Highlights
-
•
Clinical practice guidelines emphasize the need for guideline-directed medical therapy in patients with heart failure with reduced ejection fraction.
-
•
Recently, international guidelines and the American College of Cardiology Expert Consensus Decision Pathway recommended quadruple therapy for these patients, including angiotensin receptor blockers/neprilysin inhibitors, beta-blockers, mineralocorticoid receptor antagonists, and sodium–glucose co-transporter 2 inhibitors.
-
•
Strategies to optimize use of novel therapies, achieving target doses and management of side effects and tolerability, are needed to achieve this goal.
-
•
Future prospective studies aimed at guiding optimal implementation of quadruple therapy are needed.
Key Words: angiotensin receptor–neprilysin inhibitor, beta-blockers, mineralocorticoid receptor antagonists, sodium–glucose co-transporter 2 inhibitors
Abbreviations and Acronyms: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BB, beta-blocker; GDMT, guideline-directed medical therapy; eGFR, estimated glomerular filtration rate; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; MRA, mineralocorticoid receptor agonist; SGLT2i, sodium–glucose co-transporter 2 inhibitor; T2DM, type 2 diabetes mellitus
Summary
Given the high risk of adverse outcomes in patients with heart failure and reduced ejection fraction (HFrEF), there is an urgent need for the initiation and titration of guideline-directed medical therapy (GDMT) that can reduce the risk of morbidity and mortality. Clinical practice guidelines are now emphasizing the need for early and rapid initiation of therapies that have cardiovascular benefit. Recognizing that there are many barriers to GDMT initiation and optimization, health care providers should aim to introduce the 4 pillars of quadruple therapy now recommended by most clinical practice guidelines: angiotensin receptor–neprilysin inhibitors, beta-blockers, mineralocorticoid receptor antagonists, and sodium–glucose co-transporter 2 inhibitors. A large proportion of patients with HFrEF do not have clinical contraindications to GDMT but are not treated with these therapies. Early initiation of low-dose combination therapy should be tolerated by most patients. However, patient-related factors such as hemodynamics, frailty, and laboratory values will need consideration for maximum tolerated GDMT. GDMT initiation in acute heart failure hospitalization represents another important avenue to improve use of GDMT. Finally, removal of therapies that do not have clear cardiovascular benefit should be considered to lower polypharmacy and reduce the risk of adverse side effects. Future prospective studies aimed at guiding optimal implementation of quadruple therapy are warranted to reduce morbidity and mortality in patients with HFrEF.
Central Illustration
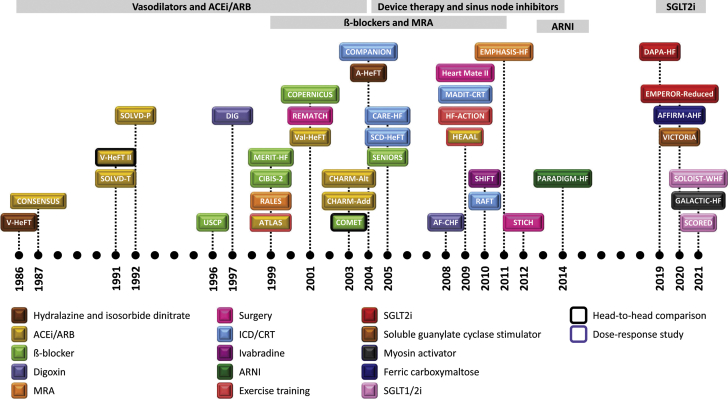
Heart failure with reduced ejection fraction (HFrEF) is a complex disease with worse morbidity and mortality than most cancers.1 Over the past 35 years, a multitude of new drug- and device-based therapies for the management of these patients have shown benefit (Figure 1) and are accordingly recommended by current practice guidelines. Although these advances have been welcomed, they have come at the expense of increasing complexity, added cost, and tolerability concerns. Given the significant co-morbidity burden among patients with HFrEF, the management of these patients has consequently become even more challenging.2,3
Figure 1.
Summary of Advances in Medical Therapies in Patients With HF and Reduced Ejection Fraction
This figure displays a temporal representation of landmark trials that have shaped heart failure therapy over the years. A-HeFT = African-American Heart Failure Trial; ACE = angiotensin-converting enzyme; AF-CHF = Rhythm Control versus Rate Control for Atrial Fibrillation and Heart Failure; AFFIRM-HF = Study to Compare Ferric Carboxymaltose With Placebo in Patients With Acute Heart Failure and Iron Deficiency; ARB = angiotensin receptor blockers; ARNI = angiotensin receptor blocker/neprilysin inhibitor; ATLAS = Assessment of Treatment with Lisinopril and Survival; CARE-HF = Cardiac Resynchronization Heart Failure Study; CHARM-Add = added arm of the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity; CHARM-Alt = alternative arm of the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity; CIBIS = Cardiac Insufficiency Bisoprolol Study; COMET = Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial; COMPANION = Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure; CONSENSUS = Cooperative North Scandinavian Enalapril Survival Study; COPERNICUS = Carvedilol Prospective Randomized Cumulative Survival; CRT = cardiac resynchronization therapy; DAPA-HF = Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; DIG = Effect of Digoxin on Mortality and Morbidity in Patients With Heart Failure; EMPEROR-Reduced = Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction; EMPHASIS-HF = Eplerenone in Patients with Systolic Heart Failure and Mild Symptoms; GALACTIC-HF = Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure; HEAAL = High-Dose Versus Low-Dose Losartan on Clinical Outcomes in Patients with Heart Failure; HEART-MATE II = Advanced Heart Failure Treated With Continuous-Flow Left Ventricular Assist Device; HF-ACTION = Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training; ICD = implantable-cardioverter-defibrillator; MADIT-CRT = Multicenter Automatic Defibrillator Implantation With Cardiac Resynchronization Therapy; MERIT-HF = Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure; MRA = mineralocorticoid receptor agonist; PARADIGM-HF = Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure; RAFT = Cardiac-Resynchronization Therapy for Mild-to-Moderate Heart Failure; RALES = Randomized Aldactone Evaluation Study; REMATCH = Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure; SCD-HeFT = Sudden Cardiac Death in Heart Failure Trial; SCORED = Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk; SENIORS = Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure; SGLT1i = sodium–glucose co-transporter 1 inhibitor; SGLT2i = sodium–glucose co-transporter 2 inhibitor; SHIFT = Ivabradine and Outcomes in Chronic Heart Failure; SOLOIST-WHF = Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure; SOLVD-P = prevention arm of the Studies of Left Ventricular Dysfunction; SOLVD-T = treatment arm of the Studies of Left Ventricular Dysfunction; STICH = Coronary-Artery Bypass Surgery in Patients with Left Ventricular Dysfunction; USCP = The Effect of Carvedilol on Morbidity and Mortality in Patients With Chronic Heart Failure; V-HeFT = Effect of Vasodilator Therapy on Mortality in Chronic Congestive Heart Failure; Val-HeFT = Valsartan Heart Failure Trial; VICTORIA = Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction.
Many of the recently updated HFrEF clinical practice guidelines advocate for the use of “foundational quadruple therapy”: a combination of angiotensin receptor blockers/neprilysin inhibitors (ARNIs), beta-blockers (BBs), mineralocorticoid receptor agonists (MRAs), and sodium–glucose co-transporter 2 inhibitors (SGLT2is).3,4 The combined use of these therapies can improve life expectancy for the average 50-year-old patient with HFrEF by a median of 6 years compared with more limited regimens.5 Despite robust evidence from well-conducted randomized clinical trials,4,6, 7, 8, 9, 10, 11 these guideline-directed medical therapies (GDMTs) with established cardiovascular benefit remain significantly underutilized in clinical practice, including those that have been shown for >2 decades to benefit patients.12
Such underutilization persists despite the absence of any absolute or relative contraindications or documented intolerances.13, 14, 15, 16 In the prospective CHAMP-HF (Change the Management of Patients with Heart Failure) registry, which enrolled 3,518 patients, only 1% of the eligible patients were prescribed triple therapy; furthermore, 86% of the patients were not prescribed ARNIs despite having no medical contraindication.12 Similar findings have been seen with the use of MRAs and SGLT2is.13,17,18 Notably, underutilization of GDMT is more pronounced among minorities and women.18 Following the discovery that the rate of heart failure (HF) hospitalization is reduced with intravenous ferric carboxymaltose in patients admitted with HF,19 and that cardiovascular death or HF hospitalization is lower in patients with recently worsening HF after use of vericiguat (a soluble guanylate cyclase stimulator),20, physicians now have multiple therapeutic strategies to leverage. If, however, these therapies continue to be underprescribed, patients will not be able to gain access to and benefit from these morbidity- and mortality-reducing therapies.21
In part, the slow uptake of these lifesaving therapies is due to a presumption that the pharmacologic management of patients with HFrEF should always follow a sequential format. Introduction of one therapy with uptitration of doses before initiating other medications takes months, if not years, and is often associated with therapeutic hesitancy. This issue is further complicated with the more recent introduction of a fourth foundational therapy, the SGTL2i class, in the regimen of these patients.22 Therapeutic hesitancy has been similarly observed with the modification of antihyperglycemic therapies in patients with type 2 diabetes mellitus (T2DM) and established atherosclerotic cardiovascular disease.23 This issue is of paramount significance because: 1) optimal therapy is not provided in a large proportion of eligible patients; and 2) when it is provided, initiation of therapy is often significantly delayed. The collective trial data indicate that benefits with optimal care accrue early; thus, not providing optimal therapy, or even delaying it, exposes patients to potentially avoidable risk.
The current clinical consensus document is a product of a think tank meeting that was held on March 23, 2021, and included HF experts from across North America to discuss how to best optimize HF care. This review provides an overview of the possible approaches to therapy sequencing optimization in HFrEF. To provide more clinical relevance, approaches for GDMT optimization in 3 common clinical scenarios are described.
Historical Consideration of Sequencing HFrEF Therapies
The historical paradigm in optimizing GDMT with all 4 drug classes of the foundational quadruple therapy is to prescribe them using the specific sequence that pivotal clinical trials used in testing them.24,25 Conventionally, this involves starting with an angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blocker (ARB), followed by the add-on of a BB and then an MRA. If the patient remains symptomatic, an ARNI is introduced (switched from ACE inhibitor/ARB) before an SGLT2i is prescribed.26 Furthermore, the doses of each therapy are increased to the guideline-recommended dosing (defined as dose target in the pivotal clinical trial) or highest tolerated dose before initiating a new therapy. This traditional paradigm does not, however, take into account several important factors:27 1) most of the landmark clinical trials did not involve patients who were already on optimized dosing of baseline HFrEF therapies at randomization; 2) this sequential algorithm tacitly assumes that a combination of low-dose therapies will not yield additive benefits; 3) the successive process may require 6 to 12 months to completely incorporate all the recommended treatments; and 4) initiation of multiple therapies up-front, specifically ARNI and SGLT2i, rather than sequentially, may facilitate stabilization of potassium and kidney function to enable initiation of MRAs in the future.
Real-world data from prospective registries around the world suggest that health care providers infrequently add GDMT and do not titrate baseline HFrEF medication doses despite the absence of clinical contraindications or circumstances in which there are no system-level barriers (eg, coverage in the U.S. Veterans Affairs system).12, 13, 14,28 Indeed, across multiple global health systems and economies, near identical patterns of infrequent drug titration and early discontinuation of core elements of GDMT have been observed.21
Significant Time Delay in Optimizing GDMT with Sequential Dosing
If a health care provider was to titrate the doses of an ACE inhibitor/ARB and BB in a sequential manner before the initiation of additional GDMT, it would take up to 12 months to achieve optimal dosing.27,29 However, pivotal trials have shown the achievement of early statistical significance.22,27 For example, the time to statistical significance, and by implication sustained clinical benefit, was achieved by day 28 in the DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial.30 Similar results have been seen in other trials, and this has triggered calls for early initiation of therapy to maximize clinical benefit.31 Of note, trials involving patients with HF and chronic kidney disease are underway to understand the safety and tolerability of dual initiation of a selective mineralocorticoid receptor modulator and SGLT2i.32
Optimal Background Therapy is a Historical Construct and Not Biologically Determined
The use of background GDMT in clinical trials was based on the conventional understanding and availabilities of therapies at the time and was not biologically determined. What is considered “background” or “baseline” optimal therapy has evolved over time. As an example, in CONSENSUS (Cooperative North Scandinavian Enalapril Survival Study), which compared enalapril versus placebo in patients with HFrEF, the enrolled participants had to be on a stable background therapy of digitalis and diuretics.33 Treatment with other drugs for HF, including nitrates, prazosin, and hydralazine, was also permitted. Inasmuch as the standard “toolbox of HFrEF therapies” has changed, more contemporary trials have not included these recommendations.
Notably, the pivotal Phase III trials of foundational quadruple therapy have shown a similar magnitude of benefit with active therapy in patients regardless of the use or dosing of background therapy.34, 35, 36, 37, 38 These results underscore the notion that the currently recommended quadruple therapies are functionally independent and not biologically associated with each other regarding their therapeutic efficacy.
To date, CIBIS (Cardiac Insufficiency Bisoprolol Study III) is one of the very few “sequencing” trials. Specifically, CIBIS III compared a bisoprolol-first then enalapril strategy versus an enalapril-first then bisoprolol strategy.39 A total of 1,010 patients were randomized to treatment, and no one strategy was superior, suggesting that either approach was reasonable. Although this cannot be extrapolated to other types of sequencing studies involving more contemporary therapies, thus far there is limited evidence that a rigid sequence approach is optimal or superior when considering the initiation of GDMT in patients with HFrEF.
False Assumption that Trials Have Optimal Use of Background GDMT
Although randomized controlled trials generally have inclusion criteria that mandate patients should be on “optimally tolerated GDMT,” it cannot be assumed that all patients in the landmark trials were on every GDMT or that these therapies were being used at the maximally recommended therapeutic doses. As recently highlighted, the use of background therapy in Phase III studies of HFrEF trials has been extremely heterogeneous.34 For instance, the background use of BB was 3% in CONSENSUS,40 8% in SOLVD (Studies of Left Ventricular Dysfunction),41 and 11% in RALES (Randomized Aldactone Evaluation Study).42 Among the more contemporary trials, the use of MRA was 52% in the PARADIGM-HF (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial,8 71.3% in EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction),10 and 71% in the DAPA-HF trial.9 Furthermore, ARNI use was 10.4% in the DAPA-HF trial and 19.4% in EMPEROR-Reduced.10 Therefore, the assumption that clinicians should only initiate foundational therapies after background therapies have been started and titrated to maximal doses is not consistent with the cohorts of these paradigm-changing clinical trials.
Low Doses of GDMT Have Therapeutic Efficacy
An additional fallacy is the assumption that only maximal doses of GDMT have therapeutic benefit, and newer therapies should only be prescribed once the maximal doses are achieved. Closer examination of the baseline trial data suggests that the vast majority of trial participants never received optimal doses of the active drug.34 For example, only 64% of the MERIT-HF (Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure) participants eventually met the target trial dose of metoprolol,43 and only 60.2% of the EMPHASIS-HF (Eplerenone in Patients with Systolic Heart Failure and Mild Symptoms) cohort achieved the target dose of eplerenone.44 Indeed, there is a paucity of evidence supporting the notion that higher versus lower doses of GDMT robustly improves outcomes; that said, the evidence for greater benefit of higher doses of GDMT is seen with BBs, in which higher doses seem to reduce the risk of death and HF hospitalizations compared with lower doses.45
Two key randomized trials come to mind when high-dose versus low-dose ACE inhibitors/ARBs are considered. The first is low- versus high-dose lisinopril in the ATLAS (Assessment of Treatment with Lisinopril and Survival) trial.46 When comparing the low-dose (2.5 to 5.0 mg daily) versus the high-dose (32.5 to 35 mg daily) group, there was no difference in mortality, although there was a 24% lower risk of HF hospitalization in the latter group. Although discontinuation rates were similar between the 2 arms, dizziness and renal insufficiency were observed more frequently in the high-dose group. The second trial is the HEAAL (High-Dose Versus Low-Dose Losartan on Clinical Outcomes in Patients with Heart Failure) study.47 HEAAL participants were randomized to receive either a high-dose (150 mg) or a low-dose (50 mg) losartan regimen. Although no difference in the risk of death was observed, there was a 13% reduction in the risk of HF hospitalization in the high-dose group. Furthermore, the impact of dose on outcomes in male and female subjects requires further clarification because post hoc analyses of HEAAL and ATLAS suggest that lower doses may be acceptable in female subjects to achieve outcome benefit.48
There have also been no dedicated trials of high- versus low-dose MRA therapies, and the pivotal MRA trials have shown that low-dose spironolactone and eplerenone (compared with placebo) still had benefit in reducing the risk of adverse outcomes.49 However, in a post hoc analysis of the EMPHASIS-HF trial, low-dose eplerenone (vs placebo) was revealed to exert a similar magnitude of benefit compared with the higher dose of eplerenone in reducing the risk of adverse HF outcomes.50
In the PARADIGM-HF trial, participants may have needed dose reduction at some point during the trial, thereby allowing some assessment of impact of dose on outcome. Overall, the magnitude of benefit with low-, moderate-, or high-dose sacubitril/valsartan relative to enalapril doses was similar.34 Although there was no direct comparison of low versus high ARNI dose, the findings suggest that ARNIs offer advantages over ACE inhibitors across the dosing ranges. SGLT2is benefit from not requiring any dose adjustments in HF. The collective trial evidence to date supports the view that uptitration of ACE inhibitor/ARB, BB, MRA, and ARNI doses may not be necessary to achieve reductions in outcomes, and that initiation of additional foundational GDMT on low-dose background therapies represents an evidence-based strategy to reduce morbidity and mortality in patients with HFrEF.
Initiation of Multiple Therapies Upfront Facilitates GDMT Optimization Later
A post hoc analysis of the PARADIGM-HF trial suggested that sacubitril/valsartan, compared with enalapril, did not lead to greater discontinuation of other GDMTs over time and may facilitate initiation and sustained use of MRAs due to stabilization of kidney function and potassium.51 Similar results have been identified in the EMPEROR-Reduced trial, in which empagliflozin, compared with placebo, was associated with a reduced risk of MRA discontinuation over time.52 These results suggest that earlier use of ARNIs and SGLT2is may provide a solution to enable further initiation, titration, and sustained use of MRA over time.
Cost-Effectiveness of Foundational Therapies
Although there are upfront costs in the utilization of foundational therapies, numerous analyses have reported the global cost-effectiveness of leveraging these medications.55, 56, 57, 58 Several elements of foundational therapy are now generic (eg, BB, MRA), thereby minimizing cost burden to patients. Furthermore, ARNIs have been shown to be associated with significant cost savings to health care systems, even in patients in whom the therapy is initiated de novo (ie, before the use of ACE inhibitors/ARBs).53,57 The vast majority of data on the system-level cost-effectiveness of SGLT2is come from the T2DM literature.54 However, emerging data from the DAPA-HF trial from 2 independent analyses show that dapagliflozin is cost-effective when used in patients with HFrEF regardless of presence of T2DM.56 Such results highlight the need for widespread system-level initiatives to utilize foundational therapies.
Current Strategies to Initiate and Titrate HFrEF Therapies
The focus on sequential therapy initiation is likely a critical reason for therapy underutilization in people with HFrEF. Updated clinical consensus documents and practice guidelines are now moving away from recommendations of strict sequencing of therapies to more rapid initiation of therapies (Table 1). Updated clinical practice guidelines acknowledge that there is limited evidence to guide optimal sequencing, rapid initiation, and titration of GDMT in patients with HFrEF.3,4 However, there are several newer strategies that have been proposed which aim to overcome some of the challenges that occur when following the conventional paradigm (Table 2).
-
1.
McMurray and Packer27 advocate for a 3-step rapid initiation method for stable outpatients. This approach starts with the initiation of a BB and an SGLT2i. This is to be followed by the subsequent initiation of an ARNI in 1 to 2 weeks, and then an MRA within an additional 1 to 2 weeks. Dose titration should be attempted once all foundational therapies have occurred. The authors recognize that the latter 2 steps can be re-ordered or changed depending on patient-specific circumstances, including therapies at baseline and therapy tolerability.
-
2.
Clinical consensus guidelines such as those created by the European Society of Cardiology highlight the needed for individualized patient profiling to identify the optimal approach to initiate and titrate HF-specific GDMT.58 Such recommendations were echoed by a recent publication by Miller et al59 in which a patient-centric or “cluster”-based approach was highlighted as a strategy to optimize GDMT. In general, patients are considered within the lens of 3 general phenotypes depending on the clinical scenario: volume overload, normo-hypertensive, or increased heart rate. In patients with volume overload, an SGLT2i (given their diuretic-like properties) with a diuretic should be initiated. In those who are normo-hypertensive, an ARNI plus an MRA at low doses should be considered. In patients with an increased heart rate, a BB plus a sinus node inhibitor (ivabradine) should be considered. Once therapy from one cluster has been initiated, the therapy classes from the other clusters can be added within 2 to 4 weeks, therefore achieving low doses of all the classes within 3 to 6 weeks.
-
3.
Greene et al60 recommend near-simultaneous initiation of low doses of all 4 classes of quadruple therapy. This should occur within 1 week, followed by gradual uptitration depending on patient factors and therapy tolerability. Furthermore, if a patient is hospitalized and in stable condition, every attempt should be made to initiate GDMT and increase the doses of pre-existing therapies.
Table 1.
Current Clinical Consensus and Clinical Practice Guideline Recommendations on Sequencing
| Quadruple Therapy | Recommendation | |
|---|---|---|
| 2021 European Society of Cardiology (preview presented at European Society of Cardiology Heart Failure 2021 congress) |
|
Recommended for all eligible patients with HFrEF to reduce the risk of mortality |
| 2021 American College of Cardiology Expert Consensus Pathway3 |
|
Each agent should be uptitrated to maximally tolerated or target dose. Initiation of a BB is better tolerated when patients are “dry” and an ACE inhibitor/ARB/ARNI when patients are “wet” |
| 2021 Canadian Cardiovascular Society4 |
|
It might be preferable to titrate doses of different classes of GDMT medications simultaneously (“in-parallel” approach), rather than fully titrate 1 medication class before initiating an additional agent (“strict sequential” approach) |
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor/neprilysin inhibitor; BB = beta-blocker; GDMT = guideline-directed medical therapy; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; MRA = mineralocorticoid receptor antagonist; NYHA = New York Heart Association; SGLT2i = sodium–glucose co-transporter 2 inhibitor.
Table 2.
Summary of Proposed Rapid Therapy Initiation Models
| Details | Timeline | Advantages | Disadvantages | |
|---|---|---|---|---|
| Packer and McMurray27 | Initiate BB and SGLT2i upfront, followed by ARNI then MRA | Four weeks to achieve initiation of GDMT | Focus on tolerability without initiating multiple medications that can induce hypotension or acute kidney injury | Coverage of therapies such as SGLT2i and ARNI may be predicated on being symptomatic on baseline HF therapies such as an ACE inhibitor, MRA, and BB |
| Miller et al59 | Cluster phenotype-based approach; if volume overloaded, add SGLT2i; if hypertensive add ARNI/MRA; if higher heart rate, add and titrate BB and SNI | 3-6 weeks of therapy initiation followed by dose titration | Based on patient clinical characteristics that may enhance tolerability | Needs multiple patient visits or touchpoints; drug coverage may predicate a sequential approach to therapy initiation |
| Greene et al60 | Rapid initiation of low doses of all categories of foundational quadruple therapy within 1 week | Dose titration across 1 month | Enables rapid initiation of GDMT upfront, which may optimize clinical benefit | If a patient has a side effect, unclear which therapy is the culprit; drug coverage may predicate a sequential approach to therapy initiation |
SNI = sinus node inhibitor; other abbreviations as in Table 1.
These recommendations have several intersecting themes (Table 2). The first consideration is that these therapies reduce morbidity and mortality very rapidly after initiation, thus emphasizing the need to add multiple classes of therapy in rapid succession.61, 62, 63 Furthermore, each therapy has a treatment effect that is independent of dose or prior initiation of other therapies.64 In addition, low doses of each of the pillars of the quadruple therapies have additive therapeutic efficacy;65 initiating low doses of all 4 categories of quadruple therapies should be prioritized over strict sequencing and dose escalation as recommended by the historical therapy paradigm. Finally, most recommendations for rapid therapy sequencing suggest that all therapy classes, in the absence of any contraindications, should be initiated within 4 weeks of identification of HFrEF.
Leveraging Hospitalizations to Titrate Therapies
Although the rapid initiation proposals predominantly focus on the outpatient setting, the hospital setting is a critical time to initiate and optimize therapies. It has been clearly shown across multiple HFrEF studies that therapies prescribed at discharge are rarely modified, titrated, or optimized up to 1 year after discharge.25,66 The CONNECT-HF (Care Optimization Through Patient and Hospital Engagement Clinical Trial for Heart Failure) study was conducted at 161 sites with 5,647 patients who were admitted with HF. Participants were randomized to clinician education, audit, and feedback of HF quality of care.67 The study showed no improvement in use of GDMT over time or clinical outcomes in patients discharged from hospital with HF (Supplemental Ref. 1). Few changes occurred in ambulatory care after hospital discharge, leading to the conclusion that health care providers should aim to leverage the hospitalization period to initiate GDMT, which seems to be a feasible approach across specialty types (Supplemental Ref. 2).
Real-World Scenarios
Although each patient with HFrEF is unique and deserves a patient-centric approach to therapy optimization, a large proportion of patients have few medical contraindications to GDMT. The following represents 3 clinical scenarios whereby rapid GDMT optimization can occur.
Chronic stable HFrEF
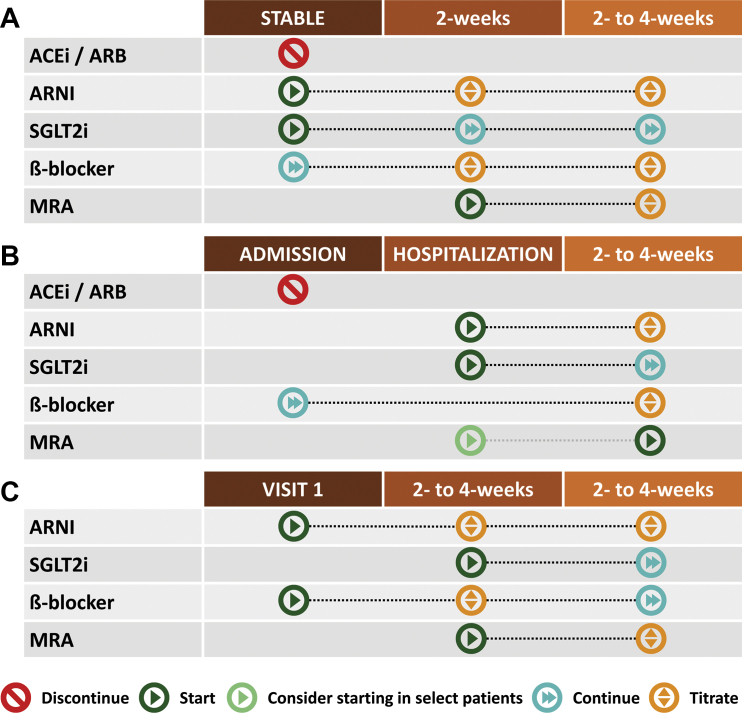
Patients with chronic stable HFrEF have a high burden of co-morbidities, including T2DM and chronic kidney disease (Supplemental Ref. 3). Here, a rapid titration strategy recommended by Greene et al can be considered, with discontinuation of any ACE inhibitor/ARB and starting low-dose ARNI 36 hours later (if the patient is on ACE inhibitor [eg, sacubitril/valsartan at 24/26 mg orally twice a day]) and immediate initiation of an SGLT2i such as empagliflozin 10 mg once daily60 or dapagliflozin 10 mg once daily (Figure 2A).9 Laboratory investigations, including kidney function and electrolytes, can be conducted, if possible, within 4 weeks, with recognition that there will be a “dip” in estimated glomerular filtration rate (eGFR) followed by stabilization of renal function over time (Supplemental Ref. 4). If the patient is not already on an MRA, this can be added early (eg, 2-4 weeks) within initiation of an ARNI and an SGLT2i. Future titration to higher doses of ARNI can occur during follow-up.
Figure 2.
Titration Strategies by Clinical Scenario in Patients With HF and Reduced Ejection Fraction
(A) Chronic stable heart failure and reduced ejection fraction; (B) acute heart failure; (C) de novo non-ischemic heart failure with reduced ejection fraction. This figure displays the time points at which each medication in the quadruple therapy regimen should be initiated and titrated in patients with acute, chronic, and de novo heart failure and reduced ejection fraction (HFrEF). Abbreviations as in Figure 1.
Acute decompensated HF with known HFrEF
Frequently, the inpatient admission for acute HF represents an optimal time to initiate and titrate GDMT.4 As previously described, what patients are prescribed within the hospital setting will be unchanged despite frequent evaluations in the outpatient setting,17 (Supplemental Ref. 5). The vast majority of patients admitted with acute HF will be on an ACE inhibitor/ARB and a BB (Supplemental Ref. 6), and therefore optimization can build on these 2 foundational therapies (Figure 2B). Current data suggest that initiation of an ARNI and potentially an SGLT2i remains relatively safe when done in the inpatient environment. Data from the PIONEER-HF (Comparison of Sacubitril/Valsartan versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode) trial identified that sacubitril/valsartan, compared with enalapril, significantly reduced N-terminal pro–B-type natriuretic peptide levels and recurrent HF hospitalization (Supplemental Ref. 7). There was no difference in rates of hypotension, hyperkalemia, or acute kidney injury with sacubitril/valsartan. As recommended by McMurray and Packer,27 the patient should be prioritized for switching the valsartan to ARNI (eg, sacubitril/valsartan at 24/26 mg orally twice a day) and adding spironolactone at 12.5 mg orally daily. There is no need to delay the initiation of an ARNI because the patient was on an ARB. The SGLT1/2 inhibitor sotagliflozin exhibited a reduction in the risk of total worsening HF events and cardiovascular death in patients with acute HF and T2DM (Supplemental Ref. 8,9). Ongoing trials are evaluating the safety and efficacy of SGLT2i among patients with acute HF both with and without T2DM (Supplemental Ref. 10,11). Therapies that do not have clear cardiovascular morbidity and mortality benefit, such as calcium-channel blockers, could be discontinued to prioritize GDMT with cardiovascular mortality benefit. This approach may help to further address the issues around polypharmacy and to prevent future hypotension. Laboratory investigations, including kidney function and electrolytes, can be conducted, if possible, within 4 weeks as an outpatient. Upon discharge, vericiguat starting at 2.5 mg orally per day can also be initiated.20
Stable outpatient with de novo nonischemic HFrEF
Among patients, with de novo nonischemic HFrEF, rapid and sequential initiation of low-dose therapies in multiple classes of quadruple therapy should be considered (Figure 2C). In this case, low-dose ARNI (eg, 24/26 mg orally twice a day), BB (eg, bisoprolol 2.5 mg orally daily), and spironolactone (12.5 mg orally once daily) can be initiated. Given that the potassium levels were borderline elevated, early patient follow-up within 2 weeks to check laboratory values, blood pressure, and heart rate should occur. If stable, the patient should be started on an SGLT2i with follow-up bloodwork to check renal function and electrolytes within 2 weeks. Furthermore, ongoing evaluation for assessment of further device therapies (eg, implantable cardioverter-defibrillators or cardiac resynchronization therapy) should commence.
Strategies to Anticipant GDMT-Related Adverse Events
Health care providers are frequently concerned with the potential of acute kidney injury, hypotension, and hyperkalemia after the initiation and titration of GDMT in patients with HFrEF (Table 3) (Supplemental Ref. 12). However, many of these issues can be anticipated. In cases of chronic kidney disease, it should be expected that the eGFR will decrease after initiation of a renin-angiotensin system inhibitor, ARNI (Supplemental Ref. 13), or SGLT2i (Supplemental Ref. 14). This action occurs because of a reduction in glomerular hyperfiltration through a drop in glomerular pressure. In terms of SGLT2i compared with other antihyperglycemic therapies in patients with chronic kidney disease, there is no increased risk of acute kidney injury on therapy initiation (Supplemental Refs. 15,16). As previously described in the landmark MRA in HFrEF trials, continuation of MRAs in patients with serum potassium levels up to 5.6 mmol/L provided mortality benefit.51 Accordingly, health care providers should continue to pursue strategies to lower serum potassium levels, such as using potassium binders while attempting to continue MRA therapies. Furthermore, clinical consensus suggests that practitioners may consider novel potassium binders, such as patiromer and sodium zirconium cyclosilicate, to enable the uptitration of ARNIs/ACE inhibitors/ARBs and MRAs if hyperkalemia persists3 (Supplemental Ref. 17).
Table 3.
Anticipated Drug-Related Side Effects and Mitigation Strategies
| Clinical Parameters to Initiate and Titrate | Follow-up Laboratory and Clinical Parameters (Within 2-4 Weeks of Initiation) | When to Consider Reducing Dose or Discontinuing | Strategies to Mitigate Adverse Side Effects | |
|---|---|---|---|---|
| ARNI/ACE inhibitor/ARB | SBP >100 mm Hg eGFR >30 mL/min/1.73 m2 K+ <5.4 mmol/L |
Symptoms of postural hypotension, serum creatinine, serum potassium | Symptomatic postural hypotension, K+ >5.4 mmol/L, serum increase creatinine >30% within 4 weeks of initiating | Recognize that early rise in serum creatinine is an anticipated effect of drug. Discontinue antihypertensive medications without cardiovascular benefit (eg, calcium-channel blockers). Can also consider novel potassium binders such as patiromer and sodium zirconium cyclosilicate to enable the uptitration of ARNI/ACE inhibitor/ARB and MRA if hyperkalemia persists |
| BB | HR >60 beats/min SBP >100 mm Hg |
No laboratory parameters needed. Heart rate and SBP | HR <50 beats/min (without PPM), symptomatic postural hypotension | If indicated as per practice guidelines, consider ICD/CRT implantation to mitigate risk of bradycardia |
| MRA | SBP >100 mm Hg eGFR >30 mL/min/1.73 m2 K+ ≤5.4 mmol/L |
Symptoms of postural hypotension, serum creatinine, serum potassium | Symptomatic postural hypotension, K+ >5.5 mmol/L, increase in serum creatinine >30% within 4 weeks of initiating | Discontinue antihypertensive medications without cardiovascular benefit (eg, calcium-channel blockers) |
| SGLT2i | SBP >100 mm Hg eGFR >25 mL/min/1.73 m2 | Symptoms of postural hypotension, serum creatinine, glycemic control (if diabetic), serum/urine ketones and lactate (if presenting in acute decompensation), genital mycotic infection | Symptomatic postural hypotension, increase in serum creatinine >30% within 4 weeks of initiating, development of ketones or elevated lactate if patient presenting acutely decompensated | Recognize that early rise in serum creatinine is an anticipated effect of drug; proper genital hygiene; if genital mycotic infection develops, consider treating with a single oral dose of fluconazole 150 mg. Counsel patient to temporarily hold SGLT2i if acutely unwell (eg, viral illness, dehydration); stop SGLT2i 2-3 days before procedure or surgery |
CRT = cardiac resynchronization therapy; eGFR = estimated glomerular filtration rate; HR = heart rate; ICD = implantable cardioverter-defibrillator; K+ = potassium; SBP = systolic blood pressure; other abbreviations as in Table 1.
With SGLT2i, there may be an increased risk of mycotic genital infections but not urinary tract infections. Patients should be counseled on adequate genital hygiene; if a genital mycotic infection does occur, treatment with a single dose of oral fluconazole 150 mg can be considered (Supplemental Ref. 12). In addition, SGLT2is increase the risk of nonhyperglycemic diabetic ketoacidosis in some patients. Patients should be counseled on temporarily discontinuing the drug if they develop acute illness that can cause limited oral intake. Therapies can be adequately restarted when there is resumption of oral intake.
Knowledge Gaps and Limitations
The aforementioned recommendations must be placed within the context of several knowledge gaps in the current literature. The patient profiles highlighted in our discussion represent commonly seen clinical scenarios, and we present one approach to initiation and titration to achieve optimal use of GDMT; however, it should be acknowledged that there are no specific contemporary randomized trials that have shown the safety and efficacy of any one titration strategy over another. Furthermore, as we bridge the gap between our understanding of the basic biology of HFrEF and the mechanisms of actions of the foundational therapies, we may enable more precision targeting of therapies to individual patients, rather than treating them as aggregates of a clinical profile (Supplemental Ref. 18). The expanding use of artificial intelligence in identifying who may optimally respond to foundational therapies also represents an emerging and expanding area of exploration (Supplemental Refs. 19,20).
Expanding on precision therapies is the concept of precision dosing; as an example, there is a growing understanding that there are likely sex-based differences in responses to dosing of some of the foundational therapies.48 Future dosing studies are needed to enable targeting of specific therapies and specific dosing to an individual patient. Furthermore, during the inpatient setting, although we have recommended a strategy of low-dose initiation of foundation therapies with subsequent uptitration over time, we recognize that there is limited titration of medication in the postdischarge setting; future quality improvement studies and educational programs will be needed to empower health care providers to titrate doses upon a patient’s discharge from hospital.
Within the EMPEROR-REDUCED and DAPA-HF clinical trials, enrollment criteria involved background optimal therapy, including ARNIs/ACE inhibitors/ARBs, BBs, and MRAs; however, the population enrolled large enough subsets of patients without those therapies to support the assertions that benefits of SGLT2i treatment are independent of the previous therapies. However, there was no heterogeneity of trial results with interaction testing based on baseline therapies; therefore, although the therapies have independent benefit, there is likely additive benefit of being on multiple agents of foundational therapies. However, future studies evaluating head-to-head comparison of foundational therapies may provide greater insight into the optimal use of HFrEF therapies.
There are additional patient-centric concerns regarding the cost related to these therapies that must be considered. The burden of pre-approval and copay may put many newer therapies out of reach for many patients with HFrEF. Because several foundational therapies are generic and as others approach patent expiry, however, cost considerations can hopefully be reduced in a significant number of patients. In addition to costs, patients themselves can be hesitant to initiate more therapies due to perceived risk of side effects and pill burden. Future quality improvement programs focusing on understanding patient-centric concerns and barriers to HFrEF GDMT are needed.
Conclusions
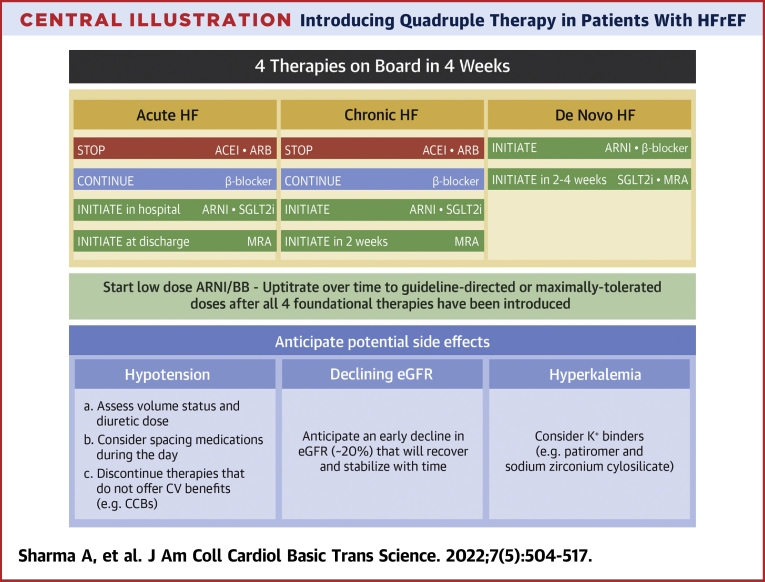
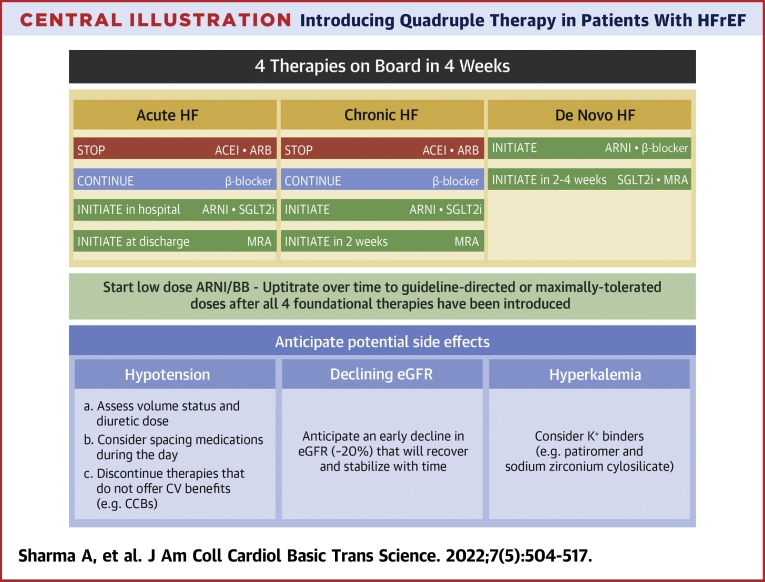
Given the high risk of adverse outcomes in patients with HFrEF and that a large proportion of these patients who do not have clinical contraindications to GDMT are not yet on these therapies, there is an urgent need to encourage the initiation and titration of GDMT to reduce morbidity and mortality. The more recently updated clinical practice guidelines now emphasize the importance of early and rapid initiation of drugs that have cardiovascular benefit(s) with the caveat that these decisions should be shared and personalized according to patient values and preferences, baseline medications, and laboratory values (Central Illustration). Removal of therapies that do not have clear cardiovascular benefit should be considered to prevent polypharmacy and reduce risk of adverse side effects. Anticipation of a drop in eGFR and a rise in potassium levels can help guide frequency of follow-up and repeat laboratory investigations. Recognizing that many barriers to GDMT initiation and optimizations exist, health care providers should aim to introduce the 4 pillars of the quadruple therapy now recommended by most clinical practice guidelines. Although implementation of other therapies is important, additional work on ensuring expanded access to quadruple therapies is critical to ensure that all eligible patients with HFrEF have access to lifesaving GDMT. Future prospective studies aimed at guiding optimal implementation of quadruple therapy are needed to reduce the morbidity and mortality in patients with HFrEF.
Central Illustration.
Introducing Quadruple Therapy in Patients With HFrEF
This figure summarizes the strategy to implement quadruple therapy in patients with acute heart failure (HF), chronic HF, and de novo HF, and the response to potential adverse effects. ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor blocker/neprilysin inhibitor; BB = beta-blocker; CCB = calcium-channel blocker; CV = cardiovascular; eGFR = estimated glomerular filtration rate; MRA = mineralocorticoid receptor agonist; SGLT2i = sodium–glucose co-transporter 2 inhibitor.
Funding Support and Author Disclosures
The funding support for the think tank meeting was provided through unrestricted grants from AstraZeneca Canada and Boehringer Ingelheim. Dr Sharma has received support from the Fonds de Recherche Santé Quebec (FRSQ) Junior 1 clinician scholars program, Canada Institute for Health Research (CIHR grant #175095), Roche Diagnostics, Boeringer Ingelheim, Novartis, and Takeda. Dr Verma holds a Tier 1 Canada Research Chair in Cardiovascular Surgery; and has received research grants and/or speaking honoraria from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, EOCI Pharmacomm Ltd, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, PhaseBio, Sanofi, Sun Pharmaceuticals, and the Toronto Knowledge Translation Working Group. He is the President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not-for-profit physician organization. Dr Bhatt discloses the following relationships: advisory board, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, and Regado Biosciences; board of directors, Boston VA Research Institute, Society of Cardiovascular Patient Care, and ToBeSoft; Chair, Inaugural Chair, American Heart Association Quality Oversight Committee; data monitoring committees, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Novartis, Population Health Research Institute; honoraria, American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); other, Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), and VA CART Research and Publications Committee (Chair); research funding, Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Lexicon, Lilly, Medtronic, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, The Medicines Company, and 89Bio; royalties, Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); site co-investigator, Abbott, Biotronik, Boston Scientific, CSI, St Jude Medical (now Abbott), Philips, and Svelte; trustee, American College of Cardiology; and unfunded research, FlowCo, Merck, and Takeda. Dr Connelly has received research grants to his institution from AstraZeneca and Boehringer Ingelheim; received support for travel to scientific meeting from Boehringer Ingelheim and honoraria for speaking engagements and ad hoc participation in advisory boards from AstraZeneca, Boehringer Ingelheim, and Janssen. Dr Swiggum has received research grants from AstraZeneca, Boehringer Ingelheim, and Novartis; advisory or consulting honorarium from Akcea Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, the Boehringer Ingelheim–Lilly Alliance, Novartis, Pfizer, and Servier. Dr Vaduganathan has received research grant support or served on advisory boards for American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, and Relypsa; speaker engagements with Novartis and Roche Diagnostics; and participates on clinical endpoint committees for studies sponsored by Galmed and Novartis. Dr Zieroth has received research grant support or served on advisory boards for and speaking engagements with Abbott, Akcea, AstraZeneca, Amgen, Alnylam, Bayer, Boehringer Ingelheim, Eli-Lilly, Merck, Novartis, Otsuka, Pfizer, Servier, and Vifor; and serves on a clinical trial steering committee or as a National Lead for studies sponsored by AstraZeneca, Boehringer Ingelheim, and Novartis. Dr Butler is a consultant to Abbott, Adrenomed, Amgen, Array, AstraZeneca, Bayer, Berlin Cures, Boehringer Ingelheim, Bristol Myers Squibb, CVRx, G3 Pharmaceutical, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Roche, Sanofi, and Vifor.
Acknowledgment
The authors acknowledge the editorial and visual assistance provided by Hwee Teoh, PhD, of HTaq Biomedical Editorial and Education Services Inc.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental references, please see the online version of this paper.
Appendix
References
- 1.Virani S.S., Alonso A., Aparicio H.J., et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A., Zhao X., Hammill B.G., et al. Trends in noncardiovascular comorbidities among patients hospitalized for heart failure. Circ Hear Fail. 2018;11(6) doi: 10.1161/CIRCHEARTFAILURE.117.004646. [DOI] [PubMed] [Google Scholar]
- 3.Maddox T.M., Januzzi J.L., Allen L.A., et al. 2021 Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(6):772–810. doi: 10.1016/j.jacc.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 4.McDonald M., Virani S., Chan M., et al. CCS/CHFS heart failure guidelines update: defining a new pharmacologic standard of care for heart failure with reduced ejection fraction. Can J Cardiol. 2021;37(4):531–546. doi: 10.1016/j.cjca.2021.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Vaduganathan M., Claggett B.L., Jhund P.S., et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396(10244):121–128. doi: 10.1016/S0140-6736(20)30748-0. [DOI] [PubMed] [Google Scholar]
- 6.Sharma A., Pagidipati N.J., Califf R.M., et al. Impact of regulatory guidance on evaluating cardiovascular risk of new glucose-lowering therapies to treat type 2 diabetes mellitus: lessons learned and future directions. Circulation. 2020;141(10):843–862. doi: 10.1161/CIRCULATIONAHA.119.041022. [DOI] [PubMed] [Google Scholar]
- 7.Zelniker T.A., Braunwald E. Cardiac and renal effects of sodium-glucose co-transporter 2 inhibitors in diabetes: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72(15):1845–1855. doi: 10.1016/j.jacc.2018.06.040. [DOI] [PubMed] [Google Scholar]
- 8.McMurray J.J.V., Packer M., Desai A.S., et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 9.McMurray J.J.V., Solomon S.D., Inzucchi S.E., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 10.Packer M., Anker S.D., Butler J., et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 11.Velazquez E.J., Morrow D.A., DeVore A.D., et al. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2018;380(6):539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 12.Greene S.J., Butler J., Albert N.M., et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF Registry. J Am Coll Cardiol. 2018;72(4):351–366. doi: 10.1016/j.jacc.2018.04.070. [DOI] [PubMed] [Google Scholar]
- 13.Vaduganathan M., Fonarow G.C., Greene S.J., et al. Contemporary treatment patterns and clinical outcomes of comorbid diabetes mellitus and HFrEF: the CHAMP-HF Registry. J Am Coll Cardiol HF. 2020;8(6):469–480. doi: 10.1016/j.jchf.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Greene S.J., Fonarow G.C., DeVore A.D., et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73(19):2365–2383. doi: 10.1016/j.jacc.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma A., Wu J., Ezekowitz J.A., et al. Eligibility of sodium–glucose co-transporter-2 inhibitors among patients with diabetes mellitus admitted for heart failure. ESC Heart Fail. 2020;7(1):275–279. doi: 10.1002/ehf2.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaduganathan M., Greene S.J., Zhang S., et al. Applicability of US Food and Drug Administration labeling for dapagliflozin to patients with heart failure with reduced ejection fraction in US clinical practice: the Get with the Guidelines-Heart Failure (GWTG-HF) Registry. JAMA Cardiol. 2021;6(3):267–275. doi: 10.1001/jamacardio.2020.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira J.P., Rossignol P., Machu J.-L., et al. Mineralocorticoid receptor antagonist pattern of use in heart failure with reduced ejection fraction: findings from BIOSTAT-CHF. Eur J Heart Fail. 2017;19(10):1284–1293. doi: 10.1002/ejhf.900. [DOI] [PubMed] [Google Scholar]
- 18.Eberly L.A., Yang L., Eneanya N.D., et al. Association of race/ethnicity, gender, and socioeconomic status with sodium-glucose cotransporter 2 inhibitor use among patients with diabetes in the US. JAMA Netw Open. 2021;4(4) doi: 10.1001/jamanetworkopen.2021.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponikowski P., Kirwan B.-A., Anker S.D., et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet. 2020;396(10266):1895–1904. doi: 10.1016/S0140-6736(20)32339-4. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong P.W., Pieske B., Anstrom K.J., et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382(20):1883–1893. doi: 10.1056/NEJMoa1915928. [DOI] [PubMed] [Google Scholar]
- 21.Savarese G., Bodegard J., Norhammar A., et al. Heart failure drug titration, discontinuation, mortality and heart failure hospitalization risk: a multinational observational study (US, UK and Sweden) Eur J Heart Fail. 2021;23(9):1499–1511. doi: 10.1002/ejhf.2271. [DOI] [PubMed] [Google Scholar]
- 22.Packer M., McMurray J.J.V. Rapid evidence-based sequencing of foundational drugs for heart failure and a reduced ejection fraction. Eur J Heart Fail. 2021;23(6):882–894. doi: 10.1002/ejhf.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma A., Aziz H., Verma S., et al. Permission to prescribe: do cardiologists need permission to prescribe diabetes medications that afford cardiovascular benefit. Curr Opin Cardiol. 2021;36(5):672–681. doi: 10.1097/HCO.0000000000000892. [DOI] [PubMed] [Google Scholar]
- 24.Yancy C.W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;62(16):1495–1539. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Seferovic P.M., Ponikowski P., Anker S.D., et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(10):1169–1186. doi: 10.1002/ejhf.1531. [DOI] [PubMed] [Google Scholar]
- 26.Ezekowitz J.A., O’Meara E., McDonald M.A., et al. 2017 Comprehensive update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can J Cardiol. 2017;33(11):1342–1433. doi: 10.1016/j.cjca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 27.McMurray J.J.V., Packer M. How should we sequence the treatments for heart failure and a reduced ejection fraction?: A redefinition of evidence-based medicine. Circulation. 2021:875–877. doi: 10.1161/CIRCULATIONAHA.120.052926. [DOI] [PubMed] [Google Scholar]
- 28.Tay W.T., Shimizu W., Anand I., et al. Prescribing patterns of evidence-based heart failure pharmacotherapy and outcomes in the ASIAN-HF registry: a cohort study. Lancet Glob Heal. 2018;6(9):e1008–e1018. doi: 10.1016/S2214-109X(18)30306-1. [DOI] [PubMed] [Google Scholar]
- 29.DeFilippis E.M., Butler J., Vaduganathan M. Waiting period before implantable cardioverter-defibrillator implantation in newly diagnosed heart failure with reduced ejection fraction: a window of opportunity. Circ Heart Fail. 2017;10(11):1–4. doi: 10.1161/CIRCHEARTFAILURE.117.004478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berg D.D., Jhund P.S., Docherty K.F., et al. Time to clinical benefit of dapagliflozin and significance of prior heart failure hospitalization in patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2021;6(5):499–507. doi: 10.1001/jamacardio.2020.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fonarow G.C., Yancy C.W. Expediting the benefits of sodium-glucose cotransporter-2 inhibitors for heart failure—there is no time for delay. JAMA Cardiol. 2021;6(5):507–508. doi: 10.1001/jamacardio.2020.7596. [DOI] [PubMed] [Google Scholar]
- 32.ClinicalTrials.gov Efficacy, safety and tolerability of AZD9977 and dapagliflozin in participants with heart failure and chronic kidney disease. https://clinicaltrials.gov/ct2/show/NCT04595370
- 33.Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med. 1987;316(23):1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 34.Marti C.N., Fonarow G.C., Anker S.D., et al. Medication dosing for heart failure with reduced ejection fraction—opportunities and challenges. Eur J Heart Fail. 2019;21(3):286–296. doi: 10.1002/ejhf.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Docherty K.F., Jhund P.S., Inzucchi S.E., et al. Effects of dapagliflozin in DAPA-HF according to background heart failure therapy. Eur Heart J. 2020;41(25):2379–2392. doi: 10.1093/eurheartj/ehaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okumura N., Jhund P.S., Gong J., et al. Effects of sacubitril/valsartan in the PARADIGM-HF trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) according to background therapy. Circ Heart Fail. 2016;9(9) doi: 10.1161/CIRCHEARTFAILURE.116.003212. [DOI] [PubMed] [Google Scholar]
- 37.Krum H., Shi H., Pitt B., et al. Clinical benefit of eplerenone in patients with mild symptoms of systolic heart failure already receiving optimal best practice background drug therapy analysis of the EMPHASIS-HF study. Circ Heart Fail. 2013;6(4):711–718. doi: 10.1161/CIRCHEARTFAILURE.112.000173. [DOI] [PubMed] [Google Scholar]
- 38.Packer M., Packer M. Are the benefits of SGLT2 inhibitors in heart failure and a reduced ejection fraction influenced by background therapy? Expectations and realities of a new standard of care. Eur Heart J. 2020;41(25):2393–2396. doi: 10.1093/eurheartj/ehaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willenheimer R., van Veldhuisen D.J., Silke B., et al. Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence. Circulation. 2005;112(16):2426–2435. doi: 10.1161/CIRCULATIONAHA.105.582320. [DOI] [PubMed] [Google Scholar]
- 40.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327(10):685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 41.SOLVD Investigators, Yusuf S., Pitt B., et al. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 42.Pitt B., Zannad F., Remme W.J., et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999 doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 43.Hjalmarson A., Goldstein S., Fagerberg B., et al. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353(9169):2001–2007. [PubMed] [Google Scholar]
- 44.Zannad F., McMurray J.J.V., Krum H., et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 45.Ajam T., Ajam S., Devaraj S., Fudim M., Kamalesh M. Effect on mortality of higher versus lower β-blocker (metoprolol succinate or carvedilol) dose in patients with heart failure. Am J Cardiol. 2018;122(6):994–998. doi: 10.1016/j.amjcard.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 46.Massie B.M., Armstrong P.W., Cleland J.G.F., et al. Toleration of high doses of angiotensin-converting enzyme inhibitors in patients with chronic heart failure: results from the ATLAS trial. Arch Intern Med. 2001;161(2):165–171. doi: 10.1001/archinte.161.2.165. [DOI] [PubMed] [Google Scholar]
- 47.Konstam M.A., Neaton J.D., Dickstein K., et al. Effects of High-Dose Versus Low-Dose Losartan on Clinical Outcomes in Patients With Heart Failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374(9704):1840–1848. doi: 10.1016/S0140-6736(09)61913-9. [DOI] [PubMed] [Google Scholar]
- 48.Ferreira J.P., Konstam M.A., McMurray J.J.V., et al. Dosing of losartan in men versus women with heart failure with reduced ejection fraction. Eur J Heart Fail. 2021;23(9):1477–1484. doi: 10.1002/ejhf.2255. [DOI] [PubMed] [Google Scholar]
- 49.Pitt B., Ferreira J.P., Zannad F. Mineralocorticoid receptor antagonists in patients with heart failure: current experience and future perspectives. Eur Hear J Cardiovasc Pharmacother. 2017;3(1):48–57. doi: 10.1093/ehjcvp/pvw016. [DOI] [PubMed] [Google Scholar]
- 50.Ferreira J.P., Abreu P., McMurray J.J.V., et al. Renal function stratified dose comparisons of eplerenone versus placebo in the EMPHASIS-HF trial. Eur J Heart Fail. 2019;21(3):345–351. doi: 10.1002/ejhf.1400. [DOI] [PubMed] [Google Scholar]
- 51.Bhatt A.S., Vaduganathan M., Claggett B.L., et al. Effect of sacubitril/valsartan vs. enalapril on changes in heart failure therapies over time: the PARADIGM-HF trial. Eur J Heart Fail. 2021;23(9):1518–1524. doi: 10.1002/ejhf.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferreira J.P., Zannad F., Pocock S.J., et al. Interplay of mineralocorticoid receptor antagonists and empagliflozin in heart failure: EMPEROR-Reduced. J Am Coll Cardiol. 2021;77(11):1397–1407. doi: 10.1016/j.jacc.2021.01.044. [DOI] [PubMed] [Google Scholar]
- 53.Grant A.D.M., Chew D.S., Howlett J.G., Miller R.J.H. Cost-effectiveness of earlier transition to angiotensin receptor neprilysin inhibitor in patients with heart failure and reduced ejection fraction. CJC Open. 2020;2(6):447–453. doi: 10.1016/j.cjco.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McEwan P., Bennett H., Khunti K., et al. Assessing the cost-effectiveness of sodium–glucose cotransporter-2 inhibitors in type 2 diabetes mellitus: a comprehensive economic evaluation using clinical trial and real-world evidence. Diabetes Obes Metab. 2020;22(12):2364–2374. doi: 10.1111/dom.14162. [DOI] [PubMed] [Google Scholar]
- 55.Isaza N., Calvachi P., Raber I., et al. Abstract 15981: cost-effectiveness of dapagliflozin in heart failure with reduced ejection fraction. Circulation. 2020;142(suppl 3) [Google Scholar]
- 56.Parizo J.T., Goldhaber-Fiebert J.D., Salomon J.A., et al. Cost-effectiveness of dapagliflozin for treatment of patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2021;6(8):926–935. doi: 10.1001/jamacardio.2021.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McMurray J.J.V., Trueman D., Hancock E., et al. Cost-effectiveness of sacubitril/valsartan in the treatment of heart failure with reduced ejection fraction. Heart. 2018;104(12):1006–1013. doi: 10.1136/heartjnl-2016-310661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosano G.M.C., Moura B., Metra M., et al. Patient profiling in heart failure for tailoring medical therapy. A consensus document of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2021;23(6):872–881. doi: 10.1002/ejhf.2206. [DOI] [PubMed] [Google Scholar]
- 59.Miller R.J.H., Howlett J.G., Fine N.M. A novel approach to medical management of heart failure with reduced ejection fraction. Can J Cardiol. 2021;37:632–643. doi: 10.1016/j.cjca.2020.12.028. [DOI] [PubMed] [Google Scholar]
- 60.Greene S.J., Butler J., Fonarow G.C. Simultaneous or rapid sequence initiation of quadruple medical therapy for heart failure-optimizing therapy with the need for speed. JAMA Cardiol. 2021;6(7):743–744. doi: 10.1001/jamacardio.2021.0496. [DOI] [PubMed] [Google Scholar]
- 61.McGuire D.K., Shih W.J., Cosentino F., et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6(2):148–158. doi: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salah H.M., Al’Aref S.J., Khan M.S., et al. Effect of sodium-glucose cotransporter 2 inhibitors on cardiovascular and kidney outcomes—systematic review and meta-analysis of randomized placebo-controlled trials: SGLT2i—Cardiovascular and Kidney Outcomes. Am Heart J. 2021;232:10–22. doi: 10.1016/j.ahj.2020.10.064. [DOI] [PubMed] [Google Scholar]
- 63.Verma S., Anker S.D., Butler J., Bhatt D.L. Early initiation of SGLT2 inhibitors is important, irrespective of ejection fraction: SOLOIST-WHF in perspective. ESC Heart Fail. 2020;7(6):3261–3267. [Google Scholar]
- 64.Zannad F., Ferreira J.P., Pocock S.J., et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396(10254):819–829. doi: 10.1016/S0140-6736(20)31824-9. [DOI] [PubMed] [Google Scholar]
- 65.Lim G.B. Benefits of combination pharmacotherapy for HFrEF. Nat Rev Cardiol. 2020;17(8):455. doi: 10.1038/s41569-020-0405-9. [DOI] [PubMed] [Google Scholar]
- 66.Crespo-Leiro M.G., Metra M., Lund L.H., et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(11):1505–1535. doi: 10.1002/ejhf.1236. [DOI] [PubMed] [Google Scholar]
- 67.DeVore A.D., Granger B.B., Fonarow G.C., et al. Care optimization through patient and hospital engagement clinical trial for heart failure: rationale and design of CONNECT-HF. Am Heart J. 2020;220:41–50. doi: 10.1016/j.ahj.2019.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.