ABSTRACT
Background
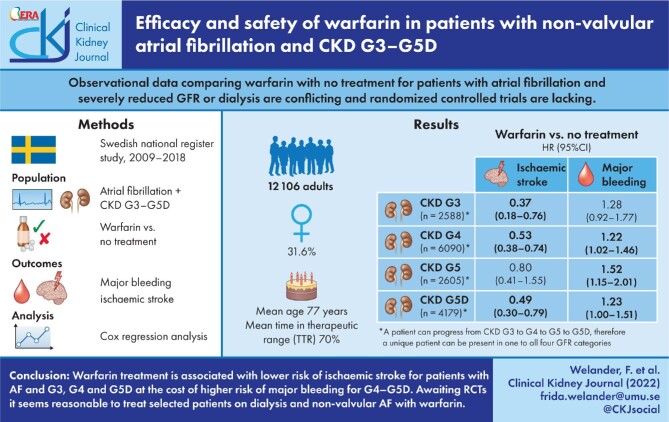
Observational data comparing warfarin with no treatment for patients with non-valvular atrial fibrillation (NVAF) and severely reduced glomerular filtration rate (GFR) are conflicting and randomized controlled trials (RCTs) are lacking. Most studies do not provide information on warfarin treatment quality, making them difficult to compare.
Methods
This national cohort study investigates the risk of ischaemic stroke and major bleeding during warfarin treatment compared with no oral anticoagulants in patients with NVAF, GFR category 3–5 (G3–G5) or on dialysis (G5D), with kidney transplant recipients excluded, between 2009 and 2018. Data extracted from high-quality Swedish national healthcare registries, including the Swedish Renal Registry, AuriculA—the Swedish national quality registry for atrial fibrillation and anticoagulation—and the Stroke Registry.
Results
At enrolment of 12 106 patients, 21.4% were G3, 43.5% were G4, 11.6% were G5 and 23.6% were G5D. The mean time in the therapeutic range was 70%. Warfarin compared with no treatment showed a lower risk for ischaemic stroke for G3 {hazard ratio [HR] 0.37 [95% confidence interval (CI) 0.18–0.76]}, G4 [0.53 (0.38–0.74)] and G5D [0.49 (0.30–0.79)] and an increased risk of major bleeding in G4 [HR 1.22 (1.02–1.46)], G5 [1.52 (1.15–2.01)] and G5D [1.23 (1.00–1.51)]. All-cause mortality was more than halved on warfarin compared with no treatment in all GFR categories.
Conclusions
Warfarin treatment is associated with a lower risk of ischaemic stroke for patients with NVAF and G3, G4 and G5D at the cost of a higher risk of major bleeding for G4–G5D. Existing observational data are conflicting, stressing the need for RCTs on warfarin compared with no treatment in G4–G5D. Awaiting RCTs, it seems reasonable to treat selected patients on dialysis and NVAF with warfarin.
Keywords: anticoagulants, atrial fibrillation, chronic kidney disease, dialysis, ischaemic stroke, major bleeding, warfarin
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Chronic kidney disease (CKD) affects 10% of the adult population and is a risk factor for atrial fibrillation (AF) [1]. Moreover, AF itself can increase the risk for CKD, meaning the association is bidirectional [2]. Approximately 20% of patients with CKD ≥G3b [glomerular filtration rate (GFR) <45mL/min/1.73m2] and up to 27% of patients on dialysis have AF [3, 4]. AF is a risk factor for thromboembolic stroke, even more so in patients with concomitant CKD [3, 5, 6].
The use of prophylactic oral anticoagulants for patients with a high risk of thromboembolic events, according to the CHA2DS2-VASc [congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke or transient ischaemic attack (TIA), vascular disease, age 65–74 years, sex category] score, is not controversial for CKD with a glomerular filtration rate (GFR) category 1–3 [G1–G3b; estimated GFR (eGFR) ≥30 mL/min/1.73 m2] [1, 7]. For these patients, warfarin and direct oral anticoagulants (DOACs) are effective. Existing data on DOACs for patients with G4–G5 (eGFR <30 mL/min/1.73 m2) are limited since these patients were excluded from the pivotal DOAC studies [8–11]. Traditionally, warfarin has been used as stroke prophylaxis in patients with AF and G4–G5, but randomized controlled trials (RCTs) are lacking. Observational studies suggest protection against ischaemic stroke with warfarin compared with no treatment, but also an increased risk of haemorrhage [5, 12, 13]. Also, data imply that the increased risk of bleeding on warfarin is reduced with optimal time in the therapeutic range (TTR) >75%, independent of GFR [14].
Data on warfarin compared with no treatment for patients on dialysis (G5D) are conflicting. No RCTs have been completed so far [15]. The spectrum of observational data on warfarin and G5D covers both the mortality benefit as well as the increased risk of ischaemic stroke with warfarin [5, 12, 13, 16, 17]. Two meta-analyses suggest no prophylactic effect regarding stroke risk and more bleeding for warfarin-treated patients on dialysis [18, 19]. In contrast one meta-analysis found no evidence of either harm or benefit of warfarin in patients on dialysis [20].
International guidelines on the use of oral anticoagulants in G5D are inconsistent. American cardiology guidelines suggest warfarin or apixaban might be reasonable for patients with AF on dialysis [21]. European Society of Cardiology Guidelines do not have a recommendation [22]. The International Society of Nephrology Kidney Disease: Improving Global Outcomes (KDIGO) guidelines do not recommend routine anticoagulation for dialysis patients with AF [1].
The aim of the present study is to investigate the risk of ischaemic stroke and major bleeding with warfarin compared with no treatment in patients with CKD G3–G5D using Swedish national healthcare registries. The hypothesis is that warfarin offers stroke protection in all GFR categories at the cost of more bleeding in G5D.
MATERIALS AND METHODS
We conducted a Swedish register–based cohort study that adheres to the Declaration of Helsinki and was approved by the Swedish Ethical Review Authority (registration 2019-03289). Personal consent was not obtained due to the register-based design.
Data sources
All Swedish inhabitants are provided with a personal registration number, making it possible to link personal data from different registers [23]. We included patients from the Swedish Renal Registry (SRR), a quality register for patients with CKD and kidney replacement therapy (KRT, i.e. patients on dialysis and kidney transplant recipients). Patients from 98% of all Swedish nephrology clinics with G3b (and from some regions with G3a) through G5D are included and their care is monitored. Approximately 80% of all CKD patients are registered in the SRR before the start of KRT and >90% of all Swedish dialysis patients are covered. eGFR is reported at least yearly [24]. The Swedish National Patient Register (NPR) is managed by the National Board of Health and Welfare, with almost 100% coverage of all somatic and psychiatric hospital admissions with information of admission and discharge dates, surgical procedures and International Classification of Diseases, Tenth Revision (ICD-10) codes. The NPR has general high validity. The positive predictive value for AF in the NPR is 97% [25]. AuriculA is the Swedish national quality registry for AF and oral anticoagulation. It was active between 2006 and 2018, including ∼50% of all Swedish warfarin users. AuriculA included whole regions with no obvious selection bias, containing data on treatment periods, dosages and international normalized ratios (INRs). The Swedish Prescribed Drug Register (PDR) has complete coverage of all dispensations at Swedish pharmacies since 2006 according to the Anatomical Therapeutic Chemical (ATC) classification. The stroke register is the national quality register for stroke care, with coverage of 94% of all acute strokes in Sweden [26]. This register has a higher validity of stroke diagnosis than the NPR since it registers the index stroke diagnosis only once and collects the correct stroke date, compared with the NPR, where the ICD-10 code for stroke is often registered a posteriori and at rehabilitation visits. The Cause of Death Register (CDR) includes all death dates of the deceased in Sweden.
Inclusion
Adult patients at Swedish nephrology clinics with CKD G3–G5D between 2009 and 2018 were obtained from the SRR and linked with the NPR for AF/flutter. Patients with valvular AF due to mitral stenosis or mechanical heart valve replacement (ICD-10 codes Z952, I050, I342, Q232) and kidney transplant recipients in the SRR or Z940 (ICD-10) and V42A (ICD-9) in the NPR were excluded. Thus a cohort of patients with G3–G5D who were not kidney transplanted and with non-valvular AF (NVAF) was obtained. Patients who underwent kidney transplantation or were diagnosed with valvular AF during follow-up were censored. Baseline characteristics and comorbidity data were obtained from the NPR, AuriculA and the SRR. The underlying kidney disease diagnosis from the SRR was only available for patients on dialysis. A full list of included variables and their sources is found in Supplementary data, Table S1. All patients were scored according to the CHA2DS2-VASc for stroke risk assessment [27].
Kidney function status
The GFR category was obtained from the SRR and categorized as G3–G5D according to the KDIGO guidelines (G3a: eGFR 45–59 mL/min/1.73 m2; G3b: 30–44; G4: 15–29; G: <15; G5D: on dialysis) [28]. G3a and G3b were merged. GFR could decrease during follow-up; i.e. G3 could switch to G4 and G5 and G5D but the patient remained in the cohort. Increasing GFR during the study was disregarded. The date of dialysis start was collected from the SRR. Baseline data were presented for all GFR categories separately at the date when each patient entered a new GFR category. Since a patient can progress from G3 to G4 to G5 to G5D, this means that patients in the GFR categories are not unique.
Treatment
Warfarin treatment periods were extracted from AuriculA. A period with no oral anticoagulants (no treatment) was defined as no treatment in AuriculA and no dispensations of either warfarin or DOAC in the PDR. If there was a dispensation of anticoagulants (warfarin or DOAC) in the PDR not matching a warfarin treatment period in AuriculA, an undefined treatment period started. An undefined period of warfarin or DOAC lasted from the last dispensation, the number of days the warfarin or DOAC dispensation covered (1 tablet per day for warfarin) and 26 weeks after the dispensation was finished. For patients included in multidrug dispensing, a period of undefined treatment with DOAC was defined as the number of days covered by the dispensation plus 1 week. A patient could be on warfarin, untreated and have undefined treatment periods during the follow-up time. Warfarin treatment quality was calculated as the TTR according to Rosendaal et al. [29].
Outcomes
Outcomes were analysed in relation to current treatment and GFR category. The stroke register was used to collect all stroke-related outcomes. Other outcomes were obtained from the NPR, except the date of death, which was collected from the CDR. Primary outcomes were ischaemic stroke or major bleeding requiring inpatient care. Secondary outcomes were all-cause stroke and systemic embolism; all-cause stroke; haemorrhagic stroke; gastrointestinal (GI), intracranial (IC) or other bleeding requiring inpatient care; myocardial infarction and all-cause mortality. Only the first type of each outcome was counted. After the occurrence of an outcome, a patient was censored for this event but remained in the cohort for other events. A full list of outcomes and their source is found in Supplementary data, Table S1.
Statistical analysis
Data were processed using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Outcomes for the separate treatment groups were presented as rates with 95% confidence intervals (CIs) as well as compared between warfarin and the no-treatment group using Cox regression analysis with time-dependent covariates presented with hazard ratios (HRs) and 95% CIs. Covariates adjusted for were sex, age, years from study start and any of the following prior events: congestive heart failure, diabetes mellitus, hypertension, stroke or transient ischaemic attack (TIA), vascular disease, major bleeding, myocardial infarction, percutaneous coronary intervention and excessive alcohol use. A sensitivity analysis was performed using Cox regression comparing warfarin and no treatment merging G3–G5 and adjusting for blood pressure, serum/plasma (S/P) albumin and β-haemoglobin in addition to mentioned covariates and for G5D adjusting for dialysis modality. All quantitative variables were used in models as restricted cubic splines.
RESULTS
Steps of inclusion
The registry outtake of patients in the SRR with AF consisted of 15 218 individuals. Patients were candidates for inclusion if their first date of GFR category (or first dialysis date) and AF diagnosis were registered before the end of the study period (31 December 2018). There were 14 097 such candidates; 225 individuals did not have ≥G3 or dialysis (in register for other reasons), 895 were registered at dates after the end of the study period and 1 patient was excluded due to a missing date of AF. The individual start date (t0) was defined as the first date when a G3–G5D and AF diagnosis coexisted and the study period had started (1 January 2009). Individuals were excluded if they received a kidney transplant (n = 1486), were diagnosed with valvular AF (n = 494) or were deceased (n = 11) prior to or on t0. The individual stop date was when an exclusion criterion occurred or at the end of the study period. This left a study population of 12 106 patients (Figure 1).
FIGURE 1:
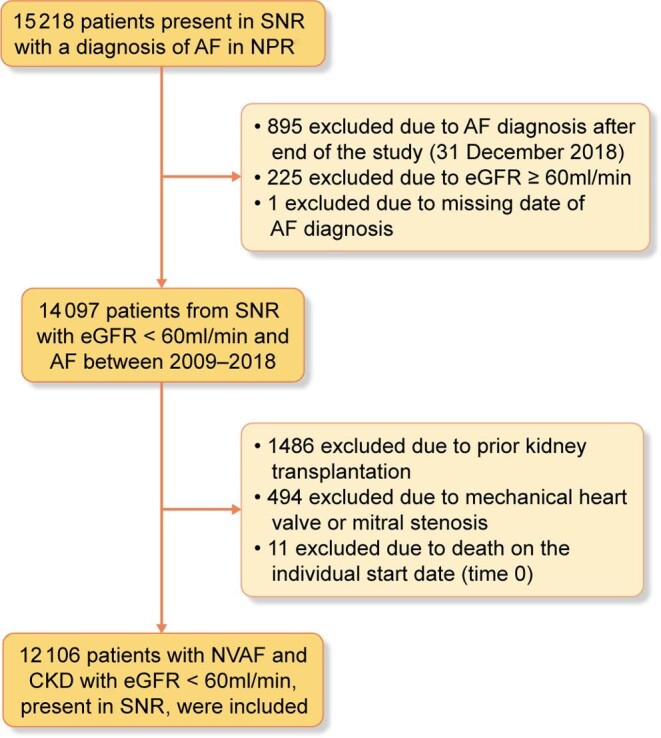
Patient flow chart.
Cohort description
A total of 12 106 patients with non-valvular AF and G3–G5D were included (Table 1). The median age at inclusion was 77.3 years and 31.6% were female. At inclusion, 21.4% were G3, 43.5% were G4, 11.6% were G5 and 23.6% were G5D. GFR could decrease during the study; a total of 6090 patients entered G4 and 2605 entered G5 (Table 1). A total of 4179 patients started dialysis (G5D): 2971 (71.1%) on haemodialysis and 1208 (28.9%) on peritoneal dialysis. Of the patients starting dialysis, 27.1% were diagnosed with hypertensive/renovascular disease, 22.7% diabetic nephropathy, 14.2% glomerulonephritis, 7.0% tubulointerstitial disease, 5.4% systemic diseases affecting the kidney, 4.9% polycystic kidney disease (adult type) and 17.6% other underlying diseases. The mean TTR for all patients was 70%, with 75% for G3, 72% for G4, 68% for G5 and 62% for G5D. A table with additional baseline parameters including blood pressure, body mass index (BMI) and laboratory workup is available (Supplementary data, Table S2). A description of baseline characteristics for patients on warfarin and no treatment when entering each GFR category is found in Supplementary data, Table S3. Of note is that women are less often treated with warfarin.
Table 1.
Baseline characteristics for 12 106 patients when entering a new GFR categorya
| Characteristics | CKD G3(n = 2588) | CKD G4(n = 6090) | CKD G5(n = 2605) | CKD G5D(n = 4179) |
|---|---|---|---|---|
| Demographics | ||||
| Age (years), median (Q1–Q3) | 76.7 (71.4–82.0) | 78.6 (72.7–83.5) | 78.4 (71.7–83.8) | 75 (68.2–80.5) |
| Female, n (%) | 583 (22.4) | 1990 (32.7) | 874 (33.6) | 1230 (29.4) |
| Medical history, n (%) | ||||
| Diabetes mellitus | 1264 (48.8) | 2840 (46.6) | 1202 (46.1) | 2009 (48.1) |
| Hypertension | 2346 (90.6) | 5517 (90.6) | 2416 (92.7) | 3782 (90.5) |
| Stroke | 485 (18.7) | 1304 (21.4) | 600 (23.0) | 839 (20.1) |
| TIA | 250 (9.7) | 593 (9.7) | 241 (9.3) | 341 (8.2) |
| COPD | 403 (15.6) | 865 (14.2) | 350 (13.4) | 551 (13.2) |
| Cancer | 727 (28.1) | 1771 (29.1) | 787 (30.2) | 1263 (30.2) |
| Congestive heart failure | 1436 (55.5) | 3569 (58.6) | 1434 (55) | 2197 (52.6) |
| Myocardial infarction | 824 (31.8) | 2075 (34.1) | 839 (32.2) | 1468 (35.1) |
| Anaemia | 861 (33.3) | 2322 (38.1) | 1136 (43.6) | 1970 (47.1) |
| Dementia | 35 (1.4) | 114 (1.9) | 56 (2.1) | 61 (1.5) |
| Liver disease | 115 (4.4) | 211 (3.5) | 84 (3.2) | 200 (4.8) |
| Excessive alcohol use | 130 (5) | 241 (4) | 103 (4) | 206 (4.9) |
| History of falls | 237 (9.2) | 728 (12) | 340 (13.1) | 551 (13.2) |
| Any previous major bleeding | 1074 (41.5) | 2606 (42.8) | 1215 (46.6) | 2165 (51.8) |
| Gastrointestinal bleeding | 361 (13.9) | 945 (15.5) | 443 (17) | 825 (19.7) |
| Intracranial bleeding | 98 (3.8) | 266 (4.4) | 123 (4.7) | 193 (4.6) |
| CHA2DS2-VASc | 5 (4–6) | 5 (4–6) | 5 (4–6) | 5 (3–6) |
| Treatment, n (%) | ||||
| Warfarin | 444 (17.1) | 1011 (16.6) | 375 (14.4) | 405 (9.7) |
| Undefinedb | 1156 (44.6) | 2260 (37) | 800 (30.7) | 943 (22.5) |
| No treatment | 990 (38.2) | 2830 (46.4) | 1433 (54.9) | 2843 (67.8) |
Since a patient can progress from CKD G3 to G4 to G5 to G5D, a unique patient can be present in one to all four GFR categories.
Undefined consists of 18% treatment periods with DOACs.
Primary and secondary outcomes
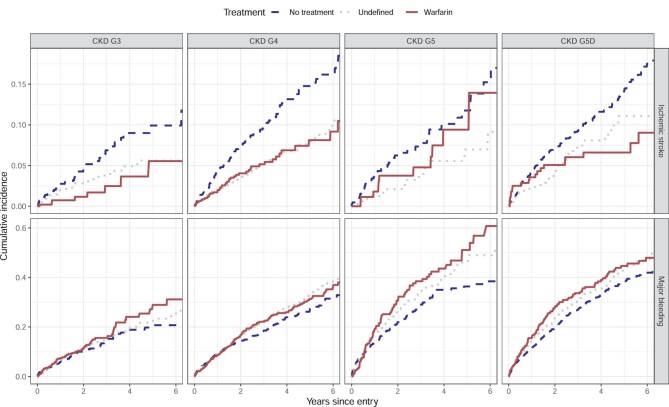
In unadjusted analysis, warfarin treatment displayed lower rates than no treatment of ischaemic stroke for G3, G4 and G5D, but no difference for G5 (Table 2 and Figure 2). All-cause mortality was lower for warfarin-treated patients compared with no treatment in all GFR categories (Table 2). In the unadjusted analysis, there was no apparent difference in major bleeding rates between warfarin and no treatment in any GFR category except G5, with higher rates of major bleeding for warfarin (Table 2 and Figure 2).
Table 2.
Number of events, exposed time and event rates sorted by treatment and GFR categorya
| Warfarin | Undefinedc | No treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Event | GFR categoryb | Time(100 years) | Events(n) | Rate (95% CI)(n/100 years) | Time(100 years) | Events (n) | Rate (95% CI)(n/100 years) | Time (100 years) | Events (n) | Rate (95% CI)(n/100 years) |
| All-cause stroke and systemic embolism | G3 (n = 2588) | 10.4 | 16 | 1.5 (0.88–2.5) | 23.7 | 59 | 2.5 (1.9–3.2) | 17.2 | 47 | 2.7 (2.0–3.6) |
| G4 (n = 6090) | 24.9 | 64 | 2.6 (2.0–3.3) | 48.2 | 132 | 2.7 (2.3–3.2) | 44.8 | 197 | 4.4 (3.8–5.1) | |
| G5 (n = 2605) | 5.2 | 20 | 3.9 (2.4–6.0) | 10.0 | 24 | 2.4 (1.5–3.6) | 15.1 | 64 | 4.2 (3.3–5.4) | |
| G5D (n = 4179) | 11.5 | 35 | 3.1 (2.1–4.2) | 16.5 | 77 | 4.7 (3.7–5.8) | 53.4 | 260 | 4.9 (4.3–5.5) | |
| All-cause stroke | G3 | 10.4 | 15 | 1.4 (0.81–2.4) | 23.8 | 48 | 2.0 (1.5–2.7) | 17.3 | 42 | 2.4 (1.7–3.3) |
| G4 | 25.0 | 59 | 2.4 (1.8–3.0) | 48.5 | 113 | 2.3 (1.9–2.8) | 44.9 | 181 | 4.0 (3.5–4.7) | |
| G5 | 5.2 | 17 | 3.3 (1.9–5.3) | 10.0 | 21 | 2.1 (1.3–3.2) | 15.2 | 55 | 3.6 (2.7–4.7) | |
| G5D | 11.6 | 28 | 2.4 (1.6–3.5) | 16.7 | 59 | 3.5 (2.7–4.6) | 54.1 | 216 | 4.0 (3.5–4.6) | |
| Ischaemic stroke | G3 | 10.4 | 9 | 0.86 (0.39–1.6) | 23.9 | 36 | 1.5 (1.1–2.1) | 17.5 | 40 | 2.3 (1.6–3.1) |
| G4 | 25.1 | 44 | 1.8 (1.3–2.4) | 48.6 | 86 | 1.8 (1.4–2.2) | 45.3 | 158 | 3.5 (3.0–4.1) | |
| G5 | 5.2 | 11 | 2.1 (1.1–3.8) | 10.0 | 14 | 1.4 (0.76–2.3) | 15.3 | 44 | 2.9 (2.1–3.9) | |
| G5D | 11.6 | 19 | 1.6 (0.99–2.6) | 16.7 | 38 | 2.3 (1.6–3.1) | 54.5 | 182 | 3.3 (2.9–3.9) | |
| Haemorrhagic stroke | G3 | 10.6 | 6 | 0.56 (0.21–1.2) | 24.5 | 13 | 0.53 (0.28–0.91) | 17.7 | 2 | 0.11 (0.014–0.41) |
| G4 | 25.7 | 15 | 0.58 (0.33–0.96) | 49.7 | 29 | 0.58 (0.39–0.84) | 46.2 | 22 | 0.48 (0.30–0.72) | |
| G5 | 5.4 | 5 | 0.93 (0.30–2.2) | 10.4 | 7 | 0.68 (0.27–1.4) | 15.7 | 11 | 0.70 (0.35–1.3) | |
| G5D | 12.2 | 9 | 0.74 (0.34–1.4) | 17.3 | 23 | 1.3 (0.84–2.0) | 56.0 | 35 | 0.63 (0.44–0.87) | |
| Major bleeding | G3 | 9.9 | 65 | 6.6 (5.1–8.4) | 23.1 | 133 | 5.8 (4.8–6.8) | 16.6 | 84 | 5.1 (4.0–6.3) |
| G4 | 23.9 | 196 | 8.2 (7.1–9.4) | 46.0 | 388 | 8.4 (7.6–9.3) | 41.9 | 308 | 7.3 (6.5–8.2) | |
| G5 | 4.6 | 75 | 16.4 (12.9–20.6) | 9.2 | 124 | 13.5 (11.2–16.1) | 13.9 | 156 | 11.2 (9.5–13.1) | |
| G5D | 9.6 | 116 | 12.1 (10.0–14.5) | 14.6 | 180 | 12.4 (10.6–14.3) | 45.5 | 472 | 10.4 (9.4–11.3) | |
| Intracranial bleeding | G3 | 10.6 | 8 | 0.76 (0.33–1.5) | 24.5 | 23 | 0.94 (0.60–1.4) | 17.6 | 4 | 0.23 (0.062–0.58) |
| G4 | 25.6 | 27 | 1.1 (0.69–1.5) | 49.4 | 71 | 1.4 (1.1–1.8) | 45.8 | 37 | 0.81 (0.57–1.1) | |
| G5 | 5.4 | 10 | 1.9 (0.89–3.4) | 10.3 | 15 | 1.5 (0.81–2.4) | 15.6 | 20 | 1.3 (0.78–2.0) | |
| G5D | 12.1 | 18 | 1.5 (0.88–2.3) | 17.2 | 34 | 2.0 (1.4–2.8) | 55.5 | 71 | 1.3 (1.0–1.6) | |
| Gastrointestinal bleeding | G3 | 10.4 | 29 | 2.8 (1.9–4.0) | 24.0 | 54 | 2.3 (1.7–2.9) | 17.3 | 42 | 2.4 (1.8–3.3) |
| G4 | 25.0 | 83 | 3.3 (2.6–4.1) | 48.2 | 178 | 3.7 (3.2–4.3) | 44.5 | 156 | 3.5 (3.0–4.1) | |
| G5 | 5.0 | 37 | 7.3 (5.2–10.1) | 9.9 | 55 | 5.6 (4.2–7.2) | 15.0 | 72 | 4.8 (3.8–6.0) | |
| G5D | 11.1 | 52 | 4.7 (3.5–6.1) | 16.5 | 72 | 4.4 (3.4–5.5) | 51.7 | 221 | 4.3 (3.7–4.9) | |
| Other bleeding | G3 | 10.2 | 37 | 3.6 (2.6–5.0) | 23.6 | 76 | 3.2 (2.5–4.0) | 17.1 | 53 | 3.1 (2.3–4.0) |
| G4 | 24.6 | 113 | 4.6 (3.8–5.5) | 47.8 | 197 | 4.1 (3.6–4.7) | 44.0 | 164 | 3.7 (3.2–4.3) | |
| G5 | 4.9 | 46 | 9.4 (6.9–12.6) | 9.6 | 72 | 7.5 (5.9–9.5) | 14.7 | 89 | 6.0 (4.8–7.4) | |
| G5D | 10.3 | 73 | 7.1 (5.6–8.9) | 15.3 | 97 | 6.3 (5.1–7.7) | 49.9 | 278 | 5.6 (4.9–6.3) | |
| All-cause mortality | G3 | 10.6 | 73 | 6.9 (5.4–8.6) | 24.6 | 294 | 11.9 (10.6–13.4) | 17.8 | 333 | 18.7 (16.7–20.8) |
| G4 | 25.7 | 317 | 12.3 (11.0–13.8) | 49.9 | 874 | 17.5 (16.4–18.7) | 46.6 | 1385 | 29.7 (28.2–31.3) | |
| G5 | 5.4 | 102 | 18.9 (15.4–22.9) | 10.4 | 319 | 30.8 (27.5–34.3) | 15.8 | 691 | 43.6 (40.5–47.0) | |
| G5D | 12.2 | 235 | 19.3 (16.9–21.9) | 17.4 | 575 | 33.1 (30.4–35.9) | 56.5 | 2140 | 37.9 (36.3–39.5) | |
| Myocardial infarction | G3 | 10.3 | 26 | 2.5 (1.7–3.7) | 24.0 | 44 | 1.8 (1.3–2.5) | 17.2 | 56 | 3.3 (2.5–4.2) |
| G4 | 24.9 | 65 | 2.6 (2.0–3.3) | 48.2 | 147 | 3.0 (2.6–3.6) | 44.5 | 205 | 4.6 (4.0–5.3) | |
| G5 | 5.1 | 34 | 6.7 (4.6–9.3) | 10.0 | 38 | 3.8 (2.7–5.2) | 14.9 | 108 | 7.3 (6.0–8.8) | |
| G5D | 10.9 | 54 | 5.0 (3.7–6.5) | 16.0 | 83 | 5.2 (4.1–6.4) | 51.4 | 336 | 6.5 (5.9–7.3) | |
Treatment time (time) is presented as 100 years. Events presented as number of events occurred. Event rate (rate) presents events per 100 person years with 95% CI. Only the first type of every outcome is counted. After the occurrence of an event a patient is censored for this event but remains in the cohort for other outcomes.
CKD G3(n = 2588), G4 (n = 6090), G5(n = 2605) and G5D (n = 4179) for all outcomes.
Undefined consists of 18% treatment periods with DOAC.
FIGURE 2:
Unadjusted Kaplan–Meyer curves for primary outcomes Ischaemic stroke (top row) and major bleeding (bottom row) with respect to treatment and GFR category. Graphs presented with years since entry on the x-axis and cumulative incidence on the y-axis.
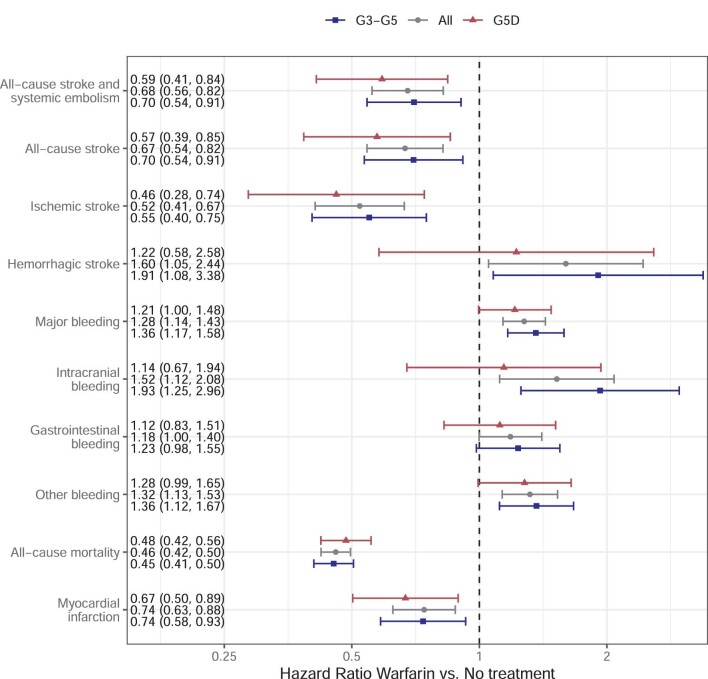
In the adjusted analysis, warfarin-treated patients had a lower risk of ischaemic stroke for G3 [HR 0.37 (95% CI 0.18–0.76)], G4 [0.53 (0.38–0.74)] and G5D [0.49 (0.30–0.79)], but no significant difference was seen in G5 [0.80 (0.41–1.55)] (Figure 3). All-cause stroke and systemic embolism and all-cause stroke follow the same pattern, although not a significantly lower risk of all-cause stroke for warfarin compared with no treatment in G3. Warfarin confers a higher risk of major bleeding in G4 [HR 1.22 (95% CI 1.02–1.46)], G5 [1.52 (1.15–2.01)] and G5D [1.23 (1.00–1.51)], but not G3 [1.28 (0.92–1.77)]. Intracranial bleeding was more common for warfarin in G3 [HR 3.60 (95% CI 1.08–11.95)], GI bleeding in G5 [1.58(1.06–2.35)] and other bleeding in G4 [1.29 (1.01–1.64)], G5 [1.59 (1.11–2.27)] and G5D [1.30 (1.01–1.69)]. Warfarin was consistently associated with lower mortality in all GFR categories [G3: HR 0.34 (95% CI 0.27–0.44), G4: 0.45 (0.40–0.51), G5: 0.44 (0.36–0.54) and G5D: 0.49 (0.43–0.56). Myocardial infarction was less common for warfarin-treated patients in G4 [HR 0.61 (95% CI 0.46–0.81)].
FIGURE 3:
Adjusted model for warfarin versus no treatment analysed with respect to GFR category in 12 106 CKD G3–G5D patients with non-valvular AF. Data presented as HR (95% CI). Analysis adjusted for sex, age, years from study start and any of the following prior events: congestive heart failure, diabetes mellitus, hypertension, stroke or TIA, vascular disease, major bleeding, myocardial infarction, PCI and excessive alcohol use.
The G3–G5 group was merged and additional variables, including blood pressure, BMI, S/P albumin and β-haemoglobin were accounted for in a sensitivity analysis (8307 complete cases). These variables were not available for the G5D group, which was adjusted for dialysis modality (haemodialysis and peritoneal dialysis) instead (Figure 4). The model shows that warfarin gives an ∼50% reduction of ischaemic stroke for G3–G5 [HR 0.55 (95% CI 0.40–0.75)], G5D [0.46 (0.28–0.74)] and all patients [0.52 (0.41–0.67)] and with a correspondingly more prevalent risk of major bleeding [G3–G5: HR 1.36 (95% CI 1.17–1.58), G5D: 1.21 (1.00–1.48) and all patients: 1.28 (1.14–1.43)].
FIGURE 4:
Sensitivity analysis with adjusted model for warfarin versus no treatment for all patients (12 106 patients), G3–G5 group (8307 patients) and dialysis group (4179 patients). The subgroups add up to more than the total individuals because GFR can decrease during the course of the study and a patient can enter a higher GFR category. Analysis adjusted for sex, age, years from study start and for any of the following prior events: congestive heart failure, diabetes mellitus, hypertension, stroke or TIA, vascular disease, major bleeding, myocardial infarction, PCI and excessive alcohol use. Also, adjustment for blood pressure, BMI, β-haemoglobin, S/P albumin is added to the G3–G5 model, while adjustment for dialysis modality is added to the G5D model.
DISCUSSION
Cardiovascular disease is the main cause of death in patients with CKD and a target for possible intervention [30]. However, side effects from various treatment strategies are common in this patient group. Unfortunately, CKD patients with a GFR <60 mL/min/1.73 m2 are often excluded from RCTs of new strategies. However, risks and benefits are of utmost importance to evaluate in this patient category.
According to the present study, warfarin offers an ∼50% risk reduction of ischaemic stroke compared with no treatment when considering the whole cohort. The cost of stroke protection is bleeding, with a 22–52% increased risk of major bleeding in G4–G5D. When separating the various GFR categories, patients in G5 are different, as in this group there is no evidence of warfarin offering protection against ischaemic stroke or thromboembolic ischaemic events along with the increased risk of major bleeding, particularly GI bleeding and other bleeding. In contrast, in G5D, warfarin seems protective against ischaemic stroke and ischaemic thromboembolic events. When analysed separately, major bleeding is more common among dialysis patients on warfarin, but gastrointestinal and intracranial bleeding are not. Previous observational data on G4–G5 concludes there is warfarin stroke protection, similar to our study if merging G3–G5 [5, 12, 13]. When comparing the TTR for G5 and G5D, it is higher in G5 than in G5D (68% versus 62%). Better quality of warfarin treatment in the G5 group than G5D is not reflected by a lower ischaemic stroke risk. This could be due in part to a bias where only the healthiest patients start dialysis (progress from G5 to G5D) and only patients with a low risk of bleeding are prescribed anticoagulants. The G5 group might consist not only of patients considered for dialysis with decreasing GFR, but also of a frailer group of patients ineligible for dialysis with a high risk of both stroke and bleeding. The risk of haemorrhagic stroke and intracranial bleeding is significantly increased in G3 but not in other GFR categories. The increased risk of intracranial bleeding on warfarin might be concealed by the increased bleeding risk due to progressive uraemia in G4–G5D. Mortality is reduced in all warfarin groups, indicating the whole cohort is biased by warfarin prescription to the healthiest patients. It should be noted that females were prescribed less warfarin in our study, similar to results in other studies [31, 32]. This should be paid attention to since being female is an independent risk factor for stroke and women do not seem to have a higher bleeding risk than men [33, 34].
Our data on warfarin protection of ischaemic stroke in dialysis patients conflict with several previous observational studies and meta-analyses but are consistent with two Danish studies [5, 12]. None of these studies presented a TTR, but it has been previously shown that Sweden and Denmark have generally high TTRs in warfarin-treated patients [35, 36]. In contrast, Genovesi et al. [37] did not see an effect of thromboembolic events and more bleeding on warfarin in haemodialysis patients. However, their mean TTR was lower (54%) than ours (62%). Other studies of haemodialysis patients have displayed TTRs of approximately 50% [38, 39]. Pokorney et al. [40, 41] showed no reduction of death or stroke with warfarin; the TTR was not presented. Previous studies have shown less INR control in the USA than in Sweden [40, 41]. In general, a TTR >70–75% is suggested to balance the risk of stroke and bleeding independent of CKD [14, 42]. Our results, with fewer ischaemic strokes among dialysis patients, could be explained by better INR control in Sweden, although a TTR >62% is desirable. Another explanation for conflicting results is study design. In contrast to similar studies using prescription data to determine if a patient was on or off anticoagulants, we used AuriculA, with accurate information whether the patient was actually on warfarin or not when the event occurred [5, 12, 40, 43]. Kai et al. [38] also showed an association between warfarin and a lower risk of ischaemic stroke in dialysis patients. The majority of warfarin-treated patients in that study (945/989) were enrolled at an anticoagulation clinic. Although they reported a low mean TTR (50%), a specialized anticoagulation clinic may be associated with closer patient follow-up and more favourable outcomes.
We intended to describe our cohort as accurately as possible. Therefore all patient time was divided into treatment periods with reliable warfarin and no treatment periods and the more uncertain periods of undefined treatment. The undefined treatment could be a stable warfarin treatment but not registered in AuriculA, as well as a patient with a dispensation of warfarin who never started the treatment. This group also contains treatment periods with DOACs, which is not part of our research question but is presented as raw data to describe the cohort with CKD and AF in full. Consequently, this is a heterogenous group of treatment periods. On the other hand, we describe the real world where changes in treatment and poor compliance are common.
The present study has many limitations. The most important one is the retrospective design with several confounders and bias, as described above. We used registers with high validity and coverage, however, data could be missing or misregistered. We tried to minimize bias by adjusting for relevant risk factors for bleeding and stroke. We also did a sensitivity analysis, dividing the cohort differently and adding possibly important covariates such as β-haemoglobin, S/P albumin, BMI and blood pressure. Results were not changed by the sensitivity analysis regarding warfarin protection of ischaemic stroke, but an increased risk for intracranial bleeding and haemorrhagic stroke on warfarin for all patients and the merged G3–G5 was observed. The covariates adjusted for also play a role in physicians’ choice to prescribe or abstain from warfarin. It is unfortunate that coverage of these covariates was not sufficient for the same adjustment in G5D. One can speculate if this adjustment would increase the HR of bleeding correspondingly in G5D. This also enlightens the problem with unmeasured confounders in observational studies, certainly present in this study as well. Patients in G3–G5 who, for some reason, are not patients at a nephrology clinic were not included. Supposedly this includes both patients with stable disease and slow progression, not yet referred to specialist care, as well as very old and/or frail patients not expected to be favoured by specialist nephrology care. Our results might not be applicable to these patients. Even though the study has limitations, we believe the accuracy of treatment periods and stroke-related outcomes, considering the GFR category continuously along with TTR, makes this observational study relevant.
Warfarin treatment is associated with a lower risk of ischaemic stroke for patients with AF and CKD G3, G4 and G5D, but not G5. Warfarin treatment is associated with a higher risk of major bleeding for G4–G5D. The results contribute to the diverging data on stroke protection of warfarin in advanced CKD and dialysis, enlightening the urgent need for RCTs. Awaiting RCTs, it might be reasonable to use warfarin in selected patients on dialysis and with non-valvular AF, with a low risk of bleeding and a high risk of ischaemic stroke as part of the prevention of cardiovascular disease and death.
Supplementary Material
ACKNOWLEDGEMENTS
The study was approved by the Swedish Ethical Review Authority.
Contributor Information
Frida Welander, Department of Public Health and Clinical Medicine, Department of Research and Development–Sundsvall, Umeå University, Umeå, Sweden.
Henrik Renlund, Uppsala Clinical Research Centre, Uppsala University, Uppsala, Sweden.
Emöke Dimény, Department of Public Health and Clinical Medicine, Umeå University, Umeå, Sweden.
Henrik Holmberg, Department of Public Health and Clinical Medicine, Umeå University, Umeå, Sweden.
Anders Själander, Department of Public Health and Clinical Medicine, Umeå University, Umeå, Sweden.
FUNDING
Support for this study was provided by Unit for Research and Development, Region Västernorrland (grant LVNFOU938547; www.rvn.se) and agreement regarding research and education of doctors, Umeå University and the Swedish Heart and Lung Foundation (grant 20200766). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
AUTHORS’ CONTRIBUTIONS
All authors were involved in the study conception. The study was designed by F.W., H.R., E.D. and AS. Statistical analysis was carried out by H.R. Interpretation of data was performed by all authors. F.W. drafted the article and all authors revised the article. All authors provided intellectual content of critical importance to the work described and approved the final version to be published.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared upon reasonable request to the corresponding author.
REFERENCES
- 1. Turakhia MP, Blankestijn PJ, Carrero J-Jet al. Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J 2018; 39: 2314–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Watanabe H, Watanabe T, Sasaki Set al. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am Heart J 2009; 158: 629–636 [DOI] [PubMed] [Google Scholar]
- 3. Soliman EZ, Prineas RJ, Go ASet al. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC). Am Heart J 2010; 159: 1102–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Genovesi S, Pogliani D, Faini Aet al. Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis 2005; 46: 897–902 [DOI] [PubMed] [Google Scholar]
- 5. Olesen JB, Lip GYH, Kamper A-Let al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 2012; 367: 625–635 [DOI] [PubMed] [Google Scholar]
- 6. Go AS, Fang MC, Udaltsova Net al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. Circulation 2009; 119: 1363–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kimachi M, Furukawa TA, Kimachi Ket al. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev 2017; 11: CD011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Connolly SJ, Ezekowitz MD, Yusuf Set al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151 [DOI] [PubMed] [Google Scholar]
- 9. Patel MR, Mahaffey KW, Garg Jet al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891 [DOI] [PubMed] [Google Scholar]
- 10. Giugliano RP, Ruff CT, Braunwald Eet al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093–2104 [DOI] [PubMed] [Google Scholar]
- 11. Granger CB, Alexander JH, McMurray JJVet al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992 [DOI] [PubMed] [Google Scholar]
- 12. Bonde AN, Lip GY, Kamper ALet al. Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: a nationwide observational cohort study. J Am Coll Cardiol 2014; 64: 2471–2482 [DOI] [PubMed] [Google Scholar]
- 13. Shah M, Avgil Tsadok M, Jackevicius CAet al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation 2014; 129: 1196–1203 [DOI] [PubMed] [Google Scholar]
- 14. Szummer K, Gasparini A, Eliasson Set al. Time in therapeutic range and outcomes after warfarin initiation in newly diagnosed atrial fibrillation patients with renal dysfunction. J Am Heart Assoc 2017; 6: e004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ravera M, Bussalino E, Fusaro Met al. Systematic DOACs oral anticoagulation in patients with atrial fibrillation and chronic kidney disease: the nephrologist's perspective. J Nephrol 2020; 33: 483–495 [DOI] [PubMed] [Google Scholar]
- 16. Abbott KC, Trespalacios FC, Taylor AJet al. Atrial fibrillation in chronic dialysis patients in the United States: risk factors for hospitalization and mortality. BMC Nephrol 2003; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan KE, Lazarus JM, Thadhani Ret al. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol 2009; 20: 2223–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dahal K, Kunwar S, Rijal Jet al. Stroke, major bleeding, and mortality outcomes in warfarin users with atrial fibrillation and chronic kidney disease: a meta-analysis of observational studies. Chest 2016; 149: 951–959 [DOI] [PubMed] [Google Scholar]
- 19. Tan J, Liu S, Segal JBet al. Warfarin use and stroke, bleeding and mortality risk in patients with end stage renal disease and atrial fibrillation: a systematic review and meta-analysis. BMC Nephrol 2016; 17: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ha JT, Neuen BL, Cheng LPet al. Benefits and harms of oral anticoagulant therapy in chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 2019; 171: 181–189 [DOI] [PubMed] [Google Scholar]
- 21. January CT, Wann LS, Calkins Het al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation 2019; 140: e125–e151 [DOI] [PubMed] [Google Scholar]
- 22. Hindricks G, Potpara T, Dagres Net al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021; 42: 373–498 [DOI] [PubMed] [Google Scholar]
- 23. Ludvigsson JF, Otterblad-Olausson P, Pettersson BUet al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009; 24: 659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swedish Renal Registry annual report 2020 . https://www.medscinet.net/snr/rapporterdocs/Svenskt%20Njurregister%20Årsrapport%202020.pdf (22 December 2021, date last accessed) [Google Scholar]
- 25. Ludvigsson JF, Andersson E, Ekbom Aet al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swedish Stroke Register. Evaluations of variables in Riksstroke, the Swedish Stroke Register. Short version in English . 2014. https://www.riksstroke.org/wp-content/uploads/2015/06/Evaluations-of-variables-in-Riksstroke-rev-15-08-03.pdf (22 December 2021, date last accessed) [Google Scholar]
- 27. Lip GY, Nieuwlaat R, Pisters Ret al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest 2010; 137: 263–272 [DOI] [PubMed] [Google Scholar]
- 28. Levey AS, Eckardt KU, Dorman NMet al. Nomenclature for kidney function and disease-executive summary and glossary from a Kidney Disease: Improving Global Outcomes (KDIGO) consensus conference. Eur Heart J 2020; 41: 4592–4598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosendaal FR, Cannegieter SC, van der Meer FJet al. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 1993; 69: 236–239 [PubMed] [Google Scholar]
- 30. Gansevoort RT, Correa-Rotter R, Hemmelgarn BRet al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013; 382: 339–352 [DOI] [PubMed] [Google Scholar]
- 31. Yong CM, Tremmel JA, Lansberg MGet al. Sex differences in oral anticoagulation and outcomes of stroke and intracranial bleeding in newly diagnosed atrial fibrillation. J Am Heart Assoc 2020; 9: e015689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Glader EL, Stegmayr B, Norrving Bet al. Sex differences in management and outcome after stroke: a Swedish national perspective. Stroke 2003; 34: 1970–1975 [DOI] [PubMed] [Google Scholar]
- 33. Friberg J, Scharling H, Gadsbøll Net al. Comparison of the impact of atrial fibrillation on the risk of stroke and cardiovascular death in women versus men (the Copenhagen City Heart Study). Am J Cardiol 2004; 94: 889–894 [DOI] [PubMed] [Google Scholar]
- 34. Lapner S, Cohen N, Kearon C. Influence of sex on risk of bleeding in anticoagulated patients: a systematic review and meta-analysis. J Thromb Haemost 2014; 12: 595–605 [DOI] [PubMed] [Google Scholar]
- 35. Wieloch M, Själander A, Frykman Vet al. Anticoagulation control in Sweden: reports of time in therapeutic range, major bleeding, and thrombo-embolic complications from the national quality registry AuriculA. Eur Heart J 2011; 32: 2282–2289 [DOI] [PubMed] [Google Scholar]
- 36. Nielsen PB, Lundbye-Christensen S, Rasmussen LHet al. Improvement of anticoagulant treatment using a dynamic decision support algorithm: a Danish cohort study. Thromb Res 2014; 133: 375–379 [DOI] [PubMed] [Google Scholar]
- 37. Genovesi S, Rossi E, Gallieni Met al. Warfarin use, mortality, bleeding and stroke in haemodialysis patients with atrial fibrillation. Nephrol Dial Transplant 2015; 30: 491–498 [DOI] [PubMed] [Google Scholar]
- 38. Kai B, Bogorad Y, Nguyen LNet al. Warfarin use and the risk of mortality, stroke, and bleeding in hemodialysis patients with atrial fibrillation. Heart Rhythm 2017; 14: 645–651 [DOI] [PubMed] [Google Scholar]
- 39. Quinn LM, Richardson R, Cameron KJet al. Evaluating time in therapeutic range for hemodialysis patients taking warfarin. Clin Nephrol 2015; 83: 80–85 [DOI] [PubMed] [Google Scholar]
- 40. Pokorney SD, Black-Maier E, Hellkamp ASet al. Oral anticoagulation and cardiovascular outcomes in patients with atrial fibrillation and end-stage renal disease. J Am Coll Cardiol 2020; 75: 1299–1308 [DOI] [PubMed] [Google Scholar]
- 41. Pokorney SD, Simon DN, Thomas Let al. Patients' time in therapeutic range on warfarin among US patients with atrial fibrillation: results from ORBIT-AF registry. Am Heart J 2015; 170: 141–148 [DOI] [PubMed] [Google Scholar]
- 42. Proietti M, Lane DA, Lip GYH. Chronic kidney disease, time in therapeutic range and adverse clinical outcomes in anticoagulated patients with non-valvular atrial fibrillation: observations from the SPORTIF trials. EBioMedicine 2016; 8: 309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Agarwal MA, Potukuchi PK, Sumida Ket al. Clinical outcomes of warfarin initiation in advanced chronic kidney disease patients with incident atrial fibrillation. JACC Clin Electrophysiol 2020; 6: 1658–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.