ABSTRACT
Background
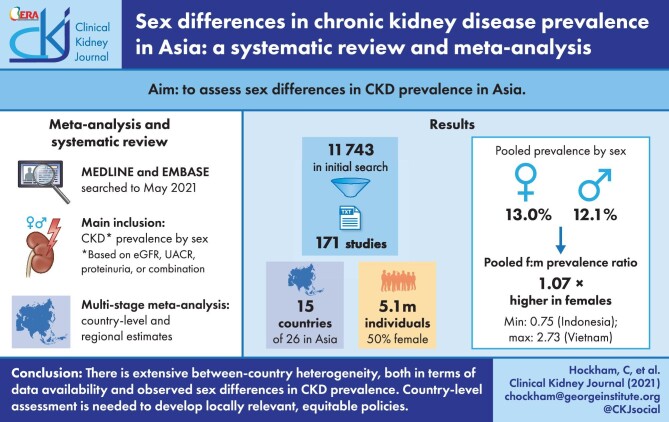
Previous reports on the prevalence of chronic kidney disease (CKD) in Asia have suggested important sex disparities but have been inconsistent in nature. We sought to synthesize available sex-disaggregated CKD prevalence data in Asia to quantify sex disparities in the region.
Methods
We systematically searched MEDLINE and Embase for observational studies involving ≥500 adults who reported sex-disaggregated CKD prevalence data in any of the 26 countries in East, Southeast and South Asia. For each study we calculated the female:male prevalence ratio (PR), with a ratio >1 indicating a higher female prevalence. For each country, log-transformed PRs were pooled using random effects meta-analysis. These were then combined using a fixed effects model, weighting by population size, to estimate a pooled PR for each of East, Southeast and South Asia and Asia overall.
Results
Sex-disaggregated data were available from 171 cohorts, spanning 15 countries and comprising 2 550 169 females and 2 595 299 males. Most studies (75.4%) came from East Asia (China, Taiwan, Japan and South Korea). Across Asia, CKD prevalence was higher in females {pooled prevalence 13.0% [95% confidence interval (CI) 11.3–14.9]} compared with males [pooled prevalence 12.1% (95% CI 10.3–14.1)], with a pooled PR of 1.07 (95% CI 0.99–1.17). Substantial heterogeneity was observed between countries. The pooled PRs for East, Southeast and South Asia were 1.11 (95% CI 1.02–1.21), 1.09 (0.88–1.36) and 1.03 (0.87–1.22), respectively.
Conclusions
Current evidence suggests considerable between-country and -region heterogeneity in the female:male PR of CKD. However, there remains a large part of the region where data on sex-specific CKD prevalence are absent or limited. Country-level assessment of the differential burden of CKD in females and males is needed to define locally relevant policies that address the needs of both sexes.
Keywords: albuminuria, CKD, epidemiology, gender, systematic review
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Chronic kidney disease (CKD) is a major cause of morbidity and mortality worldwide. In 2017, it was estimated that almost 700 million individuals worldwide were living with CKD, with 1.2 million deaths and 35.8 million disability-adjusted life years (DALYs) directly attributed to the disease [1]. Generally defined as an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.72 m2 and/or a urine albumin:creatinine ratio (UACR) ≥30 mg/g (≥3 mg/mmol) [2], CKD is associated with a wide range of adverse health outcomes. The most prominent include an increased risk of cardiovascular disease (CVD) and hypertension; however, mineral and bone disorder, anaemia and infection are also common in patients nearing kidney failure [3]. Moreover, adverse effects of CKD on health-related quality of life, including for milder CKD stages, have been reported [4–7].
Similar to other major chronic diseases (e.g. diabetes and coronary heart disease), there is accumulating evidence that females and males are differentially affected by CKD in terms of prevalence, disease progression and health outcomes [8, 9]. However, research in this area remains limited and there is uncertainty around the magnitude and direction of these differences, which themselves likely vary by outcome and geographically. In Asia, the unique aetiological profile of CKD [10–12], together with the ongoing epidemiological transition [13, 14] and vast sociocultural diversity, makes generalizations of sex- and gender-based findings from other parts of the world particularly challenging. Understanding regionally specific sex differences in CKD burden will be important to inform health policy and planning and guide future research.
The aim of this systematic review and meta-analysis is to provide a comprehensive assessment of sex differences in CKD prevalence in Asia, the region projected to experience the greatest increase in disease burden.
MATERIALS AND METHODS
Search strategy and selection criteria
This systematic review and meta-analysis was performed in accordance with Meta-Analyses of Observational Studies in Epidemiology (MOOSE) guidelines [15].
We identified relevant studies by searching MEDLINE (from 1946 to 23 January 2020) and Embase (from 1980 to 23 January 2020), using broad keywords and Medical Subject Headings (MeSH) related to kidney function, proteinuria and Asian countries (Supplementary data, S1 Search Strategy). The same search was repeated on 24 May 2021 to identify papers published since January 2020, ensuring that the final review was as comprehensive and up-to-date as possible. The persons conducting the search were C.H. and M.J., both of whom are PhD-level trained epidemiologists. In addition, reference lists of all included studies were manually searched to identify additional studies not captured in the online search. Authors of relevant studies were contacted via e-mail if further information was required.
Observational studies involving ≥500 adults (≥18 years of age), conducted in East, Southeast or South Asia and reporting sex-disaggregated CKD prevalence data were included. Single-sex studies were excluded [16]. We included studies in which the primary objective was to assess CKD prevalence or CKD prevalence was reported as part of the baseline participant characteristics. If there was overlap in the cohort used across multiple studies, the one with the broadest inclusion criteria or largest sample size was used. We excluded reviews, letters to the editor, case reports and case series and restricted our review to studies published in English but placed no restriction on language in the literature search. CKD was defined as eGFR ≥60 mL/min/1.73 m2 with albuminuria [UACR ≥30 mg/g (≥3 mg/mmol)] or proteinuria (as defined by the authors) [2] or eGFR <60 mL/min/1.73 m2.
Data extraction and quality assessment
Abstract review, data abstraction and risk of bias assessment were performed independently by two reviewers (L.B. and A.T.). Any disagreements were adjudicated by a third reviewer (C.H. and M.J.). Data from the included studies were extracted using a standardized data extraction spreadsheet. Extracted information included study design, setting, source population and selection criteria, number of females and number of males, mean age, percentage of people with diabetes, percentage of people with hypertension, definition of CKD (based on eGFR and albuminuria/proteinuria, eGFR only and albuminuria/proteinuria only), eGFR equation used and sex-specific CKD prevalence.
Risk of bias was assessed using a modified version of the Newcastle–Ottawa Quality Assessment Scale for cohort studies [17, 18]. Each study was assessed against five items: representativeness of the sample, sample size (<4000 and ≥4000 participants), comparison of patient characteristics between the CKD cohort and the non-CKD cohort, outcome ascertainment and reporting of descriptive statistics [mean and standard deviation or median and interquartile range (IQR) for continuous variables and counts and percentages for categorical variables]. For each item on the scale, the study received a score of 0, denoting either insufficient information or evidence that the study did not meet the item requirement, or 1, denoting sufficient information to indicate a low risk of bias. The scores were then summed to obtain a final risk score. Studies with a score of 1–2, 3 and 4–5 were classified as having a high, medium and low risk of bias, respectively.
Data analysis
Primary analysis
For each study, sex-specific prevalence was calculated as the percentage of females or males identified as having CKD. The prevalence in females was divided by the prevalence in males to obtain the female:male prevalence ratio (PR), with a PR >1 indicating a higher prevalence in females. Prevalence estimates and the corresponding PRs were log-transformed prior to pooling. For each country, DerSimonian–Laird random effects models were used to estimate the pooled sex-specific CKD prevalence estimates and the pooled PR [19]. These country-stratified pooled estimates were then further meta-analysed using fixed effects models, with weighting by the country's population size, to derive pooled estimates for East Asia, Southeast Asia, South Asia and Asia overall. Population estimates for 2019 were obtained from the United Nations Population Division (https://population.un.org/wpp/DataQuery/, accessed 22 October 2020).
Subgroup analyses
To examine possible causes of heterogeneity (irrespective of country), subgroup analyses were done by sample size (N < 4000 and N ≥ 4000), percentage of females in the study cohort (<median of 52.1% and ≥median), mean age of the study cohort (<65 years and ≥65 years), percentage of individuals with diabetes (<median of 31.2% and ≥median), percentage of individuals with hypertension (<median of 61.8% and ≥median), eGFR equation [Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [20], Modification of Diet in Renal Disease (MDRD) [21] and other] and risk of bias (low, medium and high). The Cochran's Q-statistic for the null hypothesis of homogeneity was used to assess heterogeneity between the subgroups.
Sensitivity analyses
We performed a series of sensitivity analyses to test the robustness of our findings. First, we removed studies that had a strong influence on the country-level pooled PR estimates. These were identified using a combination of studentized residuals (with an absolute threshold of 2 indicating an outlier), leave-one-out analyses and diagnostic plots of the Cook's distance [22]. Second, meta-analyses were repeated using only high-risk cohorts, defined as participants selected based on the presence of diabetes, hypertension, any CVD or age ≥65 years and using cohorts sampled from non-high-risk populations (e.g. general population or hospital populations attending an annual health check). Previous work has shown that diabetes, hypertension and CVD might progress differently in females compared with males [23–26]. Given that these conditions are strongly associated with CKD, it is possible that their differential effects in females and males are reflected in sex differences in CKD prevalence that are specific to multimorbidity. Analyses were also repeated using the largest study from each country [27]. We also performed sensitivity analyses for different definitions of CKD, in which we included studies that assessed CKD based on both eGFR and albuminuria/proteinuria, on eGFR alone and on albuminuria/proteinuria alone. For studies that assessed and reported on both measurements, we extracted prevalence data for each of the measures separately. Finally, analyses were repeated for advanced CKD [Kidney Disease: Improving Global Outcomes (KDIGO) Stages 4–5], but were restricted to studies with at least 10 occurrences of advanced CKD events in each of the sexes.
Meta-analyses were performed in R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria) using the metafor package (version 3.0-2) [19], with forestplots generated using the forestplot package (version 1.10-1) [28] and maps using the sf (version 1.0-4) [29] and tmap (version 3.3-2) [30] packages.
RESULTS
Search results and characteristics of included studies
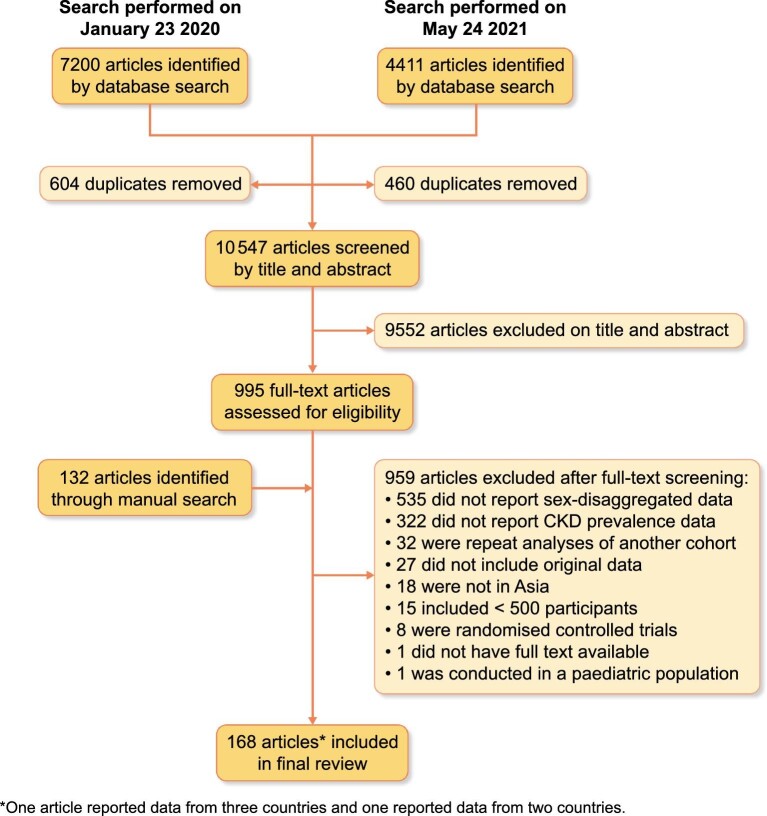
Our initial database search identified 7200 studies in January 2020 and a further 4411 in May 2021 (total 11 611), of which 995 articles qualified for full-text review. A further 132 studies were identified through manual searches. In total, 168 studies met the inclusion criteria during full-text review, of which one reported data from three countries and one from two countries (Figure 1).
FIGURE 1:
Flow diagram of study selection.
Sex-disaggregated CKD prevalence data were available for 15 of the 26 Asian countries, involving 2 550 169 females and 2 595 299 males. Most of the studies (75.4%) and participants (91.2% of females and 89.7% of males) came from East Asia (China, Taiwan, Japan or South Korea) (Table 1). Thirty-eight per cent (n = 65) were conducted in high-risk cohorts and 62.0% (n = 106) involved non-high-risk cohorts. Around a quarter (n = 43) of studies defined CKD based on either a reduced eGFR (<60 mL/min/1.73 m2) or the presence of albuminuria or proteinuria; 62.5% (n = 113) of studies defined CKD based on a reduced eGFR alone and 11.1% (n = 15) based only on the presence of albuminuria or proteinuria. Of the 156 studies that measured eGFR to ascertain CKD status, 51.3% (n = 80) used the MDRD equation, 25.0% (n = 39) used the CKD-EPI equation, 12.8% (n = 20) used a different equation and 8.3% (n = 13) did not specify the equation used. The characteristics of individual studies are provided in Supplementary data, Table S1.
Table 1.
Characteristics of included studies, summarized by country and region
| Country | Studies, n (high-risk; non-high-risk) | Participants, N (% female) | Females, median (IQR) | Males, median (IQR) | People with diabetes (%), median (IQR) | People with hypertension (%), median (IQR) | Interval of reported mean age (years) |
|---|---|---|---|---|---|---|---|
| Asia | 171 (65; 106)a | 5 139 384 (49.6) | 1866 (636–6012) | 1913 (655–5076) | 17.0 (8.6–36.6) | 40.4 (26.5–57.8) | 35.6–102.8b |
| East Asia | 129 (54; 75) | 4 648 661 (50.0) | 2123 (647–7141) | 2178 (794–7791) | 15.3 (8.4–36.1) | 43.8 (27.4–58.3) | 36.0–102.8b |
| China | 46 (18; 28) | 788 307 (45.2) | 1865 (780–6144) | 1990 (739–5136) | 18.4 (9.6–38.6) | 48.9 (33.0–63.4) | 41.8–102.8 |
| Taiwan | 21 (9; 12) | 799 479 (50.0) | 3075 (647–13 699) | 3393 (1142–12 991) | 19.5 (8.8–36.9) | 38.6 (23.3–59.8) | 36.0–74.9 |
| South Korea | 18 (7; 11) | 1 139 257 (40.8) | 5189 (858–16 741) | 4353 (1882–13 441) | 19.1 (9.3–31.5) | 30.0 (21.8–48.8) | 41.7–76.0 |
| Japan | 44 (20; 24) | 1 921 618 (57.6) | 1 891 (404–6176) | 1686 (620–4132) | 13.9 (5.4–34.4) | 45.1 (29.9–55.1) | 40.0–74.2 |
| Southeast Asia | 12 (5; 7) | 127 529 (51.9) | 1278 (755–2589) | 1446 (471–3225) | 39.0 (17.7–85.7) | 55.0 (35.6–59.9) | 48.8–65.9 |
| Indonesia | 1 (0; 1) | 1496 (63.4) | 949 | 547 | Not reported | Not reported | Not reported |
| Malaysia | 3 (2; 1) | 8519 (22.5) | 595 (560–697) | 471 (419–3118) | 40.3 (30.0–70.2) | 55.8 (53.4–58.3) | 48.8–55.4 |
| Singapore | 3 (1; 2) | 95 609 (52.1) | 1606 (1238–24 469) | 3 923 (2184–22 679) | 37.6 (27.3–40.2) | 56.6 (55.0–69.9) | 62.9–65.9 |
| Thailand | 4 (2; 2) | 13 400 (59.9) | 2 158 (1574–2589) | 1 446 (1110–1681) | 56.3 (12.4–100.0) | 42.5 (27.1–63.4) | 61.4–61.6 |
| Vietnam | 1 (0; 1) | 8505 (64.8) | 5513 | 2992 | Not reported | 30.5 | 57.2 |
| South Asia | 30 (6; 24) | 363 194 (43.4) | 1 055 (625–2430) | 1 243 (557–3136) | 14.0 (7.5–21.6) | 31.2 (16.7–41.6) | 35.6–67.5 |
| Bangladesh | 4 (3; 1) | 222 591 (43.6) | 728 (586–24 377) | 690 (573–31 529) | 61.2 (41.8–80.6) | 58.4 (37.5–79.2) | 47.0–56.7 |
| India | 11 (1; 10) | 37 549 (48.6) | 1154 (942–1982) | 1112 (849–2662) | 8.6 (6.1–17.6) | 31.2 (16.7–33.6) | 35.6–51.0 |
| Nepal | 1 (0; 1) | 20 811 (61.5) | 12 792 | 8019 | Not reported | Not reported | 39.0 |
| Pakistan | 3 (1; 2) | 5183 (52.3) | 796 (606–1148) | 1374 (822–1499) | 19.0 (18.6–20.2) | 72.1 (58.1–86.0) | 51.5–56.7 |
| Sri Lanka | 2 (1; 1) | 6949 (54.8) | 1 904 (1224–2584) | 1571 (911–2230) | 20.6 (11.8–29.3) | 52.6 (28.8–76.3) | 62.9 |
| Iran | 9 (0; 9) | 70 111 (32.8) | 760 (503–5840) | 1399 (481–4223) | 13.7 (9.4–14.0) | 25.8 (17.2–38.0) | 39.8–67.5 |
In total, 168 studies met our inclusion criteria, with 1 of these reporting data from three countries and 1 from two countries. The total number of data points is therefore 171.
The highest mean age of 102.8 years is from a study that specifically recruited individuals ≥100 years.
The risk of bias assessment indicated that 89 (52.0%) studies had a low risk of bias (score of 4–5), 61 (35.7%) had a medium risk of bias (score of 3) and 21 (12.3%) had a high risk of bias (score of 1–2).
Sex disparities in CKD prevalence
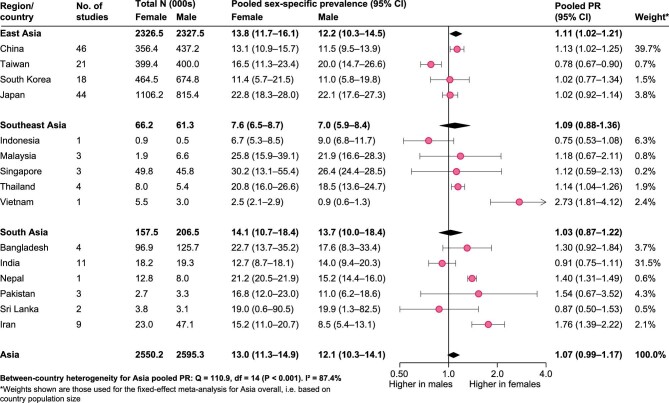
Reported sex-specific CKD prevalence estimates varied substantially between the studies (Supplementary data, Table S1). Across Asia, the pooled prevalence of CKD based on all included studies was 13.0% [95% confidence interval (CI) 11.3–14.9] in females and 12.1% (95% CI 10.3–14.1) in males (Figure 2 and Supplementary data, Figure S1). The pooled prevalence of CKD was higher in females compared with males [PR 1.07 (95% CI 0.99–1.17)]. There was substantial between-country heterogeneity (I2 = 87.4%), with the PR ranging from 0.75 (95% CI 0.53–1.08; n = 1) in Indonesia to 2.73 (95% CI 1.81–4.12; n = 1) in Vietnam. China and India, the two largest countries in Asia, had a pooled PR of 1.11 (95% CI 1.02–1.21; n = 46) and 0.91 (95% CI 0.75–1.11; n = 11), respectively. The strongest evidence for a higher CKD prevalence in females came from East Asia, with a pooled PR of 1.11 (95% CI 1.02–1.21), driven by the large population size of China. In contrast, there was no evidence for a sex difference in either Southeast Asia [PR 1.09 (95% CI 0.88–1.36)] or South Asia [PR 0.98 (95% CI 0.85–1.22)].
FIGURE 2:
Sex-specific CKD prevalence estimates and female:male PR, pooled by country (estimated using random effects meta-analysis) and by region (estimated using fixed effects meta-analysis of country-stratified pooled estimates). For the fixed effects meta-analysis, country-level estimates were weighted by country population size.
Subgroup and sensitivity analyses
In subgroup analyses of all studies (i.e. not stratifying by country), no differences in the pooled PRs were observed for the subgroups examined, including the different risk of bias categories (Supplementary data, Figure S2). Overall, results were largely consistent in sensitivity analyses in which influential outliers were removed from the country-level meta-analysis [pooled PR for Asia 1.06 (95% CI 0.99–1.14); Supplementary data, Figure S3], as well as those in which studies were restricted to either non-high-risk or high-risk population cohorts [pooled PRs for Asia 1.06 (95% CI 0.96–1.17) and 1.04 (95% CI 0.96–1.13), respectively; Supplementary data, Figures S4–S7]. There were notable differences in the pooled PR for Asia when only the largest study from each country was used [pooled PR 1.16 (95% CI 1.10–1.22)]. At the country and subregional levels, the robustness of our findings to the specific study inclusion criteria (i.e. non-high-risk or high-risk cohorts) varied between countries or sub-regions. Estimates were particularly variable in countries and regions where the evidence base was limited.
In analyses involving studies in which CKD was defined according to both eGFR and albuminuria/proteinuria (n = 43; 11 countries), the pooled PR was 0.91 (95% CI 0.81–1.03). The pooled PR for reduced kidney function (eGFR <60 mL/min/1.732) was 1.21 (95% CI 1.03–1.44; n = 140 from 15 countries), while for albuminuria/proteinuria, it was 0.94 (95% CI 0.82–1.07; n = 39 from 10 countries) (Supplementary data, Figures S8–S13).
When restricted to the nine countries where sex-disaggregated prevalence data for advanced CKD (KDIGO stages 4–5) were available, the pooled PR for advanced CKD for Asia was 1.04 (95% CI 0.72–1.48; Supplementary data, Figure S14). Once again, heterogeneity between countries was observed (I2 = 81.7%). The pooled PRs for East Asia, Southeast Asia and South Asia were 1.17 (95% CI 0.82–1.66), 1.03 (95% CI 0.67–1.60) and 0.89 (95% CI 0.45–1.78), respectively.
DISCUSSION
In a pooled analysis of 171 cohorts, comprising 2 550 169 females and 2 595 299 males from 15 Asian countries, the overall prevalence of CKD in the region was 1.07 (95% CI 0.99–1.17) times higher in females compared with males, but with considerable variation between countries. Of note, China and India, which account for 61% of the Asian population, showed contrasting sex differences; in China, the prevalence of CKD was higher in females, while in India a similar prevalence between the sexes was observed. Collectively these findings highlight the need for more widespread sex-disaggregated data collection and CKD burden estimation in Asia and targeted CKD detection and management approaches that consider the local differences in the sex-specific burden of CKD.
Our finding that the prevalence of CKD is overall higher in females compared with males is consistent with previous systematic reviews [27, 31, 32]. In 2016, a meta-analysis of 51 studies reporting sex-specific CKD prevalence found a mean prevalence of CKD of 14.6% in females and 12.8% in males, corresponding to a female:male PR of 1.14 [31]. More recently, the 2017 Global Burden of Disease (GBD) Study reported that the global age-standardized prevalence of CKD was 1.29 (95% CI 1.28–1.30) times higher in females than in males [33]. However, the global scope of these analyses comes at the cost of granularity, potentially masking any geographic variation in the observed sex differences. The only study to examine sex-specific CKD prevalence at a country level did not report any measures of uncertainty in the observed differences [32]. Our review addresses these two important limitations and provides the most comprehensive, up-to-date review of sex differences in CKD prevalence across Asia.
It is generally purported that, for any given age, the prevalence of milder forms of CKD (KDIGO Stages 1–3) is higher in females, while the opposite is true for kidney failure (KDIGO Stage 5) [8, 27, 32, 34]. However, existing studies are limited by their reliance on kidney replacement therapy registry data, which in part reflect system-level access to care or personal preferences around care rather than the burden of the disease itself [35]. In the present study, which predominantly included studies that actively measured kidney function, the prevalence of CKD Stages 4–5 for Asia was similar between the sexes [pooled PR 1.04 (95% CI 0.72–1.48)]. Given that this is based on data from only 27 studies across the region, it is possible that we had insufficient power to detect a difference in prevalence between the sexes. It is interesting, however, that in all countries where data were available, the pooled female:male PR indicated either no sex difference or a higher female prevalence. Countries where there was evidence of a higher female prevalence of advanced CKD were Japan [PR 1.24 (95% CI 1.07–1.45), n = 7], Singapore [PR 1.16 (95% CI 1.05–1.27), n = 1], Bangladesh [PR 1.48 (95% CI 1.45–1.52), n = 1] and Iran [PR 1.97 (95% CI 1.09–3.56), n = 1]. Where the female-specific prevalence of advanced stages of CKD is higher, this may be explained by differential survival rates between females and males; at each level of kidney function, the CKD mortality rate has been shown to be higher in men [33]. However, gendered factors such as adherence to guideline-recommended medications, receipt of evidence-based care and social norms around access to care may also play a role. Nevertheless, it raises important questions around the need for gender-sensitive CKD research and health planning in the region.
For instance, the extent to which women are disproportionately burdened by CKD may be particularly heightened in countries with high out-of-pocket medical costs, compounded by lower lifetime earnings and longer life expectancy in women relative to men [36]. It is also important to consider that women with CKD have a higher risk of pre-term delivery and intrauterine growth restriction (IUGR), resulting in infants being born with a low birthweight and/or who are small for their gestational age [37]. This has implications for the burden of CKD and other non-communicable diseases in future generations, with evidence that both low birthweight and being small for gestational age increase the long-term risk of kidney failure, hypertension and diabetes [38–40]. Increasing awareness of CKD among women of child-bearing age and early detection of CKD should therefore be a priority. For women already living with CKD, efforts are needed to improve treatment rates and outcomes, which have been previously shown to be suboptimal among women compared with men [41]. Furthermore, a better understanding of the sex-specific mortality rate associated with CKD in Asian populations is needed to ensure that the needs of both women and men are appropriately addressed. Across the region, sex-disaggregated data collection, analysis and reporting should be embedded in CKD monitoring and surveillance systems as they are being developed.
Reasons for the observed sex differences are likely manifold. Several recent reviews on the role of sex and gender in CKD have highlighted a complex interplay of biological, behavioural, cultural and socio-economic factors that may contribute to observed disparities [8, 9, 34]. While the data used in this study cannot shed light on why sex differences may occur, we can speculate that the observed differences are the result of interactions between diverse factors, including both gendered factors related to health-related behaviours and access to care and biological factors influencing progression of disease and the underlying pathophysiological processes involved. For instance, while not statistically significant, the suggestion of a higher female:male PR in studies with a higher percentage of participants with diabetes or hypertension provides support for the hypothesis that the effect of these conditions on the risk of CKD might vary between the sexes, as has been observed for a range of vascular diseases. Among women, pregnancy-induced hypertension or gestational diabetes leading to a higher risk of hypertension and CVD post-pregnancy, and in turn CKD, may also play a role. Local investigations into the reasons underpinning the sex differences are needed.
We observed significant between-country heterogeneity in the pooled female:male PRs, which may be attributable to several factors. First, the availability of data varied across the region and we found that pooled estimates for countries with limited data (e.g. Singapore) were generally less robust against the types of cohorts (high-risk, non-high-risk or both) used in the meta-analysis. In contrast, for pooled analyses where a large number of studies were available (e.g. Asia overall or in China), decisions about which types of cohorts to include did not markedly affect the pooled PR. It is possible, therefore, that the absence of a sex difference in some countries or subregions is simply the result of there being insufficient data to detect a difference.
Second, formulae used to estimate GFR varied between studies, the most common one being the MDRD equation [n = 80 (51.3%) studies that measured eGFR]. While the MDRD equation can yield higher prevalence estimates compared with the CKD-EPI equation (the current gold standard) [35], our subgroup analysis of studies using different eGFR equations showed no difference in the pooled PRs. However, it remains possible that differences in the performance of eGFR formulae in different country settings may have contributed to the observed between-country heterogeneity. Validation studies conducted in community-based populations with appropriate age and ethnic diversity are needed to assess the impact of different GFR estimation methods on sex-specific CKD prevalence in this region.
Third, both the MDRD and CKD-EPI equations include sex as a variable [34] and assume that, for a given serum creatinine level, kidney function will be lower in females compared with males. This has led some to argue that the ascertainment of CKD based on these equations may overestimate CKD prevalence in females compared with males, particularly in the intermediate stages [8, 31]. Indeed, this might explain in part the higher female:male PR that was observed for studies that reported on the prevalence of a reduced eGFR in our sensitivity analyses [PR 1.21 (95% CI 1.03–1.44) compared with PR 0.94 (95% CI 0.82–1.07) in studies that only assessed urine protein] and could account for some of the heterogeneity in results between countries. For example, in Thailand, where the pooled PR was 1.14 (95% CI 1.04–1.26), three of the four included studies defined CKD based only on eGFR and the fourth assessed albuminuria as well. In comparison, only 2 of the 11 studies from India defined CKD using only eGFR, 8 assessed both measures and 1 assessed only albuminuria; the pooled PR estimate was 0.91 (95% CI 0.75–1.11). However, it is worth noting that between-country heterogeneity remained high even in analyses that only included the prevalence of a reduced eGFR (I2 = 90.0%) and that considerably fewer studies defined CKD using only albuminuria/proteinuria assessments (n = 39).
Limitations of our meta-analysis are inherent to the use of published data. Data were limited, or even absent, for a large part of the region, with only 42 studies available outside of East Asia. No data on sex-specific CKD prevalence were available for 11 countries: Afghanistan, Bhutan, Brunei, Cambodia, Democratic People's Republic of Korea, Laos, Maldives, Mongolia, Myanmar, the Philippines and Timor-Leste. There was also extensive heterogeneity between studies in terms of their sampling methodology and eligibility criteria, definition of CKD, assays used and study period. Nevertheless, most studies were found to have a low or medium risk of bias. In addition, few studies fully staged CKD based on both eGFR and albuminuria categories, as recommended by the KDIGO CKD guidelines [2], precluding the assessment of sex differences in each of the separate stages. Finally, the exclusion of articles published in languages other than English may have led to some important studies being missed.
In conclusion, our findings highlight the need for ongoing country-level assessment of sex differences in CKD prevalence such that locally relevant policies can be developed that address the needs of both females and males. As the global capacity for CKD surveillance is strengthened over the next 5–10 years, it will be important for sex- and gender-disaggregated data collection and reporting to be embedded within monitoring programmes as they are being developed.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dr Shuchi Anand, Dr Dimple Kondal and Prof. Prabhakar Dorairaj for contributing original data to this work.
Contributor Information
Carinna Hockham, George Institute for Global Health, School of Public Health, Imperial College London, London, UK.
Lexia Bao, George Institute for Global Health, University of New South Wales, Sydney, NSW, Australia; University of British Columbia, Vancouver, BC, Canada.
Anushree Tiku, George Institute for Global Health, University of New South Wales, Sydney, NSW, Australia.
Sunil V Badve, George Institute for Global Health, University of New South Wales, Sydney, NSW, Australia; Department of Renal Medicine, St George Hospital, Sydney, NSW, Australia.
Aminu K Bello, Division of Nephrology, University of Alberta, Edmonton, AB, Canada.
Meg J Jardine, NHMRC Clinical Trials Centre, University of Sydney, Sydney, NSW, Australia; Concord Repatriation General Hospital, Sydney, NSW, Australia.
Vivekanand Jha, George Institute for Global Health, School of Public Health, Imperial College London, London, UK; George Institute for Global Health, University of New South Wales International, New Delhi, India; Prasanna School of Public Health, Manipal Academy of Higher Education, Manipal, India.
Tadashi Toyama, Department of Nephrology, Kanazawa University, Kanazawa, Japan.
Mark Woodward, George Institute for Global Health, School of Public Health, Imperial College London, London, UK; George Institute for Global Health, University of New South Wales, Sydney, NSW, Australia.
Min Jun, George Institute for Global Health, University of New South Wales, Sydney, NSW, Australia; Faculty of Medicine and Health, University of New South Wales, Sydney, NSW, Australia.
DATA AVAILABILITY STATEMENT
Most of the data underlying this article are available in the article and in its online supplementary material. Data not included in the article (e.g. studies that were excluded from analyses) will be shared upon reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
C.H., L.B., A.T., A.B. and T.T. have no conflicts of interest to declare. S.B. has served on the advisory boards of AstraZeneca, Bayer and Vifor Pharma; has received speaker's honoraria from Bayer, Pfizer and Vifor Pharma and has received in-kind research support from Bayer. All fees have been paid to his institution. M.J.J. is responsible for research projects that have received unrestricted funding from Amgen, Baxter, CSL Behring, Eli Lilly, Gambro and MSD; has served on advisory boards sponsored by Akebia Therapeutics, AstraZeneca, Baxter, Bayer, Boehringer Ingelheim, MSD and Vifor Pharma; serves/has served on steering committees for trials sponsored by Chinook, CSL Behring and Janssen; serves on a steering committee for an investigator-initiated trial with funding support from Dimerix and has spoken at scientific meetings sponsored by Amgen, Janssen, Roche and Vifor Pharma. Any consultancy, honoraria or travel support has been paid to her institution. V.J. has research grants from NephroPlus, Baxter Healthcare and GlaxoSmithKline and received honoraria and lecture fees from AstraZeneca, Baxter Healthcare and Visterra. All monies are paid to his employer. M.W. is a consultant to Amgen, Freeline and Kyowa Kirin. M.J. reports receiving research support from VentureWise, a wholly owned subsidiary of NPS MedicineWise, to conduct a project funded by AstraZeneca. The results presented in this article have not been published previously in whole or part.
REFERENCES
- 1. Vos T, Abajobir AA, Abate KHet al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1211–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levey AS, de Jong PE, Coresh Jet al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011; 80: 17–28 [DOI] [PubMed] [Google Scholar]
- 3. Bello AK, Alrukhaimi M, Ashuntantang GEet al. Complications of chronic kidney disease: current state, knowledge gaps, and strategy for action. Kidney Int Suppl (2011) 2017; 7: 122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park JI, Baek H, Jung HH. CKD and health-related quality of life: the Korea National Health and Nutrition Examination Survey. Am J Kidney Dis 2016; 67: 851–860 [DOI] [PubMed] [Google Scholar]
- 5. Modi GK, Yadav AK, Ghosh Aet al. Nonmedical factors and health-related quality of life in CKD in India. Clin J Am Soc Nephrol 2020; 15: 191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peng Z, Wang J, Yuan Qet al. Clinical features and CKD-related quality of life in patients with CKD G3a and CKD G3b in China: results from the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE). BMC Nephrol 2017; 18: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wyld MLR, Morton RL, Clayton Pet al. The impact of progressive chronic kidney disease on health-related quality-of-life: a 12-year community cohort study. Qual Life Res 2019; 28: 2081–2090 [DOI] [PubMed] [Google Scholar]
- 8. Carrero JJ, Hecking M, Chesnaye NCet al. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 2018; 14: 151–164 [DOI] [PubMed] [Google Scholar]
- 9. Neugarten J, Golestaneh L. Influence of sex on the progression of chronic kidney disease. Mayo Clin Proc 2019; 94: 1339–1356 [DOI] [PubMed] [Google Scholar]
- 10. Anand S, Shivashankar R, Ali MKet al. Prevalence of chronic kidney disease in two major Indian cities and projections for associated cardiovascular disease. Kidney Int 2015; 88: 178–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jha V, Prasad N. CKD and infectious diseases in Asia Pacific: challenges and opportunities. Am J Kidney Dis 2016; 68: 148–160 [DOI] [PubMed] [Google Scholar]
- 12. Bragg-Gresham J, Thakur JS, Jeet Get al. Population-based comparison of chronic kidney disease prevalence and risk factors among adults living in the Punjab, Northern India and the USA (2013–2015). BMJ Open 2020; 10: e040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cook IG, Dummer TJB. Changing health in China: re-evaluating the epidemiological transition model. Health Policy 2004; 67: 329–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yadav S, Arokiasamy P. Understanding epidemiological transition in India. Glob Health Action 2014; 7: 23248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stroup DF, Berlin JA, Morton SCet al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012 [DOI] [PubMed] [Google Scholar]
- 16. Woodward M. Rationale and tutorial for analysing and reporting sex differences in cardiovascular associations. Heart 2019; 105: 1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elm Ev, Altman DG, Egger Met al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335: 806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wells G, Shea B, O'Connell Det al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-analyses. 2009. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (Accessed 3 August 2020) [Google Scholar]
- 19. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36: 1–48 [Google Scholar]
- 20. Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levey AS, Coresh J, Greene Tet al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247–254 [DOI] [PubMed] [Google Scholar]
- 22. Cook RD. Influential observations in linear regression. J Am Stat Assoc 1979; 74: 169–74 [Google Scholar]
- 23. de Ritter R, de Jong M, Vos RCet al. Sex differences in the risk of vascular disease associated with diabetes. Biol Sex Differ 2020; 11: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peters SAE, Woodward M. Sex differences in the burden and complications of diabetes. Curr Diab Rep 2018; 18: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gillis EE, Sullivan JC. Sex differences in hypertension: recent advances. Hypertension 2016; 68: 1322–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. den Ruijter HM, Haitjema S, Asselbergs FWet al. Sex matters to the heart: a special issue dedicated to the impact of sex related differences of cardiovascular diseases. Atherosclerosis 2015; 241: 205–207 [DOI] [PubMed] [Google Scholar]
- 27. Mills KT, Xu Y, Zhang Wet al. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int 2015; 88: 950–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gordon M, Lumley T. forestplot: Advanced Forest Plot Using ‘grid’ Graphics. 2020. https://cran.r-project.org/web/packages/forestplot/index.html (Accessed 8 August 2021) [Google Scholar]
- 29. Pebesma E. Simple features for R: standardized support for spatial vector data. R J 2018; 10: 439–446 [Google Scholar]
- 30. Tennekes M. tmap: thematic maps in R. J Stat Softw 2018; 84: 1–3930450020 [Google Scholar]
- 31. Hill NR, Fatoba ST, Oke JLet al. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS One 2016; 11: e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bikbov B, Perico N, Remuzzi G. Disparities in chronic kidney disease prevalence among males and females in 195 countries: analysis of the Global Burden of Disease 2016 Study. Nephron 2018; 313–318 [DOI] [PubMed] [Google Scholar]
- 33. GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020; 395: 709–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goldberg I, Krause I.. The role of gender in chronic kidney disease. Eur Med J 2016; 1: 58–64 [Google Scholar]
- 35. Levin A, Tonelli M, Bonventre Jet al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 2017; 390: 1888–917 [DOI] [PubMed] [Google Scholar]
- 36. Carrero JJ, Hecking M, Ulasi Iet al. Chronic kidney disease, gender, and access to care: a global perspective. Semin Nephrol 2017; 37: 296–308 [DOI] [PubMed] [Google Scholar]
- 37. Piccoli GB, Cabiddu G, Attini Ret al. Risk of adverse pregnancy outcomes in women with CKD. J Am Soc Nephrol 2015; 26: 2011–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gjerde A, Lillås BS, Marti H-Pet al. Intrauterine growth restriction, preterm birth and risk of end-stage renal disease during the first 50 years of life. Nephrol Dial Transplant 2020; 35: 1157–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Terstappen F, Lely AT. Long-term renal disease after prematurity or fetal growth restriction: who is at risk? Nephrol Dial Transplant 2020; 35: 1087–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Low Birth Weight and Nephron Number Working Group . The impact of kidney development on the life course: a consensus document for action. Nephron 2017; 136: 3–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ene-Iordache B, Perico N, Bikbov Bet al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health 2016; 4: e307–e319 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Most of the data underlying this article are available in the article and in its online supplementary material. Data not included in the article (e.g. studies that were excluded from analyses) will be shared upon reasonable request to the corresponding author.