Abstract
To investigate the occurrence of the flavonoid-degrading bacterium Eubacterium ramulus in the human intestinal tract, an oligonucleotide probe designated S-S-E.ram-0997-a-A-18 was designed and validated, with over 90 bacterial strains representing the dominant described human fecal flora. Application of S-S-E.ram-0997-a-A-18 to fecal samples from 20 subjects indicated the presence of E. ramulus in each individual tested in numbers from 4.4 × 107 to 2.0 × 109 cells/g of fecal dry mass. Six fecal E. ramulus isolates were recognized by S-S-E.ram-0997-a-A-18 but exhibited different band patterns when analyzed by randomly amplified polymorphic DNA.
Quercetin is a widespread flavonoid which is of pharmacological interest (4, 7, 21, 27). It is found, usually occurring as a glycoside, in vegetables, fruits, nuts, and tea, and is therefore an integral part of the human diet. The dietary quercetin intake is 16 mg/day (15). It is known that flavonoids are subject to bacterial degradation in the intestinal tract (8, 14). Using quercetin-3-glucoside (isoquercitrin) as a carbon and energy source, Schneider et al. (29) isolated Eubacterium ramulus from human fecal dilutions. The organism is able to cleave the flavonoid ring system to 3,4-dihydroxyphenylacetic acid, acetate, and butyrate. However, there is a lack of information on the occurrence and the significance of any of the flavonoid-degrading bacteria in the human intestinal system.
In recent years there has been an increasing effort to describe complex environments by in situ hybridization with 16S rRNA-targeted oligonucleotide probes (5, 10, 22, 25, 34). The aim of this study was to determine the occurrence of E. ramulus in the human intestinal tract by whole-cell hybridization. Therefore, an oligonucleotide probe (S-S-E.ram-0997-a-A-18 [1]) targeting a hypervariable region of the 16S rRNA from E. ramulus was designed by using the Arb software package (33), the Check_Probe function of the RDP software package (24), and the EMBL database. Table 1 depicts an alignment of S-S-E.ram-0997-a-A-18 and of the 16S rRNA target sequences of E. ramulus and related organisms. The dissociation temperature of S-S-E.ram-0997-a-A-18 as determined according to De Los Reyes et al. (9) was 55.2°C ± 0.3 (mean ± standard deviation).
TABLE 1.
Aligned sequences of the oligonucleotide probe S-S-E.ram-0997-a-A-18 and the 16S rRNA sequences of E. ramulus and phylogenetically related organismsa
| Probe or organism | Sequence |
|---|---|
| S-S-E.ram-0997-a-A-18 | 3′ GGGCCACTGACTTGTACA 5′ |
| Eubacterium ramulus | 5′ CCCGGUGACAGAACAUGU 3′ |
| Eubacterium rectale | 5′ ..UUC....C.GUAC.UA 3′ |
| Eubacterium contortum | 5′ ...CC....C.GCGU... 3′ |
| Roseburia cecicola | 5′ ..UUC.....AURU.... 3′ |
| Eubacterium uniforme | 5′ ....A..CA...CUU... 3′ |
| Butyrivibrio fibrisolvens (ATCC 19171)d | 5′ ..A.A...AU..UGGGUA 3′ |
| Butyrivibrio fibrisolvens (strain 49)bd | 5′ ..UCU.....A.GU.... 3′ |
| Butyrivibrio fibrisolvens (OB strains)d | 5′ ..UCU.....A.CU.... 3′ |
| Butyrivibrio fibrisolvens (strain BU 43)e | 5′ ..A.A...AUAUCGGGUA 3′ |
| Butyrivibrio fibrisolvens (NCDO 2221)e | 5′ ..A.A...AU..UGGGUA 3′ |
| Butyrivibrio fibrisolvens (NCDO 2222)e | 5′ ..A.A...AUAC.UGGUA 3′ |
| Butyrivibrio fibrisolvens (NCDO 2398)ce | 5′ ..A.A...AUAC.GGGUA 3′ |
| Butyrivibrio fibrisolvens (NCDO 2399)e | 5′ ...AC.....A.GU.... 3′ |
| Butyrivibrio fibrisolvens (NCDO 2434)e | 5′ ..A.A...AUAC.GGGUA 3′ |
| Butyrivibrio fibrisolvens (NCDO 2435)e | 5′ ..A.A...AUA.GUGGUA 3′ |
| Pseudobutyrivibrio ruminis | 5′ ...AC.....A.GU.... 3′ |
| Eubacterium ventriosum | 5′ ...AC......GU..GUA 3′ |
| Eubacterium hadrum | 5′ ..UUC....C.GU.C.UA 3′ |
| Eubacterium fissicatena | 5′ ...AC....C.GCGUGUA 3′ |
To ensure the specificity toward the target organism, S-S-E.ram-0997-a-A-18 was checked at 46°C by fluorescent in situ hybridization (FISH) and at 53°C by dot blot hybridization with over 90 reference species (see Appendix). All bacterial species used for validation were grown at 37°C under strictly anoxic conditions with N2 and CO2 (80:20 [vol/vol]) as a gas phase (6, 16) in a complex medium (17) or on Columbia blood-agar plates (BioMérieux, Nürtingen, Germany) incubated in anaerobic jars. For whole-cell hybridization, the bacteria were fixed as described elsewhere (3, 28) and hybridized on silanized (23), Teflon-surfaced microscopic slides with 5′-end-Cy3-labeled probes according to the procedure of Roller et al. (28). As a positive control, an equimolar mixture of five bacteria-specific probes (Eub338, Eub785, Eub927, Eub1055, Eub1088 [3, 18]) was used. The fluorescing cells were viewed with either an Optiphot-2 (Nikon, Düsseldorf, Germany) or an Axioplan-2 (Zeiss, Jena, Germany), equipped with filters for epifluorescence microscopy.
The rRNA used for dot blot hybridization was extracted by the procedure of Stahl et al. (32) as modified by Doré et al. (10). Three micrograms of denaturated RNA was blotted on positively charged nylon membranes (Boehringer, Mannheim, Germany) by using a Minifold dot blot apparatus (Schleicher & Schuell, Dassel, Germany), cross-linked for 3 min with a UV Stratalinker (Stratagene, La Jolla, Calif.), and hybridized overnight with 5′-end-digoxigenin (DIG)-labeled probes as described by Boehringer, Mannheim, Germany. After the membranes were washed twice at 56°C for 20 min, the DIG-labeled DNA-RNA hybrids were detected with the DIG luminescent detection kit with CSPD (Boehringer). The bacteria-specific probe Eub338 was applied as a positive control (2).
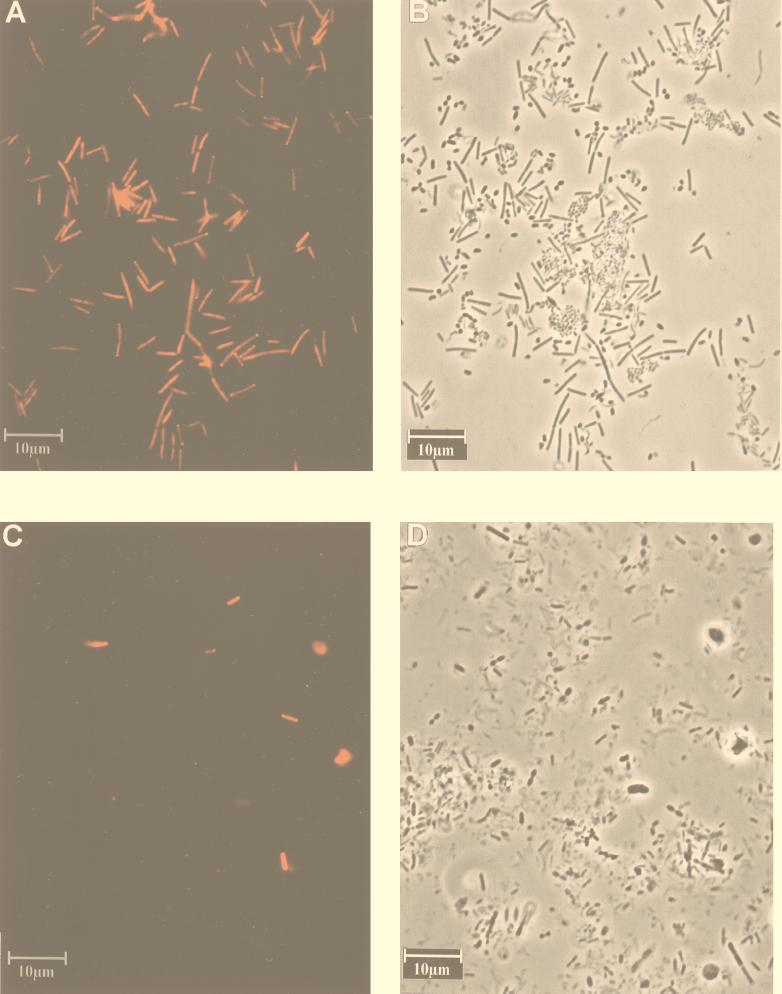
By both techniques S-S-E.ram-0997-a-A-18 hybridized exclusively with the target organism E. ramulus. All other organisms tested, including closely related bacteria, such as Butyrivibrio fibrisolvens and Eubacterium rectale, did not hybridize with S-S-E.ram-0997-a-A-18 (Fig. 1A and B). When the probe was applied to feces, some unspecific binding of the probe to undefined material was observed (Fig. 1C and D). However, this could be easily distinguished from the fluorescent cells. It must be remembered that with oligonucleotide probes in complex ecosystems, unknown bacteria unrelated to the target organism may be detected unspecifically. This, however, applies in general and is not restricted to the probe used here.
FIG. 1.
Epifluorescence and phase-contrast micrographs of a mixed culture and fecal sample. (A and B) Mixed culture of Butyrivibrio fibrisolvens, Eubacterium fissicatena, E. ramulus, E. rectale, Eubacterium uniforme, and Streptococcus pleomorphus hybridized with Cy3-labeled S-S-E.ram-0997-a-A-18. Panels A and B show the same microscopic field as viewed by epifluorescence and phase-contrast microscopy, respectively; (C and D) human fecal sample hybridized with Cy3-labeled S-S-E.ram-0997-a-A-18. Panels C and D show the same microscopic field, viewed by epifluorescence and phase-contrast microscopy, respectively.
To determine the occurrence of E. ramulus in the human intestinal tract, S-S-E.ram-0997-a-A-18 was applied to fecal samples. From 20 healthy volunteers aged 23 to 59 years, who consumed a Western diet and had not received any antibiotics at least for 3 months prior to the study, fresh fecal samples were collected and fixed as described above. The cells detected with the S-S-E.ram-0997-a-A-18 probe were enumerated and related to the bacteria detected with the Eub probe mixture (Table 2). The bacterial cells obtained with the Eub probe mix were between 1.98 × 1011 and 8.05 × 1011 per g of dry feces. E. ramulus was detected in the feces of all 20 individuals in numbers ranging from 4.40 × 107 to 2.04 × 109 cells per g of dry feces, corresponding to a mean count of 7.03 × 108 cells per g of dry feces. E. ramulus constituted, on average, 0.16% of the total fecal flora, which is approximately equivalent to Escherichia coli’s contribution to the fecal flora. Finegold et al. (11) detected E. ramulus in fecal samples at counts of 3.9 × 108 cells/g dry feces, but only 1 of 141 subjects was positive for E. ramulus. Similarly, Moore and Holdeman (26) detected E. ramulus at counts of 2.17 × 109 cells/g dry feces but in only 3 of 23 subjects. Hence, the cell counts determined in our study with the probe S-S-E.ram-0997-a-A-18 are in the same range as in these studies, but there is a major difference in the incidence of E. ramulus in humans. This discrepancy may be due to the fact that cultural methods were used in the previous studies and that some bacteria are underestimated because of their failure to grow under standard conditions. We noticed that some Eubacterium species did not grow on media that are routinely used for culturing anaerobic gut microorganisms. Langendijk et al. (20) and Doré et al. (10) demonstrated that the abundance of bifidobacteria and Bacteroides, respectively, in relation to total bacteria was overestimated when plating was used. This was proposed to be due to an underestimation of the total anaerobes by plating. However, it is also conceivable that the observed variance in the occurrence of E. ramulus is due to differences in the genetic background, lifestyle, and diet in the human populations studied.
TABLE 2.
Counts of E. ramulus and total bacteria per gram of dry feces in 20 human fecal samples determined by FISH with S-S-E.ram-0997-a-A-18 and the Eub probe mixa
| Volunteer | Mean cell count (standard deviation)b
|
||
|---|---|---|---|
| Total bacteria (1011) with Eub probe mix | E. ramulus (108) with S-S-E.ram-0997-a-A-18 | % of total bacteria | |
| DlfEH1 | 3.34 (0.23) | 8.74 (1.02) | 0.26 (0.01) |
| DlfEB1 | 8.05 (0.92) | 12.60 (1.47) | 0.16 (0.00) |
| DlfEL1 | 5.44 (0.87) | 7.82 (1.15) | 0.14 (0.00) |
| DlfEN1 | 4.84 (0.50) | 2.55 (0.44) | 0.05 (0.01) |
| DlfEG1 | 4.13 (0.19) | 3.56 (0.03) | 0.09 (0.00) |
| DlfEA1 | 4.61 (0.04) | 0.44 (0.11) | 0.01 (0.00) |
| DlfEZ1 | 6.54 (0.06) | 12.80 (0.90) | 0.20 (0.01) |
| DlfES1 | 7.74 (1.67) | 6.09 (0.39) | 0.08 (0.01) |
| DlfER1 | 7.86 (1.48) | 10.10 (0.74) | 0.13 (0.00) |
| DlfED1 | 6.96 (0.06) | 16.90 (0.52) | 0.24 (0.01) |
| DlfEA2 | 1.47 (0.25) | 1.10 (0.18) | 0.08 (0.02) |
| DlfEB2 | 1.55 (0.11) | 5.31 (0.04) | 0.34 (0.09) |
| DlfEK1 | 2.18 (0.36) | 3.76 (0.05) | 0.17 (0.02) |
| UNIF1 | 5.46 (0.06) | 3.81 (1.62) | 0.07 (0.00) |
| UNIF2 | 5.18 (0.61) | 8.38 (0.95) | 0.16 (0.02) |
| UNIF3 | 2.94 (0.32) | 1.85 (1.30) | 0.06 (0.01) |
| UNIF4 | 1.98 (0.17) | 1.74 (1.36) | 0.09 (0.02) |
| UNIF7 | 5.63 (0.04) | 20.40 (3.69) | 0.36 (0.04) |
| UNIF10 | 5.43 (0.32) | 11.30 (2.49) | 0.21 (0.02) |
| UNIF15 | 4.43 (1.52) | 1.40 (0.85) | 0.03 (0.00) |
Kleessen et al. (18).
The standard deviation was calculated from four enumerations.
Schneider et al. (29) tested five subjects, who also participated in this study (Table 2), for the occurrence of isoquercitrin-degrading bacteria in the feces. E. ramulus was isolated at dilutions of up to 10−9 from all of these subjects. The number of organisms detected with S-S-E.ram-0997-a-A-18 is in good agreement with this figure. It is notable that we were able to detect E. ramulus in each person tested so far.
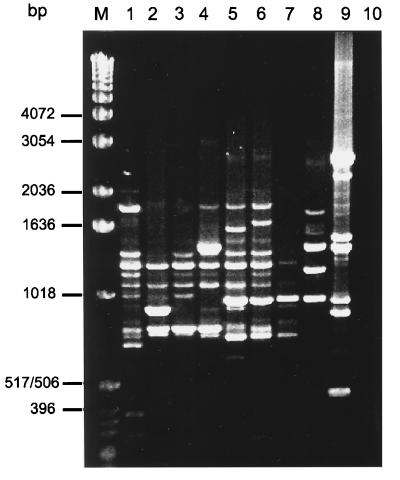
The work of Schneider et al. (29) raised the question of whether these isolates (WK1, WK5, WKBK, WKDT, WKJN, and WKRS) obtained from different individuals were identical or different strains. Since the probe technique cannot discriminate between strains of one species, randomly amplified polymorphic DNA (RAPD) analysis was used to investigate the genetic diversity of these strains. Shianna et al. (31) have shown for the protozoan parasite Cryptosporidium parvum and Klein et al. (19) have shown for Bifidobacterium strains the discriminatory potential of this method. Bacterial DNA was isolated by following protocol number five of the InViTek DNA isolation kit III (InViTek GmbH, Berlin, Germany). Cells were first incubated for 30 min in 25% sucrose in H2O, centrifuged as described above, and diluted in 2 ml of lysis buffer D (InViTek). The M13-core (5′ GAG GGT GGC GGT TCT 3′) was used as the primer in the reaction mixture (30), and amplification was done in an Omnigene thermocycler (Hybaid, Middlesex, United Kingdom) by using the following conditions: 2 min at 95°C, followed by 30 cycles at 95°C for 30 s, 50°C for 1 min, and 72°C for 1 min, with a final extension step at 72°C for 6 min. The PCR products were electrophoresed in a 1% ([wt/vol] 0.5× TBE-agarose gel [1× TBE is 90 mM Tris-borate, 2 mM EDTA [pH 8.0]) stained with ethidium bromide and documented with a video printer connected with a video camera (Biometra, Göttingen, Germany). The RAPD profiles of all isolates (Fig. 2, lanes 2 to 7) differed from that of the type strain. None of the banding patterns of the isolates were identical. Whereas some amplification products (e.g., the 1,250-bp band) occurred with all isolates, other products occurred only with three strains (e.g., the 970-bp band), and some occurred only with one strain (the 896-bp band). For comparison, E. ramulus ATCC 29099, Eubacterium rectale, and Bacteroides fragilis were also analyzed. The banding pattern of the closely related species E. rectale also showed no similarity with that of E. ramulus. This observation indicates that each human individual tested so far has his or her specific E. ramulus strain. It can be speculated that individual differences in the intestinal ecosystem promote the colonization of those strains that are optimally adapted to these conditions. This is similar to the situation described for bovine fecal isolates of Cryptosporidium parvum which could be assigned to 16 different strains (31). It therefore appears that a broad range of different strains belonging to one species can be found in fecal samples, illustrating that the intestinal flora is even more complex than previously assumed.
FIG. 2.
RAPD profiles obtained with genomic DNA of E. rectale, B. fragilis, and different strains of E. ramulus by using the M13-core as the random primer. Lanes: M, molecular mass marker (1-kb ladder; Gibco-BRL); 1, E. ramulus ATCC 29099; 2, E. ramulus WK1; 3, E. ramulus WK5; 4, E. ramulus WKBK; 5, E. ramulus WKDT; 6, E. ramulus WKJN; 7, E. ramulus WKRS; 8, E. rectale; 9, B. fragilis; 10, DNA-free control (H2O). No amplification products were observed in the absence of template DNA (H2O control).
Taken together, the data from our study show that FISH with species-specific oligonucleotide probes is a useful technique to investigate the occurrence of bacteria in fecal samples. The usefulness of this experimental approach has also been shown by Franks et al. (13). In addition, this study provides new information on the occurrence of a fecal bacterium that is capable of quercetin degradation.
Acknowledgments
We thank Bärbel Scharfenberg and Sabine Schmidt for technical assistance. We are grateful to Lynne Rogers-Blaut and Heiko Schneider for critical reading of the manuscript.
This study was supported by the Deutsche Forschungsgemeinschaft (INK 26/A1-1) and by the European Commission (FAIR-CT97-3035).
Appendix
The following 95 reference species were used for FISH and dot blot hybridization: Acidaminococcus fermentans (DSM 20731), Actinobaculum suis (DSM 20639), Bacteroides distasonis (DSM 20701), B. fragilis (DlfE05), B. fragilis (DSM 2151), B. galacturonicus (DSM 3978), B. merdae (ATCC 43184), B. ovatus (DSM 1896), B. thetaiotaomicron (DlfEBaF1), B. thetaiotaomicron (DSM 2079), B. vulgatus (DlfEBaF2), Bifidobacterium adolescentis (ATCC 15703), B. angulatum (ATCC 27535), B. animalis (ATCC 25527), B. bifidum (ATCC 29521), B. breve (ATCC 15700), B. catenulatum (ATCC 27539), B. dentium (ATCC 27678), B. infantis (ATCC 15697), B. infantis (ATCC 15702), B. infantis (ATCC 25962), B. longum (ATCC 15707), B. longum (ATCC 15708), Bifidobacterium sp. (DlfEBiF4), Bifidobacterium sp. (DlfEBiF5), B. thermophilum (ATCC 25525), Butyrivibrio fibrisolvens (DSM 3071), Clostridium acetobutylicum (ATCC 824), C. acetobutylicum (DSM 792), C. barati (DSM 601), C. bifermentans (DSM 46282), C. butyricum (DlfEClF1), C. butyricum (DSM 10702), C. cellobioparum (DSM 1351), C. clostridiiforme (DSM 933), C. coccoides (DSM 935), C. innocuum (DSM 1286), C. pasteurianum (DSM 525), C. perfringens (DlfECIF2), C. perfringens (DSM 756), C. propionicum (DSM 1682), C. sartagoformum (DSM 1292), C. sordellii (DSM 2141), C. sporosphaeroides (DSM 1294), C. tyrobutyricum (DSM 633), C. xylanolyticum (DSM 6555), Coprococcus catus (ATCC 27761), C. eutactus (ATCC 27759), Enterococcus casseliflavus (DlfEEnF1), E. durans (DSM 20633), E. faecalis (DSM 20478), E. faecium (DSM 20477), E. hirae (DSM 20160), Escherichia coli (DlfEEsF1), E. hermannii (ATCC 33650), Eubacterium aerofaciens (DSM 3979), E. barkeri (ATCC 25849), E. biforme (DSM 3989), E. contortum (DSM 3982), E. cylindroides (ATCC 27528), E. cylindroides (DSM 3983), E. dolichum (DSM 3991), E. eligens (DSM 3376), E. fissicatena (DSM 3598), E. hadrum (DlfEEuF1), E. lentum (DSM 2243), E. limosum (DSM 20543), E. rectale (ATCC 33656), E. tenue (DSM 20695), E. tortuosum (DSM 3987), E. uniforme (ATCC 35992), E. ventriosum (ATCC 27560), Fusobacterium mortiferum (ATCC 25557), F. naviforme (DSM 20699), F. necrogenes (ATCC 25556), F. nucleatum (DSM 20482), F. varium (ATCC 8501), Lactobacillus acidophilus (DlfELaF1), L. acidophilus (DSM 20079), L. fermentum (DSM 20052), L. gasseri (DSM 20243), L. murinus (DSM 20452), L. plantarum (DSM 20174), L. reuteri (DSM 20016), Megasphaera sp. (DlfEMeF3), Peptostreptococcus anaerobius (DSM 2949), P. asaccharolyticus (DSM 20463), P. prevotii (DSM 20548), Prevotella melanogenica (ATCC 25845), Pseudoramibacter alactolyticus (DSM 3980), Ruminococcus hansenii (DSM 20583), R. productus (DSM 2950), Streptococcus pleomorphus (DSM 20574), Veillonella parvula (DSM 2008), and Veillonella sp. (DlfEV257).
REFERENCES
- 1.Alm E W, Oerther D B, Larsen N, Stahl D A, Raskin L. The oligonucleotide probe database. Appl Environ Microbiol. 1996;62:3557–3559. doi: 10.1128/aem.62.10.3557-3559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann R I, Binder B J, Olson R J, Chisholm S W, Devereux R, Stahl D A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann R I, Ludwig W, Schleifer K H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjeldanes L F, Chang G W. Mutagenic activity of quercetin and related compounds. Science. 1978;197:577–578. doi: 10.1126/science.327550. [DOI] [PubMed] [Google Scholar]
- 5.Boye M, Jensen T K, Moller K, Leser T D, Jorsal S E. Specific detection of Lawsonia intracellularis in porcine proliferative enteropathy inferred from fluorescent rRNA in situ hybridization. Vet Pathol. 1998;35:153–156. doi: 10.1177/030098589803500212. [DOI] [PubMed] [Google Scholar]
- 6.Bryant M P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972;25:1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- 7.Das M, Ray P. Lipid antioxidant properties of quercetin in vitro. Biochem Int. 1988;17:203–208. [PubMed] [Google Scholar]
- 8.Das N P, Scott K N, Duncan J H. Identification of flavone metabolites in rat urine by combined gas-liquid chromatography and mass spectrometry. Biochem J. 1973;136:903–909. doi: 10.1042/bj1360903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Los Reyes F L, Ritter W, Raskin L. Group-specific small-subunit rRNA hybridization probes to characterize filamentous foaming in activated sludge systems. Appl Environ Microbiol. 1997;63:1107–1117. doi: 10.1128/aem.63.3.1107-1117.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doré J, Sghir A, Hannequart-Gramet G, Corthier G, Pochart P. Design and evaluation of a 16S rRNA-targeted oligonucleotide probe for specific detection and quantitation of human faecal Bacteroides populations. Syst Appl Microbiol. 1998;21:65–71. doi: 10.1016/S0723-2020(98)80009-X. [DOI] [PubMed] [Google Scholar]
- 11.Finegold S M, Sutter V L, Mathisen G E. Normal indigenous intestinal flora. In: Hentges D J, editor. Human intestinal flora in health and disease. New York, N.Y: Academic Press Inc.; 1983. pp. 3–31. [Google Scholar]
- 12.Forster R J, Teather R M, Deng S, Gong J. 16S rDNA analysis of Butyrivibrio fibrisolvens: phylogenetic position and relation to butyrate producing anaerobic bacteria from the rumen of white-tailed deer. Lett Appl Microbiol. 1996;23:218–222. doi: 10.1111/j.1472-765x.1996.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 13.Franks A H, Harmsen H J M, Raangs G C, Jansen G J, Schut F, Welling G W. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol. 1998;64:3336–3345. doi: 10.1128/aem.64.9.3336-3345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gugler R, Leschik M, Dengler H J. Disposition of quercetin in man after single oral and intravenous doses. Eur J Clin Pharmacol. 1975;9:229–234. doi: 10.1007/BF00614022. [DOI] [PubMed] [Google Scholar]
- 15.Hertog M G L, Feskens E J M, Hollman P C, Katan M B, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1111. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 16.Hungate R E. A roll tube method for cultivation of strict anaerobes. In: Norris J R, Ribbons D W, editors. Methods in microbiology. 3B. New York, NY: Academic Press Inc.; 1969. pp. 117–132. [Google Scholar]
- 17.Kamlage, B., L. Hartmann, B. Gruhl, and M. Blaut. The intestinal microflora does not supply gnotobiotic rats with conjugated linolic acid. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 18.Kleessen, B., J. Noack, and M. Blaut. Distribution of viable and non-viable bacteria in the gastrointestinal tract of gnotobiotic and conventional rats. Submitted for publication.
- 19.Klein G, Pack A, Bonaparte C, Reuter G. Taxonomy and physiology of probiotic lactic acid bacteria. Int J Food Microbiol. 1998;41:103–125. doi: 10.1016/s0168-1605(98)00049-x. [DOI] [PubMed] [Google Scholar]
- 20.Langendijk P S, Schut F, Jansen G J, Raangs G C, Kamphuis G R, Wilkinson M H F, Welling G W. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl Environ Microbiol. 1995;61:3069–3075. doi: 10.1128/aem.61.8.3069-3075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy J, Teurstein I, Marbach M, Radian S, Sharoni Y. Tyrosine protein kinase activity in the DMBA-induced rat mammary tumor: inhibition by quercetin. Biochem Biophys Res Commun. 1984;123:1227–1233. doi: 10.1016/s0006-291x(84)80264-8. [DOI] [PubMed] [Google Scholar]
- 22.Lin C-K, Tsen H-Y. Development and evaluation of two novel oligonucleotide probes based on 16S rRNA sequence for the identification of Salmonella in foods. J Appl Bacteriol. 1995;78:507–520. doi: 10.1111/j.1365-2672.1995.tb03093.x. [DOI] [PubMed] [Google Scholar]
- 23.Maddox P H, Jenkins D. 3-Aminopropyltriethoxysilane (APES): a new advance in section adhesion. J Clin Pathol. 1987;40:1256–1257. doi: 10.1136/jcp.40.10.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maidak B L, Olson G J, Larsen N, Overbeek R, McCaughey M J, Woese C R. The RDP (Ribosomal Database Project) Nucleic Acids Res. 1997;25:109–111. doi: 10.1093/nar/25.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manz W, Szewzyk U, Ericsson P, Amann R, Schleifer K-H, Stenström T-A. In situ identification of bacteria in drinking water and adjoining biofilms by hybridization with 16S and 23S rRNA-directed fluorescent oligonucleotide probes. Appl Environ Microbiol. 1993;59:2293–2298. doi: 10.1128/aem.59.7.2293-2298.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore W E C, Holdeman L V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974;27:961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishino H, Nishino A, Iwashima A. Quercetin inhibits the action of 12-O-tetradeconylphorbol-13-acetate, a tumor promotor. Oncology. 1984;41:120–123. doi: 10.1159/000225805. [DOI] [PubMed] [Google Scholar]
- 28.Roller C, Wagner M, Amann R, Ludwig W, Schleifer K H. In situ probing of gram-positive bacteria with high DNA G + C content using 23S rRNA-targeted oligonucleotides. Microbiology. 1994;140:2849–2858. doi: 10.1099/00221287-140-10-2849. [DOI] [PubMed] [Google Scholar]
- 29.Schneider H, Schwiertz A, Collins M D, Blaut M. Anaerobic transformation of quercetin-3-glucoside by bacteria from the human intestinal tract. Arch Microbiol. 1999;171:81–91. doi: 10.1007/s002030050682. [DOI] [PubMed] [Google Scholar]
- 30.Schönian G, Schweynoch C, Zlateva K, Oskam L, Kroon N, Gräser Y, Presber W. Identification and determination of the relationships of species and strains within the genus Leishmania using single primers in the polymerase chain reaction. Mol Biochem Parasitol. 1996;77:19–29. doi: 10.1016/0166-6851(96)02572-8. [DOI] [PubMed] [Google Scholar]
- 31.Shianna K V, Rytter R, Spanier J G. Randomly amplified polymorphic DNA PCR analysis of bovine Cryptosporidium parvum strains isolated from the watershed of the Red River of the north. Appl Environ Microbiol. 1998;64:2262–2265. doi: 10.1128/aem.64.6.2262-2265.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stahl D A, Flesher B, Mansfield H R, Montgomery L. Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl Environ Microbiol. 1988;54:1079–1084. doi: 10.1128/aem.54.5.1079-1084.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strunk O, Ludwig W. ARB, a software environment for sequence data. Munich, Germany: Technische Universität München; 1996. [Google Scholar]
- 34.Welling G W, Elfferich P, Raangs G C, Wildeboer-Veloo A C M, Jansen G J, Degener J E. 16S ribosomal RNA-targeted oligonucleotide probes for monitoring of intestinal bacteria. Scand J Gastroenterol. 1997;32.(Suppl. 222):17–19. doi: 10.1080/00365521.1997.11720711. [DOI] [PubMed] [Google Scholar]
- 35.Willems A M C G, Amat-Marco M, Collins M D. Phylogenetic analysis of Butyrivibrio strains reveals three distinct groups of species within the Clostridium subphylum of the gram-positive bacteria. Int J Syst Bacteriol. 1996;46:195–199. doi: 10.1099/00207713-46-1-195. [DOI] [PubMed] [Google Scholar]