ABSTRACT
Background
Sodium-23 magnetic resonance imaging (23Na MRI) allows the measurement of skin sodium concentration ([Na+]). In patients requiring dialysis, no data are available relating to the clinical outcomes associated with skin sodium accumulation or the determinants of increasing deposition.
Methods
This was an exploratory, observational study of adult hemodialysis (HD) and peritoneal dialysis (PD) patients. Participants underwent skin [Na+] quantification with leg 23Na MRI at the study’s beginning. Outcomes of interest were all-cause mortality and composite all-cause mortality plus major adverse cardiovascular events. Cumulative total and event-free survival were assessed using the Kaplan–Meier survival function after stratification into skin [Na+] quartiles. Cox proportional hazards regression was used to model the association between skin [Na+] and outcomes of interest. Multiple linear regression was used to model the predictors of skin [Na+].
Results
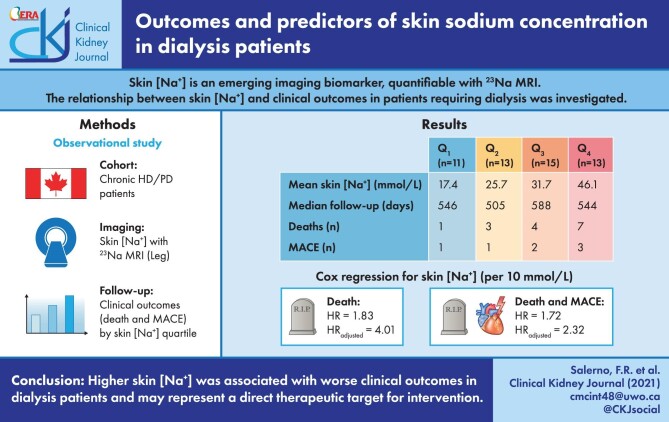
A total of 52 participants (42 HD and 10 PD) underwent the study procedures. The median follow-up was 529 days (interquartile range: 353–602). Increasing skin [Na+] quartiles were associated with significantly shorter overall and event-free survival (log-rank χ2(1) = 3.926, log-rank χ2(1) = 5.685; P for trend <0.05 in both instances). Skin [Na+] was associated with all-cause mortality {hazard ratio (HR) 4.013, [95% confidence interval (95% CI) 1.988–8.101]; P < 0.001} and composite events [HR 2.332 (95% CI 1.378–3.945); P < 0.01], independently of age, sex, serum [Na+] and albumin. In multiple regression models, dialysate [Na+], serum albumin and congestive heart failure were significantly associated with skin [Na+] in HD patients (R2adj = 0.62).
Conclusions
Higher skin [Na+] was associated with worse clinical outcomes in dialysis patients and may represent a direct therapeutic target.
Keywords: chronic hemodialysis, clinical outcomes, magnetic resonance imaging, peritoneal dialysis, sodium
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Sodium-23 magnetic resonance imaging (23Na MRI) is an emerging imaging technique in clinical nephrology [1]. The potential of 23Na MRI lies in the ability to visualize and quantify sodium concentration ([Na+]) directly at the tissue level, which is of special interest in chronic kidney disease (CKD) and dialysis population, where sodium excretion is impaired as a consequence of kidney failure. Methods to quantify skin [Na+] are attracting increasing interest to study peripheral edema and water-independent sodium accumulation [2]; however, clear evidence linking skin [Na+] with clinical outcomes is currently lacking. At present, extracellular volume overload is a well-described predictor of clinical outcomes in the kidney failure population receiving dialysis [3, 4]. Skin water-independent sodium accumulation likely plays an additional clinically significant role; this has been linked with increased left ventricular mass in non-dialysis CKD patients, independently of extracellular volume overload [5]. Furthermore, skin [Na+] has been shown to be modifiable by interventions such as loop diuretics, sodium–glucose cotransporter 2 inhibitors and ultrafiltration with hemodialysis (HD) [6–8]. Skin [Na+] may be an attractive and modifiable biomarker to assess volume status to be directly addressed therapeutically.
In this study, we explored the hypothesis that skin [Na+] is associated with mortality and major adverse cardiovascular events (MACE) in a cohort of HD and peritoneal dialysis (PD) patients who underwent interdialytic 23Na MRI of the leg. Furthermore, we aimed to define the determinants associated with increased skin [Na+].
MATERIALS AND METHODS
Study design
This was an observational, exploratory study. Study participants underwent a visit consisting of a research imaging session (1H and 23Na MRI of either the right or left leg), demographic and clinical data collection and blood biochemistry. HD patients were scanned on the day following their last HD treatment, whenever feasible. Outcomes were assessed retrospectively by clinical chart review.
The study received approval from the Western University Human Research Ethics board and was conducted in compliance with the declaration of Helsinki and all applicable regulatory requirements. This study was prospectively registered (clinicaltrials.gov Identifier: NCT03004547).
Study participants
Study patients were recruited between March 2018 and March 2020 from the London Health Sciences Centre (LHSC), London, ON, Canada. All study participants provided written informed consent. Study participants were recruited from the prevalent LHSC outpatient dialysis population and were on their established treatment modality (thrice-weekly HD or PD) for at least 3 months before recruitment. Potential study candidates were excluded if they were pregnant, breastfeeding or intending pregnancy, unable to provide consent or had contraindications to MRI.
Dialysate [Na+] prescription
Dialysate [Na+] was prescribed either according to dialysis unit protocol—within the LHSC organization, one unit used 137 mmol/L and the other 140 mmol/L—or individualized following clinical indications (e.g. to match low serum [Na+] or improve intradialytic hemodynamic stability) in selected patients. The HD standard-of-care did not differ between units, except for the dialysate [Na+] prescription.
MRI and image analysis
All magnetic resonance images were acquired using a multinuclear 3.0 Tesla GE MRI scanner (Discovery MR750, General Electric Healthcare, Milwaukee, WI, USA). To acquire 23Na spin density images, subjects were positioned in the magnet bore in the supine position, with the thickest part of their right or left calf muscle at the center of a custom-made 23Na birdcage radiofrequency coil. Calibration vials with increasing saline concentrations were placed in the RF coil over the subjects’ shins. A single-slice 23Na MR image was obtained with a radial k-space acquisition pulse sequence (density-adapted 2D projection reconstruction) [9], with the following parameters: slice-selective radiofrequency pulse; flip angle 90°; repetition time/echo time: 100/1.2 ms; number of signals averages: 100; slice thickness: 30 mm and isotropic field of view/resolution: 18/0.3 cm2; and total acquisition time: ∼30 min. During the same imaging session, additional axial 1H MR images were acquired using a standard (spoiled gradient-recalled echo) pulse sequence to identify and delineate the relevant anatomical structures.
23Na concentration maps were generated using custom software developed within MATLAB, version 9.6.0–R2019a (The MathWorks Inc., Natick, MA, USA). A region of interest encompassing the whole skin (skin [Na+]) was manually segmented after superimposing 1H and 23Na images, as detailed previously [10].
Laboratory analysis
Blood samples collected from each participant were processed and analyzed in a central laboratory (London Health Sciences Centre, London, ON, Canada) for routine clinical biomarkers. Due to the high prevalence of diabetes mellitus in the study sample, serum sodium was corrected for serum glucose concentration as according to Katz [11].
Outcomes and statistical analysis
Statistical analysis was performed using the GraphPad Prism version 9.0.0 (GraphPad Software, San Diego, CA, USA; www.graphpad.com) and the SPSS version 27.0 (IBM Corp., released 2020, IBM SPSS Statistics for MacIntosh; IBM Corp., Armonk, NY, USA). Normality was assessed both graphically and with the Shapiro–Wilk test. Continuous variables were presented as mean [standard deviation (SD)] if normally distributed and median [minimum, interquartile range (IQR), maximum] if highly skewed. Categorical variables were expressed as ratios or percentages. The study sample was stratified into four groups according to skin [Na+] quartiles. Comparisons between two subgroups were performed with the Mann–Whitney U-test; differences between quartiles were computed using the one-way analysis of variance. The following outcomes were of interest for this study: all-cause mortality and a composite of all-cause mortality and MACE, the latter defined as acute coronary syndrome, congestive heart failure, stroke and pulmonary embolism. Outcomes were determined by clinical chart review and adjudicated by F.R.S. and C.W.M. Participants who received kidney transplantation were right censored at the transplant date. Survival or event-free survival curves for each group were compared using the Kaplan–Meier function, and the log-rank test was used to analyze survival trends, assuming an incremental risk with increasing skin [Na+] quartiles. This analysis was performed for all-cause mortality and the composite endpoint of mortality and MACE. For both endpoints, univariate Cox proportional hazards regression was used to model the hazard ratios (HR) associated with skin [Na+], either as a continuous or as a categorical variable; multivariate models were created using the enter method to adjust skin [Na+] HR for confounder variables commonly associated with mortality and MACE (age, sex, serum [Na+] and serum albumin), with the idea that skin [Na+] would be associated with clinical outcomes, even after adjustment for basic demographics and traditional predictors of cardiovascular morbidity and all-cause mortality (serum albumin and serum [Na+]).
Univariate linear regression was used to analyze the relationship between skin [Na+] and main variables of interest (dialysate [Na+], corrected serum [Na+], serum albumin and age). Multiple linear regression analysis with the enter method was used to model the variables associated with skin [Na+]. The models were assembled to describe the dependence of skin [Na+] from variables associated with volume overload (hypoalbuminemia and congestive heart failure), traditional measures of sodium status (serum [Na+]), dialysis-based factors (dialysate [Na+]) and demographics (age). Independent variables were selected according to the following criteria: (i) previous published evidence (age, serum albumin and dialysate [Na+]) [8, 10, 12], (ii) pathophysiological rationale (congestive heart failure and serum [Na+]); and (iii) statistically significant difference across the skin [Na+] quartiles (serum albumin, dialysate [Na+] and serum [Na+]). To avoid model overfitting, no more than one predictor or confounder variable every 10 cases was used in the models. Missing data were handled by list-wise deletion. Unstandardized β coefficients, R2 and adjusted R2 values for goodness-of-fit were reported. An α <0.05 was considered statistically significant.
RESULTS
A total of 52 patients (42 HD and 10 PD) completed the study. Table 1 shows the main characteristics of the study sample, as overall and after quartile stratification according to skin [Na+]. Study participants tended to be older as skin [Na+] quartiles increased. Participants in Q4 tended to have a shorter dialysis vintage, a larger comorbidity burden and more anti-hypertensive medications compared with Q1–Q3. Participants in Q3 and Q4 also tended to higher dialysate [Na+], corrected serum [Na+] and lower serum albumin compared with Q1 and Q2. Out of 42 HD patients, 8 were receiving an individualized dialysate [Na+] prescription (median, range: 138 mmol/L, 135–145); 17 patients were receiving a dialysate [Na+] prescription of 137 mmol/L and 17 patients were receiving a dialysate [Na+] prescription of 140 mmol/L. Dialysate [Na+] distribution according to skin [Na+] quartiles is shown in Figure 1.
Table 1.
Demographics, anthropometrics, clinical information and medications of the overall study sample and after skin [Na+] quartile stratification
| Skin [Na+] | Skin [Na+] | Skin [Na+] | Skin [Na+] | P | ||
|---|---|---|---|---|---|---|
| Variable | Overall | Q1 | Q2 | Q3 | Q4 | |
| n | 52 | 11 | 13 | 15 | 13 | |
| Age (years) | 63.9 ± 10.2 | 59.0 ± 9.8 | 64.4 ± 13.9 | 64.2 ± 8.2 | 67.1 ± 7.8 | 0.288 |
| Sex (M/F) | 30/22 | 7/4 | 6/7 | 8/7 | 9/4 | 0.638 |
| BMI (kg/m2) | 30.1 ± 6.4 | 29.6 ± 7.2 | 30.3 ± 5.4 | 30.1 ± 5.5 | 30.4 ± 8.1 | 0.991 |
| HD/PD | 42/10 | 11/0 | 11/2 | 11/4 | 9/4 | |
| Dialysis vintage (months), median (IQR) | 22.0 (11.8–33.5) | 22.0 (19.0–29.5) | 23.0 (14.0–37.0) | 21.0 (12.5–32.0) | 16.0 (4.0–24.0) | 0.859 |
| Residual urine output (mL/24 h), median (IQR) | 250.0 (0.0–750) | 0.0 (0.0–150.0) | 250.0 (0.0–400.0) | 400.0 (200.0–1000.0) | 300.0 (100.0–900.0) | 0.219 |
| CCI, median (IQR) | 6.0 (5.0–8.0) | 5.0 (4.0–7.0) | 7.0 (5.0–8.0) | 6.0 (4.0–8.5) | 7.0 (6.0–9.0) | 0.284 |
| CKD etiology | ||||||
| Hypertensive (%) nephrosclerosis (%) | 15% | 18% | 31% | 13% | 0% | 0.184 |
| Diabetic kidney disease (%) | 44% | 27% | 54% | 27% | 69% | 0.074 |
| Glomerular disease (%) | 10% | 0% | 0% | 20% | 15% | 0.178 |
| Other (%) | 25% | 55% | 8% | 33% | 8% | <0.05 |
| Unknown (%) | 6% | 0% | 8% | 7% | 8% | 0.832 |
| Comorbidities | ||||||
| Hypertension (%) | 79% | 82% | 77% | 67% | 92% | 0.418 |
| Coronary artery disease (%) | 35% | 18% | 38% | 33% | 46% | 0.537 |
| Congestive heart failure (%) | 27% | 18% | 23% | 27% | 38% | 0.704 |
| Diabetes mellitus (%) | 60% | 36% | 69% | 53% | 77% | 0.184 |
| Medications | ||||||
| ACEi–ARB (%) | 29% | 27% | 38% | 7% | 46% | 0.107 |
| Beta blockers (%) | 52% | 45% | 69% | 33% | 62% | 0.231 |
| Calcium channel blockers (%) | 52% | 45% | 38% | 53% | 69% | 0.44 |
| Diuretic (%) | 50% | 36% | 54% | 53% | 54% | 0.792 |
| HD information (n = 42) | ||||||
| Time elapsed from last HD to MRI (h), median (IQR) | 22.5 (20.2–24.7) | 22.5 (20.1–23.2) | 23.9 (22.6–25.2) | 21.0 (20.3–33.3) | 22.8 (18.5–23.6) | 0.523 |
| spKt/V | 1.30 ± 0.23 | 1.26 ± 0.28 | 1.34 ± 0.23 | 1.32 ± 0.19 | 1.26 ± 0.26 | 0.856 |
| Dialysate [Na+] (mmol/L), median (IQR) | 138.0 (137.0–140.0) | 137.0 (137.0–137.0) | 138.0 (137.0–140.0) | 140.0 (137.5–140.0) | 140.0 (140.0–140.0) | <0.001 |
| IDWG (% body weight) | 2.1 ± 1.2 | 2.1 ± 1.0 | 2.1 ± 1.0 | 1.8 ± 1.3 | 2.3 ± 1.5 | 0.88 |
| UFR (mL/kg/h) | 7.6 ± 3.7 | 8.3 ± 4.7 | 8.2 ± 3.3 | 7.1 ± 3.5 | 6.7 ± 3.5 | 0.713 |
| PreHD SBP (mmHg) | 142.5 ± 22.8 | 137.3 ± 25.7 | 153.5 ± 21.5 | 142.1 ± 14.0 | 135.9 ± 27.4 | 0.279 |
| PreHD DBP (mmHg) | 62.8 ± 15.3 | 68.0 ± 19.4 | 59.5 ± 15.1 | 67.0 ± 8.4 | 55.1 ± 14.6 | 0.18 |
| 23Na MRI | ||||||
| Skin [Na+] (mmol/L) | 30.8 ± 11.3 | 17.4 ± 3.2 | 25.7 ± 2.5 | 31.7 ± 1.7 | 46.1 ± 8.6 | <0.001 |
| Laboratory analyses | ||||||
| Corrected serum [Na+] (mmol/L) | 137.9 ± 3.3 | 136.2 ± 5.1 | 136.3 ± 2.1 | 139.2 ± 2.1 | 139.3 ± 2.6 | <0.05 |
| Serum [K+] (mmol/L) | 4.4 ± 0.7 | 4.6 ± 0.9 | 4.2 ± 0.7 | 4.4 ± 0.7 | 4.4 ± 0.6 | 0.59 |
| Serum [HCO3−] (mmol/L) | 24.9 ± 4.0 | 22.9 ± 3.3 | 26.5 ± 3.9 | 26.4 ± 3.2 | 23.3 ± 4.6 | <0.05 |
| Serum creatinine (umol/L) | 630.5 ± 231.8 | 735.7 ± 271.2 | 614.0 ± 192.3 | 603.4 ± 240.9 | 589.3 ± 221.8 | 0.409 |
| Serum urea (mmol/L) | 19.1 ± 6.8 | 24.1 ± 6.3 | 17.0 ± 4.6 | 17.0 ± 6.8 | 19.2 ± 7.2 | <0.05 |
| Serum glucose (mmol/L) | 8.5 ± 3.9 | 8.0 ± 3.5 | 7.7 ± 2.3 | 9.0 ± 5.4 | 9.0 ± 3.5 | 0.759 |
| Serum albumin (g/L) | 39.9 ± 3.9 | 42.8 ± 3.8 | 41.1 ± 2.2 | 39.1 ± 3.6 | 37.2 ± 3.9 | <0.01 |
| Hemoglobin (g/L) | 112.4 ± 14.2 | 116.8 ± 16.8 | 113.2 ± 13.9 | 113.5 ± 13.2 | 106.5 ± 13.1 | 0.339 |
Data are expressed as mean ± SD unless otherwise noted. ACEi: angiotensin-converting enzyme inhibitors; ARB: angiotensin II receptor blocker; BMI: body mass index; CCI: Charlson comorbidity index; DBP: diastolic blood pressure; IDWG: interdialytic weight gain; preHD: pre-dialysis; SBP: systolic blood pressure; spKt/V: single-pool Kt/V; UFR: ultrafiltration rate.
FIGURE 1:
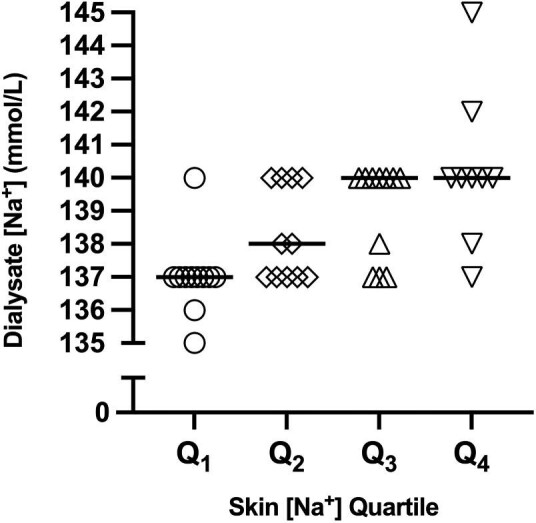
Dialysate [Na+] prescription in HD patients, according to skin [Na+] quartiles. The data are presented as median (bars) and individual values.
The distribution of PD patients was skewed toward the upper skin [Na+] quartiles. This determined a trend toward a greater residual urine output in Q3–Q4, as PD patients had a significantly greater residual urine output compared with HD patients [median (IQR): 1125 (600–1575) versus 200 (0–400) mL/24 h, Mann–Whitney U-test: P < 0.001]. HD patients were scanned after a median 22.5 (IQR: 20.2–24.8) h after their last HD session. In a minority of participants (6/42 cases), MRI was performed during the long interdialytic interval (between the third and before the first HD session of the week), after [median (IQR)] 45.9 (44.5–47.4) h from the previous HD session. In the remaining cases (36/42) MRI was performed mid-week during a short HD interval, between either the first and second or second and third HD session, after [median (IQR)] 21.8 (19.8–23.3) h from the previous HD session. No significant differences in time were observed among skin [Na+] quartiles.
Linear regression models
Univariate linear regression yielded the following results (Table 2): dialysate [Na+] was significantly and positively associated with skin [Na+] [F(1,40) = 26.79, R2 = 0.40, unstandardized β = 3.79; P < 0.001]; serum albumin was significantly and negatively associated with skin [Na+] [F(1,50) = 12.62, R2 = 0.20, unstandardized β = –1.30; P < 0.001]; corrected serum [Na+] was significantly and positively associated with skin [Na+] [F(1,50) = 10.214, R2 = 0.17, unstandardized β = 1.39; P < 0.01]. The relationship between skin [Na+] and age did not reach statistical significance [F(1,50) = 3.443, R2 = 0.06, unstandardized β = 0.28; P = 0.07].
Table 2.
Multiple linear regression models to explain skin [Na+]
| Model 1: dependent variable: skin [Na+] (HD) F(4,37) = 17.82, R2 = 0.66, R2Adj = 0.62; P < 0.001 | ||
|---|---|---|
| Independent variable | Unstandardized β | P |
| (Intercept) | –453.62 | <0.001 |
| Dialysate [Na+] | 3.33 | <0.001 |
| Serum albumin | –1.18 | <0.001 |
| Congestive heart failure | 7.07 | <0.01 |
| Corrected serum [Na+] | 0.50 | 0.18 |
| Model 2: dependent variable: skin [Na+] (HD and PD) F(4,47) = 9.573, R2 = 0.45, R2Adj = 0.40; P < 0.001 |
||
| Independent variable | Unstandardized β | P |
| (Intercept) | –124.83 | <0.05 |
| Corrected serum [Na+] | 1.29 | <0.01 |
| Serum albumin | –0.89 | <0.05 |
| Congestive heart failure | 9.33 | <0.01 |
| Age | 0.17 | 0.17 |
The results of the multiple linear regression analysis are summarized in Table 2. Using the enter method, Model 1 explained a significant amount of the variance in skin [Na+] in HD patients [F(4,37) = 17.82, P < 0.001, R2 = 0.66, R2adj = 0.62]. In particular, the following predictors were statistically significant: dialysate [Na+] (unstandardized β = 3.33; P < 0.001), serum albumin (unstandardized β = –1.18; P < 0.001) and comorbid congestive heart failure (unstandardized β = 7.07; P < 0.01).
Model 2 included variables from both HD and PD patients, and was significant as well [F(3,48) = 7.35, P < 0.001, R2 = 0.32, R2adj = 0.27]. Compared with Model 1, after the removal of dialysate [Na+], corrected serum [Na+], serum albumin (unstandardized β = –1.11; P < 0.01) and comorbid congestive heart failure (unstandardized β = 7.76; P < 0.05) were statistically significant explanatory variables of skin [Na+].
Outcomes
Follow-up and survival times, censoring and clinical events are summarized in Table 3. Of note, the median follow-up time for the overall sample was 529 days (IQR: 353–602). A total of 15 deaths and 7 MACE were observed during the follow-up period. A total of 11 participants were right-censored after a median of 215 days (IQR: 99–289) due to kidney transplantation.
Table 3.
Summary of follow-up times, survival and clinical events, in the overall study sample and after skin [Na+] quartile stratification
| Overall | Q1 | Q2 | Q3 | Q4 | |
|---|---|---|---|---|---|
| Follow-up time, median (IQR) | 529 (353–602) | 546 (515–592) | 505 (292–533) | 588 (421–685) | 544 (133–577) |
| Mean est. survival, mean (95% CI) | 847 (716–975) | 1020 (901–1140) | 873 (587–1159) | 875 (663–1087) | 657 (420–895) |
| Mean est. composite event-free survival, mean (95% CI) | 741 (602–880) | 994 (827–1160) | 754 (468–1041) | 667 (473–861) | 537 (301–774) |
| Clinical events | |||||
| Kidney transplant (n) | 11 | 3 | 2 | 3 | 3 |
| MACE (n) | 7 | 1 | 1 | 2 | 3 |
| Death (n) | 15 | 1 | 3 | 4 | 7 |
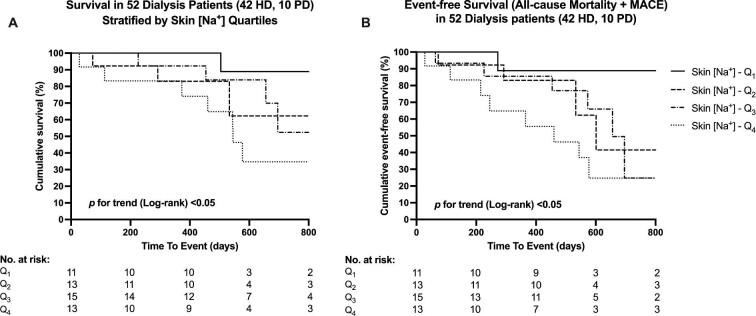
For the endpoint of all-cause mortality, survival distributions for the skin [Na+] quartiles significantly differed (log-rank χ2(1) = 3.926, P for trend < 0.05) (Figure 2A) as well as event-free survival distributions for the composite endpoint of death and MACE (log-rank χ2(1) = 5.685, P for trend <0.05) (Figure 2B). Mean survival and event-free survival times for each skin [Na+] quartile group are summarized in Table 3.
FIGURE 2:
Kaplan–Meier curves for overall survival (A) and event-free survival as a composite of all-cause mortality and MACE (B) after skin [Na+] quartile stratification.
According to univariate Cox proportional hazards regression (Table 4), skin [Na+] was significantly associated with all-cause mortality as a continuous variable {HR/10 mmol/L skin [Na+] increments: 1.832 [95% confidence interval (95% CI) 1.234–2.720]} and with composite events, both as a continuous variable and as a categorical variable [HR/10 mmol/L skin [Na+] increments: 1.722 (95% CI 1.226–2.418); HR per skin [Na+] quartile increment: 1.767 (95% CI 1.089–2.866)]. In multivariate models, skin [Na+] was associated with all-cause mortality after adjusting for age, sex, corrected serum [Na+] and serum albumin [HR/10 mmol/L skin [Na+] increments: 4.013 (95% CI 1.988–8.101); P < 0.001; HR per skin [Na+] quartile increment: 3.180 (95% CI 1.314–7.700); P < 0.05]. Similarly, skin [Na+] was also associated with composite all-cause mortality and MACE [HR per 10 mmol/L skin [Na+] increments: 2.332 (95% CI 1.378–3.945); P < 0.01; HR per skin [Na+] quartile increment: 2.038 (95% CI 1.058–3.925); P < 0.05].
Table 4.
Cox proportional hazard regression to model the association between skin [Na+] and clinical outcomes, before and after confounder adjustment
| Endpoint: all-cause mortality | Univariate | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| Variables | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Skin [Na+] (per 10 mmol/L) | 1.832 (1.234–2.720) | <0.01 | 4.013 (1.988–8.101) | <0.001 | ||
| Skin [Na+] (per quartile) | 1.709 (0.989–2.954) | 0.055 | 3.180 (1.314–7.700) | <0.05 | ||
| Age | 1.014 (0.965–1.065) | 0.584 | 0.996 (0.934–1.063) | 0.912 | 1.002 (0.941–1.067) | 0.949 |
| Sex (female versus male) | 0.225 (0.063–0.805) | <0.05 | 0.156 (0.039–0.630) | <0.01 | 0.217 (0.059–0.796) | <0.05 |
| Corrected serum [Na+] | 0.975 (0.854–1.113) | 0.708 | 0.711 (0.559–0.904) | <0.01 | 0.786 (0.621–0.995) | <0.05 |
| Serum albumin | 0.971 (0.838–1.124) | 0.692 | 1.031 (0.883–1.202) | 0.702 | 1.099 (0.919–1.313) | 0.302 |
| Endpoint: all-cause mortality + MACE | Univariate | Model 1 | Model 2 | |||
| Variables | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Skin [Na+] (per 10 mmol/L) | 1.722 (1.226–2.418) | <0.01 | 2.332 (1.378–3.945) | <0.01 | ||
| Skin [Na+] (per quartile) | 1.767 (1.089–2.866) | <0.05 | 2.038 (1.058–3.925) | <0.05 | ||
| Age | 1.023 (0.979–1.068) | 0.309 | 0.996 (0.946–1.050) | 0.894 | 1.001 (0.950–1.055) | 0.959 |
| Sex (female versus male) | 0.315 (0.112–0.885) | <0.05 | 0.291 (0.099–0.859) | <0.05 | 0.314 (0.110–0.898) | <0.05 |
| Corrected serum [Na+] | 1.046 (0.912–1.201) | 0.518 | 0.849 (0.695–1.036) | 0.107 | 0.921 (0.759–1.117) | 0.404 |
| Serum albumin | 0.926 (0.808–1.062) | 0.273 | 0.979 (0.846–1.133) | 0.775 | 1.012 (0.862–1.189) | 0.880 |
DISCUSSION
We report for the first time that increasing skin [Na+] is associated with increased mortality and MACE. Dialysate [Na+], corrected serum [Na+], serum albumin and congestive heart failure were significant clinical predictors of skin [Na+] in dialysis patients.
The main finding of this study is that skin sodium accumulation is associated with worse clinical outcomes, potentially as the result of a combination of processes depositing sodium within tissues. The simplest explanation for this finding is that noninvasive tissue sodium detection by 23Na MRI reflects the degree of tissue congestion/edema. From a mechanistic perspective, this explanation is supported by previous experimental evidence. A recent comprehensive study in rats and humans showed that tissue [Na+] in rats and skin [Na+] in humans is paralleled by water content (‘‘isotonic’’ sodium accumulation) as a direct function of extracellular volume expansion, and inversely with intracellular volume and potassium concentration [13]. Similar data have been shown in a mathematical model relating total tissue [Na+] with extracellular volume [2]. However, these observations are derived from animals/subjects without significant CKD and impaired ability to excrete sodium.
Noninvasive, human-based 23Na MRI studies confirmed the close relationship between sodium and water at the tissue level; both diuretics [6, 7] and ultrafiltration with HD [8] have been associated with tissue [Na+] reduction. At the epidemiological level, clinical outcomes are correlated with surrogate measures of extracellular volume expansion, such as fluid overload detected with bioimpedance spectroscopy [3, 4] and lung ultrasound [14].
In this study, the hypothesis of the relationship between sodium and water at the skin level is supported by the significant explanatory role of serum albumin and comorbid congestive heart failure on skin [Na+]. Indeed, these two factors are associated with positive net capillary filtration according to the Starling equation: increased plasma hydrostatic pressure (congestive heart failure) and reduced plasma oncotic pressure (hypoalbuminemia).
However, the additional, explanatory role of dialysate [Na+] suggests that sodium accumulation may also occur in excess of water, as shown by several preclinical studies [15]. Increased sodium intake in rats was associated with increased skin [Na+] and followed by lymph capillary expansion via macrophage-mediated, tonicity-sensitive vascular endothelial growth factor C secretion; dysfunction in this system was associated with increased skin [Na+] and the downregulation of the endothelial nitric oxide synthase [16]. In humans, we recently showed that chronic HD patients receiving a dialysate [Na+] of 140 mmol/L had a larger skin [Na+] compared with patients receiving a dialysate [Na+] of 137 mmol/L, based on different unit prescription protocols [17]. This evidence suggests that skin [Na+] accumulation resulting from intradialytic positive diffusive sodium balance may be an additional factor leading to worse clinical outcomes. Although we cannot discern the pathophysiological mechanisms linking water-free skin [Na+] with increased mortality and cardiovascular events, recent studies have suggested that skin [Na+] may exert `toxic' effects on the cardiovascular system, such as blood pressure and left ventricular mass, independently of extracellular volume [5, 16].
Hypoalbuminemia is a well-known predictor for all-cause mortality in dialysis patients; according to our results, we speculate that the relationship between low serum albumin and clinical outcomes is mediated by overt or subclinical tissue congestion. Indeed, we showed a continuous negative relationship between serum albumin and skin [Na+], so that even small reductions in serum albumin were associated with larger skin [Na+]—likely as the result of reduced plasma oncotic pressure [18]. Other potential mechanisms linking hypoalbuminemia with clinical outcomes in chronic dialysis patients may be related with reduced albumin synthesis due to inflammation and reduced protein–calorie intake [19], and impaired endothelial barrier with resulting transcapillary escape rate of albumin and intercompartmental albumin redistribution [20]. Unlike previous studies [8, 12], we did not find a significant correlation between age and skin [Na+] as well as clinical outcomes. This is likely the result of several combined factors: (i) small study sample, (ii) limited age range in the study population and (iii) the greater influence of other clinical and sodium-related variables, suggesting the primary relevance of volume and sodium balance in the dialysis population.
Several limitations must be considered when interpreting the present study findings. This is an observational, exploratory study, and other potentially relevant confounders associated with outcomes have not been included in the statistical models due to the small sample size and related lack of statistical power. Out of 42 HD patients, 8 were receiving individualized dialysate [Na+] prescription; although dialysate [Na+] was significantly associated with skin [Na+], it is possible the clinical factors underlying dialysate [Na+] individualization may have influenced outcomes. We assumed that an interdialytic, cross-sectional evaluation of skin [Na+] would be associated with clinical outcomes, assuming no longitudinal changes in skin [Na+] during the follow-up period. Evaluation of all-cause mortality and MACE was limited by the small number of patients included in the study, right censoring by kidney transplantation and short follow-up times.
Due to the small and unequal sample size, it was not possible to differentiate the impact of different dialytic techniques (HD versus PD) on skin [Na+] and clinical outcomes. Inherently different volume and sodium removal strategies associated with these techniques, however, might have a separate impact on skin [Na+] and clinical outcomes. Additional studies are required to confirm this hypothesis.
In conclusion, higher skin [Na+] (as quantified with 23Na MRI) was associated with worse clinical outcomes in dialysis patients. Although potential unaccounted confounding effects may be at play, these findings suggest sodium balance may play an important role in the care of dialysis patients, from both a traditional view (with expansion of extracellular volume) and as a function of our evolving understanding of water-free sodium accumulation within tissues. Future studies investigating the role of skin [Na+] as a quantitative imaging biomarker for risk stratification, therapy optimization and as a potential direct therapeutic target are necessary.
ACKNOWLEDGEMENTS
The authors would like to thank Tanya Tamasi, Justin Dorie and Patricia Jarosz for their support in this study.
Contributor Information
Fabio R Salerno, Department of Medical Biophysics, Western University, London, ON, Canada; The Lilibeth Caberto Kidney Clinical Research Unit, London Health Sciences Centre, London, ON, Canada.
Alireza Akbari, The Lilibeth Caberto Kidney Clinical Research Unit, London Health Sciences Centre, London, ON, Canada; Robarts Research Institute, Western University, London, ON, Canada.
Sandrine Lemoine, The Lilibeth Caberto Kidney Clinical Research Unit, London Health Sciences Centre, London, ON, Canada; Claude Bernard Lyon 1 University, Lyon, France.
Guido Filler, The Lilibeth Caberto Kidney Clinical Research Unit, London Health Sciences Centre, London, ON, Canada; Departments of Pediatrics, London Health Sciences Centre, London, ON, Canada.
Timothy J Scholl, Department of Medical Biophysics, Western University, London, ON, Canada; Robarts Research Institute, Western University, London, ON, Canada.
Christopher W McIntyre, Department of Medical Biophysics, Western University, London, ON, Canada; The Lilibeth Caberto Kidney Clinical Research Unit, London Health Sciences Centre, London, ON, Canada; Division of Nephrology, London Health Sciences Centre, London, ON, Canada.
FUNDING
This study was funded by the Can-SOLVE CKD Network and the Canadian Institutes of Health Research Strategy for Patient-Oriented Research.
AUTHORS’ CONTRIBUTIONS
F.R.S. finalized the study database, analyzed the images and the data, and drafted the manuscript. A.A. developed and implemented the 23Na MRI acquisition and reconstruction technique, produced the concentration maps and mentored the image analysis process. S.L. was responsible for database maintenance, and provided intellectual insight on imaging and data analysis. G.F. provided intellectual insight on result interpretation. T.J.S. provided mentoring and technical insight for the 23Na MRI development and image acquisition. C.W.M. conceived the original study idea, was medically responsible for the study, provided funding and supervision, verified the validity of the study data and reviewed the final manuscript. All authors contributed to and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest relevant to the submitted work. The results presented in this paper have not been published previously in whole or part, except in abstract format.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Kopp C, Linz P, Wachsmuth Let al. (23)Na magnetic resonance imaging of tissue sodium. Hypertension 2012; 59: 167–172 [DOI] [PubMed] [Google Scholar]
- 2. Rossitto G, Touyz RM, Petrie MCet al. Much ado about N... atrium: modelling tissue sodium as a highly sensitive marker of subclinical and localised oedema. Clin Sci (Lond) 2018; 132: 2609–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zoccali C, Moissl U, Chazot Cet al. Chronic fluid overload and mortality in ESRD. J Am Soc Nephrol 2017; 28: 2491–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dekker MJE, Marcelli D, Canaud BJet al. Impact of fluid status and inflammation and their interaction on survival: a study in an international hemodialysis patient cohort. Kidney Int 2017; 91: 1214–1223 [DOI] [PubMed] [Google Scholar]
- 5. Schneider MP, Raff U, Kopp Cet al. Skin sodium concentration correlates with left ventricular hypertrophy in CKD. J Am Soc Nephrol 2017; 28: 1867–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hammon M, Grossmann S, Linz Pet al. 23Na magnetic resonance imaging of the lower leg of acute heart failure patients during diuretic treatment. PLoS One 2015; 10: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karg MV, Bosch A, Kannenkeril Det al. SGLT-2-inhibition with dapagliflozin reduces tissue sodium content: a randomised controlled trial. Cardiovasc Diabetol 2018; 17: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dahlmann A, Dörfelt K, Eicher Fet al. Magnetic resonance-determined sodium removal from tissue stores in hemodialysis patients. Kidney Int 2015; 87: 434–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagel AM, Laun FB, Weber MAet al. Sodium MRI using a density-adapted 3D radial acquisition technique. Magn Reson Med 2009; 62: 1565–1573 [DOI] [PubMed] [Google Scholar]
- 10. Qirjazi E, Salerno FR, Akbari Aet al. (April 6 2020) Tissue sodium concentrations in chronic kidney disease and dialysis patients by lower leg sodium-23 magnetic resonance imaging. Nephrol Dial Transplant, doi: 10.1093/ndt/gfaa036 [DOI] [PubMed] [Google Scholar]
- 11. Katz MA. Hyperglycemia-induced hyponatremia––calculation of expected serum sodium depression. N Engl J Med 1973; 289: 843–844 [DOI] [PubMed] [Google Scholar]
- 12. Sahinoz M, Tintara S, Deger SMet al. Tissue sodium stores in peritoneal dialysis and hemodialysis patients determined by 23-sodium magnetic resonance imaging. Nephrol Dial Transplant 2020; 36: 1307–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rossitto G, Mary S, Chen JYet al. Tissue sodium excess is not hypertonic and reflects extracellular volume expansion. Nat Commun 2020; 11: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zoccali C, Tripepi R, Torino Cet al. Lung congestion as a risk factor in end-stage renal disease. Blood Purif 2014; 36: 184–191 [DOI] [PubMed] [Google Scholar]
- 15. Titze J, Lang R, Ilies Cet al. Osmotically inactive skin Na+ storage in rats. Am J Physiol Physiol 2003; 285: F1108–F1117 [DOI] [PubMed] [Google Scholar]
- 16. MacHnik A, Neuhofer W, Jantsch Jet al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 2009; 15: 545–552 [DOI] [PubMed] [Google Scholar]
- 17. Lemoine S, Salerno FR, Akbari Aet al. Influence of dialysate sodium prescription on skin and muscle sodium concentration. Am J Kidney Dis 2021; 78: 156–159 [DOI] [PubMed] [Google Scholar]
- 18. Bhave G, Neilson EG. Body fluid dynamics: back to the future. J Am Soc Nephrol 2011; 22: 2166–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gama-Axelsson T, Heimbürger O, Stenvinkel Pet al. Serum albumin as predictor of nutritional status in patients with ESRD. Clin J Am Soc Nephrol 2012; 7: 1446–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu Z, Tan BK, Dainty Set al. Hypoalbuminaemia, systemic albumin leak and endothelial dysfunction in peritoneal dialysis patients. Nephrol Dial Transplant 2012; 27: 4437–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.