Abstract
Objectives
The reproducibility of cefiderocol MIC determination using broth microdilution (BMD) in iron-depleted CAMHB (ID-CAMHB) was investigated, and the EUCAST disc diffusion (DD) method for cefiderocol susceptibility testing was developed and validated against reference BMD.
Methods
Cefiderocol values were determined for wild-type (WT) and non-WT isolates using BMD plates with ID-CAMHB (Thermo Scientific, Oakwood, USA) per EUCAST guidelines. DD was performed using standard EUCAST methodology on unsupplemented Mueller–Hinton agar with cefiderocol 30 μg discs. Control agents were included in all tests. MICs were correlated with zone diameters (ZD), and ZD breakpoints (BP) best corresponding to the MIC BPs were determined. Areas of technical uncertainty (ATU) were included where appropriate. External laboratory validation of cefiderocol DD was performed per the EUCAST SOP 9.2.
Results
MIC and ZD distributions for cefiderocol against WT isolates were established. Cefiderocol ZD BPs were set at susceptible ≥22 mm, resistant <22 mm for Enterobacterales and Pseudomonas aeruginosa and ATUs were decided. For Acinetobacter baumannii and Stenotrophomonas maltophilia, ZD cut-off values of ≥17 mm and ≥20 mm corresponded to MIC values of ≤2 and ≤0.5 mg/L, respectively. Cefiderocol ZDs for Escherichia coli ATCC 25922 (target 27 mm) and P. aeruginosa ATCC 27853 (target 26 mm) were within ±3 mm of the target values. For DD, there was no problematic variation between discs, media or laboratories.
Conclusions
DD is a robust and easy-to-perform method for cefiderocol susceptibility testing. For isolates with results in the ATU, an MIC test should be performed to confirm the results.
Introduction
AST is required to determine susceptibility or resistance of bacteria to specific antimicrobial agents. EUCAST guidelines recommend that AST be conducted via broth microdilution (BMD) and disc diffusion (DD).1–3
For BMD, liquid growth medium, typically cation-adjusted Mueller–Hinton broth, is used to determine the MIC, which is the lowest concentration of antimicrobial agent that inhibits visible growth of a pathogen.1,4,5
DD is a versatile, cost-effective and well-standardized method for AST that is conducted using nutrient agar medium, typically Mueller–Hinton agar, on which antimicrobial discs are applied. After a standardized incubation time, zone diameters (ZD) (circular areas of inhibited bacterial growth) are measured.2,3
EUCAST has established clinical MIC and ZD breakpoints (BPs) for multiple antimicrobial agents and bacterial species based on testing using standardized methodology, and discs and media from multiple manufacturers.1 For a species, epidemiological cut-off values (ECOFFs) distinguish between isolates with non-wild-type (non-WT) and without phenotypically detectable acquired resistance mechanisms (WT) to a specific agent.1 EUCAST ECOFFs are based on ≥5 MIC distributions, whereas tentative ECOFFs (TECOFFs), which can be used when there is not sufficient data to establish an ECOFF, can be based on 3 or 4 MIC distributions.6,7 Distributions with ECOFF values are available in the EUCAST database, where there are >30 000 MIC distributions containing more than several million MICs from worldwide sources.6
Cefiderocol is a novel siderophore cephalosporin developed for the treatment of infections caused by Gram-negative bacteria (GNB), including carbapenem-resistant GNB.8 Cefiderocol is approved in Europe for the treatment of infections caused by aerobic GNB in adults with limited treatment options8 and is approved in the USA for the treatment in adults of complicated urinary tract infections, including pyelonephritis, hospital-acquired pneumonia and ventilator-acquired pneumonia caused by susceptible GNB.9 Cefiderocol is actively transported into bacterial cells using receptor-mediated iron transport systems owing to the binding of ferric iron by a catechol moiety. Cefiderocol can also passively enter bacterial cells through outer membrane porin channels.8 Once inside the cell, cefiderocol acts like other cephalosporins, binding primarily to penicillin-binding proteins and causing cell death by inhibition of peptidoglycan cell wall biosynthesis.10In vitro activity of cefiderocol has been demonstrated against GNB harbouring carbapenemases from all four Ambler classes (for example, KPC, VIM, IMP, NDM and OXA carbapenemases) and those with derepressed AmpC and/or ESBLs plus porin/efflux pump resistance mechanisms.11–13
For cefiderocol, interpretive criteria/BPs for BMD and DD have been defined by EUCAST, CLSI and the FDA (Table S1, available as Supplementary data at JAC Online).1,14,15 Clinical BPs for cefiderocol BMD and DD have been set by EUCAST for Enterobacterales and Pseudomonas aeruginosa, but not for Acinetobacter spp. and Stenotrophomonas maltophilia.1 For Acinetobacter spp. and S. maltophilia, when treatment options are limited, EUCAST recommends the use of general non-species-specific pharmacokinetic/pharmacodynamic (PK/PD) BPs.1 For these species, ZD breakpoints were developed by EUCAST to correspond to the PK/PD BPs.16,17 When using BMD as a method of AST for cefiderocol, iron-depleted-CAMHB (ID-CAMHB) media are needed to ensure MICs used for determining clinical breakpoints and PK/PD assessment are predictive of in vivo activity.18 DD does not require iron-depleted media and standard Mueller–Hinton agar can be used,3,17 meaning DD is potentially a simpler, cheaper, and a more widely accessible method of AST for cefiderocol.3
In this report, we investigated the reproducibility of cefiderocol MIC determination using BMD in ID-CAMHB against clinical isolates with various cefiderocol MICs and validated the EUCAST DD method for cefiderocol against clinical isolates by correlating zone diameters to reference MIC values. In addition, the EUCAST quality control (QC) ranges for cefiderocol were determined and validated.
Materials and methods
Bacterial isolates
Bacterial isolates from several sources and geographical origins were included in the study. The isolates were identified to species or complex level using the Microflex system with the MALDI Biotyper 3.1 software (Bruker Daltonics) and the MBT database-5627 according to the manufacturer’s instructions.
Broth microdilution (BMD)
In vitro activity was determined using preprepared, frozen 96-well microtitre plates (Thermo Scientific, Oakwood, USA) with ID-CAMHB for cefiderocol, which had been prepared according to CLSI approved methodology,14 and standard CAMHB for the control agents (cefepime, ceftazidime, meropenem and trimethoprim/sulfamethoxazole), according to EUCAST/ISO guidelines.1,5 Cefiderocol MICs were read as the first well in which the reduction of growth corresponds to a button of <1 mm or is replaced by the presence of light haze/faint turbidity. All tests were set up blindly and each panel was inoculated with a single isolate with fresh bacterial inoculum suspensions that were prepared each day. All isolates with difficult-to-read MICs were confirmed by at least two technicians reading independently.
Disc diffusion (DD)
DD was performed using cefiderocol 30 μg discs from two manufacturers (Liofilchem, Roseto degli Abruzzi, Italy and Mast Diagnostics, Merseyside, UK) on standardized unsupplemented Mueller–Hinton agar plates prepared in-house from two manufacturers [Oxoid (Thermo Fisher Scientific, Basingstoke, UK) and BBL (Becton Dickinson, Sparks, NV, USA)] according to EUCAST methodology for non-fastidious organisms.3 DD for the control agents (cefepime 30 μg, ceftazidime 10 μg, meropenem 10 μg and trimethoprim/sulfamethoxazole 1.25/23.75 μg) was also conducted according to EUCAST guidelines.3 Colonies within zones appeared for both cefiderocol and control agents and were taken into account when reading zones after having excluded possible contaminations. EUCAST recommends that in cases of double zones, or distinct colonies within zones, that the purity is checked, and the test is repeated; if cultures are pure, colonies within zones should be taken into account when measuring the diameter.3 The cefiderocol 30 μg disc was chosen based on an initial study investigating 5, 15 and 30 μg discs (Supplementary Methods 1.1).
WT distributions and the establishment of TECOFFS and ECOFFS
WT organisms were defined as organisms without phenotypically detectable acquired resistance mechanisms against multiple β-lactam agents (Supplementary Methods 1.2). In order to set TECOFFs, WT isolates were selected from the EUCAST development laboratories’ (EDL) library (50 Escherichia coli, 50 Klebsiella pneumoniae, 50 P. aeruginosa, 50 Acinetobacter baumannii and 45 S. maltophilia) and tested by BMD and DD according to EUCAST guidelines for cefiderocol and control agents.3,4 For each species, the widths of each MIC and ZD WT distribution, i.e. number of bars (representing MIC concentration and zone diameters, respectively) were compared for cefiderocol and the control agents.
MIC–zone diameter correlations
Isolates with a range of MICs (≤0.002–>256 mg/L), including cefiderocol-susceptible and cefiderocol-resistant isolates, were tested. BMD and DD were performed in parallel from the same inoculum suspension for Enterobacterales (n = 263), including E. coli (n = 77), K. pneumoniae (n = 77), and P. aeruginosa (n = 101), A. baumannii (n = 103) and S. maltophilia (n = 75). MICs were correlated with ZD, from which the disc BP corresponding to the MIC BP was set. Isolates with elevated cefiderocol MICs were provided by Shionogi or selected from among multidrug-resistant isolates from the EDL library. For S. maltophilia, all isolates tested had cefiderocol MICs ≤0.5 mg/L. The study layout was designed according to EUCAST SOP 9.219 and used Mueller–Hinton agar from two manufacturers (BBL BD™ and Oxoid Ltd) in parallel. To optimize the space on the agar plates, two comparator discs were tested for all species, except for S. maltophilia, for which trimethoprim/sulfamethoxazole is the only relevant control agent.
Validation of disc diffusion at external laboratories
External validation of cefiderocol DD was performed across four laboratories on local clinical isolates using cefiderocol 30 μg discs (Liofilchem or Mast) on local Mueller–Hinton agar according to EUCAST SOP 9.2.19
Quality control
Quality control (QC) ranges were determined from DD tests according to EUCAST SOP 9.2,19 for E. coli ATCC 25922 and P. aeruginosa ATCC 27853 (Supplementary Methods 1.3); discs by two manufacturers (Liofilchem versus Mast) and Mueller–Hinton agar by three manufacturers (Oxoid Ltd, Difco and BBL BD™). Testing was conducted by the EDL and at four external laboratories (Supplementary Methods 1.4). These data were compared with data generated for CLSI.14,20
Results
MIC distributions in WT isolates
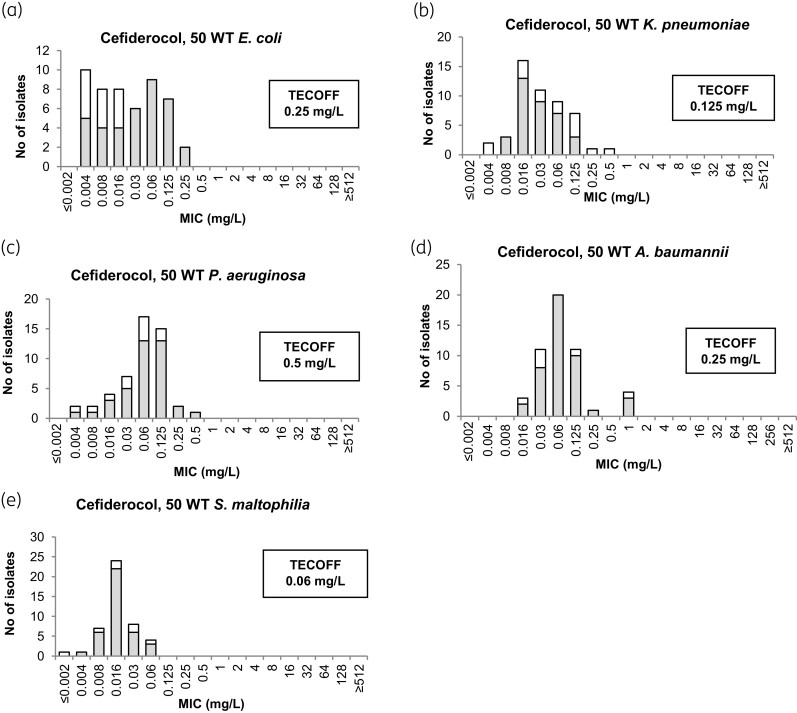
MIC distributions for cefiderocol against WT isolates are shown in Figure 1. TECOFFs are shown when determined. It was noted during these studies that cefiderocol MIC endpoints were more often difficult to read than for comparator agents and this was the case for all species tested, but MICs for the difficult-to-read isolates (shown as white bars in Figure 1) did not affect the TECOFFs. Two examples of reading of cefiderocol endpoints when trailing occurs are shown in Figure 2. In addition, growth in the positive control for ID-CAMHB was often less than in the standard CAMHB, especially for P. aeruginosa, A. baumannii and S. maltophilia. When excluding difficult-to-read isolates, the MIC distribution for cefiderocol was wider than control agents in three of the five species (Table S2). Excluding difficult-to-read isolates did not significantly impact on the calculation of the TECOFF.
Figure 1.
MIC WT distributions for cefiderocol against E. coli (a), K. pneumoniae (b), P. aeruginosa (c), A. baumannii (d) and S. maltophilia (e), based on the WT distributions from this study [suggested tentative epidemiological cut-off values (TECOFFS) are shown]; white bars correspond to isolates with difficult-to-read MICs.
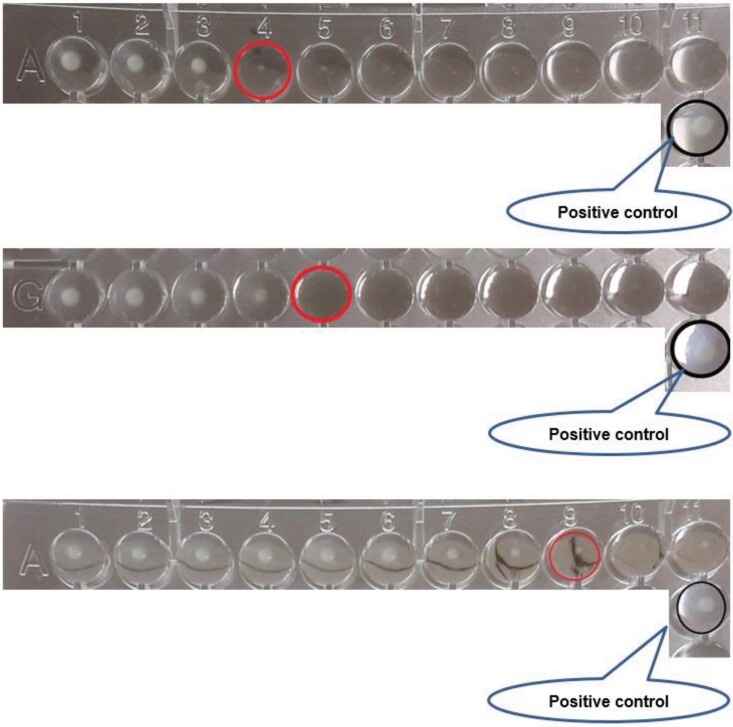
Figure 2.
Examples of reading cefiderocol endpoints when trailing occurs; examples from the EUCAST Reading Guide. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Disc potency determination
Assessment of three disc potencies (5, 15 and 30 μg discs) was conducted. As there was overlap between susceptible and resistant isolates with all three disc potencies and the 30 μg disc had already been approved for the CLSI disc diffusion methodology, it was determined that the 30 μg disc was acceptable for use with EUCAST methodology (Figure S2; Supplementary Methods 1.1).
MIC–zone diameter correlations for both WT isolates and isolates with elevated cefiderocol MICs
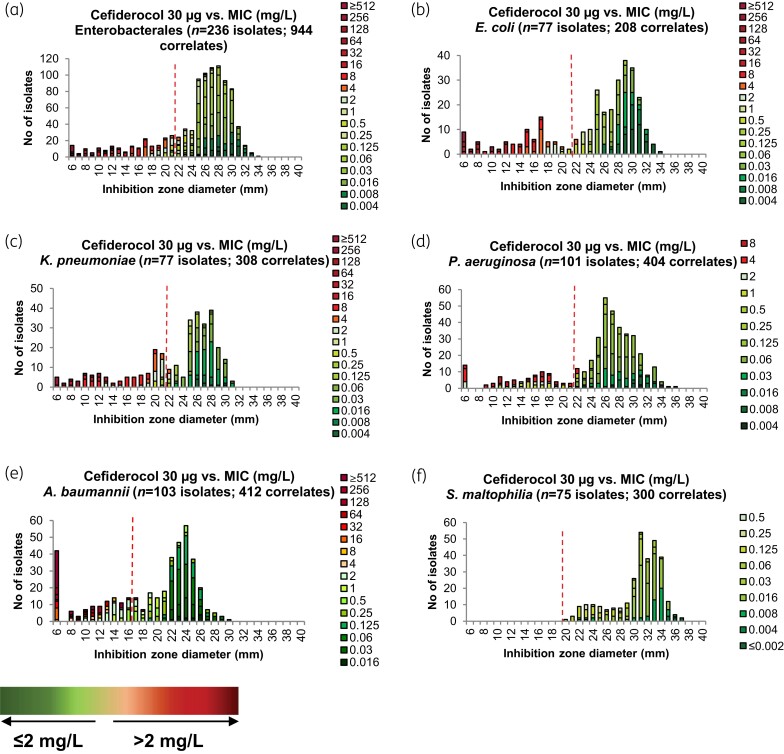
An analysis of the correlation between MICs and ZDs was performed for cefiderocol (Figure 3) and control agents (Figure S3) for Enterobacterales (n = 263), including E. coli (n = 77), K. pneumoniae (n = 77), and P. aeruginosa (n = 101), A. baumannii (n = 103) and S. maltophilia (n = 75). The correlation between zone diameters and MICs was generally good, but there was some overlap between susceptible (≤2 mg/L) and resistant (>2 mg/L) isolates in the range of 18–22 mm for Enterobacterales and of 14–22 mm for P. aeruginosa.
Figure 3.
MIC–zone diameter (ZD) correlations for cefiderocol for Enterobacterales (a), E. coli (b), K. pneumoniae (c), P. aeruginosa (d), A. baumannii (e), S. maltophilia (f). Each isolate was tested with cefiderocol discs from two manufacturers and on Mueller–Hinton media from two manufacturers, resulting in four correlates per isolate. Green = susceptible/below PK/PD MIC BP; orange/red = resistant/above PK/PD MIC BP; Red dotted line = ZD BP (Enterobacterales and P. aeruginosa) or ZD cut-off (A. baumannii and S. maltophilia).
The ZD (30 μg disc) correlates to the cefiderocol clinical BP, which was determined to be ≥22 mm for both Enterobacterales and P. aeruginosa. TECOFFs for E. coli, K. pneumoniae and P. aeruginosa were 24 mm, 22 mm and 23 mm, respectively (Figure S4). For A. baumannii, with the exception of one isolate, a cut-off value of ≥17 mm corresponded to MICs ≤2 mg/L while excluding isolates with MICs >2 mg/L.17 In our study, only S. maltophilia isolates with MIC values of ≤0.5 mg/L were included. The TECOFF for S. maltophilia was 29 mm and the smallest ZD was 20 mm. This is in line with the EUCAST-determined PK/PD BP, where the ZD of 20 mm corresponds to an MIC of ≤2 mg/L for S. maltophilia.17 For all evaluated species, an overlap between MICs on each side of the clinical BP was observed. Areas of Technical Uncertainty (ATU) were introduced to increase the robustness of the test: ATU for Enterobacterales, 18–22 mm; ATU for P. aeruginosa, 14–22 mm.
Validation for DD
The four external laboratories met the preliminary QC criteria (Figure S1) whilst generating routine ZD data on consecutive isolates of the four species. The resulting zone distributions (Figure S5) suggested that results were largely unaffected by disc and media manufacturer and that the results from the four laboratories were consistent. E. coli and P. aeruginosa data confirmed the ZD susceptible BP of ≥22 mm. For A. baumannii and S. maltophilia, data confirmed the ZD corresponding to the PK/PD susceptible BP: ≥17 mm and ≥20 mm, respectively.
Disc diffusion quality control
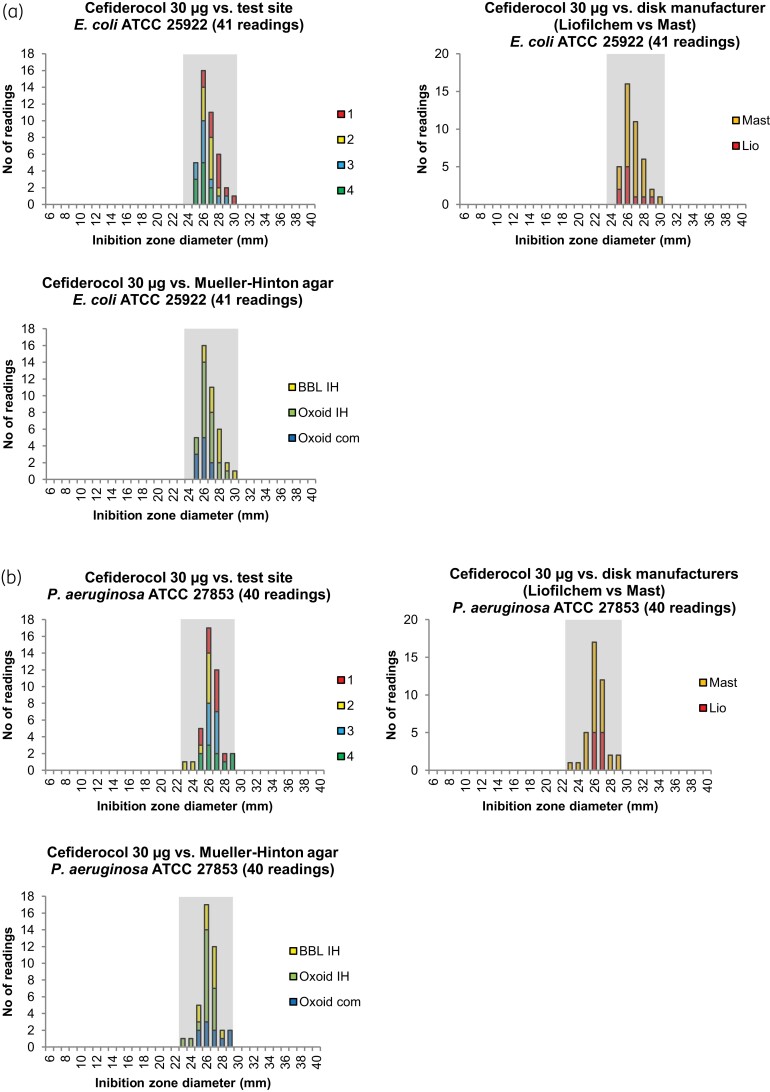
Cefiderocol 30 μg ZD distributions from testing at EDL suggested preliminary QC ranges of 24–30 mm (target 27 mm) for E. coli ATCC 25922 and 22–28 mm (target 25 mm) for P. aeruginosa ATCC 27853 (Figure S1). ZD distributions from testing at the external laboratories were within the preliminary ranges for E. coli ATCC 25922 [24–30 mm (target 27 mm)] (Figure 4a). For P. aeruginosa ATCC 27853 (Figure 4b), the disc ZD distributions were 23–29 mm (target 26 mm). Moreover, the intrinsic variability in the DD method was consistent with most other β-lactam antimicrobials, with a range of 6 mm (target ± 3 mm).6,21 The final EUCAST QC ranges (24–30 mm for E. coli ATCC 25922 and 23–29 mm for P. aeruginosa ATCC 27853) were set based on the data generated by EDL and the external laboratories and after comparison with the data generated for CLSI (Table S3).
Figure 4.
Inhibition zone diameter distributions for quality control strains from the external validation study versus test site, disc manufacturer and Mueller–Hinton manufacturer for (a) E. coli ATCC 25922 and (b) P. aeruginosa ATCC 27853. Grey shading indicates EUCAST QC ranges for E. coli ATCC 25922 (24–30 mm) and P. aeruginosa ATCC 27853 (23–29 mm). Com, commercially prepared; IH, prepared in-house. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Discussion
The results from these studies indicate that DD is a robust method for determining cefiderocol susceptibility. The DD QC results, and ensuing QC ranges, provide evidence that reproducibility is as good as for other agents, with limited effect of variability across laboratories, discs and media. A 6 mm range of intrinsic variability is within the expected ranges.6,21 For clinical isolates, correlation between MIC and ZD for Enterobacterales was consistent with the variability seen for QC strains (target ± 3 mm); however, the variability was larger for P. aeruginosa and, to avoid mischaracterization, a wider ATU (14–22 mm) was introduced.
For these studies, TECOFFs based on EUCAST guidelines were determined by using only WT isolates from EDL collections; however, it is preferable that the MIC distribution data for ECOFF determination is collected from ≥5 laboratories. Both the selection criteria for defining WT isolates and difference in number of isolates and test sites may have led to differences in ECOFFs between EUCAST and CLSI, as ECOFFs were determined by CLSI using more isolates from the SIDERO-WT multinational surveillance studies. In the studies reported here, the WT population was enhanced by excluding organisms with evidence of β-lactam resistance, through screening isolates for susceptibility to as many β-lactam antimicrobials as possible (Supplementary Methods 1.2). This simplifies the determination of WT and ECOFF and may serve as a model for other agents. However, even when limiting variation in BMD testing as much as possible, WT MIC distributions for cefiderocol were broader than for the majority of control agents. This complicates testing and the setting of ECOFFs for cefiderocol. Broader MIC distribution in WT strains with cefiderocol compared with other cephalosporins is likely due to greater variation in the expression of iron transporters and/or expression of siderophores, such as pyochelin, which can increase uptake and cause hypersusceptibility, and not due to low reproducibility of the BMD method. ECOFFs were not determined using the randomly selected isolates for cefiderocol in the surveillance studies because of widespread prevalence of β-lactamases, porin mutations and efflux over-expression.
The ATU is used as a warning to laboratory staff that the value determined is in an area where there are AST interpretive difficulties; this can be for BMD and DD methods conducted using EUCAST guidelines.22 ATU is usually defined by one MIC value or by one or several inhibition ZDs. The ATU is related to uncertainties in testing procedures.22 Although the natural and unavoidable variation in testing will influence the actions that may need to be taken, it is assumed that the test (MIC or inhibition ZD) is correctly performed and that the value obtained is correct. The ATU can be ignored or acted on, this can include repeating the test, performing an alternative test, or reporting the results in the ATU as ‘uncertain’. At present, per the EUCAST guidelines for cefiderocol, there are only ATUs for Enterobacterales (18–22 mm) and P. aeruginosa (14–22 mm).1 Moreover, the expected experimental variability margin for disc ZD is target ±3 mm, which would correspond to a range from 18–25 mm for a target of 22 mm; this was seen for the QC strains in the current study, meaning the ATU for Enterobacterales is not indicative of poor reproducibility.
The current advice for isolates that fall within the ATU for DD is that they should be retested by a different method (i.e. BMD), reported as resistant or not reported at all.1 Without re-resting, there is a risk that potentially susceptible isolates with MIC ≤2 mg/L but ZD in the 18–22 mm range are wrongly classed as resistant, which has potential negative implications for patient care due to the limited treatment options for some extensively drug resistant isolates or for patients that are not able to tolerate polymyxin-based regimens owing to renal insufficiency. This means that microbiologists need to be made aware of the urgency of obtaining a result for cefiderocol. DD for cefiderocol is as robust as DD for most other β-lactam agents. There are no specific reading challenges for cefiderocol DD. Moreover, the variation between discs and media from the different manufacturers used in this study did not impact to any extent on the DD results. Similar studies have also determined that DD offers a convenient alternative to BMD for cefiderocol AST, although other investigators have reported problems with A. baumannii complex isolates.23
EUCAST, FDA and CLSI all evaluated similar datasets to determine MIC distributions, PK/PD, and clinical exposure/response relationships, but used slightly different approaches, which has led to differences in the final BPs for specific bacterial species or antimicrobial agents. The use of different ECOFF definitions by various investigators has affected the setting of BPs.23–25 The use of formally defined ECOFFs/TECOFFs could benefit future work with similar antimicrobials (all β-lactams). The approach used in this study follows the EUCAST definitions of ECOFFs and TECOFFs, which stipulates that true WT isolates can only be defined using isolates totally devoid of interfering acquired resistance mechanisms.7,26
Conclusions
We investigated cefiderocol MIC and DD correlation, reproducibility and defined a suitable disc potency. QC criteria were established and TECOFFs for cefiderocol were determined. DD was shown to be a robust method for evaluating cefiderocol susceptibility in Enterobacterales and P. aeruginosa isolates and can be used with confidence when using media and discs that result in mean ZD values that are close to QC target values. The robustness of the method is further increased by the introduction of ATUs to strengthen the validity of results outside ATUs and to warn laboratories of potentially inconclusive results. The BMD method is more technically challenging but should be used to confirm the MIC in the event of an inconclusive result. Commercial assays for cefiderocol MIC determination can be used if they have been proven to be equivalent to reference BMD in ID-CAMHB and are quality controlled.
Supplementary Material
Acknowledgements
We thank Amra Basic, Sarah Johansson, Onur Karatuna and Jenny Åhman at the EDL for technical assistance during the antimicrobial susceptibility testing. The authors would also like to thank Mandy Wootton and Leanne Davis (University Hospital of Wales, Cardiff, UK), Anna Petersson and Simon Hintze (Clinical Microbiology Karlskrona, Sweden), Sara Petersson (Clinical Microbiology Kalmar, Sweden) and Inga Fröding (Karolinska University Hospital, Huddinge, Sweden). Medical writing support was provided by Daisy Bye, MRes, of Ashfield MedComms, an Ashfield Health company, part of UDG Healthcare plc.
Funding
This work was supported by Shionogi & Co., Ltd, Osaka, Japan. Medical writing support was funded by Shionogi & Co., Ltd., Osaka, Japan.
Transparency declarations
E.M. and G.K. are employed in the EUCAST Development Laboratory and have nothing to declare. Y.Y., M.T. and C.L. are all employees at Shionogi & Co., Ltd.
Author contributions
All authors contributed to the analysis of study data, drafting and revising the manuscript and approved the final version for submission.
Supplementary data
Additional Methods, Tables S1 to S3 and Figures S1 to S5 are available as Supplementary data at JAC Online.
References
- 1. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) . Breakpoint tables for interpretation of MICs and zone diameters, version 11.0. 2021. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf.
- 2. Matuschek E, Brown DFJ, Kahlmeter G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin Microbiol Infect 2014; 20: O255–66. doi: 10.1111/1469-0691.12373 [DOI] [PubMed] [Google Scholar]
- 3. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) . EUCAST disk diffusion method. 2021. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2021_manuals/Manual_v_9.0_EUCAST_Disk_Test_2021.pdf.
- 4. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) . EUCAST reading guide for broth microdilution. 2021. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/MIC_testing/Reading_guide_BMD_v_3.0_2021.pdf.
- 5. ISO 20776-1 . Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices – part 1: broth microdilution reference methods for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases. 2019. https://www.iso.org/standard/70464.html.
- 6. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) . MIC and zone distributions and ECOFFs. 2021. https://eucast.org/mic_distributions_and_ecoffs/.
- 7. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) SOP 10.1 MIC distributions and the setting of epidemiological cut-off (ECOFF) values. 2019. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/EUCAST_SOPs/EUCAST_SOP_10.1_MIC_distributions_and_epidemiological_cut-off_value__ECOFF__setting_20191130.pdf.
- 8. Shionogi & Co. L. Fetcroja. Summary of Product Characteristics. 2020. https://www.ema.europa.eu/documents/product-information/fetcroja-epar-product-information_en.pdf.
- 9. Shionogi & Co. L. Fetcroja (cefiderocol) Prescribing Information. 2019. https://www.shionogi.com/wp-content/themes/pdfs/fetroja.pdf.
- 10. Ito A, Kohira N, Bouchillon SKet al. . In vitro antimicrobial activity of S-649266, a catechol-substituted siderophore cephalosporin, when tested against non-fermenting Gram-negative bacteria. J Antimicrob Chemother 2016; 71: 670–7. doi: 10.1093/jac/dkv402 [DOI] [PubMed] [Google Scholar]
- 11. Longshaw C, Tsuji M, Echols Ret al. . In vitro activity of the siderophore cephalosporin, cefiderocol, against molecularly characterised, carbapenem-non-susceptible Gram-negative bacteria from Europe. JAC Antimicrob Resist 2020; 2: dlaa060. doi: 10.1093/jacamr/dlaa060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kohira N, West J, Ito Aet al. . In vitro antimicrobial activity of a siderophore cephalosporin, S-649266, against Enterobacteriaceae clinical isolates, including carbapenem-resistant strains. Antimicrob Agents Chemother 2016; 60: 729–34. doi: 10.1128/AAC.01695-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ito-Horiyama T, Ishii Y, Ito Aet al. . Stability of novel siderophore cephalosporin S-649266 against clinically relevant carbapenemases. Antimicrob Agents Chemother 2016; 60: 4384–6. doi: 10.1128/AAC.03098-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. CLSI . Performance Standards for Antimicrobial Susceptibility Testing—Thirty-First Informational Supplement: M100. 2021.
- 15. Food and Drug Administration (FDA) . Cefiderocol Injection. 2020. https://www.fda.gov/drugs/development-resources/cefiderocol-injection.
- 16. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidance on PK-PD breakpoints. 2016. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Organisms_and_agents_without_breakpoints_20160626.pdf.
- 17. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) . Breakpoints for cefiderocol, addendum (May 2020). 2020. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Addenda/Cefiderocol_addendum_20200501.pdf.
- 18. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) Guidance document on broth microdilution testing of cefiderocol. 2020. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Guidance_documents/Cefiderocol_MIC_testing_EUCAST_guidance_document_201217.pdf.
- 19. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) SOP 9.2 . Procedure for establishing zone diameter breakpoints and quality control criteria for new antimicrobial agents. 2020. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/EUCAST_SOPs/2020/EUCAST_SOP_9.2_Disk_diffusion_breakpoints_and_QC_ranges_final.pdf.
- 20. CLSI . Subcommittee meeting on Antimicrobial Susceptibility Testing. 2016. https://clsi.org/meetings/ast_old/ast-meeting-files-resources/.
- 21. The European Committee on Antimicrobial Susceptibility Testing . Routine and extended internal quality control for MIC determination and disk diffusion as recommended by EUCAST. Version 11.0. 2021. https://www.eucast.org/ast_of_bacteria/quality_control/.
- 22. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) . Area of Technical Uncertainty. 2020. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Area_of_Technical_Uncertainty_-_guidance_v2_2020.pdf.
- 23. Morris CP, Bergman Y, Tekle Tet al. . Cefiderocol antimicrobial susceptibility testing against multidrug-resistant Gram-negative bacilli: a comparison of disk diffusion and broth microdilution. J Clin Microbiol 2020; 59: e01649-20. doi: 10.1128/JCM.01649-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Albano M, Karau MJ, Schuetz ANet al. . Comparison of agar dilution of broth microdilution for testing in vitro activity of cefiderocol against Gram-negative bacilli. J Clin Microbiol 2020; 59: e00966-20. doi: 10.1128/JCM.00966-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simner PJ, Patel R. Cefiderocol antimicrobial susceptibility testing consideration: the Achilles heel of the trojan horse? J Clin Microbiol 2020; 59: e00951-20. doi: 10.1128/JCM.00951-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sato T, Yamawaki K. Cefiderocol: discovery, chemistry, and in vivo profiles of a novel siderophore cephalosporin. Clin Infect Dis 2019; 69: S538–43. doi: 10.1093/cid/ciz826 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.