Abstract
Neoplasms that secrete ectopic adrenocorticotropin (ACTH) may cause severe, life-threatening hypercortisolism. These tumors are often difficult to localize and treat, requiring a comprehensive and systematic management plan orchestrated by a multidisciplinary team. The Mount Sinai Adrenal Center hosted an interdisciplinary retreat of experts in adrenal disorders and neuroendocrine tumors (NETs) with the aim of developing a clinical pathway for the management of Cushing syndrome due to ectopic ACTH production. The result was institutional recommendations for the diagnosis, localization, surgical approaches to intrathoracic tumors and bilateral adrenalectomy, and perioperative and postoperative medical management of hypercortisolism and its sequelae. Specific recommendations were made regarding the timing and selection of therapies based on the considerations of our team as well as a review of the current literature. Our clinical pathway can be applied by other institutions directly or serve as a guide for institution-specific management.
Keywords: hypercortisolism, Cushing syndrome, ectopic ACTH
Cushing syndrome (CS) due to ectopic adrenocorticotropin (ACTH) syndrome (EAS) is a challenging problem for patients and their providers. It is often difficult to localize the source of the ectopic ACTH and manage the sequelae of extremely high cortisol levels that lead to life-threatening hypokalemia, hyperglycemia, hypercoagulability, cardiovascular events, psychosis, debility, and infections. There are frequently no curative therapies for primary and metastatic tumors. However, no guidelines for managing EAS have been established, especially regarding the role and timing of medical therapies and/or bilateral adrenalectomy in patients with severe hypercortisolism. Successful management requires close coordination between a multidisciplinary team of endocrinologists, adrenal and thoracic surgeons, radiologists, nuclear medicine physicians, anesthesiologists, and neuroendocrine oncologists at specialized centers.
Methods
With the establishment of the Adrenal Center in 2010 and the Center for Carcinoid and Neuroendocrine Tumors at the Tisch Cancer Institute in 2018, the number of EAS patients presenting to the Mount Sinai Health System has increased dramatically, with each patient managed on a case-by-case basis. Given the increasing volume of EAS cases, we conducted a 2-day retreat in December 2020 to review current practices and existing literature and devise a clinical pathway to minimize morbidity and mortality from this disease. During the 2-day retreat, invited speakers gave 15- to 45-minute presentations on specific aspects of EAS related to their clinical expertise. After the retreat, speakers provided written summaries and literature reviews, compiled in preparation for this manuscript. A draft was circulated to all participants and further reviewed for accuracy.
References from this review were identified through PubMed for articles published from January 1, 2012 to January 1, 2022, using the terms “ectopic ACTH-secretion,” “severe hypercortisolism,” “medical therapy for Cushings,” “glucocorticoid receptor blockers,” “adrenal steroidogenesis inhibitors,” “hypercoaguability in Cushings,” “infection,” and “Cushings guidelines.” English-language articles from these searches and relevant references cited in those articles were reviewed.
Incidence
The true incidence of endogenous CS from any cause is controversial but generally reported as no higher than 2.4 cases/million [1]. The proportion of patients with endogenous CS due to ectopic ACTH is typically cited as 10% with rates in larger studies ranging from 5% to 14%. A recent study from Sweden found that 26% of patients had endogenous CS due to EAS [2]. Plausible explanations for the increased proportion of CS due to ectopic ACTH include increased awareness of hypercortisolism in patients with NETs, more sensitive localization techniques, and increased patient survival due to new therapies. Nonetheless, the true incidence and prevalence of EAS likely remains underdiagnosed and underreported.
Challenges in Diagnosis and Localization
Biochemical Diagnosis
Once CS is suspected, the diagnosis is confirmed biochemically with a combination of 24-hour urine free cortisol (UFC), overnight 1-mg dexamethasone suppression test, and/or late-night salivary cortisol levels. For patients with a suspected adrenal source of hypercortisolism, the 1-mg overnight dexamethasone suppression test should be conducted first. A cortisol level < 1.8 μg/dL has been shown to exclude excess cortisol production from an adrenal incidentaloma [3]. After endogenous hypercortisolism is confirmed biochemically, ACTH independence or dependence is determined with a serum ACTH level, for which a high or inappropriately normal ACTH level in the presence of hypercortisolism suggests ACTH dependence.
Once ACTH-dependent CS is established, the source of ACTH must be determined: pituitary vs ectopic ACTH secretion. Dynamic biochemical testing with high-dose (8 mg) dexamethasone suppression test (HDDST) may be helpful in distinguishing pituitary CS from ectopic ACTH production due to low-grade carcinoid tumors. To diagnose pituitary CS, serum cortisol should suppress to <5 ug/dL or morning cortisol (drawn at 8 am) after HDDST should decrease by 50%. However, HDDST does not always distinguish pituitary from ectopic ACTH-dependent CS, with sensitivity and specificity each ranging from 60% to 100% [4, 5]. Some pituitary ACTH-producing tumors do not suppress and some benign carcinoid tumors suppress after HDDST [6]. Adding corticotrophin-releasing hormone (CRH) and desmopressin stimulation testing to HDDST increases the sensitivity of localization [7]. Patients with pituitary CS can overexpress vasopressin V2 and V1b receptors as well as CRH receptors, thus responding to vasopressin and CRH stimulation with increased plasma ACTH and cortisol [8, 9]. Biochemical testing with HDDST and/or vasopressin and CRH stimulation testing may not reliably identify the source of ACTH, with discordant results in up to 33% of patients [10]. Additionally, CRH is currently not available in the United States. Thus, accurate localization may require sophisticated imaging and inferior petrosal sinus sampling (IPSS), the gold standard for excluding ectopic ACTH production. Given the lack of CRH availability for testing and our institutional expertise with IPSS, we perform IPSS in cases where no pituitary tumor is identified, and there is no obvious source of ACTH-dependent CS.
In cases of ectopic ACTH due to high-grade and/or metastatic NETs, patients are usually acutely ill and present predominately with the antianabolic features of CS, such as proximal muscle weakness, easy bruising, pigmented striae, and thinning of the skin [11]. They may have weight loss rather than weight gain. Metabolic abnormalities include hypokalemia, metabolic alkalosis, hyperglycemia, and hypertension. The muscle debility and hypokalemia can lead to an inability to ambulate. Patients may also exhibit psychosis and severe anxiety that interfere with self-care and acceptance of medical care.
Biochemically, patients with severe EAS have significantly elevated ACTH and 24-hour UFC levels, obviating the need for extensive biochemical testing or IPSS to rule out pituitary disease. In a retrospective study of 110 patients with EAS, a 24-hour UFC above 5× the upper limit of normal (ULN) was detected in 69.9% of patients and associated with a worse prognosis [12]. Meanwhile, a smaller retrospective study of 21 patients showed ACTH levels > 200 in 57% of patients with EAS [13]. Importantly, the presence of hypokalemia is common in EAS, observed in up to 70% to 90% of patients [14, 15].
In acutely ill patients with clinical features that are highly suggestive of EAS, biochemical testing may have to be abbreviated. Specifically, the 12-hour urine cortisol may be collected, and the results extrapolated to 24 hours. In such cases, the priority is to induce rapid normalization of severe hypercortisolemia to stabilize the patient.
Imaging
In cases of suspected EAS, various imaging techniques are utilized to identify the source. EAS is associated with a variety of solid tumors, mostly of neuroendocrine origin. Small cell lung cancer and bronchial carcinoids account for approximately 50% of EAS [16]. Therefore contrast enhanced computed tomography (CT) of the chest should be used initially to localize the source of ACTH. These tumors can be occult and asymptomatic at the time of clinical presentation. The localization is especially challenging if EAS is caused by a bronchial carcinoid, given its often small size (<1.5 cm) and proximity to lung vasculature [17].
Several other NETs may produce ACTH, including thymic carcinoids, malignant thymoma, pancreatic NET, pheochromocytoma, salivary tumors, and medullary thyroid carcinoma. After excluding the more common intrathoracic sources of ectopic ACTH, abdominal imaging should be performed with multiphase contrast enhanced CT or magnetic resonance imaging (MRI), since gastrointestinal NETs and pheochromocytomas generally enhance intensely during the arterial phase.
Nuclear Imaging
Cross-sectional anatomic imaging techniques, such as CT or MRI, are first-line imaging for tumor site identification. These techniques rely primarily on spatial resolution in which the threshold of lesion detectability is determined by the size of the lesion. In contrast, molecular imaging techniques have lower spatial resolution, yet lesions below the threshold of this resolution may be unmasked if the target site accumulates sufficiently high molecular signal. In a recent systematic review, various nuclear medicine functional imaging techniques identified 79% of EAS not detected by conventional anatomic imaging [18].
Currently available nuclear medicine techniques for localizing EAS include indium octreotide planer and single-photon emission CT (SPECT) or SPECT/CT imaging, I-123 or I-131 metaiodobenzylguanidine planar and SPECT or SPECT/CT imaging, fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT or PET/MRI, flourodopa PET/CT or PET/MRI, and gallium 68 (Ga-68) DOTATATE and copper 64 DOTATATE PET/CT or PET/MRI. Planer and SPECT images with either octreotide, I-123, or I-131 metaiodobenzylguanidine generally miss lesions < 1 cm unless the signal intensity is high. More recently, PET imaging has emerged as a useful localization tool. Ga-68 DOTATATE PET/CT or PET/MRI have become the most reliable means for detecting both occult primary tumor and/or metastases. Its high affinity for the somatostatin receptor (SSTR) type 2, coupled with the spatial resolution of CT or MRI, can generally detect and localize small tumors characterized by SSTR expression. Ga-68 DOTATATE PET/CT has a sensitivity of up to 81.8% in localizing EAS [18]. However, specificity is dependent on tumor location, with lesions in the mediastinum, lung, or pancreas having a higher specificity than adrenal lesions. Cell differentiation can also play a role in detectability. Well-differentiated tumors tend to have more Ga-68 DOTATATE uptake and minimal FDG activity. Meanwhile, poorly differentiated tumors, composed of highly proliferative cells with low SSTR expression and high metabolic activity, have higher FDG uptake and can appear as false negatives on scintigraphy.
Mount Sinai Recommendations for Diagnosis and Localization
For localization in mild to moderately severe cases of ACTH-dependent CS, we recommend IPSS to distinguish pituitary CS from ectopic due to benign carcinoid tumors. In patients with severe hypercortisolism where the 24-hour UFC is greater than 5× the ULN and/or ACTH > 200 pg/mL in the absence of a demonstrable pituitary tumor on imaging, EAS is highly likely, and IPSS not required.
Most tumors causing EAS are intrathoracic, and therefore a contrast-enhanced CT of the chest is required. If CT of the chest is negative for tumor, the next imaging modality is a CT or MRI of the abdomen and pelvis as a multiphase contrast-enhanced exam. Finally, Ga-DOTATATE PET/CT or PET/MRI may identify lesions not seen with CT or MRI. In acutely ill patients where the diagnosis of EAS is highly suspicious, biochemical and radiographic workup must be accelerated and sometimes abbreviated to begin treatment.
Medical Management of Severe Hypercortisolism
Extreme hypercortisolism due to EAS is a life-threatening condition that demands a swift approach to avoid morbidity and mortality due to CS [19]. A study of 110 patients with EAS determined that the negative predictive factors for survival were hypokalemia, diabetes mellitus, distant metastases, and the severity of the hypercortisolism [12]. The mainstays of acute medical management are (1) addressing and treating the comorbidities induced by severe hypercortisolism and (2) reducing cortisol levels.
Management of Comorbidities
The rapid onset of hypercortisolism seen in EAS often leads to psychiatric, cardiovascular, thromboembolic, metabolic, and infectious complications that can be fatal, irrespective of tumor progression [20].
Psychiatric
Psychiatric symptoms including paranoia, agitation, or psychosis have been reported in up to 50% of patients with EAS and may be an impediment to compliance with medical treatment [11, 21, 22]. Psychiatric complications correlate with the severity of hypercortisolism and improve with cortisol correction. In cases of severe psychiatric complications such as refractory psychosis or interference with medical management, haloperidol or other antipsychotic agents may be administered in collaboration with a psychiatrist.
Cardiovascular
Hypertension is common in CS of any etiology, but its prevalence and severity are increased in EAS [23, 24]. Hypertension has been shown to affect > 80% of patients with EAS [14, 23-25]. The mechanism for hypercortisolism-induced hypertension is multifactorial and involves an enhanced response to angiotensin II and activation of the mineralocorticoid receptor (MR). Normally, 11β-hydroxysteroid dehydrogenase 2 converts biologically active cortisol to the inactive cortisone, which does not bind the MR. In EAS, severe hypercortisolism exhausts the ability of 11β-hydroxysteroid dehydrogenase 2 to inactivate cortisol to cortisone, thus allowing for cortisol activation of MR activity. This MR activation leads to both fluid retention and the development of cardiac fibrosis [26]. Uncontrolled hypertension and the direct cardiotoxic effects can eventually result in left ventricular hypertrophy and heart failure. Most of these patients will require MR antagonist treatment with spironolactone at high doses of 100 to 300 mg/day.
Thromboembolic
Hypercortisolism creates a hypercoagulable state that leads to an increased incidence of both arterial and venous thromboembolic events (VTE). In a population-based cohort study of 343 CS patients, mortality was twice as high in CS patients compared to controls. Patients with CS were at an increased risk of VTE, myocardial infarction, and stroke [23]. A recent meta-analysis studying the association between endogenous hypercortisolism and hypercoagulability found increased odds ratio (17.8) of spontaneous VTE in CS patients compared to the general population [27]. Active CS is associated with elevated levels of procoagulant factors VIII and IX and von Willebrand factor, impaired fibrinolysis, and increased platelet aggregation [28]. Interestingly, the increased risk of venous thromboembolism is not only found in patients with active disease but can also extend 30 to 60 days into the postoperative period [29]. One study demonstrated decreased incidence of VTE in patients treated with unfractionated heparin for at least 2 weeks postoperatively compared to patients who did not receive thromboprophylaxis [30]. While perioperative anticoagulation therapy is recommended, no guidelines exist in terms of drug choice, dosage, or duration.
Metabolic
Hypokalemia occurs in up to 70% to 90% of cases and is related to the degree of hypercortisolism [14, 15]. Cortisol-mediated MR activation leads to urinary potassium loss. Severe hypokalemia may present as muscle weakness, rhabdomyolysis, and dangerous arrhythmias including torsade de pointes.
Hyperglycemia is common in EAS and arises from a combination of factors including cortisol-mediated insulin resistance, increased hepatic gluconeogenesis, and impaired insulin secretion [23, 31]. Aggressive treatment of hypercortisolism can drastically improve hyperglycemia and can serve as a marker for general responsivity to the control of cortisol secretion. Glycemic control remains important for reducing the increased susceptibility to infection seen in hyperglycemia.
Infectious
Infections are common in patients with hypercortisolism, with sepsis being among the most life-threatening CS complications. The immunosuppressive effects of hypercortisolism leaves patients vulnerable to opportunistic infections, while also masking the classic early warning signs of sepsis such as fever or leukocytosis [20].
The lungs are both the most commonly involved system and have the highest rates of severe infections. There is evidence that patients with severe hypercortisolism (UFC > 5× ULN) develop Pneumocystis jirovecii infections [32], therefore potentially requiring prophylaxis with trimethoprim-sulfamethoxazole. Interestingly, patients can develop Pneumocystis jirovecii abruptly as cortisol is controlled, possibly due to an exaggerated immune response to infection with alleviation of hypercortisolism [32]. Prophylaxis should continue for at least 2 weeks after curative surgery or near-normalization of UCF with medical therapy to allow time for improvement in immunosuppression and minimize the risk of immune reconstitution [32, 33]. An important consideration is the potential interactions of trimethoprim-sulfamethoxazole with ketoconazole and other medications, necessitating monitoring of both liver function test and QT intervals with electrocardiogram [33]. EAS patients, especially elderly ones, can develop bowel perforation and peritonitis without the typical symptoms of rebound, guarding, loss of bowel sounds, fever, and leukocytosis [34, 35]. In cases of severe infection, the serum cortisol levels should be maintained at a stress level similar to that seen in patients with similar infections, approximately 22 to 36 μg/dL [36, 37]
Acute Management of Hypercortisolism
Medical therapies aimed at rapidly reducing cortisol levels or action should be initiated. In addition to decreasing mortality, control of hypercortisolism can make occult tumors easier to detect by increasing SSTR expression, thereby preparing the patient for definitive surgeries and cancer treatments.
Steroidogenesis inhibitors
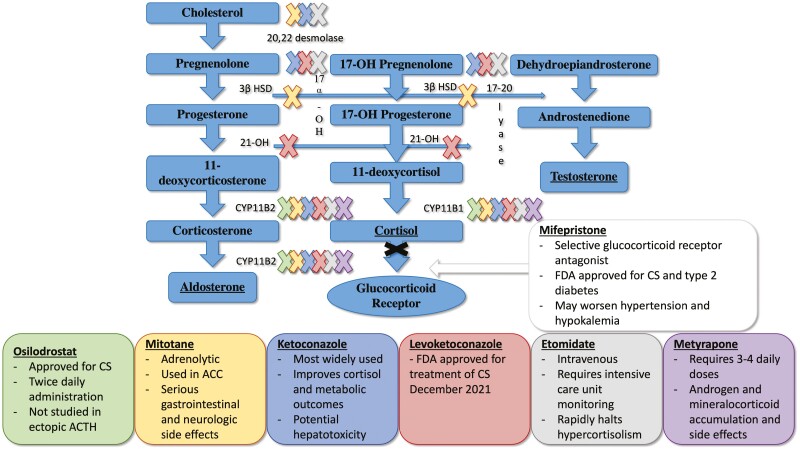
Drugs that inhibit cortisol synthesis are the mainstays of medical therapies for EAS. Etomidate is an intravenously administered hypnotic drug that inhibits 11β-hydroxylase and 20, 22 desmolase (Fig. 1). Etomidate is very effective with rapid onset, but its use is limited to intensive care units due to its sedative properties [38]. The most commonly used steroidogenesis inhibitor is ketoconazole, a drug that inhibits multiple cytochrome P450 steroidogenic enzymes needed for cortisol synthesis (Fig. 1). Ketoconazole acts rapidly, with high doses (up to 1200 mg/day) needed to counteract the severe hypercortisolism seen in some cases of EAS. The main toxicity of ketoconazole is hepatitis, although most cases are asymptomatic with mild increases ≤ 5× ULN. The increases appear to be dose dependent and reversible following discontinuation of the drug [39]. Several studies report only a minority of patients develop severe transaminitis [40]. Additionally, severe hypercortisolism can induce transaminitis, and ketoconazole may correct liver function tests [41]. Of note, the enantiomer of ketoconazole, levoketoconazole, was approved by the Food and Drug Administration in December 2021 for the treatment of CS. While it has not been specifically studied in EAS, it should be considered for medical therapy.
Figure 1.
Cortisol synthesis inhibitors and receptor blockers. Sites of inhibition are demarcated by “X,” color matched to the boxes below with the corresponding drug. Abbreviations17α-OH, 17α hydroxyprogesterone; 21-OH, 21-hydroxylase; 3β HSD, 3β-hydroxysteroid dehydrogenase; ACC, adrenal cortical carcinoma; CS, Cushing syndrome; CYP11B1, cytochrome P450 family 11 subfamily B member 1; CYP11B2, cytochrome P450 family 11 subfamily B member 2.
Metyrapone is a potent inhibitor of 11β-hydroxylase and a partial inhibitor of 18-hydroxylase, with a rapid absorption and onset of action (Fig. 1). High and frequent doses are required to reduce severe hypercortisolism associated with EAS, ranging from 1500 to 6000 mg/day in 4 divided doses. Its efficacy has been reported for decades and confirmed in recent studies [42]. In addition, its effects are rapid, and the drug is easily available at our institution as it is on formulary. Side effects may include nausea and vomiting, worsening hypokalemia/hypertension due to accumulation of aldosterone precursors with mineralocorticoid action, and hirsutism in women due to increased adrenal androgens. Another rapidly-acting 11β-hydroxylase inhibitor, osilodrostat, may produce similar results to metyrapone in EAS, but only a handful of cases have been reported to date [43, 44]. With either of the 11β -hydroxylase inhibitors, accumulation of 11-deoxycortisol occurs and can falsely elevate cortisol levels measured by immunoassays because of cross-reactivity. Therefore, when monitoring cortisol levels in patients on these drugs, the preferred assay is liquid chromatography/mass spectrometry [45].
The combination of ketoconazole and metyrapone provides rapid control of hypercortisolism in EAS, although escape from therapy is common [40, 46, 47]. In patients who require prolonged medical management of hypercortisolism, the addition of low-dose mitotane to ketoconazole and metyrapone therapy results in more durable responses [48-50]. A serious limitation of both ketoconazole and mitotane is that they can act as a CYP3A4 inhibitor and inducer, respectively [39]. This may complicate their use in patients on multiple medications, necessitating a careful review and rearrangement of medications to minimize interactions.
The glucocorticoid receptor blocking agent, mifepristone, has been studied in a small group of patients with EAS and shown to be both effective in rapidly correcting hypercortisolism as well as ameliorating psychosis and controlling hyperglycemia [51]. However, because the drug does not block cortisol actions at the MR, it can worsen the already problematic hypertension and hypokalemia, necessitating the addition of very high doses of potassium and spironolactone. Another limitation is the inability to follow cortisol levels to determine drug response.
Mount Sinai Recommendations for the Medical Management of Severe Hypercortisolism
EAS causes life-threatening complications including hypertension, hypercoagulability, hypokalemia, hyperglycemia, and infection. For hypertension and hypokalemia, patients will require MR antagonist therapy, typically spironolactone at high doses. Patients with EAS are at increased risk of developing VTE. Prophylaxis with enoxaparin should be initiated at the time of diagnosis. If patients undergo surgery, anticoagulation therapy should be resumed postoperatively for a minimum of 4 weeks. Additionally, patients with a body mass index > 35 should receive 5000 units of subcutaneous heparin intraoperatively. Hyperglycemia is often severe, requiring insulin administration even in patients without a prior history of diabetes. Patients with EAS are at high risk for infection, particularly with Pneumocystis jirovecii. We recommend prophylaxis with trimethoprim-sulfamethoxazole prior to medical or surgical therapy in patients with UFC > 5× ULN, with continuation for 2 weeks after curative surgery or near-normalization of UFC while on medical therapy.
For the acute management of severe hypercortisolism, we recommend initial treatment with metyrapone. Ketoconazole or levoketoconazole may be added if metyrapone alone is not effective. Mifepristone may be considered for acute and long-term management. In cases where imminent surgery is planned, intravenous etomidate in the intensive care unit setting should be considered. In cases of life-threatening hypercortisolism, urgent bilateral adrenalectomy may be required to stabilize the patient medically to administer more definitive therapies.
Approaches to Surgery
Surgical Approach to ACTH-secreting Lung Tumors
Most ACTH-secreting lung tumors can be removed by pulmonary resection [52]. The surgical approach can be an open thoracotomy (large incision) or minimally invasive via video-assisted thoracic surgery or robotic-assisted platforms. Determining which is most appropriate depends largely on tumor size and patient body habitus. History of pleural infection, prior procedures (surgery, thoracostomy, or pleurodesis), the patient’s tolerance of single-lung ventilation, and surgeon experience all factor into choice of approach.
The type of pulmonary resection a patient may require may involve wedge resection, segmentectomy, lobectomy, bilobectomy, or pneumonectomy, depending on location of the tumor. The decision to perform a larger anatomical resection or smaller wedge resection is primarily influenced by tumor size, location, and relationship to hilar structures. Other important considerations are patient factors and tumor biology. Most ACTH-secreting tumors represent histologies such as carcinoid and do not have aggressive oncologic behavior [16]. Data suggest parenchymal-sparing resections provide good long-term results and are preferred when anatomically feasible [53]. Anatomic features that make a lesion more amenable to wedge resection include whether the tumor is on the periphery or the edge of a fissure. Tumors located deep in the fissure, on a large ovoid surface of the lung, or at the base of the domed undersurface of the lung are more difficult to access by wedge resection and often require anatomic resection, generally in the form of lobectomy.
Anterior Approach to Bilateral Adrenalectomy
Minimally invasive adrenalectomy has become the standard of care, and approaches include anterior transabdominal, lateral transabdominal, and retroperitoneoscopic. The transabdominal approaches afford several advantages compared to retroperitoneal ones. These include easier and more direct access to the adrenals, particularly in obese patients, the ability to remove larger masses, and easier conversion to an open procedure if needed [54, 55]. Of the transabdominal approaches, the lateral approach is by far more common, as it leads to excellent operative field exposure, less dissection, and less operative time compared to the anterior approach [56]. Proponents of the anterior approach argue that it allows for early ligation of the adrenal vein as well as easy exploration of the abdominal cavity [57].
Retroperitoneal Approach to Bilateral Adrenalectomy
Transabdominal adrenalectomy is more common than a retroperitoneal approach, likely due to familiarity with the anatomy and training. Nonetheless, the retroperitoneal approach has unique merits that include avoiding the abdominal space and reducing bowel-related complications (ie, bloating, ileus, and injury). In patients with intraabdominal adhesions from prior abdominal surgeries, the retroperitoneal approach is an alternative to a transabdominal approach, as it avoids entry into the peritoneal cavity and excessive mobilization of visceral organs, thereby preventing injury to the abdominal organs [58, 59]. This approach should be considered for patients with a history of previous and extensive abdominal surgery, lower body mass index, and smaller tumors, often <5 cm [58]. Retroperitoneal laparoscopic adrenalectomy can also be performed through a lateral flank or posterior lumbar approach. The posterior lumber approach provides better visualization of the vessels of the adrenal gland, while the lateral flank approach provides more space for manipulation of the gland during adrenalectomy [60]
Mount Sinai Recommendations for Approaches to Surgery
Minimally invasive surgery with video-assisted thoracic surgery should be considered in patients with small tumor size and nonobese habitus who are able to tolerate single-lung ventilation. The type of resection, wedge vs anatomic resections, is determined largely by tumor location, size, and involvement of hilar structures. Patients with small lesions in the periphery are often good candidates for wedge resection for these types of tumors. In severe hypercortisolism due to EAS bilateral adrenalectomy should be considered in patients where medical control of CS is not feasible. The approaches to minimally invasive bilateral adrenalectomy are anterior transabdominal, lateral transabdominal, or retroperitoneoscopic. Although transabdominal approaches are the most common, retroperitoneal surgery should be considered in cases with previous and extensive abdominal surgery.
Long-term Medical Management of Metastatic Neuroendocrine Tumors With Ectopic ACTH Secretion
First-line medical therapy for metastatic well-differentiated NETs incudes somatostatin analogues in patients with somatostatin avid disease on Ga-68 or Cu-64 DOTATATE PET/CT. Case reports indicate that somatostatin analogues (octreotide, lanreotide, and especially pasireotide) can control ACTH secretion; however, in many cases, further treatments are needed [61-63].
In patients who do not respond to somatostatin analogues, some alternative therapies have been studied. Capecitabine (CAP) with temozolomide (TEM) is a chemotherapy combination with efficacy in pancreatic and thoracic NETs. In pancreatic NETs, the response rate to CAP/TEM is roughly 30% [64, 65]. CAP/TEM has not been prospectively evaluated in thoracic NETs; however, in retrospective studies, response rate is also approximately 30% [66]. Case reports of patients with ACTH-secreting NETs demonstrate improvement in CS with CAP/TEM therapy [67]. Radiolabeled somatostatin analogue therapy [peptide receptor radionuclide therapy (PRRT)] has a response rate in advanced midgut tumors of 18% [68]. Limited data exist of PRRT in patients with ACTH-secreting NETs. In 1 small case series, 13 patients with ectopic ACTH secretion received PRRT, including 8 thoracic NETs and 5 pancreatic NETs, and 10 patients (76.9%) had partial or complete control of ectopic CS [12]. In addition, locoregional liver-directed therapies, including ablative techniques such as radiofrequency ablation and microwave ablation, transarterial chemoembolization with or without chemotherapeutic drugs-eluting beads, and transarterial radioembolization with yttrium-90 microspheres have all been used in metastatic NETs [69]. Case reports describe improvement in ACTH secretion in a patient with primary pancreatic NET with liver metastases improved by hepatic artery embolization [70].
Everolimus is an oral inhibitor of mammalian target of rapamycin, which has been prospectively evaluated in both pancreatic NETs and pulmonary NETs. Although response rates are low with everolimus (approximately 2% in gastrointestinal or thoracic NETS), the drug can improve progression-free survival in advanced NETs [71, 72]. Everolimus has been used in the treatment of ACTH-secreting NETs with control of ACTH secretion [73]. Of note, ketoconazole, which is used in medical management of hypercortisolism, is an inhibitor of CYP3A4 and, when used with everolimus, necessitates a dose reduction of everolimus [74].
Multitargeted tyrosine kinase inhibitors (TKIs) are active agents in well-differentiated NETs. Sunitinib was evaluated in a randomized, double-blind, placebo-controlled Phase 3 trial in patients with advanced, well-differentiated pancreatic NETs. Response rate was 9.3% with improved progression-free survival as compared to placebo [75]. Surufatinib was also prospectively evaluated with response rate of 10% in advanced extrapancreatic NETs with improved median progression-free survival vs placebo [76]. A new drug application was recently submitted to the Food and Drug Administration for surufatinib [77]. Two multitargeted TKIs in development are promising in treatment of well-differentiated NETs. Cabozantinib has been evaluated in a prospective Phase 2 trial in patients with carcinoids and pancreatic NETs with a response rate of 15% in both groups independently [78]. Lenvatinib is another promising agent evaluated in Phase 2 trials in well-differentiated carcinoids and pancreatic NETs with response rates of 29.9% in carcinoids and 44.2% in pancreatic NETs [79]. Although these novel TKIs have not specifically been evaluated in ACTH-secreting NETs, given the promising response data, they may also be a good option in ACTH-secreting clinical syndromes.
Mount Sinai Recommendations for the Long-term Medical Management of Metastatic NETs With Ectopic ACTH Secretion
Once a patient is medically optimized and stable, antineoplastic therapy directed at the ACTH-secreting tumor should be initiated. First-line medical therapy for metastatic well-differentiated NETs incudes somatostatin analogues in patients with somatostatin avid disease on Ga-68 or Cu-64 DOTATATE PET/CT. If rapid tumor debulking is needed, locoregional liver-directed therapies are effective in liver predominant disease. CAP with TEM resulted in response rates of ~30% in both thoracic and pancreatic NETs. PRRT is also an effective agent that can induce tumor response and drive prolonged disease stability and symptom control. Everolimus is effective at stabilizing disease and controlling hormonal hypersecretion.
Conclusion
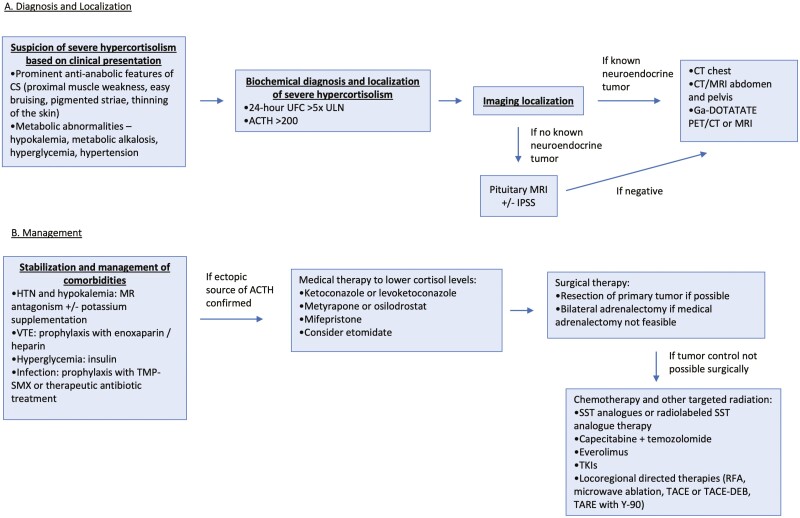
This review presents our clinical pathway for the management of CS due to EAS developed during an interdisciplinary retreat in December 2020 (Fig. 2). Biochemical demonstration of an ACTH > 200 pg/mL and a 24-hour UFC > 5× the ULN establishes the diagnosis. Source localization can be best achieved with contrast-enhanced CT of the chest, abdomen, and pelvis and/or Ga-68 DOTATATE PET/CT imaging. Severe hypercortisolism is a medical emergency that must be swiftly addressed. Specific recommendations for the type and timing of therapies were established. Patients will likely require spironolactone, potassium supplementation, anticoagulation, diabetes control, and prophylaxis against infection with trimethoprim-sulfamethoxazole. For the acute management of severe hypercortisolism, we recommend initial treatment with metyrapone ± the addition of ketoconazole or levoketoconazole. Alternate medical therapies include mifepristone and osilodrostat. If the hypercortisolism cannot be managed medically within several weeks, bilateral adrenalectomy should be performed. Specific surgical approaches to bilateral adrenalectomy and/or tumor resection depend on individual patient characteristics and the consensus of a multidisciplinary panel with experience in severe CS and EAS.
Figure 2.
The Mount Sinai clinical pathway for the diagnosis and management of severe hypercortisolism due to ectopic ACTH syndrome. Abbreviations: CS, Cushing syndrome; DEB, drug-eluting beads; HTN, hypertension; IPSS, inferior petrosal sinus sampling; MR, mineralocorticoid receptor; RFA, radiofrequency ablation; SST, somatostatin; TACE, transarterial chemoembolization; TARE, transarterial radioembolization; TKI, tyrosine kinase inhibitors; TMP-SMX, trimethoprim/sulfamethoxazole; UFC, urine free cortisol; ULN, upper limit of normal; VTE, venous thromboembolism; Y-90, yttrium-90.
Our clinical pathway can be applied directly or used by others as a platform from which they can develop their institutional methods for the treatment and management of this endocrine emergency.
Contributor Information
Eva L Alba, The Adrenal Center, Division of Endocrine, Diabetes and Bone Diseases, Department of Medicine at the Icahn School of Medicine at Mount Sinai, New York , NY, USA.
Emily A Japp, Division of Endocrinology, Diabetes, and Nutrition, Department of Medicine at the University of Maryland School of Medicine, Baltimore, MD, USA.
Gustavo Fernandez-Ranvier, Division of Metabolic, Endocrine, and Minimally Invasive Surgery, Department of Surgery at the Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Ketan Badani, Division of Urology, Department of Surgery at the Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Eric Wilck, Department of Diagnostic, Molecular and Interventional Radiology at the Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Munir Ghesani, Department of Diagnostic, Molecular and Interventional Radiology at the Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Andrea Wolf, Division of Thoracic Surgery, Department of Surgery at the Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Edward M Wolin, The Center for Carcinoid and Neuroendocrine Tumors, Tisch Cancer Institute, Division of Hematology and Oncology, Department of Medicine at the Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Virginia Corbett, Division of Hematology and Oncology, Department of Medicine at the Icahn School of Medicine at Mount Sinai, New York, NY, USA.
David Steinmetz, Division of Metabolic, Endocrine, and Minimally Invasive Surgery, Department of Surgery at the Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Maria Skamagas, The Adrenal Center, Division of Endocrine, Diabetes and Bone Diseases, Department of Medicine at the Icahn School of Medicine at Mount Sinai, New York , NY, USA.
Alice C Levine, Email: alice.levine@mountsinai.org, The Adrenal Center, Division of Endocrine, Diabetes and Bone Diseases, Department of Medicine at the Icahn School of Medicine at Mount Sinai, New York , NY, USA.
Disclosures
The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1. Ntali G, Hakami O, Wattegama M, Ahmed S, Karavitaki N. Mortality of patients with Cushing’s disease. Exp Clin Endocrinol Diabetes. 2021;129(03):203-207. doi: 10.1055/a-1197-6380 [DOI] [PubMed] [Google Scholar]
- 2. Wengander S, Trimpou P, Papakokkinou E, Ragnarsson O. The incidence of endogenous Cushing’s syndrome in the modern era. Clin Endocrinol (Oxf). 2019;91(2):263-270. doi: 10.1111/cen.14014 [DOI] [PubMed] [Google Scholar]
- 3. Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab. 2014;99(12):4462-4470. doi: 10.1210/jc.2014-3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aron DC, Raff H, Findling JW. Effectiveness versus efficacy: the limited value in clinical practice of high dose dexamethasone suppression testing in the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 1997;82(6):1780-1785. doi: 10.1210/jcem.82.6.3991 [DOI] [PubMed] [Google Scholar]
- 5. Dichek HL, Nieman LK, Oldfield EH, Pass HI, Malley JD, Cutler GB. A comparison of the standard high dose dexamethasone suppression test and the overnight 8-mg dexamethasone suppression test for the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 1994;78(2):418-422. doi: 10.1210/jcem.78.2.8106630 [DOI] [PubMed] [Google Scholar]
- 6. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet. 2015;386(9996):913-927. doi: 10.1016/S0140-6736(14)61375-1 [DOI] [PubMed] [Google Scholar]
- 7. Nieman LK, Biller BMK, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540. doi: 10.1210/jc.2008-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newell-Price J, Perry L, Medbak S, et al. a combined test using desmopressin and corticotropin-releasing hormone in the differential diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 1997;82(1):176-181. doi: 10.1210/jcem.82.1.3674 [DOI] [PubMed] [Google Scholar]
- 9. Vassiliadi DA, Tsagarakis S. Diagnosis of endocrine disease: the role of the desmopressin test in the diagnosis and follow-up of Cushing’s syndrome. Eur J Endocrinol. 2018;178(5):R201-R214. doi: 10.1530/EJE-18-0007 [DOI] [PubMed] [Google Scholar]
- 10. Biller BMK, Grossman AB, Stewart PM, et al. Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2008;93(7):2454-2462. doi: 10.1210/jc.2007-2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kamp K, Alwani RA, Korpershoek E, Franssen GJH, de Herder WW, Feelders RA. Prevalence and clinical features of the ectopic ACTH syndrome in patients with gastroenteropancreatic and thoracic neuroendocrine tumors. Eur J Endocrinol. 2016;174(3):271-280. doi: 10.1530/EJE-15-0968 [DOI] [PubMed] [Google Scholar]
- 12. Davi’ MV, Cosaro E, Piacentini S, et al. Prognostic factors in ectopic Cushing’s syndrome due to neuroendocrine tumors: a multicenter study. Eur J Endocrinol. 2017;176(4):453-461. doi: 10.1530/EJE-16-0809 [DOI] [PubMed] [Google Scholar]
- 13. Sathyakumar S, Paul TV, Asha HS, et al. Ectopic Cushing syndrome: a 10-year experience from a tertiary care center in southern India. Endocr Pract. 2017;23(8):907-914. doi: 10.4158/EP161677.OR [DOI] [PubMed] [Google Scholar]
- 14. Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing’s syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005;90(8):4955-4962. doi: 10.1210/jc.2004-2527 [DOI] [PubMed] [Google Scholar]
- 15. Campusano C, Arteaga E, Fardella C, Cárdenas I, Martínez P. [Cushing syndrome by ectopic ACTH secretion: analysis of the physiopathologic mechanism of hypokalemia. Report of two cases]. Rev Med Chil. 1999;127(3):332-336. [PubMed] [Google Scholar]
- 16. Wagner-Bartak NA, Baiomy A, Habra MA, et al. Cushing syndrome: diagnostic workup and imaging features, with clinical and pathologic correlation. Am J Roentgenol. 2017;209(1):19-32. doi: 10.2214/AJR.16.17290 [DOI] [PubMed] [Google Scholar]
- 17. Fazel P, Ganesa P, Mennel RG, Austin NA. The ectopic adrenocorticotropic hormone syndrome in carcinoid tumors. Bayl Univ Med Cent Proc. 2008;21(2):140-143. doi: 10.1080/08998280.2008.11928380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Isidori AM, Sbardella E, Zatelli MC, et al. Conventional and nuclear medicine imaging in ectopic Cushing’s syndrome: a systematic review. J Clin Endocrinol Metab. 2015;100(9):3231-3244. doi: 10.1210/JC.2015-1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Young J, Haissaguerre M, Viera-Pinto O, Chabre O, Baudin E, Tabarin A. Management of endocrine disease: Cushing’s syndrome due to ectopic ACTH secretion: an expert operational opinion. Eur J Endocrinol. 2020;182(4):R29-R58. doi: 10.1530/EJE-19-0877 [DOI] [PubMed] [Google Scholar]
- 20. Schernthaner-Reiter MH, Siess C, Micko A, et al. Acute and life-threatening complications in Cushing syndrome: prevalence, predictors, and mortality. J Clin Endocrinol Metab. 2021;106(5):e2035-e2046. doi: 10.1210/clinem/dgab058 [DOI] [PubMed] [Google Scholar]
- 21. Tsirona S, Tzanela M, Botoula E, Belenis I, Rondogianni D, Tsagarakis S. Clinical presentation and long-term outcome of patients with ectopic ACTH syndrome due to bronchial carcinoid tumors: a one-center experience. Endocr Pract. 2015;21(10):1104-1110. doi: 10.4158/EP15647.OR [DOI] [PubMed] [Google Scholar]
- 22. Okumura T, Takayama S, Nishio S, et al. ACTH-producing thymic neuroendocrine tumor initially presenting as psychosis: a case report and literature review. Thorac Cancer. 2019;10(7):1648-1653. doi: 10.1111/1759-7714.13099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dekkers OM, Horváth-Puhó E, Jørgensen JOL, et al. Multisystem morbidity and mortality in Cushing’s syndrome: a cohort study. J Clin Endocrinol Metab. 2013;98(6):2277-2284. doi: 10.1210/jc.2012-3582 [DOI] [PubMed] [Google Scholar]
- 24. Mancini T, Kola B, Mantero F, Boscaro M, Arnaldi G. High cardiovascular risk in patients with Cushing’s syndrome according to 1999 WHO/ISH guidelines. Clin Endocrinol (Oxf). 2004;61(6):768-777. doi: 10.1111/j.1365-2265.2004.02168.x [DOI] [PubMed] [Google Scholar]
- 25. Salgado LR, Fragoso MCBV, Knoepfelmacher M, et al. Ectopic ACTH syndrome: our experience with 25 cases. Eur J Endocrinol. 2006;155(5):725-733. doi: 10.1530/eje.1.02278 [DOI] [PubMed] [Google Scholar]
- 26. Kenouch S, Lombes M, Delahaye F, Eugene E, Bonvalet JP, Farman N. Human skin as target for aldosterone: coexpression of mineralocorticoid receptors and 11 beta-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab. 1994;79(5):1334-1341. doi: 10.1210/jcem.79.5.7962326 [DOI] [PubMed] [Google Scholar]
- 27. Wagner J, Langlois F, Lim DST, McCartney S, Fleseriu M. Hypercoagulability and risk of venous thromboembolic events in endogenous cushing’s syndrome: a systematic meta-analysis. Front Endocrinol (Lausanne). 2019;9:805. Accessed Jan 28, 2019. doi: 10.3389/fendo.2018.00805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van der Pas R, Leebeek FWG, Hofland LJ, de Herder WW, Feelders RA. Hypercoagulability in Cushing’s syndrome: prevalence, pathogenesis and treatment. Clin Endocrinol (Oxf). 2013;78(4):481-488. doi: 10.1111/cen.12094 [DOI] [PubMed] [Google Scholar]
- 29. Suarez MG, Stack M, Hinojosa-Amaya JM, et al. Hypercoagulability in Cushing syndrome, prevalence of thrombotic events: a large, single-center, retrospective study. J Endocr Soc. 2020;4(2):bvz033. doi: 10.1210/jendso/bvz033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boscaro M, Sonino N, Scarda A, et al. Anticoagulant prophylaxis markedly reduces thromboembolic complications in Cushing’s syndrome. J Clin Endocrinol Metab. 2002;87(8):3662-3666. doi: 10.1210/jcem.87.8.8703 [DOI] [PubMed] [Google Scholar]
- 31. Munir A, Newell-Price J. Management of diabetes mellitus in Cushing’s syndrome. Neuroendocrinology. 2010;92(1):82-85. doi: 10.1159/000314316 [DOI] [PubMed] [Google Scholar]
- 32. van Halem K, Vrolijk L, Pereira AM, de Boer MGJ. Characteristics and mortality of pneumocystis pneumonia in patients with Cushing’s syndrome: a plea for timely initiation of chemoprophylaxis. Open Forum Infect Dis. 2017;4(1):ofx002. doi: 10.1093/ofid/ofx002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Varlamov EV, Langlois F, Vila G, Fleseriu M. Management of endocrine disease: cardiovascular risk assessment, thromboembolism, and infection prevention in Cushing’s syndrome: a practical approach. Eur J Endocrinol. 2021;184(5):R207-R224. doi: 10.1530/EJE-20-1309 [DOI] [PubMed] [Google Scholar]
- 34. Sater ZA, Jha S, McGlotten R, et al. Diverticular perforation: a fatal complication to forestall in Cushing syndrome. J Clin Endocrinol Metab. 2018;103(8):2811-2814. doi: 10.1210/jc.2018-00829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shahidi M, Phillips RA, Chik CL. Intestinal perforation in ACTH-dependent Cushing’s syndrome. Biomed Res Int. 2019;2019:1-9. doi: 10.1155/2019/9721781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nieman LK, Biller BMK, Findling JW, et al. Treatment of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807-2831. doi: 10.1210/jc.2015-1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matfin G. Endocrine and Metabolic Medical Emergencies: A Clinician’s Guide. Wiley; 2018. [Google Scholar]
- 38. Preda VA, Sen J, Karavitaki N, Grossman AB. Therapy in endocrine disease: etomidate in the management of hypercortisolaemia in Cushing’s syndrome: a review. Eur J Endocrinol. 2012;167(2):137-143. doi: 10.1530/EJE-12-0274 [DOI] [PubMed] [Google Scholar]
- 39. Fleseriu M, Auchus R, Bancos I, et al. Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol. 2021;9(12):847-875. doi: 10.1016/S2213-8587(21)00235-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Castinetti F, Guignat L, Giraud P, et al. Ketoconazole in Cushing’s disease: is it worth a try? J Clin Endocrinol Metab. 2014;99(5):1623-1630. doi: 10.1210/jc.2013-3628 [DOI] [PubMed] [Google Scholar]
- 41. Young J, Bertherat J, Vantyghem MC, et al. Hepatic safety of ketoconazole in Cushing’s syndrome: results of a compassionate use programme in France. Eur J Endocrinol. 2018;178(5):447-458. doi: 10.1530/EJE-17-0886 [DOI] [PubMed] [Google Scholar]
- 42. Daniel E, Aylwin S, Mustafa O, et al. Effectiveness of metyrapone in treating Cushing’s syndrome: a retrospective multicenter study in 195 patients. J Clin Endocrinol Metab. 2015;100(11):4146-4154. doi: 10.1210/jc.2015-2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tanaka T, Satoh F, Ujihara M, et al. A multicenter, Phase 2 study to evaluate the efficacy and safety of osilodrostat, a new 11β-hydroxylase inhibitor, in Japanese patients with endogenous Cushing’s syndrome other than Cushing’s disease. Endocr J. 2020;67(8):841-852. doi: 10.1507/endocrj.EJ19-0617 [DOI] [PubMed] [Google Scholar]
- 44. Bessiène L, Bonnet F, Tenenbaum F, et al. Rapid control of severe ectopic Cushing’s syndrome by oral osilodrostat monotherapy. Eur J Endocrinol. 2021;184(5):L13-L15. doi: 10.1530/EJE-21-0147 [DOI] [PubMed] [Google Scholar]
- 45. Casals G, Hanzu FA. Cortisol measurements in Cushing’s syndrome: immunoassay or mass spectrometry? Ann Lab Med. 2020;40(4):285-296. doi: 10.3343/alm.2020.40.4.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pivonello R, De Leo M, Cozzolino A, Colao A. The treatment of Cushing’s disease. Endocr Rev. 2015;36(4):385-486. doi: 10.1210/er.2013-1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Corcuff JB, Young J, Masquefa-Giraud P, Chanson P, Baudin E, Tabarin A. Rapid control of severe neoplastic hypercortisolism with metyrapone and ketoconazole. Eur J Endocrinol. 2015;172(4):473-481. doi: 10.1530/EJE-14-0913 [DOI] [PubMed] [Google Scholar]
- 48. Kamenický P, Droumaguet C, Salenave S, et al. Mitotane, metyrapone, and ketoconazole combination therapy as an alternative to rescue adrenalectomy for severe ACTH-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 2011;96(9):2796-2804. doi: 10.1210/jc.2011-0536 [DOI] [PubMed] [Google Scholar]
- 49. Feelders RA, Newell-Price J, Pivonello R, Nieman LK, Hofland LJ, Lacroix A. Advances in the medical treatment of Cushing’s syndrome. Lancet Diabetes Endocrinol. 2019;7(4):300-312. doi: 10.1016/S2213-8587(18)30155-4 [DOI] [PubMed] [Google Scholar]
- 50. Tritos NA, Biller BMK. Advances in the medical treatment of Cushing disease. Endocrinol Metab Clin North Am. 2020;49(3):401-412. doi: 10.1016/j.ecl.2020.05.003 [DOI] [PubMed] [Google Scholar]
- 51. Wannachalee T, Turcu AF, Auchus RJ. Mifepristone in the treatment of the ectopic adrenocorticotropic hormone syndrome. Clin Endocrinol (Oxf). 2018;89(5):570-576. doi: 10.1111/cen.13818 [DOI] [PubMed] [Google Scholar]
- 52. Zhou X, Hang J, Che J, et al. Surgical treatment of ectopic adrenocorticotropic hormone syndrome with intra-thoracic tumor. J Thorac Dis. 2016;8(5):888-893. doi: 10.21037/jtd.2016.03.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen LC, Travis WD, Krug LM. Pulmonary neuroendocrine tumors: what (little) do we know? J Natl Compr Canc Netw. 2006;4(6):623-630. doi: 10.6004/jnccn.2006.0051 [DOI] [PubMed] [Google Scholar]
- 54. Carr AA, Wang TS. Minimally invasive adrenalectomy. Surg Oncol Clin N Am. 2016;25(1):139-152. doi: 10.1016/j.soc.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 55. Mazzaglia PJ, Vezeridis MP. Laparoscopic adrenalectomy: balancing the operative indications with the technical advances. J Surg Oncol. 2010;101(8):739-744. doi: 10.1002/jso.21565 [DOI] [PubMed] [Google Scholar]
- 56. Chen H, Sippel RS, O’Dorisio MS, Vinik AI, Lloyd RV, Pacak K. The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas. 2010;39(6):775-783. doi: 10.1097/MPA.0b013e3181ebb4f0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Paganini AM, Guerrieri M, Balla A, et al. Management of adrenal incidentaloma by laparoscopic transperitoneal anterior and submesocolic approach. Langenbecks Arch Surg. 2016;401(1):71-79. doi: 10.1007/s00423-015-1367-y [DOI] [PubMed] [Google Scholar]
- 58. Berber E, Tellioglu G, Harvey A, Mitchell J, Milas M, Siperstein A. Comparison of laparoscopic transabdominal lateral versus posterior retroperitoneal adrenalectomy. Surgery. 2009;146(4):621-626. doi: 10.1016/j.surg.2009.06.057 [DOI] [PubMed] [Google Scholar]
- 59. Conzo G, Tartaglia E, Gambardella C, et al. Minimally invasive approach for adrenal lesions: systematic review of laparoscopic versus retroperitoneoscopic adrenalectomy and assessment of risk factors for complications. Int J Surg. 2016;28(Suppl 1):S118-S123. doi: 10.1016/j.ijsu.2015.12.042 [DOI] [PubMed] [Google Scholar]
- 60. Meng C, Du C, Peng L, et al. Comparison of posterior retroperitoneoscopic adrenalectomy versus lateral transperitoneal laparoscopic adrenalectomy for adrenal tumors: a systematic review and meta-Analysis. Front Oncol. 2021;11:667985. Accessed May 10, 2021. doi: 10.3389/fonc.2021.667985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. von Werder K, Muller OA, Stalla GK. Somatostatin analogs in ectopic corticotropin production. Metabolism. 1996;45(8 Suppl 1):129-131. doi: 10.1016/s0026-0495(96)90107-9 [DOI] [PubMed] [Google Scholar]
- 62. Van den Bruel A, Bex M, Van Dorpe J, Heyns W, Bouillon R. Occult ectopic ACTH secretion due to recurrent lung carcinoid: long-term control of hypercortisolism by continuous subcutaneous infusion of octreotide. Clin Endocrinol (Oxf). 1998;49(4):541-546. doi: 10.1046/j.1365-2265.1998.00510.x [DOI] [PubMed] [Google Scholar]
- 63. Bertagna X, Favrod-Coune C, Escourolle H, et al. Suppression of ectopic adrenocorticotropin secretion by the long-acting somatostatin analog octreotide. J Clin Endocrinol Metab. 1989;68(5):988-991. doi: 10.1210/jcem-68-5-988 [DOI] [PubMed] [Google Scholar]
- 64. Kiesewetter B, Raderer M. How I treat neuroendocrine tumours. ESMO Open. 2020;5(4):e000811. doi: 10.1136/esmoopen-2020-000811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kunz PL, Catalano PJ, Nimeiri HS, et al. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: a trial of the ECOG-ACRIN cancer research group (E2211). J Clin Oncol. 2018;33(15S):4004. doi: 10.1200/JCO.2018.36.15_suppl.4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Al-Toubah T, Morse B, Strosberg J. Capecitabine and temozolomide in advanced lung neuroendocrine neoplasms. Oncologist. 2020;25(1):e48-e52. doi: 10.1634/theoncologist.2019-0361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lu L, Meng Q, Xing X, et al. Long-term follow-up of significant Improvement after CAPTEM treatment for rare adrenocorticotropin-producing cardiac neuroendocrine tumor. Front Endocrinol (Lausanne). 2019;10:713. Accessed Oct 22, 2019. doi: 10.3389/fendo.2019.00713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of 177lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376(2):125-135. doi: 10.1056/NEJMoa1607427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cavalcoli F, Rausa E, Conte D, Nicolini AF, Massironi S. Is there still a role for the hepatic locoregional treatment of metastatic neuroendocrine tumors in the era of systemic targeted therapies? World J Gastroenterol. 2017;23(15):2640. doi: 10.3748/wjg.v23.i15.2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lin HW, Tseng FY. Ectopic adrenal corticotropic hormone syndrome improved by transarterial embolization to hepatic metastatic lesions of pancreatic neuroendocrine carcinoma: a case report. J Clin Oncol. 2012;30(33):e360-e363. doi: 10.1200/JCO.2011.41.4326 [DOI] [PubMed] [Google Scholar]
- 71. Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968-977. doi: 10.1016/S0140-6736(15)00817-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514-523. doi: 10.1056/NEJMoa1009290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hoornaert E, Jacqmin L, Montfort L, Maiter D, Derdelinckx L. Case report: ectopic ACTH secretion due to a metastatic atypical lung carcinoid tumor: from diagnosis to treatment. Ann Endocrinol. 2019;80(2):137-139. doi: 10.1016/j.ando.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 74. Sparkes T, Lemonovich TL; AST Infectious Diseases Community of Practice. Interactions between anti-infective agents and immunosuppressants—guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9): e13510. doi: 10.1111/ctr.13510 [DOI] [PubMed] [Google Scholar]
- 75. Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501-513. doi: 10.1056/NEJMoa1003825 [DOI] [PubMed] [Google Scholar]
- 76. Xu J, Shen I, Shou Z, et al. Efficacy and safety of surufatinib in patients with well differentiated advanced extra-pancreatic neuoendocrine tumors (NETs): results from the randomized phase III study (SANET-ep). Ann Oncol. 2019;30:v851-v934. doi: 10.1093/annonc/mdz394.073 [DOI] [Google Scholar]
- 77.Tucker N. FDA approval sought for surufatinib in pancreatic and extra-pancreatic NETs. Target Oncology. Published May 3, 2021. Accessed November 9, 2021. https://www.targetedonc.com/view/fda-approval-sought-for-surufatinib-in-pancreatic-and-extra-pancreatic-nets [Google Scholar]
- 78. Chan JA, Faris JE, Murphy JE, et al. Phase II trial of cabozantinib in patients with carcinoid and pancreatic neuroendocrine tumors (pNET). J Clin Oncol. 2017;35(4 suppl):228-228. doi: 10.1200/JCO.2017.35.4_suppl.228 [DOI] [Google Scholar]
- 79. Capdevila J, Fazio N, Lopez C, et al. Lenvatinib in patients with advanced grade 1/2 pancreatic and gastrointestinal neuroendocrine tumors: results of the Phase II TALENT Trial (GETNE1509). J Clin Oncol. 2021;39(20):2304-2312. doi: 10.1200/JCO.20.03368 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.