Abstract
Male breast cancer (MaBC) is a rare clinical entity, which makes up approximately 1% of all breast cancers. However, the incidence of MaBC has been steadily increasing over the past few decades. The risk factors for MaBC include age, black race, family history of breast cancer, genetic mutations, liver cirrhosis, and testicular abnormalities. The majority of patients with MaBC present with painless lumps, and about half of the patients have at least one lymph node involved at the time of diagnosis. The treatment of MaBC models that of female breast cancer (FeBC), but this is mainly due to lack of prospective studies for MaBC patients. The treatment modality includes surgery, adjuvant radiation, endocrine therapy, and chemotherapy. However, there are some distinct features of MaBC, both clinically and molecularly, that may warrant a different clinical approach. Ongoing multinational effort is required, to conduct clinical trials for MaBC, or the inclusion of MaBC patients in FeBC trials, to help clinicians improve care for MaBC patients.
1. Introduction
Breast cancer in men is a relatively rare disease and accounts for only 1% of the breast cancer population. As with all other rare diseases, it has been challenging to conduct prospective clinical studies in male breast cancer (MaBC) as evidenced by several prematurely closed clinical trials due to the lack of enrollment. This situation is exacerbated by the exclusion of male participants in female breast cancer (FeBC) clinical trials in the past. Our current knowledge of male breast cancer is largely derived from small retrospective studies, often single-center experience, and as a result, approaches to treating MaBC are extrapolated from guidelines in FeBC management. There is rising evidence that MaBC has distinct clinical features that trace back to molecular levels (i.e., genomics and tumor subtypes), and different treatment approaches may improve morbidity and mortality. In this study, we review the epidemiology and risk factors, highlight the similarity and differences between MaBC and FeBC at molecular level (including genomics and tumor characteristics), characterize clinical features and diagnostic modalities, and summarize treatment regimens and future directions in research.
1.1. Epidemiology
MaBC is a rare disease and makes up only approximately 1% of all breast cancers in the United States and worldwide. [1] Similar to female breast cancer, the incidence rate continues to rise [2]. It is estimated that there will be 2620 new cases of male breast cancers diagnosed in the United States in 2020, compared to only 900 cases in 1991 [3]. The age-adjusted incidence rate has increased to 1.32 per 100,000 men in 2017, from 0.90 per 100,000 in 1980 as outlined by the Surveillance, Epidemiology, and End Results (SEER) [4].
1.2. Risk Factors
1.2.1. Age and Ethnicity
The incidence of MaBC varies by ethnicity and age, with the highest incidence rate of 1.89/100,000 in non-Hispanic black population, which is significantly higher than that of the non-Hispanic White population (1.3/100,000), the Asian population (0.7/100,000), and the Hispanic population (0.8/100,000) [1]. Higher incidence rates of MaBC are observed in South and Central Africa, which is possibly due to hyperestrogenism in the setting of more prevalent infectious hepatic disease [5]. In the retrospective study by O'Malley et al, non-Hispanic blacks and whites have similarly poor 5-year survival rates (57% and 66%, no confidence interval or p value available), which are noticeably worse than that of other race/ethnicities (75%) [6].
The incidence rate increases with age, with a significant increase at Age 50 (showing an incidence rate of 1.7/100,000) and plateauing at Age 80 and above (with an incidence rate of 8.3/100,000) [4]. In a large retrospective cohort study of 19,795 MaBC patients, the median age at diagnosis was 65 years, with 15% of patients diagnosed at age <50 years [7].
1.2.2. Family History
It is estimated from multiple population studies that 15–20% of male patients diagnosed with breast cancer have at least one first degree relative that developed breast cancer [8]. Similar to women, men who have either a female or male first degree relative with breast cancer have a 2- to 3-fold increased risk of developing breast cancer. The risk substantially increases with increasing numbers of affected family members [9]. Although the odds ratio is not quantified, there are case reports of association of MaBC with a family history of both Lynch syndrome and Cowden syndrome [10, 11]. However, screening mammography is not recommended for men with family history of breast cancer, as the absolute risk of MaBC in this population is still considered to be very low.
1.2.3. Klinefelter Syndrome
Klinefelter is a rare genetic disease that occurs when men gain one extra X chromosome, which clinically manifests as gynecomastia and testicular dysgenesis. Men with Klinefelter syndrome carry significantly increased risk (20- to 50-fold more) of MaBC compared with the general male population, and it is hypothesized the increased risk is primarily due to their low androgen and high gonadotrophin state [12].
1.2.4. Hormonal Imbalance
It is interesting to note that meta-analysis pooling results from 10 cohort studies and 11 case-control studies discovered an association of MaBC with increased estrogen level (rather than reduced androgen level) [13]. Similar to women, men with diseases, such as obesity and alcohol dependence that result in pathological increase in endogenous estrogen level, are at elevated risk of developing breast cancer [14, 15]. Two unique risk factors to males are liver cirrhosis and testicular dysfunction (such as undescended testes and congenital inguinal hernia), both of which also increase men's risk of developing breast cancer later in life [16, 17] Gynecomastia is frequently associated with increased estrogen level and has been proposed to be associated with increased risk of MaBC. However, in a series of prospective studies, gynecomastia does not increase the probability of developing MaBC [18–20].
1.2.5. Environmental Exposure
Several occupational hazards, including hot working environments, ionizing and electromagnetic radiation, or exposure to chemical compounds such as combustion products, have been associated with increased risks of developing MaBC [21–23]. However, these findings have been limited to case reports and case series.
1.3. Genetics
It is well characterized that mutations of both BRCA1 and BRCA2, tumor suppressor genes involved in DNA repair, are implicated in FeBC. Approximately 10–15% of FeBC are associated with these autosomal dominant mutations, which confer a cumulative 45–65% life time risk [24]. In men, BRCA2 mutations and, less frequently, BRCA1 mutations are established risk factors of breast cancer. In population studies unselected for family history, 4–33% of male breast cancer patients harbor BRCA2 mutations and 0–6% harbor BRCA1 mutations [25–39]. The calculated average BRCA2 mutations from pooled data are 10% (83/840). This broad range is, in part, explained by the differences in sample size. For instance, the study by Csokay et al only includes 18 patients. However, 6/18 patients were found to have BRCA2 mutations [28]. Most of these studies are small retrospective studies in single institutions, and they highlight the importance of multicenter/country collaboration to identify more accurate incidence rates. In two studies that are selected for family history, BRCA2 mutation frequencies ranged from 37 to 40%, and BRCA1 mutations were found in about 5% of study subjects [40, 41].
In a recent retrospective study involving the analysis of multigene panel testing of 708 MaBC patients, 97 patients (13.7%) have at least one pathogenic variant in breast cancer susceptibility gene, 11% tested positive for BRCA2, 4.1% for CHEK2, 1.5% for ATM, 1.3% for BRCA1, 0.6% for NF1, and 0.5% for PALB2 [42]. Similar results are observed in another retrospective study [43]. Mutations of CHEK2, a cell cycle check point kinase also involved in DNA repair, have been implicated in the literature as a risk factor for MaBC and, in particular, CHEK2 1100delC with as high as 10-fold increased risk [44]. However, there are also conflicting findings from other studies, where researchers did not find any association between CHEK2 and male breast cancer [45–47]. Another frequently discussed gene mutation is PALB2, which involves encoding a protein in the BRCA2-related pathway. The reported frequency ranges from 0.8 to 6.4% in MaBC patients [39, 48–50]. The risk of developing MaBC in patients with PALB2 mutation is 4- to 6.6-fold higher than those not carrying the PALB2 mutations [42, 50]. Other implicated genes in the literature also included CYP17, RAD51B, and PTEN mutations associated with Cowden syndrome [51–53]. Patients carrying these gene mutations, in particular, BRCA2, are clearly at risk of developing MaBC. The National Comprehensive Cancer Network (NCCN) recommends that these patients self-breast examination and clinical breast examinations should be carried out for MaBC screening twice each year. However, recommendations for annual mammography are unclear [54]. Gene mutations implicated in MaBC are listed in Table 1.
Table 1.
Gene mutations implicated in MaBC.
| Genes implicated | Frequency |
|---|---|
| BRCA2 | 11%42 |
| BRCA1 | 1.30%42 |
| CHEK2 | 4.10%42 |
| PALB2 | 0.8–6.4%48-51 |
| ATM | 1.50%42 |
| NF1 | 0.60%42 |
1.4. Clinical Features and Diagnostic Imaging
Similar to FeBC patients, the majority of MaBC patients (approximately 75%) present with a painless lump, most frequently in the retro-areolar area. Most patients have early nipple involvement, including retraction, discharge, or ulceration, as the rudimentary breast ducts are located directly beneath the nipple [55–58]. Because of the rarity of MaBC and the lack of established screening guidelines in men, there is often a delay in diagnosis, with one study reporting a mean duration of 21 months since the onset of symptoms [59]. For this reason, approximately 46.7% of men have disease involving at least one lymph node at the time of diagnosis, as reported in a recent study carried out by the International Male Breast Cancer Program (IMBCP) [60]. Often times, patients can present with gynecomastia, making it difficult to distinguish from MaBC. Therefore, in these cases, imaging is required for further evaluation. The American College of Radiology recommends that men younger than 25 years with a palpable mass should undergo bilateral ultrasound evaluation, and men older than 25 years should undergo bilateral mammography; however, if the mammography is indeterminate, ultrasound should be performed as an adjunct tool [61]. Most common findings on the ultrasound are irregular, hypoechoic retro-areolar masses that appear spiculated and have variable vascularity, along with mammography often showing similar spiculated and radio-dense irregular retro-areolar masses [62]. Similar to its use in diagnosis of FeBC, mammograms have a high sensitivity of 92–100% and high specificity of 90–96% in diagnosing MaBC [63]. However, there is currently no evidence that support screening for asymptomatic men.
1.5. Tumor Characteristics and Biology
The most prevalent MaBC is invasive ductal carcinoma, and because of anatomical differences (as male breasts consist of ducts without terminal lobules), the prevalence of invasive lobular carcinoma is much lower in males (1–2%) than in females (15%) [64]. The largest multicenter study of MaBC to date conducted by IMBCP, involved review of 1483 breast tissues, displayed a similar prevalence: 85% of invasive ductal carcinoma, 1.4% of invasive lobular carcinoma, and the remaining comprised of mixed ductal and lobular carcinoma (5.9%), papillary (3%), and mucinous (1.9%) [65]. Approximately 50% of these invasive cancers are histological grade 2, 22% are grade 1, and 27% are grade 3 [65]. Only about 10% of MaBC patients presented with a precursor lesion compared to 15–30% of women, which is possibly due to the rare manifestation of ductal carcinoma in situ as palpable masses and the lack of image-based screening in men [66].
As for tumor subtypes, current available data are derived from retrospective studies. The study by IMBCP examined 1483 patients diagnosed between 2001 and 2010, which discovered that 99.3% of these patients were ER positive, 81.9% PR positive, 96.9% AR positive, and 8.7% HER2 positive. The Ki67, a marker of cell proliferation, had <20% positive cells in 75% of the samples assessed, and the other 25% had >20% positive cells with a high Ki-67 expression [60]. The molecular subtyping of breast cancer can be further subdivided, according to the luminal classification, into Luminal A (ER+, PR+, HER2-, and low Ki67), Luminal B-like HER2 negative (ER+, HER2-, and high Ki67 or PR-), Luminal B-like HER2 positive (ER+, HER2+, any Ki-67, or any PR), nonluminal (HER2+, ER-, and PR-), and basal (triple negative). In this study, 42% were luminal A, 49% Luminal B-like/HER2-, 9% HER2+, and 0.3% triple negative [60]. The results of this large international study were consistent with those derived from SEER, showing that MaBC is predominantly ER/PR positive and HER2 negative. Interestingly, ER is known to have alpha and beta fractions, and the two fractions reside in different tissues of the human body-alpha is often found in endometrium and ovaries, whereas beta is often found in kidney, brain, bones, and prostate [67]. In contrast to FeBC, which contains mostly ER-alpha receptors, the majority of MaBC have ER-beta receptors, indicating possible different biological activities at the molecular level between MaBC and FeBC. This may warrant a different approach to MaBC in terms of anti-estrogen therapy compared with FeBC [68].
1.6. Prognosis
The prognosis for MaBC is generally worse than that of FeBC. The study by Wang et al investigating 16025 male and 1,800,708 female patients diagnosed with breast cancer from the National Cancer Data Base (NCDB) between 2004 and 2014 demonstrated that overall mortality and mortality at three and five years are higher in men when compared to women even after adjusting for clinical characteristics, treatment factors, age, race/ethnicities, and access to care [69]. In a study carried by Leone et al. examining 2992 MaBC patients from SEER between 2003 and 2012, age at diagnosis, tumor grade, stage, surgery, radiotherapy, ER, and marital status have been identified to carry prognostic value in MaBC [70]. Importantly, tumor subtypes have been shown to have clear impact on prognosis in two additional studies looking at the SEER OS data of 960 MaBC patients from 2010 to 2012 and 1187 MaBC patients from 2010 to 2013. These two studies show that patients with HER2+ and triple-negative (TN) tumor subtypes have significantly worse prognoses [71, 72].
1.7. Early-Stage MaBC Treatment
Given the relative rarity of MaBC (as compared to FeBC), most of the treatment modalities in MaBC are extrapolated from the current standard of care of FeBC. Although there are similarities in disease characteristics, MaBC has distinct features that warrant a specific clinical approach. A treatment algorithm is illustrated in Figure 1.
Figure 1.
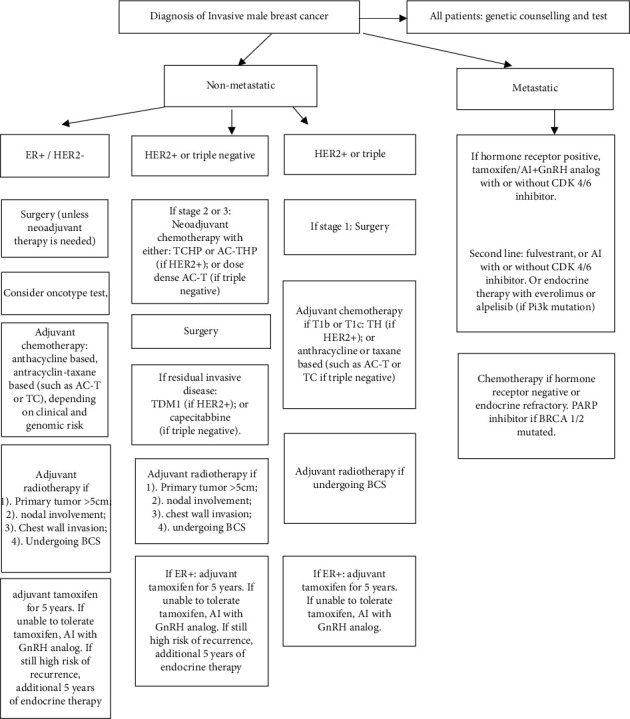
Treatment algorithm for male breast cancer.
Similar to FeBC, for early-stage disease in men, surgery plays a fundamental role. However, unlike FeBC, in which 2/3 of patients undergo breast conserving surgery (BCS) and 1/3 mastectomy, the majority of MaBC patients undergo mastectomy, leaving a small percentage of men (10–24%) who are treated with BCS. Even in MaBC patients with T1N0 disease, only 18% of patients underwent BCS [73–75]. This observation could be, in part, due to the central location of most MaBC, which can involve the nipple-areolar complex. Furthermore, MaBC patients may prefer mastectomy to avoid breast irradiation altogether, contributing to a small trend of decreasing BCS between 2004 and 2014 observed by Yadav et al. [76]. In regard to prognosis, several retrospective studies find similar overall survival rates in MaBC patients who either underwent mastectomy or BCS [73–78]. If BCS is desired in MaBC patients with large tumors or nodal involvement, neoadjuvant therapy (endocrine or cytotoxic) can potentially be employed to decrease tumor size so that BCS can be feasible. This, however, is only used in limited cases [79].
Axially lymph node dissection (ALND) has been a standard surgical procedure in MaBC patients, but is associated with significant morbidity, including lymphedema, infection, and axillary paresthesia. Sentinel lymph node biopsy (SLNB) is underutilized in MaBC patients when compared to FeBC patients, even though several case series has shown similar accuracy in predicting axillary nodal status [80–83].
In FeBC, adjuvant radiotherapy is recommended for patients with metastasis to four or more lymph nodes, any involvement of internal mammary or supraclavicular nodes, invasion of chest wall after mastectomy, and for those who undergo BCS [84]. Recommendations for FeBC patients with T1-T2 tumors with involvement of 1–3 lymph nodes require more clinical judgement, especially for the subset of patients with decreased risk of recurrence. Because there is lack of prospective data on radiotherapy in MaBC, the recommendations for FeBC also apply to MaBC, to reduce locoregional failure, disease recurrence, and breast cancer mortality. In a recent study analyzing SEER data between 1998 and 2003, there was improved OS for case-matched patients who underwent postmastectomy radiotherapy (PMRT) (83% vs 54%, p < 0.001) [85]. Subgroup analysis in the same study also demonstrated improved OS in those with 1–3 lymph nodes (79% vs 72%, p < 0.05) as well as 4+ lymph nodes involved (73% vs 53%, p < 0.001). In another meta-analysis involving 29 studies and 10,965 MaBC patients after mastectomy, PMRT also demonstrated improved locoregional control and survival [86]. However, the utilization of PMRT varies greatly from 2 to 100%, with a mean of 64%, and it generally appears underutilized in MaBC patients [86]. Radiotherapy, as part of the standard therapy for MaBC patients who underwent BCS, is also not administered systematically. In one study, only 35.4% of patients received radiotherapy after lumpectomy, and in another study, about 42% received radiotherapy [74, 75]. The reasons for the underutilization of adjuvant radiotherapy in MaBC patients remain unclear. However, there is a trend of increasing utilization of PMRT (50% in 2004 and 52% in 2014) and radiation therapy after BCS (66% in 2004 to 74.6% in 2014) as identified by Yadav et al. [76]. In recent years, innovative techniques such as hypo-fractionated regimen or controlled regional delivery via computed tomography scan planning are being developed to improve precision and minimize heart and lung toxicity in FeBC [87, 88]. However, these techniques are yet to be examined in MaBC.
The use of adjuvant chemotherapy-anthracycline-based, anthracycline-taxane-based, and cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) has been associated with improved OS in stage II and III disease [60, 76]. A phase 2 clinical trial, evaluating CMF in 31 men with node-positive breast cancer, has reported 20-year results. The study concluded that patients had significantly better OS (80% OS at 5 years, and 42% at 20 years) compared to historic rates [89]. Another retrospective study found reduced recurrence and improved OS in stage II MaBC patients with nodal involvement receiving anthracycline-based regimens [90]. Currently, MaBC patients receiving adjuvant chemotherapy tend to have larger tumors, lymph node involvement, hormone receptor-negative disease, and younger age at diagnosis [53]. In hormone receptor-positive FeBC, the 21 gene recurrence score has been used for prognosis and to predict benefit from adjuvant chemotherapy. As compared with FeBC, MaBC is more likely to have scores ≥31 (12.4% vs 7.4%) and also more likely to have scores <11 (33.8% vs 22.1%) [91]. The recurrence score is prognostic in both men and women [92].
Since over 90% of MaBC patients have hormone receptor positive disease, endocrine therapy is an important part of MaBC treatment. Tamoxifen has, so far, been the most widely used anti-estrogen therapy in both FeBC and MaBC. There are no prospective studies evaluating the efficacy of tamoxifen in MaBC. However, there are several retrospective studies showing improved OS with adjuvant tamoxifen use in early-stage MaBC, especially with node-positive disease [93, 94]. Compliance to tamoxifen in MaBC has only been investigated in a few studies. One study found 65% of men reported taking the tamoxifen after 1 year, 46% after the 2nd year, 29% after the 3rd year, 26% after the 4th year, and 18% after the 5th year [95]. The reduced compliance to tamoxifen may be secondary to the side effect profile, including hot flashes, sexual dysfunction, reduced libido, mood lability, and venous thromboembolism [96, 97]. Another commonly used endocrine therapy in postmenopausal women is an aromatase inhibitor (AI). In a matched cohort study comparing 5-year OS of FeBC and MaBC after AI, women have a significantly better OS than men (85% vs 73% p=0.028) [95]. In men, 80% of estrogen is produced by peripheral conversion of androgen via aromatase and the other 20% directly secreted by the testicles. AI work by inhibiting peripheral conversion of androgen to estrogen. However, AI as a monotherapy may not be as effective in MaBC, because the reduced serum estrogen level activates negative feedback loop to a functioning hypothalamus to secrete luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Therefore, the testicles secret additional testosterone as a substrate for estrogen production [98]. In addition, testicles secret estrogen that accounts for about 20% of the total estrogen level. This is supported by studies, showing that estradiol level is suppressed to 14.1 pg per milliliter after anastrozole in healthy men vs less than 1 pg per milliliter in postmenopausal women [99, 100]. Therefore, for AI to achieve its desired effect, it would require either surgical or chemical castration so that its use will not lead to increased testosterone production. A GnRH analog can be added to AI therapy to obliterate the secretion of LH and FSH in those who cannot tolerate tamoxifen. This has been recommended by several guidelines, including NCCN, and American Society of Clinical Oncology (ASCO) [101, 102]. However, the addition of GnRH to AI compared to AI alone in patients with MaBC showed only marginal improvement in progression-free survival (PFS) and OS in two retrospective studies (1st study: the median PFS was equal to 10.0 months, while the median OS was equal to 39.0 months and 2nd study: 1.6 months vs 6 months for PFS and 29.7 months vs 22 months for OS; both P=.05) [103, 104]. In the MALE trial, 56 hormone receptor-positive MaBC patients were randomized to receive tamoxifen, tamoxifen with GnRH, and AI with GnRH—no single arm received only AI. The study showed a consistent decrease in estradiol levels in the two combination arms, but no survival data were reported [105]. The coadministration of GnRH and AI is associated with significant sexual dysfunctions as it results in both the reduction of testosterone and estrogen. To summarize, there is consistent reduction of estradiol levels observed in both retrospective and prospective studies, but whether GnRH plus AI is superior to AI monotherapy still needs to be evaluated in prospective studies. Side effect profiles should be taken into consideration when prescribing GnRH in addition to AI.
Finally, in a very small subset of MaBC patients who are HER2 positive, mirroring treatment from FeBC is recommended with HER2-directed therapy, as there are no prospective studies that evaluate the efficacy of its use in MaBC.
1.8. Metastatic MaBC Treatment
Similar to treatment algorithm in FeBC, endocrine therapy has been proposed as a first-line treatment in hormone receptor-positive MaBC. Tamoxifen is a preferred first-line agent for MaBC. However, in patients whose disease progress while on tamoxifen treatment, AI in combination with GnRH analog should be used rather than AI alone, as discussed above. A retrospective study of 19 metastatic MaBC patients treated with AI and GNRH showed that 36.8% of patients achieved partial response, 36.8% achieved stable disease, 15.8% with progressive disease, and median PFS and OS of 12.5 months and 35.8 months, respectively [106]. Three out of 4 patients had improved response with the combination therapy, having previously been on AI monotherapy. Once the disease becomes refractory to both tamoxifen and AI with GnRH analog, fulvestrant can be considered, although its use in MaBC has not been well studied. According to one pooled analysis of 23 metastatic MaBC patients, 40% received it as first- or second-line treatment with the remaining as third line or beyond. The study concluded that 26.1% of patients achieved partial response and 47.8% of patients achieved stable disease, suggesting the potential role of fulvestrant in treating metastatic MaBC [107].
The use of CDK 4/6 inhibitors in combination with ET has doubled the PFS in women with HR+/HER2-metastatic disease, compared to those using ET alone [108]. A retrospective study investigating the use of CDK 4/6 inhibitor palbociclib (PAL) along with ET in metastatic MaBC between 2015 and 2017 showed that the mean duration of therapy is longer in the PAL group than in the non-PAL group. The maximum response rate (complete response and partial response) is higher in the PAL + ET group compared with the ET alone group (33.3% vs 12.5%) [109]. The use of real-world evidence, as conducted in this particular study, was very helpful, given that the trials that led to the approval of palbociclib for metastatic breast cancer excluded men.
The use of everolimus in combination with AI has also been demonstrated to improve median PFS, compared to administering AI alone (6.9 vs 2.8 months p < 0.001) in advanced FeBC that is HR+/HER2- [110]; however, in MaBC, its use has not been evaluated in prospective studies or large case series, but two case reports have described good response in men to the combination of everolimus with either exemestane or tamoxifen [111, 112].
Chemotherapy in advanced MaBC is primarily used in patients with hormone receptor-negative tumors, disease that becomes resistant to ET, or with visceral crisis that requires treatment with swift response [113]. One retrospective study evaluating 50 metastatic MaBC patients previously treated with ET compared anthracycline-based regimen and anthracycline-free regimen, and found no significant difference in PFS and OS [114]. In FeBC patients, it is concluded that single-agent chemotherapy has similar efficacy to multiagent chemotherapy with less toxicity [115]. There is one case series evaluating the use of single-agent eribulin in 23 MaBC. Patients received a median of 6 cycles, and nearly half of the patients achieved clinical responses [116].
A substantial percentage of both FeBC and MaBC express AR. Naturally, therapies targeting AR have been proposed as potential treatments. Although AR has an oncogenic role in prostate CA and AR antagonizing strategies with anti-androgenic drugs are effective, the role of AR in BC is unclear. Some clinical studies investigating anti-androgenic therapies, including two retrospective studies and 1 case report, have looked at CYP17A1 inhibitor cyproterone acetate with or without GnRH analog, and showed an overall 53% response rate. The two recent clinical trials show no significantly improved PFS in metastatic HR+/HER2− FeBC patients with the addition of enzalutamide to endocrine therapies [117–121]. More recently, there has been an increasing interest in AR agonist therapy after Hickey et al. undertook a large-scale study showing how AR can act as a tumor suppressor rather than driver in ER + BC, by opposing ER transcriptional activity [122]. A recent randomized phase 2 study examined the use of a nonsteroidal tissue-selective AR modulator—enobosarm—in heavily pretreated metastatic ER + FeBC, which displayed a clinical benefit rate at 24 weeks, and an objective response rate at approximately 30% in both 9 mg and 18 mg groups. This medication was well tolerated [123]. As MaBC is almost universally ER+/AR+, AR agonist therapy should be further investigated.
Given the frequency of BRCA alterations in MaBC, poly-ADP-ribose-polymerase (PARP) inhibitors can be a relevant treatment option. Its use has been studied in two phase III trials: OLYMPIAD and EMBRACA. In OLYMPIAD, a total of 295 patients (7 of which were MaBC patients with BRCA-mutated HER2-negative metastatic disease) were enrolled to receive either an oral PARP inhibitor olaparib or a physician's choice single-agent chemotherapy (TPC). There was no subgroup analysis for the MaBC patients, but PFS was significantly longer in the olaparib arm (7.0 vs 4.2 months p < 0.001), while olaparib had a better response rate (59.9% vs 28.8% p < 0.001) with a better toxicity profile than TPC [124]. In the EMBRACA trial, a total of 431 patients (9 of which were male patients with advanced breast cancer and germline BRCA mutations) were enrolled to receive either talazoparib or single-agent chemotherapy. PFS was significantly longer in the talazoparib arm (8.6 vs 5.6 months; p < 0.001), which also showed superior patient-reported outcome [125].
2. Conclusion
MaBC is a relatively rare disease with increasing incidence, yet it is understudied with most current clinical approaches extrapolated from data in FeBC. From the available data, we can conclude that MaBC has distinct molecular and clinicopathological features that may warrant different clinical approaches from FeBC. Various novel therapeutics, including PARP inhibitors and anti-androgen therapies that are undergoing investigations in FeBC, may also be successful in MaBC. We are already seeing a trend of clinical trials that now include MaBC patients to provide evidence base that will inform future treatment in MaBC. Hopefully, collective multinational effort will also facilitate the conduction of exclusively MaBC prospective trials in the near future.
Abbreviations
- AI:
Aromatase inhibitor
- GnRH:
Gonadotropin-releasing hormone
- PARP:
Poly-ADP-ribose-polymerase
- BCS:
Breast conserving surgery
- TCHP:
Docetaxel, carboplatin, trastuzumab, and pertuzumab
- AC-THP:
Adriamycin, cyclophosphamide, trastuzumab, and pertuzumab
- TDM1:
Trastuzumab.
Data Availability
The data can be obtained from the corresponding author upon request.
Conflicts of Interest
The authors declared that they have no conflicts of interest.
Authors' Contributions
Guoliang Zheng drafted and finalized the review and Dr. Jose Leone edited the review.
References
- 1.Centers for Disease Control and Prevention. Male Breast Cancer Incidence and Mortality, United States—2013–2017 No 19 . Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020. [Google Scholar]
- 2.Chen Z., Xu L., Shi W., et al. Trends of female and male breast cancer incidence at the global, regional, and national levels. Breast Cancer Research and Treatment . 2020;180(2):481–490. doi: 10.1007/s10549-020-05561-1. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Facts & Figures 2020 . Atlanta, Ga: American Cancer Society; 2020. [Google Scholar]
- 4.Howlader N. N. A., Cronin K. A., editors. SEER Cancer Statistics Review . Bethesda, MD: National Cancer Institute; 2017. [Google Scholar]
- 5.Agrawal A., Ayantunde A. A., Rampaul R., Robertson J. F. R. Male breast cancer: a review of clinical management. Breast Cancer Research and Treatment . 2007;103(1):11–21. doi: 10.1007/s10549-006-9356-z. [DOI] [PubMed] [Google Scholar]
- 6.O’Malley C. D., Prehn A. W., Shema S. J., Glaser S. L. Racial/ethnic differences in survival rates in a population‐based series of men with breast carcinoma. Cancer . 2002;94(11):2836–2843. doi: 10.1002/cncr.10521. [DOI] [PubMed] [Google Scholar]
- 7.Konduri S., Singh M., Bobustuc G., Rovin R., Kassam A. Epidemiology of male breast cancer. Breast (Edinburgh, Scotland) . 2020;54:8–14. doi: 10.1016/j.breast.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferzoco R. M., Ruddy K. J. The epidemiology of male breast cancer. Current Oncology Reports . 2016;18 doi: 10.1007/s11912-015-0487-4. [DOI] [PubMed] [Google Scholar]
- 9.Rosenblatt K. A., Thomas D. B., McTiernan A., et al. Breast cancer in men: aspects of familial aggregation. Journal of the National Cancer Institute . 1991;83(12):849–854. doi: 10.1093/jnci/83.12.849. [DOI] [PubMed] [Google Scholar]
- 10.Boyd J., Rhei E., Federici M. G., et al. Male breast cancer in the hereditary nonpolyposis colorectal cancer syndrome. Breast Cancer Research and Treatment . 1999;53(1):87–91. doi: 10.1023/a:1006030116357. [DOI] [PubMed] [Google Scholar]
- 11.Fackenthal J. D., Marsh D. J., Richardson A. L., et al. Male breast cancer in Cowden syndrome patients with germlinePTEN mutations. Journal of Medical Genetics . 2001;38(3):159–164. doi: 10.1136/jmg.38.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hultborn R., Hanson C., Kopf I., Verbiene I., Warnhammar E., Weimarck A. Prevalence of Klinefelter’s syndrome in male breast cancer patients. Anticancer Research . 1997;17(6D):4293–4297. [PubMed] [Google Scholar]
- 13.Brinton L. A. Prediagnostic sex steroid hormones in relation to male breast cancer risk. Journal of Clinical Oncology . 2015;33(18):p. 2041. doi: 10.1200/JCO.2014.59.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson K. C., Pan S., Mao Y. Risk factors for male breast cancer in Canada, 1994–1998. European Journal of Cancer Prevention The Official Journal of the European Cancer Prevention Organisation (ECP) . 2002;11(3):253–263. doi: 10.1097/00008469-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Guénel P., Cyr D., Sabroe S., et al. Alcohol drinking may increase risk of breast cancer in men: a European population-based case–control study. Cancer Causes & Control Cancer Causes & Control . 2004;15(6):571–580. doi: 10.1023/B:CACO.0000036154.18162.43. [DOI] [PubMed] [Google Scholar]
- 16.Sørensen H. T., Friis S., Olsen J. H., et al. Risk of breast cancer in men with liver cirrhosis. American Journal of Gastroenterology . 1998;93(2):231–233. doi: 10.1111/j.1572-0241.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- 17.Thomas D. B., Jimenez L. M., McTiernan A., et al. Breast cancer in men: risk factors with hormonal implications. American Journal of Epidemiology . 1992;135(7):734–748. doi: 10.1093/oxfordjournals.aje.a116360. [DOI] [PubMed] [Google Scholar]
- 18.Olsson H., Bladstrom A., Alm P. Male gynecomastia and risk for malignant tumours–a cohort study. BMC Cancer . 2002;2(1):26–6. doi: 10.1186/1471-2407-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambrogetti D., Ciatto S., Catarzi S., Muraca M. G. The combined diagnosis of male breast lesions: a review of a series of 748 consecutive cases. La Radiologia Medica . 1996;91(4):356–359. [PubMed] [Google Scholar]
- 20.O’Hanlon D. M., Kent P., Kerin M. J., Given H. F. Unilateral breast masses in men over 40: a diagnostic dilemma. The American Journal of Surgery . 1995;170(1):24–26. doi: 10.1016/s0002-9610(99)80246-3. [DOI] [PubMed] [Google Scholar]
- 21.Cocco P., Figgs L., Dosemeci M., Hayes R., Linet M. S., Hsing A. W. Case-control study of occupational exposures and male breast cancer. Occupational and Environmental Medicine . 1998;55(9):599–604. doi: 10.1136/oem.55.9.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen J.. Elevated risk for male breast cancer after occupational exposure to gasoline and vehicular combustion products. American Journal of Industrial Medicine . 2000;37(4):349–352. doi: 10.1002/(sici)1097-0274(200004)37:4<349::aid-ajim4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Sun J.-W., Li X. R., Gao H. Y., et al. Electromagnetic field exposure and male breast cancer risk: a meta-analysis of 18 studies. Asian Pacific Journal of Cancer Prevention Asian Pacific Journal of Cancer Prevention . 2013;14(1):523–528. doi: 10.7314/apjcp.2013.14.1.523. [DOI] [PubMed] [Google Scholar]
- 24.Antoniou A., Pharoah P. D. P., Narod S., et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. The American Journal of Human Genetics . 2003;72(5):1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman L. S., Gayther S. A., Kurosaki T., et al. Mutation analysis of BRCA1 and BRCA2 in a male breast cancer population. The American Journal of Human Genetics . 1997;60(2):313–319. [PMC free article] [PubMed] [Google Scholar]
- 26.Couch F. J., Farid L. M., DeShano M. L., et al. BRCA2 germline mutations in male breast cancer cases and breast cancer families. Nature Genetics . 1996;13(1):123–125. doi: 10.1038/ng0596-123. [DOI] [PubMed] [Google Scholar]
- 27.Mavraki E., Gray I. C., Bishop D. T., Spurr N. K. Germline BRCA2 mutations in men with breast cancer. British Journal of Cancer . 1997;76(11):1428–1431. doi: 10.1038/bjc.1997.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haraldsson K., Loman N., Zhang Q. X., Johannsson O., Olsson H., Borg A. BRCA2 germ-line mutations are frequent in male breast cancer patients without a family history of the disease. Cancer Research . 1998;58(7):1367–1371. [PubMed] [Google Scholar]
- 29.Csokay B., Udvarhelyi N., Sulyok Z., et al. High frequency of germ-line BRCA2 mutations among Hungarian male breast cancer patients without family history. Cancer Research . 1999;59(5):995–998. [PubMed] [Google Scholar]
- 30.Wolpert N., Warner E., Seminsky M. F., Futreal A., Narod S. A. Prevalence of BRCA1 and BRCA2 mutations in male breast cancer patients in Canada. Clinical Breast Cancer . 2000;1(1):57–63. doi: 10.3816/CBC.2000.n.005. [DOI] [PubMed] [Google Scholar]
- 31.Diez O., Cortes J., Domenech M., et al. BRCA2 germ-line mutations in Spanish male breast cancer patients. Annals of Oncology Official Journal of the European Society for Medical Oncology . 2000;11:81–84. doi: 10.1023/a:1008339009528. [DOI] [PubMed] [Google Scholar]
- 32.Pages S., Caux V., Stoppa-Lyonnet D., Tosi M. Screening of male breast cancer and of breast-ovarian cancer families for BRCA2 mutations using large bifluorescent amplicons. British Journal of Cancer . 2001;84:482–488. doi: 10.1054/bjoc.2000.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwiatkowska E., Teresiak M., Lamperska K. M., et al. BRCA2 germline mutations in male breast cancer patients in the Polish population. Human Mutation . 2001;17:p. 73. doi: 10.1002/1098-1004(2001)17:1<73::AID-HUMU12>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 34.Basham V. M. BRCA1 and BRCA2 mutations in a population-based study of male breast cancer. Breast Cancer Research . 2001;4:1–5. doi: 10.1186/bcr419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Syrjäkoski K., Kuukasjarvi T., Waltering K., et al. BRCA2 mutations in 154 Finnish male breast cancer patients. Neoplasia . 2004;6:541–545. doi: 10.1593/neo.04193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Risch H. A., McLaughlin J. R., Cole D. E. C., et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin–cohort study in Ontario, Canada. Journal of the National Cancer Institute . 2006;98:1694–1706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 37.Tai Yu C., Domchek S., Parmigiani G., Chen S. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. Journal of the National Cancer Institute . 2007;99:1811–1814. doi: 10.1093/jnci/djm203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ottini L, Rizzolo P., Zanna I., et al. BRCA1/BRCA2 mutation status and clinical-pathologic features of 108 male breast cancer cases from Tuscany: a population-based study in central Italy. Breast Cancer Research and Treatment . 2009;116:577–586. doi: 10.1007/s10549-008-0194-z. [DOI] [PubMed] [Google Scholar]
- 39.Ding Y. C., Steele L., Kuan C. J., Greilac S., Neuhausen S. L. Mutations in BRCA2 and PALB2 in male breast cancer cases from the United States. Breast Cancer Research and Treatment . 2011;126:771–778. doi: 10.1007/s10549-010-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thorlacius S., Sigurdsson S., Bjarnadottir H., et al. Study of a single BRCA2 mutation with high carrier frequency in a small population. The American Journal of Human Genetics . 1997;60:1079–1084. [PMC free article] [PubMed] [Google Scholar]
- 41.Evans D. G. R., Bulman M., Young K., et al. BRCA1/2 mutation analysis in male breast cancer families from North West England. Familial Cancer . 2008;7:113–117. doi: 10.1007/s10689-007-9153-9. [DOI] [PubMed] [Google Scholar]
- 42.Pritzlaff M., Summerour P., McFarland R., et al. Male breast cancer in a multi-gene panel testing cohort: insights and unexpected results. Breast Cancer Research and Treatment . 2017;161:575–586. doi: 10.1007/s10549-016-4085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Postula K. J. V. Abstract PD7-11: the role of multi-gene hereditary cancer panels in male patients with breast cancer. Cancer Research . 2018;78(4_Supplement) [Google Scholar]
- 44.CHEK2-Breast Cancer Consortium. Low-penetrance susceptibility to breast cancer due to CHEK2∗1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nature Genetics . 2002;31:55–59. doi: 10.1038/ng879. [DOI] [PubMed] [Google Scholar]
- 45.Syrjäkoski K., Kuukasjarvi T., Auvinen A., Kallioniemi O. P. CHEK2 1100delC is not a risk factor for male breast cancer population. International Journal of Cancer . 2004;108:475–476. doi: 10.1002/ijc.11384. [DOI] [PubMed] [Google Scholar]
- 46.Neuhausen S., Dunning A., Steele L., et al. Role of CHEK2∗ 1100delC in unselected series of non-BRCA1/2 male breast cancers. International Journal of Cancer . 2004;108:477–478. doi: 10.1002/ijc.11385. [DOI] [PubMed] [Google Scholar]
- 47.Offit K., Pierce H., Kirchhoff T., et al. Frequency of CHEK2∗1100delC in New York breast cancer cases and controls. BMC Medical Genetics . 2003;4:p. 1. doi: 10.1186/1471-2350-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casadei S., Norquist B. M., Walsh T., et al. Contribution of inherited mutations in the BRCA2-interacting protein PALB2 to familial breast cancer. Cancer Research . 2011;71:2222–2229. doi: 10.1158/0008-5472.CAN-10-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rahman N., Seal S., Thompson D., et al. PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nature Genetics . 2007;39:165–167. doi: 10.1038/ng1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Antoniou A. C., Casadei S., Heikkinen T., et al. Breast-cancer risk in families with mutations in PALB2. New England Journal of Medicine . 2014;371:497–506. doi: 10.1056/NEJMoa1400382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nahleh Z., Girnius S. Male breast cancer: a gender issue. Nature Clinical Practice Oncology . 2006;3:428–437. doi: 10.1038/ncponc0564. [DOI] [PubMed] [Google Scholar]
- 52.Gudmundsdottir K., Thorlacius S., Jonasson J. G., Sigfusson B. F., Tryggvadottir L., Eyfjord J. E. CYP17 promoter polymorphism and breast cancer risk in males and females in relation to BRCA2 status. British Journal of Cancer . 2003;88:933–936. doi: 10.1038/sj.bjc.6600839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orr N., Lemnrau A., Cooke R., et al. Genome-wide association study identifies a common variant in RAD51B associated with male breast cancer risk. Nature Genetics . 2012;44:1182–1184. doi: 10.1038/ng.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology: Genetic/familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. 2020. http://www.nccn.org . [DOI] [PubMed]
- 55.Donegan W. L., Redlich P. N. Breast cancer in men. Surgical Clinics of North America . 1996;76:343–363. doi: 10.1016/s0039-6109(05)70443-6. [DOI] [PubMed] [Google Scholar]
- 56.Treves N., Arthur I. Cancer of the male breast; a report of 146 cases. Cancer . 1955;8:1239–1250. doi: 10.1002/1097-0142(1955)8:6<1239::aid-cncr2820080622>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 57.Stierer M., Rosen H., Weitensfelder W., et al. Male breast cancer: Austrian experience. World Journal of Surgery . 1995;19:687–692. doi: 10.1007/BF00295904. [DOI] [PubMed] [Google Scholar]
- 58.Joshi M. G., Lee A. K., Loda M., et al. Male breast carcinoma: an evaluation of prognostic factors contributing to a poorer outcome. Cancer . 1996;77:490–498. doi: 10.1002/(SICI)1097-0142(19960201)77:3<490::AID-CNCR10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 59.Cutuli B., Lacroze M., Dilhuydy J. M., et al. Male breast cancer: results of the treatments and prognostic factors in 397 cases. European Journal of Cancer . 1995;31A:1960–1964. doi: 10.1016/0959-8049(95)00366-5. [DOI] [PubMed] [Google Scholar]
- 60.Cardoso F., Bartlett J. M. S., Slaets L., et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG international male breast cancer Program. Annals of Oncology Official Journal of the European Society for Medical Oncology . 2018;29:405–417. doi: 10.1093/annonc/mdx651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Expert panel on breast imaging:, niell BL, lourenco AP, moy L, baron P, didwania AD, ACR appropriateness Criteria® evaluation of the symptomatic male breast. Journal of the American College of Radiology . 2018;15:S313–S320. doi: 10.1016/j.jacr.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 62.Yen P. P. W. Benign and malignant male breast diseases: radiologic and pathologic correlation. Canadian Association of Radiologists Journal . 2015;66:198–207. doi: 10.1016/j.carj.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Woods R. W., Salkowski L. R., Elezaby M., Burnside E. S., Strigel R. M., Fowler A. M. Image-based screening for men at high risk for breast cancer: benefits and drawbacks. Clinical Imaging . 2020;60:84–89. doi: 10.1016/j.clinimag.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ottini L., Palli D., Rizzo S., Federico M., Bazan V., Russo A. Male breast cancer. Critical Reviews In Oncology-Hematology . 2010;73:141–155. doi: 10.1016/j.critrevonc.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 65.Doebar S. C., Slaets L., Cardoso F., et al. Male breast cancer precursor lesions: analysis of the EORTC 10085/TBCRC/BIG/NABCG international male breast cancer Program. Modern Pathology An Official Journal of the United States and Canadian Academy of Pathology, Inc . 2017;30:509–518. doi: 10.1038/modpathol.2016.229. [DOI] [PubMed] [Google Scholar]
- 66.Doebar S. C., van den Broek E. C., Koppert L. B., et al. Extent of ductal carcinoma in situ according to breast cancer subtypes: a population-based cohort study. Breast Cancer Research and Treatment . 2016;158:179–187. doi: 10.1007/s10549-016-3862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murphy C. E., Carder P. J., Lansdown M. R. J., Speirs V. Steroid hormone receptor expression in male breast cancer. European Journal of Surgical Oncology The Journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology . 2006;32:44–47. doi: 10.1016/j.ejso.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 68.Hotko Y. The Significance of Hormone Receptors in Male Breast Cancer. 2020. https://www.researchgate.net/publication/345426182_The_Significance_Of_Hormone_Receptors_In_Male_Breast_Cancer .
- 69.Wang F., Shu X., Meszoely I., et al. Overall mortality after diagnosis of breast cancer in men vs women. JAMA Oncology . 2019;5:1589–1596. doi: 10.1001/jamaoncol.2019.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leone J. P., Zwenger A. O., Iturbe J., Leone B. A., Vallejo C. T., Bhargava R. Prognostic factors in male breast cancer: a population-based study. Breast Cancer Research and Treatment . 2016;156:539–548. doi: 10.1007/s10549-016-3768-1. [DOI] [PubMed] [Google Scholar]
- 71.Leone J. P., Zwenger A. O., Iturbe J., Vallejo C. T., Leone B. A. Prognostic significance of tumor subtypes in male breast cancer: a population-based study. Breast Cancer Research and Treatment . 2015;152:601–609. doi: 10.1007/s10549-015-3488-y. [DOI] [PubMed] [Google Scholar]
- 72.Leone J., Zwenger A. O., Leone B. A., Vallejo C. T. Overall survival of men and women with breast cancer according to tumor subtype: a population-based study. American Journal of Clinical Oncology . 2019;42:215–220. doi: 10.1097/COC.0000000000000497. [DOI] [PubMed] [Google Scholar]
- 73.Fields E. C., DeWitt P., Fisher C. M., Rabinovitch R. Management of male breast cancer in the United States: a surveillance, epidemiology and end results analysis. International Journal of Radiation Oncology, Biology, Physics . 2013;87:747–752. doi: 10.1016/j.ijrobp.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 74.Leone J. P., Zwenger A. O., Iturbe J., Leone B. A., Vallejo C. T. Locoregional treatment and overall survival of men with T1a, b, cN0M0 breast cancer: a population-based study. European Journal of Cancer . 2017;71:7–14. doi: 10.1016/j.ejca.2016.10.038. [DOI] [PubMed] [Google Scholar]
- 75.Cloyd J. M., Hernandez-Boussard T., Wapnir I. L. Outcomes of partial mastectomy in male breast cancer patients: analysis of SEER, 1983–2009. Annals of Surgical Oncology . 2013;20:1545–1550. doi: 10.1245/s10434-013-2918-5. [DOI] [PubMed] [Google Scholar]
- 76.Yadav S., Karam D., Bin Riaz I., et al. Male breast cancer in the United States: treatment patterns and prognostic factors in the 21st century. Cancer . 2020;126:26–36. doi: 10.1002/cncr.32472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zaenger D., Rabatic B. M., Dasher B., Mourad W. F. Is breast conserving therapy a safe modality for early-stage male breast cancer? Clinical Breast Cancer . 2016;16(2):101–104. doi: 10.1016/j.clbc.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 78.Giordano S. H. Breast cancer in men. New England Journal of Medicine . 2018;378:2311–2320. doi: 10.1056/NEJMra1707939. [DOI] [PubMed] [Google Scholar]
- 79.Boughey J. C., Bedrosian I., Meric-Bernstam F., et al. Comparative analysis of sentinel lymph node operation in male and female breast cancer patients. Journal of the American College of Surgeons . 2006;203:475–480. doi: 10.1016/j.jamcollsurg.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 80.Flynn L. W., Park J., Patil S. M., Cody H. S., Port E. R. Sentinel lymph node biopsy is successful and accurate in male breast carcinoma. Journal of the American College of Surgeons . 2008;206:616–621. doi: 10.1016/j.jamcollsurg.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 81.Cimmino V. M., Degnim A. C., Sabel M. S., Diehl K. M., Newman L. A., Chang A. E. Efficacy of sentinel lymph node biopsy in male breast cancer. Journal of Surgical Oncology . 2004;86(2):74–77. doi: 10.1002/jso.20045. [DOI] [PubMed] [Google Scholar]
- 82.Gentilini O., Chagas E., Zurrida S., et al. Sentinel lymph node biopsy in male patients with early breast cancer. The Oncologist . 2007;12:512–515. doi: 10.1634/theoncologist.12-5-512. [DOI] [PubMed] [Google Scholar]
- 83.Recht A., Comen E. A., Fine R. E., et al. Postmastectomy radiotherapy: an american society of clinical oncology, american society for radiation oncology, and society of surgical oncology focused guideline update. Practical radiation oncology . 2016;6:e219–e234. doi: 10.1016/j.prro.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 84.Abrams M. J., Koffer P. P., Wazer D. E., Hepel J. T. Postmastectomy radiation therapy is associated with improved survival in node-positive male breast cancer: a population analysis. International Journal of Radiation Oncology, Biology, Physics . 2017;98(2):384–391. doi: 10.1016/j.ijrobp.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 85.Jardel P., Vignot S., Cutuli B., et al. Should adjuvant radiation therapy be systematically proposed for male breast cancer? A systematic review. Anticancer Research . 2018;38:23–31. doi: 10.21873/anticanres.12187. [DOI] [PubMed] [Google Scholar]
- 86.Macdonald G., Paltiel C., Olivotto I. A., Tyldesley S. A comparative analysis of radiotherapy use and patient outcome in males and females with breast cancer. Annals of Oncology Official Journal of the European Society for Medical Oncology . 2005;16:1442–1448. doi: 10.1093/annonc/mdi274. [DOI] [PubMed] [Google Scholar]
- 87.Coles C. E., Griffin C. L., Kirby A. M., et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet (London, England) . 2017;390:1048–1060. doi: 10.1016/S0140-6736(17)31145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strnad V., Ott O. J., Hildebrandt G., et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet (London, England) . 2016;387:229–238. doi: 10.1016/S0140-6736(15)00471-7. [DOI] [PubMed] [Google Scholar]
- 89.Walshe J. M., Berman A. W., Vatas U., et al. A prospective study of adjuvant CMF in males with node positive breast cancer: 20-year follow-up. Breast Cancer Research and Treatment . 2007;103:177–183. doi: 10.1007/s10549-006-9363-0. [DOI] [PubMed] [Google Scholar]
- 90.Patel H. Z., Buzdar A. U., Hortobagyi G. N. Role of adjuvant chemotherapy in male breast cancer. Cancer . 1989;64:1583–1585. doi: 10.1002/1097-0142(19891015)64:8<1583::aid-cncr2820640804>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 91.Massarweh S. A. Molecular characterization and mortality from breast cancer in men. Journal of Clinical Oncology . 2018;36:p. 1396. doi: 10.1200/JCO.2017.76.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang F., Reid S., Zheng W., et al. Sex disparity observed for oncotype DX breast recurrence score in predicting mortality among patients with early stage ER-positive breast cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research . 2020;26:101–109. doi: 10.1158/1078-0432.CCR-19-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Giordano S. H., Perkins G. H., Broglio K., et al. Adjuvant systemic therapy for male breast carcinoma. Cancer . 2005;104:2359–2364. doi: 10.1002/cncr.21526. [DOI] [PubMed] [Google Scholar]
- 94.Eggemann H., Ignatov A., Smith B. J., et al. Adjuvant therapy with tamoxifen compared to aromatase inhibitors for 257 male breast cancer patients. Breast Cancer Research and Treatment . 2013;137:465–470. doi: 10.1007/s10549-012-2355-3. [DOI] [PubMed] [Google Scholar]
- 95.Xu S., Yang Y., Tao W., et al. Tamoxifen adherence and its relationship to mortality in 116 men with breast cancer. Breast Cancer Research and Treatment . 2012;136:495–502. doi: 10.1007/s10549-012-2286-z. [DOI] [PubMed] [Google Scholar]
- 96.Pemmaraju N., Munsell M. F., Hortobagyi G. N., Giordano S. H. Retrospective review of male breast cancer patients: analysis of tamoxifen-related side-effects. Annals of Oncology Official Journal of the European Society for Medical Oncology . 2012;23:1471–1474. doi: 10.1093/annonc/mdr459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fisher B., Anderson S., Bryant J., et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. New England Journal of Medicine . 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 98.Doyen J., Italiano A., Largillier R., Ferrero J. M., Fontana X., Thyss A. Aromatase inhibition in male breast cancer patients: biological and clinical implications. Annals of Oncology Official Journal of the European Society for Medical Oncology . 2010;21:1243–1245. doi: 10.1093/annonc/mdp450. [DOI] [PubMed] [Google Scholar]
- 99.Hayes F. J., Seminara S. B., Decruz S., Boepple P. A., Crowley W. F. Aromatase inhibition in the human male reveals a hypothalamic site of estrogen feedback. Journal of Clinical Endocrinology & Metabolism . 2000;85:3027–3035. doi: 10.1210/jcem.85.9.6795. [DOI] [PubMed] [Google Scholar]
- 100.Mauras N., O’Brien K. O., Klein K. O., Hayes V. Estrogen suppression in males: metabolic effects. Journal of Clinical Endocrinology & Metabolism . 2000;85:2370–2377. doi: 10.1210/jcem.85.7.6676. [DOI] [PubMed] [Google Scholar]
- 101.Gradishar W. J., Anderson B. O., Abraham J., et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network: Journal of the National Comprehensive Cancer Network . 2020;18(4):452–478. doi: 10.6004/jnccn.2020.0016. [DOI] [PubMed] [Google Scholar]
- 102.Hassett M. J., Somerfield M. R., Baker E. R., et al. Management of male breast cancer: ASCO guideline. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology . 2020;38(16):1849–1863. doi: 10.1200/JCO.19.03120. [DOI] [PubMed] [Google Scholar]
- 103.Zagouri F., Sergentanis T. N., Azim H. A., Chrysikos D., Dimopoulos M. A., Psaltopoulou T. Aromatase inhibitors in male breast cancer: a pooled analysis. Breast Cancer Research and Treatment . 2015;151(1):141–147. doi: 10.1007/s10549-015-3356-9. [DOI] [PubMed] [Google Scholar]
- 104.Di Lauro L., Pizzuti L., Barba M., et al. Role of gonadotropin-releasing hormone analogues in metastatic male breast cancer: results from a pooled analysis. Journal of Hematology & Oncology . 2015;8(1):53–55. doi: 10.1186/s13045-015-0147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reinisch M., Seiler S., Hauzenberger T., et al. Efficacy of endocrine therapy for the treatment of breast cancer in men: results from the MALE phase 2 randomized clinical trial. JAMA Oncology . 2021;7:565–572. doi: 10.1001/jamaoncol.2020.7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Di Lauro, Vici P., Luigi, et al. Letrozole combined with gonadotropin-releasing hormone analog for metastatic male breast cancer. Breast Cancer Research and Treatment . 2013;141:119–123. doi: 10.1007/s10549-013-2675-y. [DOI] [PubMed] [Google Scholar]
- 107.Zagouri F., Sergentanis T. N., Chrysikos D., Dimopoulos M. A., Psaltopoulou T. Fulvestrant and male breast cancer: a pooled analysis. Breast Cancer Research and Treatment . 2015;149:269–275. doi: 10.1007/s10549-014-3240-z. [DOI] [PubMed] [Google Scholar]
- 108.Turner N. C., Ro J., Andre F., et al. Palbociclib in hormone-receptor–positive advanced breast cancer. New England Journal of Medicine . 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 109.Bartlett C. H. Real-world evidence of male breast cancer (BC) patients treated with palbociclib (PAL) in combination with endocrine therapy (ET) 2019. p. p. 1055.
- 110.Baselga J., Campone M., Piccart M., et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. New England Journal of Medicine . 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ballatore Z., Pistelli M., Battelli N., et al. Everolimus and exemestane in long survival hormone receptor positive male breast cancer: case report. BMC Research Notes . 2016;9:p. 497. doi: 10.1186/s13104-016-2301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kattan J., Kourie H. R., Hampig Raphael Kourie The use of everolimus to reverse tamoxifen resistance in men with metastatic breast cancer: a case report. Investigational New Drugs . 2014;32:1046–1047. doi: 10.1007/s10637-014-0133-2. [DOI] [PubMed] [Google Scholar]
- 113.Leon-Ferre, Giridhar K. V., Roberto A., et al. A contemporary review of male breast cancer: current evidence and unanswered questions. Cancer metastasis reviews . 2018;37:599–614. doi: 10.1007/s10555-018-9761-x. [DOI] [PubMed] [Google Scholar]
- 114.Di Lauro, Pizzuti L., Luigi, et al. Efficacy of chemotherapy in metastatic male breast cancer patients: a retrospective study. Journal of Experimental & Clinical Cancer Research Climate Research . 2015;34:1–5. doi: 10.1186/s13046-015-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dear R. F. Combination versus sequential single agent chemotherapy for metastatic breast cancer. Cochrane Database of Systematic Reviews . 2013;12 doi: 10.1002/14651858.CD008792.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Giotta F., Acito L., Candeloro G., et al. Eribulin in male patients with breast cancer: the first report of clinical outcomes. The Oncologist . 2016;21:1298–1305. doi: 10.1634/theoncologist.2016-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Labrie F., Dupont A., Belanger A., et al. Complete response to combination therapy with an LHRH agonist and flutamide in metastatic male breast cancer: a case report. Clinical and Investigative medicine. Medecine Clinique et Experimentale . 1990;13:275–278. [PubMed] [Google Scholar]
- 118.Di Lauro, Vici P., Luigi, et al. Antiandrogen therapy in metastatic male breast cancer: results from an updated analysis in an expanded case series. Breast Cancer Research and Treatment . 2014;148:73–80. doi: 10.1007/s10549-014-3138-9. [DOI] [PubMed] [Google Scholar]
- 119.Abdel Azim, Kassem L., Hamdy, et al. Durable response of androgen receptor-positive male breast cancer to goserelin. Journal of breast cancer . 2019;22:141–148. doi: 10.4048/jbc.2019.22.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Krop I., Abramson V., Colleoni M., et al. A randomized placebo controlled phase II trial evaluating exemestane with or without enzalutamide in patients with hormone receptor–positive breast cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research . 2020;26(23):6149–6157. doi: 10.1158/1078-0432.CCR-20-1693. [DOI] [PubMed] [Google Scholar]
- 121.Schwartzberg L. S., Yardley D. A., Elias A. D., et al. A phase I/Ib study of enzalutamide alone and in combination with endocrine therapies in women with advanced breast cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research . 2017;23(15):4046–4054. doi: 10.1158/1078-0432.CCR-16-2339. [DOI] [PubMed] [Google Scholar]
- 122.Hickey T. E., Selth L. A., Chia K. M., et al. The androgen receptor is a tumor suppressor in estrogen receptor–positive breast cancer. Nature Medicine . 2021;27(2):310–320. doi: 10.1038/s41591-020-01168-7. [DOI] [PubMed] [Google Scholar]
- 123.Palmieri C., Linden H., Birrell S., et al. Cancer Research . Vol. 81. Philadelphia, PA, USA: American Association Cancer Research; 2021. Efficacy and safety of enobosarm, a selective androgen receptor modulator, to target AR in women with advanced ER plus/AR plus breast cancer-final results from an international Phase 2 randomized study. [Google Scholar]
- 124.Robson M. E. OlympiAD: phase III trial of olaparib monotherapy versus chemotherapy for patients (pts) with HER2-negative metastatic breast cancer (mBC) and a germline BRCA mutation (gBRCAm) Journal of Clinical Oncology . 2017;35 [Google Scholar]
- 125.Litton J. K., Rugo H. S., Ettl J., et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. New England Journal of Medicine . 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data can be obtained from the corresponding author upon request.


