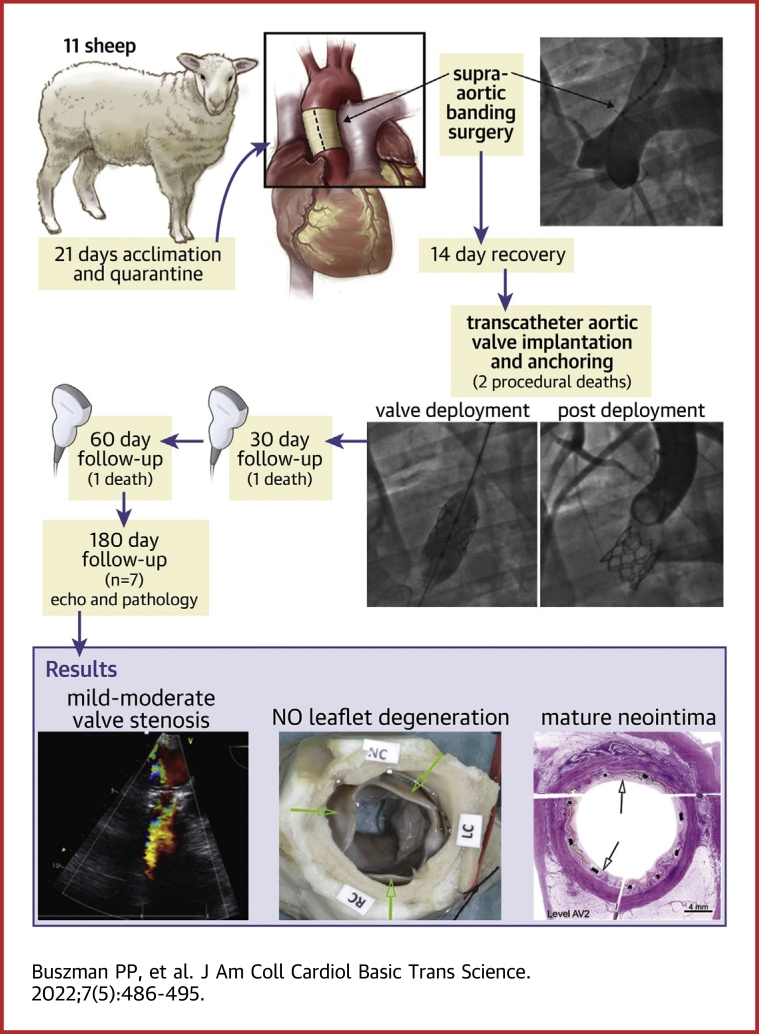
Visual Abstract
Key Words: aortic stenosis, innovation, preclinical research, TAVI
Abbreviations and Acronyms: LC, left coronary; RC, right coronary; TAVI, transcatheter aortic valve implantation; THV, transcatheter heart valve; TTE, transthoracic echocardiography; Vmax, maximum velocity
Highlights
-
•
Evaluation of long-term durability and biological response of new THVs has been limited in the preclinical setting.
-
•
We assessed a balloon expandable THV system (Myval) in the novel, ovine banding model.
-
•
TAVI and anchoring of valves was achieved in all cases with high procedural success.
-
•
Long-term (6-month) observation was feasible with cumulative 36.3% mortality.
-
•
The studied prosthesis showed good hemodynamic performance and optimal healing in pathology.
Summary
The aim of the study was to evaluate a balloon expandable transcatheter heart valve (THV) system (Myval) at 6-month follow-up in ovine banding model. Eleven THV systems were implanted via carotid approach. There were 2 procedure-related deaths and 2 premature deaths. At 6 months all valves that completed follow-up (n = 7) were functional, with no significant regurgitation, calcification, thrombi, or vegetation. Mean pressure gradient was 21.9 ± 11 mm Hg, maximum velocity = 3.3 ± 1 m/s, and ejection fraction was 53.3 ± 6%. Myval THV showed optimal hemodynamic performance and biocompatibility.
The introduction of transcatheter heart valves (THVs) and transcatheter aortic valve implantation (TAVI) was a major milestone in the treatment of calcific aortic valve stenosis.1,2 Currently, not only is TAVI the method of choice for patients who are inoperable and at high surgical risk, but also, most recently, indications have been expanded to patients in the moderate and low-risk subsets.2,3 This new reality creates more challenges for new THV technologies to further improve outcomes and overcome current limitations of THV, including paravalvular leaks, vascular complications, and long-term durability, by introducing novel solutions and upgrades to currently available technologies. Additionally, as indication expands, the demand is increasing and the access to TAVI attributed to cost in many countries remains a limitation.
Currently, long-term evaluation in the preclinical setting of new THV technologies has been limited to acute implantation feasibility studies caused by lack of calcifications and anchoring mechanism. The only available models of THV implantation in the native annulus or in descending aorta with creation of aortic valve insufficiency were associated with high procedural and follow-up mortality rates.4, 5, 6 Although anchoring was achieved in the most recent model by Carney et al,7 this model requires cardiopulmonary bypass and long creation times, and mortality remains significant. The summary of currently available models of preclinical models and methods of THV evaluation is presented in Table 1.
Table 1.
Currently Available Animal Models and Methods of THV Evaluation
| Carney et al (2022)7 | Eltchaninoff et al (2006)5 | Scherman et al (2019)11 | PMA P130009: FDA Summary of Safety and Effectiveness Data (2012)4 | |
|---|---|---|---|---|
| Model | Sheep underwent the MAA procedure and were recovered. At 60 days post-MAA TAVI was performed. | Moderate to severe aortic insufficiency was created in 14 juvenile sheep using a bioptome device. A 23-mm PHV was implanted distal to the left subclavian artery. | None | Annuloplasty rings were surgically implanted into the aortic annulus to model the semirigid environment of the diseased aortic root found in the stenotic clinical situation. |
| Methods | TAVI 60 days after model creation. | TAVI in the heterotopic position following model creation. | A modified THV valve with anchoring mechanisms and stabilization arms implanted transapically. | THVs were surgically implanted, but deployed using a delivery system for simulated use. |
| Sheep | 5 p | 14 | 5 | 34 |
| Follow-up duration, d | 140 | 150 | 150 | 70 and 140 |
| Procedural success, % | 100 | 71 | 60 (2 significant paravalvular leaks) | 97 |
| Procedural mortality | 0 | 4 | 0 | 1 |
| 30-d mortality | 2 | 4 | 0 | NA |
| 70-d mortality | NA | NA | NA | 11 |
| 90-d mortality | NA | 2 | 0 | NA |
| 140-/150-d mortality | NA | 2 | 0 | 5 |
| Cumulative mortality at terminal follow-up | 2 (40) | 8 (57.1) | 0 | 17 (50) |
Values are n or n (%) unless otherwise indicated.
MAA = modified aortic annuloplasty; NA = not available; PHV = prosthetic heart valve; TAVI = transcatheter aortic valve implantation; THV = transcatheter heart valve.
Most recently, we introduced a novel ovine model of supra-aortic banding and THV implantation that allows for valve anchoring and long-term mechanical and biological evaluation, which is associated with significantly lower mortality when compared to previous models.8,9
Herein, we evaluated 6 months of mechanical and hemodynamic performance as well as durability and biological response of a novel, hybrid honeycomb cell design THV Myval (Meril Life Sciences) in the novel ovine model of supra-aortic banding.
Methods
Study design
The study protocol was accepted by the local ethics committee for animal research and approved by the sponsor and study investigators. All animals received standard of care as outlined in the study protocol and in accordance with the act of animal welfare and the “Principles of Care of Laboratory Animals.”10 This study was conducted at the Good Laboratory Practices–certified Experimental Laboratory of the Center for Cardiovascular Research and Development of American Heart of Poland, Kostkowice.
A total of 11 blackface crossbreed sheep, ∼2 years old, weighing 50-60 kg were included. Animals went through an acclimation period of at least 21 days prior to the beginning of the study and were fed with a commercially available diet. Ascending aortic stenosis was created by fixing a surgical band around the aorta. After aortic banding creation, animals were allowed a recovery period of at least 10 days. Subsequently, a Myval THV was implanted using surgical access cut-down and transcatheter techniques. Follow-up transthoracic echocardiography (TTE) and complete blood works were performed at 30, 90, and 180 days. Animals were sacrificed at 180 days, and the THV with surrounding tissues was harvested for pathological and radiographic evaluation.
Study device
The first-generation Myval THV comprises a balloon-expandable, radiopaque, nickel-cobalt alloy (MP35N) frame with a novel, hybrid cell design and a tri-leaflet bovine pericardial tissue valve. The stent is covered with polyethylene terephthalate inner skirt and a polyethylene terephthalate outer skirt (Figure 1). The THV is a terminally sterilized, single-use device, indicated for relief of aortic stenosis in patients with symptomatic heart disease caused by severe native calcified aortic stenosis in patients at moderate or greater risk for open surgical valve replacement. Devices are stored in a glutaraldehyde solution. Myval THV is available in the following diameters: 20 mm, 21.5 mm, 23 mm, 24.5 mm, 26 mm, 27.5 mm, and 29 mm. In the current study, only valve diameters of 20 and 23 mm were used because of anatomical constraints. At the time of experiment, a 20- to 24-F, 30-cm introductory sheath was used.
Figure 1.
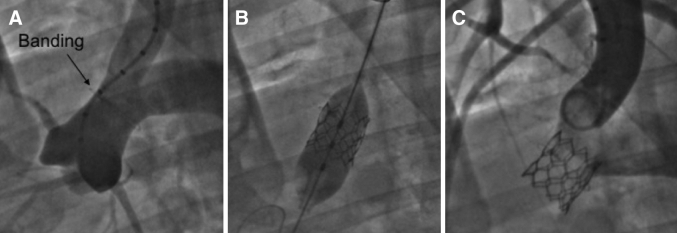
Aortography and TAVI
Aortic banding and THV implantation via carotid artery approach. (A) Preimplantation. (B) Valve implantation. (C) Aortography after implantation. TAVI = transcatheter aortic valve implantation; THV = transcatheter heart valve.
Aortic banding model
Sheep were anesthetized using a combination of intramuscular/intravenous ketamine 10 mg/kg + intramuscular xylazine 0.05-0.2 mg/kg + intramuscular atropine 0.1-0.2 mg/kg. Propofol 2-4 mg/kg was administered intravenously to facilitate intubation. Following successful intubation, sheep were placed in right lateral recumbency. Anesthesia was maintained using isoflurane 1%-2% in 100% oxygen. An orogastric tube was placed to decrease abdominal pressure and drain content from the rumen. Throughout the procedure, monitoring was performed for pulse oximetry, heart rate, and invasive blood pressure, as well as for arterial blood gases.
Aortic banding was achieved by means of a minimally invasive left-side thoracotomy. An incision was made between the fourth and fifth intercostal spaces, and the ascending aorta was exposed. The target site for the banding implantation was midway from the sino-tubular junction and bifurcation with the innominate trunk. With the help of sizer kits, the Dacron sleeve was measured, and the diameter of the aorta decreased between 2 and 3 mm to achieve 10% diameter stenosis depending on the diameter of ascending aorta. A surgical stainless steel wire was sutured in the midline of the banding tissue to allow identification under fluoroscopy. After the procedure completion, the wound was closed and the sheep moved to postoperative recovery.
TAVI
Anesthesia was induced using the same procedures outlined for aortic banding.
Sheep were placed in dorsal recumbency with the legs stretched caudally. The left carotid artery was surgically exposed and prepared, as close to the thoracic inlet as possible. A 6-F arterial sheath was placed in the carotid artery. A 0.035-inch J wire was advanced through the arterial sheath in the left ventricle, and a 5-F pigtail catheter with 10-mm markers was advanced over the J wire. Ventriculography and aortography were performed to assess the banding site and measure the target implantation site diameter. The pigtail catheter markers were used to calibrate the distance.
After all measurements are finished, the pigtail catheter and the J wire were removed.
The THV was crimped on a balloon matching the valve size (a 20-mm balloon for a 20-mm valve) and in the natural direction of blood flow from the heart (aortic position). The 6-F arterial sheath was removed and replaced with an arterial sheath 30%-40% bigger than the measured profile of the valve (20- to 26-F; Sentrant, Medtronic).
Once the large arterial sheath was inserted, heparin was administered intravenously at a dose of 300 IU/kg (3 mg/kg) to achieve an activated clotting time >300 seconds.
A super stiff Amplatz wire was advanced through the arterial sheath into the left ventricle. The valve crimped on the balloon was advanced over the Amplatz stiff wire and into the arterial sheath to the aortic banding. The valve was expanded with the help of a 50-mL syringe filled with 70:30 ratio of saline and contrast. Once the implantation was complete, the guiding wire and the balloon were removed.
Postimplantation control ventriculography and aortography were performed as outlined without changing the arterial sheath.
The arterial sheath was then removed, the carotid artery closed down, and the tissues and skin sutured in 3 layers. The sheep was then transferred to postoperative recovery.
Echocardiography
TTE was performed at 30, 90, and 180 days. Transesophageal echocardiography was performed under anesthesia at 180 days as a complement to TTE. All routine parameters (eg, left ventricle end-diastolic volume, aortic diameter, left ventricle end-systolic diameter, ejection fraction, cuspids separation) were evaluated, and valve functionality, deployment, and any other visual findings were documented in the echocardiogram reports. Visual data were not available for 1 animal at day 90 because of poor visibility.
Pathological evaluation
The independent, pathology core lab (Alizée Pathology) received fixed, explanted hearts and ascending aortas for histopathology. Hearts were trimmed and the segment of tissue containing the explants was excised, grossly examined, and radiographed. Aortic roots with valve implants were dehydrated in a graded series of ethanol, cleared in xylene, and infiltrated and embedded in Spurr’s plastic resin. After polymerization, the device with frame was sectioned radially twice to capture each cusp (left coronary [LC] cusp, right coronary[RC] cusp, and noncoronary cusp) and stained with hematoxylin and eosin. In addition, the portion of the plastic block containing each of the 3 valve cusps (radial planes) was separated from the frame, cut serially twice (thin sections), and stained with Movat’s Pentachrome and Von Kossa. The block remnants were reassembled with appropriate spacers and cut crosswise (transverse plane) at 2 levels. All ground sections were ground and micropolished to optical finish using the Exakt cutting/grinding system. Resulting sections were stained with hematoxylin and eosin. Trackable gross lesions submitted separately were processed, embedded in paraffin or Spurr’s resin as appropriate, sectioned, and stained with hematoxylin and eosin and/or Masson’s Trichrome (paraffin only). All resulting slides were evaluated via light microscopy by the study pathologist.
Statistics
This is a prospective, observational, and experimental study; therefore, no study hypothesis was made. Normally distributed data are presented as mean ± SD, whereas nonparametric as proportions and percentages. To test for temporal differences in echocardiographic parameters, repeated measures analysis of variance was performed followed by the pairwise comparison with the Bonferroni modified paired Student’s t-test. A value of P < 0.05 was considered statistically significant. MedCalc Statistical Software version 14.12.0 (MedCalc Software) was used for analysis.
Results
Study flowchart and animal mortality
A detailed study schematic is presented in Figure 2. In total 11 sheep were included in which surgical aortic banding procedure was performed as described previously. There were no deaths during the banding procedure and the following 2 weeks. Subsequently TAVI into banding site was performed. All THVs were implanted successfully. There were 2 early, procedure-related deaths that occurred within 24 hours after implantation. There were 2 additional deaths before the 6-month follow-up. In total, the procedural mortality was 18%, whereas mortality at the 6-month follow-up was 36%. In total 7 valves completed the protocoled 6-month follow-up.
Figure 2.
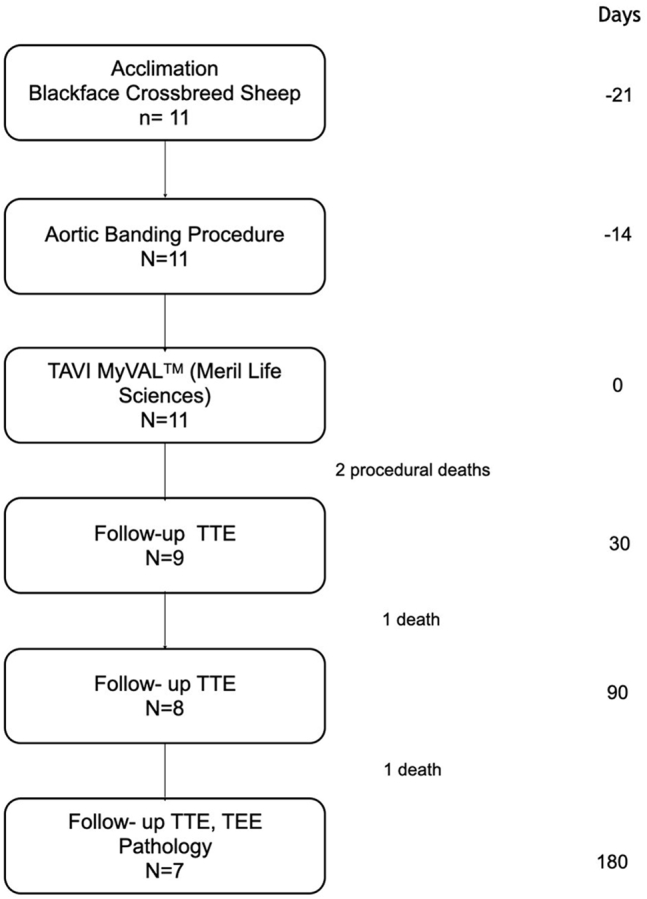
Study Schematic
Schematic depicting study stages, follow-up, animals included and mortality. TAVI = transcatheter aortic valve implantation; TEE = transthoracic echocardiography; TTE = transthoracic echocardiography.
Procedural outcomes
All prostheses (n = 11) were delivered and deployed successfully (Figure 3). There were no perivalvular leaks in any case. Full leaflet functionality and coaptation were confirmed using angiography and TTE. In 1 case, because of high banding and stenosis site, the THV was implanted toward the brachiocephalic trunk. This resulted in the subocclusion of the aorta and the animal was sacrificed. Severe vascular complication in the form of arterial inversion during sheath placement and bleeding after withdrawal was the cause of the second death.
Figure 3.
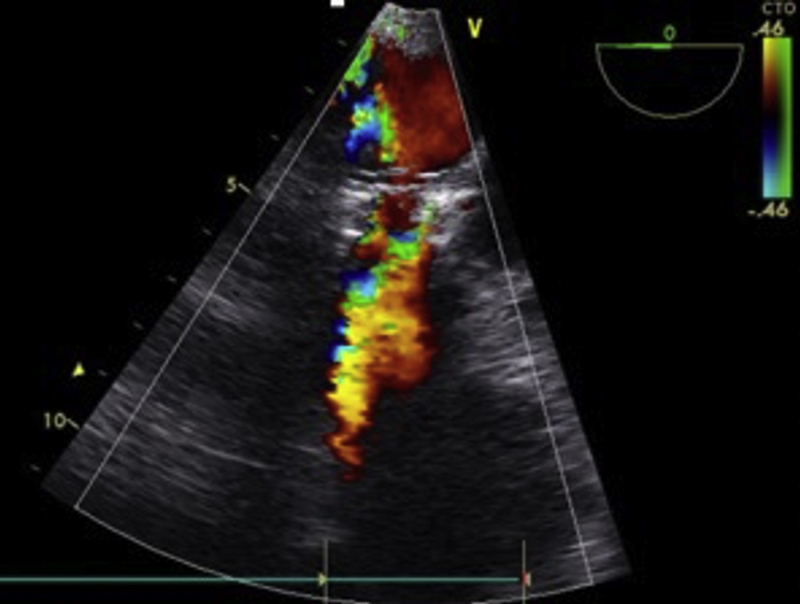
TEE at 6-Month Follow-Up
Representative image of transthoracic echocardiography (TEE) at 6-month follow-up, showing color Doppler flow through the transcatheter heart valve prosthesis.
Unscheduled deaths
Because of clinical deterioration there were 2 unscheduled deaths (preterminal sacrifices) at days 74 and 133 of follow-up. In the first animal, the native LC cusp was covered by the conduit. The LC and RC prosthetic cusps were encased in fibrous pannus and fixed in an open position. The noncoronary cusp was semiopen. Chronic native aortic insufficiency was likely the cause of chronic heart failure in this animal.
The second animal was prematurely sacrificed because of septic vegetative endocarditis and thrombosis on all implant cusps with bacteria and pseudohyphae present (likely consistent with Candida spp.).
Echocardiographic follow-up
The echocardiographic (transesophageal echocardiography and terminal TTE) findings of surviving animals are presented in Table 2. Valve functionality was confirmed in all cases. At follow-up there were no cases of severe prosthesis stenosis, regurgitation, or paravalvular regurgitation, although the mean pressure gradients were mild or moderate (Figure 3). This was caused mainly by unexpandable and tight aortic bindings that consequently caused relative valve stenosis and pannus creation. Additionally, as the animals grow, the gradient tends to increase. As the study and learning curve progressed, the bandings were later less tight and relative stenosis occurred less often. In the surviving animals there were no signs of heavy, moderate, or mild calcifications. In 1 case small or mild calcifications were noted that were no longer visible at terminal, 180-day follow-up.
Table 2.
Temporal TEE and Doppler Evaluation
| 30-Day Follow-Up (n = 9) | 90-Day Follow-Up (n = 7) | 180-Day Follow-Up (n = 7) | P Value | |
|---|---|---|---|---|
| Doppler measurements | ||||
| Maximum velocity, m/s | 3.2 ± 0.8 | 3.2 ± 1.8 | 3.3 ± 1,0 | 0.610 |
| PG max, mm Hg | 42.2 ± 21.8 | 45.9 ± 39 | 46.7 ± 27.7 | 0.479 |
| PG mean, mm Hg | 23.8 ± 13.8 | 27.1 ± 24.8 | 21.9 ± 11.5 | 0.552 |
| EF | 60.1 ± 0.7 | 63.5 ± 1.4 | 55.0 ± 2.1 | 0.010a |
| LVEDD | 48.8 ± 2.1 | 45.1 ± 1.7 | 43.0 ± 1.8 | 0.142 |
| LVESD | 28.3 ± 27.8 | 27.8 ± 1 | 28.1 ± 1.5 | 0.977 |
| Echocardiographic findings | ||||
| Mean PG >40 mm Hg | 0 | 1 | 0 | |
| Decreased LVEF | 1 | 1 | 1 | |
| Increased echogenicity of valve graft | 1 | 1 | 0 | |
| Small calcifications on leaflets | 1 | 1 | 0 | |
| Probable vegetation | 0 | 0 | 0 | |
| Valve dislocation form banding | 0 | 0 | 0 | |
| Pericardial fluid >5 mm | 2 | 1 | 0 | |
| Mild aortic regurgitation | 1 | 0 | 1 | |
| Moderate aortic regurgitation | 1 | 0 | 1 |
Values are n or mean ± SD.
EF = ejection fraction; LVEDD = left ventricular end-diastolic diameter; LVEF = left ventricular ejection fraction; LVESD = left ventricular end-systolic diameter; PG = pressure gradient; TEE = transesophageal echocardiography.
P = 0.069 for LVEF 90- versus 180-day follow-up.
Radiography
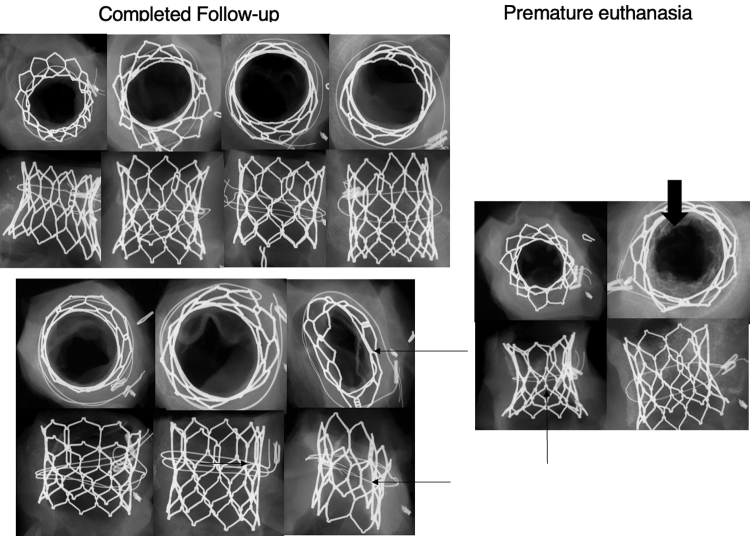
Radiographs of all valves are show in Figure 4. There were no frame fractures and no gross structural abnormalities except for flattening of the stent valve in 2 cases caused by banding. There was no radiographic evidence of calcification at day 180 in animals sacrificed at term, although minimal to mild cusp calcification was observed in a single animal and was most likely caused by the flattening of the valve.
Figure 4.
Radiographic Images of Valves After Animals Were Sacrificed
Radiographic images of valves from animals with completed follow-up (left) and those who were prematurely sacrificed (right). Thin arrows indicate relative valve stenosis caused by tight banding. Thick arrowhead shows bacterial vegetations.
Pathology
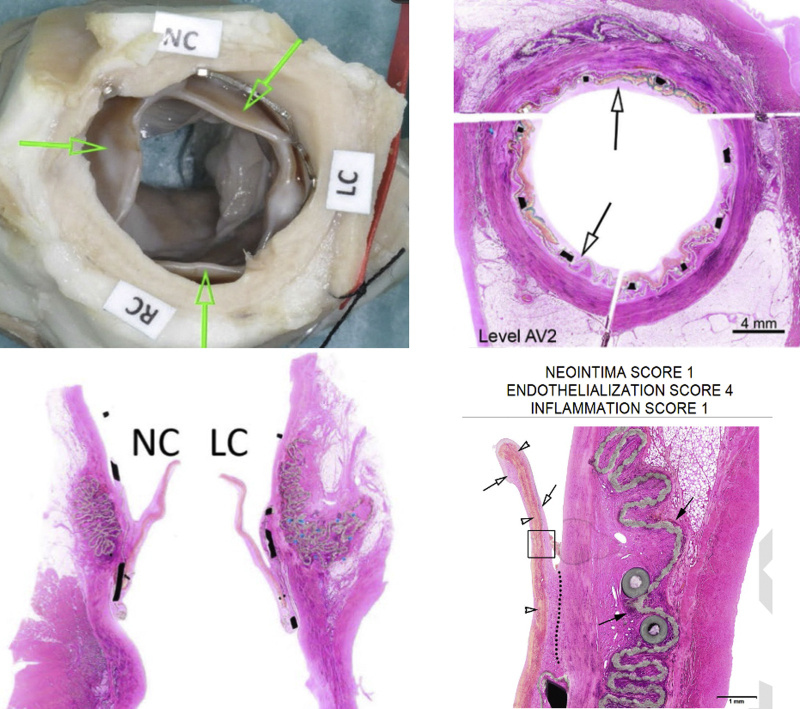
Representative macro- and microscopic pictures are shown in Figure 5. Evaluation of 7 terminally sacrificed animals (up to ∼180 days) and 2 early death animals (at 74 and 133 days) implanted with Myval THV revealed deployment and anchoring of the device within the ascending aorta varying distances above the native annulus in the banding site in all animals. Native aortic cusps remained free and functional in most animals. All animals sacrificed at term showed healing and integration features in the cusps characterized by complete coverage of the cusps by endothelialized fibrocellular neointima that frequently progressed to thicker pannus formation, resulting in cusp immobility and/or adhesion to the conduit wall. This may be caused by incomplete coverage of native valve, resulting in flow disturbances and excessive neointima proliferation downstream of the native valve.
Figure 5.
Representative Pathological Images of THV Implant at 180 Days
Representative pathological images of a Myval transcatheter heart valve implant (THV) at 180 days. (Upper left)Arrows indicate implant cusps showing neointima formation. (Upper right) Stent frame. Arrows indicate fibrocellular endothelialized neointima covering the stent. (Lower left) Normal conformation of the implant cusps with coverage by slight to mild fibrocellular neointima (noncoronary [NC] and left coronary [LC] implant cusps). (Lower right)Arrows indicate diffuse coverage of the LC implant cusp by mature fibrocellular and endothelialized neointima. Dotted line indicates slight pannus formation. Open arrowheads indicate collagenous bioprosthetic material. Solid arrows indicate polyester pad showing minimal foreign body response. AV = aortic valve; RC = right coronary.
There was no or very minimal cusp calcification and optimal local biocompatibility features characterized by no foreign body reaction to the collagenous cusp biomaterial. The conduits showed full and stable integration and healing through formation of endothelialized, fibrocellular neointima. There was minimal to mild foreign body response associated with the polyester pads/cushion that was well within the expected and acceptable range for this type of implant.
There was no thrombosis in the cusps for animals sacrificed at term and no thrombosis in the conduits.
Congestive heart failure caused by the entrapment of the native aortic valve leaflet by the prosthesis was the most like cause of the preterm sacrifice in the first animal. A bacterial/fungal and thrombotic complication associated with the implant cusps caused premature death in the second animal (septic vegetative endocarditis).
In general, the optimal biocompatibility of THV material (cusps, sutures, and stent) can be qualified as nonirritant-based ISO 10993-6 criteria.
Discussion
We report 6-month outcomes of a percutaneously implanted THV prosthesis in the novel, supra-aortic ovine banding model. To the best of our knowledge this is a first evaluation of THV prosthesis at long-term follow-up in the animal model with lowest mortality, which allows anchoring of the THV, assessment of prosthesis functionality, durability, and biological response. In 11 total animals, there were 2 procedure-related deaths and 2 premature deaths during the 6-month follow-up, one caused by infective endocarditis and the other by native valve cusp entrapment, pannus creation, and prosthesis dysfunction (the LC and RC prosthetic cusps were encased in fibrous pannus and fixed in an open position). All remaining prostheses were fully functional in echocardiographic evaluation, with no thrombosis, calcifications, or perivalvular leaks. The overall mortality is significantly lower when compared with that of previous preclinical models of THV implantation and long-term evaluation with total mortality at follow-up of 36% versus 57% as described by others4,5,7 (Table 1). As the learning curve progresses, we have shown even lower procedure-related mortality.
Long-term evaluation of transcatheter aortic valve prostheses in the preclinical setting, although desired to prove durability, resistance to calcification, and thrombus formation has been difficult to date. Lack of calcification in large animal models made the anchoring of the valve not possible, and usually implants did not sustain their position in native aortic annulus. Previous surgical models, although feasible, were associated with high or very high mortality, long creation, use of cardiopulmonary bypass, and heterotopic locations of implantations. Therefore in the current study we used a novel model of supra-aortic aortic banding prior to TAVI, which has been validated in large-series experiments.8,9 This allowed us to anchor all implanted prostheses and perform a 6-month follow-up, albeit longer follow-up periods are possible. Contrary to previous reports,11 in this model THV does not require any modification to anchor the prosthesis, thereby showing better resemblance to clinical setting.
Echocardiography showed no signs of valve hemodynamic dysfunction or valve degeneration caused by either calcification or thrombus in the surviving animals. The gradients were similar across the follow-up periods. It is notable, however, that the mean gradient of all implanted valves was suggestive of mild to moderate stenosis. In 1 case it reached the value of significant THV stenosis. None of these valves have shown leaflet deterioration in echocardiography or pathology. The reasons for elevated mean gradient at follow-up are multifactorial. First, in the early experiments, the banding itself caused 20%-30% stenosis to achieve anchoring, which is not expandable during implantation. Our later experience decreased this stenosis Additionally, the stent and leaflet attachment were covered during follow-up with neointimal pannus, which caused some additional degree of stenosis and some leaflet immobility, thus causing elevated gradient and relative stenosis. Together, with the learning curve, the gradients were lower in the final animals. Additionally, as the animals grow, the gradients tend to increase as also shown by Scherman et al11 and in the Edwards Sapien THV U.S. Food and Drug Administration–approval study.4
The pathologic evaluation of 7 terminally sacrificed animals (up to ∼180 days) and 2 early death animals (at 74 and 133 days) implanted with Myval THV revealed deployment and anchoring of the device within the ascending aortic banding. Native aortic cusps remained free and functional in most animals. All animals sacrificed at term showed healing and integration features in the cusps characterized by complete coverage of the cusps by endothelialized fibrocellular neointima that frequently progressed to thicker pannus formation.
There was no or very minimal cusp calcification and optimal local biocompatibility features characterized by no foreign body reaction to the collagenous cusp biomaterial. There was minimal to mild foreign body response associated with the polyester pads/cushion that was well within the expected and acceptable range for this type of implant.
There was no thrombosis in the cusps for animals sacrificed at term and no thrombosis in the conduits. A bacterial/fungal complication associated with the implant cusps caused premature death in 1 animal. Implant cusp immobility caused by tight banding, pannus creation, and subsequent calcification with severe heart failure was the cause of death in the other animal.
The healing results of Myval THV as evaluated in the preclinical setting, with no valve degeneration at 6-month follow-up are similar to the those reported with currently available balloon expandable THV including Edwards Sapien THV.4,5 However, in the current study, a higher proportion of valves implanted at baseline reached the terminal follow-up, mostly because of model improvements. Therefore based on these previous experiences, translation into clinical setting was very accurate, with very low long-term prosthesis failure (2.4%).12
Study limitations
The main limitation is associated with the lack of underlying disease—calcific aortic valve stenosis—and use of a healthy, surgically modified, animal as a surrogate of human disease. Additionally, heterotopic supra-annular implantation may play a role in the hemodynamic performance and biological response, such as increased pannus creation or elevated gradient.
Conclusions
The Myval THV implant showed successful delivery and optimal deployment in a supra-aortic banding position with the ability to anchor the valve for up to 6 months with low periprocedural and follow-up mortality. Valve functionality and durability was proven using echocardiographic evaluation. Mild to moderate gradients were caused by deployment of prosthesis in stenotic banding and animal growth. In pathology, healing was very advanced and there was diffuse neointima and/or pannus over the implant cusps. At term, there were no instances of excessive cusp calcification or thrombus leading to valve failure. The bioprosthetic material and frame showed optimal biocompatibility in the aortic banding model.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: TAVI has become a common method of treatment of patients with calcific aortic stenosis. Many new THVs are already available in the clinical setting; however, long-term biological response is not fully understood and experimental studies could not provide convincing hypothesis. This study adds to understanding of long-term biological response to a new THV prosthesis and provides conclusive data on long-term degeneration (ie, thrombosis, calcification) and hemodynamic performance.
TRANSLATIONAL OUTLOOK: Potentially this study could predict at early development stage of a new THV its long-term outcome in preclinical experiments. Further studies that will correlate long-term outcomes from preclinical and clinical settings of Myval THV are necessary.
Funding Support and Author Disclosures
This study was funded by Meril Life Sciences. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Cribier A., Eltchaninoff H., Bash A., et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106(24):3006–3008. doi: 10.1161/01.cir.0000047200.36165.b8. [DOI] [PubMed] [Google Scholar]
- 2.Leon M.B., Smith C.R., Mack M., et al. PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 3.Leon M.B., Smith C.R., Mack M.J., et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration Summary of Safety and Effectiveness Data, Replacement Heart Valve, Edwards Sapien Transcatheter Heart Valve. U.S. Food and Drug Administration; 2012. Application P110021. https://www.accessdata.fda.gov/cdrh_docs/pdf11/P110021b.pdf Accessed March 3, 2022.
- 5.Eltchaninoff H., Nusimovici-Avadis D., Babaliaros V., Spenser B., Felsen B., Cribier A. Five month study of percutaneous heart valves in the systemic circulation of sheep using a novel model of aortic insufficiency. EuroIntervention. 2006;1(4):438–444. [PubMed] [Google Scholar]
- 6.Andersen H.R., Knudsen L.L., Hasenkam J.M. Transluminal implantation of artificial heart valves: description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J. 1992;13(5):704–708. doi: 10.1093/oxfordjournals.eurheartj.a060238. [DOI] [PubMed] [Google Scholar]
- 7.Carney J.P., Schappa Faustich J., Lahti M.T., et al. New model for the assessment of transcatheter aortic valve replacement devices in sheep. J Invest Surg. 2022;35(2):371–377. doi: 10.1080/08941939.2020.1864796. [DOI] [PubMed] [Google Scholar]
- 8.Buszman P.P., Fernandez C., Kachel M., et al. Long-term biocompatibility and mechanical performance of a new balloon expandable transcatheter biological aortic valve system assessed based on novel ovine aortic banding model. J Am Coll Cardiol. 2020;75(11 suppl 1):1349. [Google Scholar]
- 9.Buszman P.P., Kachel M., Domaradzki W., et al. Long-term evaluation of biocompatibility and endurance of a novel, balloon expandable transcatheter polymeric aortic valve in the ovine aortic banding model. J Am Coll Cardiol. 2020;75(11 suppl 1):1350. [Google Scholar]
- 10.Institute of Laboratory Animal Resources, National Research Council . National Institutes of Health; 1996. Principles of Care of Laboratory Animals. Publication 85-23. [Google Scholar]
- 11.Scherman J., Ofoegbu C., Myburgh A., et al. Preclinical evaluation of a transcatheter aortic valve replacement system for patients with rheumatic heart disease. EuroIntervention. 2019;15(11):e975–e982. doi: 10.4244/EIJ-D-18-01052. [DOI] [PubMed] [Google Scholar]
- 12.Tarantini G., Purita P.A.M., D'Onofrio A., et al. Long-term outcomes and prosthesis performance after transcatheter aortic valve replacement: results of self-expandable and balloon-expandable transcatheter heart valves. Ann Cardiothorac Surg. 2017;6(5):473–483. doi: 10.21037/acs.2017.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]