ABSTRACT
As aging increases, monoclonal gammopathy is becoming more common and monoclonal gammopathy of renal significance (MGRS) is gaining attention due to frequent renal involvement. Within MGRS, proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID) is a special category. The disease was first described in 2004 and the research history on it is relatively short. Compared with other MGRS, the detection rate of circulating clones is lower in patients with PGNMID, which is easy to miss and misdiagnose in clinical work. In this review, the etiology and clinical features of PGNMID are discussed. It is noted that PGNMID is associated not only with MGRS, but also with malignancy, infection and other factors. PGNMID is not a disease exclusive to the elderly—young people can also develop this disease. Due to the low detection rate of circulating clones in most patients, confirmation of the disease needs to be combined with renal pathology, which emphasizes the importance of completing light and heavy chain subtype staining. Treatment options for patients with PGNMID differ by etiology. For MGRS-associated PGNMID, the current treatment is primarily empirical and more research evidence is needed to fill the treatment gap.
Keywords: etiology, proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID), treatment
INTRODUCTION
Monoclonal gammopathy is associated with an increase in ‘M protein’ (entire monoclonal immunoglobulin or fragment thereof) secreted by plasma or B cells for various reasons [1, 2]. The M proteins can be distributed in serum or urine, as well as in various organs (such as kidney, skin, liver, heart and bone marrow). When evaluating the tumor burden of patients, we need to consider all the M proteins, either in serum or deposited in organs. There is no doubt that a more aggressive treatment regimen, such as administering chemotherapy, should be pursued for patients who have clones detected in the serum or bone marrow and meet the diagnostic criteria for malignant diseases, such as symptomatic multiple myeloma or B-cell lymphoma. However, if the clones detected in the serum or bone marrow do not meet the current diagnostic criteria for malignant hematological diseases, the decision as to the patient's treatment is difficult, and depends on whether the M protein causes organ dysfunction or not [2, 3]. The concept of monoclonal gammopathy of clinical significance (MGCS) was proposed, which encompasses all types of organ lesions induced by M proteins produced by non-malignant hematological tumors or asymptomatic small B-cell clones lacking chemotherapeutic criteria [4].
The kidney is the most important excretory organ and is often involved in hematologic disorders. To better understand and manage this type of disease, we introduced the concept of monoclonal gammopathy of renal significance (MGRS) [2], which connects the ‘grey area’ between hematology and nephrology. In 2012, MGRS was first defined as a causal relationship between plasma or B-cell clones and kidney disease [2]. The subsequent literature also reported the clinicopathological manifestations and treatment prognosis of MGRS [5–9]. Unlike other types of MGRS, the positive detection rate of M protein in proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID) is relatively low (about 30%) [10, 11], which makes early diagnosis difficult. It is worth noting that a low positive detection rate for serum M protein does not mean that the renal damage is not severe. Among transplant-related MGRS, PGNMID is the more common type of transplanted kidney recurrence and has a poor prognosis [12, 13]. At present, there are still many problems worth exploring and solving for PGNMID. In this article, we discuss PGNMID in terms of etiology, clinicopathological manifestations and treatment.
CLINICOPATHOLOGIC FEATURES
In 2004, proliferative glomerulonephritis with monoclonal Immunoglobin G deposition was first defined by Nasr et al. [14]. The pathology is characterized by monoclonal immunoglobin G (IgG) (a single IgG subclass) with a single light chain subtype deposited in the glomerulus. The clinical diagnosis of PGNMID also requires the exclusion of cryoglobulinemia and any known type of M protein-related glomerular diseases (such as light chain deposition disease, fibrillary glomerulonephritis and immunotactoid glomerulopathy, and the main points of identification are shown in Supplementary data, Table S1). This disease is uncommon, with an autologous kidney biopsy rate of 0.17–0.21%, as Nasr et al. reported [10, 14]. Although the majority of patients are middle-aged, cases of adolescence have been reported in the literature [all showed heavy chain deposition of IgG3 subclass on glomerular immunofluorescence (IF)] [15, 16]. The patients often start with nephrotic syndrome; half of them have renal insufficiency and microscopic hematuria, and some have hypertension (38–67%) and hypocomplementemia (18–40%); a few patients present with gross hematuria. We have reviewed a series of case reports on PGNMID and summarized them in Table 1 [10, 14, 17–19].
Table 1.
Comparison of clinical data in review literature
| 2004 | 2009 | 2011 | 2015 | 2018 | 2020 | |
|---|---|---|---|---|---|---|
| Nasr et al. [14] | Nasr et al. [10] | Guiard et al. [17] | Bhutani et al. [18] | Gumber et al. [19] | Nasr et al. [27] | |
| Autologous renal biopsy rate (%) | 0.21 | 0.17 | NA | NA | NA | NA |
| Number of studies | 10 | 37 | 26 | 60 | 19 | 17 |
| Male/female | 5/5 | 23/14 | 10/16 | 32/28 | 12/7 | 13/4 |
| Renal biopsy age [years, mean (range)] | 58 (44–78) | 55 (20–81) | 52 (29–77) | 56M (47, 62) | 58 (25–83) | 62 (44–84) |
| Nephrotic syndrome, n (%) | 4/9 (44) | 17/35 (49) | 22 (85) | NA | NA | 9/16 (56) |
| Hypertension, n (%) | NA | 14 (38) | 16/24 (67) | NA | NA | 15 (88) |
| Hypocomplementemia, n (%) | ||||||
| Low C3 | 1 (10) | 3 (8) | 1/22 (5) | 3/43 (7) | NA | 6/12 (50) |
| Low C4 | 2 (20) | 3 (8) | 3/22 (14) | 4/43 (9) | NA | 0/12 (0) |
| Low C3 and C4 | 1 (10) | 4 (11) | 4/22 (18) | 4/43 (9) | NA | 0/12 (0) |
| Renal insufficiency, n (%) | 80 | 68 | 54 | NA | 100 | 94 |
| Scr [mg/dL, mean (range)] | 2.8 (0.9–8.0) | 2.8 (0.7–17.0) | 2.4 (0.5–9.2) | NA | 1.7 (1.2–2.8) | 2.3 (0.9–5.7) |
| Microscopic hematuria, n (%) | 6 (60) | 27/35 (77) | 21/24 (88) | NA | NA | 16 (94) |
| 24-h urine protein [g, mean (range)] | 5.8 (1.9–13.0) | 5.7 (0.4–17.0) | 5.3 (1.4–10) | 3.6M (1.9, 8.1) | 3.6M (2.3, 8.0) | 5.7 (2–12) |
| Glomerular crescent, n (%) | 1 (10) | 12 (32) | 13 (50) | 11 (18) | NA | 1 (6) |
| Complement IF staining in glomerular, n (%) | NA | NA | ||||
| C3 | 9 (90) | 36 (97) | – | 52/56 (93) | – | 17/17 (100) |
| C1q | 3 (30) | 23/36 (64) | – | 29/54 (54) | – | 2/17 (12) |
| Underlying clone and M protein evaluation | ||||||
| SIFE+, n (%) | 5 (50) | 10 (27) | 8/26 (31) | 12/59 (20) | 4 (21) | 11 (65) |
| sFLC R+, n (%) | NA | 1/4 (25) | NA | 12/56 (21) | 3 (16) | 10/12 (83) |
| PBFCM+, n (%) | NA | NA | NA | 1/9 (11) | NA | NA |
| BM+, n (%)a | 0/8 (0) | 2/22 (9) | 9/22 (41) | 10/40 (25) | 6/17 (35) | 14/16 (88) |
| Cloned cell types | – | PC (1), BC (1) | PC (2), BC (7) | PC (6), BC (3), LPC (1) | PC (3), BC (2), LPC (1) | PC (14) |
| Extrarenal disease | Non | MM (1), AL (1), solid tumor (4), autoimmune hemolytic anemia (1) | MM (2), CLL (4), NHL (3) | CLL (3), MDS (1), MGUS (4), solid tumor (9), autoimmune disease (5) | MGUS (1) | MM (5), MGUS (1) |
| Malignancy-associated PGNMID | 0/10 | 1/37 | 9/26 | 1/60c | 0/19 | 5/17 |
| MGRS-related PGNMIDb | 10/10 (100%) | 36/37 (97%) | 17/26 (65%) | 59/60 (98%) | 19/19 (100%) | 12/17 (71%) |
| Follow-up time [months, mean (range)] | 12 (2–52) | 30 (1–114) | 68 (2–216) | 21M (10, 39) | 23M (12, 45) | 72 (20–154) |
| Outcomes, n (%) | ||||||
| Kidney function | ESRD (2/9, 22) | ESRD (7/32, 22) | ESRD (6/25, 24) | NA | ESRD (21) | ESRD (8/15, 53) |
| Hematological evaluation | PD (0, 0) | New M protein (1/32, 3) | MGUS→MM (1/25, 4) | New M protein (3, 5) | PD (0, 0) | PD (0, 0) |
| Death | 0 (0) | 5/32 (16) | 1/25 (4) | NA | 1 (5) | 5/15 (33) |
NA, not applicable; M, median (interquartile range); SIFE, serum immunofixation electrophoresis; sFLCR, serum-free light chain ratio; BM, bone marrow; MM, multiple myeloma; LPC, lymphoplasmacytic clone; PC, plasma cell clone; BC, B-cell clone; PBFCM, peripheral blood flow cytometry; PD, progressive disease; AL, amyloidosis; CLL, chronic lymphocytic leukemia; NHL, non-Hodgkin lymphoma; MDS, myelodysplastic syndromes; MGUS, monoclonal gammopathy of undetermined significance; ESRD, end-stage renal disease.
The clone detected in bone marrow are consistent with renal deposition.
Contains MGRS-related and unclassified PGNMID.
All three patients had low-grade CLL (Rai stage 0) without treatment, but one patient at the time of presentation with kidney disease was found to have 80% BM involvement and renal parenchyma involvement with CLL.
As shown in Table 1, the positive detection rate of M protein was lower for PGNMID than for monoclonal immunoglobulin deposition disease (MIDD) (30% versus 100%) [3, 20–22]. Although it is challenging to detect M protein in serum, it does not mean that the tumor burden of PGNMID patients is low or the disease is not severe. To better evaluate PGNMID disease, various assay techniques can be used in clinical work to improve the detection rate of monoclonal immunoglobulin, including serum protein electrophoresis (SPEP), serum immunofixation electrophoresis (SIFE), serum free light chain (sFLC) and mass spectrometry (Supplementary data, Table S2). We can also use flow cytometry or immunohistochemistry and fluorescence in situ hybridization (FISH) on marrow specimens and extramedullary hematopoietic tissues to evaluate and identify traces of potential clones [3]. Some patients have been diagnosed with PGNMID along with solid tumors, myelodysplastic syndrome (MDS), primary renal amyloidosis, monoclonal gammopathy of undetermined significance (MGUS) or autoimmune diseases [10, 18, 23] However, based on current assays and evidence, no direct relationship between these concomitant diseases and PGNMID has been identified. Perhaps with further studies, an intrinsic link may be discovered.
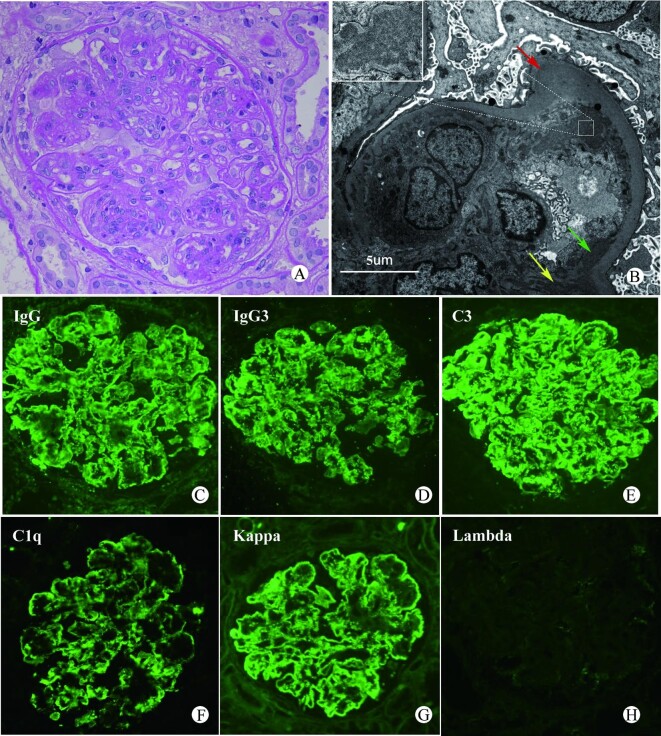
Pathological evaluation of a kidney biopsy is essential for the diagnosis of PGNMID (Figure 1). Under light microscopy, the glomeruli were predominantly proliferative changes, including membranous proliferative glomerulonephritis (MPGN)-like, mesangial proliferative glomerulonephritis (MsPGN)-like, with a few presenting membranous nephropathy (MN) changes [10, 14] and glomerular crescent seen in 10–50% of patients [10, 14, 17, 18]. Initially, in the 10 cases of PGNMID reported by Nasr et al. [14], the type of heavy chain of M protein deposited in the glomeruli was only IgG subclasses (e.g. IgG1, IgG2 or IgG3), and IgG3 heavy chain subclass was the most common type of deposition. This may be due to the considerable molecular weight of IgG3, which makes it difficult to pass through the glomerular filtration membrane. At the same time, IgG3 can self-aggregate through FC–FC interaction and may selectively accumulate in the glomerulus [10, 14]. From a retrospective study by Bhutani et al. [18], it seemed to be found that deposition of IgG3 subclass (IgG3-PGNMID) tended to show MPGN-like changes, whereas MN-like changes were common in IgG1-PGNMID, but this idea has not been confirmed. As studies progressed, PGNMID with heavy chain types of IgG4 subclass, IgA and IgM, and light chain (LC) only deposition were successively reported [10, 12, 18, 24–28]. Similarly, a few patients have been reported in the literature to show the deposition with focally variegated texture [10, 13], which is different from the typical electron microscopic presentation (granular electron-dense deposits). These atypical presentations further expanded the spectrum of pathological manifestations.
FIGURE 1:
An example of PGNMID (IgG3-κ) renal biopsy image. (A) Under light microscope, the glomerulus shows membranoproliferative pattern characterized by endocapillary and mesangial hypercellularity with lobular configuration and basement membrane double contours (periodic acid–Schiff stain, magnification ×400); (B) Electron microscopy showed subendothelial (green arrow), mesangial (yellow arrow) and subepithelial (red arrow) granular electron-dense deposits. (C–H) Under IF, IgG3, κ, C3 and C1q were deposited in mesangial area and vascular loops, whereas λ staining was negative (magnification ×400). The IgM, IgA and other IgG subclasses were negative and are not shown in the figure.
IF is an essential diagnostic tool for PGNMID; however, in clinical work, the failure of further staining for IgG subclass or LC subtypes is still encountered due to various reasons (e.g. no glomeruli to stain further), leading to diagnosis delay or misdiagnosis [29, 30]. To reduce the incidence of such events, adequate tissues should be taken at the time of renal biopsy and staining for light and heavy chain subtypes should be carried out whenever possible. If necessary, special stains such as Congo red and serum amyloid P component (SAP) can also be used for differential diagnosis. Of note, the restriction of IgG subclass or LC subtype is not sufficient to diagnose PGNMID. Fibrillary glomerulonephritis has been reported to show positive staining usually for a single IgG subclass, but LC subtypes show double positivity [31]. Similarly, membranous-like glomerulopathy with masked IgG Kappa deposits (MGMID) showed IgG1-κ-restricted deposits, but was still not diagnostic of PGNMID. MGMID is mostly seen in autoantibody-positive young women with little or no Ig staining in routine IF of renal tissue, but significantly brighter IF staining in paraffin tissue and positive SAP staining, which is distinct from PGNMID [32, 33]. Therefore, a combination of light microscopy, IF, electron microscopy findings and clinical manifestations is required for differential diagnosis in the diagnosis of PGNMID.
Interestingly, M proteins (IgG2, IgG4 and Kappa deposits) have been reported to be detected in C3 glomerulopathy (C3G) patients treated with eculizumab (all five patients were found on procedural renal biopsy 1 year after treatment) [34]. Combined with the electron microscopy results (punctate electron-dense deposits in glomerular basement membrane, tubular basement membrane and vessels), the final diagnosis of MIDD was made [34]. This situation is due to the structural characteristics of eculizumab; it is a recombinant intact immunoglobulin of human and murine origin in which CH2 and CH3 constant regions from IgG4 are fused to the IgG2 hinge region and CH1 domain, and then paired with a Kappa LC [35]. It is an exogenous monoclonal immunoglobulin, and although IF shows monoclonal immunoglobulin deposition, the deposition site is different. Therefore, careful differentiation is required in the diagnosis of PNGMID.
EXPLORATION OF THE ETIOLOGY AND MECHANISMS OF PGNMID
It is generally accepted that the pathogenesis of PGNMID is the deposition of M protein in the glomerulus, which activates the complement causing an inflammatory response that results in renal dysfunction [2, 3, 10]. However, the upstream mechanism of this entity (M protein production) has been less studied. Based on the mechanism of immunoglobulin production and combined with the clinical manifestations of PGNMID, the following hypothesis can be made about its etiology (Figure 2A): (i) malignant B cells or plasma cells in the bone marrow or serum secrete large amounts of abnormal immunoglobulins; and (ii) normal B cells or plasma cells in the bone marrow or serum are affected by various factors, resulting in the secretion of abnormal immunoglobulins. The first condition is well linked to renal damage due to hematologic malignancies.
FIGURE 2:
(A) M protein pathogenic mechanism hypothesis. (a) Abnormal B/plasma cells in bone marrow or/and blood secrete M protein. (b) Normal B/plasma cells in bone marrow or/and blood secrete M protein. The M protein can be in the circulating blood or deposited in the kidneys, skin or other organs, which is determined by the M protein load and physicochemical properties. (B) Clinical classification combined with the etiology and laboratory findings of PGNMID. Unclassified PGNMID is considered as MGRS-related PGNMID.
In the second condition, there is no primary disease of the hematological system, and abnormal immunoglobulins are secreted due to stimulation by various factors. Sirac et al. [36] have pointed out that changes in some amino acids, which may reduce the stability and folding of immunoglobulins, lead to over secretion of unstable immunoglobulins or immunoglobulin fragments. Similar cases have been reported in the literature that two patients with PGNMID (IgG3-κ) associated with parvovirus B19 infection [20]. Scholars have speculated that PGNMID is mediated by IgG3-κ-type monoclonal immunoglobulin after infection with the parvovirus B19, a process that occurs as part of the immune disorder associated with viral infection [20]. Interestingly, the clinical manifestations and laboratory findings of PGNMID were transient in these two patients and returned to normal on their own after viral clearance. In addition, PGNMID has also been reported in association with hepatitis C virus infection [37]. Renal damage due to infection has been reported previously, but the type of renal damage varies [38, 39]. Combined with previous reports that no M protein was detected in the circulation, it is speculated that immune impairment after infection may be related to the pathogenesis of PGNMID.
In addition to being associated with viral infections, PGNMID has been related to solid tumors. The patient developed nephrotic syndrome 3 months after diagnosis of squamous cell lung carcinoma (SCLC) [40]. Laboratory results did not reveal hepatitis virus infection, autoimmune disease or hematologic tumors, and the final diagnosis of PGNMID (IgG1-λ) was made in combination with renal biopsy results. It has been mentioned that the presentation of nephrotic syndrome within 12 months after cancer diagnosis supported tumor-associated glomerulopathy [41]. The patient treated with carboplatin and paclitaxel chemotherapy showed consistent improvement in tumor size and squamous cell carcinoma antigen (SCC) levels, reduced urinary protein and improved renal function. All the above evidence supports the close association of PGNMID with lung cancer, emphasizing the possibility of paraneoplastic glomerulopathy, but the specific mechanisms behind it need further investigation. Therefore, not all PGNMID is associated with hematologic tumors. Infections and solid tumors can also be responsible for the disease (Figure 3).
FIGURE 3:

Etiology and concomitant diseases of PGNMID. (A) Malignant tumor of hematology system: multiple myeloma; chronic lymphatic leukemia; non-Hodgkin lymphoma etc. (B) Not meeting the criteria for hematologic malignancy: monoclonal gammopathy of renal significance, MGRS; infection, solid tumor or unknown disease. More patients have PGNMID due to these types of causes.
TREATMENT OF PGNMID
After reviewing the literature, the etiology of PGNMID is roughly divided into two categories: primary hematologic diseases and others. Based on the hematologic assessment, primary hematologic-related PGNMID can be further divided into malignancy-related and MGRS-related PGNMID. In 2017, the International Kidney and Monoclonal Gammopathy Research Group (IKMG) updated the definition of MGRS. It emphasized that renal lesions caused by M proteins are considered MGRS when the underlying B-cell or plasma cell clones do not cause tumor complications or do not meet any current hematologic criteria for specific treatment. Just like low-grade chronic lymphocytic leukemia (CLL) and low-grade B-cell, non-Hodgkin's lymphoma with renal lesions should be diagnosed as MGRS [3, 42–44]. Therefore, if a hematologic malignancy is found at the same time as the diagnosis of PGNMID and there is a causal relationship between the two, the diagnosis of malignancy-associated PGNMID is more reasonable. PGNMID with monoclonal immunoglobulin LC deposition only (LC-PGNMID) tended to be associated with malignancy [27]. Other types of PGNMID include immune regulation disorders caused by infections, tumors and other factors, and these types of PGNMID are relatively rare in clinical practice. The literature reports that PGNMID caused by the viral infection can recover spontaneously after viral clearance [20] and no specific treatment is needed for this type of PGNMID. Depending on the clinical situation, PGNMID can be classified in the manner of Figure 2B. Of these, unclassified PGNMID is considered MGRS-related PGNMID in clinical work (in the following, both of them will be collectively referred to as MGRS-related PGNMID).
In clinical work, 65–100% of PGNMID is associated with MGRS [10, 14, 17–19, 27]. The goals of managing these patients include preserving renal function, restoring renal graft eligibility, improving life expectancy and minimizing adverse effects of treatment [45]. In 2012, IKMG [7] gave several treatment recommendations, summarized in Figure 4, which emphasizes the need to consider the patient's renal function, urinary protein and drug tolerance when treating PGNMID. In a series by Nasr et al. [10, 14], some MGRS-related PGNMID patients had a stable renal function at last follow-up after treatment with renin–angiotensin system (RAS) inhibitors or prednisone alone, even when they had nephrotic-range proteinuria or stages 3 and 4 CKD at renal biopsy. It seems that PGNMID is relatively easy to control. The following question has been raised [46]: is clone-directed therapy always necessary for MGRS-related PGNMID? There is no clear answer yet. Recently, Gumber et al. [19] mentioned that the principle of treating MGRS-related PGNMID is targeted at the potential clones. In patients without detectable clones, who received treatment targeting a hypothetical potential clone (i.e. antilymphoma regimen for B-cell clone, antimyeloma regimen for plasma cell clone and most patients treated continuously for about 6 months), there was no end-stage renal disease at the last follow-up (median 23 months after diagnosis), and the treatment was well tolerated. Further analysis of the study by Gumber et al. showed that all patients were in stages 3–5 CKD and some patients also had large amounts of proteinuria. Both studies suggest that MGRS-related PGNMID patients at a late stage of chronic kidney disease can achieve stable conditions either with conservative treatment or by targeting potential clones. Therefore, the need to target potential clones in patients with MGRS-related PGNMID is inconclusive.
FIGURE 4:

The IKMG recommended the treatment of PGNMID in 2012. (A) Patients with proteinuria of ˂1 g/day and no evidence of progressive disease. (B) Patients with proteinuria >1 g/day or progressive disease. (C) Candidate for renal transplantation (with detectable clones). (D) Patients who are ineligible for renal transplantation. Blank represents symptomatic measures and careful surveillance. Gray represents chemotherapy. In stage C, when the patient is a kidney transplant candidate and the clones can be detected, the treatment is shown in the figure. However, when the patient fails to detect clones, there is no consensus on the treatment prior to kidney transplantation.
Another important and controversial issue is the choice of chemotherapy regimen when patients with MGRS-associated PGNMID clearly need chemotherapy. This is a good clue if circulating clonal cells consistent with renal tissue deposition can be detected in MGRS-associated PGNMID patients so that we can target chemotherapy (rituximab-based regimens for potential B-cell clones and bortezomib-based regimens for potential plasma cell clones). For MGRS-associated PGNMID patients who fail to detect clonal cells in the circulation consistent with renal deposition, empirical chemotherapy is currently the mainstay. Some scholars [18, 27] have noted that the clones of PGNMID were most often related to plasma cell clones (60–100%). Based on this view, a bortezomib-based regimen is recommended. Many cases of bortezomib-based therapy have been reported in the literature, with most patients showing improvement [47–49]. It has been reported that two patients did not respond to the initial regimen of rituximab and were subsequently relieved by bortezomib [19]. Of course, there are also empirical chemotherapies based on rituximab that have good efficacy [17, 50]—thalidomide or lenalidomide (immunomodulation) [17]. While empirical chemotherapy is given, the efficacy of the treatment needs to be closely evaluated and the regimen changed if necessary. With the current study results, it is not easy to choose a specific treatment, and large samples of evidence-based evidence are still needed.
CONCLUSION
PGNMID, as a histopathological entity, was identified as monoclonal-related nephropathy when it was first proposed in 2004 [14]. As research progressed, scholars gradually realized that not all PGNMID was associated with hematological tumors and that infections and solid tumors could also cause the disease [20, 37, 40]. The detection rate of circulating clones in PGNMID patients is low and the diagnosis of the disease is based mainly on renal pathology. Restriction of IgG subclasses or LC subtypes is not sufficient to diagnose PGNMID, and it is necessary to complete the staining of light and heavy chain isoforms for comprehensive analysis and careful differentiation. For patients with PGNMID associated with malignant hematologic neoplasms, early chemotherapy is emphasized. Immune disorder-associated PGNMID can be treated for the etiology of the disease. Moreover, treatment of MGRS-associated PGNMID is mostly administered empirically based on the patient's condition.
While the interest in PGNMID is on the rise, the knowledge of it remains limited. The pathogenic mechanisms of PGNMID are unclear and effective treatment remains challenging. Further research is necessary to find biomarkers, describe their mechanisms, improve detection sensitivity and identify clones to guide treatment.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Professor Cheng Zhen from Nanjing Jinling Hospital for his support of the project.
Contributor Information
Manna Li, Department of Nephrology, the Second Affiliated Hospital of Nanchang University, Jiangxi, China.
Gaosi Xu, Department of Nephrology, the Second Affiliated Hospital of Nanchang University, Jiangxi, China.
FUNDING
This work was supported by the National Natural Science Foundation of China (grants 81970583 and 82060138) and the Nature Science Foundation of Jiangxi Province (grants 20181BAB205016 and 20202BABL206025).
AUTHORS’ CONTRIBUTIONS
M.L. contributed the idea of the article, performed the literature search and data analysis, and drafted the article. Professor G.X. critically revised the work.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1. The International Myeloma Working Group . Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003; 121: 749–757 [PubMed] [Google Scholar]
- 2. Leung N, Bridoux F, Hutchison CAet al. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood 2012; 120: 4292–4295 [DOI] [PubMed] [Google Scholar]
- 3. Leung N, Bridoux F, Batuman Vet al. The evaluation of monoclonal gammopathy of renal significance: a consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat Rev Nephrol 2019; 15: 45–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fermand J, Bridoux F, Dispenzieri Aet al. Monoclonal gammopathy of clinical significance: a novel concept with therapeutic implications. Blood 2018; 132: 1478–1485 [DOI] [PubMed] [Google Scholar]
- 5. Glavey S, Leung N. Monoclonal gammopathy: the good, the bad and the ugly. Blood Rev 2016; 30: 223–231 [DOI] [PubMed] [Google Scholar]
- 6. Zand L, Nasr S, Gertz Met al. Clinical and prognostic differences among patients with light chain deposition disease, myeloma cast nephropathy and both. Leuk Lymphoma 2015; 56: 3357–3364 [DOI] [PubMed] [Google Scholar]
- 7. Fermand JP, Bridoux F, Kyle RAet al. How I treat monoclonal gammopathy of renal significance (MGRS). Blood 2013; 122: 3583–3590 [DOI] [PubMed] [Google Scholar]
- 8. Vignon M, Javaugue V, Alexander Met al. Current anti-myeloma therapies in renal manifestations of monoclonal light chain-associated Fanconi syndrome: a retrospective series of 49 patients. Leukemia 2017; 31: 123–129 [DOI] [PubMed] [Google Scholar]
- 9. Sayed RH, Wechalekar AD, Gilbertson JAet al. Natural history and outcome of light chain deposition disease. Blood 2015; 126: 2805–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nasr SH, Satoskar A, Markowitz GSet al. Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol 2009; 20: 2055–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bridoux F, Leung N, Hutchison CAet al. Diagnosis of monoclonal gammopathy of renal significance. Kidney Int 2015; 87: 698–711 [DOI] [PubMed] [Google Scholar]
- 12. Nasr SH, Sethi S, Cornell LDet al. Proliferative glomerulonephritis with monoclonal IgG deposits recurs in the allograft. Clin J Am Soc Nephrol 2011; 6: 122–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Said SM, Cosio FG, Valeri AMet al. Proliferative glomerulonephritis with monoclonal immunoglobulin G deposits is associated with high rate of early recurrence in the allograft. Kidney Int 2018; 94: 159–169 [DOI] [PubMed] [Google Scholar]
- 14. Nasr SH, Markowitz GS, Stokes MBet al. Proliferative glomerulonephritis with monoclonal IgG deposits: a distinct entity mimicking immune-complex glomerulonephritis. Kidney Int 2004; 65: 85–96 [DOI] [PubMed] [Google Scholar]
- 15. Torrealba J, Gattineni J, Hendricks AR. Proliferative glomerulonephritis with monoclonal immunoglobulin G lambda deposits: report of the first pediatric case. Case Rep Nephrol Dial 2018; 8: 70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xing G, Gillespie R, Bedri Bet al. Proliferative glomerulonephritis with monoclonal IgG deposits in children and young adults. Pediatr Nephrol 2018; 33: 1531–1538 [DOI] [PubMed] [Google Scholar]
- 17. Guiard E, Karras A, Plaisier Eet al. Patterns of noncryoglobulinemic glomerulonephritis with monoclonal Ig deposits: correlation with IgG subclass and response to rituximab. Clin J Am Soc Nephrol 2011; 6: 1609–1616 [DOI] [PubMed] [Google Scholar]
- 18. Bhutani G, Nasr SH, Said SMet al. Hematologic characteristics of proliferative glomerulonephritides with nonorganized monoclonal immunoglobulin deposits. Mayo Clin Proc 2015; 90: 587–596 [DOI] [PubMed] [Google Scholar]
- 19. Gumber R, Cohen JB, Palmer MBet al. A clone-directed approach may improve diagnosis and treatment of proliferative glomerulonephritis with monoclonal immunoglobulin deposits. Kidney Int 2018; 94: 199–205 [DOI] [PubMed] [Google Scholar]
- 20. Fujita E, Shimizu A, Kaneko Tet al. Proliferative glomerulonephritis with monoclonal immunoglobulin G3κ deposits in association with parvovirus B19 infection. Hum Pathol 2012; 43: 2326–2333 [DOI] [PubMed] [Google Scholar]
- 21. Kourelis T, Nasr S, Dispenzieri Aet al. Outcomes of patients with renal monoclonal immunoglobulin deposition disease. Am J Hematol 2016; 91: 1123–1128 [DOI] [PubMed] [Google Scholar]
- 22. Nasr S, Valeri A, Cornell Let al. Renal monoclonal immunoglobulin deposition disease: a report of 64 patients from a single institution. Clin J Am Soc Nephrol 2012; 7: 231–239 [DOI] [PubMed] [Google Scholar]
- 23. Bridoux F, Javaugue V, Nasr SHet al. Proliferative glomerulonephritis with monoclonal immunoglobulin deposits: a nephrologist perspective. Nephrol Dial Transplant 2021; 36: 208–215 [DOI] [PubMed] [Google Scholar]
- 24. Ramos R, Poveda R, Sarrá Jet al. Renal involvement in non-malignant IgM gammopathy. Nephrol Dial Transplant 2007; 22: 627–630 [DOI] [PubMed] [Google Scholar]
- 25. Kaneko S, Usui J, Narimatsu Yet al. Renal involvement of monoclonal immunoglobulin deposition disease associated with an unusual monoclonal immunoglobulin A glycan profile. Clin Exp Nephrol 2010; 14: 389–395 [DOI] [PubMed] [Google Scholar]
- 26. Yahata M, Nakaya I, Takahashi Set al. Proliferative glomerulonephritis with monoclonal IgM deposits without Waldenstrom's macroglobulinemia: case report and review of the literature. Clin Nephrol 2012; 77: 254–260 [DOI] [PubMed] [Google Scholar]
- 27. Nasr SH, Larsen CP, Sirac Cet al. Light chain only variant of proliferative glomerulonephritis with monoclonal immunoglobulin deposits is associated with a high detection rate of the pathogenic plasma cell clone. Kidney Int 2020; 97: 589–601 [DOI] [PubMed] [Google Scholar]
- 28. Best Rocha A, Larsen C. Membranous glomerulopathy with light chain-restricted deposits: a clinicopathological analysis of 28 cases. Kidney Int Rep 2017; 2: 1141–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alpers C, Tu W, Hopper Jet al. Single light chain subclass (Kappa chain) immunoglobulin deposition in glomerulonephritis. Hum Pathol 1985; 16: 294–304 [DOI] [PubMed] [Google Scholar]
- 30. Yu XJ, Wang MJ, Yong ZHet al. Proliferative glomerulonephritis with monoclonal IgG3 lambda deposits: a case report of a rare cause of monoclonal gammopathy of renal significance. Kidney Med 2019; 1: 221–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bridoux F, Hugue V, Coldefy Oet al. Fibrillary glomerulonephritis and immunotactoid (microtubular) glomerulopathy are associated with distinct immunologic features. Kidney Int 2002; 62: 1764–1775 [DOI] [PubMed] [Google Scholar]
- 32. Larsen C, Boils C, Cossey Let al. Clinicopathologic features of membranous-like glomerulopathy with masked IgG kappa deposits. Kidney Int Rep 2016; 1: 299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Larsen C, Sharma S, Caza Tet al. Serum amyloid P deposition is a sensitive and specific feature of membranous-like glomerulopathy with masked IgG kappa deposits. Kidney Int 2020; 97: 602–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Herlitz L, Bomback A, Markowitz Get al. Pathology after eculizumab in dense deposit disease and C3 GN. J Am Soc Nephrol 2012; 23: 1229–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rother R, Rollins S, Mojcik Cet al. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol 2007; 25: 1256–1264 [DOI] [PubMed] [Google Scholar]
- 36. Sirac C, Herrera G, Sanders Pet al. Animal models of monoclonal immunoglobulin-related renal diseases. Nat Rev Nephrol 2018; 14: 246–264 [DOI] [PubMed] [Google Scholar]
- 37. Yamada T, Arakawa Y, Mii Aet al. A case of monoclonal immunoglobulin G1-lambda deposition associated with membranous feature in a patient with hepatitis C viral infection. Clin Exp Nephrol 2012; 16: 468–472 [DOI] [PubMed] [Google Scholar]
- 38. Alpers CE, Kowalewska J. Emerging paradigms in the renal pathology of viral diseases. Clin J Am Soc Nephrol 2007; 2 (Suppl 1): S6–S12 [DOI] [PubMed] [Google Scholar]
- 39. Kamar N, Izopet J, Alric Let al. Hepatitis C virus-related kidney disease: an overview. Clin Nephrol 2008; 69: 149–160 [DOI] [PubMed] [Google Scholar]
- 40. Higashihara T, Okada A, Nakamura Yet al. Proliferative glomerulonephritis with monoclonal immunoglobulin deposits without conspicuous mesangial proliferation, complicated with squamous cell lung carcinoma. Intern Med 2020; 59: 557–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jhaveri K, Shah H, Calderon Ket al. Glomerular diseases seen with cancer and chemotherapy: a narrative review. Kidney Int 2013; 84: 34–44 [DOI] [PubMed] [Google Scholar]
- 42. Poitou-Verkinder A, Francois A, Drieux Fet al. The spectrum of kidney pathology in B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma: a 25-year multicenter experience. PLoS One 2015; 10: e0119156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luciano R, Brewster U. Kidney involvement in leukemia and lymphoma. Adv Chronic Kidney Dis 2014; 21: 27–35 [DOI] [PubMed] [Google Scholar]
- 44. Kyle R, Benson J, Larson Det al. Progression in smoldering Waldenstrom macroglobulinemia: long-term results. Blood 2012; 119: 4462–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lai Z, Kumar T, Zhao Ret al. Monoclonal gammopathy of renal significance and its associated experimental models. Ann Clin Lab Sci 2019; 49: 439–447 [PubMed] [Google Scholar]
- 46. van Kruijsdijk RCM, Abrahams AC, Nguyen TQet al. Clone-directed therapy for proliferative glomerulonephritis with monoclonal immunoglobulin depositions: is it always necessary? Two case reports and literature review. J Nephrol 2020; 33: 611–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Watanabe H, Osawa Y, Goto Set al. A case of endocapillary proliferative glomerulonephritis with macrophages phagocytosing monoclonal immunoglobulin lambda light chain. Pathol Int 2015; 65: 38–42 [DOI] [PubMed] [Google Scholar]
- 48. Noto R, Kamiura N, Ono Yet al. Successful treatment with bortezomib and dexamethasone for proliferative glomerulonephritis with monoclonal IgG deposits in multiple myeloma: a case report. BMC Nephrol 2017; 18: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee H, Duggan P, Neri Pet al. Bortezomib maintenance for the treatment of monoclonal gammopathy of renal significance. Mediterr J Hematol Infect Dis 2019; 11: e2019007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Buxeda A, Said SM, Nasr SHet al. Recurrent proliferative glomerulonephritis with monoclonal immunoglobulin deposits in kidney allografts treated with anti-CD20 antibodies. Transplantation 2019; 103: 1477–1485 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.