Abstract
Polycystic ovary syndrome (PCOS), a common endocrine disorder of women, is characterized by increased ovarian androgen production and anovulatory infertility. Genome-wide association studies (GWAS) have identified more than 20 PCOS candidate loci. One GWAS candidate locus encompasses ZNF217, a zinc finger transcription factor. Immunohistochemical staining of ovarian tissue demonstrated significantly lower staining intensity for ZNF217 protein in PCOS theca interna compared to ovarian tissue from normal ovulatory women. Immunofluorescence staining of normal and PCOS theca cells demonstrated nuclear localization of ZNF217, with lower intensity in PCOS cells. Western blotting showed reduced ZNF217 protein in PCOS theca cells compared to normal theca cells, and that treatment with forskolin, which mimics the action of luteinizing hormone (LH), reduces ZNF217 expression. Lower ZNF217 expression in PCOS theca cells was confirmed by quantitative reverse transcription polymerase chain reaction. Notably, there was an inverse relationship between ZNF217 messenger RNA (mRNA) levels and theca cell androgen (dehydroepiandrosterone; DHEA) synthesis. The abundance of mRNA encoding a splice variant of DENND1A (DENND1A.V2), a PCOS candidate gene that positively regulates androgen biosynthesis, was also inversely related to ZNF217 mRNA levels. This relationship may be driven by increased miR-130b-3p, which targets DENND1A.V2 transcripts and is directly correlated with ZNF217 expression. Forced expression of ZNF217 in PCOS theca cells reduced androgen production, CYP17A1 and DENND1A.V2 mRNA, while increasing mIR-130b-3p. Conversely, knockdown of ZNF217 in normal theca cells with short hairpin RNA–expressing lentivirus particles increased DENND1A.V2 and CYP17A1 mRNA. These observations suggest that ZNF217 is part of a network of PCOS candidate genes regulating thecal cell androgen production involving DENND1A.V2 and miR-130b-3p.
Keywords: ZNF217, DENND1A.V2, CYP17A1, miR-130b-3p, polycystic ovary syndrome, theca cells
Polycystic ovary syndrome (PCOS) is a common endocrinopathy affecting women of reproductive age. It is characterized by hyperandrogenemia of ovarian origin, bilateral ovarian enlargement, and follicular growth arrest leading to anovulation and infertility [1, 2]. Based on family studies, twin studies [3], genome-wide association studies (GWAS), and case-control association studies, there is compelling evidence that PCOS is a polygenic disorder [4-10] that can be modified by epigenetic and environmental factors [11, 12]. An important milestone in PCOS genetics was achieved with the publication of GWAS on Han Chinese populations that identified multiple PCOS candidate loci [6, 10], including loci encompassing DENND1A, LHCGR, ZNF217, YAP1, INSR, THADA, C90rf3, TOX3, and RAB5B. More than 20 loci associated with PCOS have now been identified from GWAS in different ethnic populations [6, 8, 9, 13].
Although some PCOS GWAS loci encompass genes with plausible pathophysiological significance, many of the loci contain genes with no obvious link to ovarian function and androgen production [14, 15]. Moreover, the interrelationships among these putative candidate genes is not well understood [14]. We previously established that a splice variant of the PCOS GWAS candidate gene, DENND1A, named DENND1A.V2 showed increased expression in PCOS theca cells, and plays an integral role in augmented steroidogenic gene expression and excess androgen biosynthesis by PCOS theca cells [16]. Here, we report on the expression and function of another PCOS GWAS candidate gene, ZNF217, which encodes a zinc finger transcription factor with repressor (or corepressor) activity [17-21], and its role in mediating DENND1A.V2 and CYP17A1 expression and theca cell androgen production. Our observations strongly implicate ZNF217 in the pathogenesis of PCOS.
Materials and Methods
Normal and Polycystic Ovary Syndrome Theca Cell Cultures
Human theca interna tissue was obtained from follicles of women undergoing hysterectomy, following informed consent under a protocol approved by the institutional review board of the Pennsylvania State University College of Medicine and Virginia Commonwealth University. Ovarian tissue was obtained from women undergoing oophorectomy for treatment of endometrial hyperplasia or cancer (PCOS women) or benign gynecological conditions (normal ovulatory women). As a standard of care, oophorectomies were performed during the luteal phase of the cycle. Theca cells from normal cycling and PCOS follicles were isolated and grown as we have previously reported in detail [22-24]. The theca cell cultures used in these studies have been described and functionally characterized previously, and the steroidogenic properties of the normal and PCOS cells have been reported to result from the inherent properties of the cells rather than the cycle phase of the ovaries from which they were isolated [25-27]. PCOS and normal ovarian tissue came from age-matched women, 38 to 40 years old. The diagnosis of PCOS was made according to National Institutes of Health consensus guidelines [28, 29], which include hyperandrogenemia, oligoovulation, polycystic ovaries, and the exclusion of 21-hydroxylase deficiency, Cushing syndrome, adrenal and ovarian tumors, and hyperprolactinemia. All of the PCOS theca cell preparations studied came from ovaries of women with fewer than 6 menses per year and elevated serum total testosterone or bioavailable testosterone levels [25-27]. Each of the PCOS ovaries contained multiple subcortical follicles of less than 10 mm in diameter. The control (normal) theca cell preparations came from ovaries of fertile women with normal menstrual histories, menstrual cycles of 21 to 35 days, and no clinical signs of hyperandrogenism. Neither PCOS nor normal individuals were receiving hormonal medications at the time of surgery. Indications for surgery were dysfunctional uterine bleeding, endometrial cancer, and/or pelvic pain. Experiments comparing PCOS and normal theca were performed using fourth-passage (31-38 population doublings) theca cells isolated from size-matched follicles obtained from age-matched participants, in the absence of in vivo stimulation. It is important to note that these fourth-passage cells are not cell lines. The use of fourth-passage cells allowed us to perform multiple experiments from the same patient population and were propagated from frozen stocks of second-passage cells in the media described earlier. For all studies, theca cell cultures obtained from at least 4-5 independent normal and 5 independent PCOS patients were examined. The passage conditions and split ratios for all normal and PCOS cells were identical. Methods for immunohistochemistry, immunofluorescence (IF), Western blotting, quantitative reverse transcription polymerase chain reaction (qRT-PCR) for DENND1A.V2, miR-130b-3p RNA and assay of dehydroepiandrosterone (DHEA) were performed as previously reported [16, 30-32].
Quantitation of ZNF217 Expression in Human Ovarian Tissue Sections Using Immunohistochemistry
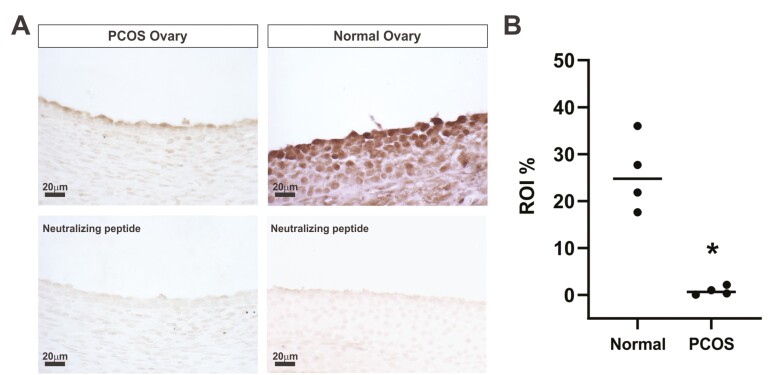
ZNF217 immunoperoxidase staining was performed on sections of paraffin-embedded ovaries from normal cycling and PCOS women, using conditions we have previously described [16]. Anti-ZNF217 antibody (Atlas Antibodies catalog No. HPA051857, RRID:AB_2681641, https://scicrunch.org/resources-legacy/Any/search?q=AB_2681641&l=AB_2681641), which binds to both ZNF217.V1 and ZNF217.V2), and the ZNF217 recombinant protein (APREST85710) were obtained from Sigma-Aldrich. Image analysis was performed using a digital camera (Olympus QColor5), attached to an Olympus BH-2 microscope. The staining area was quantified using the CellSens imaging software (Olympus). To quantify the staining area, multiple regions of interest (ROIs) were selected in the theca cell layer, and staining area was measured within the ROIs. Each image within the same experiment contained the same number of identical ROIs. Data were reported using percentage ROI area stained.
ZNF217 immunofluorescence in normal and polycystic ovary syndrome theca cells in monolayer culture
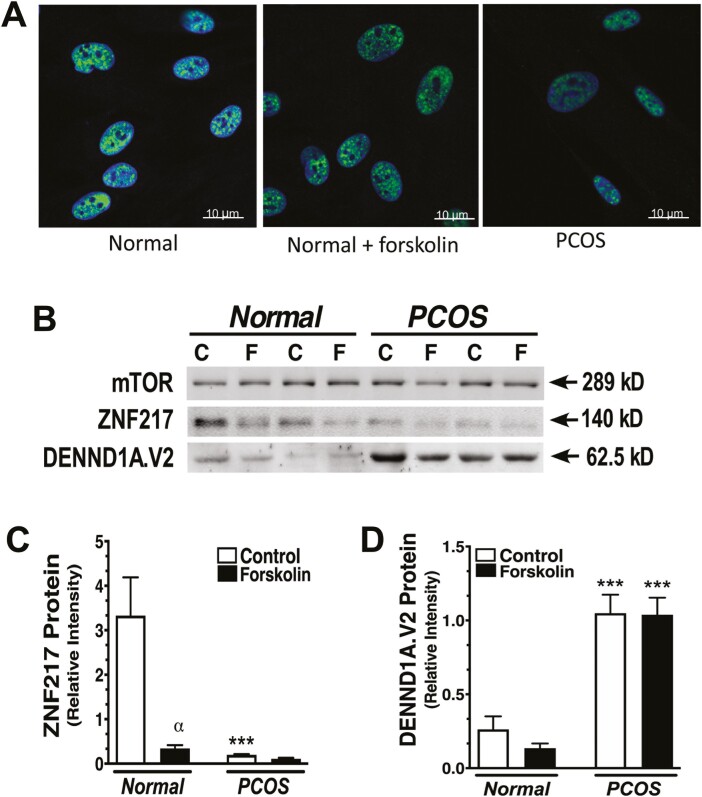
Normal (n = 4) and PCOS (n = 4) theca cell preparations were grown to subconfluence on Falcon chamber slides (VWR), and treated in the absence and presence of 20 µM forskolin for 48 hours. Theca cells were fixed with 4% formalin for 1 hour, washed with phosphate-buffered saline twice, and blocked with a blocking serum, containing 10% goat serum, 3% bovine serum albumin, and 0.2% Triton X-100. The cells were then incubated with anti-ZNF217 rabbit polyclonal antibody (dilution 1:100) as described earlier.
Western Blot Analysis of ZNF217 and DENND1A.V2 Protein
Whole-cell protein extracts were prepared from normal and PCOS theca cells treated with and without 20 μM forskolin for 48 hours, and Western blot analyses were performed using 30 μg protein per lane as we have previously described [16, 31]. A rabbit polyclonal anti-ZNF217 antibody recognizing ZNF217.V1 and V2 was obtained from Sigma-Aldrich, and a rabbit polyclonal antibody specific for the unique 33 amino acid C-terminal sequence of DENND1A.V2 (Jan M. McAllister, Penn State College of Medicine catalog No. Bunny-226n-V2, RRID:AB_2800456, https://scicrunch.org/resources-legacy/Any/search?q=AB_2800456&l=AB_2800456) generated in our laboratory, were used as primary antibodies [16, 31]. ZNF217 and DENND1A.V2 protein was visualized using electrochemiluminescence (Rockland Immunochemicals) and quantitated using a ProteinSimple FluorChem R. Protein expression was normalized to total mammalian target of rapamycin (mTOR), which is not significantly different in normal and PCOS theca cells, nor regulated by forskolin treatment [16]. The data presented are representative of multiple experiments repeated 3 times.
Quantitation of Quantitative Reverse Transcription Polymerase Chain Reaction for ZNF217, CYP17A1, and DENND1A.V2
Quantification of ZNF217 mRNA accumulation was performed using the Single Step Brilliant III Ultra-Fast qRT-PCR Reagent (Agilent) with a final concentration of 200 nM for each forward and reverse primer, 100 nM for the probe, and 50 to 100 ng total RNA per tube. Primer/probe sequences used to detect ZNF217 isoforms in exon 4 of ZNF217 isoforms 1 and 2, were the following: ZNF217 forward CDS Primer: CTGTTACCGCAGGACTGTGTGT, ZN217 Probe: TCCGTCGCAGGCGCTGCC, and ZNF217 reverse CDS Primer: TGGAGCTCAGGAACCTTGGT. qRT-qPCR for CYP17A1, DENND1A.V2, and the normalization process with TBP has been previously described [16].
Quantitative Reverse Transcription Polymerase Chain Reaction–based Analysis of miR-130b-3p Expression
The expression profile of miR-130b-3p in normal and PCOS theca cells was validated in vitro using the TaqMan MicroRNA Assays (Thermo Fisher) following the manufacturer’s instructions, as we have previously described [31]. Briefly, 10 ng RNA was reverse transcribed using the target (miRNA) specific stem-loop RT primer and the TaqMan MicroRNA reverse transcription kit (Thermo Fisher). The complementary DNA was then amplified by real-time qRT-PCR using target specific TaqMan primer-probe mix. The qRT-PCR was performed in triplicate per sample and U6 small nuclear 1 (RNU6-1) was used for normalization of the miR-130b-3p qRT-PCR expression data. The mean expression value for miR-130b-3p was divided by the mean RNU6-1 expression value to normalize each sample.
Quantitation of Dehydroepiandrosterone
DHEA was quantified by enzyme-linked immunosorbent assay (ELISA) performed on cell culture media collected from the same normal (N = 5) and PCOS (N = 5) theca cell preparations (treated with and without 20 μM forskolin) [16, 31] that were used in studies of ZNF217 mRNA expression. DHEA levels were measured using ELISA kits (DRG) according to the manufacturer’s instructions, as we previously described [16, 31].
Replication-incompetent ZNF217 Adenovirus Infections
Forced expression of ZNF217 was examined using ZNF217 adenovirus (Adv) (hZNF217-pADenoG), constructed by Applied Biological Materials. Control empty NULL nonexpressing Adv (pAdenoG Null) was also obtained from Applied Biological Materials. Recombinant Advs were propagated and expanded in HEK293T cells. The ZNF217 pAdenoG Null were used to infect normal theca cells as we previously described [16].
ZNF217 Short Hairpin RNA Knockdown
Decreased expression of ZNF217 (ie, ZNF217 knockdown) in normal theca cells was examined following infection with either control short hairpin RNA (shRNA) or ZNF217 shRNA lentiviral particles (3 pmol/106 cells) as per the standard protocol provided by Horizon Discovery.
Statistical Methods
Relative differences in staining at the ROI were measured by highlighting specific stained areas with red overlay. Data were quantified as area stained (μm2). Where indicated, statistical analyses were performed with Prism 8 by GraphPad Software. The t test was used for comparison of 2 groups of data, and 1-way analysis of variance with Bonferroni corrections when multiple groups of data are compared. Data were presented as means ± SE. Normality of data was assessed by D’Agostino and Pearson omnibus normality test. Samples were considered statistically significantly different when the P value was less than .05.
Correlations were determined in experiments where ZNF217, CYP17A1, DENND1A.V2 mRNA, miR-130b-3p, and steroid production (DHEA) were examined from multiple individual patients treated with or without forskolin. Spearman nonparametric correlation coefficient (ρ) was calculated to estimate the degree of correlation between all pairwise comparisons (ρ was considered statistically significant when P < .05), using JMP/SAS.
Results
Expression of ZNF217 Protein in Human Ovaries and Cultured Theca Cells
To examine the localization of ZNF217, we used paraffin-embedded blocks of ovarian tissue obtained from normal cycling women and our PCOS patient population. Immunohistochemical staining revealed lower staining intensity for ZNF217 in PCOS theca cells (Fig. 1A). Specificity of the anti-ZNF217antibody was confirmed by neutralization with recombinant ZNF217 peptide (see Fig. 1A, lower panels). IF images of cultured normal and PCOS theca cells established nuclear localization of ZNF217 and revealed lower signal intensity in PCOS theca cells and in normal theca cells treated with 20 μM forskolin (Fig. 2A). Quantification of Western blot data from 5 normal and 5 PCOS theca cell preparations demonstrated that ZNF217 protein (normalized by mTOR) was significantly reduced in normal cells treated with forskolin (Fig. 2B and 2C). In addition, ZNF217 protein in PCOS theca cells was significantly reduced under control culture conditions compared to normal theca cells (see Fig. 2C). Although it did not reach statistical significance, ZNF217 in forskolin-stimulated PCOS theca cells trended toward lower expression as compared to normal theca cells (see Fig. 2C). In contrast, DENND1A.V2 expression was significantly increased in PCOS theca cells compared to normal theca cells (Fig. 2D), in agreement with our prior observations [16].
Figure 1.
A, ZNF217 immunohistochemical staining of normal ovarian tissue (right) and polycystic ovary syndrome (PCOS) ovarian tissue (left). Images were taken at 40× magnification. In comparison to the theca cells of the normal cycling ovary, ZNF217 protein expression was decreased in theca layer of follicles of PCOS ovarian specimens. Antibody neutralized with the ZNF217 immunogenic peptide was used in the bottom 2 images to confirm specificity of the immunoperoxidase signal. B, Comparison of ZNF217 staining intensity in the designated region of interest (ROI, theca interna) revealed a significant decrease in the theca cell layer of PCOS ovarian tissue (N = 4) compared to normal ovarian tissue (N = 4); *P < .001.
Figure 2.
ZNF217 expression in normal and polycystic ovary syndrome (PCOS) theca cells. A, ZNF217 (green signal) is localized in punctate nuclear structures in human theca cells by immunofluorescence. The ZNF217 signal is reduced in PCOS theca cells compared to normal theca cells cultured without forskolin, and in normal theca cells treated with forskolin (20 μM, 24 hours). B, Representative Western blots comparing ZNF217 and DENND1A.V2 protein expression in 2 normal and 2 PCOS theca cell preparations cultured in the C, absence, or F, presence of forskolin (20 μM) for 24 hours. C, Quantitative Western blot data from 5 normal and 5 PCOS preparations demonstrated that ZNF217 protein was decreased by forskolin treatment in normal theca cells (αP < .001). ZNF217 protein in PCOS theca cells was decreased compared to normal theca cells (***P < .001) under control culture conditions. D, Relative DENND1A.V2 protein normalized by mammalian target of rapamycin (mTOR) was elevated both in forskolin-treated (***P < .005) and untreated (***P < .005) PCOS cells.
ZNF217 Gene Expression in Normal and Polycystic Ovary Syndrome Theca Cells and Relationships to Dehydroepiandrosterone Production, CYP17A1, DENND1A.V2, and miR-130b-3p expression
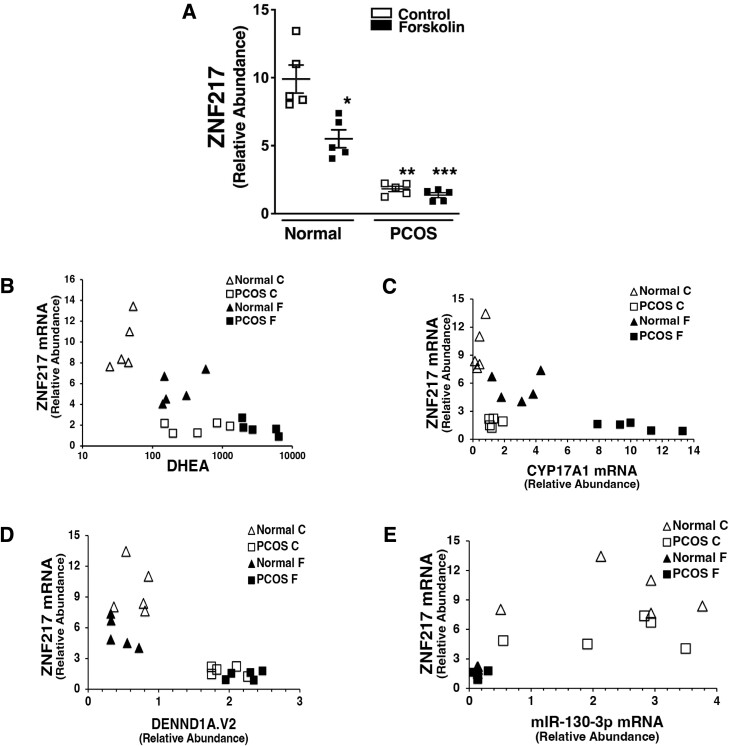
Comparison of ZNF217 expression in normal and PCOS theca cell preparations (Fig. 3A) demonstrated a significantly lower level of ZNF217 mRNA compared to normal theca cells under both forskolin-treated and untreated conditions. Moreover, forskolin treatment of normal theca cells significantly reduced ZNF217 expression. ZNF217 expression, DHEA production (Fig. 3B), CYP17A1 (Fig. 3C), and DENND1A.V2 mRNA expression (Fig. 3D), and miR-130b-3p abundance (Fig. 3E) were correlated among normal and PCOS theca cells. There was a significant negative relationship between ZNF217 mRNA levels and DHEA production (see Fig. 3B). Decreased ZNF217 expression in PCOS cells was negatively correlated with CYP17A1 gene expression, which encodes P450 17α-hydroxylase, the key enzyme in androgen biosynthesis (see Fig. 3C) [24, 33, 34]. Reduced ZNF217 expression in PCOS theca cells was associated with increased expression of CYP17A1 (see Fig. 3C). Comparison of ZNF217 mRNA expression with DENND1A.V2 mRNA abundance in PCOS and normal theca cells treated without or with forskolin revealed a negative inverse correlation (see Fig. 3D). miR-130b-3p, which targets DENND1A.V2 mRNA, was positively correlated with ZNF217 expression (see Fig. 3E).
Figure 3.
ZNF217 abundance in normal and polycystic ovary syndrome (PCOS) theca cells and the relationship between ZNF217 messenger RNA (mRNA) to dehydroepiandrosterone (DHEA) accumulation, DENND1A.V2, CYP17A1, and miR-130b-3p expression. Individual theca cell preparations from normal (n = 5) and PCOS (n = 5) women were cultured in the absence or presence of forskolin (20 μM). For quantitative reverse transcription polymerase chain reaction, mRNA was harvested from the cells 16 hours following treatment and ZNF217, CYP17A1, DENND1A.V2, miR-130b-3p expression was quantified. In parallel cultures of normal and PCOS theca cells, conditioned medium was collected at 48 hours and DHEA biosynthesis was assayed. A, PCOS theca cells showed decreased expression of ZNF217 mRNA compared to normal theca cells both in forskolin-treated (***P < .001) and untreated cultures (**P < .001. In normal theca cells, the addition of forskolin significantly decreased ZNF217 mRNA (*P < .01). B, ZNF217 mRNA abundance compared to DHEA production. PCOS theca cells cultured in the absence or presence of forskolin showed increased DHEA production and decreased ZNF217 mRNA expression compared to normal theca cells. There was an inverse negative correlation between ZNF217 mRNA expression and DHEA production (Spearman ρ = –0.7353; P < .001). C, CYP17A1 gene expression in PCOS theca cells, required for androgen biosynthesis in PCOS, was negatively correlated with decreased ZNF217 mRNA abundance (Spearman ρ = –0.67890; P = .001). D, Comparison of ZNF217 mRNA expression to DENND1A.V2 mRNA abundance in PCOS and normal theca cells treated without or with forskolin revealed an inverse negative correlation (Spearman ρ = –0.6992; P < .001). E, miR-130b-3p, which targets DENND1A.V2 transcripts, was positively correlated with ZNF217 expression (Spearman ρ = 0.7815; P < .001).
ZNF217 Overexpression in Polycystic Ovary Syndrome Theca Cells Reduces DENND1A.V2 and CYP17A1 Expression and Androgen Production, While Increasing miR-130b-3p Abundance
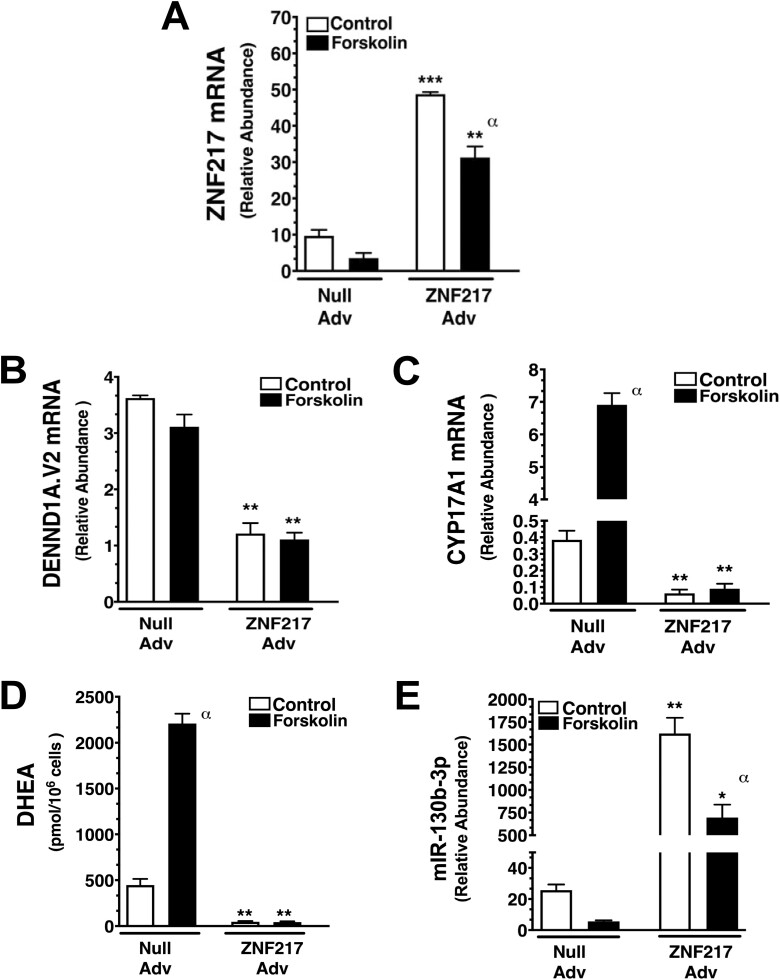
A ZNF217 Adv was generated to examine the effects of forced overexpression of ZNF217 in PCOS theca cells that have reduced ZNF217 expression (see Fig. 3A). PCOS theca cells were grown until 85% confluent and infected with ZNF217 Adv (3 pfu/cell) or empty vector (Null Adv) at 3 pfu/cell in serum-free medium for 16 hours, then cultured in the absence (control) or presence of 20 μM forskolin. After 36 hours, the infected cells were harvested, and RNA was prepared. ZNF217, DENND1A.V2, CYP17A1, and miR-130b-3p expression was quantified by qRT-PCR, as previously described [16, 31]. In parallel studies, 72 hours following treatment media were collected for DHEA measurements and cells were counted. As shown in Fig. 4A, ZNF217 mRNA was significantly increased in ZNF217 Adv-infected cells compared to Null Adv–infected cells under both basal (control) and forskolin-stimulated conditions. Of note, forskolin treatment reduced ZNF217 mRNA both in Null and ZNF217 Adv–infected cells, in agreement with data presented in Fig. 3. ZNF217 Adv infection significantly inhibited both basal and forskolin-stimulated DENND1A.V2 expression, as compared to Null Adv infection, and converted the PCOS cells to a normal phenotype of diminished DENND1A.V2 mRNA levels (Fig. 4B). CYP17A1 mRNA accumulation was significantly increased in Null Adv–infected cells following forskolin treatment (Fig. 4C). In contrast, forced ZNF217 overexpression dramatically decreased CYP17A1 mRNA accumulation under both control and forskolin-stimulated culture conditions (see Fig. 4C). In parallel studies, DHEA accumulation was also observed to be increased by forskolin treatment in Null Adv–infected cells (Fig. 4D), whereas ZNF217 Adv infection reduced DHEA biosynthesis both in control and forskolin-treated cells. ZNF217 Adv infection significantly augmented both basal and forskolin-treated miR-130b-3p accumulation, as compared to Null Adv–infected cells (Fig. 4E). Forskolin treatment significantly reduced miR-130b-3p in ZNF217 Adv–infected cells. These data are consistent with the notion that relatively high ZNF217 expression in normal theca cells is linked to lower levels of DENND1A.V2 and CYP17A1 gene expression.
Figure 4.
Forced expression of ZNF217 in polycystic ovary syndrome (PCOS) theca cells converts PCOS theca cells to a normal phenotype of reduced DENND1A.V2, CYP17A1, and dehydroepiandrosterone (DHEA) biosynthesis using a ZNF217 adenovirus (Adv). PCOS theca cells (N=5) were infected with either 3 pfu/cell of Null (empty) vector adenovirus (Null Adv) or ZNF217 adenovirus (ZNF217 Adv) overnight, then treated in the absence (Control) and presence of forskolin (20 μM) for 36 hours for RNA studies or 72 hours for DHEA studies. A, ZNF217 Adv infection augmented ZNF217 messenger RNA (mRNA) expression under unstimulated (control, ***P < .0001) and forskolin-stimulated conditions (**P < .001). ZNF217 Adv infection following forskolin stimulation resulted in decreased ZNF217 mRNA compared to control untreated cells (αP < .01). B, Following ZNF217 Adv infection, PCOS theca cells were converted to a normal phenotype of diminished DENND1A.V2 mRNA accumulation, under both control (**P < .001) and forskolin-stimulated conditions (**P < .001), compared to Null Adv (control) infected cells. C, CYP17A1 mRNA accumulation was increased following forskolin-treatment (αP < .01) in Null Adv–infected cells. In addition, ZNF217 Adv infection dramatically decreased CYP17A1 mRNA accumulation under both control (**P < .001) and forskolin-stimulated conditions (**P < .001). D, At 72 hours following infection with Null Adv, DHEA accumulation was observed to be increased by forskolin treatment (αP < .01). ZNF217 Adv infection resulted in decreased DHEA biosynthesis under both control (**P < .001) and forskolin-stimulated cells (**P < .001). E, Forced ZNF217 overexpression with ZNF217 Adv, augmented miR-130b-3p expression under unstimulated (control, **P < .001) and forskolin-stimulated conditions (*P < .1). ZNF217 Adv infection following forskolin stimulation resulted in decreased ZNF217 mRNA compared to control untreated cells (αP < .01).
ZNF217 knockdown in normal theca cells with ZNF217 short hairpin RNA lentiviral particles
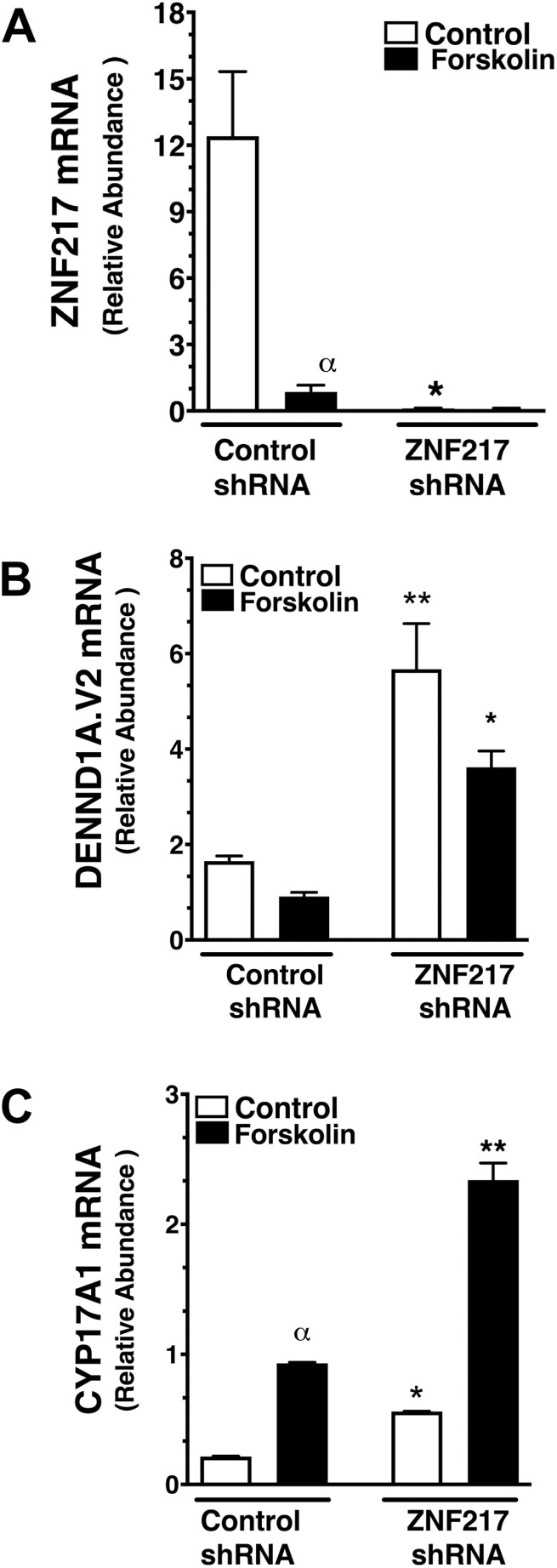
To determine whether decreased expression of ZNF217 was associated with increased DENND1A.V2 and CYP17A1 mRNA abundance and androgen production, PCOS theca cells and normal theca cells were infected with either control shRNA or ZNF217 shRNA lentiviral particles for 36 hours. As shown in Fig. 5A, ZNF217 expression was significantly suppressed following ZNF217 shRNA lentivirus infection. In agreement with results presented in Fig. 3A, forskolin treatment significantly inhibited ZNF217 expression (see Fig. 5A). As shown in Fig. 5B, DENND1A.V2 expression was significantly increased following treatment with ZNF217 shRNA, as compared to control shRNA. ZNF217 knockdown significantly increased both basal and forskolin-stimulated CYP17A1 mRNA abundance (Fig. 5C). Combined, these results provide further evidence that the decrease in ZNF217 expression in PCOS is associated with augmented CYP17A1 expression, as well as androgen biosynthesis, possibly through the intermediacy of DENND1A.V2 and a microRNA that targets the DENND1A.V2 transcript.
Figure 5.
ZNF217 knockdown in normal theca cells results in augmented DENND1A.V2 and CYP17A1 messenger RNA (mRNA) expression. Normal theca cells (N=5) were infected with equivalent amounts of control short hairpin RNA (shRNA) or ZNF217 shRNA lentiviral particles (3 pmol/106 cells) for 24 hours, then treated for 36 hours in the absence (control) or presence of 20 μM forskolin. A, In the absence of forskolin, ZNF217 expression was decreased following infection with ZNF217 lentiviral shRNA (*P < .05) as compared control shRNA. Forskolin treatment inhibited ZNF217 mRNA in control shRNA-infected cells (αP < .05). B, DENND1A.V2 expression was increased following ZNF217 shRNA lentivirus infection under both control (***P < .001) and forskolin-stimulated (***P < .001) conditions. C, CYP17 A1 mRNA was increased by forskolin-stimulation in normal theca cells infected with control shRNA (αP < .01). CYP17 A1 mRNA was increased following ZNF217 shRNA lentivirus infection under both control (*P < .01) and forskolin-stimulated (**P < .001) conditions, compared to control shRNA-infected cells.
Discussion
PCOS is currently thought to be a polygenic disorder [14]. The first PCOS candidate loci [6, 10] were identified by GWAS performed in Han Chinese populations. These loci included DENND1A, LHCGR, ZNF217, YAP1, INSR, THADA, C90rf3, TOX3, and RAB5B [10]. Subsequent GWAS, performed in various populations, revealed more than 20 PCOS candidate loci [35-37]. Despite substantial research on the genetic origins of PCOS, including follow-up association studies examining the correlation between PCOS candidate genes and various PCOS phenotypes, the mechanism(s) by which specific genes disrupt ovarian function and produce other features characteristic of PCOS is currently unknown [14, 15]. Moreover, the genetic variants that have a causal role in PCOS in different populations remain to be identified.
The functional importance of DENND1A.V2 in the PCOS phenotype of human theca cells was established by our studies demonstrating that increased DENND1A.V2 stimulates androgen synthesis in normal theca cells, whereas knockdown of DENND1A.V2 diminishes androgen production in PCOS theca cells [16, 21]. Although our prior studies provided evidence of a direct role for DENND1A.V2 in the production of the ovarian PCOS phenotype, the mechanisms of DENND1A.V2 action and the relationship of DENND1A.V2 to other PCOS candidate genes was unknown. We have expanded our studies of the functional genomics of PCOS and the interrelationship of another PCOS GWAS candidate gene, ZNF217, with respect to the genesis of hyperandrogenemia of ovarian origin, widely considered to be one of the most common characteristics of women with PCOS.
ZNF217 encodes a zinc finger DNA-binding transcription factor that usually acts as a corepressor [17]. Work by others showed that ZNF217 is expressed in granulosa cells and theca cells [19, 20]. ZNF217 has also been shown to increase estradiol synthesis in granulosa cells from normal cycling women, and to be reduced in granulosa cells collected from women with PCOS [19]. These studies agree with our immunostaining of ovarian tissue sections from normal cycling and PCOS women (see Fig. 1) that revealed lower staining intensity in PCOS theca.
Our findings suggest that decreased expression of ZNF217 in PCOS theca cells allows for the upregulation of DENND1A.V2 and CYP17A1 mRNA, resulting in enhanced androgen biosynthesis. In contrast, miR-130b-3p, which targets DENND1A.V2, was positively correlated with ZNF217 expression (see Fig. 3E). This finding raises the possibility that ZNF217 directly influences DENND1A.V2 abundance, and consequently CYP17A1 expression and androgen biosynthesis (Fig. 6). Alternatively, ZNF217 could indirectly influence DENND1A.V2 and CYP17A1 expression through the intermediacy of miR-130b-3p (see Fig. 6).
Figure 6.
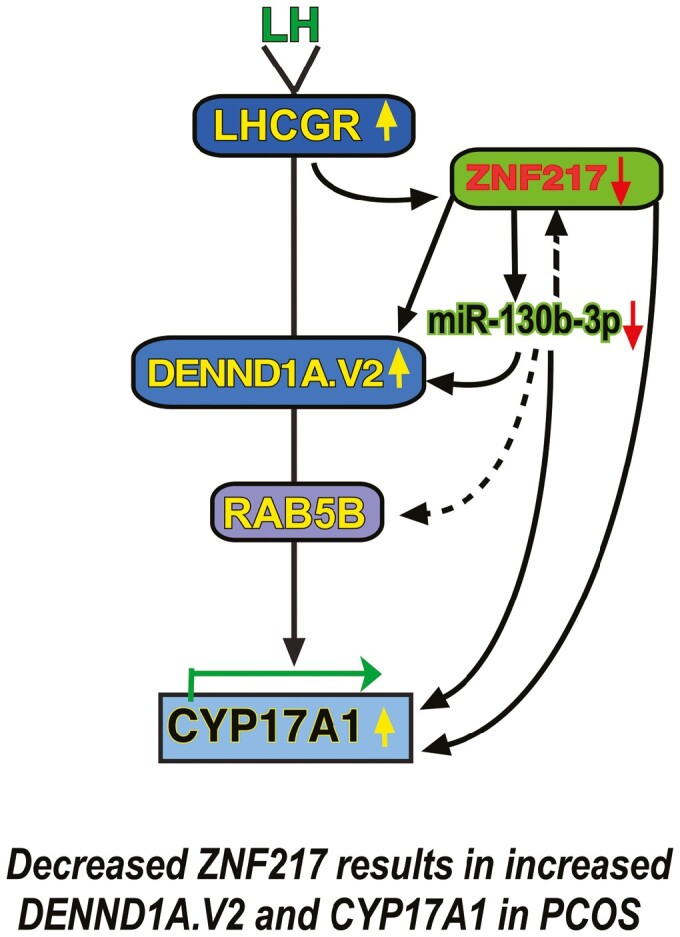
A polycystic ovary syndrome (PCOS) genome-wide association study (GWAS) candidate gene network encompassing ZNF217 and DENND1A.V2. PCOS is a manifestation of altered expression of a network of PCOS GWAS candidate genes including LHCGR, ZNF217, DENND1A, and RAB5B. Decreased ZNF217 expression in PCOS theca cells is associated with increased DENND1A.V2 and CYP17A1 expression, and downstream, androgen biosynthesis. Alternatively, ZNF217 could indirectly influence DENND1A.V2, RAB5B, and subsequently CYP17A1 expression, through the intermediacy of miR-130b-3p.
In addition to ZNF217, DENND1A, and CYP17A1, 2 other GWAS candidate genes, LHCGR and RAB5B, should be incorporated into the network of interacting genes regulating thecal androgen production. We have previously shown that RAB5B protein colocalizes with DENND1A.V2. We propose that LHCGR, an activator of adenylate cyclase, is an upstream regulator of DENND1A.V2, which has a clathrin-binding domain and, therefore, has potential interactions with LHCGR localized in plasma membrane coated pits and endocytic vesicles [30]. It is notable that databases, including TargetMiner (https://www.isical.ac.in/~bioinfo_miu/targetminer20.htm) and miRBase (https://www.mirbase.org), list ZNF217 and RAB5B as miR-130b-3p targets. Although we have no direct experimental evidence that miR-130b-3p regulates the expression of these genes in human theca cells, the potential role of miR-130b-3p in controlling expression of ZNF217 and RAB5B adds greater complexity to the proposed gene network (see Fig. 6, dashed lines), with the potential for autoregulation of ZNF217 expression and control of another gene, RAB5B, in the proposed cascade involving LHCGR and DENND1A.
Combined, our observations suggest that a reduction in ZNF217 expression is linked to increased DENND1A.V2 expression and androgen biosynthesis in PCOS theca cells. The relationships reported here and elsewhere suggest that PCOS is a manifestation of dysregulated expression of a network of genes including LHCGR, DENND1A, RAB5B, ZNF217, and miR-130b-3p (see Fig. 6). This model is concordant with the reported diminished expression of ZNF217 in granulosa cells from PCOS women [19, 20].
In summary, studies on normal and PCOS theca and granulosa cells provide a molecular framework for understanding the hormonal imbalance characteristic of PCOS, increased androgens of thecal origin, and reduced estradiol production by granulosa cells.
Glossary
Abbreviations
- Adv
adenovirus
- DHEA
dehydroepiandrosterone
- ELISA
enzyme-linked immunosorbent assay
- GWAS
genome-wide association study
- IF
immunofluorescence
- LH
luteinizing hormone
- mRNA
messenger RNA
- mTOR
mammalian target of rapamycin
- PCOS
polycystic ovary syndrome
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- ROI
region of interest
- shRNA
short hairpin RNA
Contributor Information
Jamaia S Waterbury, Department of Pathology, Penn State Hershey College of Medicine, Hershey, Pennsylvania 17033, USA.
Maria E Teves, Department of Obstetrics and Gynecology, Virginia Commonwealth University School of Medicine, Richmond, Virginia 23298, USA.
Alison Gaynor, Department of Human and Molecular Genetics, Virginia Commonwealth University School of Medicine, Richmond, Virginia 23298, USA.
Angela X Han, Department of Pathology, Penn State Hershey College of Medicine, Hershey, Pennsylvania 17033, USA.
Grace Mavodza, Department of Biochemistry and Molecular Biology, Virginia Commonwealth University School of Medicine, Richmond, Virginia 23298, USA.
Jordan Newell, Department of Pathology, Penn State Hershey College of Medicine, Hershey, Pennsylvania 17033, USA.
Jerome F Strauss, III, Department of Obstetrics and Gynecology, Virginia Commonwealth University School of Medicine, Richmond, Virginia 23298, USA; Department of Obstetrics and Gynecology, and Center for Research on Reproduction and Women’s Health, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania 19104, USA.
Jan M McAllister, Email: jmcallister@psu.edu, Department of Pathology, Penn State Hershey College of Medicine, Hershey, Pennsylvania 17033, USA.
Financial Support
This work was supported by the National Institutes of Health (grant No. R01HD083323 to J.M.M and J.F.S.).
Disclosures
The authors have nothing to disclose.
Data Availability
Original data generated and or analyzed during the present study are not available but are available from the corresponding author on reasonable request.
References
- 1. Balen A, Homburg R, Franks S. Defining polycystic ovary syndrome. BMJ. 2009;338:a2968. [DOI] [PubMed] [Google Scholar]
- 2. Sung YA, Oh JY, Chung H, Lee H. Hyperandrogenemia is implicated in both the metabolic and reproductive morbidities of polycystic ovary syndrome. Fertil Steril. 2014;101(3):840-845. [DOI] [PubMed] [Google Scholar]
- 3. Franks S, Webber LJ, Goh M, et al. Ovarian morphology is a marker of heritable biochemical traits in sisters with polycystic ovaries. J Clin Endocrinol Metab. 2008;93(9):3396-3402. [DOI] [PubMed] [Google Scholar]
- 4. Legro RS, Spielman R, Urbanek M, Driscoll D, Strauss JF III, Dunaif A. Phenotype and genotype in polycystic ovary syndrome. Recent Prog Horm Res. 1998;53:217-256. [PubMed] [Google Scholar]
- 5. Legro RS. Polycystic ovary syndrome. Long term sequelae and management. Minerva Ginecol. 2002;54(2):97-114. [PubMed] [Google Scholar]
- 6. Chen ZJ, Zhao H, He L, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43(1):55-59. [DOI] [PubMed] [Google Scholar]
- 7. Goodarzi MO, Jones MR, Li X, et al. Replication of association of DENND1A and THADA variants with polycystic ovary syndrome in European cohorts. J Med Genet. 2012;49(2):90-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strauss JF III, McAllister JM, Urbanek M. Persistence pays off for PCOS gene prospectors. J Clin Endocrinol Metab. 2012;97(7):2286-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joo YY, Actkins K, Pacheco JA, et al. International PCOS Consortium. A polygenic and phenotypic risk prediction for polycystic ovary syndrome evaluated by phenome-wide association studies. J Clin Endocrinol Metab. 2020;105(6):1918-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi Y, Zhao H, Shi Y, et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. 2012;44(9):1020-1025. [DOI] [PubMed] [Google Scholar]
- 11. Franks S, Berga SL. Does PCOS have developmental origins? Fertil Steril. 2012;97(1):2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219-231. [DOI] [PubMed] [Google Scholar]
- 13. Goodarzi MO, Louwers YV, Taylor KD, et al. Replication of association of a novel insulin receptor gene polymorphism with polycystic ovary syndrome. Fertil Steril. 2011;95(5):1736-1741.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dapas M, Dunaif A. Deconstructing a syndrome: genomic insights into PCOS causal mechanisms and classification. Endocr Rev. Published online January 13, 2022. doi: 10.1210/endrev/bnac001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dapas M, Dunaif A. The contribution of rare genetic variants to the pathogenesis of polycystic ovary syndrome. Curr Opin Endocr Metab Res. 2020;12:26-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McAllister JM, Modi B, Miller BA, et al. Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci U S A. 2014;111(15):E1519-E1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cowger JJM, Zhao Q, Isovic M, Torchia J. Biochemical characterization of the zinc-finger protein 217 transcriptional repressor complex: identification of a ZNF217 consensus recognition sequence. Oncogene. 2007;26(23):3378-3386. [DOI] [PubMed] [Google Scholar]
- 18. Vandevenne M, Jacques DA, Artuz C, et al. New insights into DNA recognition by zinc fingers revealed by structural analysis of the oncoprotein ZNF217. J Biol Chem. 2013;288(15):10616-10627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhai J, Li S, Cheng X, Chen ZJ, Li W, Du Y. A candidate pathogenic gene, zinc finger gene 217 (ZNF217), may contribute to polycystic ovary syndrome through prostaglandin E2. Acta Obstet Gynecol Scand. 2020;99(1):119-126. [DOI] [PubMed] [Google Scholar]
- 20. Zhai J, Liu J, Cheng X, et al. Zinc finger gene 217 (ZNF217) promoted ovarian hyperstimulation syndrome (OHSS) through regulating E2 synthesis and inhibiting thrombospondin-1 (TSP-1). Sci Rep. 2017;7(1):3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McAllister JM, Legro RS, Modi BP, Strauss JF III. Functional genomics of PCOS: from GWAS to molecular mechanisms. Trends Endocrinol Metab. 2015;26(3):118-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McAllister J, Simpson E. Human theca interna cells in culture. In: Methods in Toxicology. Vol 3. Academic Press; 1993:330-339. [Google Scholar]
- 23. Wickenheisser JK, Biegler JM, Nelson-Degrave VL, Legro RS, Strauss JF III, McAllister JM. Cholesterol side-chain cleavage gene expression in theca cells: augmented transcriptional regulation and mRNA stability in polycystic ovary syndrome. PLoS One. 2012;7(11):e48963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nelson-Degrave VL, Wickenheisser JK, Hendricks KL, et al. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol Endocrinol. 2005;19(2):379-390. [DOI] [PubMed] [Google Scholar]
- 25. Nelson VL, Legro RS, Strauss JF III, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13(6):946-957. [DOI] [PubMed] [Google Scholar]
- 26. Nelson-DeGrave VL, Wickenheisser JK, Cockrell JE, et al. Valproate potentiates androgen biosynthesis in human ovarian theca cells. Endocrinology. 2004;145(2):799-808. [DOI] [PubMed] [Google Scholar]
- 27. Wickenheisser JK, Quinn PG, Nelson VL, Legro RS, Strauss JF III, McAllister JM. Differential activity of the cytochrome P450 17alpha-hydroxylase and steroidogenic acute regulatory protein gene promoters in normal and polycystic ovary syndrome theca cells. J Clin Endocrinol Metab. 2000;85(6):2304-2311. [DOI] [PubMed] [Google Scholar]
- 28. Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GE, eds. Polycystic Ovary Syndrome (Current Issues in Endocrinology and Metabolism). Blackwell Scientific Publications; 1992:377-384. [Google Scholar]
- 29. Legro RS, Arslanian SA, Ehrmann DA, et al. Endocrine Society. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kulkarni R, Teves ME, Han AX, McAllister JM, Strauss JF III. Colocalization of polycystic ovary syndrome candidate gene products in theca cells suggests novel signaling pathways. J Endocr Soc. 2019;3(12):2204-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McAllister JM, Han AX, Modi BP, et al. miRNA profiling reveals miRNA-130b-3p mediates DENND1A variant 2 expression and androgen biosynthesis. Endocrinology. 2019;160(8):1964-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teves ME, Modi BP, Kulkarni R, et al. Human DENND1A.V2 drives Cyp17a1 expression and androgen production in mouse ovaries and adrenals. Int J Mol Sci. 2020;21(7):2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nelson VL, Qin KN, Rosenfield RL, et al. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86(12):5925-5933. [DOI] [PubMed] [Google Scholar]
- 34. Wickenheisser JK, Nelson-DeGrave VL, Quinn PG, McAllister JM. Increased cytochrome P450 17alpha-hydroxylase promoter function in theca cells isolated from patients with polycystic ovary syndrome involves nuclear factor-1. Mol Endocrinol. 2004;18(3):588-605. [DOI] [PubMed] [Google Scholar]
- 35. Day F, Karaderi T, Jones MR, et al. 23andMe Research Team. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018;14(12):e1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hayes MG, Urbanek M, Ehrmann DA, et al. Reproductive Medicine Network. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Commun. 2015;6:7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hayes MG, Urbanek M, Ehrmann DA, et al. Reproductive Medicine Network. Corrigendum: Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Commun. 2016;7:10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and or analyzed during the present study are not available but are available from the corresponding author on reasonable request.