Abstract
Objective
Bacterial cellulose (BC) dressing, which can maintain a moist environment and prevent the invasion of pathogens, has become a competitive dressing material for burn wound treatment. This study was conducted to evaluate the treatment efficacy of a novel China-made BC dressing for the treatment of second-degree burn wounds and skin graft donor sites.
Methods
212 patients with second-degree burn wounds or skin graft donor sites were enrolled from two research centers. They were randomly assigned to the BC dressing group (study group) or the Vaseline gauze (VG) dressing group (control group). Wound conditions were assessed before and after treatment. Dressings were changed according to the condition of the wound bed. Healing rate and healing time were recorded as primary endpoints to evaluate the efficacy of BC dressing against VG dressing. Erythema, swelling, exudation, bleeding, subeschar purulence, and pain were assessed as secondary endpoints.
Results
207 participants completed the trial and their wounds all healed within 28 days. The average healing times for superficial and deep secondary burn wounds and skin graft donor sites in the BC group were 8.12, 15.77, and 10.55 days, respectively. In the VG group, the average healing times for superficial and deep secondary burn wounds and skin graft donor sites were 9.30, 15.27, and 11.19 days, respectively. The healing time of superficial burn wounds in the BC group was statistically shorter than that in the VG group. There was no difference in the frequency of dressing changing between two groups. The BC dressing showed equal efficacy with the VG dressing at all secondary endpoints.
Conclusion
The novel BC dressing could be used for the management of second-degree burn wounds and skin graft donor sites. With a shorter healing time in superficial secondary burn wound than that of the VG dressing, the BC dressing showed noninferiority in the treatment of superficial and deep secondary burn wounds and skin graft donor sites versus the VG dressing. This study is registered with the Chinese Clinical Trial Registry (registry number: ChiCTR1800014377 (http://www.chictr.org.cn)).
1. Introduction
Burn wound due to thermal injury is one of the public health problems in modern society, usually causing irreparable harms and a variety of side effects for victims and their families. Generally, burn wounds are classified into 3 categories according to their severity or the depth of the tissues affected [1] Among them, second-degree (partial thickness) burns feature blisters covering a red base and the lesion reaches the deep skin layers, which is further classified into superficial and deep partial thickness burns. The depth of the burns directly affects the healing and scarring of the wounds [2].
The healing of second-degree burn wounds is a complex process that depends on the tissues, cell types, and matrix components [3]. Thus, the management of second-degree burns is still controversial [4]. Without the protection of the integumentary system, the burn wound bed is under high risk of bacterial infection, disrupting the natural healing process and leading to poor outcomes. Therefore, wound dressings that can maintain a moist environment and prevent the invasion of pathogens are of great importance to the treatment of second-degree burns [5]. Clinically effective wound dressings should also possess the features of excellent water retention capacity, ideal biocompatibility, comfort for the patients, and ease of application [6].
A wide variety of dressing materials, such as honey, silver sulfadiazine dressing, and chitosan, have been tested for the treatment of second-degree burn wounds [7, 8]. However, due to adherence to the wound surface, personal suffering in dressing change, and delayed healing, few of them have been well approved [9]. Bacterial cellulose (BC) is natural cellulose fermented from Acetobacter xylinum and other bacteria. Due to high purity and the absence of some typical plant components such as lignin, pectin, and hemicelluloses, BC is considered as a noncytotoxic and highly biocompatible material with several desirable characteristics, such as high permeability, absorbability, wet tensile strength, flexibility, elasticity, and biocompatibility. Today, BC is being actively investigated and developed as both an independent and composite dressing material for the treatment of burn wounds [10, 11]. With the optimization of interconnection of the fibers and nanosized pore structure, some novel BC can even be used as an antimicrobial wound dressing with a desirable sustained release functionality for targeting persistent bacterial pathogens [6]. Assembling the silver nanoparticles on the surface of BC has attracted increasing attention and has become an effective strategy of developing burn wound dressing with antibacterial property [12, 13]. However, accumulating evidence suggests that chemical contaminations may be induced during the functionalization of BC [14]. Exposure of the burn wounds to silver ions or compounds may delay the reepithelialization of wounds due to the significant cytotoxicity [15]. In the light of safety, biocompatibility, and cost, these randomized controlled trials (RCTs) were conducted to investigate the treatment efficacy of a novel BC in its original form as a dressing for second-degree burn wounds and skin graft donor sites. Vaseline gauze (VG) dressing was used as the control.
2. Materials and Methods
2.1. Patients
Since January 2018, consecutive patients of both sexes with second-degree burns who have been presenting at the Second Affiliated Hospital of Zhejiang University School of Medicine and Ningbo No. 2 Hospital were screened for the eligibility of this study. The inclusion criteria were as follows: (1) being aged 18 to 65 years, (2) being diagnosed with second-degree burns or partial-thickness skin graft donor wound within 24 hours of admission, and (3) burn wound bed area larger than 100 cm2 but less than 15% of total body surface area (TBSA) [16]. Patients with the following features were excluded: (1) impaired cardiovascular function (signs and symptoms of cardiac failure), (2) impaired hepatic function (serum total bilirubin was 1.5 times higher than the upper limit of normal value), (3) impaired renal function (increased blood creatinine and urea nitrogen), (4) hematological complications, (5) severe hypoalbuminemia (plasma albumin <25 g/L), (6) severe systemic infection (e.g., septicemia), (7) uncontrolled diabetes mellitus, (8) use of corticosteroids, (9) pregnancy or breast-feeding, (10) participation in other clinical trials three months prior, and (11) skin disorder, allergic disorder, or other conditions that could interfere in the study assessment. All eligible participants could leave the clinical trial at any time for any reason. Participants who did not complete the clinical trial were considered dropouts.
The present clinical trial was approved by the Ethics Committees of the Second Affiliated Hospital of Zhejiang University School of Medicine. Written informed consent was acquired from all the participants. The study was carried out in accordance with the Declaration of Helsinki. Demographic and clinical data were properly collected and protected by all investigators.
2.2. Sample Size and Randomization
This was a noninferiority trial [17], intended to prove that the efficacy of BC dressing was not inferior to that of the VG dressing in the management of second-degree burn wounds or partial-thickness skin graft donor sites. Hence, the ratio of participants between the two treatments was predetermined as 1:1. The sample size was estimated according to the statistical significance (α = 0.05, β = 0.2) reported previously [16], and the dropout rate (6%) and the treatment efficacy (90%) were predetermined by our preliminary data. The random allocation of patients was conducted as per the random number generated by computer program. A total of 212 patients were enrolled and randomly assigned into two BC and VG dressing groups, with 106 participants for each group. Only one wound was observed in each participant. The process of enrollment and randomization is shown in Figure 1.
Figure 1.
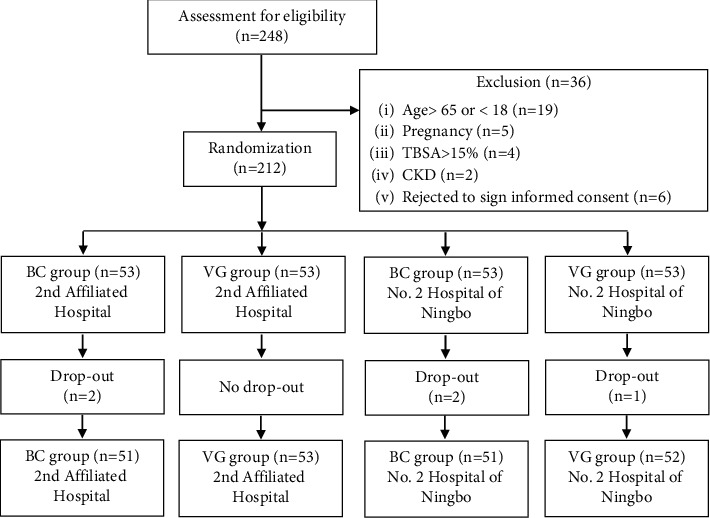
The flowchart of the randomized controlled clinical trial on wound dressing.
2.3. BC and VG Dressing
The BC dressing material tested was produced by Shenzhen Ai Jie Te Medical & Pharmaceutical Science and Technology LLC (Shenzhen, China). As commercial secrets, the recipe, production, and specifications of the BC dressing material are not disclosed here. The BC dressing had passed vigorous quality tests before being used in this clinical trial. The typical BC dressing measured 20 cm × 20 cm. VG dressing is widely used in clinical practice for the management of burn wounds as well as a control in clinical trials. It was prepared as reported previously [18]. Both BC dressing and VG dressing were tailored to fit the size and shape of the wounds in each participant.
2.4. Clinical Treatment and Evaluation
The area of a wound was measured with a disposable piece of transparent plastic with 1 cm × 1 cm grids printed on it. A marker was used to trace the boundary of the wound on the plastic. The number of grids within the boundary was used to calculate the area.
The burn wounds were cleaned with normal saline before being treated with either the BC or the VG dressing according to the randomized grouping. The donor sites were covered immediately after skin harvesting. A sterile gauze pad, covering the BC or VG dressing, was typically changed once every two days. If excessive volume of exudate was observed, the sterile gauze pad was changed on a daily basis. BC and VG dressings were not changed periodically. Instead, they were only changed according to the conditions of wounds. If the adherence between the dressing and a wound bed was strong and no collection of exudates was observed, the dressing remained in place until the wound healed. Otherwise, an opening was made to drain the exudate and the dressing was changed at the doctor's discretion.
The healing process, the conditions of the wound bed, and the changes of dressing were carefully observed and recorded until the end of the trial on day 28 of treatment. For wounds that healed completely, the time taken for complete healing was recorded. For wounds that failed to heal completely within the 28 days, the leftover area of the wound bed was measured to calculate the percentage of the wound that healed.
A wound bed area of 100 cm2 was selected from each participant. The primary endpoints were wound bed healing rate (WBHR) and the time for complete wound healing. The healing rate was calculated as previously described [19]. Standards for evaluation of efficacy are listed in Table 1.
Table 1.
Standards for evaluation of treatment.
| WBHR | Evaluation |
|---|---|
| WBHR = 100% | Complete healing |
| 50% ≤ WBHR < 100% | Significant effectiveness |
| 20% ≤ WBHR < 50%, | Effectiveness |
| WBHR < 20% | Ineffectiveness |
Note: WBHR, wound bed healing rate.
A scoring method consisting of four components was developed to characterize the conditions of wounds. The components are erythema and swelling, exudation, hemorrhage, and subeschar purulence and exudation. The score of each component ranges from 1 to 4, representing the increasing severity of the condition of a wound (Table 2).
Table 2.
The scoring method for evaluation of wound conditions.
| Wound condition | Score |
|---|---|
| No sign | 1 |
| Mild sign | 2 |
| Moderate sign | 3 |
| Sever sign | 4 |
The pain caused by the burn wounds was assessed according to the visual analogue scale (VAS) [20]. Pain was considered as severe for a score of 6–10, moderate for a score of 4-5, mild for a score of 2-3, and relieved for a score of 0-1. Data of adverse events was collected per International Committee for Harmonization guidelines for Good Clinical Practice as described previously [21]. Demographic data and the results of necessary clinical tests were filed individually.
Blood and urine samples were analyzed for screening of eligibility of the patients based on inclusion and exclusion criteria. Cardiovascular, hepatic, and renal functions were monitored during the hospitalization of each participant as a routine of our clinical practice.
2.5. Statistical Analysis
The difference in appearance between a BC dressing and a VG dressing was obvious. Hence, blinding was not applicable for both the participants and the clinical workers. To alleviate possible biases, the estimation of sample size [16], random allocation of patients [22], and statistical analysis of this study were performed by a third party, the Department of Epidemiology and Health Statistics, Wuhan University School of Health Sciences, Wuhan, China.
EpiData software (version 3.0, Odense, Denmark) was used for data entry and verification. SAS (version 9.1.3, Cary, NC) was used for the statistical analysis. Data were presented as mean ± SD, median (range) or frequency (n/n), or percentage (%). The difference between the two groups was analyzed by Student's t-test, chi-squared test, Fisher's Exact Test, or Mann–Whitney U test where appropriate. A p value less than 0.05 was considered statistically significant. All tests were two-tailed unless otherwise stated.
3. Results
3.1. Characteristics of the Participants
248 patients were screened for their eligibility for this clinical trial. Among them, 36 patients were excluded due to age, pregnancy, breast-feeding, or other conditions (Figure 1). In total, 212 patients were enrolled in this trial. In the BC group, 4 participants dropped out from the cohort: 1 participant who changed her mind, 2 who transferred to other hospitals, and 1 who did not like to be treated with BC. In the VG group, only 1 participant gave up treatment due to financial reasons. Therefore, only 5 participants dropped out from the study, yielding a dropout rate of 2.4%, less than the preset value (6%) for the estimation of sample size. Hence, we contend that the sample size is satisfactory for this clinical trial.
The average ages of the participants in the BC and VG groups were 42 and 45 years, respectively, with no statistically significant differences found (p=0.137). In this study, the incidence rate of second-degree burns in the middle-aged group was much higher than that of other age groups. The proportions of participants with a history of allergy in the two cohorts were as low as 5.88% and 5.71%, respectively. No difference was observed in the history of allergy, comorbidity, blood pressure, heart rate, and breath rate, indicating that the cohorts for the BC treatment and the VG treatment were comparable (Table 3).
Table 3.
Demographic and clinical baseline information of the participants.
| Variable | BC group (n = 102) | VG group (n = 105) | p value |
|---|---|---|---|
| Age | |||
| 18- | 14 (13.72) | 12 (11.43) | 0.137 |
| 28- | 30 (29.41) | 17 (16.19) | |
| 38- | 27 (26.47) | 37 (35.24) | |
| 48- | 23 (22.55) | 31 (29.52) | |
| 58–65 | 8 (7.85) | 8 (7.62) | |
| Education | |||
| Primary school | 28 (27.45) | 30 (28.57) | 0.105 |
| Middle school | 43 (42.16) | 57 (54.29) | |
| High school | 17 (16.67) | 16 (15.24) | |
| College and above | 14 (13.72) | 2 (1.90) | |
| Marriage | |||
| No | 17 (16.67) | 13 (12.38) | 0.381 |
| Yes | 85 (83.33) | 92 (87.62) | |
| Systolic BP (mm Hg) | 129.52 ± 17.58 | 132.87 ± 17.52 | 0.173 |
| Diastolic BP (mm Hg) | 77.98 ± 12.46 | 81.01 ± 14.67 | 0.112 |
| Breath rate (per min) | 19.22 ± 1.29 | 19.16 ± 1.57 | 0.789 |
| Heart rate (per min) | 83.51 ± 10.97 | 82.08 ± 12.54 | 0.383 |
| History of allergy | |||
| No (%) | 96 (94.12) | 99 (94.29) | 0.959 |
| Yes (%) | 6 (5.88) | 6 (5.71) | |
| Comorbidity | |||
| No (%) | 96 (94.12) | 98 (93.33) | 0.816 |
| Yes (%) | 6 (5.88) | 7 (6.67) | |
Ninety-six participants were diagnosed with superficial second-degree burn wounds, while only 24 patients suffered from deep second-degree burn wounds. All body parts were at risk of being burned. However, the right lower limb was the most frequently (31.88%) injured body part, followed by the left lower limb, with a much lower incidence rate of 18.84% than the former (Table 4). No statistical difference was observed regarding the age, education level, marriage, history of allergy, type of wounds, and the location of wounds between the two groups, which suggests that the patients in the two cohorts were comparable.
Table 4.
Characteristics of the wounds in participants.
| Variable | BC group (n = 102) | VG group (n = 105) | p value |
|---|---|---|---|
| Wound type | |||
| Superficial II° burn | 49 (48.04) | 47 (44.76) | 0.695 |
| Deep II° burn | 13 (12.74) | 11 (10.48) | |
| Skin graft donor | 40 (39.22) | 47 (44.76) | |
| Wound location | |||
| Trunk | 13 (12.75) | 14 (13.33) | 0.586 |
| Right upper limb | 8 (7.84) | 11 (10.48) | |
| Right lower limb | 31 (30.39) | 35 (33.33) | |
| Left upper limb | 13 (12.75) | 9 (8.57) | |
| Left lower limb | 23 (22.55) | 16 (15.24) | |
| Craniofacial region | 14 (13.72) | 19 (18.10) | |
| Bilateral upper limb | 0 (0.00) | 1 (0.95) | |
| Erythema and swelling | |||
| None | 14 (13.73) | 16 (15.24) | 0.539 |
| Mild | 39 (38.24) | 42 (40.00) | |
| Moderate | 33 (32.35) | 34 (32.38) | |
| Severe | 16 (15.68) | 13 (12.38) | |
| Exudate | |||
| None | 1 (0.98) | 0 (0.00) | 0.503 |
| Mild | 33 (32.35) | 29 (27.62) | |
| Moderate | 46 (45.10) | 53 (50.48) | |
| Severe | 22 (21.57) | 23 (21.90) | |
| Hemorrhage | |||
| None | 45 (44.12) | 37 (35.24) | 0.264 |
| Mild | 39 (38.24) | 47 (44.76) | |
| Moderate | 14 (13.72) | 18 (17.14) | |
| Severe | 4 (3.92) | 3 (2.86) | |
| Subeschar purulence and exudation | |||
| None | 89 (87.25) | 98 (93.33) | 0.136 |
| Mild | 12 (11.77) | 7 (6.67) | |
| Moderate | 0 (0.00) | 0 (0.00) | |
| Severe | 1 (0.98) | 0 (0.00) | |
Major laboratory test results are shown in Table 5. No statistically significant difference was found between the two groups either before or after the treatment. However, some of the parameters such as the count of white blood cells (WBC) and platelet (PLT), the concentration of hemoglobin (Hb), and total bilirubin (TBIL) improved after treatment in both groups.
Table 5.
Blood and urine test results of the participants.
| Parameter | BC | VG | p value | |||||
|---|---|---|---|---|---|---|---|---|
| Before (n = 102) | After (n = 102) | Before (n = 105) | After(n = 105) | BC (beforeversus after) | VG (beforeversus after) | BC versusVG (before) | BC versusVG (after) | |
| WBC (109/L) | 11.00 ± 5.75 | 8.43 ± 3.91 | 10.37 ± 4.21 | 9.03 ± 3.95 | 0.01 | 0.018 | 0.362 | 0.273 |
| RBC (1012/L) | 5.17 ± 4.34 | 4.34 ± 0.66 | 4.72 ± 1.99 | 4.48 ± 2.02 | 0.058 | 0.398 | 0.332 | 0.508 |
| Hb (g/L) | 143.07 ± 20.29 | 131.02 ± 19.33 | 135.82 ± 22.89 | 128.53 ± 19.90 | 0.01 | 0.015 | 0.017 | 0.363 |
| PLT (109/L) | 228.40 ± 100.30 | 306.27 ± 109.03 | 252.16 ± 110.28 | 314.80 ± 119.72 | 0.01 | 0.01 | 0.107 | 0.593 |
| ALT (U/L) | 35.85 ± 45.64 | 47.29 ± 51.09 | 29.15 ± 22.38 | 44.91 ± 53.98 | 0.093 | 0.006 | 0.179 | 0.746 |
| AST (U/L) | 36.33 ± 56.52 | 30.07 ± 25.18 | 33.62 ± 67.45 | 28.19 ± 22.76 | 0.308 | 0.438 | 0.754 | 0.575 |
| Albumin (g/L) | 35.61 ± 6.39 | 37.10 ± 6.09 | 35.14 ± 6.56 | 36.66 ± 6.88 | 0.090 | 0.104 | 0.605 | 0.628 |
| TBIL (μM) | 14.34 ± 6.30 | 8.95 ± 4.19 | 13.04 ± 7.50 | 9.50 ± 5.57 | 0.01 | 0.01 | 0.183 | 0.427 |
| BUN (mM) | 4.81 ± 3.35 | 4.34 ± 1.40 | 5.22 ± 6.67 | 4.23 ± 1.41 | 0.192 | 0.138 | 0.573 | 0.592 |
| Creatinine (μM) | 65.75 ± 16.56 | 62.21 ± 13.95 | 61.38 ± 13.62 | 59.04 ± 16.86 | 0.100 | 0.271 | 0.039 | 0.143 |
| Uric acid (μM) | 260.82 ± 97.62 | 258.52 ± 94.44 | 262.86 ± 85.75 | 266.42 ± 93.97 | 0.865 | 0.777 | 0.874 | 0.533 |
| Proteinuria | 28 (27.45) | 23 (22.55) | 29 (27.62) | 18 (17.14) | 0.419 | 0.069 | 0.982 | 0.329 |
| WBC in urine | 23 (22.55) | 7 (6.86) | 17 (16.19) | 8 (7.62) | 0.002 | 0.055 | 0.243 | 0.834 |
| RBC in urine | 26 (25.49) | 13 (12.74) | 21 (20.00) | 16 (15.24) | 0.014 | 0.365 | 0.339 | 0.605 |
Note: WBC, white blood cell; RBC, red blood cell; Hb, hemoglobin; PLT, platelet; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urine nitrogen; TBIL, total bilirubin.
3.2. Healing Effect of BC Dressing
Both BC dressing and VG dressing showed high efficacy in the management of burn wounds and skin graft donor sites; all wounds healed within 28 days. The healing time for different wound types was variable. In both groups, the healing time for superficial wounds was the shortest, followed by that of skin graft donor sites, at about 11 days. Deep second-degree wounds took the longest time to heal, about 15 days (Table 6). It is worth noting that the average healing time of superficial wounds in BC group was more than one day shorter than that of VG group (p=0.029).
Table 6.
Treatment efficacy of BC and VG dressings in partial-thickness burn wounds and donor sites.
| Variable | BC group | VG group | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Superficial II° wound (n = 49) | Deep II° wound (n = 13) | Donor site (n = 40) | Superficial II° wound (n = 47) | Deep II° wound (n = 11) | Donor site (n = 47) | Superficial II° wound | Deep II° wound | Donor site | |
| Healing time (day) | 8.12 ± 2.15 | 15.77 ± 5.04 | 10.55 ± 4.40 | 9.30 ± 3.68 | 15.27 ± 5.27 | 11.19 ± 3.88 | 0.029 | 0.408 | 0.236 |
| Healing rate (%) | 100 | 100 | 100 | 100 | 100 | 100 | 1.000 | 1.000 | 1.000 |
| Dressing changing times | 3.47 ± 1.29 | 4.69 ± 1.03 | 1.60 ± 0.96 | 3.55 ± 1.35 | 4.55 ± 1.37 | 1.47 ± 0.95 | 0.471 | 0.431 | 1.034 |
| Pain score on day 3 | 1.86 ± 1.06 | 2.31 ± 1.25 | 1.98 ± 0.83 | 2.15 ± 1.00 | 2.00 ± 0.89 | 1.89 ± 0.76 | 0.225 | 0.706 | 0.766 |
| Pain score by last treatment | 0.37 ± 0.57 | 0.42 ± 0.51 | 0.18 ± 0.45 | 0.43 ± 0.68 | 0.40 ± 0.52 | 0.04 ± 0.29 | 0.886 | 0.039 | 0.030 |
| Pain score declining | 1.49 ± 0.98 | 1.92 ± 0.95 | 1.80 ± 0.91 | 1.72 ± 0.93 | 1.64 ± 0.67 | 1.85 ± 0.75 | 0.301 | 0.925 | 0.579 |
| Wound condition score before treatment | 8.33 ± 1.61 | 9.62 ± 1.80 | 7.83 ± 1.69 | 8.68 ± 1.59 | 8.09 ± 1.64 | 7.98 ± 1.47 | 0.336 | 0.022 | 0.731 |
| Wound condition score after treatment | 4.00 ± 0.00 | 4.00 ± 0.00 | 4.00 ± 0.00 | 4.00 ± 0.00 | 4.00 ± 0.00 | 4.00 ± 0.00 | 1.000 | 1.000 | 1.000 |
| Wound condition score improvement | 4.33 ± 1.61 | 5.62 ± 1.80 | 3.83 ± 1.69 | 4.64 ± 1.57 | 4.09 ± 1.64 | 3.98 ± 1.47 | 0.379 | 0.022 | 0.731 |
| Improvement in erythema and swelling | 1.92 ± 0.76 | 2.08 ± 0.86 | 0.80 ± 0.65 | 1.87 ± 0.77 | 1.73 ± 0.79 | 0.85 ± 0.69 | 0.838 | 0.227 | 0.756 |
| Improvement in exudate | 1.96 ± 0.82 | 2.31 ± 0.63 | 1.63 ± 0.63 | 2.15 ± 0.75 | 2.09 ± 0.70 | 1.70 ± 0.59 | 0.267 | 0.457 | 0.498 |
| Improvement in hemorrhage | 0.35 ± 0.56 | 0.62 ± 0.65 | 1.35 ± 0.83 | 0.53 ± 0.65 | 0.09 ± 0.30 | 1.40 ± 0.65 | 0.142 | 0.025 | 0.717 |
| Improvement in subeschar purulence and exudation | 0.10 ± 0.31 | 0.62 ± 0.87 | 0.05 ± 0.22 | 0.09 ± 0.28 | 0.18 ± 0.40 | 0.02 ± 0.15 | 0.783 | 0.150 | 0.475 |
| Drug treatment | 40 (81.63) | 13 (100.0) | 37 (92.50) | 44 (93.62) | 11 (100.0) | 47 (100.0) | 0.076 | 1.00 | 0.093 |
| Adverse event, n (%) | 1 (2.04) | 0 (0.00) | 4 (10.00) | 1 (2.13) | 0 (0.00) | 10 (21.28) | 1.000 | 1.000 | 0.154 |
The frequencies of dressing changing in different wound types were variable. However, no difference was observed in the two groups. The BC dressing was flexible and translucent, which made it easier for doctors and nurses to observe the wound and make suitable discretion regarding the changing of dressing and alleviate unnecessary pain in patients (Figure 2). Adverse events were observed in 5 participants in the BC group, while 11 cases were reported in the VG group. The signs of adverse events included rashes, increased alanine aminotransferase (ALT) and total bilirubin (TBIL), decreased albumin, and urine occult blood. These adverse events were promptly and successfully resolved without disrupting the treatment process.
Figure 2.
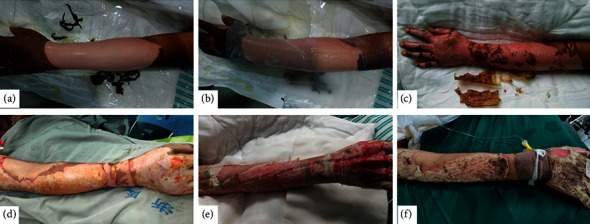
The application of BC dressing and VG dressing in second-degree burn wounds. Bacterial cellulose (BC) dressing and Vaseline gauze (VG) dressing were used in second-degree burn wounds in the two-center randomized controlled clinical study. Their treatment efficacies were compared as well. (a) Deep second-degree burn wound on left forearm. (b) BC dressing was applied. BC dressing was translucent, which allowed the wound to be checked easily. (c) Without changing BC dressing, wound healed on day 12; BC dressing dried up and fell off. (d) Deep second-degree burn wound on right forearm. (e) VG dressing was less translucent than BC dressing, making checking less convenient. 12 days later, VG was stuck to the wound bed and was not able to be changed. Hemorrhage was observed when changing outer cover of VG. (f) Wound healed on day 21, but there were difficulties when removing VG.
Wound conditions and healing process were assessed from four aspects: erythema and swelling, exudation, bleeding, and subeschar purulence and exudation. Wound conditions before treatment are listed in Table 4. Erythema and swelling presented in all wounds and were mostly mild and moderate. Wound exudation was also a significant symptom in all participants; about 22% of the participants even suffered from severe wound exudation. Around 44% of the participants in the BC group did not suffer from would bleeding, while in the VG group, only 35% did not. No difference was found regarding the bleeding in the two groups before treatment (p=0.264). In both groups, only a small proportion of the participants experienced subeschar purulence and exudation. The score of the wound conditions before and after the treatment and the comparison between the two treatment groups are shown in Table 6.
BC dressing was very helpful for the reepithelialization of wounds. From day 3 to day 7, the changes in the erythema, swelling, exudation, and bleeding were statistically significant. The score of subeschar purulence remained very low in the BC group throughout the whole process of treatment. Hence, it is reasonable to infer that the changes in subeschar purulence were not statistically significant. BC dressing was also good at pain relieving. The pain score in the BC group decreased quickly and significantly within 7 days of treatment and remained at a very low level until the healing of the wounds (Table 7).
Table 7.
Changes of wound conditions in 7 days of treatment.
| Parameter | BC group | VG group | p value (BC versus VG) | |||||
|---|---|---|---|---|---|---|---|---|
| Day 3 | Day 7 | p value | Day 3 | Day 7 | p value | Day 3 | Day 7 | |
| Erythema and swelling | 2.50 ± 0.92 | 1.30 ± 0.61 | 0.01 | 2.42 ± 0.90 | 1.31 ± 0.52 | 0.01 | 0.522 | 0.896 |
| Exudation | 2.87 ± 0.75 | 1.39 ± 0.66 | 0.01 | 2.94 ± 0.70 | 1.43 ± 0.68 | 0.01 | 0.489 | 0.696 |
| Hemorrhage | 1.77 ± 0.83 | 1.04 ± 0.20 | 0.01 | 1.88 ± 0.79 | 1.04 ± 0.19 | 0.01 | 0.369 | 0.967 |
| Subeschar purulence and exudation | 1.15 ± 0.43 | 1.08 ± 0.27 | 0.175 | 1.07 ± 0.25 | 1.07 ± 0.29 | 1.000 | 0.101 | 0.762 |
| Pain score | 1.96 ± 1.00 | 0.98 ± 0.99 | 0.01 | 2.02 ± 0.89 | 1.13 ± 0.87 | 0.01 | 0.659 | 0.239 |
4. Discussion
Results collected from well-conducted randomized controlled trials (RCTs) are regarded as top-tier evidence for decision-making in clinical practice [23], which has been used to compare the efficacy between novel, conventional, and commercial dressings for burn wounds [24, 25]. The present RCT was a prospective, comparative, quantitative clinical study conducted under controlled conditions with random allocation of treatments to comparison groups. Additionally, this study was implemented in accordance with the guidance of SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) [26]. To ensure the reliability of this study and eliminate possible confounding factors, the design and implementation of the study and the data analysis and interpretation of findings were carefully performed to assess the effect of the treatment and how far it deviated from its true value. For instance, the study was conducted in two hospitals that have similar research teams but are located in different cities. In this scenario, the participants were allocated into two independent research centers rather than single center, helpful for controlling selection and observer biases [27]. Blinding is a good strategy to alleviate introducing unconscious information bias to the participants and/or the investigators. Therefore, the study design and vigorous statistical analysis were performed by a third party with proper statistical methods. In addition, the dropout rate of this study was only 2.4%, less than the preset value of 6%. Thus, we are confident with the results and the power of this RCT.
The treatment of partial thickness burn wounds is healing-effect-oriented. The advances in material sciences have deepened understanding of wound healing and infections and driven the development of new dressings [28]. Today, a wide variety of dressings are available in the market or have been tested in clinical practice [5, 29]. The materials used for dressing include hydrocolloid, polyurethane film, hydrogel, silicon-coated nylon, biosynthetic skin substitute, antimicrobial, fiber, and pads [30]. It is well known that the treatment of second-degree burn wounds is very challenging due to significant fluid loss, repeated and painful dressing changes, and wound infection, leading to local tissue damage and complications. Due to the lack of high-quality evidence collected from RCTs, it is still risky to confirm the healing effect of a specific dressing on the treatment of second-degree burn wounds [31].
VG dressing has been used widely for the treatment of second-degree burn wounds or skin graft donor sites in clinical setting and as a reference for the evaluation of new dressing materials [18, 32–34]. BC has many intrinsic characteristics; it is nontoxic and biocompatible, and its high capacity of water retention makes it an ideal material for burn wound dressing [9]. In the present RCT, we evaluated the efficacy of a novel BC dressing against VG dressing. Based on our observation, the novel BC dressing demonstrated the following desirable characteristics: (1) It was elastic and could accommodate necessary movements of the participants. (2) It was adhesive and could conform to the wound bed perfectly, protecting the wound from infections. (3) With the reticulate structure of thin fibers coupled with superior water retention capacity [35], it could absorb exudate and maintain moisture of the wound and facilitate oxygen exchange as well, accelerating reepithelialization for burn wounds. According to the results (Table 7), with the treatment of BC dressing, the four components of the scoring method, erythema and swelling, exudate, hemorrhage, and subeschar purulence and exudation were well controlled. They disappeared within 7 days of treatment, leading to a healing rate of 100% in 28 days.
Compared to VG dressing, the average healing time of BC dressing treatment for superficial wound was about 1 day shorter (p=0.029). However, the healing times in deep wounds and skin graft donor sites of BC dressing were the same as those of the VG group. The difference in healing time of BC dressing may be because of the characteristics that were covered previously [9, 35]. However, bench-scale studies, well-designed animal experiments, and clinical trials with larger sample sizes are required to explore possible mechanism. The healing time of this study was much shorter than those in some previous reports. The efficacies of Nitrofurazone, Vaseline gauze, and ColActive Plus Ag were compared in an RCT for a wound bed with an area of about 45 cm2, which is much smaller than the area observed in this study. However, it took more than 12 days for superficial burn wounds to heal in the previous study [18]. In other RCTs, VG was used as a control to compare the efficacies of other dressings, and the healing time was longer and the healing rate was lower than in this study [32, 33].
The changing of dressings in patients with burn wounds is time-consuming. It might take 105 minutes to dress a facial burn wound and 66 minutes to change a dressing on a hand [9]. In this study, as the differences between BC and VG dressings were obvious, it was difficult to evaluate the time consumed on dressing changing objectively. Therefore, the time consumption of dressing changing was not compared. In addition, the BC dressing tested in this study was produced on a small scale for the clinical trial. Its cost was much higher than that of the VG dressing. Therefore, the cost effectiveness of the two dressings was not compared. It is arguable that when the BC dressing becomes produced on a commercial scale, its cost effectiveness will be close to that of VG dressing.
Despite the aforementioned advantages, this RCT has some limitations. Due to careful ethical considerations, the wound bed area of each participant was strictly controlled to less than 15% of the total body surface area (TBSA) and only one wound bed was observed in each participant. Limited treating area may affect the generalization and extrapolation of the findings of this study. To compare the treatment efficacies of BC dressing and VG dressing, a set of primary and secondary endpoints were observed. However, as two of the major impact factors on burn wound healing, the etiology and severity of wound infections and the response of host were not included.
In the future, a multicentered study with a much larger sample size should lead to better conclusions. For greater effectiveness, if ethically possible, the BC dressing and control dressing, such as hydrocolloids and hydrofiber dressing scan, be applied on the same participant simultaneously, though it may affect the measurement of pain and tolerance level when a normal visual analogue score scale is used.
5. Conclusions
The results of this noninferiority trial demonstrated that the novel BC dressing could be used for the management of second-degree burn wounds and skin graft donor sites. The BC dressing was superior to the VG dressing in the treatment of superficial second-degree burn wounds in terms of healing time. The BC dressing also showed noninferiority in the management of deep secondary burn wounds and skin graft donor sites versus VG dressing. The tested novel BC dressing has vast potential for future application in clinical practice.
Acknowledgments
The authors would like to express their gratitude to all the patients and colleagues who participated in this RCT for their kind cooperation, hard work, and valuable suggestions. This study was supported by the Zhejiang Medical and Health Science and Technology Plan Project (2016KYB115 and 2017KY361).
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethical Approval
This study was approved by the Ethics Committees of the Second Affiliated Hospital of Zhejiang University School of Medicine.
Consent
All participants volunteered to participate in the study and provided a signed informed consent.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Owda A. Y., Salmon N., Shylo S., Owda M. Assessment of bandaged burn wounds using porcine skin and millimetric radiometry. Sensors . 2019;19(13):p. 2950. doi: 10.3390/s19132950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parrett B. M., Pomahac B., Demling R. H., Orgill D. P. Fourth-degree burns to the lower extremity with exposed tendon and bone: a ten-year experience. Journal of Burn Care & Research . 2006;27(1):34–39. doi: 10.1097/01.bcr.0000192265.20514.c5. [DOI] [PubMed] [Google Scholar]
- 3.Chen L., Deng H., Cui H., et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget . 2018;9(6):7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jozsa G., Vajda P., Garami A., Csenkey A., Juhasz Z. Treatment of partial thickness hand burn injuries in children with combination of silver foam dressing and zinc-hyaluronic gel: case reports. Medicine . 2018;97(13) doi: 10.1097/md.0000000000009991.e9991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranjan V., Mehta M., Shah S., Sarwade P., Philipose A. Comparative study of silver-sulfadiazine-impregnated collagen dressing versus conventional burn dressings in second-degree burns. Journal of Family Medicine and Primary Care . 2019;8(1):215–219. doi: 10.4103/jfmpc.jfmpc_291_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen Z., Cai N., Xue Y., et al. Engineering sustainable antimicrobial release in silica-cellulose membrane with CaCO(3)-aided processing for wound dressing application. Polymers . 2019;11(5):p. 808. doi: 10.3390/polym11050808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baghel P. S., Shukla S., Mathur R. K., Randa R. A comparative study to evaluate the effect of honey dressing and silver sulfadiazene dressing on wound healing in burn patients. Indian Journal of Plastic Surgery . 2009;42(2):176–181. doi: 10.4103/0970-0358.59276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanescu P.-O., Radu I.-C., Leu Alexa R., et al. Novel chitosan and bacterial cellulose biocomposites tailored with polymeric nanoparticles for modern wound dressing development. Drug Delivery . 2021;28(1):1932–1950. doi: 10.1080/10717544.2021.1977423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konieczynska M. D., Villa-Camacho J. C., Ghobril C., et al. On-demand dissolution of a dendritic hydrogel-based dressing for second-degree burn wounds through thiol-thioester exchange reaction. Angewandte Chemie . 2016;128(34):10138–10141. doi: 10.1002/ange.201604827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J., Tavakoli J., Tang Y. Bacterial cellulose production, properties and applications with different culture methods-a review. Carbohydrate Polymers . 2019;219:63–76. doi: 10.1016/j.carbpol.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Bacakova L., Pajorova J., Bacakova M., et al. Versatile application of nanocellulose: from industry to skin tissue engineering and wound healing. Nanomaterials . 2019;9(2):p. 164. doi: 10.3390/nano9020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bardania H., Mahmoudi R., Bagheri H., et al. Facile preparation of a novel biogenic silver-loaded Nanofilm with intrinsic anti-bacterial and oxidant scavenging activities for wound healing. Scientific Reports . 2020;10(1):p. 6129. doi: 10.1038/s41598-020-63032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiji S., Udhayakumar S., Maharajan K., Rose C., Muralidharan C., Kadirvelu K. Bacterial cellulose matrix with in situ impregnation of silver nanoparticles via catecholic redox chemistry for third degree burn wound healing. Carbohydrate Polymers . 2020;245 doi: 10.1016/j.carbpol.2020.116573.116573 [DOI] [PubMed] [Google Scholar]
- 14.Peršin Z., Maver U., Pivec T., et al. Novel cellulose based materials for safe and efficient wound treatment. Carbohydrate Polymers . 2014;100:55–64. doi: 10.1016/j.carbpol.2013.03.082. [DOI] [PubMed] [Google Scholar]
- 15.Xu L., Bai R., Cheng X., et al. A tiered experimental approach for characterization and silver release of silver-containing wound dressings. Journal of Biomedical Nanotechnology . 2018;14(3):564–574. doi: 10.1166/jbn.2018.2534. [DOI] [PubMed] [Google Scholar]
- 16.Jahani S., Ashrafizadeh H., Babai K., Siahpoosh A., Cheraghian B. Effect of ointment-based egg white on healing of second- degree wound in burn patients: a triple-blind randomized clinical trial study. Avicenna journal of phytomedicine . 2019;9(3):260–270. [PMC free article] [PubMed] [Google Scholar]
- 17.Monsalve-Hernando C., Crespo L., Ferreiro B., et al. Phase IV noninferiority controlled randomized trial to evaluate the impact on diagnostic thinking and patient management and the test-retest reproducibility of the Gaxilose test for hypolactasia diagnosis. Medicine . 2018;97(46) doi: 10.1097/md.0000000000013136.e13136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salehi H., Momeni M., Ebrahimi M., et al. Comparing the effect of colactive plus ag dressing versus nitrofurazone and vaseline gauze dressing in the treatment of second-degree burns. Annals of burns and fire disasters . 2018;31(3):204–208. [PMC free article] [PubMed] [Google Scholar]
- 19.Savitskaya I. S., Shokatayeva D. H., Kistaubayeva A. S., Ignatova L. V., Digel I. E. Antimicrobial and wound healing properties of a bacterial cellulose based material containing B. subtilis cells. Heliyon . 2019;5(10) doi: 10.1016/j.heliyon.2019.e02592.e02592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones L. M., Uribe A. A., Coffey R., et al. Pregabalin in the reduction of pain and opioid consumption after burn injuries: a preliminary, randomized, double-blind, placebo-controlled study. Medicine . 2019;98(18) doi: 10.1097/md.0000000000015343.e15343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barret J. P., Podmelle F., Lipový B., et al. Accelerated re-epithelialization of partial-thickness skin wounds by a topical betulin gel: results of a randomized phase III clinical trials program. Burns . 2017;43(6):1284–1294. doi: 10.1016/j.burns.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Kim J., Shin W. How to do random allocation (randomization) Clinics in Orthopedic Surgery . 2014;6(1):103–109. doi: 10.4055/cios.2014.6.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhide A., Shah P. S., Acharya G. A simplified guide to randomized controlled trials. Acta Obstetricia et Gynecologica Scandinavica . 2018;97(4):380–387. doi: 10.1111/aogs.13309. [DOI] [PubMed] [Google Scholar]
- 24.Napavichayanun S., Ampawong S., Harnsilpong T., Angspatt A., Aramwit P. Inflammatory reaction, clinical efficacy, and safety of bacterial cellulose wound dressing containing silk sericin and polyhexamethylene biguanide for wound treatment. Archives of Dermatological Research . 2018;310(10):795–805. doi: 10.1007/s00403-018-1871-3. [DOI] [PubMed] [Google Scholar]
- 25.Verbelen J., Hoeksema H., Heyneman A., Pirayesh A., Monstrey S. Aquacel(®) Ag dressing versus Acticoat™ dressing in partial thickness burns: a prospective, randomized, controlled study in 100 patients. Part 1: burn wound healing. Burns . 2014;40(3):416–427. doi: 10.1016/j.burns.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Chan A.-W., Tetzlaff J. M., Gotzsche P. C., et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ . 2013;346(jan08 15) doi: 10.1136/bmj.e7586.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim C.-Y., In J. Randomization in clinical studies. Korean Journal of Anesthesiology . 2019;72(3):221–232. doi: 10.4097/kja.19049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanova L. A., Ustinovich K. B., Khamova T. V., et al. Crystal and supramolecular structure of bacterial cellulose hydrolyzed by cellobiohydrolase from scytalidium candidum 3C: a basis for development of biodegradable wound dressings. Materials . 2020;13(9):p. 2087. doi: 10.3390/ma13092087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bairagi A., Griffin B., Tyack Z., Vagenas D., McPhail S. M., Kimble R. Comparative effectiveness of Biobrane®, RECELL® Autologous skin Cell suspension and Silver dressings in partial thickness paediatric burns: BRACS randomised trial protocol. Burns & Trauma . 2019;7:p. 33. doi: 10.1186/s41038-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wasiak J., Cleland H., Campbell F., Spinks A. Dressings for superficial and partial thickness burns. Cochrane Database of Systematic Reviews . 2013;2013(3) doi: 10.1002/14651858.cd002106.pub4.Cd002106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wasiak J., Cleland H. Burns: dressings. BMJ Clinical Evidence . 2015;2015 [PMC free article] [PubMed] [Google Scholar]
- 32.Noorbala M. T., Noorbala M., Dashti-Rahmatabadi M. H., Noorbala M., Noorbala R., Mozaffary B. Comparison of hydrogel produced by radiation as applied at the research center (yazd branch) with MaxGel and routine dressing for second-degree burn Repair in yazd burn hospital. Iranian Red Crescent Medical Journal . 2016;18(8) doi: 10.5812/ircmj.24384.e24384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salehi S. H., As’adi K., Mousavi S. J., Shoar S. Evaluation of amniotic membrane effectiveness in skin graft donor site dressing in burn patients. Indian Journal of Surgery . 2015;77(S2):427–431. doi: 10.1007/s12262-013-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlsson M., Olofsson P., Steinvall I., Sjöberg F., Thorfinn J., Elmasry M. Three years’ experience of a novel biosynthetic cellulose dressing in burns. Advances in Wound Care . 2019;8(2):71–76. doi: 10.1089/wound.2018.0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portela R., Leal C. R., Almeida P. L., Sobral R. G. Bacterial cellulose: a versatile biopolymer for wound dressing applications. Microbial Biotechnology . 2019;12(4):586–610. doi: 10.1111/1751-7915.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


