Abstract
Background
Pulmonary fibrosis is a rare, but dangerous side effect of CCNU (lomustine). CCNU is a frequently used chemotherapeutic agent in the setting of recurrent or progressive glioblastoma. At present, CCNU is also administered in patients with newly diagnosed gliomas in combination with temozolomide. There is only little evidence if, and how, lung function should be monitored on treatment with CCNU.
Methods
We retrospectively collected data on patient characteristics, lung function analyses, and relevant toxicities among 166 brain tumor patients treated with CCNU at a German University Hospital and National Cancer Center.
Results
The patient collective mainly included patients with recurrent glioblastoma who received a mean number of 2.64 ± 1.57 cycles. There was overall no statistically significant change in parameters of pulmonary restriction among patients treated with CCNU. On an individual patient basis, a >10% decrease in the absolute vital capacity was primarily seen in patients with prior lung diseases and smokers. Other severe toxicities mainly included thrombocytopenia, leukopenia, nausea, and vomiting.
Conclusions
Our findings support to limit lung function analyses on CCNU to patients with gliomas and pulmonary risk factors. However, all patients should be closely followed for clinical symptoms of pulmonary restriction.
Keywords: CCNU, glioma, lung toxicity, recurrent glioblastoma
Key Points.
We analyzed serial lung function analyses in glioma patients treated with CCNU.
The risk of pulmonary function decrease is generally dispensable in these patients.
Regular lung function analysis should be restricted to pulmonary at-risk patients.
Importance of the Study.
CCNU is one of the standard therapies available for patients with recurrent glioblastoma and used with increasing frequency also for patients with newly diagnosed glioblastoma and hypermethylated MGMT promoter. While CCNU is widely used, there is uncertainty amongst therapists about the meaningfulness of repetitive lung function analyses for early detection of lung fibrosis—a rare but serious side effect of CCNU. As the first to analyze data on sequential lung function analyses in this patient collective, we aim to provide a guide in the pulmonary surveillance of patients treated with CCNU. Ultimately, we present evidence that there is a rationale for repeated pulmonary function testing only for patients at risk with prior lung diseases or other concurrent diseases. We do not see the necessity for repetitive pulmonary testing in patients that do not fulfill these criteria.
Diffuse gliomas are the most common primary brain tumors in adults.1 They can be subdivided into different CNS (Central Nervous System) WHO (World Health Organization) grades and according to the presence versus absence of an IDH (isocitrate dehydrogenase) mutation.2 IDH-mutated oligodendrogliomas (CNS WHO grades 2 and 3) and astrocytomas (CNS WHO grades 2–4) share a better prognosis as compared to IDH-wildtype gliomas including glioblastoma (CNS WHO grade 4), H3.3 G34-mutant diffuse hemispheric gliomas (CNS WHO grade 4), and H3 K27M-altered diffuse midline gliomas (CNS WHO grade 4).2
The standard of care for gliomas includes maximal safe surgical resection or biopsy followed by radio- and/or chemotherapy.3,4 There are only few chemotherapeutic agents in the treatment of gliomas, as many substances are not sufficiently able to cross the blood–brain barrier. Whereas temozolomide is mostly used in the primary setting of glioblastoma,5 CCNU (chloroethyl-cyclohexyl-nitrosourea or equally called lomustine) is frequently used in the primary therapy of lower grade oligodendrogliomas and astrocytomas,6–8 and in recurrent glioblastomas.9 Furthermore, the addition of CCNU to radiochemotherapy with temozolomide may prolong survival of patients with newly diagnosed methyl-guanine-methyl-transferase (MGMT) promoter methylated glioblastomas.10 CCNU may also be combined with procarbazine and vincristine in the so-called “PCV” regimen,6,7,11 used as monotherapy or in combination with other agents such as etoposide or teniposide.9,12 CCNU is a member of the group of nitrosoureas, which mainly act by alkylation of DNA and RNA, crosslinking of DNA and carbamoylation of amino acids.13 MGMT may revert CCNU-induced lesions such as the building of O6-chloroethylguanine.9 Common toxicities of CCNU include nausea and vomiting, myelosuppression with especially thrombocytopenia and leukopenia, increased liver enzymes or rarely secondary tumors.13 Pulmonary fibrosis is a rare, but dangerous side effect of nitrosoureas.13 The absence of relevant rates of lung fibrosis in clinical trials led to the assumption that lung function does not have to be routinely monitored in asymptomatic patients receiving CCNU.9 However, in clinical routine, there is an uncertainty if lung function should be regularly monitored or not with different local standards.
We therefore performed a cohort study based on data from a large German Brain Tumor Center to explore how lung function is affected by CCNU in the routine treatment of brain tumor patients.
Materials and Methods
Patient Cohort
The patient cohort consisted of brain tumor patients treated with CCNU at the University Hospital and National Cancer Center Heidelberg, Germany. Patients were included if they were treated with CCNU in the time between 01.03.2014 and 01.08.2020 and if they had at least 1 lung function analysis. Data on sex, age, tumor histology, IDH-mutational status, MGMT promoter methylation status, number of recurrences, CCNU regimen, dexamethasone treatment, Karnofsky Performance Status (KPS), concurrent diseases, and smoking status were retrieved from database review. The appropriate institutional review board approved the project. Informed consent was not necessary as all data were anonymized and retrospectively collected from routine patient data.
Lung Function Analyses
Lung function was measured by spirometry at the Department of ENT (Ear-Nose-Throat) at the University Hospital Heidelberg. Vital capacity (VC), forced vital capacity (FVC), and forced expiratory volume (FEV1) was measured in liters and additionally given in %-of normal before the start of CCNU treatment and before cycles 3 and 5, where available.
Assessment of Toxicities
Data on severe toxicities (grade 4) according to the Common Terminology Criteria for Adverse Events (CTCAE)14 were derived from database review.
Statistical Analyses
GraphPad Prism version 9.3.1 was used for all statistical analyses. Since the same cohort of patients were tested repetitively, we assumed dependent data and consequently applied paired calculations. Here, all the values that were only tested at 1 time point, but not at the other, are eliminated, that is, the sample size is reduced accordingly. Statistical significance was assessed by the 2-sided Student’s t-test for normally distributed data. Otherwise a Wilcoxon matched-pairs signed rank test was used for nonnormal distributions which was the case for the majority of data. All available data were depicted as column bar graphs with mean + SD. The level of significance was set at P < .05.
Results
Our cohort consisted of 166 patients treated with CCNU at the University Hospital Heidelberg. Most patients in our cohort were treated for glioblastoma (70.48%) and received CCNU in combination with etoposide (96.34%). Mean age of patients at the time of their lung function analysis was 54.56 ± 12.90 years. A KPS above 70% was present in 69.28% of patients and 64.46% of patients were men. Most patients (90.96%) were nonsmoker and 63.86% of patients took steroids. The number of recurrences was as follows: 36.75% (first recurrence), 27.10% (second recurrence), 22.29% (third recurrence), 9.64% (fourth recurrence), and 4.22% (fifth recurrence). The MGMT promoter was hypermethylated in 43.37% of patients, not hypermethylated in 31.33% and unknown in 25.30%. IDH showed a wildtype status in 54.22% of patients, 33.73% of patients were IDH-mutant and 12.05% had an unknown IDH-mutational status (Table 1). The mean number of CCNU cycles was 2.64 ± 1.57.
Table 1.
Patient characteristics
| Variable | Characteristic | Frequency (N = 166) | Percentage |
|---|---|---|---|
| Sex | Male | 107 | 64.46 |
| Female | 59 | 35.54 | |
| Age group | ≤55 | 88 | 53.01 |
| >55 | 78 | 46.99 | |
| Histology | Glioblastoma | 117 | 70.48 |
| Oligodendroglioma, IDH-mutant and 1p19q codeleted, CNS WHO grade 2 | 5 | 3.01 | |
| Oligodendroglioma, IDH-mutant and 1p19q codeleted, CNS WHO grade 3 | 2 | 1.20 | |
| Astrocytoma, IDH-mutant, CNS WHO grade 2 | 6 | 3.61 | |
| Astrocytoma, IDH-mutant, CNS WHO grade 3 | 15 | 9.04 | |
| Others | 21 | 12.65 | |
| IDH mutation | IDH-mutant | 56 | 33.73 |
| IDH-wildtype | 90 | 54.22 | |
| Not determinable | 20 | 12.05 | |
| MGMT promoter methylation | Methylated | 72 | 43.37 |
| Nonmethylated | 52 | 31.33 | |
| Unknown | 42 | 25.30 | |
| Number of recurrences | 1 | 61 | 36.75 |
| 2 | 45 | 27.10 | |
| 3 | 37 | 22.29 | |
| 4 | 16 | 9.64 | |
| 5 | 7 | 4.22 | |
| CCNU regimen | CCNU monotherapy | 1 | 0.60 |
| CCNU + etoposide | 160 | 96.34 | |
| CCNU + vincristine | 1 | 0.60 | |
| Unknown | 4 | 2.41 | |
| Dexamethasone treatment | Yes | 106 | 63.86 |
| No | 59 | 35.54 | |
| N/A | 1 | 0.60 | |
| KPS | ≤70 | 52 | 31.33 |
| >70 | 112 | 69.28 | |
| Unknown | 2 | 1.20 | |
| Concurrent disease | None | 49 | 29.52 |
| Epilepsy | 72 | 43.37 | |
| Atrial fibrillation | 6 | 3.61 | |
| Arterial hyertension | 39 | 23.49 | |
| DVT/pulmonary embolisma | 8 | 4.82 | |
| Asthma | 7 | 4.22 | |
| Othersb | 27 | 16.27 | |
| Smoking status | Smoker | 15 | 9.04 |
| Nonsmoker | 151 | 90.96 |
KPS, Karnofsky Performance Status.
aOne patient suffered both from DVT (deep vein thrombosis) and pulmonary embolism.
bIncluding 1 patient with pulmonary fibrosis.
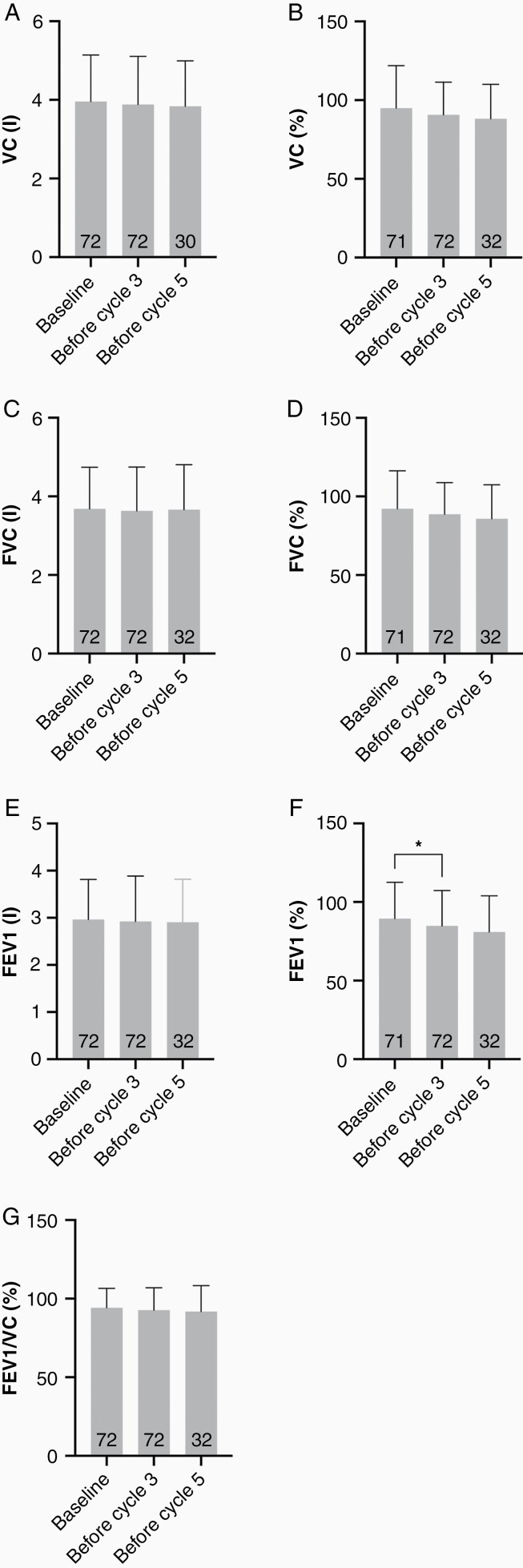
Seventy-two patients received more than 1 pulmonary function test. There was no significant change of the mean VC in liters or %-of normal at baseline, after 2 cycles and after 4 cycles of CCNU. Correspondingly, the mean FVC in liters or %-of normal remained stable at the 3 respective time points. Whereas mean FEV1 in liters was not materially changed from baseline after 2 cycles and after 4 cycles of CCNU, mean FEV1 in %-of normal decreased slightly with 89.44 ± 23.08 at baseline, 84.85 ± 22.50 after 2 cycles and 81.00 ± 22.88 after 4 cycles. Mean FEV1/VC (%) was comparable at the 3 time points. The only analysis that reached statistical significance was the comparison between FEV1% at baseline and after 2 cycles of CCNU (Figure 1 and Table 2).
Figure 1.
Lung function analysis. Vital capacity (VC) was assessed in liters (a) and %-of normal (b) at baseline, before cycle 3, and before cycle 5. Forced vital capacity (FVC) in liters (c) and %-of normal (d), forced expiratory volume (FEV1) in liters (e) and %-of normal (f), and forced expiratory volume/vital capacity in %-of normal (g) were also analyzed at baseline, before cycle 3, and before cycle 5.
Table 2.
Mean results of lung function analyses
| Parameter | Mean value ± SD at baseline | Mean value ± SD before cycle 3 | Mean value ± SD before cycle 5 |
|---|---|---|---|
| VC (l) | 3.96 ± 1.19 | 3.88 ± 1.22 | 3.84 ± 1.15 |
| VC (%) | 94.98 ± 27 | 90.68 ± 20.75 | 88.18 ± 21.85 |
| FVC (l) | 3.68 ± 1.06 | 3.63 ± 1.11 | 3.66 ± 1.14 |
| FVC (%) | 92.15 ± 24.19 | 88.64 ± 20.2 | 85.82 ± 21.66 |
| FEV1 (l) | 2.96 ± 0.85 | 2.92 ± 0.96 | 2.90 ± 0.96 |
| FEV1 (%) | 89.44 ± 23.08 | 84.85 ± 22.5 | 81.00 ± 22.88 |
| FEV1/VC (%) | 94.20 ± 12.38 | 92.64 ± 14.26 | 91.82 ± 16.54 |
FEV1, forced expiratory volume; FVC, forced vital capacity; VC, vital capacity.
We further looked at lung function analysis of glioma patients on an individual basis and studied 9 patients with a >10% decrease between the baseline and sequential absolute VC. About one-third (33.33%) of those patients were smokers and 55.55% of those patients had prior or concurrent lung diseases (pulmonary embolism, lung fibrosis, pneumonia). About one-third of patients (33.33%) with a >10% decrease in lung function had no lung diseases, but other concurrent diseases including arterial hypertension, breast cancer, and seizures. There was 1 patient (11.11%) with a significant decrease in lung function, but without other concurrent diseases, except for nicotine abuse.
Among the full cohort of 166 patients, 86.75% of patients had no toxicity greater than CTCAE grade 3. Grade 4 thrombocytopenia was present in 0.6% of patients, 2.41% of patients had grade 4 leukopenia, 0.6% had severe nausea and vomiting, or other severe toxicities, respectively (Table 3).
Table 3.
Toxicities other than lung toxicity >CTCAE grade 3
| Toxicity >CTCAE grade 3 | Frequency (N = 166) | Percentage |
|---|---|---|
| Anemia | 0 | 0.00 |
| Leukopenia | 4 | 2.41 |
| Thrombopenia | 1 | 0.60 |
| Pancytopenia | 0 | 0.00 |
| Nausea/vomiting | 1 | 0.60 |
| Other toxicity >CTCAE grade 3 | 1 | 0.60 |
| No toxicity >CTCAE grade 3 | 144 | 86.75 |
| Unknown | 15 | 9.04 |
CTCAE, Common Terminology Criteria for Adverse Events.
Discussion
In this cohort of 166 patients with recurrent and progressive glioma receiving CCNU, there was overall no increase in parameters of pulmonary restriction. Patients with >10% decrease in baseline VC had either concurrent lung diseases including nicotine abuse or other concurrent diseases, which may reduce the general physical fitness and resilience against toxic side effects rather than exerting direct effects on lung function. Other severe toxicities mainly included thrombocytopenia, leucopenia, and nausea and vomiting.
The first reports on severe pulmonary toxicity in patients treated with nitrosoureas date back to the 1970s.15–18 Most of those studies reported on interstitial pulmonary fibrosis in patients with repeated administrations of carmustine (BCNU = 1,3-bis-(2chloroethy1)-1-nitrosourea) at high-cumulative doses (1.200–1.500 mg/m² body surface), but there were also reports on patients that developed irreversible lung fibrosis after a single administration of BCNU.19 BCNU treatment led to diffuse alveolar damage and organizing interstitial pneumonia and fibrosis as seen for other agents like bleomycin.16,20 Lung toxicity (pulmonary infiltrates and/or fibrosis) has been reported to occur in up to 30% of patients in the official drug information of BCNU.21 Despite those observations, BCNU has been used in the routine treatment of glioma and pulmonary fibrosis was seen as rarely as in 0.6% of chemotherapy-naive patients in a retrospective cohort study.22 In studies based on patients with extracranial tumors, use of nitrosoureas significantly increased the risk of pulmonary fibrosis and risk estimates were especially high, when patients also received chest irradiation.23,24 If affected, lung fibrosis is a life-threatening condition22,25 and may occur up to 17 years after BCNU administration.26
Whereas the majority of studies on lung toxicity are based on BCNU, several studies also reported on lung fibrosis after administration of nimustine (ACNU),25 fotemustin,27 CCNU,28 and novel nitrosoureas such as SarCNU.29
The frequency of lung toxicity after use of CCNU ranges between 1/1.000 and 1/10.000 patients.30 In clinical trial populations using CCNU as standard of care in recurrent glioblastoma, lung fibrosis has not been a toxicity of concern.31–33 Grade 3 and 4 respiratory toxicities according to the CTCAE14 in patients treated with CCNU were not reported in the BELOB trial.32 In the REGAL trial31 and INTELLANCE-2 trial,33 grade 3 and 4 lung toxicity in patients taking CCNU were limited to pulmonary embolism. In the primary therapy of glioblastoma, 3% of patients treated with the combination of CCNU and temozolomide developed grade 3 and 4 lung infections in the CeTeG trial, whereas 2% of patients in the temozolomide arm developed grade 3 and 4 lung infections. There were no reports on pulmonary fibrosis in the CCNU and temozolomide arm.10 Several studies explored PCV in the primary therapy of lower grade glioma.6,7,11 In the study conducted by Cairncross et al., PCV therapy led to grade 3 and 4 lung toxicity in 4% of patients during chemotherapy and 1% of patients after chemotherapy as compared to 0% of patients treated with radiotherapy only.6,34 Lung toxicity was not specified for lung fibrosis. In the study led by Van Den Bent et al.,35 and Buckner et al.,8 no grade 3 and 4 lung toxicity was reported.
Reports on toxicity of PCV or CCNU in the routine clinical setting are sparse,36,37 but important, as patients treated within clinical trials may be fitter and therefore may experience less side effects. Interestingly, although some groups evaluated PCV toxicity in routine clinical settings, lung toxicity was not even reported.36,37 Our data showed that there was overall no decrease in parameters of pulmonary restriction such as the absolute or relative VC or FVC. Decreases in the VC were seen in patients with prior lung diseases, nicotine abuse and other concurrent diseases. The small, but statistically significant decrease in FEV1 is a marker of pulmonary obstruction, but not restriction and therefore not associated with pulmonary fibrosis. Regular assessment of lung function by spirometry increases the duration and expenses of practice visits, and alternatives to CCNU in the recurrent setting of glioblastoma are rare. Therefore, regular monitoring of lung function by spirometry may not be justified in patients with recurrent glioblastoma. However, clinicians should be aware of patients at increased risk of pulmonary restriction and routinely ask and clinically examine for pulmonary symptoms in patients treated with CCNU.
Other severe toxicities (CTCAE grade 4) reported in our cohort are in line with published studies mainly including thrombocytopenia, leucopenia, and emesis.9
Our study has several limitations. Data were collected retrospectively and numbers of patients with several lung function analyses were limited. Most patients in the analysis were diagnosed with recurrent glioblastoma with limited patient prognosis, therefore long-term pulmonary toxicities may have been lost and results cannot be translated to younger people with lower grade tumors. Most patients were treated with CCNU and etoposide and results may not be translated to patients treated with PCV. The combination of nitrosoureas and etoposide/teniposide may be used in the treatment of recurrent glioblastomas (adapted from the German Neuro-Oncology Working Group 01 trial12), but standard of care at tumor recurrence is poorly defined9 and different CCNU regimens are used according to local treatment standards. The mean number of administered cycles of CCNU in the present data set was low when compared to other studies.8 Therefore, the cumulative dose of CCNU may not have been high enough to induce lung toxicity. We did not perform sequential chest X-ray examinations or blood gas analyses, which are part of the CTCAE criteria for grade 1–3 lung fibrosis.
In conclusion, our findings support to limit lung function analyses under CCNU to patients at risk with either prior lung diseases or other concurrent diseases including nicotine abuse or symptoms of worsening lung function.
Acknowledgments
We cordially thank Jenny Frech for excellent support regarding database access and acquisition of data.
Contributor Information
Corinna Seliger, Department of Neurology and National Center for Tumor Diseases, University Hospital Heidelberg, Heidelberg, Germany.
Christina Nürnberg, Department of Neurology and National Center for Tumor Diseases, University Hospital Heidelberg, Heidelberg, Germany; Clinical Cooperation Unit Neurooncology, German Cancer Consortium (DKTK), German Cancer Research Center (DKFZ), Heidelberg, Germany.
Wolfgang Wick, Department of Neurology and National Center for Tumor Diseases, University Hospital Heidelberg, Heidelberg, Germany; Clinical Cooperation Unit Neurooncology, German Cancer Consortium (DKTK), German Cancer Research Center (DKFZ), Heidelberg, Germany.
Antje Wick, Department of Neurology and National Center for Tumor Diseases, University Hospital Heidelberg, Heidelberg, Germany.
Funding
Fellowship of the German Cancer Research Center Clinician Scientist Program, supported by the Dieter Morszeck Foundation to C.N. We received funding for the current project from the German Research Foundation (SFB 1389-A03 to W.W.).
Conflict of interest statement. None declared.
Authorship Statement.
C.S., W.W., and A.W. conceptualized the study. C.S. retrieved the data. C.N. statistically analyzed the data. C.S. and A.W. interpreted the data. C.S. wrote the manuscript, all authors revised it.
References
- 1. Ostrom QT, Patil N, Cioffi G, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro Oncol. 2020;22(12 suppl 2):iv1–iv96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weller M, Le Rhun E, Preusser M, Tonn JC, Roth P. How we treat glioblastoma. ESMO Open. 2019;4(suppl 2):e000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 6. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 8. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weller M, Le Rhun E. How did lomustine become standard of care in recurrent glioblastoma? Cancer Treat Rev. 2020;87:102029. [DOI] [PubMed] [Google Scholar]
- 10. Herrlinger U, Tzaridis T, Mack F, et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet. 2019;393(10172):678–688. [DOI] [PubMed] [Google Scholar]
- 11. Buckner JC, Gesme D Jr, O’Fallon JR, et al. Phase II trial of procarbazine, lomustine, and vincristine as initial therapy for patients with low-grade oligodendroglioma or oligoastrocytoma: efficacy and associations with chromosomal abnormalities. J Clin Oncol. 2003;21(2):251–255. [DOI] [PubMed] [Google Scholar]
- 12. Weller M, Muller B, Koch R, Bamberg M, Krauseneck P; Neuro-Oncology Working Group of the German Cancer Societyociety. Neuro-Oncology Working Group 01 trial of nimustine plus teniposide versus nimustine plus cytarabine chemotherapy in addition to involved-field radiotherapy in the first-line treatment of malignant glioma. J Clin Oncol. 2003;21(17):3276–3284. [DOI] [PubMed] [Google Scholar]
- 13. Drug Information for Lomustine: http://www.bccancer.bc.ca/drug-database-site/DrugIndex/Lomustine_monograph.pdf. Accessed December 20, 2021.
- 14.CTCAE-criteria: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. Accessed December 20, 2021.
- 15. Weiss RB, Shah S, Shane SR. Pulmonary toxicity from carmustine (BCNU): a case report. Med Pediatr Oncol. 1979;6(3):255–259. [DOI] [PubMed] [Google Scholar]
- 16. Holoye PY, Jenkins DE, Greenberg SD. Pulmonary toxicity in long-term administration of BCNU. Cancer Treat Rep. 1976;60(11):1691–1694. [PubMed] [Google Scholar]
- 17. Crittenden D, Tranum BL, Haut A. Pulmonary fibrosis after prolonged therapy with 1,3-bis (2-chloroethyl)-1-nitrosourea. Chest. 1977;72(3):372–373. [DOI] [PubMed] [Google Scholar]
- 18. Bailey CC, Marsden HB, Jones PH. Fatal pulmonary fibrosis following 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) therapy. Cancer. 1978;42(1):74–76. [DOI] [PubMed] [Google Scholar]
- 19. Lieberman A, Ruoff M, Estey E, et al. Irreversible pulmonary toxicity after single course of BCNU. Am J Med Sci. 1980;279(1):53–56. [DOI] [PubMed] [Google Scholar]
- 20. Sostman HD, Matthay RA, Putman CE. Cytotoxic drug-induced lung disease. Am J Med. 1977;62(4):608–615. [DOI] [PubMed] [Google Scholar]
- 21.Product Information for Carmustine: https://www.ema.europa.eu/en/documents/product-information/carmustine-obvius-epar-product-information_en.pdf. Accessed December 20, 2021.
- 22. Jungk C, Chatziaslanidou D, Ahmadi R, et al. Chemotherapy with BCNU in recurrent glioma: analysis of clinical outcome and side effects in chemotherapy-naive patients. BMC Cancer. 2016;16(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer. A report from the Childhood Cancer Survivor Study. Cancer. 2002;95(11):2431–2441. [DOI] [PubMed] [Google Scholar]
- 24. Jacoulet P, Depierre A, Moro D, et al. Long-term survivors of small-cell lung cancer (SCLC): a French multicenter study. Groupe d’Oncologie de Langue Francaise. Ann Oncol. 1997;8(10):1009–1014. [DOI] [PubMed] [Google Scholar]
- 25. Matsumoto M, Yamashita J, Okamoto S, et al. [High dose ACNU and radiation therapy with autologous bone marrow rescue for a patient with cerebellar medulloblastoma: case report]. No Shinkei Geka. 1984;12(6):731–736. [PubMed] [Google Scholar]
- 26. O’Driscoll BR, Hasleton PS, Taylor PM, et al. Active lung fibrosis up to 17 years after chemotherapy with carmustine (BCNU) in childhood. N Engl J Med. 1990;323(6):378–382. [DOI] [PubMed] [Google Scholar]
- 27. Bertrand M, Wemeau-Stervinou L, Gauthier S, Auffret M, Mortier L. [New toxicity of fotemustine: diffuse interstitial lung disease]. Ann Dermatol Venereol. 2012;139(4):277–281. [DOI] [PubMed] [Google Scholar]
- 28. Tucci E, Verdiani P, Di Carlo S, Sforza V. Lomustine (CCNU)-induced pulmonary fibrosis. Tumori. 1986;72(1):95–98. [DOI] [PubMed] [Google Scholar]
- 29. Webster M, Cairncross G, Gertler S, et al. Phase II trial of SarCNU in malignant glioma: unexpected pulmonary toxicity with a novel nitrosourea: a phase II trial of the National Cancer Institute of Canada Clinical Trials Group. Invest New Drugs. 2005;23(6):591–596. [DOI] [PubMed] [Google Scholar]
- 30.Product Information for Lomustine: https://www.medicines.org.uk/emc/medicine/6252-gref. Accessed December 20, 2021.
- 31. Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 33. Van Den Bent M, Eoli M, Sepulveda JM, et al. INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFR amplified glioblastoma. Neuro Oncol. 2020;22(5):684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Intergroup Radiation Therapy Oncology Group Trial 9402, Cairncross G, Berkey B, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24(18):2707–2714. [DOI] [PubMed] [Google Scholar]
- 35. van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24(18):2715–2722. [DOI] [PubMed] [Google Scholar]
- 36. Keogh RJ, Aslam R, Hennessy MA, et al. One year of procarbazine lomustine and vincristine is poorly tolerated in low grade glioma: a real world experience in a National Neuro-oncology Centre. BMC Cancer. 2021;21(1):140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Irfan N, Samuel E, Rafi Ranjha F, et al. Toxicity profile of procarbazine lomustine and vincristine chemotherapy in low-grade glioma—retrospective review. Cureus. 2020;12(10):e11070. [DOI] [PMC free article] [PubMed] [Google Scholar]