Abstract
Background
Non-cystic fibrosis bronchiectasis (BE) is a chronic structural lung condition that facilitates chronic colonization by different microorganisms and courses with recurrent respiratory infections and frequent exacerbations. One of the main pathogens involved in BE is Pseudomonas aeruginosa.
Objectives
To determine the molecular mechanisms of resistance and the molecular epidemiology of P. aeruginosa strains isolated from patients with BE.
Methods
A total of 43 strains of P. aeruginosa were isolated from the sputum of BE patients. Susceptibility to the following antimicrobials was analysed: ciprofloxacin, meropenem, imipenem, amikacin, tobramycin, aztreonam, piperacillin/tazobactam, ceftazidime, ceftazidime/avibactam, ceftolozane/tazobactam, cefepime and colistin. The resistance mechanisms present in each strain were assessed by PCR, sequencing and quantitative RT–PCR. Molecular epidemiology was determined by MLST. Phylogenetic analysis was carried out using the eBURST algorithm.
Results
High levels of resistance to ciprofloxacin (44.19%) were found. Mutations in the gyrA, gyrB, parC and parE genes were detected in ciprofloxacin-resistant P. aeruginosa strains. The number of mutated QRDR genes was related to increased MIC. Different β-lactamases were detected: blaOXA50, blaGES-2, blaIMI-2 and blaGIM-1. The aac(3)-Ia, aac(3)-Ic, aac(6″)-Ib and ant(2″)-Ia genes were associated with aminoglycoside-resistant strains. The gene expression analysis showed overproduction of the MexAB-OprM efflux system (46.5%) over the other efflux system. The most frequently detected clones were ST619, ST676, ST532 and ST109.
Conclusions
Resistance to first-line antimicrobials recommended in BE guidelines could threaten the treatment of BE and the eradication of P. aeruginosa, contributing to chronic infection.
Introduction
Non-cystic fibrosis bronchiectasis (BE) is a persistent and progressive respiratory disease characterized by irreversible dilation of one or both bronchi. The dilation is a result of a destructive process in the bronchial walls, with damage to the epithelial lining due to the recurrent bacterial infections and continuous inflammation. The symptoms of this disease include sputum production, constant cough, dyspnoea and periodic exacerbations that result in decreased lung function and a worse quality of life.1Pseudomonas aeruginosa is a Gram-negative opportunistic microorganism that causes severe healthcare infections globally, such as sepsis, urinary tract infections, surgical site infections and respiratory tract infections. This microorganism is one of the most frequent pathogens in BE and chronic respiratory infections.2 Unfortunately, P. aeruginosa diagnosis and eradication therapy have a high rate of failure. Thus, BE patients colonized by P. aeruginosa receive frequent antimicrobial agents, favouring the emergence and spread of MDR/XDR P. aeruginosa strains and challenging the efficacy of antimicrobial agents. The extensive dissemination of MDR/XDR strains and high-risk clones worldwide adds further concern. Previous studies found that the high-risk clones are associated with certain clonal complexes (CCs) and that their distribution varies depending on the region.3 However, no previous studies have reported high-risk clones from BE patients.
The most important antipseudomonal agents include quinolones (e.g. ciprofloxacin), β-lactams (e.g. cefepime, ceftazidime, piperacillin/tazobactam, imipenem and meropenem) and aminoglycosides (e.g. amikacin and tobramycin). A wide range of mechanisms of resistance have been described for the different antimicrobial types: (1) acquisition of mutations in QRDRs; (2) production of β-lactamases (e.g. ESBLs and carbapenemases); (3) aminoglycoside-modifying enzymes (AMEs); (4) up-regulation of efflux systems such as MexAB-OprM, MexCD-OprJ, MexEF-OprN and MexXY with specific exportable substrates including quinolones, cephalosporins, carbapenems and aminoglycosides; and (5) loss or decreased production of the OprD protein used as an entrance channel by carbapenems.4 Recent information shows that resistance to antimicrobial agents is increasing, even to first-line antimicrobial agents, which may lead to therapeutic failure and chronic infection.5 The objective of our study was to determine the molecular mechanisms of resistance and the molecular epidemiology of P. aeruginosa strains isolated from patients with BE.
Materials and methods
Forty-three clinical P. aeruginosa strains were isolated from sputum samples of different consecutive patients with chronic BE during their stable phase, in a prospective observational study carried out in the Hospital Clínic of Barcelona (Spain). This prospective observational study (NCT04803695) was conducted at the pulmonology service of a tertiary care hospital and at the CELLEX research laboratories of the Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) in Barcelona, Spain. Thirty-eight patients were included from June 2017 to February 2020 and followed up for 1 year prospectively. One strain was isolated per patient but in five patients with chronic P. aeruginosa infection we isolated two different P. aeruginosa morphotypes (one mucoid and one non-mucoid for one patient, and one small and one large colony for each of the other four: strains 17, 18, 20, 21, 22, 23, 26, 27, 29 and 30). However, they were different in resistance pattern and/or mechanisms of resistance. A visit was performed every 3 months during the stable phase. During each visit: (1) one sputum sample was obtained; and (2) lung function was assessed with an EasyOne World Spirometer (NDD Medical Technologies, Zurich, Switzerland) and classified according to the American Thoracic Society/European Respiratory Society Guidelines.1
Antimicrobial susceptibility testing
The strains from sputum were cultured at 37°C for 24 h and were prepared in 0.9% NaCl at a density adjusted to a 0.5 McFarland (Becton Dickinson, Germany) turbidity standard. Antibiotic susceptibility testing was performed using the Kirby–Bauer method and Etest in accordance with the instructions of the manufacturers (bioMérieux and Liofilchem). MICs were determined by the standard agar dilution method with Mueller–Hinton II agar (Becton Dickinson). Colistin susceptibility was tested by broth microdilution method using MICRONAUT plates (MERLIN Diagnostika GmbH, Bornheim, Germany). The ATCC 27853 strain was used as a control. The following antibiotics were tested: aztreonam, ciprofloxacin, meropenem, imipenem, amikacin, tobramycin, piperacillin/tazobactam, ceftazidime, ceftazidime/avibactam, ceftolozane/tazobactam, cefepime and colistin. Replicates of each susceptibility test were performed. All results were interpreted in accordance with EUCAST guidelines v9.0 (http://www.eucast.org/clinical_breakpoints/).6
Mechanisms of resistance
Using PCR and sequencing, we tested the main mechanisms of resistance to ciprofloxacin (mutations in the QRDR), amikacin and tobramycin (the presence of AMEs), aztreonam, meropenem, imipenem, piperacillin/tazobactam, ceftazidime, ceftazidime/avibactam, ceftolozane/tazobactam and cefepime (production of β-lactamases) and colistin (mcr genes). Mutations in oprD and post-transcriptional regulator genes (nalC, nalD, mexR, nfxB, mexT, mexS and mexZ) were also determined by PCR and sequencing. Gene expression analysis was conducted by quantitative RT–PCR (RT–qPCR). The primers and conditions are shown in Table 1. The PCR products were sequenced by Sanger methods (GENEWIZ, Germany), and were analysed by alignment with the template sequence in GenBank.
Table 1.
Primers used in this study
| Amplified product | Primer pair | Sequence (5′ to 3′) | Amplicon size (bp) | Annealing temperature (°C) | Reference |
|---|---|---|---|---|---|
| gyrA | gyrA-F | AGTCCTATCTCGACTACGCGAT | 341 | 55 | 17 |
| gyrA-R | AGTCGACGGTTTCCTTTTCCAG | ||||
| gyrB | gyrB-F | TGCGGTGGAACAGGAGATGGGCAAGTAC | 697 | 55 | 17 |
| gyrB-R | CTGGCGGAAGAAGAAGGTCAACAGCAGGGT | ||||
| parC | parC-F | CGAGCAGGCCTATCTGAACTAT | 235 | 55 | 17 |
| parC-R | GAAGGACTTGGGATCGTCCGGA | ||||
| parE | parE-F | CGGCGTTCGTCTCGGGCGTGGTGAAGGA | 592 | 65 | 4 |
| parE-R | TCGAGGGCGTAGTAGATGTCCTTGCCGA | ||||
| nalC | nalC-F | TCAACCCTAACGAGAAACGCT | 814 | 69 | 4 |
| nalC-R | TCCACCTCACCGAACTGC | ||||
| nalD | nalD-F | GCGGCTAAAATCGGTACACT | 789 | 55 | 4 |
| nalD-R | ACGTCCAGGTGGATCTTGG | ||||
| mexR | mexR20 | CCAGTAAGCGGATAC | 1016 | 51 | 4 |
| mexRINT | GGATGATGCCGTTCACCTC | ||||
| mexT | mexT-F | TGCATCACGGGGTGAATAAC | 1398 | 55 | 4 |
| mexT-R | GGTAGCGCCAGGAGAAGTG | ||||
| mexS | mexS-F | ATACAGTCACAACCCATGA | 1153 | 50 | 4 |
| mexS-R | TCAACGATCTGTGAATCT | ||||
| mexZ | mexZ2060 | CCAGCAGGAATAGGGCGACCAGGGC | 1059 | 64 | 4 |
| mexZ1026 | CAGCGTGGAGATCGAAGGCAGCCGG | ||||
| oprD | oprD-F | GGCAGAGATAATTTCAAAACCAA | 1384 | 64 | 26 |
| oprD-R | GTTGCCTGTCGGTCGATTAC | ||||
| oxa50 | oxa50-F | AATCCGGCGCTCATCCATC | 619 | 54 | 32 |
| oxa50-R | GGTCGGCGACTGAGGCGG | ||||
| ges | ges-F | GTTTTGCAATGTGCTCAACG | 371 | 55 | 26 |
| ges-R | TGCCATAGCAATAGGCGTAG | ||||
| imi | imi-F | ATAGCCATCCTTGTTTAGCTC | 818 | 55 | 26 |
| imi-R | TCTGCGATTACTTTATCCTC | ||||
| gim | gim-F | TCGACACACCTTGGTCTGAA | 477 | 55 | 26 |
| gim-R | AACTTCCAACTTTGCCATGC | ||||
| aac(3)-Ia | aac(3)Ia-F | CCCTGACCAAGTCCAATCCATGC | 435 | 55 | 28 |
| aac(3)Ia-R | GGTGGCGGTACTTGGGTCGATA | ||||
| aac(3)-Ic | aac(3)Ic-F | CTCTCAAGACGTTGGTGTAATGC | 143 | 55 | 28 |
| aac(3)Ic-R | CAGCGATTGCGATGAAGCCAGA | ||||
| aac(6″)-Ib | aac(6″)Ib-F | GGTATGCCCAGTCGTACGTTGC | 281 | 55 | 28 |
| aac(6″)Ib-R | TGGACCATMTGGGGTGGTTACG | ||||
| ant(2″)-Ia | ant(2″)Ia-F | ATGAGCGAAATCTGCCGCTCTG | 150 | 55 | 28 |
| ant(2″)Ia-R | GCCCGCCGAGCATTTCAACTAT | ||||
| mcr1 | mcr1-F | AGTCCGTTTGTTCTTGTGGC | 1626 | 58 | 29 |
| mcr1-R | AGAT CCTTGGTCTCGGCTTG | ||||
| mexB | mexB-F | CAACATCCAGGACCCACTCT | 167 | 60 | 7 |
| mexB-R | AGGAAATCTGCACGTTCTGC | ||||
| mexD | mexD-F | CTACCCTGGTGAAACAGC | 250 | 58 | 8 |
| mexD-R | AGCAGGTACATCACCATCA | ||||
| mexF | mexF-F | TGTACGCGAACGACTTCAAC | 163 | 60 | 7 |
| mexF-R | GAGGTGTCGCTGACCTTGAT | ||||
| mexY | mexY-F | TCAGGCCGACCTTGAAGTAG | 159 | 60 | 7 |
| mexY-R | TCTCGGTGTTGATCGTGTTC | ||||
| rpsL | rpsL-F | TACTTCGAACGACCCTGCTT | 163 | 60 | 7 |
| rpsL-R | TTTCCTCGTACATCGGTGGT |
RNA extraction and reverse transcription
Strains were grown in 10 mL of LB broth at 37°C for 18–24 h up to the late exponential phase and collected by centrifugation. Total RNA extraction was carried out using the QIAGEN RNeasy purification kit. After checking the RNA extraction quality on a 1% agarose gel and measuring the RNA content (Nanodrop, Thermo Fisher Scientific, France), RNA extracts were stored at −20°C until further use. Prior to cDNA synthesis, genomic DNA (gDNA) was removed from 1 μg of total RNA using the gDNA wipeout buffer included in the QuantiTect Reverse Transcription kit (QIAGEN). The reverse transcription was performed in a volume of 20 μL including 14 μL of template RNA (extract concentrations adjusted to contain 1 μg of RNA), 1 μL of Reverse Transcription Master Mix, 4 μL of RT buffer 5× (containing dNTPs and Mg2+) and 1 μL of RT primer mix. Reverse transcription was performed in a Veriti PCR Thermal Cycler (Applied Biosystems, France) for 30 min at 42°C followed by a 3 min incubation at 95°C to inactivate the reverse transcriptase. All reactions including RNA handling were carried out on ice. The rpsL gene was used as reference to normalize the relative amount of mRNA.7
Real-time PCR assay
This work was focused on the expression of the four major P. aeruginosa efflux pump genes (mexB, mexD, mexF and mexY). Normalization of expression results was carried out using rpsL (reference gene to normalize the relative amount of mRNA) and using the PA01 strain as a control. A LightCycler 96 (Roche Diagnostics, Meylan, France) was used for all quantitative PCRs. All PCR amplification reactions were performed in 96-well plates in a 10 μL final volume containing 2.5 μL of diluted (1:20) template cDNA, 1 μL of each primer (corresponding to a final concentration of 0.5 μM), 5 μL of QuantiTect SYBR Green PCR Master Mix (including MgCl2 to reach a final concentration of 2.5 mM) (QIAGEN) and 0.5 μL of RNase/DNase free water (QIAGEN). The cycling program was set as follows: (1) activation: 1 cycle at 95°C for 15 min; (2) amplification: 45 cycles including a 15 s denaturation at 95°C, a 25 s annealing at 60°C and a 15 s elongation at 72°C; and (3) melting curve: 1 cycle including 5 s at 95°C, 1 min at 65°C and a final increase at 97°C with a transition rate of 0.11°C/s. Each reaction was carried out in duplicate and the experiment was repeated on two different sets of RNA extracts (biological replicate).4,7,8
Evaluation of real-time PCR results
Using the ΔΔCt method, overexpression of mexB, mexD, mexF and mexY was considered when the corresponding mRNA level was at least 2-fold higher than that of ATCC PA01 (the rpsL gene was used as reference to normalize the relative amount of mRNA), negative if less than 1-fold higher and borderline if between 1- and 2-fold higher.7,9
Statistical analysis
Differences in the expression of each gene of interest were tested using the single sample t-test versus cut-off values of 0.5 for underexpression and 2 for overexpression.9
Molecular typing
Molecular epidemiology was analysed by MLST (https://pubmlst.org/paeruginosa/). Allelic profiles of seven P. aeruginosa housekeeping genes (acsA, aroE, guaA, mutL, nuoD, ppsA and trpE) were analysed by PCR and confirmed in 2% agarose gel. Next, PCR products were sequenced by GENEWIZ. Phylogenetic analysis was carried out using the eBURST algorithm (http://www.phyloviz.net/goeburst).10,11
Ethics
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki (current version, Fortaleza, Brazil, October 2013) and its later amendments and it was conducted in accordance with the requirements of the 2007 Spanish Biomedical Research Act or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Hospital Clínic ethical committee reference number: HCB/2018/0236.
Results
Antimicrobial susceptibility
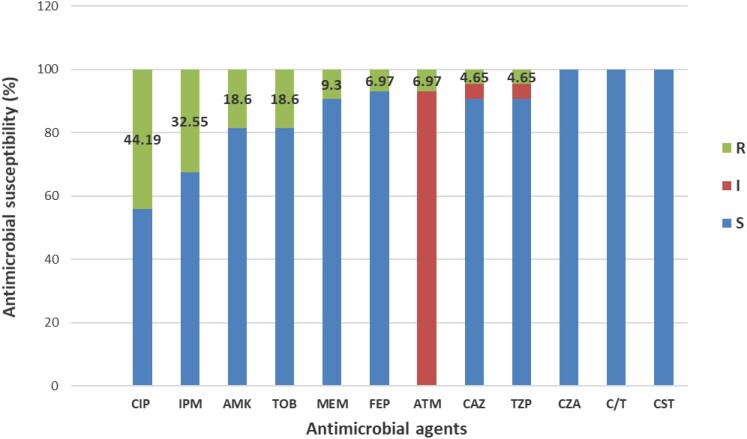
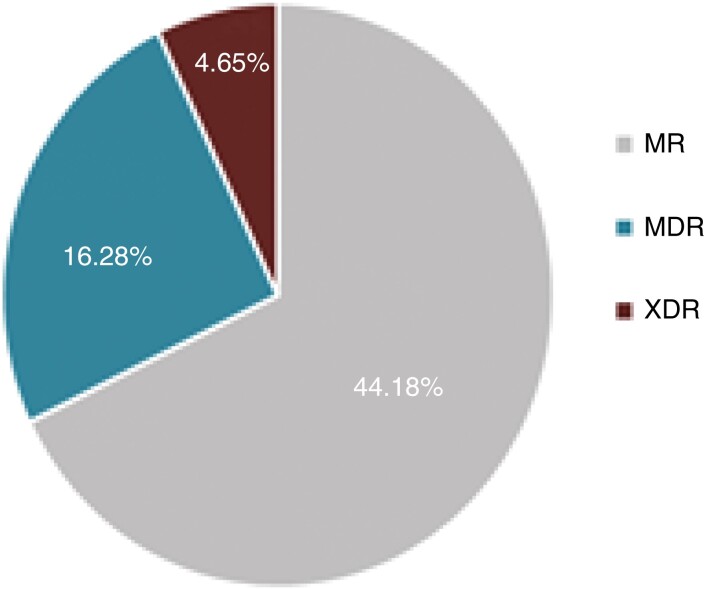
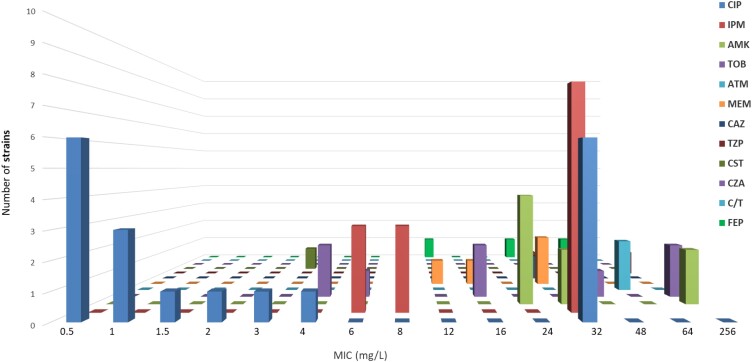
A total of 43 strains of P. aeruginosa were isolated from the sputum of 38 BE patients during their stable phase with mean ± SD forced expiratory volume in 1 s (FEV1) at inclusion of 58.92% ± 19.26%. Overall, 7 strains were obtained from BE patients with intermittent P. aeruginosa colonization versus 36 from patients with chronic P. aeruginosa colonization. P. aeruginosa isolates were resistant to ciprofloxacin (44.19%), imipenem (32.55%), amikacin (18.6%), tobramycin (18.6%), meropenem (9.3%), cefepime (6.97%), aztreonam (6.97%), piperacillin/tazobactam (4.65%) and ceftazidime (4.65%). The strains showed three different antimicrobial profiles: moderately resistant (MR; 44.18%), MDR (16.28%) and XDR (4.65%) (Figures 1 and 2). Ciprofloxacin and imipenem had the highest MICs (Figure 3). All strains showed resistance to at least one antimicrobial agent.
Figure 1.
Antimicrobial susceptibility of all P. aeruginosa strains analysed by Etest. CIP, ciprofloxacin; IPM, imipenem; AMK, amikacin; TOB, tobramycin; ATM, aztreonam; MEM, meropenem; CAZ, ceftazidime; TZP, piperacillin/tazobactam; CST, colistin; CZA, ceftazidime/avibactam; C/T, ceftolozane/tazobactam; FEP, cefepime; R, resistant; I, intermediate; S susceptible.
Figure 2.
Antimicrobial profile of all P. aeruginosa strains analysed. MR, moderately drug resistant; XDR, isolates resistant to all the antimicrobial agents except ≤2.
Figure 3.
Number of resistant P. aeruginosa strains with each MIC value. CIP, ciprofloxacin; IPM, imipenem; AMK, amikacin; TOB, tobramycin; ATM, aztreonam; MEM, meropenem; CAZ, ceftazidime; TZP, piperacillin/tazobactam; CST, colistin; CZA, ceftazidime/avibactam; C/T, ceftolozane/tazobactam; FEP, cefepime.
Mechanisms of resistance
Ciprofloxacin-resistant P. aeruginosa strains contained mutations in the gyrA, gyrB, parC and parE genes. The most frequent mutations were T83I in GyrA (21.05%), S466F in GyrB (21.05%), S87W in ParC (21.05%) and D539E in ParE (36.84%). A large number of mutated genes in the QRDR were associated with increased MIC (Table 2). Several β-lactamases were detected; sequencing showed allelic variants blaGES-2 (44.18%), blaIMI-2 (11.62%), blaGIM-1 (2.32%) and blaOXA50 (97.67%), an intrinsic β-lactamase in P. aeruginosa. Allelic variants of OXA-50 were determined, OXA-396 and OXA-1034 being the most frequent. The variants were widely distributed among the different clones, and no specific correlation with clones was found. The aac(3)-Ia (41.6%), aac(3)-Ic (25%), aac(6″)-Ib (8.33%) and ant(2″)-Ia (25%) genes were associated with aminoglycoside-resistant strains. The mcr-1 gene was detected in one strain and confirmed by sequencing, although not associated with resistance (Table 3). OprD absence and different mutation patterns found in the oprD gene were associated with resistance to carbapenems. Five different mutation patterns (MP1 to MP5) were detected. In 9.3% of strains, the OprD porin was inactivated (Table 3).
Table 2.
Relationship between the number of mutated genes and ciprofloxacin MIC
| QRDR mutations | Number of strains | MIC (mg/L) | Mean MIC (mg/L) |
|---|---|---|---|
| One mutation | |||
| gyrB | 1 | 1.5 | 0.75 |
| parC | 1 | 0.5 | |
| parE | 2 | 0.5–0.5 | |
| Two mutations | |||
| gyrB + parC | 1 | 0.5 | 9.06 |
| gyrB + parE | 2 | 0.5–32 | |
| parC + parE | 2 | 0.5–2 | |
| gyrA + parE | 2 | 1.0–4 | |
| gyrA + parC | 1 | 32 | |
| Three mutations | |||
| gyrA + gyrB + parE | 2 | 1–32 | 20 |
| gyrA + parC + parE | 2 | 32–32 | |
| gyrB + parC + parE | 1 | 3 | |
| Four mutations | |||
| gyrA + gyrB + parC + parE | 1 | 32 | 32 |
Table 3.
Resistance patterns and mechanisms of resistance found in P. aeruginosa strains
| Strain | Resistance pattern | QRDR mutations | Resistance genes | |||||
|---|---|---|---|---|---|---|---|---|
| gyrA | gyrB | parC | parE | β-lactamases | aminoglycosides | colistin | ||
| 1 | CIP-ATM-CST | N366D | K66Q,K69M | bla OXA50(OXA-395) | mcr-1 | |||
| 2 | CIP-ATM | L41W | N366D | K380Q | bla OXA50(OXA-396) | |||
| 3 | CIP-ATM-IPM-TOB | T83I | S87W | R378G,Y536T,A537P,D539E | bla OXA50(OXA-1034),blaGES-2 | aac(6″)-Ib | ||
| 4 | CIP-ATM-AMK | K46E | bla OXA50(OXA-395) | aac(3)-Ia | ||||
| 5 | ATM-MEM | bla OXA50(OXA-1034),blaIMI-2,blaGIM-1 | ||||||
| 6 | CIP-TZP-AMK-ATM-CAZ-MEM-IPM | N57Q,D58R,W59L,N60E | S466F | S373I,N374Y,A375D,R378H | bla OXA50(OXA-396),blaGES-2 | aac(3)-Ia | ||
| 7 | ATM-IPM | bla OXA50(OXA-396),blaGES-2 | ||||||
| 8 | AMK-ATM-TOB | bla OXA50(OXA-1032) | aac(3)-Ia | |||||
| 9 | ATM-MEM | bla OXA50(OXA-905),blaGES-2 | ||||||
| 10 | ATM | blaOXA50(OXA-395) | ||||||
| 11 | ATM | bla OXA50(OXA-396) | ||||||
| 12 | ATM | bla OXA50(OXA-1034) | ||||||
| 13 | CIP-ATM | Q443H | G376A,R378H,D539E | bla OXA50(OXA-395) | ||||
| 14 | ATM | bla OXA50(OXA-1034) | ||||||
| 15 | ATM-MEM | bla OXA50(OXA-396),blaGES-2 | ||||||
| 16 | CIP-TZP-ATM-CAZ-MEM-IPM-TOB | T83I | Q443H | D35W,S87W | D539E | bla OXA50(OXA-396),blaGES-2 | ant(2″)-Ia | |
| 17 | AMK-ATM-TOB | bla OXA50(OXA-1032),blaGES-2 | ant(2″)-Ia | |||||
| 18 | AMK-ATM-TOB-IPM | bla OXA50 (OXA-905),blaGES-2 | ant(2″)-Ia | |||||
| 19 | CIP-ATM | S466F | I33N | Y536T,A537P,D539E | bla OXA50(OXA-395) | |||
| 20 | ATM | bla OXA50(OXA-396) | ||||||
| 21 | ATM | bla OXA50(OXA-1034) | ||||||
| 22 | ATM | bla OXA50(OXA-395) | ||||||
| 23 | ATM | blaOXA50(OXA-1034) | ||||||
| 24 | CIP-ATM-AMK-MEM-IPM | N366D,S466F | bla OXA50(OXA-396),blaGES-2,blaIMI-2 | aac(3)-Ia | ||||
| 25 | CIP-ATM-MEM | K69M | R379Q,D539E | bla OXA50(OXA-396),blaGES-2 | ||||
| 26 | ATM | bla OXA50(OXA-1032) | ||||||
| 27 | ATM-MEM-IPM | bla OXA50(OXA-905),blaGES-2,blaIMI-2 | ||||||
| 28 | CIP-ATM-MEM-IPM | T83I | K46E,S87W | R378H,D539E | bla OXA50(OXA-395),blaGES-2 | |||
| 29 | CIP-ATM-AMK-MEM-IPM | K120Q | A375Y,R378G | bla OXA50(OXA-396),blaGES-2,blaIMI-2 | ||||
| 30 | AMK-ATM | bla OXA50(OXA-1034) | aac(3)-Ic | |||||
| 31 | ATM | bla OXA50(OXA-395) | ||||||
| 32 | CIP-TZP-ATM-CAZ-MEM-IPM-TOB | bla OXA50(OXA-1034),blaGES-2 | aac(3)-Ic | |||||
| 33 | CIP-ATM-IPM-TOB | T83V | A375Y,G376A | bla OXA50(OXA-396),blaGES-2 | aac(3)-Ic | |||
| 34 | CIP-ATM-TZP-TOB-MEM-IPM | R378Q,D539E | bla OXA50(OXA-396),blaGES-2 | aac(3)-Ia | ||||
| 35 | CIP-ATM | S466F | A368L,E369D,S373I,N374Y | bla OXA50(OXA-1032) | ||||
| 36 | ATM-CAZ | bla OXA50(OXA-905),blaGES-2 | ||||||
| 37 | ATM | bla OXA50(OXA-395) | ||||||
| 38 | CIP-ATM | D539E | bla OXA50(OXA-396) | |||||
| 39 | ATM | bla OXA50(OXA-1034) | ||||||
| 40 | CIP-ATM | D87G | R378G | bla OXA50(OXA-395) | ||||
| 41 | ATM-MEM-IPM | bla OXA50(OXA-1034),blaGES-2, blaIMI-2 | ||||||
| 42 | ATM-MEM-IPM | bla OXA50(OXA-396),blaGES-2 | ||||||
| 43 | CIP-ATM | T83I | M34Y,D35G,S87W | bla OXA50(OXA-396) | ||||
Bold signifies the most frequent mutation related to antimicrobial resistance.
Gene expression analysis
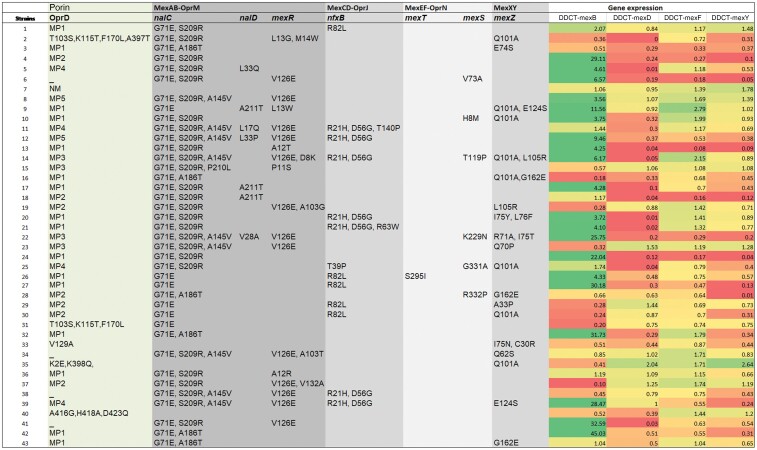
Gene expression analysis showed overexpression in the MexAB-OprM efflux system. The mexB gene was expressed at significantly higher levels (46.5%; P < 0.001 by t-test) than the mexD, mexF and mexY genes. Although there is no evidence that the amino acid changes listed in post-transcriptional regulators are involved in the overexpression, these results are consistent with the high number of mutations in post-transcriptional regulatory genes associated with mexB overexpression (nalC: G71E, S209R, A186T, A145V; nalD: L33Q, A211T, L17Q, L33P, V28A; mexR: L13G, M14W, V126E, A12T, D8K, P11S, A103G, A103T, A12R, V132A). Interplay between mexB and mexF was observed in two strains. Interplay between mexD and mexY was observed in one strain. Expression of the MexCD-OprJ operon was considerably lower (Figure 4).
Figure 4.
Mutations detected in OprD, regulators of efflux systems and gene expression heat map for efflux pumps MexAB-OprM, MexCD-OprJ, MexEF-OprN and MexXY. MP, mutation pattern; NM, no mutation; _, absence. MP1 = D43N, S57E, S59R, E202Q, I210A, E230K, S240T, N262T, A267S, A281G, K296Q, Q301E, R310G, V359L, 372(V-DSSSSYAGL-)383. MP2 = K2E, D43N, S57E, S59R, E202Q, I210A, E230K, S240T, N262T, A267S, A281G, K296Q, Q301E, R310G, V359L, 372(V-DSSSSYAGL-)383. MP3 = K2E, T103S, K115T, F170L, E185Q, P186G, V189T, R310E, A315G. MP4 = S57E, S59R, V127L, E185Q, P186G, V189T, E202Q, I210A, E230K, S240T, N262T, T276A, A281G, K296Q, Q301E, R310E, A315G, L347M, 372(V-DSSSSYAGL-)383. MP5 = T103S, K115T, F170L, E185Q, P186G, V189T, R310E, A315G.
Molecular epidemiology
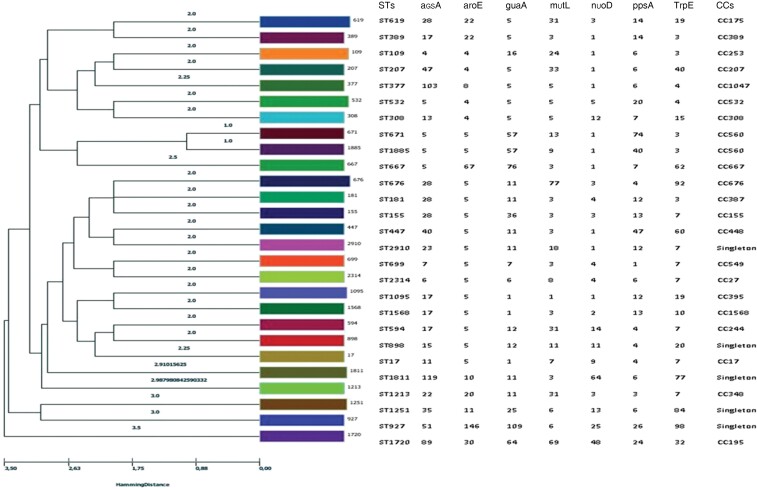
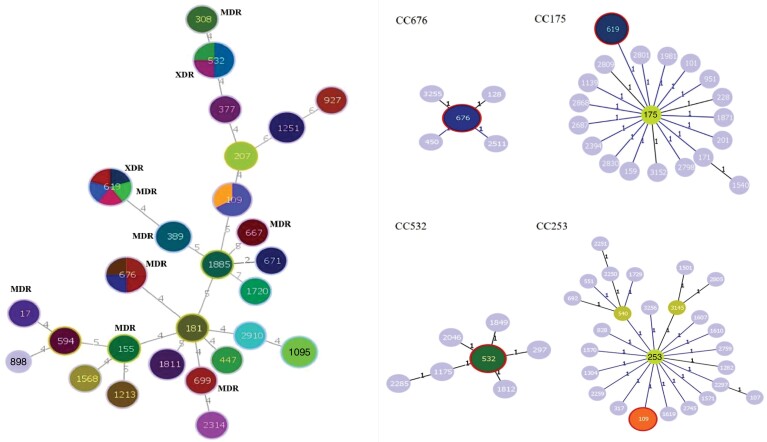
A wide variety of clones were found but the Hamming distance showed high genetic proximity between them (Figure 5). Twenty-seven STs were identified in our strains. The most frequent clones detected were ST619 (11.4%), ST676 (9.09%), ST532 (9.09%) and ST109 (6.8%), followed by ST1811, ST1251, ST1095 and ST389 (4.65%) and ST181, ST1213, ST155, ST1885, ST308, ST594, ST1568, ST898, ST1720, ST17, ST671, ST447, ST699, ST667, ST377, ST2910, ST2314, ST927 and ST207 (2.32%). The four most frequent clones were distributed in four different CCs: CC175, CC676, CC532 and CC253 (Figures 5 and 6). The XDR profile was associated with the most frequently found clones, ST619 and ST532, while the MDR profile had a broader distribution, being found in ST619, ST676, ST308, ST17, ST155, ST667 and ST699. Resistance to ciprofloxacin was widely extended and found in 13 different clones: ST619, ST676, ST109, ST308, ST17, ST155, ST181, ST377, ST667, ST671, ST1213, ST1568 and ST1720. Resistance to the other antimicrobial agents was distributed in all clones except resistance to piperacillin/tazobactam (ST619 and ST532), ceftazidime (ST619 and ST532) and colistin (ST181) (Figure 6).
Figure 5.
Genetic distance among the different STs. Hierarchical clustering of all STs found in P. aeruginosa strains, including alleles for the different housekeeping genes and CCs.
Figure 6.
Minimum spanning tree of the 43 P. aeruginosa strains based on the MLST allelic profile and main CCs. Each circle represents a clone. The size of the circle corresponds to the number of isolates ascribed to that particular clone and each different colour inside the circle represents a different antimicrobial profile associated with each clone.
Discussion
Several papers have focused on Pseudomonas resistance in BE. However, to the best of our knowledge, this is the first study reporting the mechanisms of resistance combined with the ST and CCs in P. aeruginosa from BE patients. We found a high prevalence of ciprofloxacin-resistant strains (ciprofloxacin being the first-line treatment for P. aeruginosa eradication)12 and an association between a higher number of mutations in the QRDR and a higher ciprofloxacin MIC. Finally, we identified two new emerging high-risk clones in BE.
Although some studies have reported high rates of MDR in strains of P. aeruginosa from BE patients, for instance during exacerbations, their associated mechanisms of resistance have not been analysed previously.13 Mensa et al.14 found a similar average resistance (20%) to that found herein (15%) towards most antipseudomonal antibiotics in Spain. Consistent with our results, they found that colistin and ceftolozane/tazobactam showed activity close to 95%. However, they included P. aeruginosa strains from other types of infection and excluded those from BE patients.
We found a higher incidence of antimicrobial resistance [ciprofloxacin (44.19% versus 38.4%), tobramycin (18.6% versus 16.3%), amikacin (18.6% versus 4%) and imipenem (32.55% versus 15.6%)] compared with that found by Barrio-Tofiño et al.,3,15 who also described mechanisms of resistance and molecular epidemiology, but like others did not exclusively use respiratory samples, nor were they exclusively from patients with BE.
Several reports have indicated that mutations in gyrA (75%) and parC (98%) genes are the primary target for quinolone resistance in P. aeruginosa.16 In our study, the most frequent mutations were T83I in GyrA (21.05%) and S87W in ParC (21.05%). We identified two other amino acid changes in GyrA (T83V and D87G) that could be characteristic of P. aeruginosa strains from BE patients since different amino acid changes have been described in other respiratory infections such as in positions 83 (T83I) in GyrA and 87 (S87L or S87T) in ParC.4,17
Despite not being the main QRDR target, mutations in the gyrB (3%–29%) and parE (2%–7%) genes are still important, since those amino acid changes that we described in GyrB (S466F) were previously reported to greatly increase the ciprofloxacin MIC.4,16 We found ParE amino acid substitution that differed from those previously reported in the literature (D419N, E459D, A473V and S457R), D539E being the most frequent in our strains. In addition, we found that a greater number of different mutated genes in the QRDR were associated with an increased MIC, as reported in Table 2.4,16
Different β-lactamases were detected [blaOXA50, MBL (GIM-1) and serine carbapenemases (GES-2 and IMI-2)]. blaOXA50 plays an important role in our strains since the classic β-lactamase inhibitors show weak activity against blaOXA50.18,19 MBLs were barely found in our strains. Nevertheless, we found one strain with a GIM-1 instead of VIM and IMP, which are the most prevalent types in P. aeruginosa.19,20 Although the worldwide prevalence of GES-type serine carbapenemase is rather low,19,20 almost half of our strains carried the GES carbapenemase, being characteristic of strains from Spain.14 This incidence of GES-2 explains the aztreonam resistance found in our strains since other authors have reported that GES is active against aztreonam.21 We also highlight the presence of IMI-2 in our strains, a carbapenemase of chromosomal origin that is present at low levels in P. aeruginosa.19,22,23 However, an IMI of plasmid origin has recently been described in Escherichia coli, which could facilitate gene transfer exchange between different species.24 Our strains could carry this plasmid.
Previous studies have reported that the loss or mutation of OprD is associated with non-susceptibility to imipenem. In contrast, the mechanism leading to meropenem resistance is multifactorial (OprD inactivation plus hyperexpression of MexAB-OprM).4,14,25,26 We described five different mutation patterns and also OprD absence in strains resistant to imipenem (Figure 4), and multifactorial resistance mechanisms [overexpression of MexAB-OprM and serine carbapenemases (Table 3)] in strains resistant to meropenem. However, it is difficult to establish clear causality since each strain combines multiple resistance mechanisms.
The most commonly described AMEs in P. aeruginosa are the acetyltransferases AAC(3′) and AAC(6′) (conferring resistance to both tobramycin and amikacin in the first case and to both or only tobramycin in the second case) and the nucleotidyltransferase ANT(2′)-I (conferring resistance to gentamicin and tobramycin).18,27,28 We detected the presence of these AMEs in our aminoglycoside-resistant strains, the most frequent being AAC(3′)-Ia (Table 2). AMEs have high clinical impact since, like β-lactamases with a higher hydrolytic profile, class B β-lactamases (MBLs) and ESBLs, they are usually associated with transferable genetic elements (plasmids or transposons).14
Our study confirms that ceftazidime/avibactam, ceftolozane/tazobactam and colistin are an ultimate line of attack against MDR Gram-negative pathogens in chronic respiratory diseases. However, the recent emergence of plasmid-mediated mcr-1 colistin resistance is a challenge to public global health since it increases the potential dissemination of the mcr-1 gene.29 In a previous study of samples from ICU patients with different sources of infection, 10% of colistin-resistant isolates were positive for the mcr-1 gene. We detected the mcr-1 gene in only one P. aeruginosa strain but it was not associated with resistance.30
In our study, MexAB-OprM, a pump with a wide substrate profile, was the pump with the highest prevalence and overexpression. Our finding coincides with that of Serra et al.7 and others4,31 who also found a high prevalence and overexpression of mexB and mexY genes in their clinical P. aeruginosa strains. We only found one strain with overexpression of MexXY associated with intrinsic resistance to aminoglycosides. The simultaneous overexpression of MexB and MexF (observed in two strains) and the low level of expression of MexCD-OprJ (<5%) are consistent with previous studies (Figure 4).7,9,25,32
High-risk P. aeruginosa clones associated with MDR/XDR strains (e.g. ST175, ST111 and ST235) are widely disseminated around the world.33,34 However, in our study these clones were not identified except for ST235 and ST308. Therefore, our strains presented different clonal distribution compared with previous studies of P. aeruginosa strains from other infections and samples. A multicentre study of P. aeruginosa bacteraemia in Spain revealed that 90% of XDR isolates belonged to the aforementioned high-risk clones.3,32,35 Although we found that 21% of isolates had the MDR/XDR resistance profile, similar to the ∼30% recently described (Figure 2),3,18,26 our study included two emerging high-risk clones among the most frequent of our P. aeruginosa strains, ST619 and ST532, which were also associated with the MDR/XDR phenotype and had not been described before in P. aeruginosa strains from BE.11,36 The high frequency of these emerging high-risk clones in BE patients is a matter of concern since it favours the spread of resistance. Here we stress that ST619 is found within the same CC (CC175) as ST175, a clone with a high prevalence in Spain. So this CC is even more important in the dissemination of MDR/XDR strains. Our findings are quite different from previous studies, as besides the new emerging high-risk clones, we did not find the ST179 reported previously as being associated with other MDR P. aeruginosa causing chronic respiratory infections in Spanish hospitals.26,37,38 In addition, we barely (2.3%) found ST308, which is associated with MDR/XDR strains producing carbapenemases, also described by Ruiz et al.26
This study has some limitations. First, the number of strains was low because our strains came exclusively from BE patients. Other studies with more strains describe the mechanisms of resistance and epidemiology but in strains from different infections. Second, we did not assess the virulence of our P. aeruginosa strains. Previous studies have shown the association between some type III secretion system (TTSS) genotypes and antibiotic resistance patterns. Despite its aforementioned limitations this study provides novel information about resistance to first-line treatment, essentially analysis of antibiotic resistance genes and antimicrobial resistance associated with clonal distribution in P. aeruginosa strains from BE, with potential clinical implications.
Conclusions
The high level of resistance to first-line recommended antimicrobial agents for P. aeruginosa eradication in BE, the combination of multiple resistance mechanisms found in each strain and the identification of two emerging high-risk clones, not described before in BE, threatens the treatment and eradication of P. aeruginosa in BE patients. In view of our results and although there are still therapeutic options for P. aeruginosa in BE such as colistin, new antipseudomonal therapies are urgently needed. Other IV antimicrobial agents such as ceftolozane/tazobactam, not currently included in BE guidelines, could become therapeutic candidates for BE patients with MDR P. aeruginosa. Secondly, since diagnostic accuracy is a key aspect for the adequacy of antimicrobial treatment, further investigations are needed to determine whether improvements in microbial diagnostics could positively influence Pseudomonas eradication rates and decrease the emergence of new resistant strains as well as the spread of current ones.
Acknowledgements
We thank Dr Joaquim Ruiz, Dr Elisenda Bañon and Mireya Fuentes for their professional advice.
Funding
This study was funded by ISCIII-FEDER with the FIS (PI1800145) to A.T./L.F.B., intramural CIBERES (ES18PI01) to A.T./L.F.B., CIBER de enfermedades respiratorias -CIBERES (CB 06/06/0028, an initiative of ISCIII), SEPAR 2016 (Grant: 208) and SEPAR 2018 (Grant: 628) to L.F.B., PFIS-FSE to R.L.A. (FI19/00090) and SGR-Generalitat de Catalunya, IDIBAPS and ICREA Academy Award to A.T.
Transparency declarations
A.T. has received grants from Medimmune, Cubist, Bayer, Theravance and Polyphor and personal fees as an Advisory Board member from Bayer, Roche, The Medicines Company and Curetis. He has received personal speaker’s bureau fees from GSK, Pfizer, AstraZeneca and the Biotest Advisory Board, unconnected to the study submitted here. All other authors: none to declare.
Author contributions
R.C. assessed the mechanisms of resistance, conducted the MLST including the analysis of gene sequences, and performed the gene expression analysis and the phylogenetic analysis. R.C., L.F.B. and N.V. participated in the study of antimicrobial susceptibility. R.C., L.F.B. and A.T. participated in the protocol development, study design and study management. R.C. and L.B.F. participated in data interpretation and writing of the manuscript. L.F.B., R.A., L.B., V.A.S. and P.O. participated in the recruitment of patients. R.C., N.V., L.F.B., L.M. and J.V. participated in the identification of microorganisms. L.F.B., N.V., R.L.A., V.A., L.B.F., R.A. and A.T. obtained the respiratory specimens and critically reviewed the manuscript. All authors participated in data collection and reviewed the manuscript.
Data availability
All data generated or analysed during this study are included in this published article.
References
- 1. Fernández-Barat L, Alcaraz-Serrano V, Amaro Ret al. Pseudomonas aeruginosa in bronchiectasis. Semin Respir Crit Care Med 2021; 42: 587–94. [DOI] [PubMed] [Google Scholar]
- 2. McDonnell MJ, Jary HR, Perry Aet al. Non cystic fibrosis bronchiectasis: a longitudinal retrospective observational cohort study of Pseudomonas persistence and resistance. Respir Med 2015; 109: 716–26. [DOI] [PubMed] [Google Scholar]
- 3. Del Barrio-Tofinõ E, Zamorano L, Cortes-Lara Set al. Spanish nationwide survey on Pseudomonas aeruginosa antimicrobial resistance mechanisms and epidemiology. J Antimicrob Chemother 2019; 74: 1825–35. [DOI] [PubMed] [Google Scholar]
- 4. Solé M, Fàbrega A, Cobos-Trigueros Net al. In vivo evolution of resistance of Pseudomonas aeruginosa strains isolated from patients admitted to an intensive care unit: mechanisms of resistance and antimicrobial exposure. J Antimicrob Chemother 2015; 7: 3004–13. [DOI] [PubMed] [Google Scholar]
- 5. Treepong P, Kos VN, Guyeux Cet al. Global emergence of the widespread Pseudomonas aeruginosa ST235 clone. Clin Microbiol Infect 2018; 24: 258–66. [DOI] [PubMed] [Google Scholar]
- 6. EUCAST . Breakpoint tables for interpretation of MICs and zone diameters. http://www.eucast.org/clinical_breakpoints/.
- 7. Serra C, Bouharkat B, Touil-Meddah ATet al. MexXY multidrug efflux system is more frequently overexpressed in ciprofloxacin resistant French clinical isolates compared to hospital environment ones. Front Microbiol 2019; 10: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al Rashed N, Joji RM, Saeed NKet al. Detection of overexpression of efflux pump expression in fluoroquinolone-resistant Pseudomonas aeruginosa isolates. Int J Appl Basic Med Res 2020; 10: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tomás M, Doumith M, Warner Met al. Efflux pumps, OprD porin, AmpC β-lactamase, and multiresistance in Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob Agents Chemother 2010; 54: 2219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carriço JA, Crochemore M, Francisco APet al. Fast phylogenetic inference from typing data. Algorithms Mol Biol 2018; 13: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feil EJ, Li BC, Aanensen DMet al. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol 2004; 186: 1518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Polverino E, Goeminne PC, McDonnell MJet al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. [DOI] [PubMed] [Google Scholar]
- 13. Menéndez R, Méndez R, Polverino Eet al. Risk factors for multidrug-resistant pathogens in bronchiectasis exacerbations. BMC Infect Dis 2017; 17: 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mensa J, Barberán J, Soriano Aet al. Antibiotic selection in the treatment of acute invasive infections by Pseudomonas aeruginosa: Guidelines by the Spanish Society of Chemotherapy. Rev Esp Quimioter 2018; 31: 78–100. [PMC free article] [PubMed] [Google Scholar]
- 15. Kaehne A, Milan SJ, Felix LMet al. Head-to-head trials of antibiotics for bronchiectasis. Cochrane Database Syst Rev 2018: CD012590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rehman A, Patrick WM, Lamont IL. Mechanisms of ciprofloxacin resistance in Pseudomonas aeruginosa: new approaches to an old problem. J Med Microbiol 2019; 68: 1–10. [DOI] [PubMed] [Google Scholar]
- 17. Akasaka T, Tanaka M, Yamaguchi Aet al. Type II topoisomerase mutations in fluoroquinolone-resistant clinical strains of Pseudomonas aeruginosa isolated in 1998 and 1999: role of target enzyme in mechanism of fluoroquinolone resistance. Antimicrob Agents Chemother 2001; 45: 2263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horcajada JP, Montero M, Oliver Aet al. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin Microbiol Rev 2019; 32: e00031-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nicolau CJ, Oliver A. Carbapenemases in Pseudomonas spp. Enferm Infecc Microbiol Clin 2010; 28Suppl 1: 19–28. [DOI] [PubMed] [Google Scholar]
- 20. Botelho J, Grosso F, Peixe L. Unravelling the genome of a Pseudomonas aeruginosa isolate belonging to the high-risk clone ST235 reveals an integrative conjugative element housing a blaGES-6 carbapenemase. J Antimicrob Chemother 2018; 73: 77–83. [DOI] [PubMed] [Google Scholar]
- 21. Poirel L, Brinas L, Fortineau Net al. Integron-encoded GES-type extended-spectrum β-lactamase with increased activity toward aztreonam in Pseudomonas aeruginosa. Antimicrob Agents Chemother 2005; 49: 3593–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Queenan AM, Bush K. Carbapenemases: the versatile β-lactamases. Clin Microbiol Rev 2007; 20: 440–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walther-Rasmussen J, Høiby N. Class A carbapenemases. J Antimicrob Chemother 2007; 60: 470–82. [DOI] [PubMed] [Google Scholar]
- 24. Rojo-Bezares B, Martín C, López Met al. First detection of blaIMI-2 gene in a clinical Escherichia coli strain. Antimicrob Agents Chemother 2012; 56: 1146–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Riera E, Cabot G, Mulet Xet al. Pseudomonas aeruginosa carbapenem resistance mechanisms in Spain: impact on the activity of imipenem, meropenem and doripenem. J Antimicrob Chemother 2011; 66: 2022–7. [DOI] [PubMed] [Google Scholar]
- 26. Horna G, Amaro C, Palacios Aet al. High frequency of the exoU+/exoS+ genotype associated with multidrug-resistant ‘high-risk clones’ of Pseudomonas aeruginosa clinical isolates from Peruvian hospitals. Sci Rep 2019; 9: 10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poole K. Pseudomonas aeruginosa: resistance to the max. Front Microbiol 2011; 2: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Castanheira M, Deshpande LM, Woosley LNet al. Activity of plazomicin compared with other aminoglycosides against isolates from European and adjacent countries, including Enterobacteriaceae molecularly characterized for aminoglycoside-modifying enzymes and other resistance mechanisms. J Antimicrob Chemother 2018; 73: 3346–54. [DOI] [PubMed] [Google Scholar]
- 29. Ye H, Li Y, Li Zet al. Diversified mcr-1-harbouring plasmid reservoirs confer resistance to colistin in human gut microbiota. mBio 2016; 7: e00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abd El-Baky RM, Masoud SM, Mohamed DSet al. Prevalence and some possible mechanisms of colistin resistance among multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa. Infect Drug Resist 2020; 13: 323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vatcheva-Dobrevska R, Mulet X, Ivanov Iet al. Molecular epidemiology and multidrug resistance mechanisms of Pseudomonas aeruginosa isolates from Bulgarian hospitals. Microb Drug Resist 2013; 19: 355–61. [DOI] [PubMed] [Google Scholar]
- 32. El Zowalaty ME, Al Thani AA, Webster TJet al. Pseudomonas aeruginosa: arsenal of resistance mechanisms, decades of changing resistance profiles, and future antimicrobial therapies. Future Microbiol 2015; 10: 1683–706. [DOI] [PubMed] [Google Scholar]
- 33. Oliver A, Mulet X, López-Causapé Cet al. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist Updat 2015; 10: 41–59. [DOI] [PubMed] [Google Scholar]
- 34. García-Castillo M, Del Campo R, Morosini MIet al. Wide dispersion of ST175 clone despite high genetic diversity of carbapenem-nonsusceptible Pseudomonas aeruginosa clinical strains in 16 Spanish hospitals. J Clin Microbiol 2011; 49: 2905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 2009; 22: 582–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Founou RC, Founou LL, Allam Met al. First report of a clinical multidrug-resistant Pseudomonas aeruginosa ST532 isolate harbouring a ciprofloxacin-modifying enzyme (CrpP) in South Africa. J Glob Antimicrob Resist 2020; 22: 145–6. [DOI] [PubMed] [Google Scholar]
- 37. Juan C, Zamorano L, Mena Aet al. Metallo-β-lactamase-producing Pseudomonas putida as a reservoir of multidrug resistance elements that can be transferred to successful Pseudomonas aeruginosa clones. J Antimicrob Chemother 2010; 65: 474–8. [DOI] [PubMed] [Google Scholar]
- 38. Gomila M, Del Carmen Gallegos M, Fernández-Baca Vet al. Genetic diversity of clinical Pseudomonas aeruginosa isolates in a public hospital in Spain. BMC Microbiol 2013; 13: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.