a segmented numeric version of the visual analog scale in which a respondent selects a whole number (integers 0–10) that best reflects the intensity of his/her pain. For every patient diagnosed with calciphylaxis, NRPS 0 was the baseline score; NPRS of 1–3 was the average score of patients during the each course of treatment. The change in pain was classified as relief (declining or unchanged NPRS score after analgesic withdrawal), duration (unchanged average NPRS score), and deterioration (the average score of NPRS was rising).
Information collected on skin lesions included number, size, distribution, and with/without ulceration. By distributions, skin lesions were classified as central (involving central areas within subcutaneous adipose tissue such as the abdomen or thighs), peripheral (restricted to peripheral sites having limited adipose tissue, such as the digits) [28] or systemic (distributed widely all over the body). The changes in skin lesions were classified as improvement (complete remission of wound or reduction of size and/or number of skin lesions), nonspecific changes (interrupted progression of skin lesions), and progression (deterioration of wound).
The primary outcome was clinical improvement which included two aspects: improvement or nonspecific changes of skin lesions and pain relief. The secondary outcomes included survival and adverse events rate.
Statistical analysis
Continuous variables are presented as means with standard deviations or medians with interquartile ranges, and categorical variables are presented as counts with percentages. Differences between baseline and course of treatment were examined using one-way ANOVA, Kruskal-Wallis, or Fisher’s exact tests as appropriate. All statistical tests were two-sided, with a value of p < 0.05 defined as significant. All statistical analyses were performed using SPSS software, version 23.0 for Windows 10.
Results
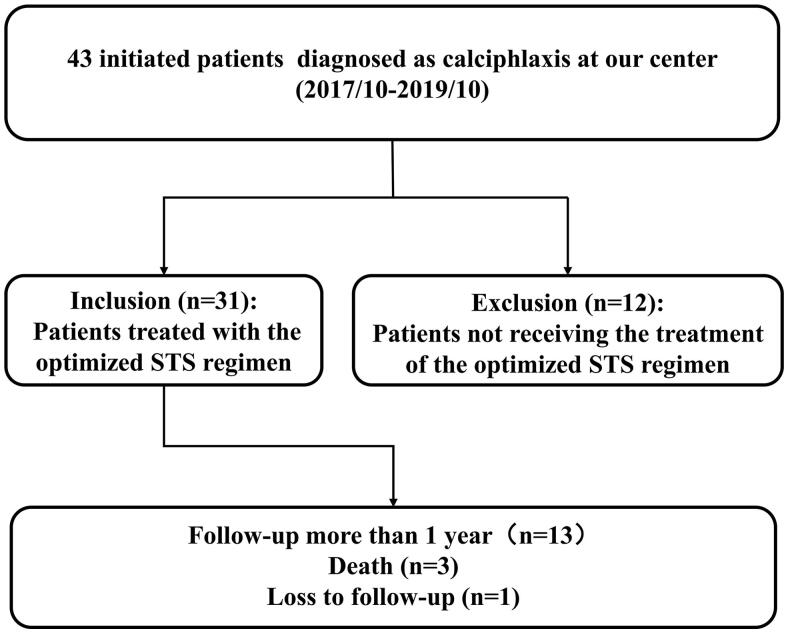
A total of 43 patients were diagnosed with calciphylaxis at our center between October 2017 and October 2019. Of these, 31 patients were included in the study, based on the inclusion and exclusion criteria. (Figure 1). During a median follow-up of 9 months (interquartile range 4‒19), 27 patients (87.10%) survived; additionally, 13 patients (41.94%) survived after a one-year follow-up period.
Figure 1.
Flow chart of cohort study participants.
The demographics and clinical characteristics of the patients at baseline were summarized in Table 1. The mean age at diagnosis of calciphylaxis and onset of hemodialysis was 51.10 ± 14.85, 47.45 ± 14.83 respectively. 71% of patients were men and the median time of dialysis initiation to calciphylaxis diagnosis was 74 months (interquartile range 48–120). The primary cause of kidney disease was diabetic nephropathy (25.81%); all other causes were less represented. All patients were undergoing maintenance hemodialysis except for one patient with CKD Stage 3. The percentage of patients diagnosed with calciphylaxis who were overweight (BMI ≥ 25) was 35.5%. The most common comorbidity was hypertension (87.10%), followed by diabetes (45.16%), chronic heart failure (29.03%), autoimmunity disease (12.90%), and tumor (6.45%). Previous medication history included phosphate binders (64.52%), activated vitamin D and analogs (51.60%), cinacalcet (41.94%), and antithrombotic drugs (48.40%, including one patient on warfarin).
Table 1.
Patient demographics and clinical characteristics.
| Characteristic | Value |
|---|---|
| Age at diagnosis of calciphylaxis (years) | 51.10 ± 14.85 |
| Gender, n (%) | |
| Male | 22 (71%) |
| Female | 9 (29%) |
| BMI | 23.14 ± 3.52 |
| Age at onset of hemodialysis (years) | 47.45 ± 14.83 |
| Duration of dialysis (months) | 74 [48,120] |
| CKD/ESKD/dialysis modality, n (%) | |
| CKD, non-dialysis | 1 (3.23%) |
| Hemodialysis | 30 (96.77%) |
| Peritoneal dialysis | 0 (0%) |
| Vascular access type, n (%) | |
| Arteriovenous fistula | 28 (93.33%) |
| long-term tunneled cuffed catheter | 2 (6.67%) |
| Type of primary kidney disease, n (%) | |
| Others or Unknown | 18 (58.1% |
| Diabetic Nephropathy | 8 (25.8%) |
| Chronic Glomerulonephritis | 4 (12.9%) |
| Hypertensive Nephropathy | 1 (3.2%) |
| Comorbidities, n (%) | |
| Hypertension | 27 (87.10%) |
| Diabetes | 14 (45.16%) |
| Chronic Heart Failure | 9 (29.03%) |
| Autoimmunity Disease | 4 (12.90%) |
| Tumor | 2 (6.45%) |
| Medication history, n (%) | |
| Phosphate binder | 20 (64.52%) |
| Activated vitamin D and its analogues | 16 (51.6%) |
| Cinacalcet | 13 (41.94%) |
| Antithrombotic Drugs | 15 (48.4%) |
Continuous variables are presented as mean ± standard deviation or median [inter-quartile range] when non-normally distributed; categorical variables, as number (percentage).
BMI: body mass index.
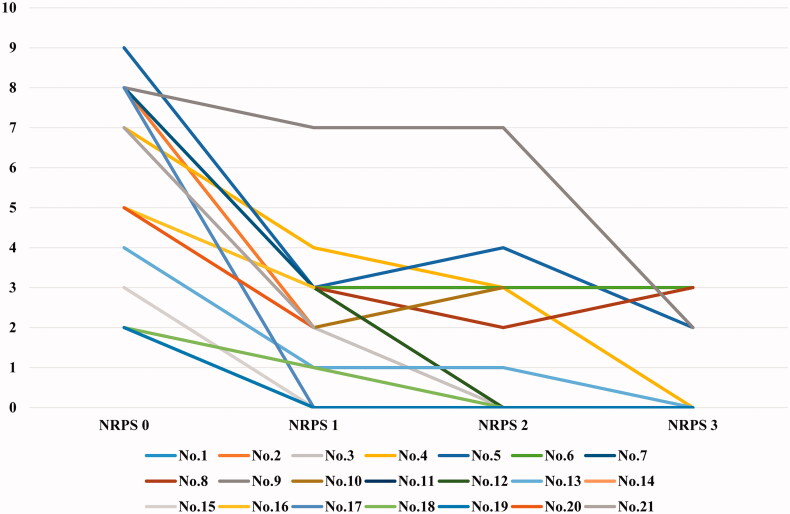
Before initiation of the optimized STS regimen, 21 patients (approximately 70%) experienced somatic pain and 20% of them experienced intractable pain with depression or suicidal tendencies. The average NPRS showed a downward trend during treatment (Figure 2). Additionally, more than half of patients who experienced pain reduced the dosage of analgesics during the second week of the first course, and 8 patients (nearly 40%) stopped taking analgesics by the end of the first course of treatment. As treatment continued, three other patients successfully withdrew from analgesics. Overall, all patients achieved pain relief.
Figure 2.
NRPS changes in patients by the course of treatment.
Twenty-seven patients (87.10%) exhibited typical skin lesions, and 66.70% of them showed ulceration with a median number of wounds being 3 (range: 1–4). The remaining patients showed atypical clinical manifestation such as local hyperpigmentation or induration. The lesions were distributed predominantly peripherally (18/27, 66.67%) rather than centrally (3/27, 11.11%) or systemically (6/27, 22.22%). The distribution of calcium deposition confirmed by single-photon emission computed tomography/computed tomography was classified as nonspecific (7.41%), extremities (48.15%), trunk (14.81%), combination 1 (extremities and trunk without internal organ involvement, 14.81%), internal organs (3.70%) and combination 2 (internal organ and extremities or/and trunk, 11.11%). Twenty-two patients (70.97%) underwent skin biopsy, and the positive results were as well as the negative results. (Table 2)
Table 2.
Characteristics of skin lesions in patients with calciphylaxis.
| Skin lesions or nodules | Value |
|---|---|
| Presence, n (%) | 27 (87.10%) |
| Median number | 3 [1,4] |
| Distribution, n (%) | |
| Peripheral | 18 (66.67%) |
| System | 6 (22.22%) |
| Central | 3 (11.11%) |
| Ulceration/necrosis, n (%) | |
| Yes | 17 (62.96%) |
| No | 10 (37.04%) |
| Presence in the body parts by SPECT/CT, n (%) | |
| Extremities | 13 (48.15%) |
| Trunk | 4 (14.81%) |
| Compound 1 | 4 (14.81%) |
| Compound 2 | 3 (11.11%) |
| Nonspecific findings | 2 (7.41%) |
| Internal organs | 1 (3.70%) |
| Skin Biopsy, n (%) | 22 (70.97%) |
| Positive, n (%) | 11 (50%) |
| Negative, n (%) | 11 (50%) |
Compound 1means the presence of calcium deposition in both extremities and trunk with no internal organ involvement; compound 2 means the presence of calcium deposition in both extremities and trunk with internal organ involvement.
Continuous variables are presented as mean ± standard deviation or median [interquartile range] when non-normally distributed; categorical variables, as number (percentage).
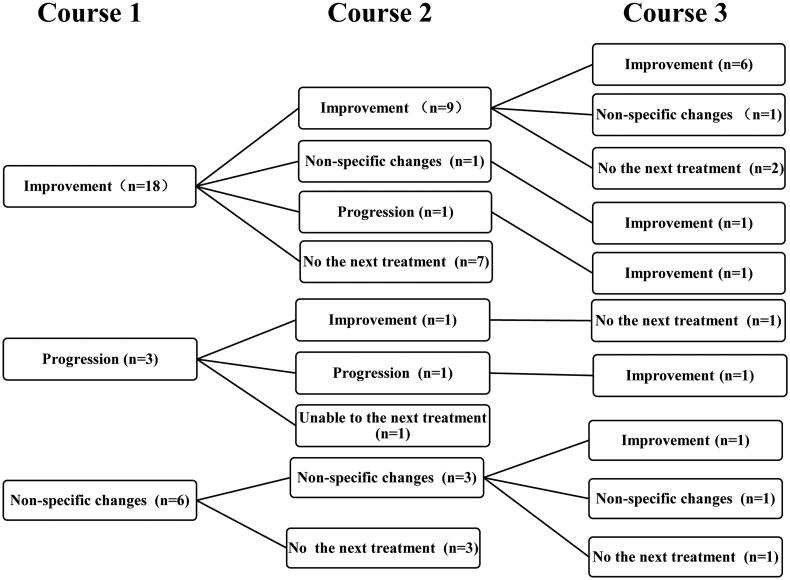
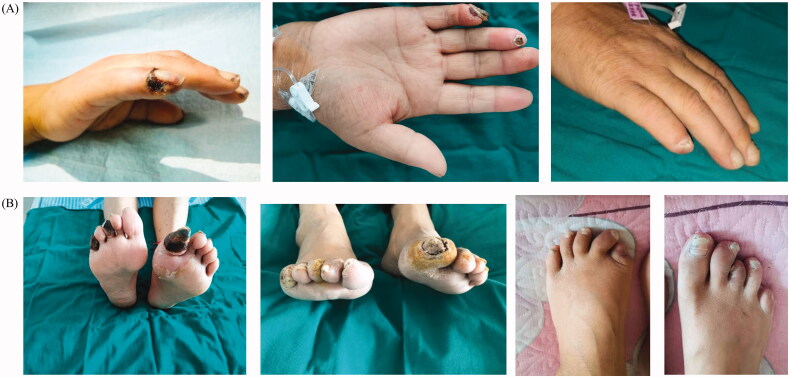
Skin lesions with typical or atypical manifestation showed improvement in 80.65% of the patients, which meant that over 80% of the patients achieved clinical improvement including pain relief. Two-thirds of patients with typical skin lesions improved after the first course of treatment. Although the remaining one-third of patients showed no improvement in the skin lesions after the first course of treatment, three (33.33%) showed an improvement after subsequent course of treatment (Figure 3). Furthermore, 6 patients (22.22%) with ulcers/necrosis were completely cured after 3 courses of treatment (Figure 4) and all patients with atypical lesions had no progression during the treatment.
Figure 3.
Prognosis trees of the skin lesions in the study cohort during the three treatment courses. Notes: Course 1, 2, 3 represented the first, second, third treatment course respectively; the prognosis of skin lesions were classified into improvement, non-specific changes and progression; some patients who unable to continue treatment or we cannot gather their information before a data-collection deadline were belong to ‘No the next treatment’; the value was the patients number in different category.
Figure 4.
Changes in the lesions before and after treatment. (A) Hand lesions; (B) Feet lesions.
Table 3 summarized trends in the biochemical and pain-related measurements during the optimized treatment courses. The levels of phosphorus and NPRS reduced significantly during the courses of STS treatment (p = 0.035 and p < 0.001, respectively), compared with the baseline. Serum calcium levels also showed a decline, but this difference was not statistically significant.
Table 3.
Comparison of biochemical and clinical characteristics at baseline and after each course of treatment.
| Baseline Condition | One Course | Two Course | Three Course | P | |
|---|---|---|---|---|---|
| Number | 31 | 31 | 21 | 16 | — |
| Hb (g/L)a | 111.32 ± 21.14 | 105.97 ± 18.61 | 105.95 ± 24.07 | 112.63 ± 22.17 | 0.598 |
| WBC (*109/L)a | 7.96 ± 3.23 | 7.25 ± 2.91 | 7.75 ± 3.02 | 7.21 ± 1.57 | 0.736 |
| CRP (mg/L)b | 2.53 (1.51,6.29) | 2.67 (0.82,5.78) | 4.27 (1.93,18.66) | 3.96 (1.81,11.62) | 0.423 |
| PCT (ng/ml)b | 0.29 (0.17,0.68) | 0.28 (0.13,0.53) | 0.56 (0.23,0.85) | 0.32 (0.22,1.25) | 0.414 |
| ALB (g/L)a | 38.14 ± 5.41 | 38.36 ± 5.09 | 37.33 ± 6.39 | 39.63 ± 8.06 | 0.719 |
| ALP (U/L)b | 111 (80,185) | 100 (80,196) | 145 (99,252.5) | 124 (88,176) | 0.37 |
| Ca (mmol/L)a | 2.31 ± 0.28 | 2.22 ± 0.27 | 2.23 ± 0.31 | 2.23 ± 0.25 | 0.587 |
| P (mmol/l)a | 1.93 ± 0.60 | 1.59 ± 0.67 | 1.54 ± 0.49 | 1.36 ± 0.48 | 0.009 |
| PTH (pg/mL)b | 399.7 (114.5,903.5) | 266.7 (101.8,651.7) | 392.9 (58.5,573.4) | 411.9 (51.3,846.0) | 0.626 |
| NPRSb | 5 (0,7) | 2 (0,3) | 0 (0,3) | 0 (0,1.5) | 0.001 |
Superscript a: means that testing correlation between the index and baseline condition by one-way ANOVA test.
Superscript b: means that testing correlation between the index and baseline condition by Kruskal-Wallis test.
NPRS means numerical pain rating scale (range: 1–10).
P-values are shown here for comparison and categories of column proportion differ significantly from each other at the 0.05 level.
Normal reference ranges: Hb: 130–175 g/L,WBC: 3.5–9.5*109/L, CRP: 0–3.0 mg/L, PCT: 0–0.5 ng/ml, ALB: 40–55 g/L, ALP: 45–125 U/L, Ca: 2.11–2.52 mmol/L, P:0.85–1.51 mmol/l, PTH:12–88 pg/ml.
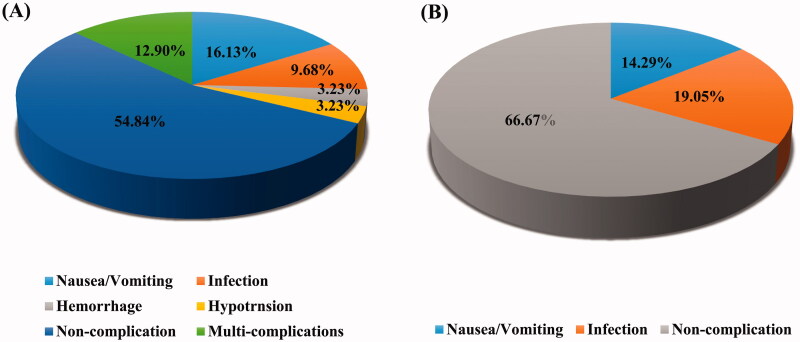
During the study period, two patients died from cardiovascular events; only one patient died from infection, the leading cause of death in calciphylaxis [28]. During the entire treatment session, more than half of the patients (54.84%) never experienced adverse events, only one withdrew from treatment and no one died from complications. The most common adverse event was nausea/vomiting (16.13%), followed by multi-complications (12.90%), and infection (9.68%). (Figure 5A)
Figure 5.
Distribution of different complications with optimized STS therapy. (A) during the whole study period; (B) during the second treatment.
Another important finding was that the incidence of infection showed a declining trend and there was no significant difference in the distribution of other adverse events between the three courses (Table 4). Furthermore, the most adverse events occurred during the course when the STS dose was increased; the adverse events were relieved when the dose was not increased any further or when it was decreased to the dose administered a day prior. In accordance with this result, the rate of adverse events decreased significantly by 33.33% in the second treatment (Figure 5B).
Table 4.
Comparison of adverse events in each treatment course.
| One Course | Two Course | Three Course | p Value | |
|---|---|---|---|---|
| Patients | 31 | 21 | 16 | — |
| Nausea/Vomiting1 | 9 (28%)a | 3 (14.3%)a | 0 (0%)a | 0.203 |
| Hypotension1 | 2 (6.25%)a | 0 (0%)a | 0 (0%)a | 0.304 |
| Infection1 | 9 (28%)a | 4 (19.05%)a | 1 (6.25%)a | 0.048 |
Superscript 1: means that testing correlation between the index and baseline serum potassium by Fisher's Exact test. P-values are shown here for comparison and categories of column proportion differ significantly from each other at the 0.05 level.
Subscript a: means that a subset of Courses categories whose column proportion do not differ significantly from each other at the 0.05 level.
Discussion
This study determined the response to the optimized STS regimen used for the treatment of calciphylaxis in Chinese patients. This was achieved by evaluating the healing of skin lesions, pain relief, and survival. Although the sample size in this study was consistent with previous studies [25,26,32], our study systematically assessed the importance of STS primarily in resolving clinical symptoms. In this study, we focused on evaluating patients’ clinical conditions and quality of life, rather than solely on the rate of survival and mortality. The improvements of skin lesions and pain relief was reflected not only in the clinical data but also helped patients overcome depression, distress, suicidal tendency, and even helped avert a repeated amputation in one case.
As for baseline demographics and clinical characteristics, results were partially consistent with those of previous studies such as common primary renal disease distribution, interval range from the initiation of dialysis to the development of calciphylaxis, and certain risk factors including obesity, diabetes, and some oral drugs [2,6,10,25,26,32,33]. However, compared to previous studies, our study included relatively younger patients and more men [6,33,34]. Skin lesions showed a dominant peripheral distribution, unlike the results of previous studies, which showed central dominance [2,6,10]. Only one patient had used warfarin, which increased the risk of calciphylaxis [6,9,33–35]. These differences might be attributed to factors such as variations in medication habits, ethnicity, genetic makeup, and susceptibility; however, the actual cause is still unknown. These differences indicate the necessity to systemically collect and analyze patient information in our country.
The single dose of STS in the optimized STS therapy was much lower than the normal dose (25 g, each hemodialysis session) [28]. However, one-week accumulated dose in our study was up to 70 g, similar to 75 g per week in previous reports compared to the substantial disparity between the single doses in the two approaches (10 g vs. 25 g). To our knowledge, there is no guideline for a standard dose of STS for CUA treatment at present. A recent pharmacokinetic study showed that non-renal clearance of STS equaled hemodialysis clearance in patients on dialysis [36]. The dose of STS varied markedly depending on the hemodialysis frequency and duration [37]. Most patients were treated with STS halfway in the off-dialysis period, which may slow down the clearance of STS. Furthermore, we assumed that Chinese patients have a smaller body surface area and lighter weight; thus, they could potentially have a higher sensitivity to STS. In our study, there was no accumulated effect with time shown in the comparison in adverse event occurrence between the three treatment courses. This finding provides more confidence to prolong the treatment for patients and continue follow-up to assess the long-term response to the optimized STS regimen.
A case of calciphylaxis treated by STS was firstly reported in 2004 [19]. While two registered phase 3 clinical trials investigating its safety and efficacy have been completed (Current Controlled Trials no, ISRCTN73380053; and ClinicalTrials.gov no, NCT03150420), no result of the randomized controlled trial (RCT) has been reported to date. Recently, our research team registered an Internet domain name (http://www.calciphylaxis.com.cn/), developed the Chinese calciphylaxis registration system, and started registering diagnosed patients nationally. We expect to obtain more patient samples to objectively evaluate the optimized STS regimen in the next RCT study.
Although many reports have discussed the treatment mechanisms such as calcium-chelating properties, anti-oxidative effects, the induction of a high anion-gap acidosis [15–17], and STS-induced changes in serum inhibitors of vascular calcification [38], the exact mechanism of STS remains elusive and the concentrations required for these effects are unknown.
There were several limitations in our study such as the lack of a control group, retrospective study design, a limited sample size, and limited follow-up time for partly patients. In addition, STS is part of a multi-interdisciplinary approach, and the precise positive effect of STS cannot be isolated. The basic mechanism and concentration-effect studies are valuable strategies for determining future rational doses of STS. Our center is involved in an ongoing epidemiological investigation of the Chinese population, a systemic mechanism study, and pharmacokinetic research.
In summary, this study confirmed the effectiveness of an optimized STS regimen as a promising approach for the treatment of patients in China with calciphylaxis. The treatment was relatively safe, associated with satisfactory outcomes, and well tolerated by patients for short to medium treatment duration. To our knowledge, this was the first systematic study on the curative effect of STS for calciphylaxis in the Chinese population. Our center offers referential data to help Chinese doctors assess and modify their line of treatment based on our own ethnic, demographic, and clinical data, rather than relying solely on available literature. In addition, compared with many cases or case series reports, our research provided a more objective assessment of the outcomes and prognosis of patients with calciphylaxis during the courses of treatment.
Funding Statement
This work was supported by the National Natural Science Foundation of China [grant numbers 81570612 and 81870497]; and Jiangsu Province Key Research and Development Program-social Development [grant number BE2021737].
Ethical approval
The off-label drug use for the indication and STS treatment approach was approved by the Pharmaceutical and Drug Administration Committee of Zhongda Hospital at Southeast University School of Medicine in Nanjing (Figure S1).
This study complied with the guidelines of the Declaration of Helsinki, and was approved by the institutional ethical review board of Zhongda Hospital, Southeast University School of Medicine, Nanjing in 2020 (Figure S2) (approval identifier: 2020ZDSYLL037-P01). The study has been registered in the Chinese Clinical Trial Registry (registration number: ChiCTR2000039084).
Author contributions
Research idea and study design: Xin Yang, and Xiaoliang Zhang; data acquisition: Xin Yang, Yuqiu Liu, Wen Shi, and Jieyi Si; data analysis/interpretation: Xin Yang, and Xiaotong Xie; supervision or mentorship: Xiaoliang Zhang, Bicheng Liu, and Xiaomin Li. Each author contributed important intellectual content during manuscript drafting and revision. Xin Yang takes responsibility for honest, accurate, and transparent reporting of the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Fu JM, Koo KD.. Non-uraemic calciphylaxis with acral necrosis. Lancet Diabetes Endocrinol. 2014;2(1):914. [DOI] [PubMed] [Google Scholar]
- 2.Brandenburg VM, Kramann R, Rothe H, et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nationwide registry. Nephrol Dial Transpl. 2017;32(1):126–132. [DOI] [PubMed] [Google Scholar]
- 3.Bazari H, Jaff MR, Mannstadt M, et al. Case records of the Massachusetts general hospital. Case 7-2007. A 59-year-old woman with diabetic renal disease and nonhealing skin ulcers. N Engl J Med. 2007;356(10):1049–1057. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh T, Winchester DS, Davis MDP, et al. Early clinical presentations and progression of calciphylaxis. Int J Dermatol. 2017;56(8):856–861. [DOI] [PubMed] [Google Scholar]
- 5.Daudén E, Oñate M.. Calciphylaxis. Dermatol Clin. 2008;26(4):557–568. [DOI] [PubMed] [Google Scholar]
- 6.Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27(11):3421–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17(4):498–503. [DOI] [PubMed] [Google Scholar]
- 8.Fine A, Zacharias J.. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61(6):2210–2217. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91(10):1384–1394. [DOI] [PubMed] [Google Scholar]
- 10.Weenig RH, Sewell LD, Davis MDP, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56(4):569–579. [DOI] [PubMed] [Google Scholar]
- 11.Gabel CK, Nguyen ED, Chakrala T, et al. Assessment of outcomes of calciphylaxis. J Am Acad Dermatol. 2021;85(4):1057–1064. [DOI] [PubMed] [Google Scholar]
- 12.Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39(11):795–802. [DOI] [PubMed] [Google Scholar]
- 13.Mochel MC, Arakaki RY, Wang G, et al. Cutaneous calciphylaxis: a retrospective histopathologic evaluation. Am J Dermatopathol. 2013;35(5):582–586. [DOI] [PubMed] [Google Scholar]
- 14.McMullen ER, Harms PW, Lowe L, et al. Clinicopathologic features and calcium deposition patterns in calciphylaxis: Comparison with gangrene, peripheral artery disease, chronic stasis, and thrombotic vasculopathy. Am J Surg Pathol. 2019;43(9):1273–1281. [DOI] [PubMed] [Google Scholar]
- 15.Pasch A, Schaffner T, Huynh-Do U, et al. Sodium thiosulfate prevents vascular calcifications in uremic rats. Kidney Int. 2008;74(11):1444–1453. [DOI] [PubMed] [Google Scholar]
- 16.Hayden MR, Tyagi SC, Kolb L, et al. Vascular ossification-calcification in metabolic syndrome, type 2 diabetes mellitus, chronic kidney disease, and calciphylaxis-calcific uremic arteriolopathy: the emerging role of sodium thiosulfate. Cardiovasc Diabetol. 2005;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'neill WC, Hardcastle KI.. The chemistry of thiosulfate and vascular calcification. Nephrol Dial Transplant. 2012;27(2):521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Araya CE, Fennell RS, Neiberger RE, et al. Sodium thiosulfate treatment for calcific uremic arteriolopathy in children and young adults. Clin J Am Soc Nephrol. 2006;1(6):1161–1166. [DOI] [PubMed] [Google Scholar]
- 19.Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43(6):1104–1108. [DOI] [PubMed] [Google Scholar]
- 20.Guerra G, Shah RC, Ross EA.. Rapid resolution of calciphylaxis with intravenous sodium thiosulfate and continuous venovenous haemofiltration using low calcium replacement fluid: case report. Nephrol Dial Transplant. 2005;20(6):1260–1262. [DOI] [PubMed] [Google Scholar]
- 21.Mataic D, Bastani B.. Intraperitoneal sodium thiosulfate for the treatment of calciphylaxis. Ren Fail. 2006;28(4):361–363. [DOI] [PubMed] [Google Scholar]
- 22.Meissner M, Bauer R, Beier C, et al. Sodium thiosulphate as a promising therapeutic option to treat calciphylaxis. Dermatology. 2006;212(4):373–376. [DOI] [PubMed] [Google Scholar]
- 23.Subramaniam K, Wallace H, Sinniah R, et al. Complete resolution of recurrent calciphylaxis with long-term intravenous sodium thiosulfate. Australas J Dermatol. 2008;49(1):30–34. [DOI] [PubMed] [Google Scholar]
- 24.Brucculeri M, Cheigh J, Bauer G, et al. Long-term intravenous sodium thiosulfate in the treatment of a patient with calciphylaxis. Semin Dial. 2005;18(5):431–434. [DOI] [PubMed] [Google Scholar]
- 25.Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8(7):1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zitt E, Konig M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28(5):1232–1240. [DOI] [PubMed] [Google Scholar]
- 27.Gabel CK, Nguyen ED, Dobry AS, et al. Assessment of outcomes of calciphylaxis lesions treated with intralesional sodium thiosulfate. J Am Acad Dermatol. 2021;85(3):770–773. [DOI] [PubMed] [Google Scholar]
- 28.Nigwekar SU, Thadhani R, Brandenburg VM.. Calciphylaxis. N Engl J Med. 2018;378(18):1704–1714. [DOI] [PubMed] [Google Scholar]
- 29.Schlieper G, Brandenburg V, Ketteler M, et al. Sodium thiosulfate in the treatment of calcific uremic arteriolopathy. Nat Rev Nephrol. 2009;5(9):539–543. [DOI] [PubMed] [Google Scholar]
- 30.Mao M, Lee S, Kashani K, et al. Severe anion gap acidosis associated with intravenous sodium thiosulfate administration. J Med Toxicol. 2013;9(3):274–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Zhang X, Xie X, et al. Risk factors for calciphylaxis in chinese hemodialysis patients: a matched case-control study. Ren Fail. 2021;43(1):406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruderman I, Toussaint ND, Hawley CM, et al. The australian calciphylaxis registry: reporting clinical features and outcomes of patients with calciphylaxis. Nephrol Dial Transplant. 2021;36(4):649–656. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi M, Takamatsu I, Kanno Y, et al. Japanese Calciphylaxis Study Group . A case-control study of calciphylaxis in japanese end-stage renal disease patients. Nephrol Dial Transplant. 2012;27(4):1580–1584. [DOI] [PubMed] [Google Scholar]
- 34.Nigwekar S, Zhang Y, Corapi K, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nigwekar SU, Bhan I, Turchin A, et al. Statin use and calcific uremic arteriolopathy: a matched Case-Control study. Am J Nephrol. 2013;37(4):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farese S, Stauffer E, Kalicki R, et al. Sodium thiosulfate pharmacokinetics in hemodialysis patients and healthy volunteers. Clin J Am Soc Nephrol. 2011;6(6):1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh RP, Derendorf H, Ross EA.. Simulation-based sodium thiosulfate dosing strategies for the treatment of calciphylaxis. Clin J Am Soc Nephrol. 2011;6(5):1155–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schurgers LJ, Barreto DV, Barreto FC, et al. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol. 2010;5(4):568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]