Abstract
Aspergillus spp. are frequently occurring seed-colonizing fungi that complete their disease cycles through the development of asexual spores, which function as inocula, and through the formation of cleistothecia and sclerotia. We found that development of all three of these structures in Aspergillus nidulans, Aspergillus flavus, and Aspergillus parasiticus is affected by linoleic acid and light. The specific morphological effects of linoleic acid include induction of precocious and increased asexual spore development in A. flavus and A. parasiticus strains and altered sclerotium production in some A. flavus strains in which sclerotium production decreases in the light but increases in the dark. In A. nidulans, both asexual spore production and sexual spore production were altered by linoleic acid. Spore development was induced in all three species by hydroperoxylinoleic acids, which are linoleic acid derivatives that are produced during fungal colonization of seeds. The sporogenic effects of these linoleic compounds on A. nidulans are similar to the sporogenic effects of A. nidulans psi factor, an endogenous mixture of hydroxylinoleic acid moieties. Light treatments also significantly increased asexual spore production in all three species. The sporogenic effects of light, linoleic acid, and linoleic acid derivatives on A. nidulans required an intact veA gene. The sporogenic effects of light and linoleic acid on Aspergillus spp., as well as members of other fungal genera, suggest that these factors may be significant environmental signals for fungal development.
Aspergillus spp. are common seed-infesting fungi that reproduce asexually by forming mitotically derived spores called conidia from specialized multicellular structures called conidiophores. These asexual spores are an efficient form of dissemination and serve as the primary inocula of members of this genus. Depending on whether the species has a teleomorph, Aspergillus spp. also may produce sexual fruiting bodies called cleistothecia, in which meiotically derived ascospores are formed (e.g., Aspergillus nidulans), or asexual bodies called sclerotia (e.g., Aspergillus flavus and Aspergillus parasiticus). Timely production of asexual spores, cleistothecia, and/or sclerotia increases the fitness of Aspergillus spp. in the field.
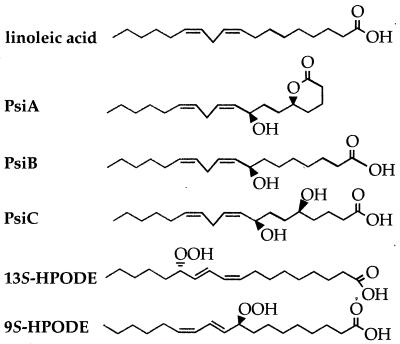
Identification of environmental factors that trigger morphological differentiation would be useful for designing strategies to control Aspergillus colonization of seeds and seed products. Two extracellular signals that have been reported to affect asexual development and sexual development in Aspergillus spp. are light (2, 18, 20, 22, 23) and psi factor (6). Psi factor is composed of three hydroxylated linoleic acid derivatives, psiA, psiB, and psiC (Fig. 1), that govern the relative development of cleistothecia or conidiophores in A. nidulans (5, 6, 15). Purified psiB and psiC promote sexual sporulation and inhibit conidiation, whereas psiA is an antagonist of psiB and psiC and has lower biological activity (5). The conservation of psi factor function in other Aspergillus spp. has not been investigated.
FIG. 1.
Chemical structures of linoleic acid, the components of A. nidulans sporogenic psi factor (psiA, psiB, and psiC), and the plant lipoxygenase products 9S-HPODE and 13S-HPODE.
Formation of conidia, cleistothecia, and sclerotia is also dependent on light (2, 18, 20, 22, 23). In wild-type A. nidulans strains, red light delays sexual development and induces conidium production (22). Little is known about the mechanisms of light dependency; however, mutation of the velvet (veA) gene eliminates light-dependent conidiation (22, 23). The sporogenic effects of light and psi factor depend on an intact veA locus; veA1 mutants are severely muted in their responses to light (22) and psi factor (6).
The fatty acids in seeds are primarily linoleic acid (18:2), palmitic acid (16:0), and oleic acid (18:1) (3), and these compounds could act as potential sporogenic factors. Linoleic acid has sporogenic effects in other fungal species, such as Alternaria tomato (11), Sclerotinia fructicola (12), and Neurospora crassa (17, 19). Seeds also produce the lipoxygenase-derived linoleic acid derivatives 13S-hydroperoxylinoleic acid and 9S-hydroperoxylinoleic acid when they are subjected to abiotic or biotic stress, including fungal colonization (reviewed in reference 8). Both of these acids resemble psi factor in structure (Fig. 1) and can alter secondary metabolism in A. parasiticus and A. nidulans (4). The results of this study support the hypothesis that plant-derived fatty acids function as Aspergillus sporogenic factors and that the sporogenic activity of these fatty acids is subject to light regulation, specifically in the presence of an intact veA gene in A. nidulans. We tested this hypothesis by adding different fatty acids to A. nidulans, A. flavus, and A. parasiticus cultures and then examining the development of colonies under continuous white light or continuous dark conditions.
MATERIALS AND METHODS
Fungal strains and growth conditions.
Aspergillus strains (Table 1) were maintained as silica stock preparations at room temperature or as glycerol stock preparations at −80°C. All strains were grown on YGT medium, which contained 0.5% (wt/vol) yeast extract, 2% (wt/vol) glucose, and 1 ml of a trace element solution per liter of medium (7). For solid medium 1.5% (wt/vol) agar was added. A. nidulans strains were cultured at 37°C, and A. flavus and A. parasiticus were cultured at 28°C. Cultures requiring white light were grown in an incubator equipped with General Electric 15-W broad-spectrum fluorescent light bulbs (catalog no. F15T12CW) positioned 20 cm from the agar surface (23), and the light intensity was 66 microeinsteins/m2/s.
TABLE 1.
Fungal strains used in this study
| Strain | Genotype | Pertinent phenotypea | Sourceb |
|---|---|---|---|
| A. nidulans WIM126 | yA2 pabaA1 veA+ | Wild-type sporulation | L. Yager |
| A. nidulans FGSC4 | veA+c | Wild-type sporulation | FGSC |
| A. nidulans WIM145 | yA2 pabaA1 acoC193 veA+ | Wild-type sporulation, psi overproducer | L. Yager |
| A. nidulans TTA11 | yA2 pabaA1 | veA1 type sporulation | T. Adams |
| A. nidulans FGSC26 | biA1 | veA1 type sporulation | FGSC |
| A. flavus 12S | Wild type | S strain | P. Cotty |
| A. flavus 70S | Wild type | S strain | P. Cotty |
| A. flavus 13L | Wild type | L strain | P. Cotty |
| A. flavus 92087-F | Wild type | L strain | P. Cotty |
| A. parasiticus SRRC145 | Wild type | SRRC |
Wild-type sporulation means that the strain is responsive to light; asexual development can occur only in the light, while sexual development can occur both in the light and in the dark. However, light delays initiation of sexual development. veA1 type sporulation means that the strain is not responsive to light, which results in asexual development when the organism is cultured in the light or the dark. S strains produce many small sclerotia, while L strains produce larger sclerotia and lower numbers of sclerotia than S strains.
FGSC, Fungal Genetic Stock Center, Kansas City, Mo.; SRRC, Southern Regional Research Center, USDA Agricultural Research Service, New Orleans, La.
Glasgow wild type.
Preparation of psi extracts.
A 250-ml portion of liquid YGT medium in a 1-liter Erlenmeyer flask was inoculated with 2 × 103 A. nidulans WIM145 conidia per ml and agitated at 250 rpm at 42°C. After 96 h mycelial pellets were removed by filtration, and the medium was extracted twice with an equal volume of ethyl acetate. The combined extracts were dried, and the residue was redissolved in a volume of ethyl acetate equal to 2% of the volume of the extracted supernatant (6).
Bioassay of psi extracts.
Ethyl acetate extracts were dried on 12.5-mm-diameter filter paper discs (Schleicher & Schuell, Keene, N.H.) and placed on agar surfaces onto which 100 μl containing 105 A. nidulans FGSC4, WIM126, TTA11, or FGSC26 conidia or 100 μl containing 105 A. flavus 12S conidia had been spread. Cultures were grown under continuous white light. For A. nidulans WIM126, cleistothecial primordia were visualized by treating the culture plates with a laccase chromogenic substrate (4-amino-2,6-dibromophenol and the coupling agent 3,5-dimethylaniline) which stained the Hülle cells and the cleistothecial primordia green (10). This treatment was not used to identify FGSC4 and FGSC26 cleistothecia, as these strains are yA+ and the asexual structures would have produced laccase and stained green. Instead, visual and microscopic observations were made. The yellowish color of the Hülle cells that aggregated around cleistothecia strongly contrasted with the green conidia of FGSC4 and could be observed with the unaided eye. The sexual stage was then verified by optical microscopy. Crude psi extracts promoted sexual sporulation in A. nidulans wild-type veA+ strains FGSC4 and WIM126 but had little effect on veA1 strains TTA11 and FGSC26. This response was used as a reference to assess fatty acid effects on sporulation in A. nidulans.
Bioassay of the lipid metabolites.
The fatty acids used in this study included oleic acid (18:1), palmitic acid (16:0), eicosatrienoic acid (20:3), arachidonic acid (20:4), eicosapentaenoic acid (20:5), linoleic acid (18:2), linolenic acid (18:3), myristic acid (14:0), and ricinoleic acid (18:1), all of which were obtained from Sigma Chemical Co. (St. Louis, Mo.). 13(S)-Hydroperoxy-cis-9,trans-11-octadecadienoic acid (13S-HPODE), 9(S)-hydroperoxy-trans-10,cis-12-octadecadienoic acid (9S-HPODE), and 13(S)-hydroxy-cis-9,trans-11-octadecadienoic acid were synthesized from linoleic acid as described previously (9). Fatty acids (0.01 to 2 mg) dissolved in 50 μl of methanol were dried on 12.5-mm-diameter paper filter discs. A paper filter disc treated with methanol was used as the solvent control. After drying, the fatty acid-containing discs and the methanol-containing discs were laid on agar surfaces at the time when fungi were inoculated (105 conidia per plate). Typically, four discs (control disc and 0.1-, 0.5-, and 1-mg fatty acid discs) were placed equidistant on an agar surface, and the relative positions of the different discs on the plates were changed randomly. The cultures were incubated in both light and darkness. Cores (diameter, 17 mm) were removed from the areas around the discs, and the interior core corresponding to the area covered by each paper disc (diameter, 12.5 mm) was discarded, which left a 4.5-mm-wide annulus; the conidia and ascospores in this annulus were counted with a hemacytometer. Sclerotia were collected from the cores and washed twice with water containing 0.01% Tween 80 (Sigma) to remove conidia; then they were freeze dried, and sclerotium production was determined gravimetrically (dry weight per core).
For each fungal strain, tests for each fatty acid were performed by using three replicates, with one petri plate per replication. Each fatty acid test was conducted at a different time (e.g., the effect of oleic acid on A. nidulans WIM126 in the light was one test). The linoleic acid test with A. flavus 12S was repeated three times over the course of 1 year in order to examine reproducibility; the rest of the tests were performed only once. Spore suspensions were prepared once a month and stored at 4°C, which prevented spores from germinating.
Statistical analysis.
An analysis of variance and Fisher’s protected least-significant-difference test were used for the statistical analysis. The data generated from each fatty acid treatment were analyzed individually in order to determine the effects of the fatty acid compared with the control. Comparisons between fatty acid treatments were not made.
RESULTS
Light affects Aspergillus development.
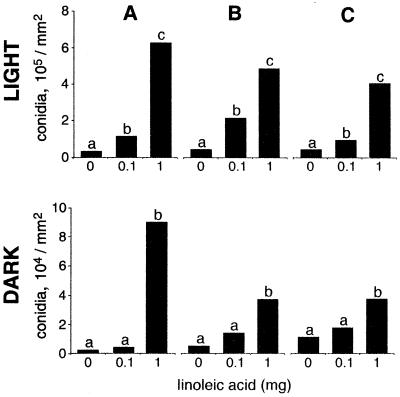
We determined if light regulated development in A. parasiticus and A. flavus. Our results showed that for all treatments conidial production and sclerotial production were statistically different with respect to light (Fig. 2).
FIG. 2.
Replication over time of linoleic acid effects on conidial development in A. flavus 12S in either complete darkness or continuous white light. A, first test; B, second test; C, third test. On each occasion the test was performed with three replicates. The results of the three tests are shown chronologically. The only differences found in the analysis were differences with respect to light and linoleic acid treatments. Within each test, bars with the same letter are not significantly different (P ≤ 0.05).
Fatty acids effects on Aspergillus development are reproducible.
We repeated the linoleic acid test with A. flavus 12S three times over the course of a year by using a different spore suspension each time both in the light and in the dark (Fig. 2). Neither replication within a test nor test repetition at different times had a significant effect on the fungus. Therefore, the rest of the fatty acid treatments were performed in triplicate but were not repeated over time.
Sclerotial development and asexual spore development are affected by 18:2 fatty acids in A. flavus.
We tested the effects of 0.1- to 1-mg portions of fatty acids on the development of a sclerotial strain of A. flavus (strain 12S) grown with continuous light, and the physiological levels of the three main fatty acids ranged from 2 to 30 mg for palmitic acid, from 4 to 120 mg for oleic acid, and from 9 to 75 mg for linoleic acid in corn (3, 21) and peanut (1, 3) seeds (the values for the HPODEs are not known).
Linoleic acid and two of its derivatives, 13S-HPODE and 9S-HPODE, caused an increase in conidial development (visualized as a green halo of conidia [Fig. 3A]), whereas oleic acid and palmitic acid did not have any effect. Sclerotial production was significantly (P ≤ 0.05) reduced by the 13S-HPODE treatments, and sclerotial weight decreased from an average of 4.3 mg of sclerotia/core for the control treatment to 3.5 mg of sclerotia/core for the preparation treated with 1 mg of 13S-HPODE. Oleic acid, palmitic acid, and 9S-HPODE did not have significant effects on sclerotial production.
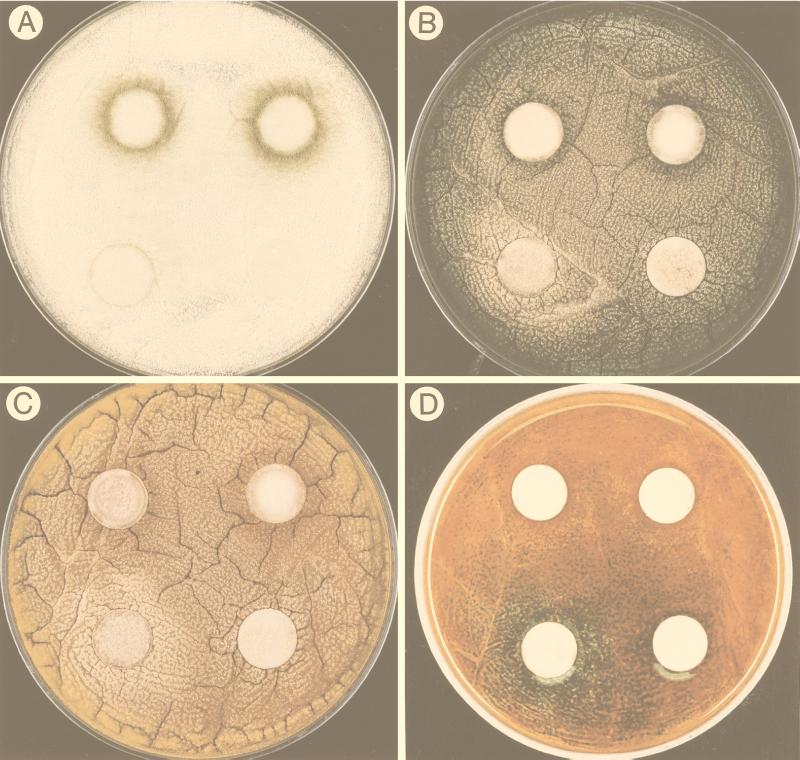
FIG. 3.
Photographs showing the effect of linoleic acid on Aspergillus development. The pictures were taken 40 h after inoculation. In each photograph the lower right disc was the solvent control, the lower left disc contained 0.1 mg of linoleic acid, the upper left disc contained 0.5 mg of linoleic acid, and the upper right disc contained 1 mg of linoleic acid. (A) Precocious and increased conidiation in A. flavus 12S with increasing amounts of linoleic acid. (B) The 0.1-mg linoleic acid treatment induced sexual development (visualized by a halo of yellow Hülle cells) in A. nidulans FGSC4, whereas the 0.5- and 1-mg treatments induced asexual development (visualized by the production of green conidia). (C) The 0.1-mg linoleic acid treatment induced sexual development (visualized by a halo of yellow Hülle cells) in A. nidulans WIM126, whereas the 0.5- and 1-mg treatments induced asexual development (visualized by the production of brown conidia). (D) Plate shown in panel C after treatment with the laccase substrate that stains sexual primordia green.
We also tested other fatty acids (0.1 and 1 mg) that differed in saturation, chain length, and position of double bonds to determine their effects on sporulation in A. flavus 12S. Specifically, we tested eicosatrienoic acid (20:3), arachidonic acid (20:4), eicosapentaenoic acid (20:5), linolenic acid (18:3), myristic acid (14:0), ricinoleic acid (18:1), 13(S)-hydroxy-cis-9, trans-11-octadecadienoic acid, and psi factor extract. Visual observations of A. flavus spore halo diameters (conidial development) indicated that 1-mg portions of all of all the fatty acids except myristic acid increased conidial development in A. flavus 12S but had lower biological activities than linoleic acid, 13S-HPODE, and 9S-HPODE, as determined by the smaller halo diameters (data not shown).
Conservation of linoleic acid developmental effects on other Aspergillus strains.
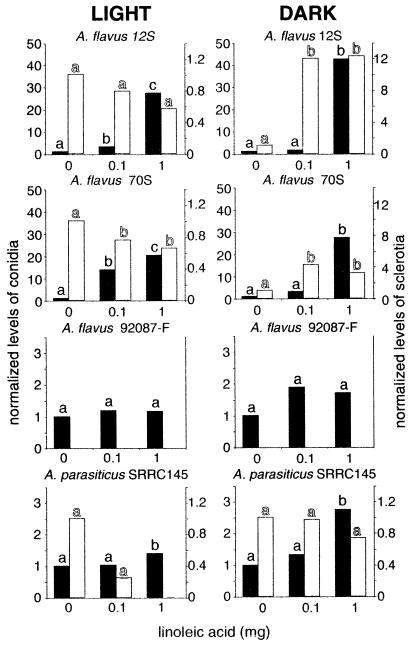
Asexual spore production was significantly (P ≤ 0.05) induced by linoleic acid in all of the A. parasiticus and A. flavus strains tested after 40 h of growth. In A. parasiticus SRRC145, there was a 1.6-fold increase with the 1-mg linoleic treatment compared to the control in the light and a 4-fold increase in the dark; in A. flavus sclerotial strain 70S, there was a 6-fold increase in the light and a 23-fold increase in the dark; and in asclerotial A. flavus strains 92087-F and 13L, there was a 2.2-fold increase in the light and there were 6.4- and 2.3-fold increases in the dark, respectively. However, after 120 h induction was significant only in the sclerotial A. flavus strains and A. parasiticus (Fig. 4). Independent of fatty acid treatments, conidial production was approximately 10-fold greater under continuous white light than in the dark (Fig. 2).
FIG. 4.
Effects of different amounts of linoleic acid (on discs) on conidial production (solid bars) and sclerotial production (open bars) in A. flavus 12S, 70S, and 92087-F and A. parasiticus SRRC145, as determined with 120-h cultures grown in continuous light or in complete darkness. A. flavus 92087-F did not produce sclerotia. Bars with the same letter are not significantly different (P ≤ 0.05). In each experiment data were normalized with respect to the control value, which was 1. For example, in the graph representing linoleic acid treatment of A. flavus 12S in the light, the control preparation contained 9 × 106 conidia and the 1-mg linoleic acid treatment preparation contained 2.5 × 108 conidia, which is 28-fold greater than the control value. The sclerotial mean control value for this treatment was 3.4 mg. The other mean values for the controls were as follows: for A. flavus 12S in the dark, 2 × 106 conidia and 0.6 mg of sclerotia; for A. flavus 70S in the light, 2 × 107 conidia and 4.1 mg of sclerotia; for A. flavus 70S in the dark, 2 × 106 conidia and 1.2 mg of sclerotia; for A. flavus 92087-F in the light, 1 × 109 conidia; for A. flavus 92087-F in the dark, 5 × 108 conidia; for A. parasiticus SRRC145 in the light, 5 × 108 conidia and 0.4 mg of sclerotia; and for A. parasiticus SRRC145 in the dark, 5 × 107 conidia and 4.1 mg of sclerotia.
Linoleic acid and light interacted to influence sclerotial production in A. flavus 12S and 70S. For example, under white light conditions, linoleic acid decreased sclerotial production in A. flavus 70S (Fig. 4). In the same A. flavus strain in the dark, linoleic acid increased sclerotial production (Fig. 4). A. parasiticus SRRC145 sclerotial production was not significantly affected by any treatment.
Linoleic acid and hydroperoxylinoleic acids act as sporogenic factors in A. nidulans veA+ strains, and veA is important for an A. nidulans developmental response.
Treatment of the wild-type veA+ strains A. nidulans WIM126 and FGSC4 with linoleic acid, 13S-HPODE, 9S-HPODE, palmitic acid, and oleic acid showed that only the polyunsaturated fatty acids affected development. Both asexual spore production and sexual spore production were affected (Fig. 3 and 5). No effects on development were observed when the saturated fatty acid palmitic acid or the monounsaturated fatty acid oleic acid was applied to the fungi (Fig. 5 and data not shown).
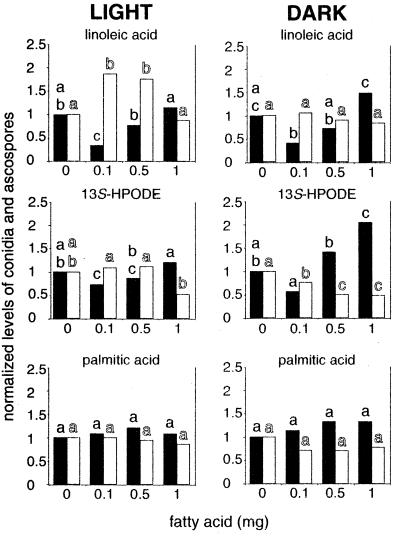
FIG. 5.
Effects of different amounts of linoleic acid, 13S-HPODE, and palmitic acid (on discs) on conidial production (solid bars) and ascospore production (open bars) in A. nidulans WIM126, as determined with 5-day cultures grown in continuous light or in complete darkness. Most of the experiments were performed in triplicate; the exception was the 13S-HPODE experiment, in which five replicates were used. 9S-HPODE effects were similar to linoleic acid effects, and oleic acid effects were similar to palmitic acid effects (data not shown). Bars with the same letter are not significantly different (P ≤ 0.05). Data were calculated as explained in the legend to Fig. 4, and in each experiment the data were normalized with respect to the control value, which was 1. The absolute values for the controls were as follows: for the linoleic acid treatment in the light, 2 × 105 conidia and 9 × 104 ascospores; for the linoleic acid treatment in the dark, 6 × 104 conidia and 3 × 105 ascospores; for the 13S-HPODE treatment in the light, 4 × 105 conidia and 2 × 104 ascospores; for the 13S-HPODE treatment in the dark, 1 × 105 conidia and 3 × 105 ascospores; for the palmitic acid treatment in the light, 2 × 105 conidia and 1 × 105 ascospores; and for the palmitic acid treatment in the dark, 5 × 104 conidia and 3 × 105 ascospores.
The fatty acid effects were light dependent in A. nidulans. Sexual development is normally delayed and reduced in wild-type veA+ strains exposed to white light (22). We found that ascospore production was twofold higher in the dark than in the light in the absence of fatty acids. We also found that low amounts of two of the fatty acids, linoleic acid and 9S-HPODE, stimulated sexual development in white light; specifically, the 0.1-mg linoleic acid treatment and the 0.01- and 0.1-mg 9S-HPODE treatments increased sexual sporulation (Fig. 5). In contrast, higher amounts of 9S-HPODE (1 to 2 mg) and 13S-HPODE (1 mg) decreased sexual sporulation (Fig. 5). The reduction in sexual development was more pronounced in the dark.
In 120-h cultures, conidial production was approximately fivefold greater in the light than in the dark. Under both dark and light regimens, low amounts of linoleic acid (0.1 mg), 13S-HPODE (0.1 mg), and 9S-HPODE (0.01 and 0.1 mg) tended to decrease conidial production. However, conidial production significantly increased in the 1-mg 13S-HPODE treatment in the dark. Increases in asexual sporulation were typically associated with decreases in sexual sporulation (Fig. 3 and 5). None of the other fatty acids tested with A. nidulans WIM126 grown under continuous illumination significantly affected conidiation.
We found that the responses of two A. nidulans veA1 strains, TTA11 and FGSC26, to treatment with linoleic acid were weak (data not shown). The only statistically significant difference was a 10-fold increase in ascospore production in the presence of 0.1 mg of linoleic acid in FGSC26 cultures grown in the dark.
DISCUSSION
We found that linoleic acid and two of its lipoxygenase derivatives stimulate morphological differentiation in A. nidulans, A. flavus, and A. parasiticus. The sporogenic effect of linoleic acid has been described previously for members of other fungal genera, including A. tomato (11), S. fructicola (12), and N. crassa (17). In N. crassa, oscillation of the mole percentage of linoleic and linolenic acids in the growing tips of surface cultures coincides with oscillation of the circadian rhythm of conidiation (19). These findings, together with our results, suggest that linoleic acid and/or its derivatives may be conserved signaling molecules that modulate fungal sporulation. Because A. nidulans psi factors also are derived from linoleic acid, our results suggest that seed fatty acids might regulate fungal development by mimicking and/or interfering with signals that regulate fungal sporogenesis.
The different effects of linoleic acid, 9S-HPODE, and 13S-HPODE on A. nidulans development may reflect the ability of each of these compounds to substitute for psi factor or to be metabolized to psi factor. Linoleic acid is the precursor of psi factor (5, 6, 15). It is not known how the presence of a hydroperoxide on the 9th and 13th carbons affects psi formation and/or psi activity. As 9S-HPODE but not 13S-HPODE stimulates sexual development in A. nidulans, it is possible that 9S-HPODE can substitute for psi factor. An examination of the Aspergillus metabolic products of 9S-HPODE and 13S-HPODE might provide insight into this issue. We also found that 13S-HPODE but not linoleic acid or 9S-HPODE had a statistically significant effect on sclerotial production in A. flavus 12S when it was grown in the light (Fig. 4 and data not shown) (dark-grown cultures were not treated with the HPODEs). We previously observed that these two metabolites have different effects on mycotoxin production in A. parasiticus and A. nidulans (4). These effects suggest that the location of the side groups on linoleic acid may determine functional specificity in the genus Aspergillus (Fig. 1). Our finding that ricinoleic acid but not oleic acid induced A. flavus sporulation also supports the hypothesis that the side groups on a fatty acid backbone play a role. The only difference between these two monounsaturated fatty acids is the presence of a hydroxyl group on the 12th carbon in ricinoleic acid.
Lipid metabolites and light may interact to influence fungal development. Morphological differentiation is light dependent in all three Aspergillus spp. In general, light-grown cultures of A. flavus, A. parasiticus, and A. nidulans strains produced 5- to 10-fold more conidia than dark-grown cultures produced, but the difference was obscured in linoleic acid treatments of asclerotial strains of A. flavus. Sclerotial formation was also light regulated in sclerotial A. flavus strains 70S and 12S, which produced more sclerotia in the light than in the dark control. The interactive effect of linoleic acid and light was demonstrated by the fact that linoleic acid induced sclerotial production in dark-grown cultures yet inhibited sclerotial production in light-grown cultures of these two strains. Other researchers have described light effects (both stimulatory and inhibitory) on sclerotial production in A. flavus, A. parasiticus, and other Aspergillus spp. (2, 18, 20), and we suggest that the composition of the medium, particularly the availability of lipid metabolites, could partially explain the different results. In A. nidulans veA+ strains, cleistothecial formation is favored in dark-grown cultures and is delayed in light-grown cultures, and the light effects on asexual sporulation are just the opposite. This phenomenon in A. nidulans, which was described in detail by Mooney and Yager (16, 22), requires an intact veA gene. Red light responsiveness is a characteristic observed in veA+ strains (22), and veA likely interacts with FluG (23), a protein required for normal sporulation (14). The light dependence of conidiation and sclerotium formation in A. flavus and A. parasiticus raises the possibility that these species contain veA homologs that function similarly.
Larroche (13) proposed that competition for nutrients and environmental factors governs growth and development in fungi. An examination of our data and the data of other workers suggests that competition for the same factors regulates the relative development of conidia and cleistothecia-sclerotia in members of the genus Aspergillus and possibly other fungal genera. With the goal of further characterizing the role that linoleic acid and its derivatives play in fungal development, current studies are directed towards identification of the genes required for formation of these polyunsaturated fatty acids in both Aspergillus spp. and seed crops. A better understanding of the molecular mechanisms that govern Aspergillus lipid metabolism could contribute to the design of control strategies to reduce the survival and spread of seed-colonizing aspergilli.
ACKNOWLEDGMENTS
This study was supported by the Texas Grain & Grass Gene Initiative, by the Texas Cotton Biotechnology Initiative, and by grant 58-6435-5-083 from the USDA Agricultural Research Service.
We thank Bruce McDonald and Jim Starr for advice concerning the statistical analysis of the data.
REFERENCES
- 1.Ahmed E M, Young C T. Composition, quality, and flavor of peanuts. In: Patte H E, Young C T, editors. Peanut science and technology. Yoakum, Tex: American Peanut Research and Education Society, Inc.; 1982. pp. 655–688. [Google Scholar]
- 2.Bennett J W, Fernholz F A, Lee L S. Effect of light on aflatoxins, anthraquinones, and sclerotia in Aspergillus flavus and A. parasiticus. Mycologia. 1978;70:104–116. [PubMed] [Google Scholar]
- 3.Bewley J D, Black M. Seeds: physiology of development and germination. New York, N.Y: Plenum Press; 1985. p. 16. [Google Scholar]
- 4.Burow G B, Nesbitt T C, Dunlap J, Keller N P. Seed lipoxygenase products modulate Aspergillus mycotoxin biosynthesis. Mol Plant- Microbe Interact. 1997;10:380–387. [Google Scholar]
- 5.Champe S P, El-Zayat A A E. Isolation of a sexual sporulation hormone from Aspergillus nidulans. J Bacteriol. 1989;171:3982–3988. doi: 10.1128/jb.171.7.3982-3988.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Champe S P, Rao P, Chang A. An endogenous inducer of sexual development in Aspergillus nidulans. J Gen Microbiol. 1987;133:1383–1388. doi: 10.1099/00221287-133-5-1383. [DOI] [PubMed] [Google Scholar]
- 7.Cove D J. Studies of mutants altered in nitrate assimilation. Mol Gen Genet. 1976;146:147–159. doi: 10.1007/BF00268083. [DOI] [PubMed] [Google Scholar]
- 8.Gardner H W. Biological roles and biochemistry of the lipoxygenase pathway. HortScience. 1995;30:197–205. [Google Scholar]
- 9.Gardner H W. Analysis of plant lipoxygenase metabolites. In: Christie W W, editor. Advances in lipid methodology—four. Dundee, Scotland: The Oily Press; 1997. pp. 1–43. [Google Scholar]
- 10.Hermann T E, Kurtz M B, Champe S P. Laccase localized in Hülle cells and cleistothecial primordia of Aspergillus nidulans. J Bacteriol. 1983;154:955–964. doi: 10.1128/jb.154.2.955-964.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyeon B. Chemical studies on the factors controlling sporulation of fungi. Chem Regul Plants. 1976;11:69–76. [Google Scholar]
- 12.Katayama M, Marumo S. R(−)-glycerol monolinoleate, a minor sporogenic substance of Sclerotinia fructicola. Agric Biol Chem. 1978;42:1431–1433. [Google Scholar]
- 13.Larroche C. Microbial growth and sporulation behaviour in solid state fermentation. J Sci Ind Res. 1996;55:408–423. [Google Scholar]
- 14.Lee B N, Adams T H. FluG and FlbA function interdependently to initiate conidiophore development in Aspergillus nidulans through BrlA-beta activation. EMBO J. 1996;15:299–309. [PMC free article] [PubMed] [Google Scholar]
- 15.Mazur P, Nakanishi K, El-Zayat A A E, Champe S P. Structure and synthesis of sporogenic psi factors from Aspergillus nidulans. J Chem Soc Chem Commun. 1991;20:1486–1487. [Google Scholar]
- 16.Mooney J L, Yager L N. Light is required for conidiation in Aspergillus nidulans. Genes Dev. 1990;4:1473–1482. doi: 10.1101/gad.4.9.1473. [DOI] [PubMed] [Google Scholar]
- 17.Nukima M, Sassa T, Ikeda M, Takahashi K. Linoleic acid enhances perithecial production in Neurospora crassa. Agric Biol Chem. 1981;45:2371–2373. [Google Scholar]
- 18.Rai J N, Tewari J P, Sinha A K. Effect of environmental conditions on sclerotia and cleistothecial production in Aspergillus. Mycopathol Mycol Appl. 1967;31:209–224. doi: 10.1007/BF02053418. [DOI] [PubMed] [Google Scholar]
- 19.Roeder P E, Sargent M L, Brody S. Circadian rhythms in Neurospora crassa: oscillations in fatty acids. Biochemistry. 1982;21:4909–4916. doi: 10.1021/bi00263a012. [DOI] [PubMed] [Google Scholar]
- 20.Rudolph E D. The effects of some physiological and environmental factors on sclerotial aspergilli. Am J Bot. 1962;49:71–78. [Google Scholar]
- 21.Watson S A. Structure and composition. In: Watson S A, editor. Corn chemistry and technology. St. Paul, Minn: American Association of Cereal Chemists; 1987. pp. 53–82. [Google Scholar]
- 22.Yager L N. Early developmental events during asexual and sexual sporulation in Aspergillus nidulans. In: Bennett J W, Klich M A, editors. Aspergillus: biology and industrial applications. Boston, Mass: Butterworth-Heinemann; 1992. pp. 19–41. [PubMed] [Google Scholar]
- 23.Yager L N, Lee H, Nagle D L, Zimmerman J E. Analysis of fluG mutations that affect light-dependent conidiation in Aspergillus nidulans. Genetics. 1998;149:1777–1786. doi: 10.1093/genetics/149.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]