Abstract
Background
We report characteristics and outcomes of adults admitted to Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance (SOS) Network hospitals with COVID-19 in 2020.
Methods
Patients with laboratory-confirmed COVID-19 admitted to 11 sites in Ontario, Quebec, Alberta, and Nova Scotia up to December 31, 2020 were enrolled in this prospective observational cohort study. Measures included age, sex, demographics, housing, exposures, Clinical Frailty Scale, comorbidities; in addition, length of stay, intensive care unit (ICU) admission, mechanical ventilation, and survival were assessed. Descriptive analyses and multivariable logistic regressions were conducted.
Results
Among 2,011 patients, mean age was 71.0 (range 19–105) years. 29.7% were admitted from assisted living or long-term care facilities. The full spectrum of frailty was represented in both younger and older age groups. 81.8% had at least one underlying comorbidity and 27.2% had obesity. Mortality was 14.3% without ICU admission, and 24.6% for those admitted to ICU. Older age and frailty were independent predictors of lower ICU use and higher mortality; accounting for frailty, obesity was not an independent predictor of mortality, and associations of comorbidities with mortality were weakened.
Conclusions
Frailty is a critical clinical factor in predicting outcomes of COVID-19, which should be considered in research and clinical settings.
Keywords: age, frailty, frail elderly, COVID-19, SARS-CoV-2, outcomes, hospitalization, surveillance
INTRODUCTION
The COVID-19 pandemic has had dramatic impacts around the world, including in Canada. The first case in Canada was identified on January 25th, 2020, and by mid-March 2020, COVID-19 activity had been detected in all ten Canadian provinces.(1)
The Serious Outcomes Surveillance (SOS) Network of the Canadian Immunization Research Network (CIRN) was established just prior to the 2010 H1N1 influenza pandemic, and has since provided infrastructure for active influenza surveillance in sentinel Canadian hospitals to help inform the Public Health Agency of Canada (PHAC) influenza response.(2–4) In addition to providing estimates of seasonal influenza vaccine effectiveness and informing the public health response to seasonal influenza, the CIRN SOS Network was designed to enable rapid pandemic surveillance response capacity to complement PHAC activities and the need for immediate collation of epidemiological data from across Canada. Beyond providing surveillance numbers, the SOS Network collects detailed clinical data, including on measures of health relevant to older adults (e.g., frailty and comorbidities). Given this established hospital surveillance infrastructure, it has been possible to pivot SOS Network’s efforts to provide COVID-19 surveillance. This capacity supplements the routine COVID-19 surveillance performed by acute care Infection Prevention and Control and Infectious Diseases practitioners and aids the PHAC’s pandemic response efforts.
With regard to COVID-19, the overarching objective of the SOS Network is to provide Canadian public health decision-makers with detailed, real-time information about the burden of COVID-19 leading to hospitalization in Canadian adults, and to characterize the clinical and epidemiologic characteristics, burden of severe disease, and outcomes of adults admitted with laboratory-confirmed COVID-19. Here we aimed to describe the characteristics and outcomes of adults admitted to hospital with COVID-19 during 2020, representing the first wave and beginning of the second wave of the pandemic in Canada.
METHODS
Eleven sites in four provinces participate in COVID-19 surveillance: Nova Scotia (Halifax/Dartmouth), Québec (Québec and Sherbrooke), Ontario (Ottawa, Hamilton, Toronto, Sudbury), and Alberta (Edmonton), representing a total of over 6,000 adult in-patient beds. Patients with admission dates until December 31, 2020 were included in the present analyses. Outcomes were included up to the data extraction date of March 25, 2021.
Case Definition
Network monitors and/or site health-care staff screened all eligible hospital admissions for COVID-19 using the most current PHAC/SOS COVID-19 case definition.(5) SOS surveillance for COVID-19 has expanded beyond usual influenza seasonal surveillance to take place year-round. For case capture purposes, the following categories of COVID-19 cases are enrolled: “confirmed” (laboratory test positive), “probable” (symptoms and epidemiological exposure criteria met, initial positive test but not confirmed, or inconclusive test result [e.g., poor sample quality]), and “clinical suspicion” (clinical impression of COVID-19 in setting of negative test results presumed false negative, or test not done). Here we report characteristics and outcomes for patients with laboratory-confirmed COVID-19.
Laboratory Testing
Samples from probable COVID-19 cases are collected as standard of care. Nasopharyngeal (NP) or oropharyngeal (OP) swabs are collected and tested using standardized real-time reverse transcriptase polymerase chain reaction (RT-PCR) methods at the local laboratory, with further confirmation at a reference laboratory or the National Microbiology Laboratory, according to local protocol.(6–8)
Measures
Research monitors at each site collect clinical data using standardized data collection forms. Data are gathered from the best available source, including chart review and interviews with patients, families, and clinicians.
Clinical characteristics including age, sex, ethnicity, residential situation (e.g., congregate living, homelessness, long term care [LTC]), admission and discharge diagnoses, length of stay, date of symptom onset, comorbidities, and influenza immunization status. Obesity was defined as BMI ≥30 or obesity noted in chart. Pre-illness level of frailty was measured using the well-validated Clinical Frailty Scale (CFS), which categorizes frailty as follows: 1=very fit, 2=well, 3=managing well, 4=vulnerable/pre-frail, 5=mildly frail, 6=moderately frail, 7=severely frail, 8=very severely frail, 9=end of life.(9,10) Treatment for COVID-19 was recorded, whether following the hospital medical guidelines or under investigation by a COVID-19 clinical trial. Additional data are collected regarding COVID-19 exposure and contact history (e.g., travel, close contact with another confirmed case).
Outcomes included hospital length of stay, intensive care unit (ICU) admission, need for mechanical ventilation, and all-cause in-hospital death.
Reporting
In addition to weekly reporting to PHAC, reporting to provincial public health is provided to complement their efforts and assessment of COVID-19 activity within their jurisdictions.
Analysis
Descriptive statistics are presented as mean (SD) for continuous variables and as frequency (%) for categorical variables. Time span variables, such as length of stay (LOS), are reported as median (interquartile range [IQR]). Independent samples t-tests were used to compare groups on continuous variables and chi-squared tests of independents were used to compare groups on categorical variables. Non-parametric independent samples median tests were used to compared time span variables by age group and frailty. Separate logistic regressions were conducted for each predictor to assess associations with ICU admission, mechanical ventilation, and mortality. We presented unadjusted ORs and ORs adjusted for age, sex, and frailty. The amount of missing data ranged from 60% for known direct exposure to none for mortality and province. Missing data were handled through multiple imputation (MI) (see Table 1 in Appendix A). MI is superior to listwise deletion as it reduces bias and maintains power.(11–13) All variables were included in the imputation model. We imputed 100 data sets and the results were pooled using the rules outlined by Rubin.(11,14) Imputed datasets were created using the multivariate imputation by chained equations (MICE) package in R.(15) Analysis done with listwise deletion and more information about missing data can be found in Appendix A. Analyses were done using SPSS version 26 (IBM SPSS Statistics, Armonk, NY) and R version 4.03 (R Foundation for Statistical Computing; https://www.r-project.org/foundation/).(16)
TABLE 1.
Clinical and demographic characteristics: Mean (SD) or N (%)
| Characteristic |
Full Sample
Total N = 2031 |
Age < 65 yrs
N = 651 |
Age 65 yrs +
N = 1380 |
p |
|---|---|---|---|---|
| Age | 71.0 (17.4) | 50.2 (11.6) | 80.9 (8.9) | <.001 |
|
| ||||
| Female sex | 928 (45.7%) | 280 (43.0%) | 648 (47.0%) | .10 |
|
| ||||
| Ethnicity: | <.001 | |||
| White | 1503 (74.0%) | 387 (59.4%) | 1116 (80.9%) | |
| Black | 96 (4.7%) | 65 (10.0%) | 31 (2.3%) | |
| South Asian | 86 (4.2%) | 42 (6.5%) | 43 (3.1%) | |
| West Asian | 76 (3.8%) | 40 (6.1%) | 37 (2.7%) | |
| Chinese | 102 (5.0%) | 33 (5.1%) | 69 (5.0%) | |
| Other | 168 (8.3%) | 84 (13.0%) | 84 (6.1%) | |
|
| ||||
| Housing: | <.001 | |||
| Private dwelling | 1355 (66.7%) | 577 (88.6%) | 778 (56.4%) | |
| Assisted living facility | 438 (21.5%) | 13 (1.9%) | 425 (30.8%) | |
| Long term care facility | 167 (8.2%) | 18 (2.8%) | 149 (10.8%) | |
| Homeless / shelter | 43 (2.1%) | 33 (5.0%) | 10 (0.7%) | |
| Other | 28 (1.4%) | 11 (1.7%) | 18 (1.3%) | |
|
| ||||
| Province: | <.001 | |||
| Alberta | 130 (6.4%) | 62 (9.5%) | 68 (4.9%) | |
| Ontario | 1003 (49.4%) | 341 (52.4%) | 662 (47.9%) | |
| Quebec | 868 (42.7%) | 230 (35.3%) | 638 (46.2%) | |
| Nova Scotia | 30 (1.5%) | 18 (2.8%) | 12 (0.9%) | |
|
| ||||
| Clinical Frailty Scale: | <.001 | |||
| 1–2 | 354 (17.4%) | 282 (43.4) | 72 (5.2%) | |
| 3 | 491 (24.2%) | 236 (36.2%) | 255 (18.5) | |
| 4 | 314(15.5%) | 69 (10.5%) | 245 (17.8%) | |
| 5 | 259 (12.7%) | 28 (4.3%) | 231 (16.7%) | |
| 6 | 317 (15.6%) | 19 (2.9%) | 298 (21.6%) | |
| 7–9 | 297 (14.6%) | 18 (2.7%) | 279 (20.2%) | |
|
| ||||
| Obese (Calculated BMI ≥30 or obesity noted in medical record) | 553 (27.2%) | 249 (38.3%) | 304 (22.0%) | <.001 |
|
| ||||
| Comorbidities: | ||||
| None of respiratory, cardiovascular, immunosuppressed, or immunocompromised | 371 (18.2%) | 249 (38.2%) | 121 (8.8%) | <.001 |
| Cardiovascular | 1499 (73.8%) | 312 (47.8%) | 1187 (86.0%) | <.001 |
| Respiratory | 613 (30.2%) | 161 (24.7%) | 452 (32.8%) | <.001 |
| Immunosuppressed | 132 (6.5%) | 49 (7.5%) | 83 (6.0%) | .29 |
| Immunocompromised | 42 (2.1%) | 11 (1.7%) | 31 (2.3%) | .37 |
| Travel history | 130 (6.4%) | 46 (7.1%) | 84 (6.1%) | .45 |
| Known direct exposure | 1040 (51.2%) | 324 (49.8%) | 716 (51.9%) | .39 |
|
| ||||
| Occupation: | <.001 | |||
| Health-care worker | 116 (5.7%) | 98 (15.1%) | 18 (1.3%) | |
| Other occupation | 324 (15.9%) | 272 (41.7%) | 52 (3.8%) | |
| Not working | 301 (14.8%) | 208 (32.0%) | 93 (6.8%) | |
| Retired | 1291 (63.5%) | 74 (11.3%) | 1217 (88.2%) | |
|
| ||||
| Goals of Care: | <.001 | |||
| Full resuscitation | 1117 (55.0%) | 599 (92.0%) | 518 (37.5%) | |
| Escalation of care with exceptions | 190 (9.4%) | 21 (3.1%) | 170 (12.3%) | |
| Ward based medical care, but no CPR, ICU, or intubation | 454 (22.3%) | 22 (3.4%) | 432 (31.3%) | |
| Comfort Care | 270 (13.3%) | 10 (1.5%) | 260 (18.9%) | |
APPENDIX A1.
Frequencies for missing data
| Variable | N present | N missing | % missing |
|---|---|---|---|
| Days COVID-19 test sample to admission | 2031 | 0 | 0.0 |
|
| |||
| Mortality | 2031 | 0 | 0.0 |
| Province | 2031 | 0 | 0.0 |
| Sex | 2030 | 1 | 0.0 |
|
| |||
| Age | 2029 | 2 | 0.1 |
|
| |||
| Days symptom onset to admission | 1743 | 288 | 14.2 |
|
| |||
| Days symptom onset to COVID-19 test sample | 1743 | 288 | 14.2 |
|
| |||
| ICU | 1647 | 384 | 18.9 |
|
| |||
| Mechanical ventilation | 1643 | 388 | 19.1 |
|
| |||
| LOS | 1640 | 391 | 19.3 |
|
| |||
| Housing | 1633 | 398 | 19.6 |
|
| |||
| Antibiotics or anti-virals | 1625 | 406 | 20.0 |
|
| |||
| Direct acting antiviral or immunie modulatory medications | 1622 | 409 | 20.1 |
|
| |||
| Clinical Frailty Scale | 1602 | 429 | 21.1 |
|
| |||
| Cardiovascular | 1584 | 447 | 22.0 |
|
| |||
| At least one comorbidity | 1583 | 448 | 22.1 |
|
| |||
| Respiratory | 1583 | 448 | 22.1 |
|
| |||
| Immunosuppressed | 1583 | 448 | 22.1 |
|
| |||
| Immunocompromised | 1583 | 448 | 22.1 |
|
| |||
| Goals of care | 1571 | 460 | 22.6 |
|
| |||
| Travel history | 1463 | 568 | 28.0 |
|
| |||
| Ethnicitya | 1419 | 612 | 30.1 |
|
| |||
| Occupation | 1346 | 685 | 33.7 |
|
| |||
| Known direct exposure | 810 | 1221 | 60.1 |
Research Ethics Board regulations did not allow collection of ethnicity data at some sites.
Ethics
The protocol for active COVID-19 surveillance has been approved by each local site’s Research Ethics Board.
RESULTS
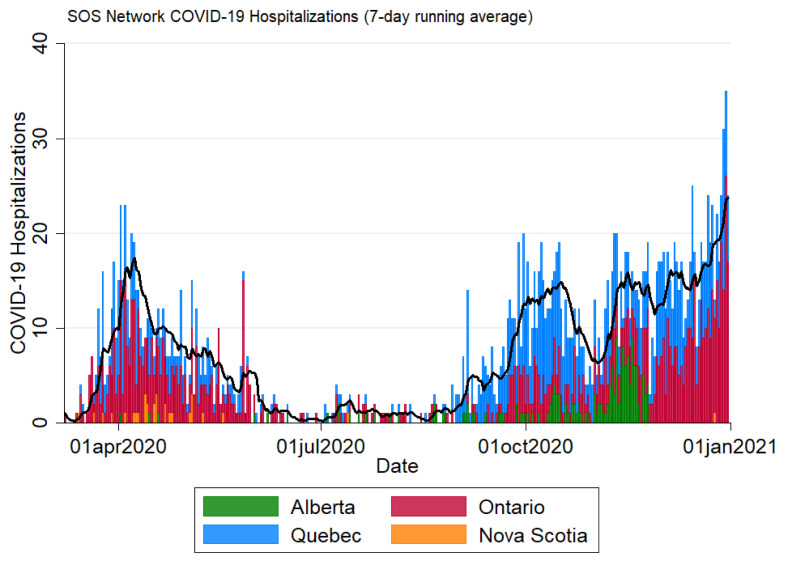
During 2020, 2,011 patients with lab-confirmed COVID-19 were enrolled in the SOS Network (Figure 1). Mean age was 71.0, with a range of 19–105 years. 45.7% were women and 74.0% were white. The majority of patients (66.7%) were admitted from private dwellings; 21.5% were from assisted living facilities, 8.2% were admitted from LTC, and 2.1% from homeless shelters (Table 1). In terms of exposure, 6.4% had a documented travel history and 51.2% had a known COVID-19 exposure.
FIGURE 1.
Epidemic curve of admissions to Serious Outcomes Surveillance (SOS) Network hospitals, colour-coded by province
Overall, all-cause in-hospital mortality was high in this hospitalized cohort. Of those not admitted to ICU, 14.3% (226/1,583) died, compared with 24.6% (110/448) of those admitted to ICU, and 29.6% (73/247) of those who received mechanical ventilation.
Most patients (1,380/2,031 = 67.9%) were 65 or older, although a substantial number (651/2,031 = 32.1%) were under 65. Median age was 74 (IQ = 60–85). Table 2 presents age-stratified outcomes. Length of stay generally increased with age. Mortality showed a strong correlation with age: 4.5% of those <65 died compared with 28.2% aged 85 or older. Odds of dying increased by 5% each year of age, OR = 1.05, 95%CI = 1.04–1.06. Notably, ICU admission and mechanical ventilation rates were similar in those aged <65 and those aged 65–74, but decreased once age was ≥75 years (all p < .001).
TABLE 2.
Medians (interquartile range (IQR)) or frequency (per cent) for outcomes by age
| Outcome |
Whole sample
N = 2,031 |
Age <65
N = 651 |
Age 65–74 N = 377 |
Age 75–84 N = 494 |
Age 85+
N = 509 |
p |
|---|---|---|---|---|---|---|
| Length of stay (days)b | 11 (6–25) | 7 (4–14) | 12 (6–27) | 13 (7–28) | 19 (9–36) | <.001a (<.001, <.001) |
|
| ||||||
| ICU admission | 448 (22.1%) | 203 (31.2%) | 121 (32.1%) | 91 (18.4%) | 33 (6.4%) | <.001 |
|
| ||||||
| Mechanical ventilation | 247 (12.2%) | 112 (17.6%) | 77 (20.5%) | 42 (8.5%) | 16 (3.1%) | <.001 |
|
| ||||||
| Death | 336 (16.5%) | 29 (4.5%) | 63 (16.7%) | 100 (20.3%) | 144 (28.2%) | <.001 |
|
| ||||||
| Days symptom onset to admission | 4 (1–8) | 5 (2–9) | 5 (1–9) | 4 (1–7) | 2 (0–6) | <.001a (<.001, <.001) |
|
| ||||||
| Days Symptom onset to COVID-19 test sample | 2 (0–6) | 2 (0–6) | 3 (0–7) | 2 (0–6) | 1 (0–4) | <.001a (<.001, <.001) |
|
| ||||||
| Days COVID-19 test sample to admission | 0 (0–4) | 1 (0–5) | 0 (0–3) | 0 (0–2) | 0 (−1–3) | <.001a (<.001, <.001) |
|
| ||||||
| Goals of Care: | <.001 | |||||
| Full resuscitation | 1,117 (55.0%) | 599 (92.1%) | 244 (64.7%) | 187 (37.8%) | 88 (17.2%) | |
| Escalation of care with exceptions | 190 (9.4%) | 21 (3.1%) | 35 (9.3%) | 75 (15.1%) | 60 (11.7%) | |
| Ward based medical care, but no CPR, ICU, or intubation | 454 (22.3%) | 22 (3.4%) | 67 (17.8%) | 166 (33.7%) | 199 (39.0%) | |
| Comfort Care | 270 (13.3%) | 10 (1.5%) | 31 (8.2%) | 66(13.4%) | 163 (32.0%) | |
No known pooling method for test between medians. We present the median and IQR range of p values over the 100 multiply imputed datasets.
For length of stay, only those whose admission date was not more than 14 days prior to symptom onset and who were alive at discharge were included (N = 1,593).
At baseline, most patients were not frail (17.4% were very fit or well, 24.2% were managing well, and 15.5% were vulnerable/pre-frail), 12.8% were mildly frail, 15.6% moderately frail, and 14.6% severely frail (Table 1). Length of stay and mortality increased dramatically with frailty, while ICU admission and mechanical ventilation were reduced at higher levels of frailty (all p < .001, Table 3).
TABLE 3.
Medians (interquartile range (IQR)) or frequency (per cent) for outcomes by frailty
| Outcome |
CFS = 1–2 N=354 |
CFS = 3
N=491 |
CFS = 4
N=314 |
CFS = 5
N=259 |
CFS = 6
N=317 |
CFS = 7–9 N=297 |
p |
|---|---|---|---|---|---|---|---|
| Length of stayb | 6 (4–11) | 8 (5–18) | 14 (7–26) | 17 (8–33) | 19 (10–39) | 18 (10–35) | <.001a (<.001, <.001) |
|
| |||||||
| ICU admission | 99 (27.9%) | 157 (32.0%) | 96 (30.5%) | 39 (15.0%) | 36 (11.4%) | 21 (7.0%) | <.001 |
|
| |||||||
| Mechanical ventilation | 57 (16.0%) | 92 (18.8%) | 54 (17.3%) | 20 (7.6%) | 14 (4.3%) | 11 (3.8%) | <.001 |
|
| |||||||
| Death | 10 (2.9%) | 36 (7.4%) | 49 (15.4%) | 51 (19.5%) | 77 (24.1%) | 114 (38.3%) | <.001 |
|
| |||||||
| Goals of Care: | <.001 | ||||||
| Full resuscitation | 337 (95.3%) | 399 (81.3) | 176 (56.0%) | 93 (35.8%) | 77 (24.1%) | 36 (12.1%) | |
| Escalation of care with exceptions | 7 (1.8%) | 36 (7.4%) | 41 (13.0%) | 39 (15.1%) | 44 (14.0%) | 23 (7.8%) | |
| Ward based medical care, but no CPR, ICU, or intubation | 7 (2.0%) | 47 (9.5%) | 77 (24.6%) | 94 (36.3%) | 108 (34.0%) | 122 (40.9%) | |
| Comfort Care | 12 (1.5%) | 20 (6.4%) | 33 (12.7%) | 88 (27.8%) | 116 (39.2%) | ||
No known pooling method for test between medians. We present the median and IQR range of p values over the 100 multiply imputed datasets.
For length of stay, only those whose admission date was not more than seven days prior to symptom onset were included (N = 1,593).
The majority of patients had at least one underlying comorbidity, the most common being cardiovascular (73.8%) and respiratory (30.2%). Only 6.5% were reported to be immunosuppressed and 2.1% were immunocompromised. 27.2% were reported to be obese (Table 1). Individuals with underlying comorbidities experienced more severe outcomes and death than those without. The death rates for cardiovascular, respiratory, immunosuppressed, and immunocompromised were 19.8%, 20.1%, 17.4%, and 21.4%, respectively. The mortality rate was 6.5% for those with none of these comorbidities. The mortality rate for obese participants was 15.0% (Table 4).
TABLE 4.
Demographic and clinical characteristics of patients admitted to ICU and experiencing mortality
| Characteristic |
Admitted to ICU
N = 448 |
Mortality
N = 336 |
|---|---|---|
| Female sex | 151 (33.6%) | 155 (46.1%) |
|
| ||
| Ethnicity: | ||
| White | 309 (69.0%) | 277 (82.6%) |
| South Asian | 19 (4.3%) | 9 (2.7%) |
| Black | 25 (5.5%) | 7 (2.2%) |
| West Asian | 14 (3.1%) | |
| Chinese | 23 (5.0%) | 17 (5.0%) |
| Other | 59 (13.1%) | 25 (7.5%) |
|
| ||
| Housing: | ||
| Private dwelling | 395 (88.3%) | 155 (46.1%) |
| Assisted living facility | 20 (4.5%) | 133 (39.6%) |
| Long term care facility | 20 (4.5%) | 44 (13.0%) |
| Homeless / shelter | 12 (2.7%) | Both groups < 5a |
| Other | ||
|
| ||
| Province: | ||
| Alberta | 33 (7.4%) | 19 (5.7%) |
| Ontario | 239 (53.4%) | 146 (43.5%) |
| Quebec | 169 (37.7%) | 167 (49.7%) |
| Nova Scotia | 7 (1.6%) | < 5 |
| Obese (Calculated BMI ≥30 or obesity noted in medical record) | 173 (38.7%) | 83 (24.6%) |
| Comorbidities: | ||
| None of respiratory, cardiovascular, immunosuppressed, or immunocompromised | 76 (17.0%) | 24 (7.0%) |
| Cardiovascular | 332(74.2%) | 297 (88.5%) |
| Respiratory | 143 (32.0%) | 123 (36.5%) |
| Immunosuppressed | 32 (7.1%) | 23 (6.9%) |
| Immunocompromised | 13 (2.9%) | 9 (2.8%) |
Cells collapsed so no single cell presented with n < 5.
Antibiotic or antiviral use to treat the current episode of illness (including before admission, Emergency Department presentation, and within seven days of the admission or positive COVID-19 result) was reported in 1,464/2,031 (72.1%). During this period in the first wave of the pandemic, direct-acting antiviral or immune modulatory medications were used in only 192/2,031 (9.5%).
In logistic regression models adjusted for age, sex, and frailty, higher frailty was inversely associated with ICU admission and mechanical ventilation, while older age was inversely associated with ICU admission. In the adjusted ICU and mechanical ventilation models, living in LTC was not an independent predictor of outcomes (Table 5). In multivariable models of death, increasing age and frailty were strongly associated with increased mortality. Obesity was associated with ICU admission and mechanical ventilation, but not death. Having at least one comorbidity and cardiovascular disease was associated with increased odds of ICU admission, mechanical ventilation, and death. Respiratory disease was associated with increased odds of death.
TABLE 5.
Odds ratios (95% CI) from logistic regressions
| ICU Admission | Mechanically Ventilated | Mortality | |
|---|---|---|---|
| Unadjusted: | |||
| Age | 0.97 (0.97, 0.98)*** | 0.98 (0.97, 0.99)*** | 1.05 (1.04, 1.06)*** |
| Sex (Female) | 0.53 (0.41, 0.67)*** | 0.52 (0.38, 0.71)*** | 1.02 (0.81, 1.29) |
| CFS | 1–3 | ||
| 1.01 (0.74, 1.38) CFS 4 | 0.98 (0.67, 1.42) | 3.13 (2.01, 4.86)*** | |
| 0.41 (0.26, 0.62)*** CFS 5 | 0.38 (0.22, 0.67)** | 4.16 (2.67, 6.48)*** | |
| 0.24 (0.17, 0.34)*** CFS 6–9 | 0.20 (0.12, 0.33)*** | 7.69 (5.43, 10.90)*** | |
| Non-white | 1.38 (1.05, 1.80)* | 1.31 (0.92, 1.86) | 0.55 (0.40, 0.76)*** |
| Long-term care facility | 0.46 (0.27, 0.78)** | 0.46 (0.23, 0.92)* | 1.92 (1.31, 2.80)** |
| Obesity | 2.00 (1.53, 2.61)*** | 2.05 (1.46, 2.88)*** | 0.85 (0.64, 1.13) |
| Cormorbidity: | |||
| At least onea | 1.12 (0.82, 1.52) | 1.33 (0.87, 2.05) | 3.43 (2.18, 5.39)*** |
| Cardiovascular | 1.03 (0.78, 1.35) | 1.21 (0.84, 1.73) | 3.14 (2.19, 4.52)*** |
| Respiratory | 1.11 (0.87, 1.43) | 0.91 (0.66, 1.27) | 1.41 (1.09, 1.82)** |
|
| |||
| Adjusted:b | |||
| Age | 0.98 (0.98, 0.99)*** | 0.99 (0.98, 1.00) | 1.03 (1.02, 1.04)*** |
| Sex (Female) | 0.57 (0.45, 0.74)*** | 0.58 (0.42, 0.79)** | 0.80 (0.62, 1.03) |
| CFS | 1–3 | ||
| 1.27 (0.90, 1.79) CFS 4 | 1.13 (0.75, 1.69) | 2.19 (1.38, 3.48)** | |
| 0.57 (0.36, 0.90)* CFS 5 | 0.48 (0.26, 0.87)* | 2.50 (1.53, 4.06)*** | |
| 0.37 (0.24, 0.55)*** CFS 6–9 | 0.27 (0.15, 0.47)*** | 4.32 (2.85, 6.55)*** | |
| Non-white | 1.01 (0.75, 1.36) | 0.98 (0.67, 1.43) | 0.86 (0.60, 1.22) |
| Long-term care facility | 1.08 (0.61, 1.94) | 1.22 (0.56, 2.69) | 0.90 (0.60, 1.36) |
| Obesity | 1.71 (1.29, 2.27)*** | 1.78 (1.25, 2.54)** | 1.36 (0.98, 1.89) |
| Cormorbidity: | |||
| At least onea | 2.05 (1.43, 2.94)*** | 2.33 (1.43, 3.77)** | 1.65 (1.00, 2.71)* |
| Cardiovascular | 1.91 (1.38, 2.64)*** | 2.15 (1.40, 3.31)*** | 1.62 (1.09, 2.42)* |
| Respiratory | 1.20 (0.92, 1.57) | 0.97 (0.68, 1.37) | 1.33 (1.01, 1.74)* |
At least one comorbidity of cardiovascular, respiratory, immunosuppressed, or immunocompromised.
Adjusted for age, sex, and Clinical Frailty Scale (CFS).
p<.05.
p<.01.
p<.001.
DISCUSSION
Among the first 2,011 patients enrolled in the SOS Network with laboratory-confirmed COVID-19, there was a wide range in age, frailty, and comorbidities. Many patients had underlying frailty and comorbidities, but a substantial proportion did not. Older age and increasing frailty were independent predictors of higher in-hospital mortality and lower odds of mechanical ventilation or admission to ICU, and obesity was also an independent risk factor for mechanical ventilation and death. Presence of underlying respiratory, cardiovascular and immunocompromising comorbidities remained independently associated with outcomes, though the strength of this association was much attenuated when adjusting for frailty.
The age range (19–105) in our hospitalized cohort was wide. Predictably, older patients were at highest risk of severe outcomes, but not exclusively so. Mortality was high at 16.5% in the sample as a whole, and was higher in the subset of patients admitted to ICU and receiving mechanical ventilation. Our findings are comparable to a published report from the Canadian Nosocomial Infection Surveillance Program(17) (though here we add consideration of frailty), and to published reports of patients hospitalized with COVID-19 in other jurisdictions. A Swedish study reported mortality of 24% in a cohort of 250 hospitalized older adults, with older age and higher frailty being associated with worse outcomes.(18) A Scottish study of 224 adults hospitalized with COVID-19 found mortality of 23%, with age ≥70 and CFS > 3 being independent predictors of mortality.(19)
We found that, while obesity was associated with ICU admission and mechanical ventilation, it was not independently associated with death, while underlying cardiovascular and respiratory conditions were. Obesity has been identified as an important risk factor in other studies.(20,21) Our findings contrast with other studies reporting a stronger increased risk associated with chronic conditions,(22) and indeed we did find stronger associations between comorbidities and mortality prior to adjusting for age and frailty in the multivariable models; this is consistent with other studies where frailty has been considered. For example, a Swedish study also reported that frailty is the strongest predictive factor for mortality in older adults.(23)
During this first wave of COVID-19 hospital admissions in Canada, and early into the second wave in some areas, most patients (72.1%) were treated with antibiotics or antiviral medications, while only 9.5% had received targeted direct-acting antiviral or immune modulatory medications. As data on the effectiveness of these targeted therapies evolve, it is likely that targeted therapies will gain more widespread use.
Our results are similar to aggregate data on hospital admission and outcomes communicated by the PHAC. During the time period of our enrolments, PHAC reported that 32% of hospitalizations occurred in patients aged 80+, while 30% were adults <60. ICU admissions peaked in the 60–69 age group and then declined. 71% of deaths occurred in those aged 80+.(1)
Interestingly, over half of patients were not frail at baseline. Frailty is increasingly recognized as a predictor of outcomes from COVID-19.(18,19,24) Indeed, some (including the UK’s National Institute for Health and Care Excellence) have recommended that frailty be considered in resource allocation decisions.(25,26) While this was not done in the SOS Network sites during this time period to our knowledge, we did find that ICU use and mechanical ventilation declined with frailty. This phenomenon has also been observed in influenza,(27) and likely reflects that these aggressive interventions may not be felt to be consistent with goals of care. Our findings support this, as goals of care were progressively less aggressive as age and frailty increased. Interestingly, a French study reported that older age and frailty were not independently associated with mortality among patient admitted to geriatrics units, suggesting that the field requires more research.(28)
A minority of patients was admitted from LTC or congregate living situations. Although homeless individuals are appropriately recognized as a marginalized and high-risk group for COVID-19 infection and outcomes,(29) differential outcomes were not seen among the small number of patients identified as experiencing homelessness in our study. This is similar to a Belgian study in which 14 (5.8%) of the cohort of 238 admitted patients had similar clinical courses to non-homeless patients.(30)
Just over eight per cent (8.2%) of our cohort were admitted from a LTC facility. LTC and assisted living residents experienced higher mortality (44/167=26.3% and 133/438=30.4%, respectively) than that seen for patients admitted from private dwellings (155/1,355=11.4%). However, LTC residence was not an independent predictor of mortality once age and frailty were taken into account. This may reflect differential decisions to transfer residents who were felt to have better clinical chances of survival to hospital while pursuing treatment or palliative care on site for others. In the bigger picture, LTC has in many ways been the epicentre of COVID-19 mortality in Canada (and other industrialized countries).(31) Although reporting has been suboptimal, estimates suggest that up to 85% of early Canadian COVID-19 deaths have occurred among LTC residents.(32,33) It is also interesting to put our findings regarding LTC facility residents in policy context. In some included jurisdictions (e.g., Nova Scotia), efforts were made to re-allocate resources to the LTC in cases of outbreaks, effectively creating an on-site hospital (which would not be captured in our data), whereas other jurisdictions may have prioritized pathways that would admit LTC facility residents to hospital. This suggests that our estimates of rates of frailty, LTC residence, and mortality among people with COVID-19 are conservative, compared with the overall Canadian experience.
Given interest in understanding how to best prioritize vaccines, demographic characteristics of the admitted patients are of particular interest. Among patients aged 65+, 80.9% were white and 56.4% lived in a private dwelling. For the younger adult group, relatively more patients (40.6%) were racialized and 88.6% lived in private dwellings. Only 6.4% had a travel history, while 51.2% had a known exposure. 5.7% (15.1% of those <65) were health-care workers. Although the majority of our enrolled population was white (which may also reflect the demographics of participating sites), the relative over-representation of racialized persons in the younger cohort is consistent with reports from many other jurisdictions.(34–36) This, along with the over-representation among frontline and health-care workers (also bringing into consideration important socioeconomic and racial contributing factors),(37) supports calls for these groups to be considered when decisions are made regarding prioritization of COVID-19 vaccination.(38)
Our study is not without limitations. COVID-19 surveillance is being conducted in trying and demanding clinical settings, and data collection for some variables that are not part of core reporting to PHAC have been delayed, resulting in missing data for some variables (e.g., 448 cases missing comorbidity data), though we were able to address this in the analyses using Multiple Imputation. Our study was conducted in the real world of the pandemic response as it unfolded across Canada, and laboratory testing protocols, case definitions, and clinical management were modified at the sites as knowledge and resources evolved. SOS surveillance is conducted at 11 participating hospital sites, which each reflect the demographics of their catchment communities, but may not reflect the demographics of COVID-19 admission in Canada as a whole. Given the logistical limitations of collecting data during the pandemic, we were not able to collect data on longer term outcomes, such as worsening frailty and function, which we have previously found to be important sequelae of influenza and are likely important outcomes of COVID-19.(39,40)
Early Canadian experience with hospital-based COVID-19 surveillance demonstrates the importance of frailty and age as independent predictors of lower ICU and mechanical ventilation use and higher mortality. Even so, admitted patients represented a wide spectrum of both frailty and age, and poor outcomes were not limited to frail patients. This clinical impact across the spectrum of frailty is important for informing public health messaging and ongoing COVID-19 control efforts. Continued surveillance, along with understanding of frailty in research and clinical settings, will be critical to informing Canada’s COVID-19 response.
APPENDIX A2.
Clinical and demographic characteristics: Mean (SD) or N (%)a
| Characteristic |
Full Sample
Total N = 2031 |
Age < 65 yrs
N = 651 |
Age 65 yrs +
N = 1378 |
P |
|---|---|---|---|---|
| Age | 71.0 (17.4) | 50.2 (11.6) | 80.9 (8.9) | <.001 |
|
| ||||
| Female sex | 928 (45.7%) | 280 (43.0%) | 646 (46.9%) | .10 |
|
| ||||
| Ethnicity b : | <.001 | |||
| Caucasian | 1133 (79.8%) | 302 (65.2%) | 830 (86.9%) | |
| Black | 53 (3.7%) | 38 (8.2%) | 15 (1.6%) | |
| South Asian | 46 (3.2%) | 25 (5.4%) | 21 (2.2%) | |
| West Asian | 42 (3.0%) | 24 (5.2%) | 18 (1.9%) | |
| Chinese | 45 (3.2%) | 18 (3.9%) | 27 (2.8%) | |
| Other | 100 (7.0%) | 56 (12.1%) | 44 (4.6%) | |
|
| ||||
| Housing: | <.001 | |||
| Private dwelling | 1072 (65.6%) | 484 (88.6%) | 588 (54.1%) | |
| Assisted living facility | 381 (23.3%) | 11 (2.0%) | 369 (34.0%) | |
| Long term care facility | 131 (8.0%) | 16 (2.9%) | 115(10.6%) | |
| Homeless / shelter | 28 (1.7%) | One group < 5 | ||
| Other | 21 (1.3%) | 9 (1.6%) | 12 (1.1%) | |
|
| ||||
| Province: | <.001 | |||
| Alberta | 130 (6.4%) | 62 (9.5%) | 68 (4.9%) | |
| Ontario | 1003 (49.4%) | 341 (52.4%) | 661 (48.0%) | |
| Quebec | 868 (42.7%) | 230 (35.3%) | 637 (46.2%) | |
| Nova Scotia | 30 (1.5%) | 18 (2.8%) | 12 (0.9%) | |
|
| ||||
| Clinical Frailty Scale: | <.001 | |||
| 1–2 | 288 (18.0%) | 237 (43.6%) | 51 (4.8%) | |
| 3 | 387 (24.2%) | 197 (36.2%) | 190 (18.0%) | |
| 4 | 239 (14.9%) | 56 (10.3%) | 183 (17.3%) | |
| 5 | 198 (12.4%) | 22 (4.0%) | 176 (16.7%) | |
| 6 | 251 (15.7%) | 16 (2.9%) | 234 (22.1%) | |
| 7–9 | 239 (14.9%) | 16 (2.9%) | 223 (21.1%) | |
| Obese (Calculated BMI ≥30 or obesity noted in medical record) | 414 (28.8%) | 194 (39.5%) | 220 (23.3%) | <.001 |
|
| ||||
| Comorbidities: | ||||
| None of respiratory, cardiovascular, immunosuppressed, or immunocompromised | 267 (16.9%) | 179 (36.0%) | 88 (8.1%) | <.001 |
| Cardiovascular | 1195 (75.4%) | 251 (50.4%) | 943 (86.9%) | <.001 |
| Respiratory | 508 (32.1%) | 134 (27.0%) | 374 (34.5%) | <.01 |
| Immunosuppressed | 96 (6.1%) | 36 (7.2%) | 60 (5.5%) | .19 |
| Immunocompromised | 21 (1.3%) | One group < 5 | .22 | |
| Travel history | 68 (4.6%) | 26 (5.3%) | 42 (4.3%) | .40 |
| Known direct exposure | 409 (50.5%) | 157 (51.5%) | 251 (49.8%) | .64 |
|
| ||||
| Occupation: | <.001 | |||
| Health-care worker | 61 (4.5%) | 56 (13.9%) | 5 (0.5%) | |
| Other occupation | 198 (14.7%) | 170 (42.2%) | 28 (3.0%) | |
| Not working | 179 (13.3%) | 129 (32.0%) | 50 (5.3%) | |
| Retired | 908 (67.5%) | 48 (11.9%) | 859 (91.2%) | |
|
| ||||
| Goals of Care: | <.001 | |||
| Full resuscitation | 850 (54.1%) | 494 (92.0%) | 356 (34.5%) | |
| Escalation of care with exceptions | 137 (8.7%) | 16 (3.0%) | 121 (11.7%) | |
| Ward based medical care, but no CPR, ICU, or intubation | 352 (22.4%) | 18 (3.4%) | 333 (32.2%) | |
| Comfort Care | 232 (14.8%) | 9 (1.7%) | 223 (21.6%) | |
Percentages are per cent of valid data (i.e., missing not included).
Research Ethics Board regulations did not allow collection of ethnicity data at some sites.
APPENDIX A3.
Medians (interquartile range (IQR)) or frequency (per cent) for outcomes by agea
| Outcome |
Whole sample
N = 2031 |
Age <65
N = 651 |
Age 65–74 N = 377 |
Age 75–84 N = 493 |
Age 85+
N = 508 |
p |
|---|---|---|---|---|---|---|
| Length of stay (days)b | 10 (5–22) | 7 (4–14) | 12 (6–26) | 12 (6.75–25.25) | 20 (8–33.5) | <.001 |
|
| ||||||
| ICU admission | 358 (21.7%) | 169 (30.4%) | 102 (32.6%) | 69 (17.7%) | 18 (4.6%) | <.001 |
|
| ||||||
| Mechanical ventilation | 197 (12.0%) | 94 (16.9%) | 66 (21.1%) | 30 (7.7%) | 7 (1.8%) | <.001 |
|
| ||||||
| Death | 336 (16.5%) | 29 (4.5%) | 63 (16.7%) | 100 (20.3%) | 143 (28.1%) | <.001 |
|
| ||||||
| Days symptom onset to admission | 4 (1–8) | 6 (2–9) | 5 (1–9) | 4 (1–7) | 2 (0–6) | <.001 |
|
| ||||||
| Days Symptom onset to COVID-19 test sample | 2 (0–6) | 2 (0–6) | 3 (0–7) | 2 (0–6) | 1 (0–4) | <.001 |
|
| ||||||
| COVID-19 test sample Days to Admission | 0 (0–4) | 1 (0–5) | 0 (0–3.5) | 0 (0–2) | 0 (−1–3) | <.001 |
|
| ||||||
| Goals of Care: | <.001 | |||||
| Full resuscitation | 850 (54.1%) | 494 (92.0%) | 184 (63.2%) | 127 (34.0%) | 45 (12.2%) | |
| Escalation of care with exceptions | 137 (8.7%) | 16 (3.0%) | 25 (8.6%) | 57 (15.2%) | 39 (10.6%) | |
| Ward based medical care, but no CPR, ICU, or intubation | 352 (22.4%) | 18 (3.4%) | 54 (18.6%) | 134 (35.8%) | 145 (39.4%) | |
| Comfort Care | 232 (14.8%) | 9 (1.7%) | 28 (9.6%) | 56 (15.0%) | 139 (37.8%) | |
Percentages are percent of valid data (i.e., missing not included).
For length of stay, only those whose admission date was not more than 14 days prior to symptom onset and who were alive at discharge were included (N = 1166).
APPENDIX A4.
| Outcome |
CFS = 1–2 N=288 |
CFS = 3
N=387 |
CFS = 4
N=239 |
CFS = 5
N=198 |
CFS = 6
N=251 |
CFS = 7–9 N=239 |
p |
|---|---|---|---|---|---|---|---|
| Length of stayc | 6 (4–10) | 8 (4–16) | 13 (7–25) | 17 (7.75–30) | 18 (10–40) | 20 (10–36) | <.001 |
|
| |||||||
| ICU admission | 72 (25.1%) | 125 (32.4%) | 78 (32.6%) | 27 (13.7%) | 27 (10.8%) | 14 (5.9%) | <.001 |
|
| |||||||
| Mechanical ventilation | 41 (14.3%) | 74 (19.2%) | 45 (18.8%) | 14 (7.1%) | 9 (3.6%) | 7 (3.0%) | <.001 |
|
| |||||||
| Death | 10 (3.5%) | 35 (9.0%) | 46 (19.2%) | 48 (24.2%) | 73 (29.1%) | 111 (46.4%) | <.001 |
|
| |||||||
| Goals of Care: | <.001 | ||||||
| Full resuscitation | 267 (95.7%) | 300 (82.0) | 128 (55.9%) | 62 (32.6%) | 52 (22.5%) | 24 (10.4%) | |
| Escalation of care with exceptions | 29 (4.5%) | 28 (12.2%) | 27 (14.2%) | 30 (13.0%) | 15 (6.5%) | ||
| Ward based medical care, but no CPR, ICU, or intubation | 5 (1.8%) | 34 (9.3%) | 57 (24.9%) | 73 (38.4%) | 73 (31.6%) | 92 (39.8%) | |
| Comfort Care | 10 (1.6%) | 16 (7.0%) | 28 (14.7%) | 76 (32.9%) | 100 (43.3%) | ||
Cells collapsed so no single cell presented with n < 5. Chi-squared based on full data.
Percentages are percent of valid data (i.e., missing not included).
For length of stay, only those whose admission date was not more than 7 days prior to symptom onset were included (N = 1132).
APPENDIX A5.
Admitted to ICU and mortality by demographic information and comorbiditya
| Characteristic |
Admitted to ICU
N=358 |
Mortality
N=336 |
|---|---|---|
| Female sex | 118 (33.1%) | 155 (46.1%) |
|
| ||
| Ethnicity: | ||
| White | 223 (75.1%) | 255 (85.0%) |
| South Asian | 13 (4.4%) | 7 (2.3%) |
| Black | 10 (3.4%) | 5 (1.7%) |
| West Asian | 7 (2.4%) | |
| Chinese | 10 (3.4%) | 13 (4.3%) |
| Other | 34 (11.4%) | 20 (6.7%) |
|
| ||
| Housing: | ||
| Private dwelling | 314 (88.7%) | 153 (46.1%) |
| Assisted living facility | 17 (4.8%) | 132 (39.8%) |
| Long term care facility | 15 (4.2%) | 43 (13.0%) |
| Homeless / shelter | 8 (2.2%) | Both groups < 5 |
| Other | ||
|
| ||
| Province: | ||
| Aberta | 33 (9.2%) | 19 (5.7%) |
| Ontario | 167 (46.6%) | 146 (43.5%) |
| Quebec | 151 (42.2%) | 167 (49.7%) |
| Nova Scotia | 7 (2.0%) | < 5 |
|
| ||
| Obese (Calculated BMI ≥30 or obesity noted in medical record) | 133 (41.2%) | 74 (25.4%) |
|
| ||
| Comorbidities: | ||
| None of respiratory, cardiovascular, immunosuppressed, or immunocompromised | 53 (15.6%) | 22 (6.7%) |
| Cardiovascular | 258 (75.7%) | 292 (88.8%) |
| Respiratory | 116 (34.1%) | 121 (36.8%) |
| Immunosuppressed | 23 (6.8%) | 23 (7.0%) |
| Immunocompromised | 7 (2.1%) | 9 (2.7%) |
Cells collapsed so no single cell presented with n < 5.
APPENDIX A6.
Odds ratios and 95% CIs from logistic regressions
| Characteristic | ICU Admission | Mechanically Ventilated | Mortality | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Unadjusted | Adjusted a | Unadjusted | Adjusted a | Unadjusted | Adjusted a | |
| Age NICU=1594 Nmv = 1594 Nmort=1600 |
0.98*** (0.97, 0.98) | 0.99** (0.98, 1.00) | 0.98*** (0.97, 0.99) | 0.99 (0.98, 1.00) | 1.06*** (1.05, 1.07) | 1.04*** (1.02, 1.05) |
|
| ||||||
| Sex (Female) NICU=1594 Nmv = 1594 Nmort=1600 |
0.51*** (0.40, 0.66) | 0.56*** (0.43, 0.73) | 0.47*** (0.34, 0.65) | 0.53*** (0.38, 0.75) | 1.00 (0.78, 1.28) | 0.76* (0.58, 1.00) |
|
| ||||||
| CFS NICU=1594 Nmv = 1594 Nmort=1600 |
1–3*** | *** | *** | *** | *** | *** |
| 1.18 (0.86, 1.62) CFS 4 |
1.43* (1.01, 2.01) | 1.14 (0.78, 1.66) | 1.26 (0.83, 1.91) | 3.33*** (2.14, 5.18) | 2.18** (1.37, 3.46) | |
| 0.39*** (0.25, 0.60) CFS 5 |
0.52** (0.32, 0.84) | 0.37** (0.21, 0.67) | 0.45* (0.24, 0.84) | 4.47*** (2.87, 6.97) | 2.37** (1.45, 3.88) | |
| 0.22*** (0.16, 0.32) CFS 6–9 |
0.33*** (0.22, 0.51) | 0.17*** (0.10, 0.29) | 0.22*** (0.12, 0.40) | 8.36*** (5.87, 11.91) | 4.16*** (2.72, 6.35) | |
|
| ||||||
| Non-white NICU=1371 Nmv = 1371 Nmort=1375 |
1.49* (1.10, 2.02) | 1.05 (0.76, 1.47) | 1.57* (1.07, 2.30) | 1.15 (0.76, 1.73) | 0.66* (0.46, 0.93) | 1.22 (0.83, 1.81) |
|
| ||||||
| Long-term care facility NICU=1582 Nmv = 1582 Nmort=1587 |
0.47** (0.27, 0.81) | 1.15 (0.62, 2.12) | 0.41* (0.19, 0.90) | 1.21 (0.52, 2.85) | 2.13*** (1.45, 3.15) | 1.00 (0.66, 1.52) |
|
| ||||||
| Obesity NICU=1395 Nmv = 1395 Nmort=1399 |
2.08*** (1.60, 2.71) | 1.78*** (1.34, 2.36) | 2.14*** (1.55, 2.97) | 1.86*** (1.32, 2.62) | 0.85 (0.63, 1.14) | 1.47* (1.04, 2.03) |
|
| ||||||
| Cormorbidity: | ||||||
| At least oneb NICU=1528 Nmv = 1528 Nmort=1534 |
1.18 (0.84, 1.65) | 2.29*** (1.56, 3.38) | 1.48 (0.93, 2.36) | 2.74*** (1.64, 4.60) | 3.21*** (2.04, 5.07) | 1.42 (0.87, 2.34) |
| Cardiovascular NICU=1529 Nmv = 1529 Nmort=1535 |
1.06 (0.79, 1.41) | 2.01*** (1.43, 2.82) | 1.30 (0.88, 1.91) | 2.37*** (1.53, 3.70) | 3.00*** (2.07, 4.33) | 1.48 (0.99, 2.23) |
| Respiratory NICU=1528 Nmv = 1528 Nmort=1534 |
1.10 (0.85, 1.43) | 1.17 (0.88, 1.54) | 0.92 (0.66, 1.29) | 0.96 (0.67, 1.37) | 1.30* (1.01, 1.69) | 1.23 (0.93, 1.62) |
Adjusted for age, sex, and Clinical Frailty Scale (CFS).
At least one comorbidity of cardiovascular, respiratory, immunosuppressed, or immunocompromised.
p<.05.
p<.01.
p<.001.
ACKNOWLEDGEMENTS
The SOS Network is part of the Canadian Immunization Research Network. Funding was provided by the Public Health Agency of Canada and the Canadian Institutes of Health Research. Study sponsors were not involved in the study design, analyses, interpretation or reporting. The authors are solely responsible for final content and interpretation.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood the Canadian Geriatrics Journal’s policy on conflicts of interest disclosure and declare the following interests: MKA reports grant funding from the Public Health Association of Canada, CIHR, Canadian Frailty Network, Sanofi Pasteur and GSK group of companies, and payments from Pfizer, Sanofi Pasteur and Seqirus outside the submitted work. AM reports payments from GSK, Seqirus and Sanofi Pasteur, outside the submitted work. JEM reports payments from RestorBio, Sanofi, GSK, Merck and Medicago outside of the submitted work. TFH reports grants from Pfizer and GSK. ML reports payments from Sanofi, Medicago, Sequirus, and Pfizer outside the submitted work. SAM reports grants and payments from Pfizer, GSK, Merck, Novartis and Sanofi, outside the submitted work. JG, JJL, GB, LV, ME, DM-C, AA, KW, ST, SS, AMc and KK report no conflicts of interest.
FUNDING
The Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance Network is funded by the Canadian Institutes of Health Research (FRN #96974), Public Health Agency of Canada and the COVID-19 Immunity Task Force (CITF).
REFERENCES
- 1.Public Health Agency of Canada. COVID-19 daily epidemiology update [Internet] Ottawa: PHAC; 2020. [cited 2020 November 18]. Available from: https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html. [Google Scholar]
- 2.Nichols MK, Andrew MK, Hatchette TF, et al. Influenza vaccine effectiveness to prevent influenza-related hospitalizations and serious outcomes in Canadian adults over the 2011/12 through 2013/14 influenza seasons: a pooled analysis from the Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance (SOS Network) Vaccine. 2018;36(16):2166–75. doi: 10.1016/j.vaccine.2018.02.093. [DOI] [PubMed] [Google Scholar]
- 3.McNeil S, Shinde V, Andrew M, et al. Interim estimates of 2013/14 influenza clinical severity and vaccine effectiveness in the prevention of laboratory-confirmed influenza-related hospitalisation, Canada, February 2014. Euro Surveill. 2014;19(9):20729. doi: 10.2807/1560-7917.ES2014.19.9.20729. [DOI] [PubMed] [Google Scholar]
- 4.LeBlanc JJ, Li Y, Bastien N, Forward KR, Davidson RJ, Hatchette TF. Switching gears for an influenza pandemic: validation of a duplex reverse transcriptase PCR assay for simultaneous detection and confirmatory identification of pandemic (H1N1) 2009 influenza virus. J Clin Microbiol. 2009;47(12):3805–13. doi: 10.1128/JCM.01344-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Public Health Agency of Canada. National case definition: Coronavirus disease (COVID-19) [Internet] Ottawa: PHAC; 2020. [cited 2020 October 20]. Available from: https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals/national-case-definition.html. [Google Scholar]
- 6.LeBlanc JJ, Gubbay JB, Li Y, et al. Real-time PCR-based SARS-CoV-2 detection in Canadian laboratories. J Clin Virol. 2020;128:104433. doi: 10.1016/j.jcv.2020.104433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeBlanc JJ, Heinstein C, MacDonald J, Pettipas J, Hatchette TF, Patriquin G. A combined oropharyngeal/nares swab is a suitable alternative to nasopharyngeal swabs for the detection of SARS-CoV-2. J Clin Virol. 2020;128:104442. doi: 10.1016/j.jcv.2020.104442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patriquin G, Davis I, Heinstein C, MacDonald J, Hatchette TF, LeBlanc JJ. Exploring alternative swabs for use in SARS-CoV-2 detection from the oropharynx and anterior nares. J Virol Methods. 2020;285:113948. doi: 10.1016/j.jviromet.2020.113948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geriatric Medicine Research. Clinical Frailty Scale [Internet] Halifax: Dalhousie University; n.d.. [cited 2021, 4 May]. Available from: https://www.dal.ca/sites/gmr/our-tools/clinical-frailty-scale.html. [Google Scholar]
- 11.Enders CK. Applied Missing Data Analysis. New York: Guilford Press; 2010. [Google Scholar]
- 12.Faris PD, Ghali WA, Brant R, Norris CM, Galbraith PD, Knudtson ML. Multiple imputation versus data enhancement for dealing with missing data in observational health care outcome analyses. J Clin Epidemiol. 2002;55(2):184–91. doi: 10.1016/S0895-4356(01)00433-4. [DOI] [PubMed] [Google Scholar]
- 13.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7(2):147–77. doi: 10.1037/1082-989X.7.2.147. [DOI] [PubMed] [Google Scholar]
- 14.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: Wiley; 1987. [DOI] [Google Scholar]
- 15.van Buuren S, Groothuis-Ousdhoorn K. mice: multivariate imputation by chained equations in R. J Statist Software. 2011;45(3):1–67. [Google Scholar]
- 16.Foundation R. R: a language and environment for statistical computing. Vienna: R Foundation; 2020. Available from: https://www.r-project.org. [Google Scholar]
- 17.Mitchell R, Choi KB, Pelude L, Rudnick W, Thampi N, Taylor G. Patients in hospital with laboratory-confirmed COVID-19 in a network of Canadian acute care hospitals, Mar. 1 to Aug. 31, 2020: a descriptive analysis. CMAJ Open. 2021;9(1):E149–E56. doi: 10.9778/cmajo.20200246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagg S, Jylhava J, Wang Y, et al. Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc. 2020;21(11):1555–59. doi: 10.1016/j.jamda.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maguire D, Woods M, Richards C, et al. Prognostic factors in patients admitted to an urban teaching hospital with COVID-19 infection. J Transl Med. 2020;18(1):354. doi: 10.1186/s12967-020-02524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrell RJ, O’Regan R, O’Neill E, et al. Sociodemographic variables as predictors of adverse outcome in SARS-CoV-2 infection: an Irish hospital experience. Irish J Med Sci. 2021;190(3):893–903. doi: 10.1007/s11845-020-02407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soeroto AY, Soetedjo NN, Purwiga A, et al. Effect of increased BMI and obesity on the outcome of COVID-19 adult patients: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14(6):1897–904. doi: 10.1016/j.dsx.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mesas AE, Cavero-Redondo I, Alvarez-Bueno C, et al. Predictors of in-hospital COVID-19 mortality: a comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PLoS One. 2020;15(11):e0241742. doi: 10.1371/journal.pone.0241742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tehrani S, Killander A, Astrand P, Jakobsson J, Gille-Johnson P. Risk factors for death in adult COVID-19 patients; frailty predicts fatal outcome in older patients. Int J Infect Dis. 2021;102:415–21. doi: 10.1016/j.ijid.2020.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kundi H, Cetin EHO, Canpolat U, et al. The role of frailty on adverse outcomes among older patients with COVID-19. J Infect. 2020;81(6):744–51. doi: 10.1016/j.jinf.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institute for Health & Care Excellence. COVID-19 rapid guideline: critical care in adults [Internet] London, UK: NICE; 2020. [cited 2020 October 21]. Available from: https://www.nice.org.uk/guidance/ng159. [PubMed] [Google Scholar]
- 26.Rockwood K, Theou O. Using the Clinical Frailty Scale in allocating scarce health care resources. Can Geriatr J. 2020;23(3):210–15. doi: 10.5770/cgj.23.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrew MK, Shinde V, Ye L, et al. The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis. 2017;216(4):405–14. doi: 10.1093/infdis/jix282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinmeyer Z, Vienne-Noyes S, Bernard M, et al. Acute care of older patients with COVID-19: clinical characteristics and outcomes. Geriatrics. 2020;5(4):65. doi: 10.3390/geriatrics5040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller JP, Phillips G, Hutton J, et al. COVID-19 and emergency care for adults experiencing homelessness. Emerg Med Aust. 2020;32(6):1084–86. doi: 10.1111/1742-6723.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrooyen L, Delforge M, Lebout F, Vanbaelen T, Lecompte A, Dauby N. Homeless people hospitalized with COVID-19 in Brussels. Clin Microbiol Infect. 2021;27(1):151–52. doi: 10.1016/j.cmi.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrew M, Searle SD, McElhaney JE, et al. COVID-19, frailty and long-term care: implications for policy and practice. J Infect Dev Countr. 2020;14(5):428–32. doi: 10.3855/jidc.13003. [DOI] [PubMed] [Google Scholar]
- 32.Comas-Herrera A, Zalakaín J, Litwin C, Hsu AT, Lane N, Fernández JL. Mortality associated with COVID-19 outbreaks in care homes: early international evidence. London, UK: International Long-term Care Policy Network CPEC-LSE; 2020. May 3, Available at: www.ltccovid.org. [Google Scholar]
- 33.Walsh M, Semeniuk I. Long-term care connected to 79 per cent of COVID-19 deaths in Canada. The Globe and Mail. 2020. Apr 28, Available from: https://www.theglobeandmail.com/politics/article-long-term-care-connected-to-79-per-cent-of-covid-19-deaths-in-canada/
- 34.Moore JT, Ricaldi JN, Rose CE, et al. Disparities in incidence of COVID-19 among underrepresented racial/ethnic groups in counties identified as hotspots during June 5–18, 2020–22 States, February–June 2020. Morbidity and Mortality Weekly Report (MMWR) 2020;69(33):1122–26. doi: 10.15585/mmwr.mm6933e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otu A, Ahinkorah BO, Ameyaw EK, Seidu AA, Yaya S. One country, two crises: what Covid-19 reveals about health inequalities among BAME communities in the United Kingdom and the sustainability of its health system? Int J Equity Health. 2020;19(1):189. doi: 10.1186/s12939-020-01307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raine S, Liu A, Mintz J, Wahood W, Huntley K, Haffizulla F. Racial and ethnic disparities in COVID-19 outcomes: social determination of health. Int J Environ Res Public Health. 2020;17(21):8115. doi: 10.3390/ijerph17218115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bui DP, McCaffrey K, Friedrichs M, et al. Racial and ethnic disparities among COVID-19 cases in workplace outbreaks by industry sector—Utah, March 6–June 5, 2020. Morbidity and Mortality Weekly Report (MMWR) 2020;69(33):1133–38. doi: 10.15585/mmwr.mm6933e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt H, Pathak P, Sönmez T, Ünver MU. Covid-19: how to prioritize worse-off populations in allocating safe and effective vaccines. BMJ. 2020:371. doi: 10.1136/bmj.m3795. [DOI] [PubMed] [Google Scholar]
- 39.Lees C, Godin J, McElhaney JE, et al. Frailty hinders recovery from influenza and acute respiratory illness in older adults. J Infect Dis. 2020;222(3):428–37. doi: 10.1093/infdis/jiaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrew MK, MacDonald S, Godin J, et al. Persistent functional decline following hospitalization with influenza or acute respiratory illness. J Am Geriatr Soc. 2021;69(3):696–703. doi: 10.1111/jgs.16950. [DOI] [PMC free article] [PubMed] [Google Scholar]