ABSTRACT
Background
Cancer risk is increased by 2- to 4-fold in kidney transplant recipients (KTRs) compared with the general population. Little attention, however, has been given to KTRs with ultra long-term survival >20 years.
Methods
We studied 293 of 1241 KTRs (23.6%), transplanted between 1981 and 1999, who showed kidney allograft survival >20 years. These long-term survivors were analysed for cancer development, cancer type, cancer-associated risk factors and patient and allograft outcomes.
Results
By 10, 20 and 30 years post-transplantation, these long-term KTRs showed a cancer rate of 4.4%, 14.6% and 33.2%, and a non-melanoma skin cancer (NMSC) rate of 10.3%, 33.5% and 76.8%, respectively. By recipients’ ages of 40, 60 and 80 years, KTRs showed a cancer rate of 3.4%, 14.5% 55.2%, and a NMSC rate of 1.7%, 31.6% and 85.2%, respectively. By 30 years post-transplantation, post-transplant lymphoproliferative disorder (PTLD) showed the highest incidence of 8.5%, followed by renal cell carcinoma (RCC) with 5.1%. Risk factors associated with the development of cancer were only recipient age (P = 0.016). Smoking history was associated with the risk of lung cancer (P = 0.018). Risk factors related to the development of NMSC included recipient age (P = 0.001) and thiazide diuretics (P = 0.001). Cancer increased the risk of death by 2.4-fold (P = 0.002), and PTLD increased the risk of kidney allograft loss by 6.5-fold (P = 0.001). No differences were observed concerning the development of donor-specific antibodies (P > 0.05).
Conclusions
In long-term KTRs, cancer is a leading cause of death. PTLD remains the most common cancer type followed by RCC. These results emphasize the need for focused long-term cancer surveillance protocols.
Keywords: cancer, kidney transplantation, non-melanoma skin cancer, PTLD
INTRODUCTION
Since kidney transplantation has become the standard treatment for end-stage kidney disease, the number of kidney transplant recipients (KTRs) dependent on maintenance immunosuppression has substantially increased [1–3]. The improved life expectancy of KTRs compared with dialysis patients and the improvement of long-term survival contribute to this observation [4, 5]. Five-year deceased-donor patient and kidney allograft survival rates in Europe improved to 87.1% and 77.8%, respectively [6, 7]. In addition, the Collaborative Transplant Study (CTS) reports an estimated 20-year allograft survival as high as 41% for first deceased-donor KTRs between 1990 and 2019, and an estimated allograft half-life of 16.8 years [7]. This improvement is due to more potent immunosuppressive drugs that strongly decreased acute rejection rates [8, 9]. As a result of these improved therapy options, increasing incidences of cancer have been observed [8–10]. Several studies have reported that the incidence of post-transplant cancer ranges between 2% and 31% depending on the follow-up time and cancer type, and increases up to 50% in KTRs followed up for a longer time [11, 12].
In short-term studies, cancer-associated risk factors in KTRs included recipient age, duration under immunosuppression and the duration of dialysis treatment [11, 12]. In contrast to the general population, the overall level of immunosuppression appears to be the principal factor that increases post-transplant cancer risk [5]. Calcineurin inhibitors (CNIs) themselves may promote cancer progression, principally via the production of transforming growth factor-beta [13]. The use of azathioprine has been associated with neoplastic development, particularly an increased risk of non-melanoma skin cancer (NMSC) [5] via inhibition of DNA repair mechanisms [14]. Interestingly, some studies suggest that the risk of developing cancer is not higher with mycophenolate mofetil (MMF) and may be associated with a trend for decreased risk [5, 15, 16]. In addition, impaired immune responses to oncogenic viruses like Epstein–Barr virus (EBV), human papillomavirus (HPV), human herpesvirus-8 (HHV8) or polyomavirus BK contribute to the increased cancer risk after kidney transplantation [11, 12].
Considering this development, surprisingly little attention has been given to ultra long-term survivors [1–3]. The literature has mainly focused on the first decade post-transplant, and none of the registry reports reveals further data on ultra long-term survivors [6, 7, 17–19]. Short-term studies showed an increased risk of a wide range of cancers associated with kidney transplantation: a 2-fold higher risk of colorectal, lung, prostate, stomach, esophagus, pancreas, ovarian and breast cancer; a 3-fold higher risk of testicular and bladder cancer; a 5-fold higher risk of melanoma, leukaemia, hepatobiliary, cervical and vulvovaginal cancer; a 15-fold higher risk of renal cell carcinoma (RCC); and a 20-fold higher risk of Kaposi's sarcoma, NMSC and post-transplant lymphoproliferative disorder (PTLD) than the general population [20]. Although recently, colleagues from the Swiss Transplant Cohort Study (STCS) suggested a very low incidence of PTLD with a cumulative incidence at 5 years of 0.96% [21, 22], long-term outcomes were not studied.
Due to the lack of studies in ultra long-term KTRs, we analysed whether the distribution of cancer rates and types, known from registry analyses for the short- and medium-term, is also true for the ultra long-term. We attempted to address the following questions: (i) What is the cancer rate among KTRs with kidney allograft survival >20 years? (ii) What are the cancer types among KTRs with kidney allograft survival >20 years? (iii) What are the risk factors for cancer among KTRs with kidney allograft survival >20 years? (iv) What are the patient and kidney allograft outcomes among KTRs with kidney allograft survival >20 years?
MATERIALS AND METHODS
Patients
Our study was approved by the cantonal ethic commission review board of Zurich, Switzerland (KEK-ZH-Number 2019-02082) and has been conducted in compliance with the declaration of Helsinki. We performed a retrospective data analysis at our single transplant centre at the University Hospital Zurich. We studied a total of 304 of 1241 KTRs (24.5%), who were transplanted between 1 January 1981 and 31 December 1999, and showed kidney allograft survival of at least 20 years. A total of 11 of 304 KTRs (3.6%) with kidney allograft survival >20 years were lost-to-follow-up, and were not included in our analysis, leaving 293 KTRs (23.6%) for analysis.
Post-transplant care was carried out according to our standardized scheme with appointments in the outpatient clinic quarterly or yearly. KTRs were either followed three-monthly (n = 95) in our transplant centre or three-monthly by external nephrologists, and only annually in our transplant centre (n = 198).
For data collection, we reviewed medical records from the electronic database of the hospital registry. The end of follow-up and data collection was 31 December 2020. To evaluate characteristics 20 years after transplantation, we identified the 20 years post-transplantation visit for each KTR, defined as the closest and most complete visit to the date of transplantation plus 20 years. We analysed patient outcomes concerning the development of cancer, cancer type, patient survival, kidney allograft survival and the development of de novo donor-specific antibodies (DSA). A total of 231 KTRs underwent regular screening for anti-human leukocyte antigen (HLA) antibodies starting in the year 2009. The anti-HLA antibody testing was performed annually using a Luminex-based assay (One Lambda, Canoga Park, CA, USA) and at any other time in case of unexplained deterioration of allograft function. Patient characteristics at transplantation and at 20 years after kidney transplantation are shown in Tables 1 and 2.
Table 1.
Basic characteristics of KTRs with >20 years of kidney allograft survival
| Number (%) | All >20 years KTRs, n = 293 | KTRs with cancer, n = 74 | KTRs without cancer, n = 219 | P-value |
|---|---|---|---|---|
| Recipient age at transplantation (years)a | 39 (17–68) | 41 (17–62) | 37 (17–68) | 0.203 |
| Recipient male sex, n (%) | 183 (62) | 46 (62) | 137 (63) | 1 |
| Donor age (years)a | 32 (3–73) | 31 (5–72) | 33 (3–68) | 0.702 |
| Donor male sex, n (%) | 186 (66) | 50 (70) | 136 (62) | 0.748 |
| Number of kidney transplantations, n (%) | ||||
| 1 | 265 (90) | 71 (96) | 194 (89) | 0.069 |
| ≥2 | 28 (10) | 3 (4) | 25 (11) | |
| Causes of ESRD, n (%) | ||||
| Glomerulonephritis | 107 (37) | 33 (45) | 74 (34) | 0.307 |
| Diabetic nephropathy | 6 (2) | 1 (1) | 5 (2) | 1 |
| Vasculitis | 10 (3) | 0 (0) | 10 (5) | 0.126 |
| Polycystic kidney disease | 35 (12) | 11 (15) | 24 (11) | 0.420 |
| Uropathy/CAKUT | 53 (18) | 12 (16) | 41 (19) | 0.734 |
| Analgesic nephropathy | 13 (4) | 2 (3) | 11 (5) | 0.530 |
| Hypertensive nephropathy | 4 (1) | 0 (0) | 4 (2) | 0.575 |
| Other | 29 (10) | 7 (9) | 22 (10) | 1 |
| undetermined | 36 (12) | 8 (11) | 28 (13) | 0.839 |
| Time on dialysis (months)a | 26 (0–168) | 30 (0–168) | 24 (0–104) | 0.036a |
| Pretransplant cancer, n (%) | 4 (1) | 0 (0) | 4 (1) | 0.575 |
aMedian (range). CAKUT, congenital anomalies of kidney and urinary tract.
Table 2.
Basic characteristics at 20 years after kidney transplantation
| All KTRs, | KTRs with cancer, | KTRs without cancer, | ||
|---|---|---|---|---|
| n = 293 | n = 74 | n = 219 | P-value | |
| Immunosuppression, n (%) | ||||
| Calcineurin-inhibitor | ||||
| Ciclosporin | 235 (80) | 58 (78) | 177 (81) | 0.736 |
| Tacrolimus | 45 (15) | 12 (16) | 33 (15) | 0.853 |
| Antimetabolite | ||||
| MMF/MPA | 158 (54) | 40 (54) | 118 (54) | 1 |
| Azathioprine | 113 (39) | 30 (41) | 83 (38) | 0.681 |
| Steroid | 100 (34) | 36 (49) | 73 (33) | 0.132 |
| Other medication | ||||
| Acetylsalicylic acid, n (%) | 81 (28) | 21 (28) | 60 (27) | 0.886 |
| Proton pump inhibitors, n (%) | 71 (24) | 16 (22) | 55 (25) | 0.759 |
| Statin, n (%) | 162 (55) | 40 (54) | 122 (56) | 0.911 |
| Antihypertensives, n (%) | ||||
| 0 | 58 (20) | 19 (26) | 39 (19) | 0.176 |
| 1 | 93 (32) | 19 (26) | 74 (34) | 0.404 |
| 2 | 96 (33) | 22 (30) | 74 (34) | 0.685 |
| >2 | 46 (16) | 14 (19) | 32 (15) | 0.472 |
| Diuretics, n (%) | ||||
| Thiazide diuretics | 24 (8) | 9 (12) | 15 (7) | 0.150 |
| Loop diuretics | 60 (20) | 21 (28) | 39 (18) | 0.150 |
| Smoking, n (%) | 43 (15) | 14 (19) | 19 (9) | 0.040* |
| Diabetes mellitus, n (%) | 61 (21) | 20 (27) | 41 (19) | 0.264 |
| Insulin therapy, n (%) | 24 (8) | 8 (11) | 16 (7) | 0.466 |
Maintenance immunosuppression
The primary immunosuppression consisted of a triple-drug combination of a CNI, tacrolimus or cyclosporine, antimetabolite [MMF, mycophenolic acid (MPA) or azathioprine] and steroids. Tacrolimus trough levels were maintained at 4–6 µg/L, and cyclosporine trough levels at 50–80 µg/L over the long-term. The daily dosage of MMF was 500–2000 mg/day, the daily dose of MPA was 360–1440 mg/day and the daily dose of azathioprine was 0.5–1.5 mg/kg body weight. Steroids were maintained at a dose of 5 mg prednisone/day or withdrawn according to the immunologic risk.
Assessment of cancer rates and types
As part of transplant follow-up, all KTRs undergo a regular cancer screening. This includes annual skin cancer screening. In addition, a physical examination, sonography of the transplanted kidney and sonography of the patient's own kidneys take place as part of the annual check-up. A urinalysis is performed at each visit, and a urological workup is initiated in the case of non-glomerular haematuria. Annual gynaecological and urological examinations and colon cancer screening occur according to the general population's recommendations.
Statistical methods
Statistical analysis was performed using the IBM SPSS Version 25 (SPSS, Chicago, IL, USA). For comparisons of study groups, the Mann–Whitney U-test was used for nonparametric independent samples. Outcomes were measured with the Kaplan–Meier models, and log-rank tests measured overall strata comparisons. Clinical characteristics were compared across groups using the Fisher's exact test for categorical variables. Univariate and multivariate Cox proportional hazards models were used to investigate factors associated with the development of cancer. Cox proportional hazards models with the time of cancer as the time-dependent covariate were used to study cancer's impact on patients and kidney allograft outcomes. A significance level of P < 0.05 was considered statistically significant.
RESULTS
Overall patient characteristics
A total of 293 KTRs were included in this study. KTRs were followed over at least 20 years. During the whole study period, a total of 74 KTRs (25.3%) developed cancer (excluding NMSC). In addition, a total of 145 KTRs (49.5%) developed NMSC. A total of 48 of the 74 patients with cancer (64.9%) also developed NMSC. A total of 4 of 293 KTRs (1.4%) had pre-transplant cancer, 2 KTRs with seminoma, 1 KTR with breast cancer and 1 KTR with Hodgkin lymphoma; of those, none developed post-transplant cancer. Recipient age at transplantation and previous time on dialysis was associated with higher cancer risk in long-term follow-ups (P < 0.05). The characteristics of KTRs are shown in Table 1 and Supplementary data, Table S1.
Cancer rates among KTRs with ultra long-term kidney allograft survival
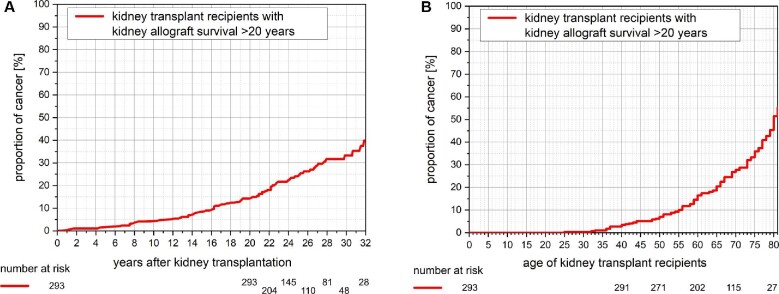
KTRs' characteristics at 20 years post-transplantation are shown in Table 2. Throughout 10, 20 and 30 years post-transplantation, KTRs showed an incidence of cancer of 4.4%, 14.6% and 33.2%, respectively (Figure 1A). Similarly, by recipients’ ages of 40, 60 and 80 years, KTRs showed an incidence of cancer of 3.4%, 14.5% and 55.2%, respectively (Figure 1B). Upon multivariate Cox regression analysis, recipient age further increased the risk of cancer {hazard ratio 1.026 [95% confidence interval (95% CI) 1.005–1.047]; P = 0.016}. Smoking (95% CI 0.809–2.592; P = 0.213) and time on dialysis (95% CI 0.992–1.007; P = 0.953) did not impact cancer risk upon multivariate Cox regression analysis.
Figure 1:
(A) Kaplan–Meier plot of cancer development among KTRs with kidney allograft survival >20 years by time after transplantation. The incidence of cancer increases after kidney transplantation with a proportion of 4.4%, 14.6% and 33.2% at 10, 20 and 30 years after transplantation, respectively. (B) Kaplan–Meier plot of cancer development among KTRs with kidney allograft survival >20 years by recipient age. The incidence of cancer increases by recipient age with a proportion of 3.4%, 14.5% and 55.2% at 40, 60 and 80 years of age, respectively.
NMSC rates among KTRs with ultra long-term kidney allograft survival
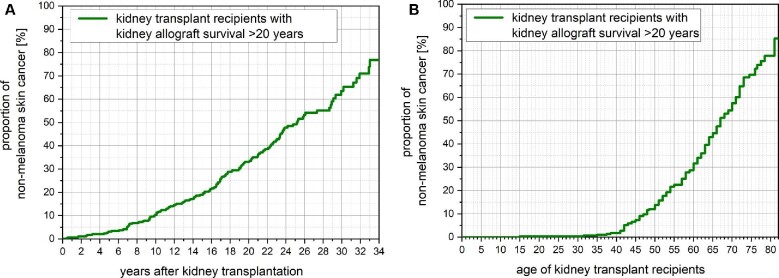
KTRs' characteristics at 20 years post-transplantation are shown in Supplementary data, Table S2. By 10, 20 and 30 years post-transplantation, KTRs showed an NMSC rate of 10.3%, 33.5% and 76.8%, respectively (Figure 2A). Similarly, by recipients’ ages of 40, 60 and 80 years, KTRs showed an incidence of NMSC of 1.7%, 31.6% and 85.2%, respectively (Figure 2B). Upon multivariate Cox regression analysis, recipient age further increased the risk of NMSC [hazard ratio 1.040 (95% CI 1.025–1.057); P < 0.001]. Interestingly, the use of thiazide diuretics showed an increased risk of NMSC [hazard ratio 2.772 (95% CI 1.662–4.624); P = 0.001] upon multivariate Cox regression analysis (Supplementary data, Figure S2). NMSC was significantly more common among KTRs who developed cancer (64% versus 45%; P = 0.003).
Figure 2:
(A) Kaplan–Meier plot of the development of NMSC among KTRs with kidney allograft survival >20 years by time after transplantation. The incidence of NMSC increases after kidney transplantation with a proportion of 10.3%, 33.5% and 76.8% at 10, 20 and 30 years after transplantation, respectively. (B) Kaplan–Meier plot of the development of NMSC among KTRs with kidney allograft survival >20 years by recipient age. The incidence of NMSC increases by recipient age with a proportion of 1.7%, 31.6% and 85.2% at 40, 60 and 80 years of age, respectively.
Patient and kidney allograft outcomes among KTRs with ultra long-term kidney allograft survival
Upon time-dependent Cox regression analysis using the time of cancer as the time-dependent covariate, KTRs with cancer showed an inferior patient survival rate with a 25-year patient survival rate of 78.2% versus 85.6% and a 28-year patient survival rate of 56.1% versus 80.3% [hazard ratio 2.352 (95% CI 1.406–3.933); P = 0.002] (Supplementary data, Figure S1A). No differences were observed concerning death-censored kidney allograft survival [hazard ratio 1.688 (95% CI 0.810–3.177); P = 0.194]; Supplementary data, Figure S1B or the development of donor-specific antibodies [hazard ratio 1.172 (95% CI 0.576–2.387); P = 0.667].
Upon time-dependent Cox regression analysis using the time of NMSC as the time-dependent covariate no differences were observed concerning patient survival [hazard ratio 1.096 (95% CI 0.670–1.792); P = 0.716], death-censored kidney allograft survival [hazard ratio 1.205 (95% CI 0.668–2.173); P = 0.537] or the development of donor-specific antibodies [hazard ratio 0.965 (95% CI 0.565–1.646); P = 0.895].
Cancer types among KTRs with ultra long-term kidney allograft survival
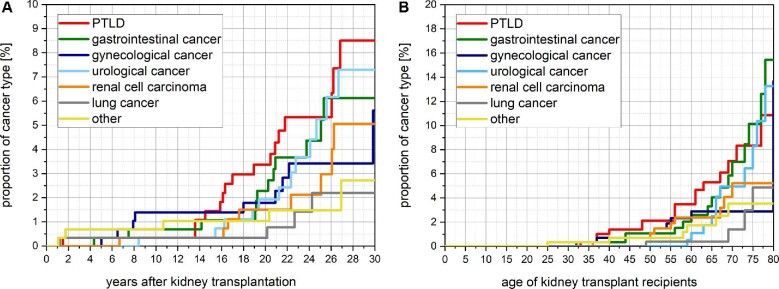
Different cancer types are shown in Table 3. The onset of each cancer type concerning the time post-transplantation and the KTR's age is shown in Figure 3A and B.
Table 3A.
Cancer types
| KTRs with cancer, n = 74 | |
|---|---|
| Total number of cancers (without NMSC), n (%) | 95 |
| PTLD, n (%) | 18 (24) |
| RCC, n (%) | 10 (14) |
| Remaining native kidneys | 7 (10) |
| Clear cell RCC | 3 (4) |
| Papillary RCC | 3 (4) |
| Chromophobe RCC | 1 (1) |
| Transplanted kidney | 2 (3) |
| Papillary RCC | 2 (3) |
| Functionless transplanted kidney | 1 (1) |
| Clear cell papillary RCC | 1 (1) |
| Gastrointestinal cancer, n (%) | 15 (20) |
| Anal cancer | 1 (1) |
| Colorectal cancer | 5 (7) |
| Esophageal cancer | 2 (3) |
| Liver cancer | 2 (3) |
| Pancreatic cancer | 3 (4) |
| Stomach cancer | 2 (3) |
| Lung cancer, n (%) | 3 (4) |
| Gynaecological cancer, n (%) | 11 (15) |
| Breast cancer | 6 (8) |
| Cervical cancer | 3 (4) |
| Ovarian cancer | 1 (1) |
| Uterine cancer | 1 (1) |
| Urological cancer, n (%) | 18 (24) |
| Bladder cancer | 3 (4) |
| Cancer of the penis | 1 (1) |
| Prostate cancer | 12 (16) |
| Testicular cancer | 2 (3) |
| Others, n (%) | 8 (11) |
| Adrenal cancer | 1 (1) |
| Angiosarcoma | 1 (1) |
| Neuroendocrine tumour | 2 (3) |
| Tongue cancer | 2 (3) |
| Thyroid cancer | 2 (3) |
| Skin cancer (without NMSC), n (%) | |
| Kaposi's sarcoma | 3 (4) |
| Merkel cell carcinoma, n (%) | 1 (1) |
| Melanoma, n (%) | 8 (11) |
| NMSC | |
| among KTRs with cancer, n (%) | 48 (64) |
| Basal cell carcinoma (BCC) onlya | 12 (16) |
| Squamous cell carcinoma (SCC) onlya | 19 (26) |
| Both BCC and SCC* | 17 (23) |
aThe different types of NMSC listed are mutually exclusive.
Figure 3:
(A) Kaplan–Meier plot of the development of different cancer types among KTRs with kidney allograft survival >20 years by time after transplantation. The highest incidence is observed for PTLD with a proportion of 0.3%, 3.4% and 8.5% at 10, 20 and 30 years after transplantation, respectively. (B) Kaplan–Meier plot of the development of different cancer types among KTRs with kidney allograft survival >20 years by recipient age. The highest incidence is observed for PTLD with a proportion of 1.4%, 3.5 and 10.9% at 40, 60 and 80 years of age, respectively.
Table 3B.
NMSC types
aThe different types of NMSC listed are mutually exclusive.
By 10, 20 and 30 years post-transplantation, KTRs showed a PTLD rate of 0.34%, 3.36% and 8.51%, respectively (Figure 2A). PTLD is thereby the cancer with the highest incidence as a single cancer type. By recipients’ ages of 40, 60, and 80 years, KTRs showed an incidence of PTLD of 1.39%, 3.50% and 10.87%, respectively. Characteristics of KTRs with late-onset PTLD are shown in Table 4.
Table 4.
Basic and PTLD characteristics of KTRs with late-onset PTLD
| KTRs with late-onset PTLD, (n = 18) | |
|---|---|
| Recipient age at transplantation (years)a | 34 (18–57) |
| Number of kidney transplantations, n (%) | |
| 1 | 17 (94.4) |
| 2 | 1 (5.6) |
| Causes of ESRD, n (%) | |
| Glomerulonephritis | 8 (44.4) |
| Diabetic nephropathy | 1 (5.6) |
| Hypertensive nephropathy | 1 (5.6) |
| Polycystic kidney disease | 2 (11.1) |
| Uropathy/CAKUT | 2 (11.1) |
| Interstitial nephritis | 1 (5.6) |
| Hyperoxaluria | 1 (5.6) |
| Undetermined | 2 (11.1) |
| Time on dialysis (months)a | 31 (4–108) |
| Time of PTLD post-transplant (months)a | 201 (153–363) |
| Morphological classification of PTLD, n (%) | |
| Polymorphic PTLD | 1 (5.6) |
| Monomorphic PTLD | 16 (88.9) |
| Diffuse large B-cell lymphoma | 14 (77.8) |
| Plasma cell neoplasm | 2 (11.1) |
| Classic Hodgkin lymphoma | 1 (5.6) |
| Localization of PTLD, n (%) | |
| Nodal | 6 (33.3) |
| Extranodal | 12 (66.7) |
| CNS | 3 (16.7) |
| Gingiva/pharynx | 3 (16.7) |
| Small intestine | 4 (22.2) |
| Stomach | 1 (5.6) |
| Skin | 1 (5.6) |
| PTLD EBV status, n (%) | |
| EBV positive | 7 (38.9) |
| EBV negative | 11 (61.1) |
aMedian (range). CAKUT, congenital anomalies of kidney and urinary tract; CNS, central nervous system.
By 10, 20 and 30 years post-transplantation, KTRs showed a RCC rate of 0.34%, 1.50% and 5.06%, respectively (Figure 2A). RCC is thereby the cancer type with the second-highest incidence as a single cancer entity. By recipients’ ages of 40, 60 and 80 years, KTRs showed RCC incidence of 0.69%, 2.36% and 5.23%, respectively.
Upon multivariate Cox regression analysis, recipient age further increased the risk of cancer among KTRs developing prostate cancer and lung cancer [hazard ratio 1.098 (95% CI 1.039–1.161); P = 0.002] and [hazard ratio 1.097 (95% CI 1.005–1.210); P = 0.043], respectively. Upon multivariate Cox regression analysis, smoking history further increased cancer risk among KTRs developing lung cancer [hazard ratio 6.510 (95% CI 1.879–48.587); P = 0.018]. Time on dialysis did not impact the risk of any cancer type upon multivariate Cox regression analysis.
Patient and kidney allograft outcomes among KTRs with different cancer types
Upon time-dependent Cox regression analysis using the time of the specific cancer type as the time-dependent covariate, no specific impact could be detected for any cancer type. Upon time-dependent Cox regression analysis using the time of PTLD as the time-dependent covariate, PTLD increased the risk of kidney allograft loss by >6-fold [hazard ratio 6.187 (95% CI 2.606–14.691]; P = 0.001]. No impact on death-censored kidney allograft survival was observed for other cancer types.
DISCUSSION
Cancer after solid organ transplantation has been increasingly recognized in registry analyses for the short- and medium-term post-transplantation. Less is known, however, about the ultra long-term cancer rates.
Firstly, our results suggest that cancer rates continuously increase in the ultra long-term after kidney transplantation. According to several registry analyses, an increased overall risk of cancer and NMSC has been associated with solid organ transplantation than the general population [23]. All these analyses found that specific cancer rates varied depending on the organ transplanted, with an average latency after transplantation of ∼3–5 years [23, 24]. However, despite the often very large numbers of patients, these registry analyses usually have significant drawbacks in that they incorrectly assess cancer risk over the ultra long-term due to the short follow-up period and, most importantly, limited data quality. Specifically, these registry analyses also mix two patient groups, namely those patients who in all likelihood already have a very early cancer stage at the time of transplantation, which may then progress rapidly due to the initiated immunosuppression, and those who develop cancer in response to long-term exposure to immunosuppression. In the latter, however, a follow-up period of usually 10–15 years after transplantation does not seem to be sufficient. Here, our analysis shows for the first time that the accumulation of risk factors such as duration under immunosuppression, increasing age and individual risk factors leads to a cancer rate of over 30% and an NMSC rate of over 60% after 30 years under immunosuppression.
Secondly, PTLD remains the most common cancer type in the ultra long-term, reaching a PTLD rate as high as 8.5% at 30 years post-transplantation. Recently, colleagues from the STCS suggested a very low 5-year incidence of PTLD of <1% [21, 22]. However, long-term outcomes from our study show that PTLD remains the most common cancer type in the ultra long-term. The morphological classification of PTLD is thereby in line with previous observations [25]. One reason for the high incidence of PTLD in the long-term appears to be that PTLD occurs after a shorter period of immunosuppression and at an earlier age than other cancer types, so more KTRs reach this event during their post-transplant course. Due to the high PTLD rate, clinical vigilance is critical in case of nonspecific symptoms such as fever, unexplained weight loss, fatigue, lymphadenopathy or gastrointestinal disturbances, and should call for more comprehensive diagnostics in this cohort.
In line with registry analyses, RCC is the second most common cancer among KTRs and arises more likely in the non-functioning native kidneys or transplanted kidneys than in the functioning transplanted kidneys [26, 27]. Our data confirm the predominance of papillary RCC over clear cell RCC even in the ultra long-term after kidney transplantation, as suggested in a previous study [27]. The high RCC rate reinforces surveillance ultrasonography and computed tomography scanning as routine cancer screening among KTRs.
Thirdly, our results suggest that long-term exposure to maintenance immunosuppression is the most relevant risk factor contributing to the strikingly high cancer rates in the ultra long-term after kidney transplantation. The immunosuppressive medication facilitates cancer and NMSC through various mechanisms, including decreased immune surveillance of atypical cells, impaired cell repair mechanisms and proliferation of carcinogenic viruses [27]. Interestingly, >10% of cancer diagnoses in our study may be attributed to coexisting viral infections with carcinogenic viruses like EBV-positive PTLD, HHV-8-associated Kaposi's sarcoma, HPV-associated cervical or anal cancer, and hepatitis B- or C-associated liver cancer [28, 29].
Very surprisingly, thiazide diuretics, which have been linked to an increased risk of NMSC through its photosensitizing properties, also appear to be associated with an increased NMSC risk after kidney transplantation [30, 31]. This observation suggests that the cumulative effect of impaired immune surveillance, direct carcinogenic effects of azathioprine and the photosensitizing activity of thiazide diuretics, may act synergistically in this cohort and put KTRs at the greatest risk for NMSC. This finding may have direct implications on KTR aftercare. Thiazide diuretics should be used with caution among KTRs, mainly when other first-line antihypertensive medications can be used and a direct added value is missing.
Fourthly, cancer is contributing as a primary cause of death in the ultra long-term. Previous registry analyses demonstrated that KTRs are at an increased risk of cancer death compared with the general population. However, this cohort faces lower life expectancy and a higher risk of non-cancer death [32]. Although the risk of non-cancer death is higher than cancer death, cancer death is expected to increase steadily over the next decade due to improved long-term survival [33].
Interestingly, the development of de novo DSA is not associated with the presence or absence of cancer or NMSC in the ultra long-term. Although the development of cancer might be a failure of immune surveillance, not all cancers are naturally immunogenic, cancers may have the ability to thwart an effective anti-tumour immune response and mechanisms to allow an alloimmunity against the kidney allograft most likely differ significantly from mechanisms to allow an adequate anti-tumour response.
Our study has a few limitations. Firstly, the results are limited by a single-centre retrospective design. Although all KTRs studied in our work had kidney allograft survival of at least 20 years, our cohort also represents a selection of KTRs who survived a cancer event <20 years after transplantation. Secondly, our study mainly included adults of Caucasian origin, limiting the generalizability of our findings to other cohorts. Thirdly, since life expectancy in Switzerland is considered to be among the highest in the world by far, the results of our work are likely to have limited applicability to other world regions.
Our study has several strengths, particularly analysing thoroughly characterized KTRs at least 20 years exposed to immunosuppression. A standardized immunosuppressive protocol, close clinical monitoring and protocol cancer screening post-transplant enabled us to obtain a very high data density, quality and almost no loss-to-follow-up.
In conclusion, for the first time, we show cancer risk among KTRs with a cumulative exposure to immunosuppression of at least 20 years that causes a disproportionate increase in overall cancer risk in the ultra long-term. Not surprising but impressive in terms of scale are the PTLD and RCC rates among KTRs in the ultra long-term, which should raise awareness among clinicians. An individually tailored approach to cancer prevention, screening and surveillance is required for KTRs with ultra long-term exposure to immunosuppression.
Supplementary Material
Contributor Information
Julia D Fuhrmann, Division of Nephrology, University Hospital Zurich, Zurich, Switzerland.
Kristyna Valkova, Division of Nephrology, University Hospital Zurich, Zurich, Switzerland.
Seraina von Moos, Division of Nephrology, University Hospital Zurich, Zurich, Switzerland.
Rudolf P Wüthrich, Division of Nephrology, University Hospital Zurich, Zurich, Switzerland.
Thomas F Müller, Division of Nephrology, University Hospital Zurich, Zurich, Switzerland.
Thomas Schachtner, Division of Nephrology, University Hospital Zurich, Zurich, Switzerland.
CONFLICT OF INTEREST STATEMENT
The authors hereby declare that the results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Traynor C, Jenkinson A, Williams Yet al. Twenty-year survivors of kidney transplantation. Am J Transplant 2012; 12: 3289–3295 [DOI] [PubMed] [Google Scholar]
- 2. McCaughan JA, Courtney EA. The clinical course of kidney transplant recipients after 20 years of graft function. Am J Transplant 2015; 15: 734–740 [DOI] [PubMed] [Google Scholar]
- 3. Choi H, Lee W, Lee HSet al. The risk factors associated with treatment-related mortality in 16,073 kidney transplantation-a nationwide cohort study. PLoS One 2020; 15: e0236274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hariharan S, Johnson CP, Bresnahan BAet al. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 2000; 342: 605–612 [DOI] [PubMed] [Google Scholar]
- 5. Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation 2005; 80: 254–264 [DOI] [PubMed] [Google Scholar]
- 6. Coemans M, Süsal C, Döhler Bet al. Analyses of the short- and long-term graft survival after kidney transplantation in Europe between 1986 and 2015. Kidney Int 2018; 94: 964–973 [DOI] [PubMed] [Google Scholar]
- 7. Collaborative Transplant Study . CTS Outcome Graphs. 2021; Graph K-15103E-0221. https://www.ctstransplant.org/public/graphics/sample.shtml (5 April 2021, date last accessed) [Google Scholar]
- 8. Rama I, Grinyo JM. Malignancy after renal transplantation: the role of immunosuppression. Nat Rev Nephrol 2010; 6: 511–9 [DOI] [PubMed] [Google Scholar]
- 9. Marcén R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs 2009; 69: 2227–2243 [DOI] [PubMed] [Google Scholar]
- 10. Kasiske BL, Snyder JJ, Gilbertson DTet al. Cancer after kidney transplantation in the United States. Am J Transplant 2004; 4: 905–913 [DOI] [PubMed] [Google Scholar]
- 11. Morath C, Mueller M, Goldschmidt Het al. Malignancy in renal transplantation. J Am Soc Nephrol 2004; 15: 1582–1588 [DOI] [PubMed] [Google Scholar]
- 12. Au EH, Chapman JR, Craig JCet al. Overall and site-specific cancer mortality in patients on dialysis and after kidney transplant. J Am Soc Nephrol 2019; 30: 471–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maluccio M, Sharma V, Lagman Met al. Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. Transplantation 2003; 76: 597. [DOI] [PubMed] [Google Scholar]
- 14. Swann PF, Waters TR, Moulton DCet al. Role of postreplicative DNA mismatch repair in the cytotoxic action of thioguanine. Science 1996; 273: 1109. [DOI] [PubMed] [Google Scholar]
- 15. Robson R, Cecka JM, Opelz Get al. Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant 2005; 5: 2954. [DOI] [PubMed] [Google Scholar]
- 16. O'Neill JO, Edwards LB, Taylor DO. Mycophenolate mofetil and risk of developing malignancy after orthotopic heart transplantation: analysis of the transplant registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006; 25: 1186. [DOI] [PubMed] [Google Scholar]
- 17. ANZDATA Registry . 43rd Report, Chapter 7: Kidney Transplantation, Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia. 2020http://www.anzdata.org.au (5 April 2021, date last accessed) [Google Scholar]
- 18. United States Renal Data System . 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Kidney Disease, 2020 [Google Scholar]
- 19. Canadian Institute for Health Information . Treatment of End-Stage Organ Failure in Canada, Canadian Organ Replacement Register, 2010 to 2019: End-Stage Kidney Disease and Kidney Transplants – Data Tables. Ottawa, ON: CIHI, 2020 [Google Scholar]
- 20. Sprangers B, Nair V, Launay-Vacher Vet al. Risk factors associated with post-kidney transplant malignancies: an article from the Cancer-Kidney International Network. Clin Kidney J 2018; 11: 315–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lengwiler E, Stampf S, Zippelius Aet al. Solid cancer development in solid organ transplant recipients within the Swiss Transplant Cohort Study. Swiss Med Wkly 2019; 149: w20078. [DOI] [PubMed] [Google Scholar]
- 22. Steiner R, Kridel R, Giostra Eet al. Low 5-year cumulative incidence of post-transplant lymphoproliferative disorders after solid organ transplantation in Switzerland. Swiss Med Wkly 2018; 148: w14596. [DOI] [PubMed] [Google Scholar]
- 23. Engels EA, Pfeiffer RM, Fraumeni JF Jret al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011; 306: 1891–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pedotti P, Cardillo M, Rossini Get al. Incidence of cancer after kidney transplant: results from the North Italy transplant program. Transplantation 2003; 76: 1448–1451 [DOI] [PubMed] [Google Scholar]
- 25. Caillard S, Agodoa LY, Bohen EMet al. Myeloma, Hodgkin disease, and lymphoid leukemia after renal transplantation: characteristics, risk factors and prognosis. Transplantation 2006; 81: 888–895 [DOI] [PubMed] [Google Scholar]
- 26. Denton MD, Magee CC, Ovuworie Cet al. Prevalence of renal cell carcinoma in patients with ESRD pre-transplantation: a pathologic analysis. Kidney Int 2002; 61: 2201–2209 [DOI] [PubMed] [Google Scholar]
- 27. Cornelis F, Buy X, André Met al. De novo renal tumors arising in kidney transplants: midterm outcome after percutaneous thermal ablation. Radiology 2011; 260: 900–907 [DOI] [PubMed] [Google Scholar]
- 28. Chen ML, Wang SH, Wei JCet al. The impact of human papillomavirus infection on skin cancer: a population-based cohort study. Oncologist 2021; 26: e473–e483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen CJ, You SL, Hsu WLet al. Epidemiology of virus infection and human cancer. Recent Results Cancer Res 2021; 217: 13–45 [DOI] [PubMed] [Google Scholar]
- 30. Pedersen SA, Gaist D, Schmidt SAJet al. Hydrochlorothiazide use and risk of nonmelanoma skin cancer: a nationwide case-control study from Denmark. J Am Acad Dermatol 2018; 78: 673–681 [DOI] [PubMed] [Google Scholar]
- 31. Kreutz R, Algharably EAH, Douros A. Reviewing the effects of thiazide and thiazide-like diuretics as photosensitizing drugs on the risk of skin cancer. J Hypertens 2019; 37:1950–1958 [DOI] [PubMed] [Google Scholar]
- 32. Acuna SA, Fernandes KA, Daly Cet al. Cancer mortality among recipients of solid-organ transplantation in Ontario, Canada. JAMA Oncol 2016; 2: 463–469 [DOI] [PubMed] [Google Scholar]
- 33. Vajdic CM, McDonald SP, McCredie MRet al. Cancer incidence before and after kidney transplantation. JAMA 2006; 296: 2823–2831 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.