ABSTRACT
Time-restricted eating (TRE) is a popular dietary strategy that emphasizes the timing of meals in alignment with diurnal circadian rhythms, permitting ad libitum energy intake during a restricted (∼8–10 h) eating window each day. Unlike energy-restricted diets or intermittent fasting interventions that focus on weight loss, many of the health-related benefits of TRE are independent of reductions in body weight. However, TRE research to date has largely ignored what food is consumed (i.e., macronutrient composition and energy density), overlooking a plethora of past epidemiological and interventional dietary research. To determine some of the potential mechanisms underpinning the benefits of TRE on metabolic health, future studies need to increase the rigor of dietary data collected, assessed, and reported to ensure a consistent and standardized approach in TRE research. This Perspective article provides an overview of studies investigating TRE interventions in humans and considers dietary intake (both what and when food is eaten) and their impact on selected health outcomes (i.e., weight loss, glycemic control). Integrating existing dietary knowledge about what food is eaten with our recent understanding on when food should be consumed is essential to optimize the impact of dietary strategies aimed at improving metabolic health outcomes.
Keywords: diet, nutrition, timing, energy intake, fasting
Statement of Significance: Time-restricted eating (TRE) is a dietary strategy that focuses on the timing of meals, but frequently neglects the quality and quantity of food consumed. This Perspective challenges researchers in the field of TRE to incorporate rigorous dietary assessment to unravel the complex relations between the type of food consumed and the timing of meals.
Introduction
Dietary advice for improving metabolic health in individuals with noncommunicable diseases such as obesity and type 2 diabetes (T2D) has traditionally focused on what food is consumed, with an emphasis on dietary quality and energy intake (EI). Decades of research from nutrition scientists have provided robust evidence of the metabolic responses to diets of differing macronutrient profiles (i.e., Mediterranean, low-carbohydrate, high-fat, high-protein) as well reduced EIs (i.e., very-low-energy diets). Recently, there has been growing recognition that the timing of meals is critical for metabolic health and well-being, and that manipulating the feeding–fasting cycle carries important consequences for a number of physiological and metabolic processes (1–5). Time-restricted eating (TRE), often called the 16:8 diet, is a popular dietary strategy placing emphasis on the timing of food but permits ad libitum EI during a restricted (∼8–10 h) eating window each day. Several recent reviews have highlighted the potential for TRE to induce improvements in body weight (i.e., reduce obesity) and other cardiometabolic health markers (6–9). Many of the benefits of TRE on metabolic health are independent of weight loss, but instead are underpinned by the timing of meals in alignment with circadian rhythms. To date, many of the interventional studies of TRE have largely ignored what food is consumed and its quality and quantity (i.e., macronutrient composition and energy density), with a sole focus on when food is consumed. This Perspective article provides an overview of studies investigating TRE in humans highlighting both what and when food is consumed. Our intent is to incorporate the decades of dietary intake research of what is eaten (i.e., the premise of dietetics as a profession) into future TRE investigations. Integrating dietary composition and quality with timing is key to unravel the complex relations between the types of foods consumed and the timing of meals to determine their unique roles underpinning improvements in metabolic health. Before providing an analysis of the dietary components of TRE studies to date, we provide important working definitions and a brief background of the evolution of TRE interventions.
Current Dietary Strategies for Improving Metabolic Health
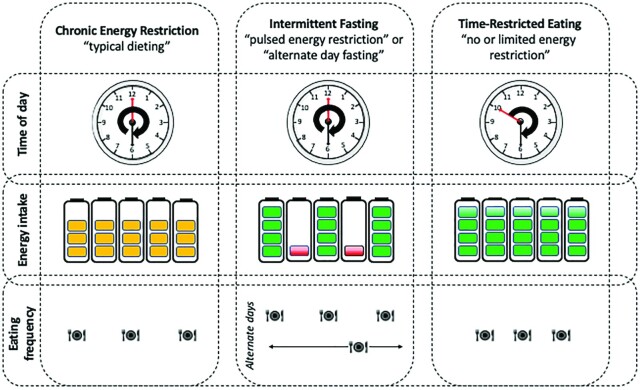
The majority of evidence-based dietary interventions prescribed to improve metabolic health and/or weight loss can be broadly classified as follows: 1) chronic energy restriction (CER), in which daily EI is reduced by up to 40%, but meal frequency and timing remain unchanged; 2) intermittent fasting (IF), where 1 day or several days of fasting are interspersed with normal ad libitum eating patterns, and meal frequency and timing remaining unchanged on the days of food intake (e.g., alternate-day fasting and the 5:2 diet); or 3) TRE, in which food is consumed ad libitum but the eating duration (i.e., the time between the first and last EI of the day) is typically reduced from a 12–16-h “eating window” to <8–12 h (7) (Figure 1).
FIGURE 1.
Categorization of popular diet practices. For CER (1), during which daily energy intake is reduced by up to 40%, but meal frequency and timing remain unchanged; IF (2), where 1 d or several days of fasting are interspersed with normal ad libitum eating patterns, such that total weekly energy intake is reduced, and meal frequency and timing remain unchanged on the days of food intake; or TRE (3), in which food is consumed ad libitum throughout a set time period, and energy intake may or may not be reduced. In TRE, the daily eating duration (i.e., the time between the first and last energy intake) is typically reduced from a 12–14-h/d “eating window” to ∼8-10 h/d. CER, chronic energy restriction; IF, intermittent fasting; TRE, time-restricted eating.
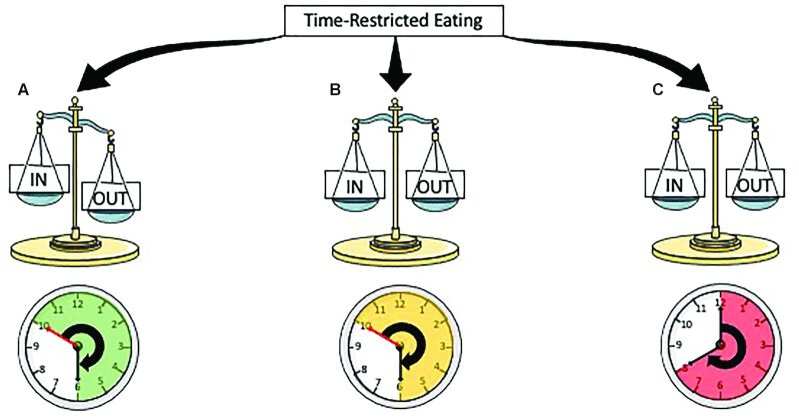
Importantly, we and others (10, 11) regard TRE to be a distinct dietary intervention rather than a modified form of IF. Specifically, TRE interventions do not intend to reduce EI, in contrast with all IF regimes. Furthermore, CER and IF are not chrono-nutritive therapies per se, in that they do not restrict food consumption to specified times of day to play off chronobiology. Instead, their therapeutic value and any positive health outcomes are mainly derived from chronic or intermittent periods of energy restriction. TRE is a chrono-nutritional strategy offering a less food-focused approach, where the timing of meals is closely aligned with typical metabolite and hormonal profiles over 24-h periods, in an ∼8–10-h eating window. A requirement for TRE to be considered a chrono-nutritional strategy is the alignment of meals with typical circadian oscillations of hormonal profiles, with insulin sensitivity declining during the day and cortisol and growth hormone peaking in the morning and evening, respectively (12, 13). Indeed, the TRE literature to date suggests that later or self-selected TRE periods are less effective in improving markers of metabolic health (Figure 2). Where TRE interventions have induced energy restriction, it is likely that the alignment of EI with circadian patterns of hormones and metabolites is less important than for energy-matched TRE.
FIGURE 2.
The 3 different approaches to TRE. (A) TRE reduces energy intake as a result of an appropriately timed window of daily energy intake, which reduces time-of-day discretionary foods consumption and induces weight loss; (B) TRE does not result in a change in energy intake, but there is an appropriately timed window of energy intake, which contributes to improvements in metabolic health independent of any weight loss; or (C) TRE does not change energy intake and, due to an inappropriately timed eating window, little or no health benefits are observed. TRE, time-restricted eating.
Studies of TRE to date have exploited several different approaches, with such variations, in part, underpinning inconsistencies in their success or failure to improve health outcomes (Figure 2). Many short-term (<3 mo) TRE protocols have been associated with moderate energy restriction (14–19), resulting in weight loss and associated health benefits. Depending on the duration of the feeding–fasting cycle, TRE can inadvertently reduce EI and/or alter macronutrient intakes via reductions in discretionary “time-of-day” foods such as alcohol and confectionary, that are typically consumed in the evening (i.e., outside the “eating window” of TRE protocols). TRE protocols that do not restrict EI but align the timing of meals and the eating window to cycles in hormone and metabolite oscillations also elicit improvements in health outcomes. From the well-controlled (all food/meals provided) early and mid-TRE human studies, this is the case (20–23), but far less evidence is available from free-living interventions (24). Further work is needed to corroborate that circadian-aligned TRE intakes lead to beneficial outcomes irrespective of EI. Additionally, “what” participants are consuming throughout the TRE period can have a significant impact on outcomes; yet, for the most part, dietary intake has been poorly reported in TRE studies.
TRE: from preclinical to human intervention studies
The concept of TRE and its basis in chronobiology originates from preclinical studies of mice in which food availability was synchronized to the diurnal rhythms of a cluster of genes responsible for regulating 24-h circadian cycles and compared with energy-matched ad libitum food availability throughout the day. When food was only available during the animals’ waking hours (overnight) (25), or restricted to a shorter window (26), mice gained less weight and body fat, and displayed improved glucose tolerance and concentrations of inflammatory markers. These animal studies of time-restricted feeding (TRF) provide evidence that ad libitum food intake is associated with disrupted circadian rhythms and adverse health outcomes (25, 26). Furthermore, when metabolically challenged with high-fat, high-sucrose diets during TRE, mice lost more body weight and improved circulating metabolites compared with ad libitum intake (i.e., no TRE) (26). The mechanisms underlying the beneficial effect of TRF are complex and are likely to act on multiple pathways that impinge on the circadian clock and improve robustness of oscillation of clock components and downstream targets (4). An evaluation of the mechanistic bases of preclinical data conducted in rodents has been reviewed previously (27–29). The translation of preclinical TRF data has several limitations: the length of time spent feed deprived (i.e., fasting) for most animal models varies substantially compared with humans, with the time of eating for mice/rodents generally confined to nocturnal hours. In contrast, humans consume the majority of daily energy during the day, with light being a major photopic signal to the body's central pacemaker, the superchiasmatic nucleus, influencing circadian oscillations. In humans, the few energy-matched studies in which meal timing has been rigorously controlled confirm earlier observations in animal models, providing “proof of concept” that the timing of meals has profound consequences on physiology, and can be used as an intervention to treat or prevent obesity and other metabolic conditions.
The results of several well-controlled studies in humans (i.e., interventions where all meals were quantified and provided to participants) provide strong evidence that TRE is an efficacious intervention for improving metabolic health outcomes (20–23). In a proof-of-concept study using a crossover design, Sutton et al. (20) reported that 5 wk of isoenergetic early TRE (08:00–14:00 h, 3 meals of 33% EI with 50% from carbohydrate) improved measures of insulin sensitivity, blood pressure, B-cell responsiveness, and markers of oxidative stress in men with prediabetes compared with when they consumed the same dietary intake over 12 h (08:00–20:00 h). That study (20) provided the first evidence to suggest that some of the health benefits of TRE may be independent of EI and weight loss. These researchers also collected preliminary data on the feasibility and acceptability of early TRE, with participants reporting that the challenge of eating within a 6-h window was more difficult than the requirement to fast for 18 h each day (20). In another short-term intervention of early TRE (4 d, eating window of 08:00–14:00 h, 3 meals of 33% EI with 50% from carbohydrate), isoenergetic TRE reduced 24-h glucose concentrations and glycemic variability in individuals with prediabetes compared with a 12-h eating window (08:00–20:00 h) (21). Similarly, reduced nocturnal glucose concentrations are observed after only 5 d of isoenergetic TRE (10:00–18:00 h, 3 meals of 25:35:40% EI with 30% from carbohydrate) in men with obesity (23). The mechanisms by which early- and mid-TRE protocols induce beneficial outcomes in the absence of energy restriction are likely related to a combination of improved circadian glucose homeostasis, reduced oxidative stress, improved B-cell function, increased autophagic flux, and increased ketone body production (30). From the evidence to date, both the start and finish time and the duration of the eating window are important considerations for translation to practice in maximizing health outcomes from TRE interventions.
In contrast to the robust laboratory-controlled TRE studies, many of the human studies of TRE conducted in free-living conditions have simultaneously manipulated both the time of day and the duration of the eating window, making it difficult to determine which of these perturbations to normal eating habits is responsible for any changes in health outcomes (20–23, 31). Based on the results of studies where EI has been estimated (14–17, 32), the notion that a shortened eating window might lead to a reduction in daily EI has gained credibility. However, the majority of free-living TRE interventions have neither quantified or estimated EI, and have not consistently reported improved health outcomes after TRE protocols (18, 24, 33–39). Most studies that fail to report the dietary intake of their participants are either late or delayed TRE (e.g., first eating occasion after 12:00 h) or have required participants to self-select their individual eating windows, with the caveat that there have been more late-TRE and self-selected TRE studies conducted to date. Further, many studies investigating TRE omit, or conduct only limited, dietary analysis (i.e., baseline and end of intervention only).
The lack of dietary information reported in many studies is, in part, due to a focus on TRE interventions being about the timing of food intake, and not the types or amounts of food consumed. However, the lack of detailed dietary intake assessments and reported data makes it unclear whether the health benefits from TRE are derived from changing the timing of food intake, reducing the energy content of food consumed, altering the types of foods consumed (i.e., macronutrient composition), or a combined effect. Of course, changing both the timing and amount/types of food consumed may have synergistic or additive effects and further work will be required to tease out potential mechanisms responsible for some of the improved metabolic health outcomes observed after TRE protocols.
Improving the quality of dietary assessment in future TRE interventions
The current literature of human interventions of TRE is limited and impacted by a lack of reliable and comprehensive dietary analysis. To present the dietary analysis of TRE studies to date, we conducted a literature search using PubMed, Google Scholar, and cross-checking of citations of other research studies to summarize human TRE studies (Table 1). We divided investigations into early-TRE (eating window finishing by 17:00 h), mid-TRE (delayed first eating occasion and eating window finishing by 19:00 h), and late-TRE (TRE beginning from 12:00 h), as well as those that had self-selected (i.e., unspecified) TRE eating windows. Of the 26 free-living TRE interventions summarized in Table 1, more than one-third had no analysis of either dietary intake or dietary quality. Most studies that report reductions in body weight had a concomitant decrease in total EI (14, 16, 40, 41), but many provided little or no dietary analysis (18, 35, 37–39). One might assume that participants undertaking TRE protocols that did not induce a reduction in body weight and/or changes in body composition, and neglected to conduct any analysis of dietary intake, failed to change their dietary intake (quality or quantity) (33, 34, 36). However, the interventions that have provided meals matched for EI, and in which only the timing of eating is altered, demonstrate that a reduced EI may not be necessary for improvements to a selected metabolic health outcome (20–23). Whether standard dietary advice regarding food quality induces additive and superior health outcomes to appropriately timed TRE has yet to be investigated (i.e., improving both what and when individuals consume food). To reach consensus between TRE interventions, traditional dietary records for a minimum of 3 d (2 weekdays and 1 weekend day), undertaken at least 3 times (baseline, midpoint, and end of intervention) for the determination of energy and macronutrients should be a minimum requirement, and provide valuable information regarding the most effective protocol of TRE. While frequent (i.e., daily), comprehensive, and extended dietary analysis (i.e., macronutrients, micronutrients, dietary patterns, core food-group analysis, level of processed food, and timing of all meals/snacks) throughout a TRE intervention would provide valuable information, it is important to be mindful of the dietary analysis skills of research teams, the time burden to participants of daily records, along with the impact that dietary recording has on dietary intake (42).
TABLE 1.
Summary of TRE interventions in humans, divided into early (eating window finished before/by 17:00 h), mid (delayed breakfast and end of eating window by 19:00 h), or late (delayed start of eating window to after 12:00 h), and studies whereby the TRE window was self-selected1
| Study (reference) | Participants (number, sex, age, BMI) | Design | Intervention | Major findings | Diet recording methodology and related outcomes |
|---|---|---|---|---|---|
| “Early” TRE (eating window finishes before/by 17:00 h) | |||||
| Hutchison et al. 2019 (24) | 15, M 55 y, 34 kg/m2 | 1 wk RXT(2-wk w/o) | eTRE: 9 h, 08:00–17:00 h vs. dTRE: 9 h, 12:00–21:00 h | ↓ Glucose AUC in eTRE and dTRE↓ Fasting glucose by CGM (eTRE) | No diet recording or diet analysis; no data on timing of when participants ate meals |
| Jamshed et al. 2019 (21)and Ravussin et al.2019 (22)2 | 11, M + F32 y, 30 kg/m2 | 4 d RXT(3.5–5-wk w/o) | TRE: 8 h, 08:00–14:00 h vs. Control 12 h, 08:00–20:00 h | ↓ 24-h glucose and glycemic variability via CGM | Meals provided with matched energy at each meal (33% EI), same macronutrients (50% CHO, 30% fat, 15% protein) across day; same as Sutton et al. (20) |
| Sutton et al. 2019 (20)2 | 8, M56 y, 32 kg/m2, prediabetes | 5 wk RXT(∼7-wk w/o) | TRE: 8 h, 08:00–14:00 hvs. Control 12 h, 08:00–20:00 h | ↑ Insulin sensitivity↔ Body weight | Meals provided with matched energy at each meal (33% EI), same macronutrients (50% CHO, 30% fat, 15% protein) across day; same as Jamshed/Ravussin et al. (21, 22) |
| Zeb et al. 2020 (33) | 56, Myoung (no age) | 25 d Pre-post | TRE: 8 h, 07:30–15:30 h | ↓ CHO, TGs, AST, ALT, and albumin↑ HDL | No diet recording or diet analysis; no data on timing of when participants ate meals |
| “Mid” TRE (delayed breakfast and early dinner) | |||||
| Gabel et al. 2018 (32) andGabel et al. 2020 (69) | 23, M + F49 y, 34.5 kg/m2 | 12 wk Pre-post | TRE: 8 h, 10:00–18:00 h vs. historical control | ↔ Body weight, fat/lean mass, fasting glucose↓ SBP | 7-d food record at baseline and at week 12; decreased energy intake (∼1420 kJ/d, −20%), NC in macronutrient intake; self-reported timing of intake |
| Martens et al. 2020 (50) | 22, M + F67 y, 25 kg/m2 | 6 wk RXT(2-wk w/o) | TRE: 8 h, starting between 10:00 and 11:00 h | ↔ Vascular endothelial function, body weight, fat/lean mass, BP↓ Hunger | Energy intake (via 24-h diet record ASA24, once per week) was unchanged, diet quality (through HEI) unchanged; self-reported timing of intake |
| Parr et al. 2020 (43) | 19, M + F50 y, 34 kg/m2, type 2 diabetes | 4 wk (+2 wk baseline) Pre-post | TRE: 9 h, 10:00–19:00 h | ↔ Body weight, fat/lean mass, HbA1c, fasting glucose | Food records throughout entire 2-wk baseline and 4-wk study; N/C to dietary intake with TRE (vs. baseline); photos to capture dietary timing; reduced EI on adherent TRE days vs. nonadherent (reduced CHO, alcohol) |
| Parr et al. 2020 (23)2 | 11, M38 y, 32 kg/m2 | 5 dRXT (2-wk w/o) | TRE: 8 h, 10:00–18:00 h vs. Control: 15 h, 07:00 h–22:00 h | ↔ 24-h glucose concentrations or AUC (CGM), insulin↓ Nocturnal glucose concentrations | Meals provided; 25:30:45% EI; same macronutrients at each meal (30% CHO, 50% fat, 20% protein); self-reported timing of intake at structured times |
| Peeke et al. 2021 (19)3 | 60, M + F44 y, 38 kg/m2 | 8 wkRCT | TRE: 10 h (self-selected from 07:00–17:00 h to 10:00–20:00 h) vs. Control 12 h | ↓ Body weight (−10.7 kg) in TRE vs. CON (−8.9 kg), fasting glucose (when FBG >5.5 mmol/L) | Controlled meals/energy intake (reduced energy intake by 500–100 kJ/d) via Jenny Craig Rapid Results Program and purchasing 8 wk of food; no reporting of timing of intake |
| “Late” TRE (after 12:00 h start) | |||||
| Cienfuegos et al. 2020 (16)and Cienfuegos et al.2021 (70) | 58, M + F47 y, 36 kg/m2 | 8 wkRCT | TRE: 4 h (from 15:00 h) and 6 h (from 13:00 h) vs. Control, ad libitum | ↓ Body weight (3.9 and 3.4%) in TRE groups vs. Control (0.1%)↔ Fasting glucose, HbA1c↔ Body weight, pre- vs. postmenopausal women | 7-d food record at baseline and week 8; household measures and self-reported times.Decreased EI in both groups (∼−2090 kJ/d) compared with Control (∼−420 kJ/d); N/C to sugar, saturated fat, cholesterol, fiber, or sodium intakes |
| Isenmann et al. 2021 (63)4 | 35, M + F27 y, 26 kg/m2 | 14 wk (+2 wk baseline)RCT | TRE: 8 h, 12:00–20:00 h)vs. MBD | ↓ Body weight (∼5%) in both TRE and MBD groups↓ Body fat↔ Lean mass | Food records throughout entire 2-wk baseline (phase 1) and 8-wk phase 2, encouraged for 6-wk phase 3; N/C to dietary intake with TRE or MBD (vs. baseline) |
| Kotarsky et al. 2021 (60)4 | 21, M + F44 y, 30 kg/m2 | 8 wkRCT | TRE: 8 h, 12:00–20:00 h)vs. Control, normal diet pattern | ↓ Body weight in TRE (3.3%) vs. Control (0.2%) | 3-d diet records collected at weeks 1, 4, and 7; participants were excluded after more than 1 noncompliant (to the timing of eating) day; decreased EI in both groups (∼1250 kJ/d) due to decreased CHO intake |
| Lowe et al. 2020 (34) | 105 M + F (online), including 46 (in person)46 y, 31 kg/m2 | 12 wk RCT | TRE: 8 h, 12:00–20:00 h vs. CMT (06:00–10:00 h breakfast, 11:00–15:00 h lunch, 17:00–22:00 h dinner) | ↔ Body weight (−0.9 vs. CMT: −0.6 kg), ↓ appendicular lean mass index in TRE vs. CMT | No diet recording or diet analysis; no data on timing of when participants ate meals |
| Moro et al. 2016 (59)4 | 34, M 29 y, 27 kg/m2 | 8 wk RCT | TRE: 8 h, 13:00–20:00 h vs. Control: 12 h, 08:00–20:00 h | ↓ Fat mass (−16%) vs. Control (−2%), fasting glucose, fasting insulin, ↔ lean mass | Participants were instructed to consume 3 meals, based on their baseline (7-d recording) dietary intake; TRE was 40%, 25%, and 35% EI at the 3 meals (13:00, 16:00, and 20:00) vs. Control of 25% at 08:00, 40% at 13:00 and 35% at 20:00; ND between groups for EI or macronutrient intake |
| Schroder et al. 2021 (35) | 32, F39 y, 33 kg/m2 | 3 mo Non-RCT | TRE: 8 h, 12:00–20:00 h vs. Control: no change to habitual intake/patterns | ↓ Body weight (−3.4 kg) vs. Control (+1.3 kg) | No diet recording or diet analysis; no data on timing of when participants ate meals |
| Smith et al. 2017 (36) | 20, F21 y, ∼65 kg (no BMI data) | 4 wk Pre-post | TRE: 8 h, 12:00–20:00 h | ↓ Body weight (0.6 kg) | Self-reported adherence to the diet prescription but no analysis of diet energy intake or data on the timing of when participants ate meals |
| Stote et al. 2007 (31)2 | 15, M + F45 y, 23 kg/m2 | 8 wk RXT (11-wk w/o) | TRE: 4 h, 17:00–21:00 h vs. Control (3 meals/d) | ↓ Body weight (1.4 kg), ↑ blood pressure vs. Control | Meals provided (∼9890 kJ/d TRE and 10,160 kJ/d in Control), same macronutrient intake (50% CHO, 35% fat, 15% protein) |
| Tinsley et al. 2017 (61)4 | 18 MNormal weight | 8 wk RCT | TRE: 4 h (between 16:00 and 00:00 h) for 4 d/wk vs. Control | ↔ Body weight, fat mass | −2720 kJ/d energy reduction each day of TRE (nontraining days) |
| Tinsley et al. 2019 (62)4 | 40 F22 y, 23 kg/m2 | 8 wk RCT | TRE: 8 h, 12:00–20:00 h vs. Control (13 h) | ↑ Body weight (both groups), ↓ fat mass (∼4%) TRE vs. CON, ↑ muscle strength and endurance (both groups) | Weighed diet records on selected weekday and weekend days during pre- and 2 separate weeks during intervention period; increased EI in all groups (∼84–840 kJ/d) |
| Participant choice TRE (no specified “window”) | |||||
| Anton et al. 2019 (37) | 10, M + F77 y, 34 kg/m2 | 4 wkPre-post | TRE: 8 h, self-selected | ↓ Body fat (−0.6 kg, −0.7%) | Food diaries collected for adherence (84%, in weeks 2–4); no analysis of dietary intake |
| Antoni et al. 2018 (17) | 13, F46 y, 29 kg/m2 | 10 wkPre-post | TRE: 90 min earlier dinner and 90 min later breakfast, self-selected | ↔ Body weight (−0.7 vs. −0.5 kg), ↓ body fat percentage | Validated food diaries used for the entire intervention period; diet timing via self-report in food diaries; decreased EI by ∼2930 kJ/d |
| Cai et al. 2019 (41) | 271, M + F34 y, 26 kg/m2 NAFLD | 12 wkRCT | TRE: 8 h, self-selected, vs. ADF vs. Control | ↓ Body weight (−3.6 kg) in TRE (and −4.5 kg in ADF) vs. Control | All groups were prescribed energy-restricted diet intake, with the TRE group being provided 1 meal in the 8-h period; no reporting of baseline energy intake, self-reported intake during intervention (weeks 4 and 12), with eating times |
| Chow et al. 2020 (18) | 20, M + F45 y, 34 kg/m2 | 12 wk Pre-post | TRE: 8 h, self-selected (achieved 10 h) vs. Control 15 h | ↓ Body weight (3.7% ∼3.6 kg) in TRE vs. Control | Energy intake logged using MCC app to obtain meal timing; number of eating occasions reported, as a surrogate measure of diet intake; TRE eating window selected ∼10:40–18:40 h with 55% adherence |
| Gill and Panda, 2015 (14) | 8, M + F27 y, 33 kg/m2 | 16 wkPre-post | TRE: 10 h, self-selected | ↓ Body weight (−3.3 kg) | Custom mobile app (MCC) to take photos of food for entire period; annotated and analyzed using FDDNS or CalorieKing. EI decreased by 20.26% (−4.92 to 35.6% 95% CI) |
| Kesztyüs et al. 2019 (38) | 40, M + F49 y, 31 kg/m2 | 12 wkPre-post | TRE: 8 h, self-selected | ↓ Body weight (−1.7 kg), ↓ waist circumference↓ HbA1c | Self-reported intake of main diet components rated on 6-point Likert scale (never–several times a day) at baseline and postintervention; no diet intake reporting or analysis; self-reported timing of eating (time of first and last meal) using a diary |
| Kesztyüs et al. 2021 (39) | 63, M + F 48 y, 26 kg/m2 | 12 wkPre-post | TRE: 8–9 h, self-selected | ↓ Body weight (−1.3 kg), ↓ waist circumference (−1.7 cm), ↑ HRQoL | Self-reported adherence (∼72%) via time of first and last meal; no diet intake reporting or analysis |
| LeCheminant et al. 2013 (15) | 27, M21 y, 24 kg/m2 | 2 wkRXT(1-wk w/o) | TRE: 06:00–19:00 h vs. ad libitum | ↓ Body weight (−0.4 kg) vs. ad libitum (+0.6 kg) | 3-d diet recall (2 weekdays, 1 weekend) during each week using 24-h multi-pass recallReduced EI in TRE vs. ad libitum, no differences in macronutrient intake; self-reported timing of intake |
| McAllister et al. 2020 (71) | 22, M22 y, 28 kg/m2 | 4 wkRCT | TRE: 8 h, self-selectedvs. either ad libitum or prescribed isoenergetic | ↔ Body weight↓ Body fat, ↓ BP | Self-reported time of first and last meal, diet intake logged using MyFitnessPal; trend (P = 0.054) for higher diet intake in the ad libitum TRE group compared with isoenergetic |
| Phillips et al. 2021 (49) | 213 M + F (observation), 40 y, 25 kg/m254, M + F (RCT)43 y, ∼28 kg/m2 | 1-mo observation6-moRCT | TRE: 12 h, self-selected vs. SDA (10-min nutrition counseling) | ↓ Body weight (TRE: 1.6% vs. SDA: 1.1%) | Diet intake logged using MCC app (for timing), text coded for dietary quality analysis using NOVA (unprocessed to processed) categories; no analysis of energy intake |
| Pureza et al. 2020 (72) | 58, F31 y, 33 kg/m2 | 3 wkPre-post | TRE: 12 h, self-selected vs. unrestricted (Control) | ↓ Body weight (−1 kg to 2 kg in both groups), ↓ body fat in TRE | No measurement of diet timing but energy reduction (prescribed) was similar in both groups (−2680 kJ/d) |
| Wilkinson et al. 2020 (40) | 19, M + F59 y, 33 kg/m2MetS | 12 wkPre-post | TRE: 10 h, self-selected | ↓ Body weight [−3 kg (−3%)], fat mass, BP↔ Fasting glucose, insulin, HbA1c | Diet intake logged using MCC app (for timing), estimated ∼9% (840 kJ/d) energy reduction but no analysis of macronutrient intake |
Arrows indicate significant reductions (↓) or no significant changes (↔). ADF, alternate-day fasting; ALT, alanine transaminase; ASA24, Automated Self-Administered 24-hour dietary assessment tool; AST, aspartate aminotransferase; BP, blood pressure; CGM, continuous glucose monitor; CHO, carbohydrate; CMT, consistent meal timing; dTRE, delayed time-restricted eating; EI, energy intake; eTRE, early time-restricted eating; FBG, fasting blood glucose; FDDNS, Food and Nutrient Database for Dietary Studies; HbA1c, glycated hemoglobin; HEI, Healthy Eating Index; HRQoL, health-related quality of life; MBD, macronutrient-based diet; MCC, MyCircadianClock; MetS, metabolic syndrome; NAFLD, nonalcoholic fatty liver disease; N/C, no change; ND, no difference; RCT, randomized controlled trial; RXT, randomized crossover trial; SBP, systolic blood pressure; SDA, standard dietary advice; TG, triglyceride; TRE, time-restricted eating; w/o, washout.
Provided meals (isoenergetic).
Prescribed diet (hypoenergetic).
Exercise protocol with TRE/Control.
The other, less studied, yet important dietary component is the change in macronutrient and energy distribution across meals, as well as the number of meals and snacks consumed during a day. Typically, in Western cultures/societies, breakfast is the most carbohydrate-centric meal, yet contributes the least to total daily EI. In the evening, dinner is generally higher in protein compared with other meals, as well as being the largest meal with regard to total EI. Due to lack of detailed dietary data reported in previous TRE interventions, it is currently unknown if TRE protocols change the distribution or intake of macronutrients at meals across the day.
In addition to failing to report EI, most studies of TRE that have manipulated the size of meals throughout the day do not specify what proportions of macronutrient have been provided/consumed at each meal. The TRE studies that have utilized meal photo timing have provided a comprehensive analysis of the number of eating occasions (as a surrogate measure of total EI) and reported a reduction (14, 18) or similar number (43), in response to the reduced eating window. Evidence from studies by Jakubowicz and colleagues (44, 45) has shown that larger morning meals (high in carbohydrate) with small evening meals (high in protein) are effective for reducing body weight and improving glycemic control. However, in these studies, it is difficult to determine whether it is the EI or the macronutrient distribution that led to changes in several physiological outcomes. Neglecting to consider what is being consumed and how frequently in a TRE intervention, while focusing solely on when food is consumed, is overlooking a crucial component in understanding the full benefit of TRE. This is particularly important when translating TRE research into practice. Without the information of what food has been consumed, the TRE advice provided to individuals is limited to simply the eating window. Although this may help keep the message simple, in practice, individuals will naturally ask what foods they can consume within a specified time. It would be ideal to elucidate the best TRE eating window along with the ideal meal timing and macronutrient composition for optimal results (i.e., combining the what with the when).
The quality of ingested nutrients plays a crucial role when determining any effects of dietary intervention on metabolic health outcomes. For example, carbohydrate-rich foods that have a widely different glycemic index induce different glucose/insulin responses (46). Thus, the quality of the ingested food is also important from a metabolic health perspective (47, 48), with dietary guidelines recommending changes to both the quality (i.e., increased grains vs. refined foods; whole foods vs. processed foods) and the quantity (i.e., reduced portion sizes) of food. Only 2 of the 25 TRE studies reviewed (Table 1) have utilized a measure of dietary quality to assess TRE and compared this with either a 10-min nutrition-counseling session (standard dietary advice) (49) or no advice (50). Using the qualitative NOVA classification (51) from free-text annotations of food photos collected throughout a 6-mo intervention, Phillips and colleagues (49) reported that participants receiving standard dietary advice significantly increased their intake of unprocessed or minimally processed foods by 7% and compensated by a reduced intake of processed food, with no changes to fluids consumed. Martens and colleagues (50) used the Healthy Eating Index (52) to obtain an outcome of dietary quality from weeks 3–5 of a 6-wk intervention compared with 6 wk of no advice (following normal diet). Importantly, for the comparisons in both studies, the TRE condition did not improve or change dietary quality, which was described as “adhering to the protocol” (49) or not adversely affecting dietary intake (50). Detailed dietary analysis that has been performed in several studies has indicated that time-of-day foods, such as late evening snacks and alcohol consumption, are reduced with TRE (14, 43). If TRE can induce such changes to dietary intake and quality without structured advice, then more rigorous dietary analysis is crucial in future TRE interventions.
TRE: not just another weight-loss intervention
The primary outcomes of TRE interventions to date have been weight loss, glycemic control, and selected biomarkers of cardiometabolic health, with the majority of studies reporting positive effects on these and several other measures (6, 8, 9, 53, 54). However, it is not currently known whether it is the modest energy restriction induced by TRE protocols or the alignment of meal timing with circadian oscillations that induce many of the health benefits of TRE. Not only does circadian phase influence the metabolic response to food intake but food intake itself is under control by the endogenous circadian system (i.e., independent of the sleep/wake and fasting/feeding cycle) (55).
In energy-restricted diets that induce weight loss, there is a concomitant reduction in lean mass, typically accounting for at least 25% of the total weight lost (56). The loss of lean tissue during energy restriction can be mitigated by exercise in the face of adequate protein intake (57), but high-protein, energy-restricted diets are not effective in isolation (58). In the few TRE studies that have measured body composition, the magnitude of change in lean mass has been small (∼1.0 kg) (34) or negligible (32, 43, 50), usually reflecting a modest loss in body weight, or possibly typical measurement error. Several investigations have combined TRE with exercise training to maximize improvements in body composition (reduced fat mass and maintained or increased lean mass) (59–63). Whether a restricted eating window is optimal to promote adequate rates of protein synthesis to maintain protein balance in the absence of an exercise stimulus is an important question that warrants further research (64). Indeed, whether TRE confers additive benefits to disordered metabolism above and beyond those induced by exercise training remains to be determined experimentally (65).
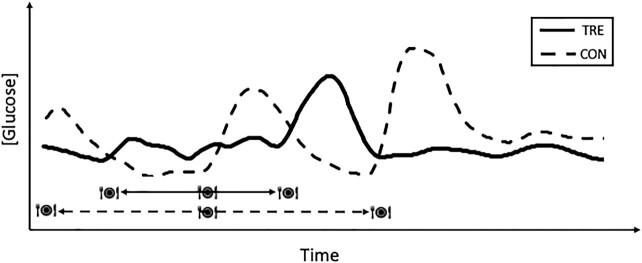
Dietary interventions are often implemented with the aim of improving glycemic control. In addition to weight loss, TRE interventions improve fasting glucose concentrations (19, 19, 24, 59), 24-h glucose profiles (determined by continuous glucose monitoring) (21, 40), glycated hemoglobin (HbA1c) (38), reduce glucose AUC in response to an oral-glucose-tolerance test (24, 50), reduce nocturnal glucose concentrations (23) (Figure 3), and enhance insulin sensitivity (16, 20). Typically, but not always, changes in glucose parameters have been evident in cohorts with elevated glucose concentrations (>5.6 mmol/L) at baseline (i.e., impaired fasting glucose, T2D, metabolic syndrome) compared with a lack of change observed in those studies in which these parameters were in the normal range before the intervention (16, 32, 34, 40). Furthermore, most of the improvements in glycemic control measures come from studies of early- or mid-TRE (Table 1). As highlighted by Zhao et al. (66), the distribution of carbohydrate intake across the eating window is vital when attempting to modify glycemic control, a factor that should be considered in future studies, and emphasizes the need for rigorous dietary assessment in TRE interventions. The range of improvements in glycemic control across the limited TRE literature to date provides scope for specific TRE interventions with such markers as primary outcome variables, especially in populations such as individuals with T2D (43), where glucose management is important to minimize diabetes-associated complications and improve health and quality of life.
FIGURE 3.
Representative schematic of glucose concentrations changing over a 24-h period comparing the effects of 3 meals during a control day (meals over >12 h; dashed line) with a time-restricted eating pattern (meals within 8 h; solid line). CON, control; TRE, time-restricted eating. Adapted from reference 23 with permission.
Adherence to TRE
A major benefit of TRE protocols compared with other dietary interventions is the ability for individuals to adhere to such practices without overt changes on the quality or quantity of dietary intake. This removes some of the stigma and psychological barriers often associated with dietary modification. It has been suggested that, over the long term, TRE may be easier to tolerate and implement than other dietary approaches (67) as the focus is on when rather than on what to eat. While not all aspects of TRE may encourage adherence [reviewed previously (67)], TRE may offer an option of an alternative dietary strategy to improve metabolic health. Adherence to TRE in free-living environments has varied from 5 d (43) to 6 d/wk (16, 32) over 4- to 10-wk intervention periods, 55% over 12 wk (18), ∼62% over 10 wk (17), and up to ∼84% over 6 wk (50) or 12 wk (34). In a subanalysis, Martens et al. (50) measured improved adherence (from 84% to 95% over 6 wk) when the eating window was extended from 8 h to 8.5 h/d (50). In a supported 8-wk intervention, immediately followed by 6 wk of free-living TRE (12:00–20:00 h) in habitual (3–4 sessions/wk) exercisers, Isenmann et al. (63) reported a drop in adherence from 98% (supported) to 71% (self-implemented). Participants in that study (63) rated the ease of TRE implementation as similar to that for a group that followed a traditional macronutrient-based diet. While no explanation for these observations was provided, participants in other studies have indicated that if the evening mealtime could be delayed slightly, it would improve their adherence (17, 43).
Adherence to TRE in studies discussed and summarized in Table 1 is typically from self-report. Studies incorporating objective time-stamped photos are still limited as they rely on participants to accurately capture their meal timing. In support of the self-reported adherence are qualitative responses from participants that mid-TRE as a dietary intervention is achievable on most days of the week (17, 23, 43), with early-TRE deemed subjectively feasible based on positive health outcomes (20). Although the implementation of these early-TRE protocols is challenging with regard to the impact on social and family life, to date, early-TRE interventions have not been investigated in free-living conditions. In several studies, investigators have chosen TRE eating windows based on participants’ personal preferences (i.e., 12:00–20:00 h) due to both social considerations and the importance of evening meals with family or friends (34–36, 59, 60). Taken together, there is an underlying narrative of what can be achieved in the real world versus what is most efficacious with regard to optimal meal timing to align with circadian rhythms. There is also unlikely to be a single eating window that will be equally beneficial for every individual, as circadian preferences vary between “larks” (morning chronotypes) and “owls” (night chronotypes) (68), leading to difficulties in making generic recommendations.
Conclusions and Future Directions
TRE has become a popular dietary strategy to improve measures of metabolic health, possibly due to a lack of focus on weight loss per se. Indeed, we believe that TRE protocols can be adapted to tackle a variety of pre-existing metabolic conditions dependent on the goals or desired health outcomes of the individual. Further research expanding the use of TRE interventions in different clinical populations under free-living conditions is essential to evaluate long-term adherence and feasibility before recommending additions to national and international diet guidelines. In this regard, we acknowledge that TRE is not the only option or dietary strategy in a health professional's toolbox to be used to improve or manage the diverse range of chronic metabolic conditions seen in society. However, we hope this Perspective article has highlighted the necessity for future studies of TRE to increase the rigor of dietary data collected, assessed, and reported to ensure there is a consistent and standardized approach across TRE interventions. Almost the entire body of dietary literature to date, along with the profession of nutrition science, has focused on what we eat; new knowledge from TRE interventions is shifting that narrative so that now it is vital that we also consider that the timing of meals plays an important role in determining metabolic health outcomes. Without consideration of both what and when is eaten, we cannot begin to understand the potential synergies between these 2 variables and their potential impact on reducing the burden of chronic metabolic diseases at the population level.
Acknowledgments
We acknowledge the funding bodies for the grants, and the wider research team for the continual sharing of ideas and progression of this work. The authors’ responsibilities were as follows—EBP and BLD: conceptualized the article; EBP: drafted the manuscript and Table 1; EBP, BLD, and JAH: edited and revised all sections of the manuscript and had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Supported by a 2018 Early Career Fellowship award from the European Society for Clinical Nutrition (ESPEN) to EBP, a Diabetes Australia Research Program (DARP; 2019) grant to EBP, and by a Novo Nordisk Foundation Challenge grant (NNF14OC0011493) to JAH.
Author disclosures: The authors report no conflicts of interest.
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Abbreviations used: CER, chronic energy restriction; EI, energy intake; IF, intermittent fasting; TRE, time-restricted eating; TRF, time-restricted feeding; T2D, type 2 diabetes.
Contributor Information
Evelyn B Parr, Exercise and Nutrition Research Program, Mary MacKillop Institute for Health Research, Australian Catholic University, Melbourne, Victoria, Australia.
Brooke L Devlin, Department of Dietetics, Nutrition, and Sport, La Trobe University, Bundoora, Victoria, Australia.
John A Hawley, Exercise and Nutrition Research Program, Mary MacKillop Institute for Health Research, Australian Catholic University, Melbourne, Victoria, Australia.
References
- 1. Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell. 2015;161(1):84–92. [DOI] [PubMed] [Google Scholar]
- 2. Di Francesco A, Di Germanio C, Bernier M, de Cabo R. A time to fast. Science. 2018;362(6416):770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hawley JA, Sassone-Corsi P, Zierath JR. Chrono-nutrition for the prevention and treatment of obesity and type 2 diabetes: from mice to men. Diabetologia. 2020;63(11):2253–9. [DOI] [PubMed] [Google Scholar]
- 4. Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer Fet al. Meal frequency and timing in health and disease. Proc Natl Acad Sci. 2014;111(47):16647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381(26):2541–51. [DOI] [PubMed] [Google Scholar]
- 6. Gabel K, Cienfuegos S, Kalam F, Ezpeleta M, Varady KA. Time-restricted eating to improve cardiovascular health. Curr Atheroscler Rep. 2021;23(5):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaix A, Manoogian ENC, Melkani GC, Panda S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr. 2019;39(1):291–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Regmi P, Heilbronn LK. Time-restricted eating: benefits, mechanisms, and challenges in translation. Iscience. 2020;23(6):101161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waldman HS, Renteria LI, McAllister MJ. Time-restricted feeding for the prevention of cardiometabolic diseases in high-stress occupations: a mechanistic review. Nutr Rev. 2020;78(6):459–64. [DOI] [PubMed] [Google Scholar]
- 10. Lee MB, Hill CM, Bitto A, Kaeberlein M. Antiaging diets: separating fact from fiction. Science. 2021;374(6570):eabe7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clayton DJ, Mode WJA, Slater T. Optimising intermittent fasting: evaluating the behavioural and metabolic effects of extended morning and evening fasting. Nutr Bull. 2020;45(4):444–55. [Google Scholar]
- 12. Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88(3):934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol. 1992;262:E467–75. [DOI] [PubMed] [Google Scholar]
- 14. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22(5):789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. LeCheminant JD, Christenson E, Bailey BW, Tucker LA. Restricting night-time eating reduces daily energy intake in healthy young men: a short-term cross-over study. Br J Nutr. 2013;110(11):2108–13. [DOI] [PubMed] [Google Scholar]
- 16. Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, Lin S, Oliveira ML, Varady KA. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 2020;32(3):366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Antoni R, Robertson TM, Robertson MD, Johnston JD. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. J Nutr Sci. 2018;7:E22 [Google Scholar]
- 18. Chow LS, Manoogian ENC, Alvear A, Fleischer JG, Thor H, Dietsche K, Wang Q, Hodges JS, Esch N, Malaeb Set al. Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study. Obesity. 2020;28(5):860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peeke PM, Greenway FL, Billes SK, Zhang D, Fujioka K. Effect of time restricted eating on body weight and fasting glucose in participants with obesity: results of a randomized, controlled, virtual clinical trial. Nutr Diabetes. 2021;11(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212–21..e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11(6):1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity. 2019;27(8):1244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parr EB, Devlin BL, Radford BE, Hawley JA. A delayed morning and earlier evening time-restricted feeding protocol for improving glycemic control and dietary adherence in men with overweight/obesity: a randomized controlled trial. Nutrients. 2020;12(2):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hutchison AT, Regmi P, Manoogian ENC, Fleischer JG, Wittert GA, Panda S, Heilbronn LK. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring). 2019;27:724–32. [DOI] [PubMed] [Google Scholar]
- 25. Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JAJet al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20(6):991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zarrinpar A, Chaix A, Panda S. Daily eating patterns and their impact on health and disease. Trends Endocrinol Metab. 2016;27(2):69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chaix A, Zarrinpar A. The effects of time-restricted feeding on lipid metabolism and adiposity. Adipocyte. 2015;4(4):319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Panda S. Circadian physiology of metabolism. Science. 2016;354(6315):1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cienfuegos S, McStay M, Gabel K, Varady KA. Time restricted eating for the prevention of type 2 diabetes. J Physiol. 2021; 10.1113/JP281101Published online: 21 August 2021. [DOI] [PubMed] [Google Scholar]
- 31. Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, Strycula P, Najjar SS, Ferrucci L, Ingram DKet al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85(4):981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, Panda S, Varady KA. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging. 2018;4(4):345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zeb F, Wu X, Chen L, Fatima S, Haq I, Chen A, Majeed F, Feng Q, Li M. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br J Nutr. 2020;123(11):1216–26. [DOI] [PubMed] [Google Scholar]
- 34. Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, Philip E, Vittinghoff E, Heymsfield SB, Olgin JEet al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020;180(11):1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schroder JD, Falqueto H, Mânica A, Zanini D, de Oliveira T, de Sá CA, Cardoso AM, Manfredi LH. Effects of time-restricted feeding in weight loss, metabolic syndrome and cardiovascular risk in obese women. J Transl Med. 2021;19(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith ST, LeSarge JC, Lemon PWR. Time-restricted eating in women—a pilot study. WURJ Health Nat Sciences. 2017;8:1–6. [Google Scholar]
- 37. Anton SD, Lee SA, Donahoo WT, McLaren C, Manini T, Leeuwenburgh C, Pahor M. The effects of time restricted feeding on overweight, older adults: a pilot study. Nutrients. 2019;11(7):1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kesztyüs D, Cermak P, Gulich M, Kesztyüs T. Adherence to time-restricted feeding and impact on abdominal obesity in primary care patients: results of a pilot study in a pre–post design. Nutrients. 2019;11(12):2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kesztyüs D, Vorwieger E, Schönsteiner D, Gulich M, Kesztyüs T. Applicability of time-restricted eating for the prevention of lifestyle-dependent diseases in a working population: results of a pilot study in a pre-post design. Ger Med Sci. 2021; 19:Doc04.; 10.3205/000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda Set al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31(1):92–104.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cai H, Qin Y-L, Shi Z-Y, Chen J-H, Zeng M-J, Zhou W, Chen R-Q, Chen Z-Y. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: a randomised controlled trial. BMC Gastroenterol. 2019;19(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dao MC, Subar AF, Warthon-Medina M, Cade JE, Burrows T, Golley RK, Forouhi NG, Pearce M, Holmes BA. Dietary assessment toolkits: an overview. Public Health Nutr. 2019;22(3):404–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parr EB, Devlin BL, Lim KHC, Moresi LNZ, Geils C, Brennan L, Hawley JA. Time-restricted eating as a nutrition strategy for individuals with type 2 diabetes: a feasibility study. Nutrients. 2020;12(11):3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity. 2013;21(12):2504–12. [DOI] [PubMed] [Google Scholar]
- 45. Jakubowicz D, Wainstein J, Ahrén B, Bar-Dayan Y, Landau Z, Rabinovitz HR, Froy O. High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: a randomised clinical trial. Diabetologia. 2015;58(5):912–9. [DOI] [PubMed] [Google Scholar]
- 46. Radulian G, Rusu E, Dragomir A, Posea M. Metabolic effects of low glycaemic index diets. Nutr J. 2009;8(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, Chung ST, Costa E, Courville A, Darcey Vet al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):67–77, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wirt A, Collins CE. Diet quality—what is it and does it matter?. Public Health Nutr. 2009;12(12):2473–92. [DOI] [PubMed] [Google Scholar]
- 49. Phillips N, Mareschal J, Schwab N, Manoogian E, Borloz S, Ostinelli G, Gauthier-Jaques A, Umwali S, Gonzalez Rodriguez E, Aeberli Det al. The effects of time-restricted eating versus standard dietary advice on weight, metabolic health and the consumption of processed food: a pragmatic randomised controlled trial in community-based adults. Nutrients. 2021;13(3):1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Martens CR, Rossman MJ, Mazzo MR, Jankowski LR, Nagy EE, Denman BA, Richey JJ, Johnson SA, Ziemba BP, Wang Yet al. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. GeroScience. 2020;42(2):667–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Monteiro CA, Cannon G, Moubarac J-C, Levy RB, Louzada MLC, Jaime PC. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;21(1):5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, Wilson MM, Reedy J. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moon S, Kang J, Kim SH, Chung HS, Kim YJ, Yu JM, Cho ST, Oh C-M, Kim T. Beneficial effects of time-restricted eating on metabolic diseases: a systemic review and meta-analysis. Nutrients. 2020;12(5):1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pellegrini M, Cioffi I, Evangelista A, Ponzo V, Goitre I, Ciccone G, Ghigo E, Bo S. Effects of time-restricted feeding on body weight and metabolism. a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21(1):17–33. [DOI] [PubMed] [Google Scholar]
- 55. Scheer F, Morris CJ, Shea SA. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors: body clock controls hunger. Obesity. 2013;21(3):421–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Parr EB, Coffey VG, Hawley JA. ‘Sarcobesity’: a metabolic conundrum. Maturitas. 2013;74(2):109–13. [DOI] [PubMed] [Google Scholar]
- 57. Parr EB, Coffey VG, Cato LE, Phillips SM, Burke LM, Hawley JA. A randomised trial of high dairy protein, variable carbohydrate diets and exercise on body composition in adults with obesity. Obesity. 2016;24(5):1035–45. [DOI] [PubMed] [Google Scholar]
- 58. Englert I, Bosy-Westphal A, Bischoff SC, Kohlenberg-Müller K. Impact of protein intake during weight loss on preservation of fat-free mass, resting energy expenditure, and physical function in overweight postmenopausal women: a randomized controlled trial. Obesity Facts. 2021;14(3):259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, Palma A, Gentil P, Neri M, Paoli A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kotarsky CJ, Johnson NR, Mahoney SJ, Mitchell SL, Schimek RL, Stastny SN, Hackney KJ. Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiol Rep. 2021;9. 10e14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, Morgan GB, Grandjean PW. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur J Sport Sci. 2017;17(2):200–7. [DOI] [PubMed] [Google Scholar]
- 62. Tinsley GM, Moore ML, Graybeal AJ, Paoli A, Kim Y, Gonzales JU, Harry JR, VanDusseldorp TA, Kennedy DN, Cruz MR. Time-restricted feeding plus resistance training in active females: a randomized trial. Am J Clin Nutr. 2019;110(3):628–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Isenmann E, Dissemond J, Geisler S. The effects of a macronutrient-based diet and time-restricted feeding (16:8) on body composition in physically active individuals—a 14-week randomised controlled trial. Nutrients. 2021;13(9):3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lees MJ, Hodson N, Moore DR. A muscle-centric view of time-restricted feeding for older adults. Curr Opin Clin Nutr Metab Care. 2021;24(6):521–7. [DOI] [PubMed] [Google Scholar]
- 65. Parr EB, Heilbronn LK, Hawley JA. A time to eat and a time to exercise. Exerc Sport Sci Rev. 2020;48(1):4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhao L, Hutchison AT, Heilbronn LK. Carbohydrate intake and circadian synchronicity in the regulation of glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2021;24:342–8. [DOI] [PubMed] [Google Scholar]
- 67. O'Connor SG, Boyd P, Bailey CP, Shams-White MM, Agurs-Collins T, Hall K, Reedy J, Sauter ER, Czajkowski SM. Perspective: time-restricted eating compared with caloric restriction: potential facilitators and barriers of long-term weight loss maintenance. Adv Nutr. 2021;12(2):325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, Merrow M. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11(6):429–38. [DOI] [PubMed] [Google Scholar]
- 69. Gabel K, Marcell J, Cares K, Kalam F, Cienfuegos S, Ezpeleta M, Varady KA. Effect of time restricted feeding on the gut microbiome in adults with obesity: a pilot study. Nutr Health. 2020;26(2):79–85. [DOI] [PubMed] [Google Scholar]
- 70. Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Lin S, Varady KA. Changes in body weight and metabolic risk during time restricted feeding in premenopausal versus postmenopausal women. Exp Gerontol. 2021;154:111545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McAllister MJ, Pigg BL, Renteria LI, Waldman HS. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: a 4-week randomized pre-post pilot study. Nutr Res. 2020;75:32–43. [DOI] [PubMed] [Google Scholar]
- 72. Pureza I, Melo ISV, Macena ML, Praxedes DRS, Vasconcelos LGL, Silva-Júnior AE, Florêncio T, Bueno NB. Acute effects of time-restricted feeding in low-income women with obesity placed on hypoenergetic diets: randomized trial. Nutrition. 2020;77:110796. [DOI] [PubMed] [Google Scholar]