Abstract
Objective
To investigate the prevalence of depression and anxiety in patients undergoing maintenance hemodialysis (MHD) in Hohhot, a large city on the northern border of China, and to identify independent risk factors for depression and anxiety in these patients.
Methods
Patients receiving MHD for >3 months were enrolled in the four largest hemodialysis centers between September 2020 and December 2020. Depression and anxiety were assessed using the Zung self-rated depression scale (SDS) and Zung self-rated anxiety scale (SAS), respectively, with demographic and other data collected for logistic regression analyses.
Results
Among 305 MHD patients included in this study, the prevalence of depression was 55.1%, including 27.5%, 21.0%, and 6.6% with mild, moderate and severe cases, respectively. The prevalence of anxiety was 25.9%, with 20.0%, 4.6%, and 1.3% having mild, moderate, and severe cases, respectively. An independent protective factor for depression was family income of ≥1415 US dollars/month relative to <157 US dollars/month (odds ratio [OR] 0.209, 95% confidence interval [CI] 0.065–0.673), and predictors of depression included ≥3 comorbidities (OR 18.527, 95% CI 1.674–205.028) and severe pruritus (OR 15.971, 95% CI 5.173–49.315). Independent predictors of anxiety included infrequent exercise (OR 3.289, 95% CI 1.411–7.664) and severe pruritus (OR 5.912, 95% CI 1.733–20.168). The correlation between depression and anxiety in these patients was significant (rs = 0.775, p < 0.001).
Conclusion
MHD patients in Northern China had high prevalence rates of depression (55.1%) and anxiety (25.9%). Lower family income, more comorbidities, and a higher degree of pruritus were predictors of depression, while infrequent exercise and severe pruritus were predictors of anxiety. Depression correlated significantly with anxiety. Attention should be given to family income, comorbidity, exercise, and pruritus severity for improved management of depression and anxiety among MHD patients.
Keywords: Chronic kidney disease, chronic renal failure, end stage renal disease, maintenance hemodialysis, depression, anxiety
Introduction
Chronic kidney disease (CKD) is defined by the presence of kidney damage for more than 3 months, originating from various etiologies. A substantial proportion of patients with CKD will eventually progress to having the end-stage renal disease (ESRD), necessitating treatment with renal replacement therapy (RRT). CKD is a serious public health concern globally. According to data from the Global Burden of Disease (GBD) Chronic Kidney Disease Collaboration, the global prevalence of CKD in 2017 reached as high as 9.1%, with a total of 697.5 million people having CKD, among whom one-third resided in China and India (132.3 million and 115.1 million, respectively) [1]. Additionally, CKD also brings forward high mortality. In 2017, the number of deaths related to CKD worldwide was 1.23 million, with age-standardized mortality of 0.16‰ [1]. Currently, hemodialysis remains the mainstay of RRT for patients with ESRD in most countries [2,3]. The population of maintenance hemodialysis (MHD) patients is rapidly increasing in China; data from the Chinese National Renal Data System (CNRDS) revealed that the number of patients undergoing MHD in China increased from 0.235 million in 2011 to 0.447 million in 2016 (90.2% within 5 years), and the prevalence increased from 174 cases per million in 2011 to 298 per million in 2016 (71.3% within 5 years).
Patients undergoing MHD suffer from psychiatric illnesses of varying severity with adverse influences, especially depression and anxiety. A meta-analysis including observational studies found by searching MEDLINE and Embase reported that the prevalence rates of interview-based and self- or clinician-rated depression in patients undergoing MHD were 22.8% and 39.3%, respectively [4]. Patients undergoing MHD in Greece also had high prevalence rates of depression and anxiety, as a multicenter study identified that 29.4% and 35.9% of patients undergoing MHD had depression and anxiety, respectively [5]. On the other hand, another meta-analysis of cohort study indicated that depression substantially increases the risk of mortality in patients with CKD, while effective anti-depressive treatments in patients with CKD may reduce mortality [6]. A separate meta-analysis suggested that depression is an independent risk factor for mortality in patients undergoing chronic dialysis [7]. A study from Japan suggested that anxiety and depression significantly impair the quality of life in older patients undergoing hemodialysis [8]. Furthermore, several studies indicated that depression and anxiety are robust indicators of suicidal ideation [9,10], and researchers have tried to reduce the risk of suicidality [11,12].
In China, existing studies focusing on depression and anxiety in patients undergoing MHD were mostly performed in a single center with few patients included, and most have been done in developed coastal regions or central areas with very few regional or nationwide reports. Compared with the central areas and coastal regions, the northern border of China has unique geographical, social, economic, cultural and ethnic characteristics. The population of this area consumes a high salt diet, drinks alcohol frequently, has a high prevalence of obesity and has suboptimal economics and limited medical resources, and the area has the geographic features of a cold and dry climate, with a large minority subpopulation. From this perspective, data from central and coastal China are not likely to be representative of those from the northern border of China. However, most data about the mental status of MHD patients were derived in studies in the central and the coastal areas of China, even though the area of the northern border accounts for 20% of China’s population. Therefore, it is epidemiologically important to gain more understanding of the psychological health of the MHD population living on the northern border of China. Consequently, the current study aimed to investigate the prevalence of depression and anxiety in patients undergoing MHD in Hohhot, the capital of the northern border region of China, and to identify independent predictors of depression and anxiety, for the purpose of providing guidance for the prevention and treatment of depression and anxiety in hemodialysis patients in this region.
Materials and methods
Sample and setting
Prior to study initiation, 1313 patients were undergoing MHD in 10 hemodialysis units in Hohhot in August 2020, based on the records from the Hohhot Hemodialysis Quality Control Center. According to the results from Gerogianni et al. [5], the prevalence of depression in patients undergoing MHD in Greece was 29.4%. We extrapolated their data to our expected cohort because the design of their study was found to be rigorous. We further set the confidence degree 1-α at 0.95 and set the permissible error at 1/6 of the population rate, resulting in a calculated minimal sample size of 332 for the analyses of depression, based on the calculation formula for enumeration data sample size estimation. Similarly, the expected prevalence of anxiety was 35.9% according to findings from Gerogianni et al. [5]. Using the same framework described above (confidence degree at 0.95, permissible error at 1/6 of the population rate), the calculated minimal sample size was 247 for the analyses of anxiety. Consequently, we set out to enroll 332 patients undergoing MHD in this study.
According to the hemodialysis quality control center, 75.5% of patients undergoing MHD in Hohhot were treated in the four largest dialysis rooms in the city, including the First Hospital of Hohhot (353 patients), the Affiliated Hospital of Inner Mongolia Medical University (320 patients), the People’s Hospital of Inner Mongolia Autonomous Region (210 patients), and the Inner Mongolia International Mongolian Hospital (108 patients). To ensure the representativeness of enrollees and study feasibility, we randomly sampled eligible patients undergoing MHD from the four largest dialysis units, with the patient number from each dialysis room corresponding to the number of patients undergoing MHD patients in each unit.
Inclusion and exclusion criteria
This study was a multicenter and cross-sectional one. Patients receiving regular hemodialysis for more than 3 months were selected between September 2020 and December 2020 from the four hemodialysis units. After determining patients’ eligibility based on inclusion and exclusion criteria, enrollees were asked to complete a questionnaire survey, conducted in a separate room by trained investigators. At the beginning of the interview, the investigator introduced the study to eligible patients and helped them sign the informed consent form. Enrollees completed the survey in a mini program of the WeChat application on their cellphone called the questionnaire star. Enrollees’ personal information was kept anonymized, with their names coded using initials only. Those who were severely depressed were referred for further treatment. This study was approved by the Ethics Committee of the Affiliated Hospital of Inner Mongolia Medical University (No. 2020025).
The inclusion criteria were as follows: age between 18 and 85 years; receiving MHD for more than 3 months; having clear consciousness and being able to communicate normally; and being willing to participate and providing informed consent. The exclusion criteria were as follows: unable to complete the survey because of poor linguistic competence; visual, hearing or mental impairment; age younger than 18 years or older than 80 years; serious diseases precluding their participation in this study, including heart failure of New York Heart Association functional class IV, recent or frequent attack of angina pectoris, or having hypoxemia, defined by a blood oxygen saturation less than 90% at rest; and refusal to participate in this study.
Data collection and measurement
Socio-demographic profile
We collected demographic data including patients’ name initials, age, gender, occupation, nationality, employment status, education level, marital status, type of health insurance, monthly family income, and exercise status.
Comorbidities and laboratory indices
Patient's clinical history includes clinical features, comorbidities and physical parameters, such as the duration of receiving dialysis, the dialysis hours per week, ESRD origins, number of comorbidities, blood pressure on non-dialysis days, pruritus severity, and sleep status. The pruritus severity was divided into three grades, “No,” “Sustainable” and “Unbearable.” Laboratory indices including hemoglobin were recorded.
Assessment of depression and anxiety
We used the Zung self-rating depression scale (SDS) and Zung self-rating anxiety scale (SAS) to assess depression and anxiety, respectively. The SDS was developed by W.K. Zung in 1965, and the SAS was developed by W.K. Zung in 1971. Both scales were translated into Chinese versions shown to have a high-reliability coefficient for different Chinese populations [13]. Each scale contains 20 items each scored on a four-point Likert scale that assesses the frequency or severity of symptoms of either depression or anxiety: “1” indicates no or little time, “2” represents a small amount of time, “3” refers to a lot of time, and “4” represents most or all of the time. Reverse scoring questions were scored as “4, 3, 2, and 1.” The self-assessment scale evaluation method involved first explaining the evaluation method, meaning and requirements to the participants, after which time the participants completed the electronic questionnaires according to the actual situation. Higher total scores represent more severe depression or anxiety. The wording of questionnaire items from the contents of the SDS and SAS was adjusted in the Inner Mongolia Mental Health Center, in order to adapt the content to the language and habits of local citizens. The raw scores were converted into standard scores by multiplying by 1.25 for further evaluation. The thresholds for identifying depression and anxiety were 50 points [14,15], with scores higher than 50 defined as having depression or anxiety [16]. Scores of 50–59, 60–69, and >70 indicated mild, moderate, and severe depression or anxiety, respectively, based upon a study aimed at the Chinese MHD population [17].
Statistical analysis
All analyses were performed using SPSS version 20.0. Normally distributed quantitative data were expressed as while quantitative data with a skewed distribution were expressed as median with 25% and 75% quartiles in parentheses. Categorical variables were expressed using frequencies or rates. Univariate binary logistic regression analysis was first performed with 26 independent variables, with variables found to be significant (p < 0.05) included in the subsequent binary multiple logistic regression analyses, to explore independent predictors of depression or anxiety. Spearman correlation analysis between depression and anxiety scores was performed using enrollees’ standard scores of SDS or SAS. A two-tailed test was used in statistical analyses and a P value <0.05 was considered statistically significant.
Results
Enrollee characteristics
A total of 305 (23.2%) of the 1313 patients undergoing MHD were enrolled in this study, among whom 108, 95, 42, and 60 cases were recruited from the Affiliated Hospital of Inner Mongolia Medical University, First Hospital of Hohhot, People's Hospital of Inner Mongolia Autonomous Region, and Inner Mongolia International Mongolian Hospital, respectively. The patients’ demographic data are presented in Table 1. Enrollees had a mean age of 52 years (22–84 years), and 120 (39.4%) were aged between 45 and 59 years. Approximately 55.1% (n = 168) were male, with the majority being of Han ethnicity (n = 265, 86.9%). Most enrollees lived in an urban area (n = 264, 86.9%), and most (78.4%) were married. We found that 56.4% of enrollees had a senior high school, vocational school, or higher level of education. In addition, 57.7% were absent from work, while 28.2% had a family monthly income between 157 and 472 US dollars.
Table 1.
Patients’ characteristics (N = 305).
| Characteristics | n (%) |
|---|---|
| Gender | |
| Male | 168 (55.1) |
| Female | 137 (44.9) |
| Age group (years) | |
| 18–44 | 93 (30.5) |
| 45–59 | 120 (39.4) |
| 60–79 | 86 (28.2) |
| 80–85 | 6 (1.9) |
| Race | |
| Han ethnicity | 265 (86.9) |
| Mongol ethnicity | 27 (8.9) |
| Hui ethnicity | 5 (1.6) |
| Other ethnicity | 8 (2.6) |
| Education level | |
| Primary school and below | 43 (14.1) |
| Junior high school | 90 (29.5) |
| Senior high/vocational school | 75 (24.6) |
| Junior college | 51 (16.7) |
| Bachelor or above | 46 (15.1) |
| Marital status | |
| Married | 239 (78.4) |
| Never married | 39 (12.8) |
| Divorced or separate | 17 (5.6) |
| Widowed | 10 (3.2) |
| Occupation | |
| Mental worker | 76 (24.9) |
| Manual worker | 53 (17.4) |
| Others | 176 (57.7) |
| Family monthly income (US dollars/month) | |
| <157 | 74 (24.3) |
| 157–472 | 86 (28.2) |
| 472–943 | 85 (27.9) |
| 943–1415 | 28 (9.2) |
| ≥1415 | 32 (10.4) |
| Daily sleep duration (hours) | |
| ≥7 | 211 (69.2) |
| <7 | 94 (30.8) |
| Number of co-morbid illnesses | |
| 0 | 157 (51.5) |
| 1 | 110 (36.1) |
| 2 | 28 (9.2) |
| ≥3 | 10 (3.2) |
| Employment status | |
| Yes | 50 (16.4) |
| No | 255 (83.6) |
| Health care type | |
| New rural cooperative medical care | 101 (33.1) |
| Medical insurance for urban residents | 44 (14.4) |
| Medical insurance for urban workers | 122 (40.0) |
| Provincial health care | 38 (12.5) |
| Exercise status | |
| Often | 75 (24.6) |
| Occasionally | 155 (50.8) |
| Never | 75 (24.6) |
| Dialysis duration (years) | |
| ≤2 | 74 (24.3) |
| 3—5 | 95 (31.1) |
| 6—9 | 82 (26.9) |
| ≥10 | 54 (17.7) |
| Duration of dialysis per week (hours) | |
| <11.5 | 67 (22.0) |
| ≥11.5 | 238 (78.0) |
| Primary disease | |
| Diabetes mellitus | 42 (13.8) |
| Glomerulonephritis | 63 (20.7) |
| Primary hypertension | 95 (31.1) |
| Tubulointerstitial disease | 0 (0.0) |
| Autoimmune disease | 12 (3.9) |
| Polycystic kidney | 19 (6.2) |
| Unknown | 59 (19.4) |
| Drug-induced kidney injury | 15 (4.9) |
| With diabetes mellitus or not | |
| Yes | 52 (17.0) |
| No | 253 (83.0) |
| With heart disease or not | |
| Yes | 87 (28.5) |
| No | 218 (71.5) |
| Pruritus severity | |
| No | 60 (19.7) |
| Sustainable | 202 (66.2) |
| Unbearable | 43 (14.1) |
| Systolic blood pressure (mmHg) | |
| >160 | 72 (23.6) |
| 141–159 | 76 (24.9) |
| 130–140 | 96 (31.5) |
| 89–129 | 61 (20.0) |
| Diastolic blood pressure (mmHg) | |
| ≥100 | 42 (13.8) |
| 90–99 | 90 (29.5) |
| 80–89 | 88 (28.9) |
| <80 | 85 (27.8) |
| Mean arterial pressure (mmHg) | |
| ≤105 | 154 (50.5) |
| >105 | 151 (49.5) |
| *Hemoglobin (g/L) | |
| <100 | 98 (32.1) |
| 100–130 | 151 (49.5) |
| >130 | 37 (12.1) |
Notes: *Total percentage did not equal to 100% because of missing data.
With regard to clinical history, 69.2% had a daily sleep duration longer than 7 h, and 26.6% were overweight or obese. Nearly half (47.0%) had one or more co-morbid diseases, and 83.6% quit their work after starting MHD. Other comorbidities, treatment information, and laboratory indicators are shown in Table 1.
Prevalence of depression and anxiety among study participants
The prevalence of depression in patients undergoing MHD in this study was 55.1%, with 27.5%, 21.0, and 6.6% having mild, moderate, and severe depression, respectively. The prevalence of anxiety in these patients was 25.9%, with 20.0%, 4.6%, and 1.3% having mild, moderate, and severe anxiety, respectively.
Univariate analysis of predictors of depression and anxiety
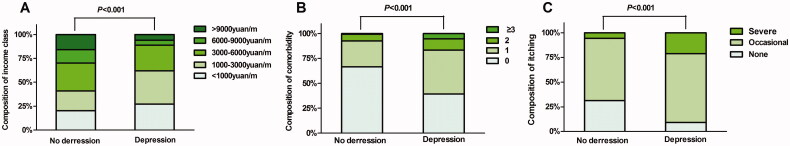
Univariate binary logistic regression analysis was first performed to assess determinants of depression or anxiety, respectively. During the analysis of depression, “No depression” was taken as one variable, while mild, moderate and severe depression were combined into the other. During the analysis of anxiety, “No anxiety” was taken as one variable, while mild, moderate and severe anxiety were combined into the other. On univariate analysis with depression as the dependent variable, nine variables were found to be statistically significant, including monthly family income (p < 0.001), daily sleep duration (p < 0.001), number of comorbidities (p < 0.001), type of medical insurance (p = 0.023), exercise status (p = 0.002), heart disease history (p < 0.001), pruritus severity (p < 0.001), systolic blood pressure (p = 0.026) and diastolic blood pressure (p = 0.046; Table 2). Figure 1 shows the results for the relationships between monthly family income, number of comorbidities, pruritus severity, and depression.
Table 2.
Univariate analysis of factors associated with depression or anxiety (N = 305).
| Characteristics | No depression n (%) | Depression n (%) | P | OR | 95%CI | No anxiety n (%) | Anxiety n (%) | P | OR | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | 0.801 | 0.892 | ||||||||
| Male | 74 (24.3) | 94 (30.8) | 1.000 | 125 (41.0) | 43 (14.1) | 1.000 | ||||
| Female | 63 (20.7) | 74 (24.2) | 0.943 | 0.599–1.485 | 101 (33.1) | 36 (11.8) | 0.344 | 0.619–1.733 | ||
| Age group (years) | 0.592 | 0.162 | ||||||||
| 18–44 | 40 (13.1) | 53 (17.4) | 1.000 | 72 (23.6) | 21 (6.9) | 1.000 | ||||
| 45–59 | 62 (20.3) | 58 (19.3) | 0.705 | 0.409–1.217 | 93 (30.5) | 27 (8.9) | 0.995 | 0.521–1.903 | ||
| 60–79 | 31 (10.2) | 55 (18.0) | 0.848 | 0.471–1.526 | 56 (18.4) | 30 (9.8) | 1.837 | 0.951–3.546 | ||
| 80–85 | 4 (1.3) | 2 (0.7) | 0.511 | 0.089–2.926 | 5 (1.6) | 1 (0.3) | 0.686 | 0.076–6.197 | ||
| Race | 0.092 | 0.265 | ||||||||
| Han ethnicity | 116 (38.0) | 149 (48.9) | 1.000 | 196 (64.3) | 69 (22.6) | 1.000 | ||||
| Mongol ethnicity | 16 (5.2) | 11 (3.7) | 0.494 | 0.209–1.167 | 21 (6.9) | 6 (2.0) | 0.812 | 0.315–2.094 | ||
| Hui ethnicity | 1 (0.3) | 4 (1.3) | 4.689 | 0.517–42.508 | 2 (0.6) | 3 (1.0) | 4.261 | 0.697–26.039 | ||
| Other ethnicities | 4 (1.3) | 4 (1.3) | 0.391 | 0.077–1.971 | 7 (2.3) | 1 (0.3) | 0.406 | 0.049–3.358 | ||
| Education level | 0.241 | 0.053 | ||||||||
| Primary school and below | 15 (4.9) | 28 (9.2) | 1.000 | 27 (8.9) | 16 (5.2) | 1.000 | ||||
| Junior high school | 36 (11.8) | 54 (17.7) | 1.048 | 0.506–2.167 | 61 (20.0) | 29 (9.5) | 0.802 | 0.375–1.716 | ||
| Senior high/vocational school | 32 (10.5) | 43 (14.1) | 0.917 | 0.433–1.942 | 62 (20.3) | 13 (4.3) | 0.354 | 0.150–0.836 | ||
| Junior college | 27 (8.9) | 24 (7.8) | 0.733 | 0.324–1.661 | 38 (12.5) | 13 (4.2) | 0.577 | 0.239–1.395 | ||
| Bachelor or above | 27 (8.9) | 19 (6.2) | 0.458 | 0.193–1.091 | 38 (12.5) | 8 (2.6) | 0.355 | 0.133–0.948 | ||
| Marital status | 0.650 | 0.650 | ||||||||
| Married | 111 (36.4) | 128 (42.0) | 1.000 | 177 (58.1) | 62 (20.3) | 1.000 | ||||
| Never married | 17 (5.6) | 22 (7.2) | 1.003 | 0.507–1.985 | 29 (9.5) | 10 (3.3) | 0.984 | 0.454–2.136 | ||
| Divorced or separate | 5 (1.6) | 12 (4.0) | 1.854 | 0.683–5.037 | 14 (4.6) | 3 (1.0) | 0.612 | 0.170–2.200 | ||
| Widowed | 4 (1.3) | 6 (1.9) | 1.298 | 0.366–4.602 | 6 (2.0) | 4 (1.2) | 1.903 | 0.520–6.968 | ||
| Occupation | 0.053 | 0.307 | ||||||||
| Mental worker | 44 (14.4) | 32 (10.5) | 1.000 | 59 (19.3) | 17 (5.6) | 1.000 | ||||
| Manual worker | 19 (6.2) | 34 (11.2) | 1.688 | 0.820–3.476 | 35 (11.5) | 18 (5.9) | 1.785 | 0.815–3.908 | ||
| Others | 74 (24.3) | 102 (33.4) | 1.994 | 1.136–3.500 | 132 (43.3) | 44 (14.4) | 1.157 | 0.611–2.190 | ||
| Family monthly income (US dollars/month) | <0.001 | 0.506 | ||||||||
| <157 | 28 (9.2) | 46 (15.1) | 1.000 | 52 (17.0) | 22 (7.3) | 1.000 | ||||
| 157–472 | 28 (9.2) | 58 (19.0) | 1.126 | 0.602–2.104 | 61 (20.0) | 25 (8.2) | 0.969 | 0.490–1.916 | ||
| 473–943 | 40 (13.1) | 45 (14.8) | 0.624 | 0.333–1.1770 | 64 (21.0) | 21 (6.9) | 0.776 | 0.385–1.563 | ||
| 944–1414 | 19 (6.2) | 9 (3.0) | 0.185 | 0.063–0.539 | 24 (7.9) | 4 (1.3) | 0.394 | 0.122–1.755 | ||
| ≥1415 | 22 (7.2) | 10 (3.2) | 0.196 | 0.072–0.532 | 25 (8.2) | 7 (2.2) | 0.662 | 0.250–1.755 | ||
| Daily sleep duration (hours) | <0.001 | 0.014 | ||||||||
| ≥7 | 106 (34.8) | 105 (34.4) | 1.000 | 165 (54.1) | 46 (15.1) | 1.000 | ||||
| <7 | 31 (10.2) | 63 (20.6) | 2.413 | 1.468–3.967 | 61 (20.0) | 33 (10.8) | 1.940 | 1.137–3.313 | ||
| Number of co-morbid diseases | <0.001 | <0.001 | ||||||||
| 0 | 91 (29.9) | 66 (21.6) | 1.000 | 131 (43.0) | 26 (8.5) | 1.000 | ||||
| 1 | 36 (11.8) | 74 (24.3) | 2.423 | 1.468–4.000 | 73 (23.9) | 37 (12.2) | 2.554 | 1.433–4.550 | ||
| 2 | 9 (3.0) | 19 (6.2) | 2.330 | 1.033–5.257 | 18 (5.9) | 10 (3.3) | 2.799 | 1.161–6.749 | ||
| ≥3 | 1 (0.3) | 9 (2.9) | 18.173 | 2.242–147.302 | 4 (1.2) | 6 (2.0) | 7.558 | 1.992–28.670 | ||
| Employment status | 0.475 | 0.737 | ||||||||
| Yes | 26 (8.5) | 24 (7.9) | 1.000 | 38 (12.5) | 12 (3.9) | 1.000 | ||||
| No | 111 (36.4) | 144 (47.2) | 1.252 | 0.675–2.320 | 188 (61.6) | 67 (22.0) | 1.129 | 0.557–2.287 | ||
| Health care type | 0.023 | 0.576 | ||||||||
| New rural cooperative medical care | 35 (11.5) | 66 (21.6) | 1.000 | 70 (23.0) | 31 (10.1) | 1.000 | ||||
| Medical insurance for urban residents | 19 (6.2) | 25 (8.2) | 0.870 | 0.429–1.768 | 33 (10.8) | 11 (3.6) | 0.753 | 0.337–1.680 | ||
| Medical insurance for urban workers | 61 (20.0) | 61 (20.0) | 0.604 | 0.355–1.029 | 93 (30.5) | 29 (9.5) | 0.704 | 0.389–1.275 | ||
| Provincial health care | 22 (7.2) | 16 (5.3) | 0.311 | 0.137–0.707 | 30 (9.8) | 8 (2.7) | 0.602 | 0.248–1.462 | ||
| Exercise Status | 0.002 | <0.001 | ||||||||
| Often | 43 (14.1) | 32 (10.5) | 1.000 | 62 (20.3) | 13 (4.3) | 1.000 | ||||
| Occasionally | 71 (23.3) | 84 (27.5) | 1.750 | 0.981–3.123 | 121 (39.7) | 34 (11.1) | 0.418 | 0.660–2.722 | ||
| Never | 23 (7.5) | 52 (17.1) | 2.705 | 1.390–5.263 | 43 (14.1) | 32 (10.5) | 0.001 | 1.672–7.535 | ||
| Dialysis duration (years) | 0.972 | 0.848 | ||||||||
| ≤2 | 33 (10.8) | 41 (13.5) | 1.000 | 52 (17.0) | 22 (7.3) | 1.000 | ||||
| 3–5 | 42 (13.8) | 53 (17.3) | 1.072 | 0.582–1.974 | 72 (23.6) | 23 (7.5) | 0.755 | 0.381–1.497 | ||
| 6–9 | 39 (12.8) | 43 (14.1) | 0.925 | 0.491–1.744 | 62 (20.3) | 20 (6.6) | 0.762 | 0.375–1.549 | ||
| ≥10 | 23 (7.5) | 31 (10.2) | 0.994 | 0.491–2.013 | 40 (13.1) | 14 (4.6) | 0.827 | 0.377–1.817 | ||
| Duration of dialysis per week (hours) | 0.554 | 0.250 | ||||||||
| <11.5 | 24 (7.9) | 43 (14.1) | 1.000 | 46 (15.1) | 21 (6.9) | 1.000 | ||||
| ≥11.5 | 113 (37.0) | 125 (41.0) | 0.849 | 0.493–1.462 | 180 (59.0) | 58 (19.0) | 0.706 | 0.389–1.280 | ||
| Primary disease | 0.688 | <0.001 | ||||||||
| Diabetes mellitus | 15 (4.9) | 27 (8.9) | 1.000 | 27 (8.9) | 15 (4.9) | 1.000 | ||||
| Glomerulonephritis | 25 (8.2) | 38 (12.5) | 1.000 | 0.458–2.185 | 48 (15.8) | 15 (4.9) | 2.554 | 1.433–4.550 | ||
| Primary hypertension | 44 (14.4) | 51 (16.7) | 0.949 | 0458–1.964 | 68 (22.2) | 27 (8.9) | 2.799 | 1.161–6.749 | ||
| Tubulointerstitial disease | 0 (0.0) | 0 (0.0) | 0.786 | 0.215–2.876 | 0 (0.0) | 0 (0.0) | 7.558 | 1.992–28.670 | ||
| Autoimmune disease | 5 (1.6) | 7 (2.3) | 0.393 | 0.120–1.288 | 9 (2.9) | 3 (1.0) | ||||
| Polycystic kidney | 13 (4.3) | 6 (1.9) | 0.928 | 0.420–2.051 | 17 (5.6) | 2 (0.6) | ||||
| Unknown | 26 (8.5) | 33 (10.8) | 0.550 | 0.160–1.886 | 45 (14.8) | 14 (4.6) | ||||
| Drug-induced kidney injury | 9 (3.0) | 6 (2.0) | 12 (3.9) | 3 (1.0) | ||||||
| With diabetes mellitus or not | 0.803 | 0.379 | ||||||||
| Yes | 19 (6.2) | 33 (10.8) | 1.000 | 36 (11.8) | 16 (5.2) | 1.000 | ||||
| No | 118 (38.7) | 135 (44.3) | 0.927 | 0.509–1.687 | 190 (62.3) | 63 (20.7) | 0.746 | 0.388–1.435 | ||
| With heart disease or not | <0.001 | 0.001 | ||||||||
| Yes | 24 (7.9) | 63 (20.6) | 1.000 | 53 (17.4) | 34 (11.1) | 1.000 | ||||
| No | 113 (37.0) | 105 (34.5) | 0.279 | 0.165–0.471 | 173 (56.7) | 45 (14.8) | 0.405 | 0.236–0.697 | ||
| Pruritus severity | <0.001 | <0.001 | ||||||||
| No | 44 (14.4) | 16 (5.3) | 1.000 | 55 (18.0) | 5 (1.7) | 1.000 | ||||
| Sustainable | 86 (28.2) | 116 (38.0) | 2.806 | 1.480–5.528 | 146 (47.9) | 56 (18.3) | 4.219 | 1.606–11.085 | ||
| Unbearable | 7 (2.3) | 36 (11.8) | 6.133 | 2.578–14.592 | 25 (8.2) | 18 (5.9) | 7.920 | 2.642–23.741 | ||
| Systolic blood pressure (mmHg) | 0.026 | 0.050 | ||||||||
| >160 | 27 (8.9) | 45 (14.7) | 1.000 | 52 (17.0) | 20 (6.6) | 1.000 | ||||
| 141–159 | 38 (12.5) | 38 (12.5) | 0.426 | 0.221–0.821 | 63 (20.7) | 13 (4.2) | 0.521 | 0.259–1.047 | ||
| 130–140 | 47 (15.3) | 49 (16.1) | 0.458 | 0.231–0.911 | 73 (24.0) | 23 (7.5) | 0.341 | 0.155–0.751 | ||
| 89–129 | 25 (8.2) | 36 (11.8) | 0.785 | 0.395–1.560 | 38 (12.5) | 23 (7.5) | 0.635 | 0.306–1.320 | ||
| Diastolic blood pressure (mmHg) | 0.046 | 0.045 | ||||||||
| ≥100 | 13 (4.3) | 29 (9.5) | 1.000 | 33 (10.8) | 9 (3.0) | 1.000 | ||||
| 90–99 | 44 (14.4) | 46 (15.1) | 0.413 | 0.195–0.873 | 75 (24.6) | 15 (4.9) | 0.733 | 0.292–1.844 | ||
| 80–89 | 43 (14.1) | 45 (14.8) | 0.428 | 0.202–0.907 | 62 (20.3) | 26 (8.6) | 1.538 | 0.646–3.662 | ||
| <80 | 37 (12.1) | 48 (15.7) | 0.696 | 0.329–1.472 | 56 (18.4) | 29 (9.4) | 1.899 | 0.801–4.499 | ||
| Mean arterial pressure (mmHg) | 0.393 | 0.034 | ||||||||
| ≤105 | 68 (22.3) | 86 (28.2) | 1.000 | 106 (34.8) | 48 (15.7) | 1.000 | ||||
| >105 | 69 (22.6) | 82 (26.9) | 0.983 | 0.626–1.543 | 120 (39.3) | 31 (10.2) | 0.570 | 0.339–0.961 | ||
| Hemoglobin (g/L) | 0.877 | 0.736 | ||||||||
| <100 | 49 (17.1) | 49 (17.1) | 1.000 | 75 (26.2) | 23 (8.1) | 1.000 | ||||
| 100–130 | 64 (22.4) | 87 (30.5) | 0.878 | 0.527–1.463 | 111 (38.8) | 40 (14.0) | 1.175 | 0.651–2.121 | ||
| >130 | 15 (5.2) | 22 (7.7) | 0.961 | 0.450–2.052 | 26 (9.1) | 11 (3.8) | 1.380 | 0.592–3.214 | ||
Figure 1.
Univariate analysis of the relationship between monthly family income (A), number of comorbidities (B), pruritus severity (C), and depression, using univariate logistic regression.
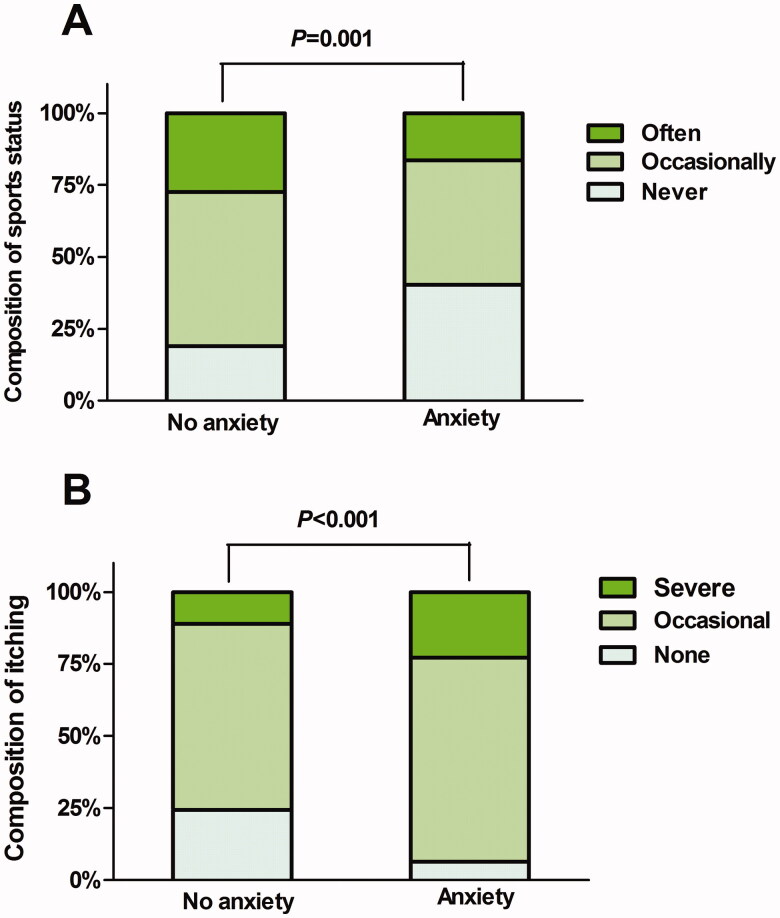
On univariate analysis with anxiety as the dependent variable, eight variables reached statistical significance, including daily sleep duration (p < 0.014), number of comorbidities (p < 0.001), exercise status (p < 0.001), ESRD origins (p < 0.001), heart disease history (p = 0.001), pruritus severity (p < 0.001), diastolic blood pressure (p = 0.045) and mean blood pressure (p = 0.034). Figure 2 shows the results for the relationships between exercise status, pruritus severity, and anxiety.
Figure 2.
Univariate analysis of the relationship between exercise status (A), pruritus severity (B) and depression, using univariate logistic regression.
Multivariate logistic regression analysis results for depression predictors
Variables with a P value <0.05 from univariate analysis were included in the multivariate logistic regression analysis (Table 3). We found that lower monthly family income, more comorbidities, and a higher degree of pruritus were independent predictors of depression in patients undergoing MHD. Higher monthly family income was protective against depression; the risk of depression in patients with a monthly family income between 944 and 1414 US dollars was about one-fifth that of patients with a monthly family income of less than 157 US dollars (95% confidence interval [CI] 0.063–0.769). The risk of depression in patients with a monthly family income of more than 1415 US dollars was about one-fifth that of patients with a monthly family income of less than 157 US dollars (95% CI 0.065–0.673). In addition, patients with one, two, and three comorbidities had 3.352 (95% CI 1.551–7.243), 4.146 (95% CI 1.232–13.949), and 18.527 (95% CI 1.674–205.028) times higher risks of depression than those without any comorbidity. Patients with self-reported intermittent and severe pruritus had 4.364 (95% CI 2.075–9.137) and 15.971 (95% CI 5.173–49.315) times higher risks of depression than those without pruritus.
Table 3.
Multivariate analysis of factors predicting depression (N = 305).
| 95% CI |
|||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Β | S.E. | Wald | P | OR | Lower | Upper |
| Family monthly income (US dollars/month) | |||||||
| <157 | 1.000 | ||||||
| 157–472 | 0.297 | 0.392 | 0.572 | 0.449 | 1.346 | 0.624 | 2.904 |
| 473–943 | −0.485 | 0.421 | 1.329 | 0.249 | 0.616 | 0.27 | 1.404 |
| 944–1414 | −1.516 | 0.639 | 5.623 | 0.018 | 0.22 | 0.063 | 0.769 |
| ≥1415 | −1.564 | 0.596 | 6.885 | 0.009 | 0.209 | 0.065 | 0.673 |
| Daily sleep duration (hours) | |||||||
| ≥7 | 1.000 | ||||||
| <7 | −0.042 | 0.074 | 0.323 | 0.57 | 0.959 | 0.830 | 1.108 |
| Number of co-morbid illnesses | |||||||
| 0 | 1.000 | ||||||
| 1 | 1.21 | 0.393 | 9.47 | 0.002 | 3.352 | 1.551 | 7.243 |
| 2 | 1.422 | 0.619 | 5.278 | 0.022 | 4.146 | 1.232 | 13.949 |
| ≥3 | 2.919 | 1.227 | 5.665 | 0.017 | 18.527 | 1.674 | 205.028 |
| Health care type | |||||||
| New rural cooperative medical care | 1.000 | ||||||
| Medical insurance for urban residents | −0.108 | 0.45 | 0.058 | 0.81 | 0.897 | 0.372 | 2.167 |
| Medical insurance for urban workers | −0.476 | 0.375 | 1.614 | 0.204 | 0.621 | 0.298 | 1.295 |
| Provincial health care | −0.48 | 0.551 | 0.761 | 0.383 | 0.619 | 0.21 | 1.821 |
| Exercise status | |||||||
| Often | 1.000 | ||||||
| Occasionally | 0.424 | 0.376 | 1.267 | 0.260 | 1.528 | 0.730 | 3.195 |
| Never | 0.607 | 0.430 | 1.987 | 0.159 | 1.834 | 0.789 | 4.263 |
| With heart disease or not | |||||||
| Yes | 1.000 | ||||||
| No | −0.06 | 0.434 | 0.019 | 0.891 | 0.942 | 0.403 | 2.204 |
| Pruritus condition | |||||||
| No | 1.000 | ||||||
| Sustainable | 1.473 | 0.377 | 15.275 | <0.001 | 4.364 | 2.085 | 9.137 |
| Unbearable | 2.771 | 0.575 | 23.202 | <0.001 | 15.971 | 5.173 | 49.315 |
| Systolic blood pressure(mmHg) | |||||||
| >160 | 1.000 | ||||||
| 141–159 | −0.385 | 0.452 | 0.726 | 0.394 | 0.68 | 0.281 | 1.65 |
| 130–140 | −0.508 | 0.478 | 1.126 | 0.289 | 0.602 | 0.236 | 1.537 |
| 89–129 | −0.187 | 0.524 | 0.127 | 0.722 | 0.83 | 0.297 | 2.317 |
| Diastolic blood pressure (mmHg) | |||||||
| ≥100 | 1.000 | ||||||
| 90–99 | −0.415 | 0.482 | 0.741 | 0.389 | 0.66 | 0.256 | 1.7 |
| 80–89 | −0.807 | 0.494 | 2.666 | 0.103 | 0.446 | 0.169 | 1.175 |
| <80 | −0.696 | 0.551 | 1.599 | 0.206 | 0.498 | 0.169 | 1.467 |
Notes: CI, confidence interval; OR, odds ratio.
Multivariate logistic regression analysis results for anxiety predictors
Variables with a P value <0.05 from univariate analysis were included in the multivariate logistic regression analysis (Table 4). We found two independent predictors of anxiety, including infrequent exercise status and a higher degree of pruritus. The risks for anxiety in patients undergoing MHD who occasionally exercised (OR 1.635, 95% CI 0.750–3.565) and who never exercised (OR 3.289, 95% CI 1.411–7.664) were higher than those for patients who often exercised. Patients with occasional pruritus (OR 3.849, 95% CI 1.403–10.556) or severe pruritus (OR 5.912, 95% CI 1.733–20.168) had a significantly higher risk of anxiety than those without pruritus.
Table 4.
Multivariate analysis of factors predicting anxiety (N = 305).
| 95% CI |
|||||||
|---|---|---|---|---|---|---|---|
| Characteristics | β | S.E. | Wald | P | OR | Lower | Upper |
| Daily sleep duration (hours) | |||||||
| ≥7 | 1.000 | ||||||
| <7 | 0.302 | 0.339 | 0.793 | 0.373 | 1.352 | 0.696 | 2.627 |
| Number of co-morbid illnesses | |||||||
| 0 | 1.000 | ||||||
| 1 | 0.623 | 0.410 | 2.312 | 0.128 | 1.865 | 0.835 | 4.166 |
| 2 | 0.408 | 0.655 | 0.388 | 0.534 | 1.503 | 0.417 | 5.423 |
| ≥3 | 1.261 | 0.986 | 1.634 | 0.201 | 3.527 | 0.511 | 24.372 |
| Exercise status | |||||||
| Often | 1.000 | ||||||
| Occasionally | 0.492 | 0.894 | 1.592 | 0.216 | 1.635 | 0.750 | 3.565 |
| Never | 1.191 | 0.432 | 7.608 | 0.006 | 3.289 | 1.411 | 7.664 |
| Primary disease | |||||||
| Diabetes mellitus | 1.000 | ||||||
| Glomerulonephritis | −0.228 | 0.558 | 0.167 | 0.683 | 0.796 | 0.267 | 2.375 |
| Primary hypertension | 0.073 | 0.518 | 0.020 | 0.888 | 1.076 | 0.390 | 2.967 |
| Autoimmune disease | 0.113 | 0.558 | 0.167 | 0.683 | 0.796 | 0.207 | 6.068 |
| Polycystic kidney | −1.303 | 0.916 | 2.026 | 0.155 | 0.272 | 0.045 | 1.634 |
| Drug-induced kidney injury | −0.975 | 0.894 | 1.188 | 0.276 | 0.377 | 0.065 | 2.177 |
| Unknown | −0.270 | 0.572 | 0.222 | 0.637 | 0.764 | 0.249 | 2.343 |
| With heart disease or not | |||||||
| Yes | 1.000 | ||||||
| No | −0.287 | 0.423 | 0.461 | 0.497 | 0.750 | 0.327 | 1.719 |
| Pruritus condition | |||||||
| No | 1.000 | ||||||
| Sustainable | 1.348 | 0.515 | 6.856 | 0.009 | 3.849 | 1.403 | 10.556 |
| Unbearable | 1.777 | 0.626 | 8.056 | 0.005 | 5.912 | 1.733 | 20.168 |
| Diastolic blood pressure (mmHg) | |||||||
| ≥100 | 1.000 | ||||||
| 90–99 | −0.159 | 0.519 | 0.093 | 0.760 | 0.853 | 0.309 | 2.360 |
| 80–89 | 0.346 | 0.575 | 0.362 | 0.547 | 1.413 | 0.458 | 4.357 |
| <80 | 0.283 | 0.659 | 0.184 | 0.668 | 1.327 | 0.365 | 4.834 |
| Mean arterial pressure (mmHg) | |||||||
| ≤105 | 1.000 | ||||||
| >105 | −0.352 | 0.460 | 0.586 | 0.444 | 0.703 | 0.285 | 1.733 |
Notes: CI, confidence interval; OR, odds ratio.
Correlation analysis between depression and anxiety
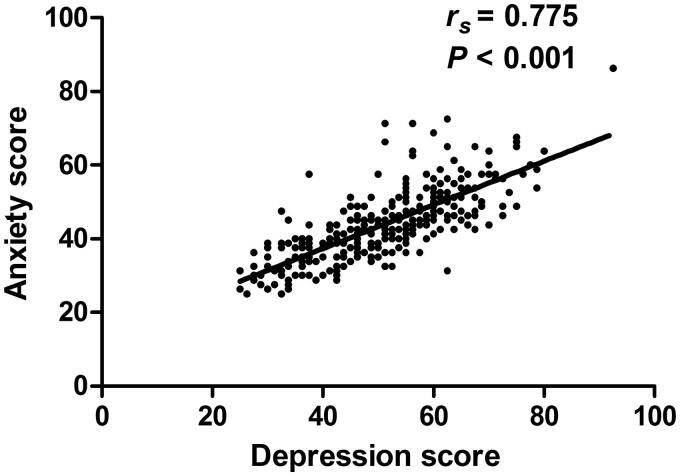
Among the enrollees, 76 patients (24.9%) suffered from both depression and anxiety. Spearman correlation analysis showed a significant correlation between depression and anxiety (rs = 0.775, p < 0.001; Figure 3).
Figure 3.
Correlation results between depression and anxiety. The x-axis represents the standard scores for depression, and the y-axis represents the standard scores for anxiety.
Discussion
In this study, we investigated the prevalence of depression and anxiety in patients undergoing MHD in a large city in Northern China and identified predictors for depression and anxiety in these patients. We found that MHD patients in Northern China had high prevalence rates of depression (55.1%) and anxiety (25.9%). Lower family income, more comorbidities, and a higher degree of pruritus were predictors of depression, and infrequent exercise and a higher degree of pruritus were predictors of anxiety. Depression was correlated with anxiety obviously.
Prior studies showed that patients undergoing MHD have a significantly higher prevalence of depression or anxiety than the general population. A meta-analysis showed that the prevalence of clinical interview-identified depression in patients undergoing MHD was 22.8% and that the prevalence of self- or clinician-rated depression in patients undergoing MHD was 39.3% [4]. Another meta-analysis suggested the prevalence of depression among Iranian patients undergoing MHD was 62.0% [18], while a multicenter study identified the prevalence rates of depression and anxiety to be 29.4% and 35.9%, respectively, in Greek patients undergoing MHD [5]. Reports have shown that the prevalence rates of depression and anxiety among Kuwaiti patients undergoing MHD were 21.7% and 21.4%, respectively [19], and those among patients in Saudi Arabia were 23.3% and 21.1%, respectively [20]. The prevalence rates of depression and anxiety in Chinese patients undergoing MHD in the present study ranged from 21.7% to 62.0% and from 21.1% to 35.9%, respectively, and these rates may be higher than those in other populations [21,22].
We did not find any literature regarding a nationwide epidemiological survey on the prevalence of depression or anxiety in patients undergoing MHD in China in the literature search. Few regional studies existed, while multiple single-center reports were found for depression alone, anxiety alone or the two together. A study from Kashgar, Xinjiang, China showed that the prevalence rates of depression and anxiety in patients undergoing MHD were 66.7% and 53.3%, respectively [23]. Most single-center studies from coastal and central China revealed a 40%–50% prevalence of depression and a 15%–30% prevalence of anxiety in a patient undergoing MHD. We found that the prevalence rates of depression and anxiety in patients undergoing MHD on the northern border of China were 55.1% and 25.9%, respectively. This finding for depression prevalence was mildly higher than those reported in coastal and central China, while our finding for anxiety prevalence was similar to those reported in coastal and central China. We considered that these differences in depression and anxiety prevalence may reflect true differences in their prevalence or be due to differences in the assessment approaches for depression and anxiety, as studies that used other scales and survey results from the same population gathered using different scales showed different results. Such scales included the symptom check list-90, the Center for Epidemiologic Studies depression scale, the state-trait anxiety inventory, and the Beck depression inventory. We believe that true differences may exist in the depression and anxiety prevalence of our study population.
We showed that suboptimal economic status might be an important contributor to the higher prevalence of depression on the northern border of China, based on the multivariate regression analyses result that family monthly income independently predicted depression. Monthly family income is relatively lower on the northern border than in central and coastal China. Our findings of high prevalence rates of depression and anxiety on the northern border of China suggest that medical workers and healthcare-relevant personnel should pay attention to the prevention and treatment of depression and anxiety in patients undergoing MHD, in order to reduce the associated harm. We plan to establish an intervention group to treat MHD patients with psychological disorders and foster a partnership with the regional mental health centers.
From the multivariate analysis in the present study, three factors can independently increase the incidence rate of depression, including a lower monthly family income, more comorbidities, and a higher degree of pruritus. Existing studies suggest that family income has a close relationship with depression [24–26], with a lower family income corresponding to a higher incidence of depression. However, most studies were cross-sectional studies, and very few cohort studies or randomized controlled trials (RCTs) exist. It is likely that enhancing economic stability can provide patients with better medical accessibility and better medical care, thereby reducing depression. Multiple studies suggest that the number and severity of comorbidities are linearly associated with depression in patients undergoing MHD [25,27], but we did not find any cohort studies or RCTs addressing this issue. We considered that this relationship exists not only in patients with ESRD but also in patients with other chronic diseases. For example, a number of comorbidities are positively correlated with the occurrence of depression in patients with other conditions, such as diabetes [28]. It is likely that the number and severity of comorbidities significantly increase patients’ suffering, reduce their quality of life, increase difficulties encountered during treatment, and increase medical costs, all of which could contribute to the relationship between the number of comorbidities and the development of depression.
Pruritus is common in patients undergoing MHD. Among 219 patients undergoing MHD, Inbar et al. found that 66% reported experiences of pruritus and 48% had ongoing pruritus during the study [29]. Contemporary studies mostly concur that pruritus is closely associated with the prognosis of patients undergoing MHD. Among 1773 Japanese patients undergoing MHD, Narita et al. found that severe pruritus was an independent risk factor for mortality after 2 years of follow-up [30]. Another prospective study in Germany also found that among 860 patients undergoing MHD, severe pruritus independently predicted mortality after 4 years of follow-up [31]. However, similar studies have not been conducted in China. From the above information, we believe that pruritus in patients undergoing MHD is an independent risk factor for depression. Interestingly, there are reports suggesting that treatment with anti-pruritus drugs can alleviate anxiety in patients undergoing MHD [32]. Nonetheless, clinicians serving in dialysis units in China pay little attention to pruritus, and the management of pruritus in patients undergoing MHD should be emphasized in the future.
In the present study, two independent predictors of anxiety were found, including infrequent exercise and a higher degree of pruritus. Others also found that exercise status is closely associated with anxiety. A prior cross-sectional study reported that exercise correlates with anxiety and depression [33]. Another small-scale study indicated that the institution of an exercise program can improve anxiety and quality of life in patients undergoing MHD [34].
We further discovered factors that were associated with depression or anxiety on univariate analysis but not on multivariate analysis, including occupation, daily sleep duration, health insurance type, history of heart disease, and systolic and diastolic blood pressure. Some of the above factors, such as sleep duration, were identified by others as independent risk factors for depression or anxiety in patients undergoing MHD. We hope that in the future, prospective studies can be undertaken to further explore the validity of our findings.
Several limitations of this study must be noted. First, although this was a multi-center study, the number of enrolled centers and the distribution area of enrolled centers were limited. Second, this cross-sectional study could not determine the causal relationship between the independent variable and the dependent variable. More studies including more centers across broader areas are needed to investigate the prevalence of depression an anxiety in MHD patients, and prospective studies are needed to identify risk factors or new therapeutic approaches for depression and anxiety.
In conclusion, we found that the prevalence of depression in patients undergoing MHD on the northern border of China was 55.1%, including 27.5%, 21.0%, and 6.6% with mild, moderate, and severe cases, respectively, and the prevalence of anxiety in the same area was 25.9%, including 20.0%, 4.6%, and 1.3% with mild, moderate, and severe cases, respectively. Multivariate regression analyses confirmed that high monthly family income was an independent protective factor for depression in patients undergoing MHD, while an increasing number of comorbidities and higher pruritus severity were independent risk factors for depression. Infrequent exercise and higher pruritus severity were two independent risk factors for anxiety in patients undergoing MHD. Depression was correlated with anxiety. The findings of this article indicate that MHD patients have high prevalence rates of depression and anxiety and that efforts to increase family income, manage comorbidities and pruritus, and promote exercise among patients may ameliorate depression and anxiety in these patients in the future.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 81960143, 81960130), the Program for Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region (No. NJYT-19-B34), the Inner Mongolia Health Science and Technology Project in 2022 (no., 202201293) “Going far” Talent Program of Inner Mongolia Medical University (No. ZY0130015), the Trinity College Students Innovation and Entrepreneurship Cultivation Project of Inner Mongolia Medical University (No. SWYT2020008) and the Head Start Program of the Affiliated Hospital of Inner Mongolia Medical University (No. FYQMJH2020026).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Collaboration GCKD . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):933–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho Y, Bello AK, Levin A, et al. Peritoneal dialysis use and practice patterns: an international survey study. Am J Kidney Dis. 2021;77(3):315–325. [DOI] [PubMed] [Google Scholar]
- 3.Htay H, Bello AK, Levin A, et al. Hemodialysis use and practice patterns: an international survey study. Am J Kidney Dis. 2021;77(3):326–335. [DOI] [PubMed] [Google Scholar]
- 4.Palmer S, Vecchio M, Craig JC, et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013;84(1):179–191. [DOI] [PubMed] [Google Scholar]
- 5.Gerogianni G, Lianos E, Kouzoupis A, et al. The role of socio-demographic factors in depression and anxiety of patients on hemodialysis: an observational cross-sectional study. Int Urol Nephrol. 2018;50(1):143–154. [DOI] [PubMed] [Google Scholar]
- 6.Palmer SC, Vecchio M, Craig JC, et al. Association between depression and death in people with CKD: a meta-analysis of cohort studies. Am J Kidney Dis. 2013;62(3):493–505. [DOI] [PubMed] [Google Scholar]
- 7.Farrokhi F, Abedi N, Beyene J, et al. Association between depression and mortality in patients receiving long-term dialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63(4):623–635. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu U, Aoki H, Sakagami M, et al. Walking ability, anxiety and depression, significantly decrease EuroQol 5-Dimension 5-Level scores in older hemodialysis patients in Japan. Arch Gerontol Geriatr. 2018;78(5):96–100. [DOI] [PubMed] [Google Scholar]
- 9.Chen CK, Tsai YC, Hsu HJ, et al. Depression and suicide risk in hemodialysis patients with chronic renal failure. Psychosomatics. 2010;51(6):528–534. [DOI] [PubMed] [Google Scholar]
- 10.Martiny C, de Oliveira e Silva AC, Neto JP, et al. Factors associated with risk of suicide in patients with hemodialysis. Compr Psychiatry. 2011;52(5):465–468. [DOI] [PubMed] [Google Scholar]
- 11.De Berardis D, Fornaro M, Valchera A, et al. Eradicating suicide at its roots: preclinical bases and clinical evidence of the efficacy of ketamine in the treatment of suicidal behaviors. IJMS. 2018;19(10):2888–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orsolini L, Latini R, Pompili M, et al. Understanding the complex of suicide in depression: from research to clinics. Psychiatry Investig. 2020;17(3):207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song S, Yang X, Hua Yang H, et al. Psychological resilience as a protective factor for depression and anxiety among the public during the outbreak of COVID-19. Front Psychol. 2021;11(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunstan DA, Scott N.. Clarification of the cut-off score for zung's self-rating depression scale. BMC Psychiatry. 2019;19(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunstan DA, Scott N.. Norms for zung's self-rating anxiety scale. BMC Psychiatry. 2020;20(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunstan DA, Scott N, Todd AK.. Screening for anxiety and depression: reassessing the utility of the zung scales. BMC Psychiatry. 2017;17(1):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hao W, Tang Q, Huang X, et al. Analysis of the prevalence and influencing factors of depression and anxiety among maintenance dialysis patients during the COVID-19 pandemic. Int Urol Nephrol. 2021;53(7):1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravaghi H, Behzadifar M, Behzadifar M, et al. Prevalence of depression in hemodialysis patients in Iran: a systematic review and meta-analysis. Iran J Kidney Dis. 2017;11(2):90–98. [PubMed] [Google Scholar]
- 19.Al-Shammari N, Al-Modahka A, Al-Ansari E, et al. Prevalence of depression, anxiety, and their associations among end-stage renal disease patients on maintenance hemodialysis: a multi-center population-based study. Psychol Health Med. 2021;26(9):1134–1142. [DOI] [PubMed] [Google Scholar]
- 20.Turkistani I, Nuqali A, Badawi M, et al. The prevalence of anxiety and depression among end-stage renal disease patients on hemodialysis in Saudi Arabia. Ren Fail. 2014;36(10):1510–1515. [DOI] [PubMed] [Google Scholar]
- 21.Gelenberg AJ. The prevalence and impact of depression. J Clin Psychiatry. 2010;71(3):e06. [DOI] [PubMed] [Google Scholar]
- 22.Baxter AJ, Scott KM, Vos T, et al. Global prevalence of anxiety disorders: a systematic review and meta-regression. Psychol Med. 2013;43(5):897–910. [DOI] [PubMed] [Google Scholar]
- 23.Maimaituxun ZL, Junqiao W, Wenmei Z, et al. Study on anxiety and depression status and its influencing factors among patients with maintenance hemodialysis in Kashi Region of Xinjiang. J Nurses Train. 2019;34(4):299–304. [Google Scholar]
- 24.Sezer S, Uyar ME, Bal Z, et al. The influence of socioeconomic factors on depression in maintenance hemodialysis patients and their caregivers. CN. 2013;80(11):342–348. [DOI] [PubMed] [Google Scholar]
- 25.SW A-J, Sous A, Jorf F, et al. Depression in patients treated with haemodialysis: a cross-sectional study. Lancet. 2018;391(Suppl 2):S41. [DOI] [PubMed] [Google Scholar]
- 26.Jiuzhi Z. The mood in hemodialysis patients with end-stage renal disease and its influencing factors. Journal of International Psychiatry. 2020;47(3):593–595. [Google Scholar]
- 27.Kuo-Chin H, Wu CC, Chen HS, et al. Serum IL-6, albumin and comorbidities are closely correlated with symptoms of depression in patients on maintenance haemodialysis. Nephrol Dial Transplant. 2011;26(2):658–664. [DOI] [PubMed] [Google Scholar]
- 28.Qiu S, Sun H, Liu Y, et al. Prevalence and correlates of psychological distress among diabetes mellitus adults in the Jilin province in China: a cross-sectional study. PeerJ. 2017;5(e2869):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zucker I, Yosipovitch G, David M, et al. Prevalence and characterization of uremic pruritus in patients undergoing hemodialysis: uremic pruritus is still a major problem for patients with end-stage renal disease. J Am Acad Dermatol. 2003;49(5):842–846. [DOI] [PubMed] [Google Scholar]
- 30.Narita I, Alchi B, Omori K, et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006;69(9):1626–1632. [DOI] [PubMed] [Google Scholar]
- 31.Grochulska K, Ofenloch RF, Mettang T, et al. Mortality of haemodialysis patients with and without chronic itch: a follow-up study of the German Epidemiological Hemodialysis Itch Study (GEHIS). Acta Derm Venereol. 2019;99(4):423–428. [DOI] [PubMed] [Google Scholar]
- 32.Inui S, Shirakawa Y, Itami S.. Effect of nalfurafine hydrochloride on pruritus and anxiety level in hemodialysis patients. J Dermatol. 2012;39(10):886–887. [DOI] [PubMed] [Google Scholar]
- 33.Zhang M, Kim JC, Li Y, et al. Relation between anxiety, depression, and physical activity and performance in maintenance hemodialysis patients. J Ren Nutr. 2014;24(4):252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suh M, Jung H, Kim S, et al. Effects of regular exercise on anxiety, depression, and quality of life in maintenance hemodialysis patients. Ren Fail. 2002;24(3):337–345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.