ABSTRACT
The ability to maintain a high core body temperature is a defining characteristic of all mammals, yet their diverse habitats present disparate thermal challenges that have led to specialized adaptations. Marine mammals inhabit a highly conductive environment. Their thermoregulatory capabilities far exceed our own despite having limited avenues of heat transfer. Additionally, marine mammals must balance their thermoregulatory demands with those associated with diving (i.e. oxygen conservation), both of which rely on cardiovascular adjustments. This review presents the progress and novel efforts in investigating marine mammal thermoregulation, with a particular focus on the role of peripheral perfusion. Early studies in marine mammal thermal physiology were primarily performed in the laboratory and provided foundational knowledge through in vivo experiments and ex vivo measurements. However, the ecological relevance of these findings remains unknown because comparable efforts on free-ranging animals have been limited. We demonstrate the utility of biologgers for studying their thermal adaptations in the context in which they evolved. Our preliminary results from freely diving northern elephant seals (Mirounga angustirostris) reveal blubber’s dynamic nature and the complex interaction between thermoregulation and the dive response due to the dual role of peripheral perfusion. Further exploring the potential use of biologgers for measuring physiological variables relevant to thermal physiology in other marine mammal species will enhance our understanding of the relative importance of morphology, physiology, and behavior for thermoregulation and overall homeostasis.
KEYWORDS: Biologging, blood flow, blubber, heat flux, seals, thermoregulation
Introduction
Few endothermic organisms live at ambient temperatures at or near body temperature. The marine environment is particularly challenging for thermoregulation due to the high thermal conductivity of water [1]. Marine endotherms must maintain thermal balance while immersed in a medium that conducts heat roughly 25 times faster than air [2]. Additionally, the marine environment provides limited thermal refugia except perhaps in the vertical dimension or when hauling out. This thermally challenging environment has provided intense evolutionary pressure as endothermic tetrapods transitioned from terrestrial to aquatic living on multiple occasions [3,4]. While the underlying physiological mechanisms of marine mammals resemble those of humans, their thermoregulatory capabilities far exceed our own.
Technological innovations enable physio-logging of marine mammals
Marine mammals display many extreme adaptations suitable for investigations aligned with the Krogh Principle .1 The increased thermal challenges often translate to greater signal-to-noise ratio that may facilitate mechanistic studies linking cause and effect, especially through the application of biologging devices. However, challenging logistics, limited animal access, and animal welfare issues may limit such studies in addition to technological challenges associated with high-pressure cycles in a corrosive environment [5]. As a result, most physiology research was carried out on dead (and/or excised tissue samples) or captive marine mammals. Field research, on the other hand, consisted primarily of observational studies of marine mammals on land (Figure 1).
Figure 1.
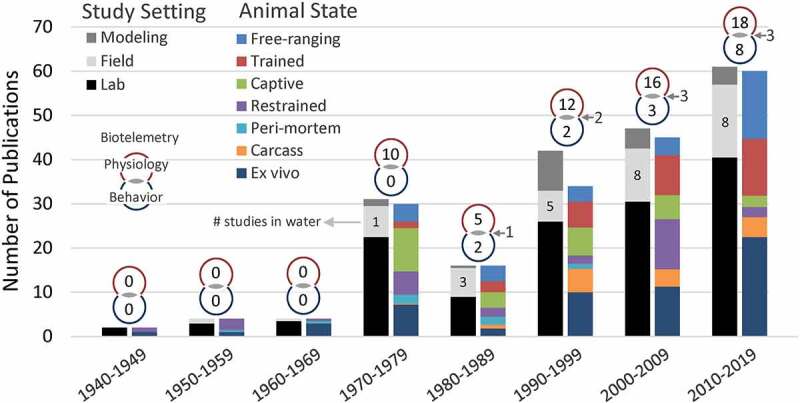
Number of publications (total = 207) by decade from 1940 to 2019 relevant to the thermal physiology of marine mammals. Refer to the supplementary file for the studies included. Studies are classified by setting (i.e. where it was performed) (left bar) and by the state or condition of the animal (right bar; modeling studies that did not make measurements on an animal were not included). Numbers inside the field bar (light gray) indicate how many of those field studies (if ≥1) involved animals in water (vs. land). Venn-diagrams on top of the bars indicate how many studies during that decade used biotelemetry for remote measurements of physiological variables (e.g. body temperature; top), or behavioral variables (e.g. diving behavior; bottom), or both. Biotelemetry is defined broadly here and includes remote sensing of physiologically relevant data (e.g. infrared thermography) and the use of data loggers (i.e. biologgers) to record continuous measurements on captive animals. Categories for study settings were defined as: lab (i.e. using an experimental approach in a captive and controlled setting), field (i.e. using wild animals in their natural setting), biophysical modeling (e.g. heat transfer models). Categories for animal state were defined as: ex vivo (i.e. excised tissue measurements), carcass (i.e. in situ measurements on a dead animal), peri-mortem (i.e. measurements taken at or near death), restrained (including sedated), captive (and unrestrained or freely-behaving given surrounding constraints), trained (i.e. accustomed to experimental protocol or performing a task on command), free-ranging (i.e. freely-behaving wild animals). Classification into these categories was inclusive (i.e. one study could be classified into multiple categories) and a study’s contribution to multiple categories was weighted evenly. Only research articles were included; book and encyclopedia chapters, theses, reviews, or comments were excluded. Papers that did not include any discussion of thermoregulation even if relevant parameters were measured (e.g. blubber lipid content, body composition, movement patterns in relation to sea surface temperature) were excluded.
Recent biologging innovations are now revealing novel insights into the behavior and physiology of these difficult-to-study species (Figure 1). The application of biologging to study physiology remotely, a.k.a. physio-logging [6], has also enabled the extension of earlier, traditional controlled-access laboratory experiments (e.g.[7–11]) and modeling efforts (e.g. [12–15]) with field studies on free-ranging animals that are critical for determining the ecological relevance of findings derived from laboratory studies and modeling efforts (Figure 2). To demonstrate this, we present preliminary results from our research on the thermal physiology of freely diving elephant seals. By capturing fine-scale changes in their thermal responses while diving, our findings provide novel evidence of blubber’s dynamic nature and the complex interaction between thermoregulation and the dive response due to the dual role of peripheral perfusion.
Figure 2.
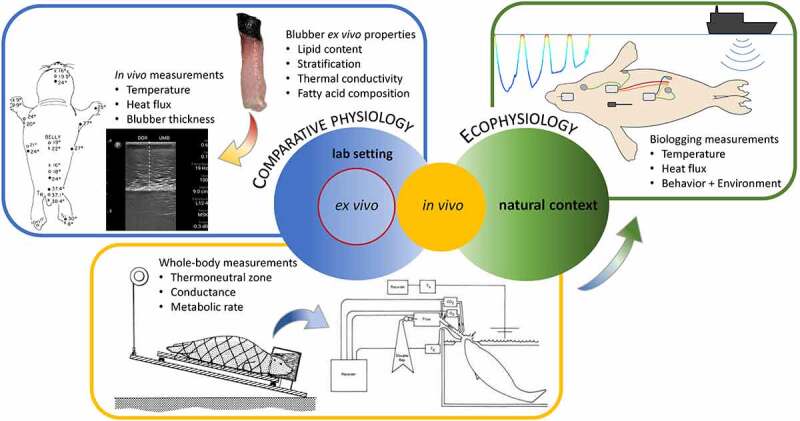
Graphical depiction of various approaches for understanding the thermal physiology of marine mammals. Both comparative physiology studies performed in the laboratory and ecophysiology studies (i.e. physiology studies conducted in the field where the ecological context is taken into consideration) have contributed to our understanding of marine mammal thermal physiology. However, most have relied heavily on species that are more readily accessible (e.g. seals). The blue box highlights how knowledge gained from ex vivo studies on blubber is relevant to understanding its function in vivo and its influence on other thermal measurements (e.g. skin temperature and heat flux). In vivo studies in the laboratory (yellow box) have provided insights into whole-body thermal dynamics using experimental methods that simulate reality to varying degrees (e.g. forced submersion experiments to trained dives). To understand the ecological relevance of their thermal limits, field studies (green box) using biologgers can record thermal responses during natural behavior and use these baseline measurements to compare those taken under disturbed conditions. Arrows depict efforts to translate findings using different approaches (ex vivo to in vivo, red to yellow) or in different contexts (lab setting to the natural context, blue to green). Skin and blubber biopsy sample from Megaptera novaeangliae and image (courtesy of L. Pallin) collected under scientific research permits NMFS 23095, ACA 2020–016, and UCSC IACUC Friea2004. Ultrasound image from Mirounga angustirostris collected under scientific research permits NMFS 21388 and UCSC IACUC Costad2009-1. Seal outline (© Jessica Kendall-Bar) was modified to depict instrumentation for research conducted under NMFS 19108 and 21388 and UCSC IACUC Costad2009-3. Seal outline depicting body temperature measurements adapted with permission from Miller & Irving 1975. Metabolism and temperature regulation in young harbor seals Phoca vitulina richardi. American Journal of Physiology 229: 506–511. Schematic of an experimental approach reprinted from Gallivan and Ronald, 1979. Temperature regulation in freely diving harp seals (Phoca groenlandica). Canadian Journal of Zoology, 57: 2256–2263. © Canadian Science Publishing. Figure depicting methods used for forced submersion experiments reprinted with permission from Zapol et al. 1979. Regional blood flow during simulated diving in the conscious Weddell seal. Journal of Applied Physiology, 47: 968:973. © American Physiological Society.
Marine mammals: Masters of endothermy in water
An evolutionary perspective of their thermal adaptations
Extant marine tetrapods display diverse adaptations spanning morphology, physiology, and behavior that allow them to thrive in challenging thermal habitats. Species with longer evolutionary histories spend a greater proportion of time in the water. This has led to a convergence of morphological adaptations [3,16], including those pertinent to thermal physiology, such as a large size (i.e. small surface-area-to-volume ratio) and little to no hair [17]. Over time, the loss of hair or fur was possible due to an evolutionary transition to blubber as the primary insulation [18,19]. While mammals typically have subcutaneous fat, most marine mammals have a specialized blubber layer composed of adipose tissue reinforced by collagen and elastin [20,21]. The fully aquatic marine mammals, such as whales and dolphins (i.e. cetaceans), are the prime example of transitioning to this morphological adaptation [22]. They rely solely on blubber for insulation.
In contrast, amphibious marine mammals have retained fur in addition to having a blubber layer because of the unique challenge of dealing with the contrasting thermal properties of air and water. These species include seals and sea lions (i.e. pinnipeds) that forage at sea but must return to land to breed or molt. The relative importance of their insulation layers depends on the frequency and duration of time at sea. Some species can strategically partition their time between land and water to meet their thermal demands [23,24]. Other species are constrained by long seasonal periods in either environment. They must therefore be able to maintain thermal balance in both [25,26].
In water, blubber is advantageous due to its hydrodynamic qualities and greater functional resistance to pressure compared to fur. For this reason, deep divers do not rely on their fur for insulation while at depth. The pinnipeds’ broad spectrum of diving capabilities is reflected in their relative blubber thicknesses and fur densities [27]. These species provide interesting case studies for comparing and understanding the role of blubber in their thermal flexibility [28,29].
What makes blubber the ideal insulator also makes it challenging for in vivo studies. Blubber is internal, which allows for blood to bypass this insulation when needed (Figure 3a). A complex microvasculature network exists within the skin and blubber of marine mammals [23,24,30–32]. Superficial vessels are associated with arteriovenous anastomoses. These structures allow fine-scale adjustment to blubber’s insulative and transmissive properties by either constricting or dilating to regulate peripheral perfusion. By effectively modifying their conductance, marine mammals control the characteristics of the thermal gradient (Figure 3b) and can maintain a high core body temperature while immersed in water [33,34]. Therefore, understanding the thermoregulatory strategies of diving marine mammals requires examining the physiological mechanisms that influence heat transfer at their periphery.
Figure 3.
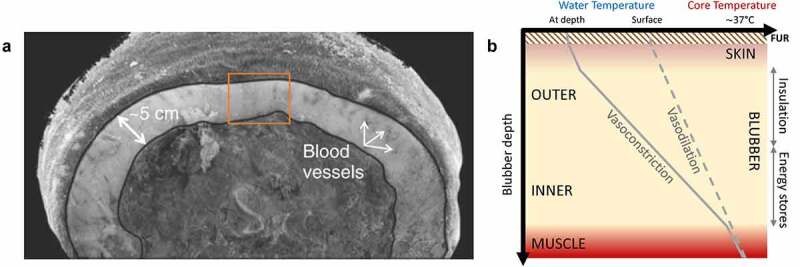
A cross-section from an adult Weddell seal (Leptonychotes weddellii) with blood vessels visible within the blubber layer. The orange box denotes the area represented in (b). Adapted with permission from Springer Nature: Marine Mammals by Randall W. Davis © 2019 (a). A conceptual figure depicting blubber’s various thermal states dependent on peripheral perfusion, and blubber’s functional roles due to its stratification. The temperature gradient across the blubber layer will vary based on the degree of peripheral perfusion during a dive. A larger gradient will generally occur at depth where lower water temperatures and peripheral vasoconstriction associated with the dive response lead to cooling of the periphery. The inner and outer blubber layers primarily serve different functions (energy store and insulation, respectively). This stratification is due to the relative proportional composition of fatty acids which confers different physical and biochemical properties (b).
Modes and spatial heterogeneity of heat transfer
Marine mammals experience fewer avenues of heat transfer in water: there is no evaporative heat loss, and radiation is minimal compared to other avenues of heat loss. Thus, conduction and convection, both of which depend on the temperature differential, are the principal mechanisms through which heat transfer occurs. The temperature gradient and the thickness of the blubber layer determine the conductive heat transfer through the blubber and ultimately how much core body heat reaches the skin. Convective heat transfer causes the heat at the skin’s surface to dissipate to the surrounding water. Unlike the thermal conductivity of blubber, which is a single readily measured property, the convective heat transfer coefficient depends on several factors, including fluid properties and type of fluid flow [35]. However, often neglected is the variability introduced by internal forced convection, which occurs due to active transfer of heat through the circulation of blood [36–38].
Many studies have referred to conduction through the blubber layer when both conductive and convective mechanisms were considered. To account for this, the blubber conductivity, which describes how readily heat transfers through a material, was adjusted using a correction (“perfusion”) factor (e.g. [15,39]). The values of marine mammal blubber conductivity are typically measured from excised blubber and genuinely reflect its intrinsic property. In live blubber, variable peripheral perfusion will contribute to heat transfer through active transport resulting in a different heat transfer rate than would be expected by conduction alone. Circulating blood thus sensibly changes the conductivity of the blubber layer [40–42]. Analyzing convective heat transfer is more complex than conduction. By incorporating a variable blubber conductivity relative to peripheral perfusion, the biophysical problem is simplified.
Kvadsheim and Folkow [38] measured the total heat transfer (conductive and convective) with heat flux sensors in harp seals (Pagophilus groenlandicus) resting in the water to determine the circulatory effects of heat transfer through the blubber. This was compared to the calculated conductive heat transfer based on the blubber temperature gradient measured with thermistors placed in the deep blubber and subcutaneously. Convective heat transfer was minimal in cold water, and heat loss was mainly from the trunk. An increasing proportion of convective heat loss from the flippers as water temperatures increased to ~23-24°C documented the role of flippers as heat dissipators when seals were heat-stressed. This has also been demonstrated for the flippers and flukes of cetaceans [41,43] and the hands and feet of humans [44]. The lack of insulation in these appendages and their large surface-area-to-volume ratio make these sites ideal as thermal windows, where rapid changes in heat transfer are induced through physiological mechanisms (i.e. regulating blood flow [44–46]).
This experiment demonstrated how ambient temperatures influence vasomotor control in seals, albeit to varying degrees across the body, which aligns with the expansive literature on the effects of temperature on vasoconstriction and vasodilation in humans [47–51]. While equivalent mechanisms underlie peripheral perfusion, marine mammals may have greater thermal capabilities than humans, in part, due to a greater density and distribution of arteriovenous anastomoses. Arteriovenous anastomoses are primarily found in the glabrous skin of humans and play a significant role in regulating body temperature with minimal energy expenditure within the thermoneutral zone [46,50]. In contrast, marine mammals have arteriovenous anastomoses along their body surface – although some more than others [30,31] – which allows the well-insulated trunk to substantially contribute to total body heat flux [38,52,53]. The different thermal responses of glabrous (i.e. non-hairy) and hairy skin regions of humans are analogous to the different roles of flippers or fins and the body trunk of marine mammals [46]. However, the importance of skin temperature as a feedback signal in marine mammals is unclear especially since water dampens the variation in temperature across the skin compared to in air [41,53–60]. Given the limited modes of heat transfer in water and the additional constraints that diving imposes on regulating their body temperature (Figure 4), marine mammals are likely to rely on arteriovenous anastomoses and adjustments in peripheral perfusion as a primary mechanism for thermoregulation.
Figure 4.
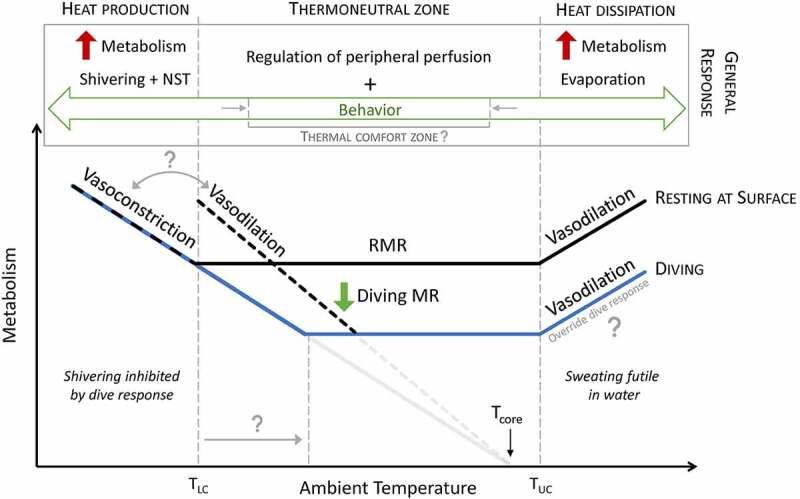
Schematic figure depicting the relationship between ambient temperature and metabolism (black line) and the general thermal responses within the thermoneutral zone, above the upper critical temperature (TUC), and below the lower critical temperature (TLC). For marine mammals in water, evaporative heat loss becomes futile, and diving imposes additional constraints on heat production due to the need to conserve oxygen. Therefore, marine mammals must compensate for these limited modes of heat exchange through vasomotor changes and behavior. Several questions remain to address how diving (and the associated metabolic response, blue line) modulates their thermal responses (represented by the question marks).
Perspectives from the field of diving physiology
In addition to the challenges mentioned above faced by endotherms in the marine environment, free-ranging marine mammals must balance the physiological demands of thermoregulation with exercise and diving. The cardiovascular system is integral to the physiological responses associated with the dive response, exercise, and thermoregulation. These responses could either be in sync or conflict, requiring marine mammals to coordinate their activities or compromise their performance [27]. The dive response involves a suite of cardiovascular adjustments, including apnea, bradycardia, and peripheral vasoconstriction. Together they reduce blood flow and oxygen consumption of non-essential tissues to conserve their “on-board” oxygen supply for vital organs, such as the heart and brain [61]. Therefore, blood flow regulation is essential for the redistribution of oxygen and heat within the body. While endotherms are generally considered homeotherms, homeothermy may not be the most efficient strategy for marine mammals due to the physiological demands of diving [62]. Any regional or systemic drop in body temperature would reduce tissue’s metabolic requirements (i.e. oxygen consumption) due to the temperature dependence of metabolic energy production (quantified by the Q10 temperature coefficient). This would extend the aerobic dive limit by decreasing the rate at which on-board oxygen stores are depleted. Hypothermia-induced metabolic suppression and regional heterothermy have been posited as separate hypotheses to explain how marine mammals increase their aerobic dive capacity [63,64]. The role of hypothermia and peripheral cooling has been primarily discussed in the diving physiology literature [63–66]. Still, the thermoregulatory implications of these diving strategies have not been adequately investigated. By integrating and building on knowledge gained in the separate considerations of diving physiology and thermal physiology, current research is advancing our understanding of the complex interactions between the dive response and thermoregulation.
Insights from ex vivo and in vivo lab studies
Unlike research in diving physiology that has embraced physio-logging to simultaneously record dive profiles along with physiological responses (e.g. heart rate and blood oxygen saturation) in freely diving species [65,67–74], progress in thermal physiology has heavily relied on ex vivo studies (e.g. [23,30,31,75–77]) or those on captive animals in the laboratory (e.g. [7,37,78–80]) (Figure 1). Similar to the differences between studying nude humans in the lab and humans in their routine states (e.g. clothed and working [29]), these laboratory findings demonstrated the maximum capabilities of marine mammals. Although these studies could not address whether or how often animals reached these maximum capabilities in their natural environments, they have, nonetheless, provided key insights that would not have been possible through field studies.
Structure meets function in blubber
Ex vivo studies have enhanced our understanding of the role of blubber by investigating the physical (e.g. adipocyte morphology, thickness, thermal conductivity [81–85]) and biochemical (e.g. lipid content, fatty acid composition, and stratification [19,76,86–88]) properties of blubber (Figure 2) . These have shown that most marine mammals have stratified blubber layers. The outer layer primarily functions as insulation where lipids with lower melting points confer flexibility particularly at lower temperatures. In contrast, the composition of the inner layer makes it more readily metabolized and thus primarily serves as an energy store (Figure 3b). The relative importance of either function is also dependent on age and season [84,85,89], as well as species-specific thermal habitats and life-history strategies [32,75]. For example, blubber as an energy store may be more important for older animals during the breeding fast (especially for capital breeders) [90–92]. In contrast, blubber as an insulator may be more important for younger, smaller animals that have a greater body surface-area-to-volume ratio and/or are nutritionally dependent [7,84,93,94].
The tradeoffs between blubber’s dual role as insulation and an energy store are directly influenced by its relative thickness. Many studies using captive species have described the topographical distribution of the blubber layer and its significance [79,88,90,92,95–98]. In addition to seasonal and ontogenetic changes in blubber thickness, other changes in the blubber layer occur on much shorter time scales due to the physiological responses associated with diving, particularly the regulation of peripheral perfusion. Blubber is considered a dynamic insulator [99,100], and understanding its context-dependent roles can only be addressed in vivo.
A dynamic insulator used as a buffer zone
Many early studies investigated the thermoregulatory capabilities and whole-body thermal dynamics of marine mammals in laboratory settings (Figure 2). As early as the 1950s, experiments were performed in vivo to define a species’s lower critical temperatures and whether an elevated metabolic rate is required to maintain thermal balance in an aquatic environment [9,10,29,34,37,54,78,93,101–104]. Irving and Hart [34] found differences in the blubber gradient explained how seals exhibited similar metabolic rates in air and water of the same temperature despite the greater cooling effects of water (Figure 5). They concluded that peripheral cooling is essential to allow homeothermy in aquatic “bare-skinned” mammals. Such labile control of insulation through changes in vascular heat transfer would be advantageous over the static insulation provided by thick fur.
Figure 5.
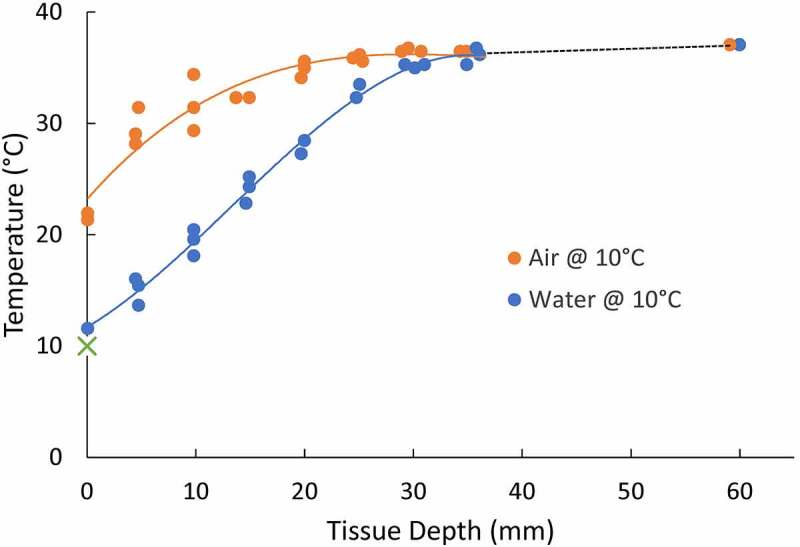
Tissue temperature gradients of a harbor seal (Phoca vitulina) in air (orange) and water (blue) at 10°C (green “x” on y-axis). Modified from Irving and Hart, 1957. The metabolism and insulation of seals as bare-skinned mammals in cold water. Canadian Journal of Zoology, 35: 497–511. © Canadian Science Publishing.
Fewer studies have investigated the upper critical temperatures of marine mammals while immersed. This is partly because they cannot respond to heat stress in water with evaporative cooling [12,105] (Figure 4). Without such an early warning indicator, there is the risk of surpassing their thermal tolerance limit, resulting in severe heat injury or death. Still, an experiment on captive bottlenose dolphins (Tursiops truncatus) resting in water examined how dolphins avoid hyperthermia when moving from cooler pelagic waters to warmer coastal waters [106]. Water temperature was progressively increased and then maintained above skin temperature to prevent heat loss from the body. Heat flux values showed the rate of heat gain decreasing over time. Simultaneously, rectal temperatures declined up to 1.3°C of baseline temperature. This reduction is surprising given the anticipated increase of 1.6°C based on basal metabolic heat production. These results imply that dolphins can redistribute core body heat to their periphery via increased peripheral blood flow. By using their blubber layer as a buffer zone to store heat, dolphins can achieve short-term heat tolerance (~1 hr) in coastal waters. Subcutaneous thermistors, alongside experiments investigating the phase change properties of blubber, could provide further evidence of blubber’s dynamic role and its influence on peripheral heat flow and metabolic rates.
While similar experiments with large cetaceans would be challenging, modeling indicates that the regulation of peripheral perfusion is critical for long-term thermal homeostasis. Models based solely on the insulative properties of the blubber layer suggest that large whales are over-insulated for their size and metabolism [15]. While blood flow and metabolism were estimated, models that included peripheral circulation provided reasonable predictions of in situ temperature profiles of freshly harpooned fin and sei whales in the mid-1900s [107]. These results suggest that whales must maintain some blood flow to the skin, even in the coldest waters they encounter, to maintain thermal balance. In warmer waters, the level of blood flow would increase exponentially.
These studies have provided key insights into the physiological responses to water temperature and thermal limits of species spanning three orders of magnitude in size and adapted to vastly different thermal habitats. However, the ecological relevance is limited since few marine mammals remain stationary in water for prolonged periods. Thus, an essential extension of these studies will require investigating how exercise and diving affect thermal dynamics.
Thermal effects of exercise and diving
The paradoxical interaction between the physiological demands of diving and exercise has interested many researchers [108–112]. Studies have found that diving mammals seem to avoid the classic exercise response [1, 2,113] and are rather well-adapted for exercise while breath-holding. A reduction in heart rate associated with the dive response reduces metabolism and conserves oxygen but is contrary to the expected elevated heart rate during exercise [100,114,115]. Additionally, peripheral vasoconstriction reduces blood flow to the periphery during the dive, thus reducing oxygen supply to locomotory muscles [63]. To meet their exercising muscles’ oxygen demands during breath-hold dives, marine mammals swim efficiently and utilize their larger muscle myoglobin oxygen stores [116,117]. Studies have shown that the dive response is modulated by exercise [112,118–120]. Yet, whether the dive response is modulated by thermoregulatory demands and the associated consequences of exercise and diving on thermal homeostasis is unknown.
To investigate the thermal effects of exercise, the spatial patterns of heat flux in Steller sea lions (Eumetopias jubatus) were examined while stationary in the water, swimming naturally in a swim flume, and swimming under increased load [55]. Areas with less insulation (hips and shoulders) had the highest heat flux values compared to other more insulated areas (middle and axillary girths) regardless of the level of physical exertion. It was concluded that the exercising Steller sea lions were not under thermal stress. Additionally, they used certain areas to preferentially dissipate heat (which parallels findings from [121]). Studies of captive dolphins have shown that the dorsal fin serves as a flexible site for heat dissipation [41,78,102,122], similar to the lesser insulated body surface areas of the Steller sea lions.
In addition to activity level, other factors influencing the use of thermal windows to control thermal balance are body size and water temperature. For example, Hawaiian spinner dolphins (Stenella longirostris) rely on activity-induced thermogenesis to maintain their body temperature in waters near their lower critical temperature, which are not uncommon in their habitat range [78,102]. On the other hand, a larger delphinid species, the Pacific bottlenose dolphin, experienced elevated core temperatures after intense exercise, leading to a broader range in body temperatures than the stenothermic Hawaiian spinner dolphins [78]. Despite these differences, the authors concluded that blood flow to the dorsal fin is independent of that to the body trunk for both species. Thus, the interacting effects of exercise and thermoregulation are species- and context-specific based on thermoneutral zones and capacities for thermogenesis.
Unlike studying exercise in a swim flume or large pool, using animals in a captive setting limits our ability to investigate how much the dive response modulates thermal responses because pools are shallow compared to the depths commonly reached by diving marine mammals [27]. Noren et al. [109] took advantage of trained animals in a channel connected to the open ocean and recorded heat flux during dives using hand-held devices. Reduced heat flux at depth (15 m) compared to the surface suggests that heat dissipation is limited until the end of the dive where anticipatory tachycardia occurs. In an exceptional situation, elevated heat flux values at the dorsal fin were observed at depth on a dolphin that had been vigorously active prior to the dive. This indicates there was a momentary override of the dive response to dissipate heat through a thermal window. These findings beg the questions: how often is thermoregulation prioritized during natural diving conditions, and how much volitional control is involved in coordinating these potentially conflicting demands? Translating research with trained dolphins to the natural context can provide a sense of how close they operate to their thermal limits and their physiological capacity to adapt when faced with additional stressors (Figure 2).
Psychological influences of physiological responses
Researchers have used animals trained or forced to dive or haul out to investigate how anticipatory behavior and psychological influences modulate physiological responses (Figure 2). A diving physiology study compared the dive response in trained versus naïve harbor seals (Phoca vitulina) under forced submersions to investigate the effects of anticipation [123]. Compared to naïve seals, trained seals accustomed to 3-minute forced submersions had significantly higher heart rates. They thus maintained greater blood flow to the muscle (measured using laser Doppler flowmetry). In one instance, the submersion was prolonged to 5 minutes without any cues. The heart rate and muscle blood flow profiles show abrupt reductions occurred at precisely 3 minutes into the submersion and were maintained at levels comparable to naïve seals until the end of the submersion (Figure 6). Although forced submersion is far from seals’ natural diving behavior, this and other studies (e.g. [63,124–126]) demonstrated that animals can modify their physiological response when the need arises.
Figure 6.
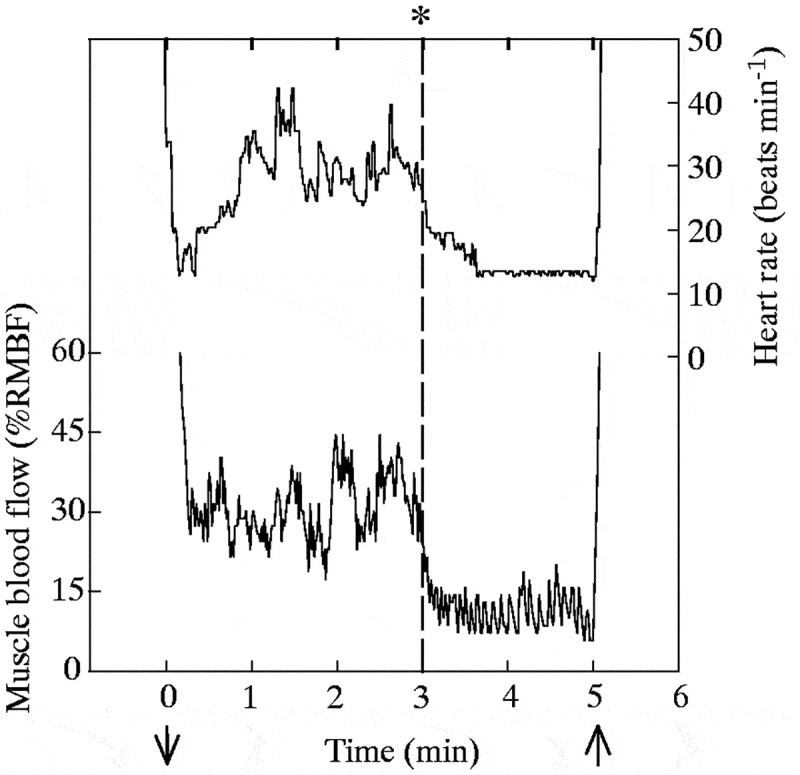
Heart rate and muscle blood flow (as a percentage of mean resting muscle blood flow, %RMBF) of a trained harbor seal (Phoca vitulina) during a 5-minute submersion. The seal was accustomed to 3-minute submersions after which its heart rate and muscle blood flow declined to levels similar to naïve seals during submersion. The abrupt change at 3 minutes (dashed line) demonstrates the psychological influences on the physiological responses associated with the dive response. Reprinted with permission from Jobsis et al. 2001. Effects of training on forced submersion responses in harbor seals. The Journal of Experimental Biology, 204: 3877-3885. © The Company of Biologists Ltd.
To investigate the degree of control seals exert over heat dissipation through thermal windows, Erdsack et al. [127] used infrared thermography on trained harbor seals. Seals that hauled out voluntarily remained out of the water for several hours and developed thermal windows within minutes regardless of the environmental conditions. Significant amounts of heat dissipation occurred through these thermal windows, which could take several minutes to close once the animal returned to the water resulting in large energy losses. In contrast, when seals hauled out on command during training sessions, they anticipated transitioning between water and land several times. None developed thermal windows, which may be explained by the influence of their psychological state on the autonomic nervous system that regulates peripheral perfusion [128]. By suppressing the formation of thermal windows, amphibious marine mammals minimize excessive heat loss upon returning to water, and thus overall thermoregulatory costs. In addition to demonstrating how physiological demands are balanced to minimize energetic costs, these examples exemplify the value of laboratory studies because sometimes there are not simple parallel approaches in the field.
Thermal responses during natural diving behavior
Unlike captive studies where animal behavior is manipulated or limited to some extent, wild animals exert control over their activities. During normal, undisturbed diving, it is reasonable to assume that marine mammals that do not frequently haul out are ultimately capable of maintaining thermal balance. Investigating the mechanisms that underlie their physiological thermoregulation requires using remote methods, i.e. biologgers with physiological sensors (Figure 2).
Temperature regulation as a diving strategy
Studies in diving physiology have used state-of-the-art biologgers to record the impressive breath-hold dives of phocids (true seals) [129–132]. The addition of physiological sensors to time-depth recorders tested the hypothesis that hypothermia while diving enhances aerobic dive capacity and provided insights into body temperature regulation strategies. By measuring arterial and venous blood temperatures as a proxy for core body temperature, Meir and Ponganis [65] demonstrated that a high mean core body temperature (36–37°C) is maintained throughout routine diving behavior (10–30 min) of juvenile northern elephant seals (Mirounga angustirostris). Additionally, there was no strong relationship between mean blood temperatures and duration of individual dives. These findings suggest that core hypothermia is not a common strategy in routine dives.
Other studies measuring muscle temperature have reported varying degrees of tissue cooling. For example, there was little to no decline in muscle temperatures in adult Weddell seals despite reductions in aortic temperatures during long dives [130]. On the other hand, a decrease of 4°C occurred in the muscle of a diving immature elephant seal [131]. Both authors concluded that the working muscle was either hypometabolic or maintained some level of perfusion to allow dissipation of locally generated heat. They also noted that peripheral cooling extends beyond the insulation into muscle [9,131]. The degree to which this occurs and where core body temperature measurements are taken influence how the data are interpreted to align with different thermal strategies [66,133]. As emphasized in these studies, the heterogeneity of body temperature precludes obtaining a representative whole-body temperature from just one location [133]. Multiple measurements at different locations enable a more holistic understanding of heat distribution throughout the body and the mechanisms contributing to heat transfer.
Peripheral perfusion patterns: Insights from modeling
Even if multiple simultaneous measurements are possible, numerous factors are impossible to control in the wild, making it difficult to decipher which variables are independent or dependent. To develop a mechanistic understanding of thermal dynamics in a swimming and diving animal, modeling approaches can simulate background variation while testing specific hypotheses regarding variables or parameters of interest. With a time-series of in situ measurements to compare model outputs, Boyd [33] modeled peripheral circulation as “on-off” states in a heat balance model to gain insight into the processes affecting the temporal dynamics of the temperature gradient between the water and skin of diving Antarctic fur seals (Arctocephalus gazella). During dives, the temperature gradient decreased and then increased toward the end of the dive. Transient increases occurred at the bottom of the dive and were attributed to changes in activity. Their model tracked this variability in the temperature gradient reasonably well. Discrepancies between the model output and in situ measurements were likely due to an overly simplistic representation of the regulation of perfusion as an abrupt switch between two states rather than a more graded process.
Fine-scale changes in peripheral heat flow while diving
Unlike fur seals which maintain a large temperature gradient between their skin and water [33], marine mammals relying on blubber will experience more profound variations in their insulation due to changes in peripheral perfusion in response to water temperature and as a result of the dive response. Laboratory experiments have demonstrated the former by measuring physiological variables while exposing marine mammals to temperature-controlled water baths [9,34,78,106]. To explore the latter, we recorded heat flux and peripheral temperatures in freely diving juvenile northern elephant seals using custom-made biologgers.
Heat flux showed consistent within-dive patterns: seals generally lost heat throughout most of the dive and gained heat throughout the short post-dive intervals (Figure 7a). A departure from this pattern occurred during shallow diving bouts. Instead of gaining heat at the surface, the seals lost heat throughout the ascent and surface interval (Figure 7a). This thermal response during shallow diving bouts may occur because their shallow dives are in relatively warm water (>10°C). As a result, they experience smaller changes in water temperature within a dive cycle. Higher arterial temperatures during short, shallow diving bouts observed by Meir and Ponganis [65] also suggest a greater need to dissipate heat than conserve heat while in relatively warm waters to maintain thermal homeostasis. When they dive deeper into cold waters (~5°C), their skin temperature drops to within a few degrees of water temperature. During the latter portion of their ascent, their skin is colder than the surrounding water, which reverses the temperature gradient resulting in a transition to gaining heat. How these different heat flux patterns ultimately affect their thermal balance – and the relative importance of activity and body size on thermal dynamics – is currently being investigated.
Figure 7.
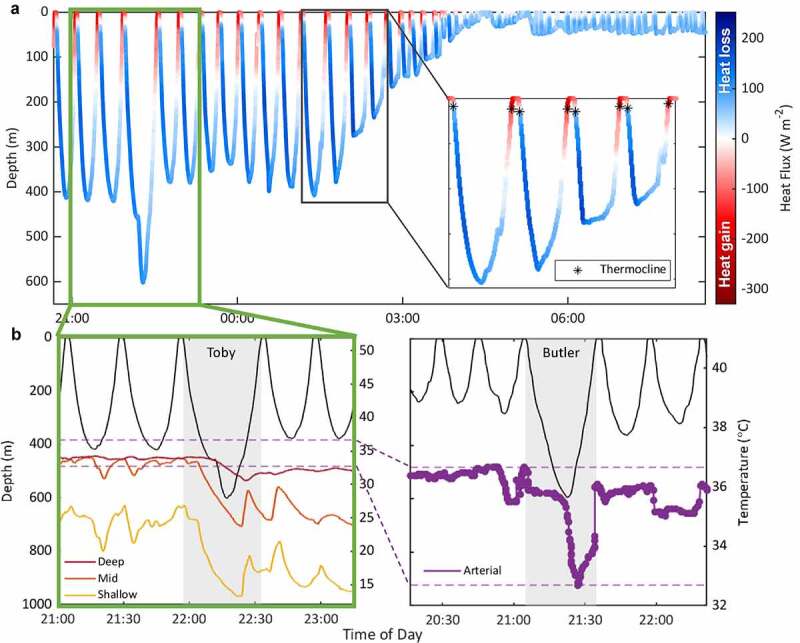
The dive profile of Toby, a translocated juvenile northern elephant seal (Mirounga angustirostris), with the color indicating the direction of heat flux (red = heat gain, blue = heat loss) throughout the dive. The inset shows four deep dives (>200 m) and how the transition from heat gain to heat loss occurs near the thermocline (denoted with asterisks) on the descent, but the opposite transition occurs at deeper depths during the ascent. Heat flux values [W m−2] in (a) are preliminary values pending post-deployment calibrations. Raw voltage output has been corrected for the added thermal resistance of the sensor and attachment mechanism (determined experimentally) as well as the unique sensor’s calibration constant (provided by the manufacturer). A section of the dive profile denoted by the green box containing the seal’s deepest dive in (a) is shown in greater detail (b, left) with temperature profiles at three depths within the blubber layer (deep = red, mid = orange, shallow = yellow). The drop in blubber temperature during the deep dive (b, left) is analogous to the drop in arterial temperature (b, right) recorded in Butler, a translocated juvenile northern elephant seal by [65], although arterial temperature declined more abruptly. Both blubber and arterial temperatures rewarmed before the end of the dive, and arterial temperatures remained comparatively warmer (note the difference in temperature range of the y-axes; the range of arterial temperatures is shown on both figures with the purple dashed lines). The figure showing Butler’s data is modified from Meir and Ponganis, 2010. Blood Temperature Profiles of Diving Elephant Seals. Physiological and Biochemical Zoology, 83(3): 531–540. © The University of Chicago Press.
Another variation in pattern is the transition from heat gain to heat loss occurring near the thermocline on the descent, while the opposite transition occurred at deeper and more variable depths on the ascent (Figure 7a). Whether dive conditions and an individual’s dive capacity contribute to the earlier transition remains to be tested but aligns with temperature profiles on deep dives suggesting increased blood flow and perfusion of the periphery during the ascent (Figure 7b). Arterial blood temperature [65] and blubber temperatures on long dives (≥30 mins) declined but always rewarmed before the end of the dive. This is consistent with the anticipatory tachycardia and the resulting redistribution of blood flow that marine mammals experience during the dive ascent [112,131,134,135].
Blubber temperatures showed a distinct gradient, with greater variation across dives than within dives (Figure 8b). While the minimum temperature varied among individuals, deep blubber temperatures fell as low as 22.9°C during deep dives (>200 m), resulting in a blubber gradient (ΔTavg) of 14.2°C. During shallow diving (<100 m) and extended surface intervals, the blubber gradient dropped to <5°C. The transition from a large blubber gradient at low temperatures to a smaller gradient at higher temperatures occurred more abruptly than the opposite transition (Figure 8b). Simultaneous changes in diving behavior were not apparent in either case. This suggests that these thermal responses were not simply due to passive warming or cooling from changing water temperatures but were actively regulated through vasomotor changes.
Figure 8.
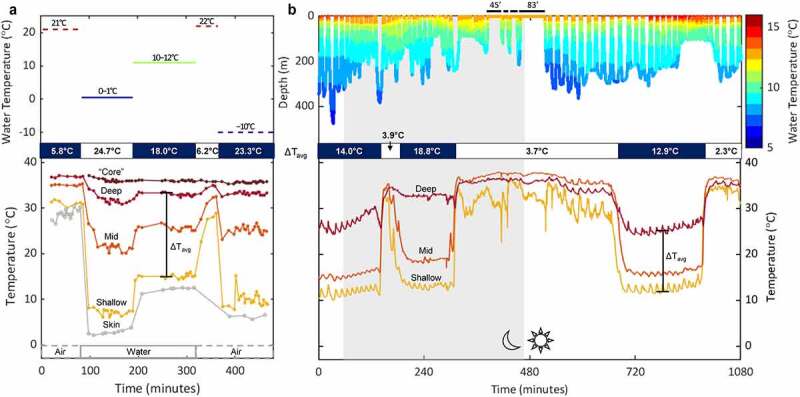
Skin and tissue temperature measurements of a harbor seal (Phoca vitulina) in air and water at temperatures indicated by the dashed and solid line segments, respectively (a). Tissue temperatures were measured at 3 mm (shallow), 13 mm (mid), 23 mm (deep), and 42 mm (“core”), which included the blubber layer (<25 mm thick) and deeper tissue. The seal was allowed to equilibrate for at least one hour at each ambient temperature while recording measurements every 4 minutes. Adapted from Hart and Irving, 1959. The energetics of harbor seals in air and water with special considerations of seasonal changes. Canadian Journal of Zoology, 37: 447–457. © Canadian Science Publishing. The dive profile of a translocated juvenile northern elephant seal (Mirounga angustirostris) that dove continuously for 7 days (18 hours shown in figure) and experienced water temperatures between 5–16°C (b, top). Four consecutive extended surface intervals are marked with thick black bars along with the duration (in minutes) of the first and last extended surface intervals. Temperature profiles at three depths within the blubber layer (deep = red, mid = orange, shallow = yellow) reveal how the temperature gradient within the blubber layer (ΔTavg = average(Tdeep−Tshallow)) undergoes large variations across dives (b, bottom). The cyclical nature of diving also results in smaller fluctuations within dives that is not observed in the resting harbor seal in (a) .
Similarly, Hart and Irving [9] attributed the rapid changes observed in subcutaneous temperatures of captive harbor seals in response to changing ambient temperatures to active regulation (Figure 8a). It is worth comparing blubber temperature data from captive harbor seals (Figure 8a) to wild northern elephant seals (Figure 8b) to highlight how similar measurements taken 50 years apart yielded valuable insights into peripheral perfusion as a fundamental physiological mechanism in marine mammal thermoregulation. When animals are exposed to constant air or water temperature within their thermal limits, their thermal responses will stabilize after some time. On the other hand, the cyclical nature of diving results in more complex changes mainly in the peripheral body, which will consequently affect heat transfer. While minimum water temperature (at depths >100 m) differed by less than 5°C across dives, shallow blubber temperatures varied as much as 26°C. By measuring similar physiological variables on a freely diving seal, our study replicates past controlled experiments in an uncontrolled setting, incorporating an essential behavioral component (i.e. diving). Our field research builds on previous laboratory findings and extends our understanding of their thermal responses to the natural context.
Our data from freely diving elephant seals further demonstrates the utility of combining multiple continuous measurements at fine resolution to provide insight into the complex physiological responses to the interacting demands of diving and thermoregulation in marine mammals. Together, the heat flux patterns and temperature profiles indicate that allowing peripheral cooling prevents conflict with the dive response, especially during deep-diving bouts. During the short surface intervals (2–3 mins), reperfusion of the periphery associated with a relaxation of the dive response increases blubber temperatures slightly (mean increase of <1°C at all blubber depths, max of 5°C in the shallow blubber). Still, longer periods of shallow diving or extended surface intervals seem to be required to return the deep blubber close to normothermia (35.3 ± 2.1°C). Whether these cyclical changes in perfusion contribute to maintaining whole-body thermal balance or increase thermoregulatory costs is still unknown. Nonetheless, our data supports the idea that regional heterothermy – more specifically, peripheral hypothermia – is preferred over strict homeothermy as a thermal strategy and may offer a compromise between thermal and oxygen demands for continuously diving marine mammals, like elephant seals.
Considerations for future progress in the field
Understanding how marine mammals survive in such a thermally challenging environment is a complex subject that requires a multi-pronged approach, particularly given the difficulty of studying marine mammals. Here we focused on laboratory or field studies that have provided insight into the role of peripheral perfusion as a physiological mechanism that directly regulates or indirectly influences heat distribution within and across the body. We acknowledge that this is most relevant for marine mammals that primarily rely on blubber for insulation. Integrating in vivo and field approaches will deepen our understanding of how thermal responses interact with conflicting physiological demands, such as the dive response. Used together, these approaches will provide ecologically relevant insight into an important but understudied aspect of marine mammal physiology and energetics (Figure 2).
Outstanding gaps: The many roles of peripheral perfusion
Future work should address other conflicting physiological demands that require peripheral perfusion and their interactions with thermoregulation. For instance, molting is a necessary phenomenon that requires perfusion of the skin to replace old skin and fur. The form and phenology of molting vary widely, from annual catastrophic molting to continuous gradual molting. It often depends on whether animals can find thermal refugia to minimize the energetic costs of increased heat loss associated with skin perfusion [136–139]. Similarly, wound healing requires perfusion of the injured site, potentially leading to a tradeoff in short-term energetic costs and long-term health and survival. Haul-out periods for amphibious species, or seasonal residency at warmer latitudes for long-distance migrators, allow for temporal separation of these physiological demands that would otherwise increase thermoregulatory costs. Finally, unlike fur, blubber is a living tissue that serves as an energy store, and depositing or metabolizing lipid stores also requires perfusion of this layer [32,140,141]. Thus, in addition to the dual role associated with thermoregulation and diving that was the primary focus of this review, peripheral perfusion is critical for many other physiological processes. How these conflicting demands interact with each other warrants further investigation.
Challenging but insightful measurements
While temperature measurements are commonly used in biologging studies and have various applications (e.g. stomach temperature telemetry in foraging ecology studies [142,143]), heat flux has generally been limited to studies directly interested in thermal physiology. Nevertheless, heat flux data have proven to be quite informative. Still, the nature of the measurements is more complex than temperature measurements. The sensor provides thermal resistance at the measurement site, resulting in heat flux values that do not accurately represent heat flux from adjacent skin surfaces [144]. Therefore, it is critical to determine a correction factor for the sensor and the attachment mechanism [38,41,145–148]. Additionally, observations of high variability in heat flux values both among and within individuals are common, which should caution the quantitative interpretation of heat flux values [41,149].
Some studies have also noted large changes in heat flux associated with small temperature changes between the skin and water. Whether this discrepancy has a physiological underpinning warrants further investigation [109,110,145]. Changes in convective heat transfer via blood flow may explain these periodic increases in heat flow at localized skin surface areas [15,56,150]. However, obtaining a direct measure of peripheral blood flow is difficult. Blood flow measurements have only been done on a few captive animals [123,124,151–156]. While many direct and indirect methods of measuring blood flow exist and improve human applications (see [47,157] for methodological reviews), their sensitivity to motion is a significant barrier to their use on free-ranging animals.
As demonstrated in freely diving juvenile elephant seals, blubber temperature is more readily measured than blood flow and is thus a good proxy for peripheral perfusion. Using indirect measures, such as peripheral temperature and derivatives, to infer peripheral perfusion is not a novel approach (examples primarily from the seabird literature, e.g. [140,141,158–160]) and even has diagnostic potential in biomedical applications [161]. Unlike the skin surface, blubber is not directly affected by external influences, such as convective heat loss from water flow. Instead, blubber temperatures reflect internal processes that serve to maintain homeostasis.
Integrating behavioral and physiological thermoregulation
While this review focused on the critical role of peripheral perfusion, it is essential to underscore that behavior is generally the first line of defense for maintaining thermal homeostasis. Schlader [162] noted less than a decade ago that behavioral thermoregulation is often neglected in human studies, even more so than in animal studies. Animals outside their thermoneutral zone employ more energetically costly strategies such as sweating or shivering to restore thermal balance (Figure 4). However, conflicting physiological demands may prevent marine mammals from employing these mechanisms. For example, the need to conserve water may explain why California sea lions (Zalophus californianus) neither pant nor sweat despite having functional sweat glands, unlike elephant seals. Rather, both species seem to rely on behavior to deal with the warm air temperatures within their home range and prevent heat stress [163,164]. Similarly, diving marine mammals cannot employ sweating or shivering while diving [101]. Therefore, they must either avoid exceeding their upper and lower critical temperatures or utilize behavioral strategies that increase their sensible heat exchange effectiveness (Figure 4).
Natural conditions may allow behavioral interventions to avoid reaching the boundaries of the thermoneutral zone and remain in their thermal comfort zone [162,165] (Figure 4). Within this zone, marine mammals combine physiological mechanisms and behavioral strategies on different timescales to maintain thermal balance. For example, sea otters minimize thermoregulatory costs by spending a significant amount of time floating at the surface and balancing thermal substitution from activity and the heat increment of feeding [166]. Marine mammals that haul out can switch between the two media as a form of behavioral thermoregulation [23,26,57,164]. Deeper diving marine mammals may modify their diving behavior to take advantage of the steep gradients in water temperature to adjust their thermal balance (as observed in ectothermic and/or regional endothermic vertebrates, pelagic tuna and sharks [167–171]). While regulating peripheral perfusion provides a faster mechanism for adjusting heat transfer, behavioral strategies, such as extended surface intervals, may allow for slower but larger adjustments in overall heat balance that would otherwise conflict with the dive response, especially during non-routine behavior.
To truly integrate our understanding of behavioral and physiological thermoregulation, we need to study wild animals minimally disturbed and behaving naturally (Figure 2). Most behavioral thermoregulatory studies of wild marine mammals were carried out on animals on the beach [25,57,164,172–174] or at the water’s surface near the coastline [174] (Figure 1). With the rapid integration of inertial measurement units into biologgers, sophisticated interpretations of their non-observable behavior at-sea are now possible [175,176]. These can be combined with measurements relevant to thermal physiology to determine how activity level and activity-induced thermogenesis influence peripheral dynamics and heat balance.
Studies of how behavior and intense activity modify thermal balance have implications for understanding the thermoregulatory costs of marine mammals exposed to anthropogenic disturbances. Disturbances, such as fisheries interactions, ship traffic, or sonar, can lead to altered diving behavior or haul-out patterns (Figure 2). For example, simulated sonar has been shown to cause marine mammals to prolong their dives [177]. If the dive response is modulated by dive conditions, these unanticipated prolongations will reduce heart rate and peripheral perfusion. Such cardiovascular adjustments would result in secondary consequences for heat dissipation or potentially a complete override of thermoregulatory demands.
To investigate these issues related to conservation, we need to study their thermoregulation in an ecophysiologically relevant context (Figure 2). By performing controlled exposure experiments on wild animals [178–180], we can quantify biologically significant and context-dependent responses to disturbances (e.g. [181]). Minimizing or avoiding activities known to increase the susceptibility of marine mammals to thermal imbalance will help reduce the cumulative impacts of anthropogenic stressors on marine mammals. However, we must first establish a baseline understanding of their thermal physiology during natural behavior before understanding the pathophysiology associated with disturbed behavior.
Supplementary Material
Acknowledgments
We would like to thank R. S. Beltran, N. Frasson, R. R. Holser, T. R. Keates, J. M. Kendall-Bar, and H. Robb for their comments and helpful discussions that greatly improved the manuscript.
Funding Statement
This work was supported by the Office of Naval Research [N00014-18-1-2822]; Office of Polar Programs [1644256]; International Association of Gas and Oil Producers Joint Industry Program on Marine Life [JIP 22 07-23].
Note
The Krogh Principle states that “for a large number of problems there will be some animal of choice or a few such animals on which it can be most conveniently studied” [182]. We consider marine mammals to be “Krogh organisms” for studying thermal physiology because they have noteworthy, or even extreme, thermal adaptations (e.g. morphological traits and physiological mechanisms) [183].
Data availability statement
The data used to create Figures 7 and Figure 8 are openly available in Dryad at https://doi.org/10.7291/D1M09M where additional information regarding methods for data collection is also present.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here
References
- [1].Dejours P. Water and air physical characteristics and their physiological consequences. In: Dejours, P., Bolis, L., Taylor, C.R., Weibel, E.R, editors. Comp Physiol. life water L. Padova: Liviana Press; 1987, p. 3–11. [Google Scholar]
- [2].Pendergast DR, Lundgren CEG. The underwater environment: cardiopulmonary, thermal, and energetic demands. J Appl Physiol. 2009;106(1):276–283. [DOI] [PubMed] [Google Scholar]
- [3].Reidenberg JS. Anatomical adaptations of aquatic mammals. Anat Rec. 2007;290(6):507–513. [DOI] [PubMed] [Google Scholar]
- [4].Pyenson ND, Kelley NP, Parham JF. Marine tetrapod macroevolution: physical and biological drivers on 250Ma of invasions and evolution in ocean ecosystems. Palaeogeogr Palaeoclimatol Palaeoecol. 2014;400:1–8. [Google Scholar]
- [5].Sikes RS, Gannon WL. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal. 2011;92(1):235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fahlman A, Aoki K, Bale G, et al. The New Era of Physio-Logging and Their Grand Challenges. Front Physiol. 2021;12:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hansen S, Lavigne DM. Ontogeny of the Thermal Limits in the Harbor Seal (Phoca vitulina). Physiol Zool. 1997;70(1):85–92. [DOI] [PubMed] [Google Scholar]
- [8].Boily P, Lavigne DM. Thermoregulation of juvenile grey seals, Halichoerus grypus, in air. Can J Zool. 1996;74(2):201–208. [Google Scholar]
- [9].Hart JS, Irving L, Mackenzie B. The energetics of harbor seals in air and in water with special consideration of seasonal changes. Can J Zool. 1959;37(4):447–457. [Google Scholar]
- [10].Morrison P, Rosenmann M, Estes JA. Metabolism and Thermoregulation in the Sea Otter. Physiol Zool. 1974;47(4):218–229. [Google Scholar]
- [11].Gallivan GJ, Best RC, Kanwisher JW. Temperature Regulation in the Amazonian Manatee Trichechus inunguis. Physiol Zool. 1983;56(2):255–262. [Google Scholar]
- [12].Kvadsheim PH, Gotaas ARL, Folkow LP, et al. An Experimental Validation of Heat Loss Models for Marine Mammals. J Theor Biol. 1997;184(1):15–23. [Google Scholar]
- [13].Boily P, Kvadsheim PH, Folkow LP. Cutaneous Heat Flux Models do not Reliably Predict Metabolic Rates of Marine Mammals. J Theor Biol. 2000;207(3):317–323. [DOI] [PubMed] [Google Scholar]
- [14].Watts P, Hansen S, Lavigne DM. Models of heat loss by marine mammals: thermoregulation below the zone of irrelevance. J Theor Biol. 1993;163(4):505–525. [Google Scholar]
- [15].Hokkanen JEI. Temperature regulation of marine mammals. J Theor Biol. 1990;145(4):465–485. [DOI] [PubMed] [Google Scholar]
- [16].Kelley NP, Pyenson ND. Evolutionary innovation and ecology in marine tetrapods from the Triassic to the Anthropocene. Science. 2015;348(80):aaa3716. [DOI] [PubMed] [Google Scholar]
- [17].Ridgway Sam H. Homeostasis in the Aquatic Environment in the textbook Mammals of the Sea - Biology and Medicine. Springfield, Illinois: Thomas, Springfield, Mass; 1972. p. 690–747. [Google Scholar]
- [18].Liwanag HEM, Berta A, Costa DP, et al. Morphological and thermal properties of mammalian insulation: the evolution of fur for aquatic living. Biol J Linn Soc. 2012;106(4):926–939. [Google Scholar]
- [19].Liwanag HEM, Berta A, Costa DP, et al. Morphological and thermal properties of mammalian insulation: the evolutionary transition to blubber in pinnipeds. Biol J Linn Soc. 2012;107(4):774–787. [Google Scholar]
- [20].Parry DA. The Structure of Whale Blubber, and a Discussion of its Thermal Properties. Q J Microsc Sci. 1949;90:13–25. [PubMed] [Google Scholar]
- [21].Pabst DA, Rommel SA, McLellan WA. Functional Anatomy of Marine Mammals. In: RJ III, Sa R, editors. Biol. Mar. Mamm. Washington D.C: Smithsonian Institution Press; 1999. p. 15–72. [Google Scholar]
- [22].Wang Z, Chen Z, Xu S, et al. Obesity” is healthy for cetaceans? Evidence from pervasive positive selection in genes related to triacylglycerol metabolism. Sci Rep. 2015;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tarasoff FJ, Fisher HD. Anatomy of the hind flippers of two species of seals with reference to thermoregulation. Can J Zool. 1970;48(4):821–829. [Google Scholar]
- [24].Khamas WA, Smodlaka H, Leach-Robinson J, et al. Skin histology and its role in heat dissipation in three pinniped species. Acta Vet Scand. 2012;54(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gentry RL. Thermoregulatory Behavior of Eared Seals. Behaviour. 1973;46(1–2):73–93. [DOI] [PubMed] [Google Scholar]
- [26].Codde SA, Allen SG, Houser DS, et al. Effects of environmental variables on surface temperature of breeding adult female northern elephant seals, Mirounga angustirostris, and pups. J Therm Biol. 2016;61:98–105. [DOI] [PubMed] [Google Scholar]
- [27].Favilla AB, Costa DP. Thermoregulatory Strategies of Diving Air-Breathing Marine Vertebrates: a Review. Front Ecol Evol. 2020;8. doi: 10.3389/fevo.2020.555509 [DOI] [Google Scholar]
- [28].Ladds MA, Rosen DAS, Slip DJ, et al. Proxies of energy expenditure for marine mammals: an experimental test of “the time trap. Sci Rep. 2017;7(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rutishauser MR, Costa DP, Goebel ME, et al. Ecological Implications of Body Composition and Thermal Capabilities in Young Antarctic Fur Seals (Arctocephalus gazella). Physiol Biochem Zool. 2004;77(4):669–681. [DOI] [PubMed] [Google Scholar]
- [30].Molyneux GS, Bryden MM. Arteriovenous Anastomoses in the Skin of Seals: i. The Weddell Seal Leptonychotes weddelli and the Elephant Seal Mirounga leonina (Pinnipedia: phocidae). Anat Rec. 1978;191(2):239–251. [DOI] [PubMed] [Google Scholar]
- [31].Bryden MM, Molyneux GS. Arteriovenous anastomoses in the skin of seals: II. The California Sea Lion Zalophus californianus and the Northern Fur Seal Callorhinus ursinus (Pinnipedia: otariidae). Anat Rec. 1978;96:285–300. [DOI] [PubMed] [Google Scholar]
- [32].McClelland SJ, Gay M, Ann D, et al. Microvascular Patterns in the Blubber of Shallow and Deep Diving Odontocetes. J Morphol. 2012;273(8):932–942. [DOI] [PubMed] [Google Scholar]
- [33].Boyd IL. Skin temperatures during free-ranging swimming and diving in Antarctic fur seals. J Exp Biol. 2000;203(12):1907–1914. [DOI] [PubMed] [Google Scholar]
- [34].Irving L, Hart JS. The metabolism and insulation of seals as bare-skinned mammals in cold water. Can J Zool. 1957;35(4):497–511. [Google Scholar]
- [35].Massoud M. Engingeering Thermofluids: thermodynamics, Fluid mechanics, and Heat Transfer. Berlin: Springer; 2005. [Google Scholar]
- [36].Xu X, Tikuisis P. Thermoregulatory modeling for cold stress. Compr Physiol. 2014;4:1057–1081. [DOI] [PubMed] [Google Scholar]
- [37].Gallivan GJ, Ronald K. Temperature regulation in freely diving harp seals (Phoca groenlandica). Can J Zool. 1979;57(11):2256–2263. [DOI] [PubMed] [Google Scholar]
- [38].Kvadsheim PH, Folkow LP. Blubber and flipper heat transfer in harp seals. Acta Physiol Scand. 1997;161(3):385–395. [DOI] [PubMed] [Google Scholar]
- [39].Brodie PF, Energetics C. an Overview of Intraspecific Size Variation. Ecology. 1975;56:152–161. [Google Scholar]
- [40].Kanwisher J, Sundnes G. Thermal regulation in cetaceans. In: Norris KS, editor. Whales Dolphins and Porpoises. Los Angeles: University of California Press; 1966. p. 397–407. [Google Scholar]
- [41].Meagher EM, McLellan WA, Westgate AJ, et al. Seasonal patterns of heat loss in wild bottlenose dolphins (Tursiops truncatus). J Comp Physiol B. 2008;178(4):529–543. [DOI] [PubMed] [Google Scholar]
- [42].Ducharme MB, Tikuisis P. In vivo thermal conductivity of the human forearm tissues. J Appl Physiol. 1991;70(6):2682–2690. [DOI] [PubMed] [Google Scholar]
- [43].Scholander PF, Schevill WE. Counter-Current Vascular Heat Exchange in the Fins of Whales. J Appl Physiol. 1955;8(3):279–282. [DOI] [PubMed] [Google Scholar]
- [44].Taylor NAS, Machado-Moreira Ca, Van Amj DH, et al. Hands and feet: physiological insulators, radiators and evaporators. Eur J Appl Physiol. 2014;114(10):2037–2060. [DOI] [PubMed] [Google Scholar]
- [45].Andrade DV. Thermal windows and heat exchange. Temperature. 2015;2(4):451. doi: 10.1080/23328940.2015.1040945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Romanovsky AA. Skin temperature: its role in thermoregulation. Acta Physiol. 2014;210(3):498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Low DA, Jones H, Cable NT, et al. Historical reviews of the assessment of human cardiovascular function: interrogation and understanding of the control of skin blood flow. Eur J Appl Physiol. 2020;120. 10.1007/s00421-019-04246-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kellogg DL. In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Physiol. 2006;100(5):1709–1718. [DOI] [PubMed] [Google Scholar]
- [49].Savage MV, Brengelmann GL. Control of skin blood flow in the neutral zone of human body temperature regulation. J Appl Physiol. 1996;80(4):1249–1257. [DOI] [PubMed] [Google Scholar]
- [50].Walløe L. Arterio-venous anastomoses in the human skin and their role in temperature control. Temperature. 2016;3(1):92–103. doi: 10.1080/23328940.2015.1088502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wong BJ, and Hollowed CG. Current concepts of active vasodilation in human skin. Temperature. 2017;4(1):41–59. doi: 10.1080/23328940.2016.1200203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Na Ø. Variations in the body surface temperature of the harp seal. Acta Physiol Scand. 1968;73(4):35A–36A. [Google Scholar]
- [53].Mauck B, Bilgmann K, Jones DD, et al. Thermal windows on the trunk of hauled-out seals: hot spots for thermoregulatory evaporation? J Exp Biol. 2003;206(10):1727–1738. [DOI] [PubMed] [Google Scholar]
- [54].Worthy GAJ. Insulation and thermal balance of fasting harp and grey seal pups. Comp Biochem Physiol. 1991;100(4):845–851. [DOI] [PubMed] [Google Scholar]
- [55].Willis K, Horning M, Rosen DAS, Trites AW . Spatial variation of heat flux in Steller sea lions: evidence for consistent avenues of heat exchange along the body trunk. J Exp Mar Biol Ecol. 2005;315(2): 163–175. [Google Scholar]
- [56].Hampton IFG, Whittow GC, Szekerczes J, et al. Heat transfer and body temperature in the Atlantic bottlenosed dolphin, Tursiops truncatus. Int J Biometerology. 1971;15(2–4):247–253. [DOI] [PubMed] [Google Scholar]
- [57].Norris AL, Houser DS, Crocker DE. Environment and activity affect skin temperature in breeding adult male elephant seals (Mirounga angustirostris). J Exp Biol. 2010;213(24):4205–4212. [DOI] [PubMed] [Google Scholar]
- [58].Guerrero AI, Rogers TL, Sepúlveda M, et al. Conditions influencing the appearance of thermal windows and the distribution of surface temperature in hauled-out southern elephant seals. Conserv Physiol. 2021;9. doi: 10.1093/conphys/coaa141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nienaber J, Thomton J, Horning M, Polasek L, Mellish J-A . Surface temperature patterns in seals and sea lions: a validation of temporal and spatial consistency. J Therm Biol. 2010;35(8):435–440. [Google Scholar]
- [60].Kastelein RA, Koene P, and Nieuwstraten SH, et al. Skin surface temperature changes in a harbour porpoise (phocoena phocoena) while on land. Read, Andrew J., P. R., Wiepkema, and Paul E., Nachtigall. In: Biol. Harb. porpoise (Woerden, The Netherlands: De Spil Publishers; ). 1997. p. 225–264. [Google Scholar]
- [61].Costa DP. Diving Physiology of Marine Vertebrates. Encycl Life Sci. 2007; 1–7. doi: 10.1002/9780470015902.a0004230. [DOI] [Google Scholar]
- [62].Levesque DL, Nowack J, Stawski C. Modelling mammalian energetics: the heterothermy problem. Clim Chang Responses. 2016;3(1):1–11. [Google Scholar]
- [63].Scholander PF, Irving L, Grinnell SW. On the Temperature and Metabolism of the seal during diving. J Cell Comp Physiol. 1942;19(1):67–78. [Google Scholar]
- [64].Butler PJ. Metabolic regulation in diving birds and mammals. Respir Physiol Neurobiol. 2004;141(3):297–315. [DOI] [PubMed] [Google Scholar]
- [65].Meir JU, Ponganis PJ. Blood Temperature Profiles of Diving Elephant Seals. Physiol Biochem Zool. 2010;83(3):531–540. [DOI] [PubMed] [Google Scholar]
- [66].Ponganis PJ, Van Dam RP, Levenson DH, et al. Regional heterothermy and conservation of core temperature in emperor penguins diving under sea ice. Comp Biochem Physiol Part A. 2003;135(3):477–487. [DOI] [PubMed] [Google Scholar]
- [67].Ponganis PJ, Kooyman GL, and Winter LM, et al. Heart rate and plasma lactate responses during submerged swimming and trained diving in California sea lions, Zalophus californianus. J Comp Physiol B. 1997;167(1):9–16. [DOI] [PubMed] [Google Scholar]
- [68].Meir JU, Champagne CD, Costa DP, et al. Extreme hypoxemic tolerance and blood oxygen depletion in diving elephant seals. Am J Physiol - Regul Integr Comp Physiol. 2009;297(4):927–939. [DOI] [PubMed] [Google Scholar]
- [69].McDonald BI, Ponganis PJ. Insights from venous oxygen profiles: oxygen utilization and management in diving California sea lions. J Exp Biol. 2013;216(17):3332–3341. [DOI] [PubMed] [Google Scholar]
- [70].Tift MS, Hückstädt LA, McDonald BI, et al. Flipper stroke rate and venous oxygen levels in free-ranging California sea lions. J Exp Biol. 2017;220:1533–1540. [DOI] [PubMed] [Google Scholar]
- [71].Meir JU, Robinson PW, Ignacio Vilchis L, et al. Blood oxygen depletion is independent of dive function in a deep diving vertebrate, the northern elephant seal. PLoS One. 2013;8(12):8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Williams CL, Ponganis PJ. Diving physiology of marine mammals and birds: the development of biologging techniques. Philos Trans R Soc B. 2021;376. doi: 10.1098/rstb.2020.0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ponganis PJ. Bio-logging of physiological parameters in higher marine vertebrates. Deep Res Part II Top Stud Oceanogr. 2007;54(3–4):183–192. [Google Scholar]
- [74].Ponganis PJ. A Physio-Logging Journey . Heart Rates of the Emperor Penguin and Blue Whale. Front Physiol. 2021:1. 10.3389/fphys.2021.721381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Worthy GAJ, Edwards EF. Morphometric and biochemical factors affecting heat loss in a small temperate cetacean (Phocoena phocoena) and a small tropical cetacean (Stenella attenuata). Physiol Zool. 1990;63(2):432–442. [Google Scholar]
- [76].Koopman HN. Phylogenetic, ecological, and ontogenetic factors influencing the biochemical structure of the blubber of odontocetes. Mar Biol. 2007;151(1):277–291. [Google Scholar]
- [77].Rommel SA, Pabst DA, McLellan WA, et al. Anatomical evidence for a countercurrent heat exchanger associated with dolphin testes. Anat Rec. 1992;232(1):150–156. [DOI] [PubMed] [Google Scholar]
- [78].McGinnis SM, Whittow GC, Ohata CA, et al. Body heat dissipation and conservation in two species of dolphins. Comp Biochem Physiol. 1972;43(2):417–423. [DOI] [PubMed] [Google Scholar]
- [79].Rosen DAS, Renouf D. Seasonal Changes in Blubber Distribution in Atlantic Harbor Seals: indications of Thermodynamic Considerations. Mar Mammal Sci. 1997;13(2):229–240. [Google Scholar]
- [80].Miller K, Irving L. Metabolism and temperature regulation in young harbor seals Phoca vitulina richardi. Am J Physiol. 1975;229(2):506–511. [DOI] [PubMed] [Google Scholar]
- [81].Horgan P, Booth D, Nichols C, et al. Insulative capacity of the integument of the dugong (Dugong dugon): thermal conductivity, conductance and resistance measured by in vitro heat flux. Mar Biol. 2014;161(6):1395–1407. [Google Scholar]
- [82].Montie EW, Garvin SR, Fair PA, et al. Blubber morphology in wild bottlenose dolphins (Tursiops truncatus) from the Southeastern United States: influence of geographic location, age class, and reproductive state. J Morphol. 2008;269(4):496–511. [DOI] [PubMed] [Google Scholar]
- [83].Singleton EM, McLellan WA, Koopman HN, et al. Lipid composition and thermal properties of the blubber of Gervais’ beaked whale (Mesoplodon europaeus) across ontogeny. Mar Mammal Sci. 2017;33(2):695–705. [Google Scholar]
- [84].Dunkin RC, McLellan WA, Blum JE, et al. The ontogenetic changes in the thermal properties of blubber from Atlantic bottlenose dolphin Tursiops truncatus. J Exp Biol. 2005;208(8):1469–1480. [DOI] [PubMed] [Google Scholar]
- [85].Ball HC, Stavarz M, Oldaker J, et al. Seasonal and Ontogenetic Variation in Subcutaneous Adipose Of the Bowhead Whale (Balaena mysticetus). Anat Rec. 2015;298(8):1416–1423. [DOI] [PubMed] [Google Scholar]
- [86].Pearson LE . Blubber and Beyond: the role of lipids in thermoregulation and energy reserves of phocid seals. Doctoral dissertation, University of Alaska Fairbanks. 2015;1-170. [Google Scholar]
- [87].Bagge LE, Koopman HN, Rommel SA, et al. Lipid class and depth-specific thermal properties in the blubber of the short-finned pilot whale and the pygmy sperm whale. J Exp Biol. 2012;215:4330–4339. [DOI] [PubMed] [Google Scholar]
- [88].Samuel AM, Worthy GAJ. Variability in fatty acid composition of bottlenose dolphin (Tursiops truncatus) blubber as a function of body site, season, and reproductive state. Can J Zool. 2004;82(12):1933–1942. [Google Scholar]
- [89].Noren SR, Wells RS. Blubber Deposition during Ontogeny in Free-Ranging Bottlenose Dolphins: balancing Disparate Roles of Insulation and Locomotion. J Mammal. 2009;90(3):629–637. [Google Scholar]
- [90].Cornick LA, Quakenbush LT, Norman SA, et al. Seasonal and developmental differences in blubber stores of beluga whales in Bristol Bay, Alaska using high-resolution ultrasound. J Mammal. 2016;97(4):1238–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Castellini MA, Trumble SJ, Mau TL, et al. Body and blubber relationships in Antarctic pack ice seals: implications for blubber depth patterns. Physiol Biochem Zool. 2009;82:113–120. [DOI] [PubMed] [Google Scholar]
- [92].Mellish J-AE, Horning M, York AE . Seasonal and Spatial Blubber Depth Changes in Captive Harbor Seals (Phoca vitulina) and Steller’s Sea Lions (Eumetopias jubatus). J Mammal. 2007;88(2): 408–414. [Google Scholar]
- [93].Donohue MJ, Costa DP, Goebel ME, et al. The ontogeny of metabolic rate and thermoregulatory capabilities of northern fur seal, Callorhinus ursinus, pups in air and water. J Exp Biol. 2000;203(6):1003–1016. [DOI] [PubMed] [Google Scholar]
- [94].Le P, Hem L, Mo H, et al. To each its own: thermoregulatory strategy varies among neonatal polar phocids. Comp Biochem Physiol -Part A Mol Integr Physiol. 2014;178:59–67. [DOI] [PubMed] [Google Scholar]
- [95].Ryg M, Smith TG, Øritsland NA. Thermal Significance of the Topographical Distribution of Blubber in Ringed Seals (Phoca hispida). Can J Fish Aquat Sci. 1988;45(6):985–992. [Google Scholar]
- [96].Koopman HN. Topographical distribution of the blubber of harbor porpoises (Phocoena phocoena). J Mammal. 1998;79(1):260–270. [Google Scholar]
- [97].Tighe RL, Bonde RK, Avery JP. Seasonal response of ghrelin, growth hormone, and insulin-like growth factor I in the free-ranging Florida manatee (Trichechus manatus latirostris). Mamm Biol. 2016;81(3):247–254. [Google Scholar]
- [98].Beck GG, Smith TG. Distribution of blubber in the northwest Atlantic harp seal,Phoca groenlandica. Can J Zool. 1995;73(11):1991–1998. [Google Scholar]
- [99].Iverson SJ, and Koopman HN. Blubber. Würsig Bernd G., J. G. M. Thewissen, and Kit M. Kovacs, editors.Encycl. Mar. Mamm. Third, Elsevier Inc (London, England: Academic Press; ); 2018. p. 107–110. 10.1016/B978-0-12-804327-1.00069-8 [DOI] [Google Scholar]
- [100].Davis RW. Marine Mammals: adaptations for an Aquatic Life. Cham Switzerland: Springer; 2019. 10.1007/978-3-319-98280-9. [DOI] [Google Scholar]
- [101].Kvadsheim PH, Folkow LP, Blix AS. Inhibition of shivering in hypothermic seals during diving. Am J Physiol Regul Integr Comp Physiol. 2005;289(2):326–331. [DOI] [PubMed] [Google Scholar]
- [102].Hampton IFG, Whittow GC. Body temperature and heat exchange in the Hawaiian spinner dolphin, Stenella longirostris. Comp Biochem Physiol. 1976;55A(2):195–197. [DOI] [PubMed] [Google Scholar]
- [103].Noren DP. Thermoregulation of Weaned Northern Elephant Seal (Mirounga angustirostris) Pups in Air and Water. Physiol Biochem Zool. 2002;75(5):513–523. [DOI] [PubMed] [Google Scholar]
- [104].Kasting NW, Adderley SAL, and Safford T, et al. Thermoregulation in Beluga (Delphinapterus leucas) and Killer (Orcinus orca) Physiological Zoology. 1989;62(3):687-701. 10.1086/physzool.62.3.30157921. [DOI] [Google Scholar]
- [105].Commission IUPS . Glossary of terms for thermal physiology. Jpn J Physiol. 2001;51:245–280. [Google Scholar]
- [106].Heath ME, Ridgway SH. How dolphins use their blubber to avoid heat stress during encounters with warm water. Am J Physiol. 1999;276:R1188–94. [DOI] [PubMed] [Google Scholar]
- [107].Brodie P, Paasche A. Thermoregulation and energetics of fin and sei whales based on postmortem, stratified temperature measurements. Can J Zool. 1985;63(10):2267–2269. [Google Scholar]
- [108].Whittow GC. Thermoregulatory adaptations in marine mammals: interacting effects of exercise and body mass. A review. Mar Mammal Sci. 1987;3(3):220–241 [Google Scholar]
- [109].Noren DP, Williams TM, Berry P, et al. Thermoregulation during swimming and diving in bottlenose dolphins (Tursiops truncatus). J Comp Physiol B. 1999;169(2):93–99. [DOI] [PubMed] [Google Scholar]
- [110].Williams TM, Noren D, Berry P, et al. The Diving Physiology of Bottlenose Dolphins (Tursiops Truncatus): III. Thermoregulation at Depth. J Exp Biol. 1999;202(20):2763–2769. [DOI] [PubMed] [Google Scholar]
- [111].Castellini MA, Murphy BJ, Fedak M, et al. Potentially conflicting metabolic demands of diving and exercise in seals. J Appl Physiol. 1985;58(2):392–399. [DOI] [PubMed] [Google Scholar]
- [112].Noren SR, Kendall T, Cuccurullo V, et al. The dive response redefined: underwater behavior influences cardiac variability in freely diving dolphins. J Exp Biol. 2012;215(16):2735–2741. [DOI] [PubMed] [Google Scholar]
- [113].Looney DP, Long ET, Potter AW, et al. Divers risk accelerated fatigue and core temperature rise during fully-immersed exercise in warmer water temperature extremes. Temperature. 2019;6(2):150–157. doi: 10.1080/23328940.2019.1599182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Williams TM, Davis RW, Fuiman LA, Francis J, Le Boeuf BJ, Horning M, et al . Sink or Swim: strategies for cost-efficient diving by marine mammals. Science. 2000;288(5463):133–136. [DOI] [PubMed] [Google Scholar]
- [115].Sparling CE, Fedak MA . Metabolic rates of captive grey seals during voluntary diving. J Exp Biol. 2004;207(10): 1615–1624. [DOI] [PubMed] [Google Scholar]
- [116].Noren SR, Williams TM. Body size and skeletal muscle myoglobin of cetaceans: adaptations for maximizing dive duration. Comp Biochem Physiol - A Mol Integr Physiol. 2000;126(2):181–191. [DOI] [PubMed] [Google Scholar]
- [117].Ponganis PJ, Meir JU, Williams CL. In pursuit of Irving and Scholander: a review of oxygen store management in seals and penguins. J Exp Biol. 2011;214(20):3325–3339. [DOI] [PubMed] [Google Scholar]
- [118].Davis RW, Williams TM. The marine mammal dive response is exercise modulated to maximize aerobic dive duration. J Comp Physiol A. 2012;198(8):583–591. [DOI] [PubMed] [Google Scholar]
- [119].Williams TM, Fuiman LA, Kendall T, et al. Exercise at depth alters bradycardia and incidence of cardiac anomalies in deep-diving marine mammals. Nat Commun. 2015;6. doi: 10.1038/ncomms7055. [DOI] [PubMed] [Google Scholar]
- [120].McDonald BI, Johnson M, Madsen PT. Dive heart rate in harbour porpoises is influenced by exercise and expectations. J Exp Biol. 2018;221:jeb168740. [DOI] [PubMed] [Google Scholar]
- [121].Savastano DM, Gorbach AM, Eden HS, et al. Adiposity and human regional body temperature. Am J Clin Nutr. 2009;90(5):1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Barbieri MM, McLellan WA, Wells RS, et al. Using infrared thermography to assess seasonal trends in dorsal fin surface temperatures of free-swimming bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Mar Mammal Sci. 2010;26(1):53–66. [Google Scholar]
- [123].Jobsis PD, Ponganis PJ, Kooyman GL. Effects of training on forced submersion responses in harbor seals. J Exp Biol. 2001;204(22):3877–3885. [DOI] [PubMed] [Google Scholar]
- [124].Zapol WM, Liggins GC, Schneider RC, et al. Regional blood flow during simulated diving in the conscious Weddell seal. J Appl Physiol. 1979;47(5):968–973. [DOI] [PubMed] [Google Scholar]
- [125].Hammel HT, Elsner RW, Heller HC, et al. Thermoregulatory responses to altering hypothalamic temperature in the harbor seal. Am J Physiol Regul Integr Comp Physiol. 1977;232(1):R18–26. [DOI] [PubMed] [Google Scholar]
- [126].Elmegaard SL, Johnson M, Madsen PT, et al. Cognitive control of heart rate in diving harbor porpoises. Curr Biol. 2016;26(22):R1175–6. [DOI] [PubMed] [Google Scholar]
- [127].Erdsack N, Hanke FD, Dehnhardt G, et al. Control and amount of heat dissipation through thermal windows in harbor seals (Phoca vitulina). J Therm Biol. 2012;37(7):537–544. [Google Scholar]
- [128].Ootsuka Y, and Tanaka M. Control of cutaneous blood flow by central nervous system. Temperature. 2015;2(3):392–405, doi: 10.1080/23328940.2015.1069437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Hill RD, Schneider RC, and Liggins GC, et al. Heart rate and body temperature during free diving of Weddell seals. Am Physiol Soc.1987;253(2) :R344–51. [DOI] [PubMed] [Google Scholar]
- [130].Ponganis PJ, Kooyman GL, Castellini MA, et al. Muscle temperature and swim velocity profiles during diving in a Weddell seal, Leptonychotes Weddellii. J Exp Biol. 1993;183(1):341–348. [DOI] [PubMed] [Google Scholar]
- [131].Andrews RD. The cardiorespiratory, metabolic, and thermoregulatory physiology of juvenile northern elephant seals (Mirounga angustirostris). Doctoral dissertation, University of British Columbia. 1999;1-103. [Google Scholar]
- [132].Hochachka PW. Pinniped diving response mechanism and evolution: a window on the paradigm of comparative biochemistry and physiology. Comp Biochem Physiol - A Mol Integr Physiol. 2000;126(4):435–458. [DOI] [PubMed] [Google Scholar]
- [133].McCafferty DJ, Gallon S, Nord A. Challenges of measuring body temperatures of free-ranging birds and mammals. Anim Biotelemetry. 2015;3(1):1–10. [Google Scholar]
- [134].Thompson D, Fedak MA. Cardiac responses of grey seals during diving at sea. J Exp Biol. 1993;174(1):139–154. [DOI] [PubMed] [Google Scholar]
- [135].Elmegaard SL, McDonald BI, Madsen PT. Drivers of the dive response in trained harbour porpoises (Phocoena phocoena). J Exp Biol. 2019;222:0–7. [DOI] [PubMed] [Google Scholar]
- [136].Walcott SM, Kirkham AL, Burns JM. Thermoregulatory costs in molting Antarctic Weddell seals: impacts of physiological and environmental conditions. Conserv Physiol. 2020;8(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Durban JW, Pitman RL. Antarctic killer whales make rapid, round-trip movements to subtropical waters: evidence for physiological maintenance migrations? Biol Lett. 2012;8(2):274–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Pitman RL, Durban JW, Joyce T, et al. Skin in the game: epidermal molt as a driver of long-distance migration in whales. Mar Mammal Sci. 2019; 1–30. 10.1111/mms.12661 [DOI] [Google Scholar]
- [139].Thometz NM, Hermann-Sorensen H, Russell B, et al. Molting strategies of Arctic seals drive annual patterns in metabolism. Conserv Physiol. 2021;9. 10.1093/conphys/coaa112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Lewden AS, Enstipp MR, Picard B, et al. High peripheral temperatures in king penguins while resting at sea: thermoregulation versus fat deposition. J Exp Biol. 2017;3084–3094. 10.1242/jeb.158980 [DOI] [PubMed] [Google Scholar]
- [141].Lewden AS, Enstipp MR, Bonnet B, et al. Thermal strategies of king penguins during prolonged fasting in water. J Exp Biol. 2017;220:4600–4611. [DOI] [PubMed] [Google Scholar]
- [142].Kuhn CE. Measuring at sea feeding to understand the foraging behavior of pinnipeds. Doctoral dissertation, University of California, Santa Cruz. 2006;1-129. [Google Scholar]
- [143].Wilson RP, Putz K, Gremillet D, et al. Reliability of stomach temperature changes in determining feeding characteristics of seabirds. J Exp Biol. 1995;198(5):1115–1135. [DOI] [PubMed] [Google Scholar]
- [144].Ducharme MB, Frim J, Tikuisis P. Errors in heat flux measurements due to the thermal resistance of heat flux disks. J Appl Physiol. 1990;69(2):776–784. [DOI] [PubMed] [Google Scholar]
- [145].Meagher EM, McLellan WA, Westgate AJ, et al. The relationship between heat flow and vasculature in the dorsal fin of wild bottlenose dolphins Tursiops truncatus. J Exp Biol. 2002;205(22):3475–3486. [DOI] [PubMed] [Google Scholar]
- [146].Willis K, Horning M. A novel approach to measuring heat flux in swimming animals. J Exp Mar Biol Ecol. 2005;315(2):147–162. [Google Scholar]
- [147].Westgate AJ, McLellan WA, Wells RS, et al. A new device to remotely measure heat flux and skin temperature from free-swimming dolphins. J Exp Mar Biol Ecol. 2007;346(1–2):45–59. [Google Scholar]
- [148].Hindle AG, Horning M, Mellish JAE . Estimating total body heat dissipation in air and water from skin surface heat flux telemetry in Weddell seals. Anim Biotelemetry. 2015;3:1–11. [Google Scholar]
- [149].Erdsack N, McCully Phillips SR, Rommel SA, et al. Heat flux in manatees: an individual matter and a novel approach to assess and monitor the thermal state of Florida manatees (Trichechus manatus latirostris). J Comp Physiol B. 2018;188(4):717–727. [DOI] [PubMed] [Google Scholar]
- [150].Cuyler LC, Wiulsrod R, Oritsland NA. Thermal infrared radiation from free living whales. Mar Mammal Sci. 1992;8(2):120–134 [Google Scholar]
- [151].Bevan RM, and Butler PJ. The Effects of Temperature on the Oxygen Consumption, Heart Rate and Deep Body Temperature during Diving in the Tufted Duckaythya Fuligula. J Exp Biol. 1992;163(1):139–151. [Google Scholar]
- [152].West NH, Butler PJ, Bevan RM. Pulmonary Blood Flow at Rest and during Swimming in the Green Turtle, Chelonia mydas. Physiol Zool. 1992;65(2):287–310. [Google Scholar]
- [153].Hochscheid S, Bentivegna F, Speakman JR. Regional blood flow in sea turtles: implications for heat exchange in an aquatic ectotherm. Physiol Biochem Zool. 2002;75(1):66–76. [DOI] [PubMed] [Google Scholar]
- [154].Cherepanova V, Neshumova T, Elsner R. Muscle blood flow in diving mammals. Comp Biochem Physiol. 1993;106A(1):1–6. [DOI] [PubMed] [Google Scholar]
- [155].Ponganis PJ, Stockard TK, Levenson DH, et al. Cardiac output and muscle blood flow during rest-associated apneas of elephant seals. Comp Biochem Physiol - A Mol Integr Physiol. 2006;144(1):105–111. [DOI] [PubMed] [Google Scholar]
- [156].Ponganis PJ, Kreutzer U, Stockard TK, et al. Blood flow and metabolic regulation in seal muscle during apnea. J Exp Biol. 2008;211(20):3323–3332. [DOI] [PubMed] [Google Scholar]
- [157].Chaseling GK, Crandall CG, Gagnon D. Skin blood flow measurements during heat stress: technical and analytical considerations. Am J Physiol - Regul Integr Comp Physiol. 2020;318(1):R57–69. [DOI] [PubMed] [Google Scholar]
- [158].Schmidt A, Alard F, Handrich I. Changes in body temperatures in king penguins at sea: the result of fine adjustments in peripheral heat loss? Am J Physiol Regul Integr Comp Physiol. 2006;291(3):608–618. [DOI] [PubMed] [Google Scholar]
- [159].Niizuma Y, Gabrielsen GW, Sato K, et al. Brünnich’s guillemots (Uria lomvia) maintain high temperature in the body core during dives. Comp Biochem Physiol Part A. 2007;147(2):438–444. [DOI] [PubMed] [Google Scholar]
- [160].Enstipp MR, Bost C-A, Le Bohec C, et al. The dive performance of immature king penguins following their annual molt suggests physiological constraints. J Exp Biol. 2019;222(20):1–14. [DOI] [PubMed] [Google Scholar]
- [161].Sagaidachnyi A, Fomin A, Usanov D, et al. Real-time technique for conversion of skin temperature into skin blood flow: human skin as a low-pass filter for thermal waves. Comput Methods Biomech Biomed Engin. 2019;22(12):1009–1019. [DOI] [PubMed] [Google Scholar]
- [162].Schlader Z. The relative overlooking of human behavioral temperature regulation: an issue worth resolving. Temperature. 2014;1(1):20–21 doi: 10.4161/temp.29235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Whittow GC, Matsuura DT, Lin YC. Temperature Regulation in the California Sea Lion (Zalophus californianus). Physiol Zool. 1972;45(1):68–77. [Google Scholar]
- [164].White FN, Odell DK. Thermoregulatory Behavior of the Northern Elephant Seal, Mirounga angustirostris. J Mammal. 1971;52(4):758–774. [Google Scholar]
- [165].Kingma BR, Frijns AJ, and Schellen L, et al. Beyond the classic thermoneutral zone: including thermal comfort. Temperature. 2014;1:142–149 doi: 10.4161/temp.29702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Costa DP, Kooyman GL. Oxygen consumption, thermoregulation, and the effect of fur oiling and washing on the sea otter,Enhydra lutris. Can J Zool. 1982;60(11):2761–2767. [Google Scholar]
- [167].Costa DP, Field Physiology: SB. Physiological Insights from Animals in Nature. Annu Rev Physiol. 2004;66(1):209–238. [DOI] [PubMed] [Google Scholar]
- [168].Adamczak SK, McLellan WA, Read AJ, et al. The impact of temperature at depth on estimates of thermal habitat for short-finned pilot whales. Mar Mammal Sci. 2020; 1–14. 10.1111/mms.12737. [DOI] [Google Scholar]
- [169].Bernal D, Brill RW, Dickson KA, et al. Sharing the water column: physiological mechanisms underlying species-specific habitat use in tunas. Rev Fish Biol Fish. 2017;27:843–880. [Google Scholar]
- [170].Royer MA. Thermoregulation Strategies of Deep Diving Ectothermic Sharks. Doctoral dissertation, University of Hawaii at Manoa. 2020;1-119. [Google Scholar]
- [171].Thygesen UH, Sommer L, Evans K, et al. Dynamic optimal foraging theory explains vertical migrations of bigeye tuna. Ecology. 2016;97(7):1852–1861. [DOI] [PubMed] [Google Scholar]
- [172].Beentjes MP. Behavioral thermoregulation of the New Zealand sea lion (Phocarctos hookeri). Mar Mammal Sci. 2006;22(2):311–325. [Google Scholar]
- [173].Chaise LL, McCafferty DJ, Krellenstein A, et al. Environmental and physiological determinants of huddling behavior of molting female southern elephant seals (Mirounga leonina). Physiol Behav. 2019;199:182–190. [DOI] [PubMed] [Google Scholar]
- [174].Liwanag HEM. Energetic Costs and . Energetic Costs and Thermoregulation in Northern Fur Seal (Callorhinus ursinus) Pups: the Importance of Behavioral Strategies for Thermal Balance in Furred Marine Mammals. Physiol Biochem Zool. 2010;83(6): 898–910. [DOI] [PubMed] [Google Scholar]
- [175].Williams HJ, Taylor LA, Benhamou S, et al. Optimizing the use of biologgers for movement ecology research. J Anim Ecol. 2020;89(1):186–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Goldbogen JA, Cade DE, and Boersma AT, et al. Using Digital Tags With Integrated Video and Inertial Sensors to Study Moving Morphology and Associated Function in Large Aquatic Vertebrates. Animal Biotelemetry. 2017;300(11):1935–1941. [DOI] [PubMed] [Google Scholar]
- [177].Fregosi S, Klinck H, Horning M, Costa DP, Mann D, Sexton K, et al . An animal-borne active acoustic tag for minimally invasive behavioral response studies on marine mammals. Anim Biotelemetry. 2016;4:9.1 [Google Scholar]
- [178].Southall BL, Nowacek DP, Miller PJO, et al. Experimental field studies to measure behavioral responses of cetaceans to sonar. Endanger Species Res. 2016;31:293–315. [Google Scholar]
- [179].Nowacek DP, Christiansen F, Bejder L, et al. Studying cetacean behaviour: new technological approaches and conservation applications. Anim Behav. 2016;120:235–244. [Google Scholar]
- [180].Harris CM, Thomas L, Falcone EA, et al. Marine mammals and sonar: dose-response studies, the risk-disturbance hypothesis and the role of exposure context. J Appl Ecol. 2018;55(1):396–404. [Google Scholar]
- [181].Pabst DA, McLellan WA, Meagher EM, et al., Measuring temperatures and heat flux from dolphins in the eastern tropical pacific: is thermal stress associated with chase and capture in the ETP-tuna purse seine fishery? La Jolla. 2002; [Google Scholar]
- [182].Krogh A. The progress of physiology. Am J Psychol. 1929;90:243–251. [Google Scholar]
- [183].Green S, Dietrich MR, Leonelli S, et al. ‘Extreme’ organisms and the problem of generalization: interpreting the Krogh principle. Hist Philos Life Sci. 2018;40(4):40–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to create Figures 7 and Figure 8 are openly available in Dryad at https://doi.org/10.7291/D1M09M where additional information regarding methods for data collection is also present.


