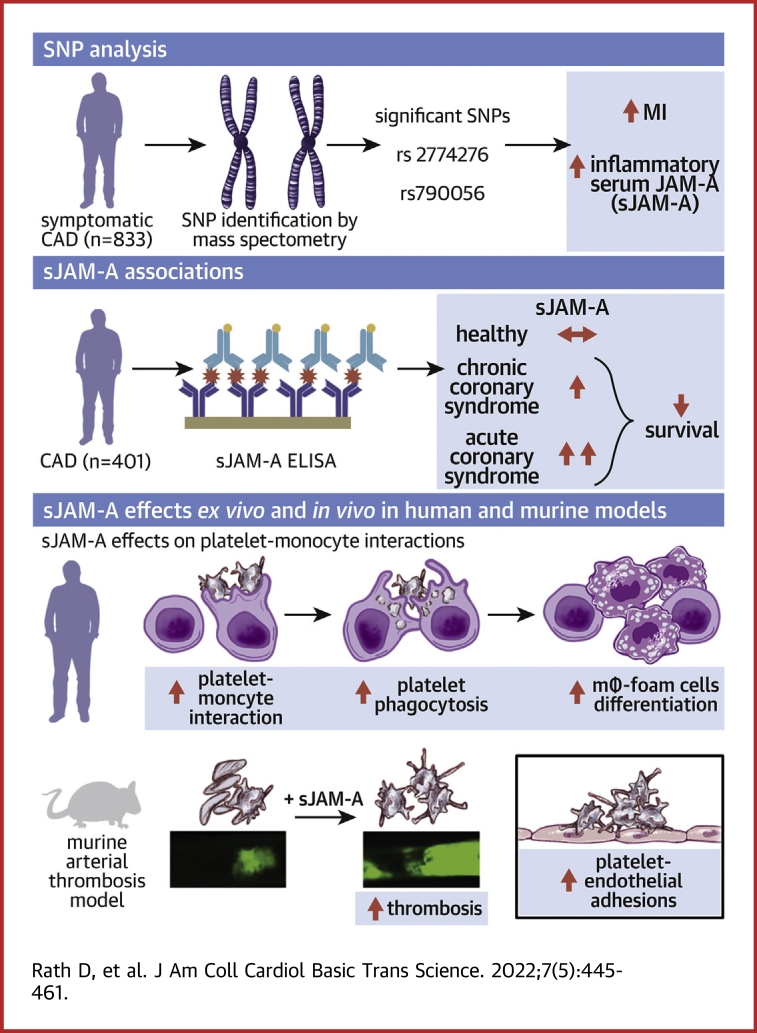
Visual Abstract
Key Words: coronary artery disease, JAM-A, platelet, SNV, thrombo-inflammation
Abbreviations and Acronyms: ACS, acute coronary syndrome; ACM, all-cause mortality; ADP, adenosine diphosphate; CAD, coronary artery disease; CCS, chronic coronary syndrome; CE, combined endpoint; HC, homozygous carriers; IS, ischemic stroke; JAM-A, junctional adhesion molecule-A; MI, myocardial infarction; sJAM-A, soluble junctional adhesion molecule-A; smJAM-A, soluble murine junctional adhesion molecule-A; SNV, single-nucleotide variation; TRAP, thrombin receptor activating peptide
Highlights
-
•
Genetic predisposition through F11R-SNVs determine circulatory sJAM-A levels in CAD patients. Homozygous carriers of the minor alleles-(rs2774276, rs790056) show enhanced levels of sJAM-A and worse event-free survival for MI in a 3-year follow-up time period.
-
•
Activated platelets shed transmembrane-JAM-A generating sJAM-A. Serum sJAM-A levels are elevated in ACS patients, correlate with peak troponin I, and influence probability of recurrent MI.
-
•
Platelet transmembrane-JAM-A and sJAM-A as unique homophilic interaction partners exaggerate thrombotic functions.
-
•
sJAM-A-activated apoptotic platelets form aggregates with monocytes and are phagocytosed, which fosters monocyte differentiation into macrophages-(mΦ) and foam cells prompting thrombo-inflammation.
-
•
Therapeutic strategies interfering with this homophilic interface between transmembrane-JAM-A and sJAM-A may regulate thrombotic and thrombo-inflammatory platelet response in cardiovascular pathologies where circulatory sJAM-A levels are elevated.
Summary
Genetic predisposition through F11R-single-nucleotide variation (SNV) influences circulatory soluble junctional adhesion molecule-A (sJAM-A) levels in coronary artery disease (CAD) patients. Homozygous carriers of the minor alleles (F11R-SNVs rs2774276, rs790056) show enhanced levels of thrombo-inflammatory sJAM-A. Both F11R-SNVs and sJAM-A are associated with worse prognosis for recurrent myocardial infarction in CAD patients. Platelet surface-associated JAM-A correlate with platelet activation markers in CAD patients. Activated platelets shed transmembrane-JAM-A, generating proinflammatory sJAM-A and JAM-A-bearing microparticles. Platelet transmembrane-JAM-A and sJAM-A as homophilic interaction partners exaggerate thrombotic and thrombo-inflammatory platelet monocyte interactions. Therapeutic strategies interfering with this homophilic interface may regulate thrombotic and thrombo-inflammatory platelet response in cardiovascular pathologies where circulatory sJAM-A levels are elevated.
Inflammatory factors are chronic mediators and therefore therapeutic targets in atherosclerotic cardiovascular complications. Junctional adhesion molecule-A (JAM-A) belonging to the immunoglobulin superfamily exists as a transmembrane adhesion receptor or soluble form (soluble junctional adhesion molecule-A [sJAM-A]), when shed from endothelium, hematopoietic progenitors, and circulatory cells1 by metalloproteinases ADAM10 and ADAM-17. JAM-A is a proinflammatory mediator in atheroprogression.2,3 Cytokines up-regulate JAM-A expression in human aortic and venous endothelial cells.4 Consequently, transmembrane-JAM-A mediates proinflammatory interactions among activated endothelium, monocytes,5 and platelets4 through trans-homophilic and heterophilic associations, which facilitates their recruitment to the inflamed endothelium.1 Enhanced F11R transcript and JAM-A protein expression are therefore detected at atherosclerotic plaques2 from cardiovascular disease patients and Apoe−/− mice.1,3 Therefore, therapeutic strategies antagonizing JAM-A are implicated in preventing atherothrombosis.2,6
Circulatory sJAM-A levels may depend on the expression status of transmembrane-JAM-A in cellular sources, influenced by single-nucleotide variation (SNV) at the F11R locus,7 and the extent of its release.1,3,8, 9, 10 A meta-analysis of >5,000 peripheral blood samples has shown that minor allele carriers of intronic F11R-SNV-rs2774276 and 5′ upstream SNV-rs790056 show significantly enhanced F11R gene expression in peripheral blood samples (cis-eQTLs),11 as well as several other tissues including aorta and the tibial artery.12 Both SNVs have been associated with low systolic blood pressure, whereas F11R-SNVs rs2481084 and rs3737787 have been associated with lower odds of central obesity. Enhanced circulatory sJAM-A levels are observed in end-stage renal failure10 and hypertension,8,9 and independently associated with the presence and severity of angiographically defined CAD.3 However, a link between distinct F11R-SNVs and circulatory sJAM-A among CAD patients remained undefined.
F11R was cloned from platelets.13 Transmembrane-JAM-A regulates platelet activation14 and signaling through αIIbβ3, by complex formation with β3-integrin.15 Genetic deficiency of F11r potentiates thrombotic response in Jam-Agt/gt mice.15, 16, 17 Since circulating platelets in CAD patients may shed receptors in their hyperactive state, they might act as a potential novel source of sJAM-A like leukocytes, and the inflamed endothelium, which needed verification. Moreover, as circulatory sJAM-A levels are elevated in hypertensive8 and CAD patients,3 its impact on thrombo-inflammatory platelet functions warranted validation. Current investigation uncovered F11R-SNVs’ influence on circulatory sJAM-A levels in CAD patients and their potential association with long-term cardiovascular prognosis. Moreover, we revealed the novel thrombo inflammatory potential of sJAM-A mediated through homophilic interaction with platelet transmembrane-JAM-A, adding an unexpected dimension to the previously recognized regulatory influence of transmembrane-JAM-A on platelet outside-in-signaling16,17 and its inflammatory attributes in early stages of atheroprogression.18,19 The current paper validates the contribution of transmembrane-JAM-A and sJAM-A as a functional duo in influencing thrombotic and thrombo-inflammatory platelet functions, which had not been explored before but is of vital significance in the pathophysiology of CAD.
Methods
Methodological details for experimental studies are provided in the Supplemental Appendix.
CAD patient cohort
F11R-SNV (rs2774276, rs2481084, rs3737787, rs790056) analysis was performed in consecutive symptomatic CAD20 patients (n = 833). Chronic coronary syndrome (CCS) and acute coronary syndrome (ACS) were defined as explained previously.20 All patients were admitted to the Department of Cardiology and Angiology, University Hospital Tübingen, and gave written informed consent. The study was approved by the institutional ethics committee (270/2011BO1, 237/2018BO2), and complied with the Declaration of Helsinki and good clinical practice guidelines. Baseline patient characteristics are given in Table 1.
Genotyping of F11R-SNV variants in CAD patients
Genotyping was performed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry using the MassARRAY Compact system (Sequenom)20 Genotype data for all SNVs were in Hardy-Weinberg equilibrium. Study personnel assessing outcome was blinded to the clinical manifestations of patients. Of note, genotyping quality for rs790056 and rs3737787 was too low in 2 and 3 patients, respectively. Hence, rs790056 and rs3737787 were only analyzed in 831 and 830 patients, respectively.
Serum sJAM-A levels in CAD patients
sJAM-A levels were subsequently determined by enzyme-linked immunosorbent assay (ELISA) in 401 CAD patients (Table 2). Among these, 159 (39.7%) were specifically matched patients from the F11R-SNV cohort. Because homozygous carriers of the minor alleles (rs2774276, rs790056) showed a significant impact on prognosis, this matched subset was determined by first choosing 51 and 35 of the homozygote minor allele carriers of F11R-SNV-rs2774276 and F11R-SNV-rs790056, respectively. Corresponding heterozygous as well as homozygous major allele carriers were then selected using library optmatch 0.9-1321 of statistical software R version 3.5.0 (R Foundation for Statistical Computing)22 and a 1:1-matching for ACS/CCS and left ventricular ejection fraction class (normal, mild/moderate/severe impairment).
Table 2.
Baseline Characteristics of CAD Patients Stratified According to sJAM-A ≤1.71 ng/mL and >1.71 ng/mL
| sJAM-A ≤ Median 1.71 ng/mL (n = 201) | sJAM-A > Median 1.71 ng/mL (n = 200) | P Value | |
|---|---|---|---|
| Age, y | 69.8 ± 11.0 | 69.0 ± 10.9 | 0.552 |
| Male | 141 (70.1) | 148 (74.0) | 0.380 |
| LVEF, % | 52.3 ± 11.0 | 49.7 ± 12.4 | 0.041 |
| Risk factors | |||
| Arterial hypertension | 164 (81.6) | 163 (81.5) | 0.826 |
| Hyperlipidemia | 115 (57.2) | 94 (47.0) | 0.024 |
| Diabetes mellitus type II | 59 (29.4) | 65 (32.5) | 0.519 |
| Smoking | 70 (34.8) | 69 (34.5) | 0.916 |
| Medication on admission | |||
| ASA | 107 (53.2) | 102 (51.0) | 0.642 |
| Clopidogrel | 25 (12.4) | 22 (11.0) | 0.655 |
| Prasugrel | 3 (1.5) | 5 (2.5) | 0.471 |
| Ticagrelor | 9 (4.5) | 3 (1.5) | 0.096 |
| ACE inhibitors | 80 (39.8) | 90 (45.0) | 0.306 |
| AT-1 antagonists | 43 (21.4) | 36 (18.0) | 0.399 |
| Calcium-channel blockers | 40 (19.9) | 45 (22.5) | 0.517 |
| Beta-blockers | 118 (58.7) | 112 (56.0) | 0.596 |
| Statins | 105 (52.2) | 91 (45.5) | 0.177 |
| Reason of admission, CCS vs ACS | |||
| ACS | 98 (48.8) | 119 (59.5) | 0.034 |
| Type of coronary intervention | |||
| PCI | 169 (84.1) | 174 (87.0) | 0.412 |
| CABG | 1 (0.5) | 1 (0.5) | 0.997 |
| None | 31 (15.4) | 25 (12.5) | 0.402 |
| Medication at discharge | |||
| ASA | 193 (96.0) | 181 (90.5) | 0.062 |
| Clopidogrel | 104 (51.7) | 97 (48.5) | 0.575 |
| Prasugrel | 26 (12.9) | 38 (19.0) | 0.096 |
| Ticagrelor | 45 (22.4) | 40 (20.0) | 0.579 |
| Simvastatin | 132 (65.7) | 130 (65.0) | 0.998 |
| Atorvastatin | 33 (16.4) | 33 (16.5) | 0.947 |
| Rosuvastatin | 6 (3.0) | 6 (3.0) | 0.979 |
| Pravastatin | 3 (1.5) | 2 (1.0) | 0.664 |
| Fluvastatin | 7 (3.5) | 5 (2.5) | 0.578 |
| Lovastatin | 0 (0.0) | 0 (0.0) | — |
Values are mean ± SD or n (%). Bold values indicate statistical significance.
Abbreviations as in Table 1.
ELISA and cytometric bead array for inflammatory mediators
Serum samples were used to estimate soluble L-selectin and P-selectin in matched CAD patients (with F11R-SNVs) with ELISA (Duo set Kits, R&D System). Similarly, a human proinflammatory chemokine panel (Legendplex, Biolegend) was used to estimate serum levels of inflammatory mediators by cytometric bead array.
Survival endpoints and prognostic association
All patients were followed up for all-cause mortality (ACM), myocardial infarction (MI), and ischemic stroke (IS) for 1,080 days after study enrollment. A combined endpoint (CE) was defined as a composite of ACM and/or MI and/or IS. Further endpoints were defined as single events of ACM and MI. Acute MI and IS were defined as described previously.20 Follow-up was performed by telephonic interview and/or review of patients’ charts on readmission by investigators blinded to laboratory results. In the F11R-SNV cohort, 75 of 833 (9%) patients were lost to follow-up. In the sJAM-A cohort, 10 of 401 (2.5%) patients were lost to follow-up.
Statistical analysis
Experimental data were analyzed using GraphPad Prism software with analysis of variance for ≥3 groups and Student’s t-test for 2 groups. Data represent mean ± SEM.
Clinical cohort
Statistical analyses were performed using SPSS version 27.0 (IBM), GraphPad Prism software, and libraries clusrank_1.0-2, cmprsk_2.2-11, crrSC_1.1, htestClust_0.2.0, and rms_6.1-1 of R statistical software. Data are presented as median with 25th and 75th percentiles (Q1, Q3), mean ± SD, or count and percentage. Chi-square tests, Student’s t-test, Mann-Whitney U tests, clustered Wilcoxon rank sum test using the Rosner-Glynn-Lee method,23 and logistic regression along with the Huber-White method24 were applied as appropriate to analyze baseline characteristics. The latter 2 methods were used to correct for the correlation structure in case of matched patients (ie, when analyzing the 401 patients with sJAM-A levels). Correlations of non-normally distributed data were assessed by the usual Spearman’s rank correlation coefficient or an analogue for matched data,25 as appropriate. Clustered Wilcoxon rank sum test was also used to investigate the association between sJAM-A levels and F11R-SNV variants in the subset of matched patients. Cox proportional hazard (PH) regression and the Fine-Gray method26 for competing risks as a secondary method were applied to investigate associations between survival endpoints and F11R-SNVs (recessive model) or serum sJAM-A-(≤ median vs > median), using clinical factors as covariables. Here, in the case of matched patients, we applied the Huber-White method (Cox regression) or an extension of the Fine-Gray method for clustered data.27 The time-dependent covariate method was used to check the proportional hazard assumption of the Cox model. Survival functions were estimated by Kaplan-Meier curves. The log-rank test was applied to compare survival functions between homozygous carriers of the minor allele and carriers of the major allele (ie, recessive model). All statistical tests were 2-tailed, and statistical significance was defined as P < 0.05. Where indicated, the Holm-Bonferroni method28 was applied to adjust P values for multiple testing.
Results
Prognostic association of F11R-SNVs in CAD and in influencing proinflammatory sJAM-A levels
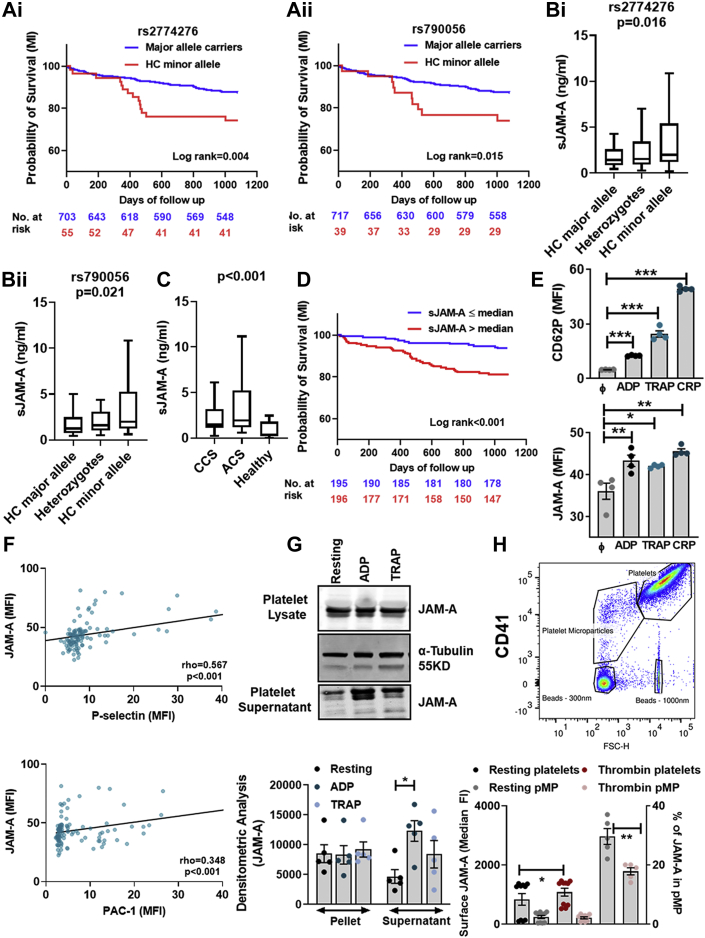
Several SNVs of extramyocardial functional significance have been associated with long-term mortality after acute myocardial infarction (AMI), including those affecting platelet responsiveness to therapeutic interventions.29 Currently, F11R-SNV analysis in CAD patients revealed that homozygous carriers (HC) of the minor alleles (rs2774276, rs790056) had significantly worse event-free survival for MI when compared with major allele carriers (unadjusted P = 0.004; P = 0.015; Holm-adjusted P = 0.016; P = 0.045, respectively) (Table 3, Figure 1A, i-ii). In multivariable Cox regression analysis, rs2774276 was independently associated with time to MI (Holm-adjusted P = 0.048), whereas rs790056 was not significantly correlated after correction for multiple testing (Holm-adjusted P = 0.075). However, when applying the Fine-Gray’s method with all-cause death as competing risk, rs790056 was significantly associated with MI (Holm-adjusted P = 0.039) (Table 4). This implies that rs790056 has a stronger relationship with MI directly than with the underlying etiology of the disease.
Table 3.
Event Rates and Incidence Rates/100 Person-Years in the Overall Cohort
| Endpoint | Number of Events (Carriers of Major Allele/HC of Minor Allele) | IR/100 Person-y | P Value |
|---|---|---|---|
| F11R-SNV-rs2774276 (n = 703/55) | |||
| Combined endpoint | 175/16 | 8.3/9.7 | 0.49 |
| Myocardial infarction | 81/14 | 3.8/8.5 | 0.003 |
| All-cause mortality | 97/6 | 4.6/3.6 | 0.547 |
| F11R-SNV-rs790056 (n = 717/39) | |||
| Combined endpoint | 179/12 | 8.3/10.3 | 0.417 |
| Myocardial infarction | 85/10 | 4.0/8.5 | 0.011 |
| All-cause mortality | 97/6 | 4.5/5.1 | 0.742 |
| Endpoint | Number of events sJAM-A (≤1.71 ng/mL n = 195/ >1.71 ng/mL n = 196) | IR/100 Person-y | P Value |
|---|---|---|---|
| Combined endpoint | 33/53 | 5.6/9.1 | 0.012 |
| Myocardial infarction | 13/35 | 2.2/6.0 | <0.001 |
| All-cause mortality | 14/24 | 2.4/4.1 | 0.071 |
Bold values indicate statistical significance.
IR = incidence rate; other abbreviations as in Table 1.
Figure 1.
F11R-SNVs May Influence sJAM-A Levels and Be Associated With Prognosis in CAD Patients
Kaplan-Meier curves showing probability of recurrent MI stratified according to (Ai)F11R-SNV-rs2774276 genotype and (Aii)F11R-SNV-rs790056 genotype. (B) sJAM-A serum levels in CAD patients (n = 51 each for rs2774276, HC-major, HC-minor, and heterozygotes; n = 35 each for rs790056, HC-major, HC-minor, and heterozygotes), stratified according to F11R-SNV genotype. P values show differences between HC of minor allele vs major allele carriers. (C) Serum sJAM-A levels in CAD (n = 401) patients with CCS (n = 184) vs ACS (n = 217) compared with healthy subjects (n = 24). Between ACS vs CCS and CAD vs healthy: P < 0.001. (D) Kaplan-Meier curves showing probability of recurrent MI stratified according to sJAM-A ≤ median vs > median (≤1.71 ng/mL vs >1.71 ng/mL) in CAD patients (n = 391). (E) Significantly enhanced platelet surface-associated JAM-A upon activation compared with CD62P. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 vs resting (ϕ) platelets. Correlation among (F) P-selectin, PAC-1, and platelet surface-associated JAM-A and/or sJAM-A in CAD (n = 111) patients. (G) Western blot and corresponding densitometric analysis for JAM-A in resting and ADP or TRAP-activated platelet lysate and shed sJAM-A detected in activated platelet supernatant. Loading controls for cell lysates as α–tubulin immunoblots. (H) Flow cytometry dot plot showing gating strategy for CD41+ platelets and pMPs. Bar diagram shows increased pMP generation in thrombin-activated platelets, and surface-associated JAM-A on platelets and pMPs. ∗P < 0.050; ∗∗P < 0.001 Data show mean ± SEM for n = 5 donors. ACS = acute coronary syndrome; CAD = coronary artery disease; CCS = chronic coronary syndrome; HC = homozygous carriers; JAM-A = junctional adhesion molecule-A; MI = myocardial infarction; sJAM-A = soluble junctional adhesion molecule-A; SNV = single nucleotide variation; TRAP = thrombin receptor activating peptide.
Table 4.
Results of Multivariable Cox PH Regression Analyses as Well as Fine and Gray’s Proportional Subdistribution Hazards Regression Model for Myocardial Infarction With Clinical Factorsa as Covariates
| Cox PH Model |
Fine-Gray Model |
|||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| F11R-SNA-rs2774276 (recessive genetic model) | 2.09 (1.18-3.70) | Unadjusted 0.012 Holm-adjusted 0.048 |
2.37 (1.32-4.24) | Unadjusted-0.004 Holm-adjusted-0.015 |
| Age | 1.05 (1.03-1.07) | <0.001 | 1.04 (1.02-1.06) | <0.001 |
| Gender (female/male) | 0.72 (0.45-1.17) | 0.189 | 0.72 (0.45-1.16) | 0.180 |
| LVEF% | 0.79 (0.65-0.96) | 0.020 | 0.83 (0.68-1.03) | 0.085 |
| Reason of admission (ACS/CCS) | 1.67 (1.11-2.52) | 0.016 | 1.53 (1.02-2.32) | 0.042 |
| F11R-SNA-rs790056 (recessive genetic model) | 2.13 (1.10-4.11) | Unadjusted 0.025 Holm-adjusted 0.075 |
2.38 (1.20-4.70) | Unadjusted-0.013 Holm-adjusted-0.039 |
| Age | 1.05 (1.03-1.07) | <0.001 | 1.04 (1.02-1.06) | <0.001 |
| Gender (female/male) | 0.73 (0.45-1.18) | 0.202 | 0.73 (0.45-1.17) | 0.190 |
| LVEF% | 0.78 (0.64-0.96) | 0.016 | 0.83 (0.68-1.02) | 0.073 |
| Reason of admission (ACS/CCS) | 1.71 (1.13-2.58) | 0.011 | 1.57 (1.04-2.37) | 0.032 |
| sJAM-A ≤ median vs > median (1.71 ng/mL) | 2.95 (1.59-5.46) | Unadjusted <0.001 Holm-adjusted 0.003 |
2.91 (1.57-5.38) | Unadjusted <0.001 Holm-adjusted 0.003 |
| Age | 1.02 (0.99-1.05) | 0.150 | 1.01 (0.99-1.04) | 0.260 |
| Gender (female/male) | 0.81 (0.43-1.55) | 0.530 | 0.84 (0.44-1.60) | 0.600 |
| LVEF% | 0.83 (0.64-1.07) | 0.149 | 0.86 (0.66-1.11) | 0.240 |
| Reason of admission (ACS/CCS) | 0.96 (0.52-1.75) | 0.883 | 0.94 (0.52-1.70) | 0.840 |
Bold values indicate statistical significance.
Abbreviations as in Table 1.
Clinical variables included into the model: age, gender, LVEF%, and reason of admission (ACS/CCS).
Among CCS patients, 25.0% of HC of F11R-SNV-rs2774276 minor allele vs 8.9% of major allele carriers experienced MI (log-rank test P = 0.007), whereas, in the ACS group, 25.9% of HC of F11R-SNV-rs2774276 minor allele and 14.3% of major allele carriers succumbed to recurrent MI (log-rank test P = 0.127). There was no significant variation in interventions and medications that the patients received on discharge (Table 1). In Cox PH regression analysis (Supplemental Table 1), none of these treatments were significantly associated with outcome, whereas rs2774276 remained significantly associated with time to MI (Holm-adjusted P = 0.020). In contrast, rs790056 closely failed to be independently and significantly associated in Cox regression and Fine-Gray analysis after correction for multiple testing (Holm-adjusted P = 0.069 and P = 0.063, respectively).
Table 1.
Baseline Characteristics of CAD Patients Stratified According to F11R-SNV-rs2774276 and to F11R-SNV-rs790056 Major Allele Carriers vs HCs of Minor Allele (Recessive Genetic Model)
| Carriers of Major Allele | HC of Minor Allele | P Value | |
|---|---|---|---|
| F11R-SNV-rs2774276 | |||
| n | 777 | 56 | |
| Age, y | 68.4 ± 11.6 | 70.0 ± 10.0 | 0.309 |
| Male | 549 (70.7) | 41 (73.2) | 0.716 |
| LVEF, % | 51.0 ± 10.7 | 52.0 ± 10.5 | 0.528 |
| Risk factors | |||
| Arterial hypertension | 649 (83.5) | 45 (80.4) | 0.926 |
| Hyperlipidemia | 454 (58.4) | 30 (53.6) | 0.621 |
| Diabetes mellitus type II | 257 (33.1) | 16 (28.6) | 0.593 |
| Smoking | 319 (41.1) | 20 (35.7) | 0.525 |
| Medication on admission | |||
| ASA | 430 (55.3) | 32 (57.1) | 0.850 |
| Clopidogrel | 94 (12.1) | 9 (16.1) | 0.400 |
| Prasugrel | 15 (1.9) | 2 (3.6) | 0.418 |
| Ticagrelor | 35 (4.5) | 2 (3.6) | 0.732 |
| ACE inhibitors | 332 (42.7) | 24 (42.9) | 0.983 |
| AT-1 antagonists | 139 (17.9) | 12 (21.4) | 0.522 |
| Calcium-channel blockers | 154 (19.8) | 14 (25.0) | 0.364 |
| Beta-blockers | 442 (56.9) | 31 (55.4) | 0.782 |
| Statins | 367 (47.2) | 30 (53.6) | 0.383 |
| Reason of admission, CCS vs ACS | |||
| ACS | 383 (49.3) | 28 (50.0) | 0.918 |
| Type of coronary intervention | |||
| PCI | 658 (84.7) | 50 (89.3) | 0.352 |
| CABG | 8 (1.0) | 0 (0.0) | 0.445 |
| None | 111 (14.3) | 6 (10.7) | 0.364 |
| Medication at discharge | |||
| ASA | 733 (94.3) | 52 (92.9) | 0.323 |
| Clopidogrel | 406 (52.3) | 30 (53.6) | 0.934 |
| Prasugrel | 120 (15.4) | 10 (17.9) | 0.664 |
| Ticagrelor | 145 (18.7) | 12 (21.4) | 0.646 |
| Simvastatin | 591 (76.1) | 46 (82.1) | 0.388 |
| Atorvastatin | 38 (4.8) | 2 (3.6) | 0.641 |
| Rosuvastatin | 24 (3.1) | 1 (1.8) | 0.571 |
| Pravastatin | 2 (0.3) | 1 (1.8) | 0.068 |
| Fluvastatin | 27 (3.5) | 2 (3.6) | 0.985 |
| Lovastatin | 1 (0.1) | 0 (0.0) | 0.787 |
| F11R-SNV- rs790056 | |||
| n | 791 | 40 | |
| Age, y | 68.4 ± 11.6 | 70.4 ± 10.2 | 0.294 |
| Male | 562 (71.0) | 28 (70.0) | 0.857 |
| LVEF, % | 50.9 ± 10.7 | 53.9 ± 10.0 | 0.092 |
| Risk factors | |||
| Arterial hypertension | 656 (82.9) | 35 (87.5) | 0.102 |
| Hyperlipidemia | 458 (57.9) | 24 (60.0) | 0.514 |
| Diabetes mellitus type II | 260 (32.9) | 12 (30.0) | 0.888 |
| Smoking | 324 (41.0) | 14 (35.0) | 0.619 |
| Medication on admission | |||
| ASA | 436 (55.1) | 24 (60.0) | 0.586 |
| Clopidogrel | 96 (12.1) | 7 (17.5) | 0.329 |
| Prasugrel | 17 (2.1) | 0 (0.0) | 0.346 |
| Ticagrelor | 35 (4.4) | 2 (5.0) | 0.875 |
| ACE inhibitors | 338 (42.7) | 17 (42.5) | 0.950 |
| AT-1 antagonists | 141 (17.8) | 10 (25.0) | 0.260 |
| Calcium-channel blockers | 159 (20.1) | 9 (22.5) | 0.728 |
| Beta-blockers | 450 (56.9) | 22 (55.0) | 0.779 |
| Statins | 373 (47.2) | 23 (57.5) | 0.215 |
| Reason of admission, CCS vs ACS | |||
| ACS | 392 (49.6) | 17 (42.5) | 0.384 |
| Type of coronary intervention | |||
| PCI | 671 (84.8) | 35 (87.5) | 0.645 |
| CABG | 8 (1.0) | 0 (0.0) | 0.523 |
| None | 112 (14.2) | 5 (12.5) | 0.768 |
| Medication at discharge | |||
| ASA | 746 (94.3) | 37 (92.5) | 0.351 |
| Clopidogrel | 413 (52.2) | 23 (57.5) | 0.574 |
| Prasugrel | 124 (15.7) | 6 (15.0) | 0.880 |
| Ticagrelor | 147 (18.6) | 8 (20.0) | 0.856 |
| Simvastatin | 603 (76.2) | 32 (72.5) | 0.691 |
| Atorvastatin | 37 (4.7) | 3 (7.5) | 0.430 |
| Rosuvastatin | 24 (3.0) | 1 (2.5) | 0.836 |
| Pravastatin | 3 (0.4) | 0 (0.0) | 0.694 |
| Fluvastatin | 28 (3.5) | 1 (2.5) | 0.716 |
| Lovastatin | 1 (0.1) | 0 (0.0) | 0.821 |
Values are mean ± SD or n (%).
ACE = angiotensin-converting enzyme; ASA = aspirin; ACS = acute coronary syndrome; CAD = coronary artery disease; CCS = chronic coronary syndrome; CE = combined endpoint; HC = homozygous carriers; JAM-A = junctional adhesion molecule-A; SNV = single nucleotide variation
Because F11R-SNVs influence JAM-A expression in cells,11 they may regulate sJAM-A levels arising from shedding of transmembrane-JAM-A from cellular sources. Due to the observed prognostic association of F11R-SNV minor alleles, we analyzed serum sJAM-A levels among matched CAD patients. Serum sJAM-A levels were consistently increased with the presence and number of minor alleles For F11R-SNV-rs790056, median (Q1, Q3) serum sJAM-A levels in HC of major alleles, heterozygotes, and HC of minor alleles were 1.24 ng/mL (0.75, 2.48 ng/mL), 1.60 ng/mL (1.02, 3.21 ng/mL), and 2.00 ng/mL (1.25, 5.26 ng/mL), respectively (Clustered Wilcoxon rank sum test for HC of minor allele vs major allele carriers: P = 0.021). Similarly, for F11R-SNV-rs2774276 median (Q1, Q3) serum sJAM-A levels in HC of major alleles, heterozygotes, and HC of minor alleles were 1.45 ng/mL (0.86, 2.60 ng/mL), 1.57 ng/mL (0.95, 3.80 ng/mL), and 1.99 ng/mL (1.19, 5.40 ng/mL), respectively (Clustered Wilcoxon rank sum test for HC of minor allele vs major allele carriers: P = 0.016) (Figure 1B).
Association of sJAM-A with thrombo-ischemic complications in CAD
Encouraged by these findings, we further extended our analysis to explore a potential prognostic association of serum sJAM-A in CAD patients (n = 401). Median sJAM-A levels (Q1, Q3), were significantly (P < 0.001) elevated in CAD patients (1.71 ng/mL; 1.18, 4.43 ng/mL) compared with healthy subjects without manifestation of cardiovascular disease (n = 24; 0.37 ng/mL; 0.20, 1.80 ng/mL) (Figure 1C). Moreover, significantly elevated serum sJAM-A levels were observed in ACS compared with CCS patients (1.94 ng/mL; 1.21, 5.21 ng/mL vs 1.51 ng/mL; 1.14, 3.19 ng/mL; P < 0.001) (Figure 1C), suggesting a potential link with thrombo-ischemic events. Additionally, peak troponin I correlated moderately and significantly with sJAM-A levels in CAD patients (rho = 0.302; P < 0.001).
Elevated (> median, ie, >1.71 ng/mL) sJAM-A levels measured upon admission were significantly associated with CE (P = 0.008) but not ACM (P = 0.058). Patients with higher (> median, ie, >1.71 ng/mL) sJAM-A levels showed a significantly increased risk for recurrent MI (P < 0.001; Holm-adjusted P = 0.001, respectively) (Figure 1D). sJAM-A was independently associated with time to MI in multivariable analysis (Table 4, Supplemental Table 1). Notably, we could measure sJAM-A levels only upon admission, which left the potential influence of subsequent antiplatelet therapy to speculation. However, we verified the influence of medication at admission on baseline sJAM-A levels. Although aspirin (ASA), clopidogrel, and prasugrel showed no significant impact, we observed reduced sJAM-A levels (median; Q1, Q3) (0.90 ng/mL; 0.69, 1.82 ng/mL vs 1.72 ng/mL; 1.19, 4.46 ng/mL; P = 0.014) in 12 patients with ticagrelor at admission. Because of the association of transmembrane-JAM-A and αIIbβ3-intergrin in a signaling complex in resting platelets,17 we compared sJAM-A levels in ACS patients (n = 6) administered with glycoprotein GPIIb/IIIa antagonists in 22 patients after thrombus aspiration who did not receive GPIIbIIIa antagonists. We did not observe a significant difference in sJAM-A levels (median; Q1, Q3) (4.25 ng/mL; 2.84, 5.82 ng/mL vs 4.63 ng/mL; 1.29, 8.03 ng/mL; P = 0.740). Because inflamed endothelium is a primary source of circulatory sJAM-A, we furthermore validated the effect of statins, which reduce endothelial inflammation30 on sJAM-A levels. Patients receiving statins (n = 196) at admission showed lower sJAM-A levels (median; Q1, Q3) when compared with those without statin treatment (1.56 ng/mL; 1.14, 3.79 ng/mL vs 1.82 ng/mL; 1.21, 4.96 ng/mL; P = 0.110). However, this effect was not statistically significant.
Activated platelets shed transmembrane-JAM-A to generate thrombo-inflammatory sJAM-A
Among healthy donors, platelet surface-associated JAM-A (cumulative signals from transmembrane-JAM-A + surface-bound circulatory sJAM-A) was significantly increased following in vitro activation for 30 minutes induced by (Figure 1E) adenosine diphosphate (ADP) (P2Y12), thrombin receptor (PAR-1)-activating peptide (TRAP), and collagen-related peptide-GPVI, although requiring a stronger stimulus than degranulation (CD62P) (Supplemental Figure S1). Moreover, basal platelet surface-associated JAM-A in CAD patients significantly correlated with basal platelet activation markers-CD62P surface expression (rho = 0.567; P < 0.001) and PAC-1 binding (rho = 0.348; P < 0.001) (Figure 1F), revealing an unpredicted association of JAM-A with thrombotic propensity. Upon prolonged activation (60 minutes), activated platelets shed transmembrane-JAM-A, in ADP and TRAP-activated platelet supernatant (Figure 1G). Platelet activation with thrombin generates platelet-derived microparticles (pMPs) (Figure 1H), which carry thrombo-inflammatory mediators.31 We detected surface-associated JAM-A on resting and thrombin activated platelets and pMPs (Figure 1H), which can engage in thrombo-inflammatory interaction with the endothelium and leukocytes.1,5
sJAM-A substantiates thrombotic functions
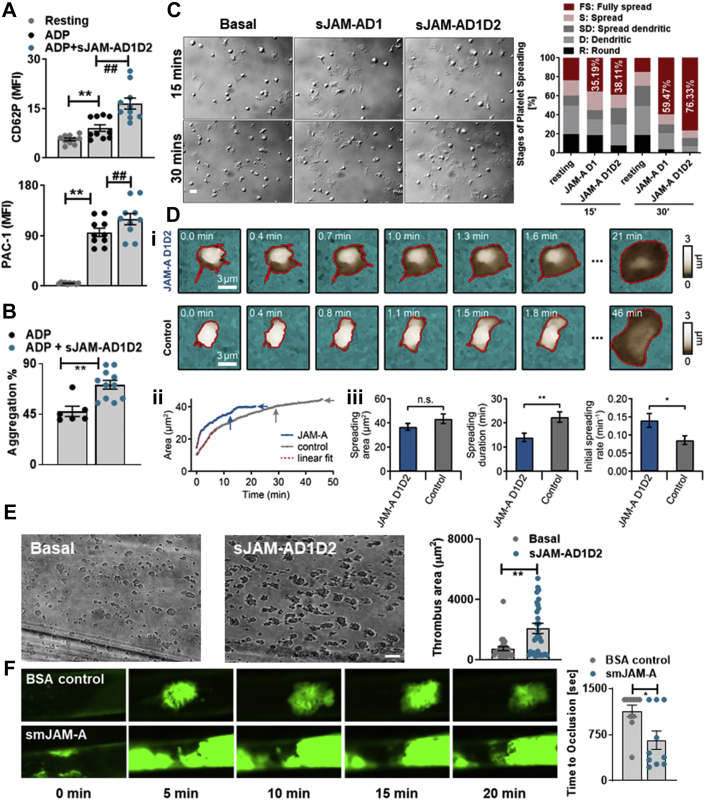
To assess the pathophysiological relevance of increased sJAM-A levels in CAD patients, we analyzed the effect of recombinant-JAM-A, mimicking circulatory sJAM-A, on thrombotic functions. JAM-A consists of a membrane-proximal-D2 and a membrane-distal-D1 domain.32 We generated a native recombinant sJAM-A molecule with both domains (sJAM-AD1D2) and a truncated version (sJAM-AD1) with only the D1 domain.32 Although sJAM-A alone did not induce platelet degranulation (CD62P exposure), or aggregation (Supplemental Figure S2), pretreatment with sJAM-A synergistically enhanced ADP-induced CD62P surface expression, αIIbβ3-integrin activation (Figure 2A), and aggregation (Figure 2B). Priming with sJAM-A also enhanced the percentage of fully spread platelets on fibrinogen (Figure 2C) and accelerated the kinetics of spreading, deciphered by high-resolution live imaging of single platelet response (Figure 2D, i-iii). sJAM-A enhanced thrombus formation (Figure 2E) ex vivo. Similarly, administration of soluble murine junctional adhesion molecule-A (smJAM-A) mimicking pathological elevation in circulatory sJAM-A levels enhanced thrombus formation and occluded the injured carotid artery in vivo (Figure 2F, Videos 1 and 2) compared with control mice, which mostly did not show complete vessel occlusion. Current results reveal that sJAM-A may exert a unique prothrombotic impact besides its proinflammatory attributes.
Figure 2.
sJAM-A Exerts a Prothrombotic Effect
Synergistic effect of sJAM-AD1D2 (30 μg/mL) on ADP (10 μmol/L) induced platelet (A) CD62P surface expression, PAC-1 binding (n = 5). ∗∗P < 0.01; ##P < 0.01. (B) Aggregation (n = 5 with technical replicates). ∗∗P < 0.01. (C) Relative stages of platelets spreading on fibrinogen (n = 5) over time ±sJAM-AD1 (10 μg/mL) or sJAM-AD1D2 (30 μg/mL). Bar = 2 μm. (D) Kinetics of platelet spreading on fibrinogen, and sJAM-AD1D2 accelerating the process. (Di) SICM topography images of spreading platelets ±sJAM-AD1D2 (30 μg/mL). Bar = 3 μm. (Dii) The spreading area as a function of time. Spreading was quantified by the final spreading area (horizontal arrows) and spreading duration until 90% of final spreading area (vertical arrows). The relative initial spreading rate was determined by fitting the spreading area (solid traces) up to 60% of the relative area with a linear fit (dashed red line). (Diii) Spreading area, spreading duration, and relative initial spreading rate ±sJAM-AD1D2. Data show mean ± SEM for sJAM-AD1D2-treated (n = 11) and control platelets (n = 8). ∗P < 0.05; ∗∗P < 0.01 (Student’s t-test). (E) Thrombus coverage (n = 5) ±sJAM-AD1D2 (30 μg/mL). ∗∗P < 0.01. Bar = 50 μm. (F) Thrombus (platelets labelled with GP Ibβ-X488 appearing in green) build-up over time in smJAM-A (50 μg/mouse) and BSA-control (50 μg/mouse) administered mice (n = 5 mice/group). Bar diagram shows time to vessel occlusion ±smJAM-A. ∗∗P < 0.01. Data represent mean ± SEM. Abbreviations as in Figure 1.
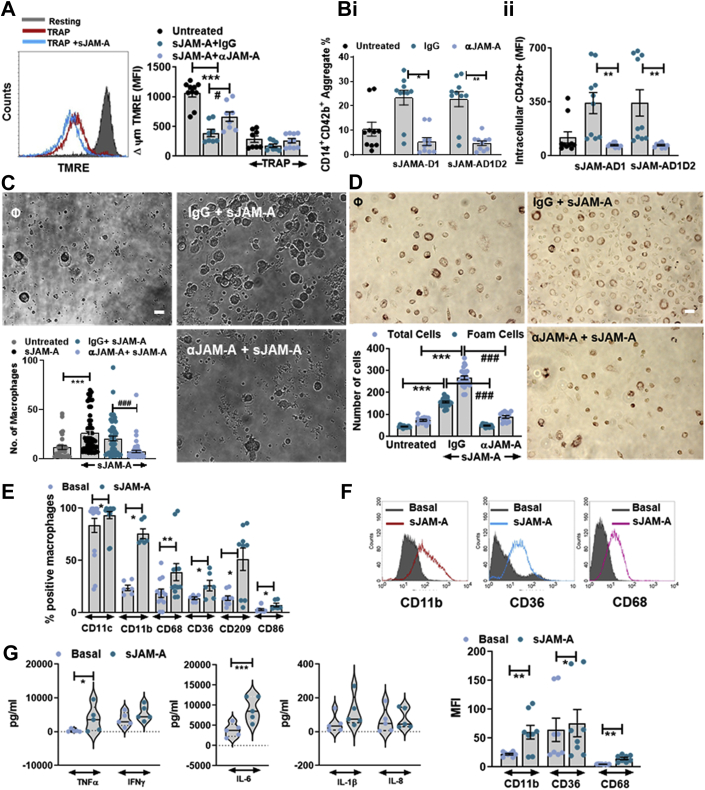
sJAM-A exerts its prothrombotic effects through homophilic interaction with platelet transmembrane-JAM-A
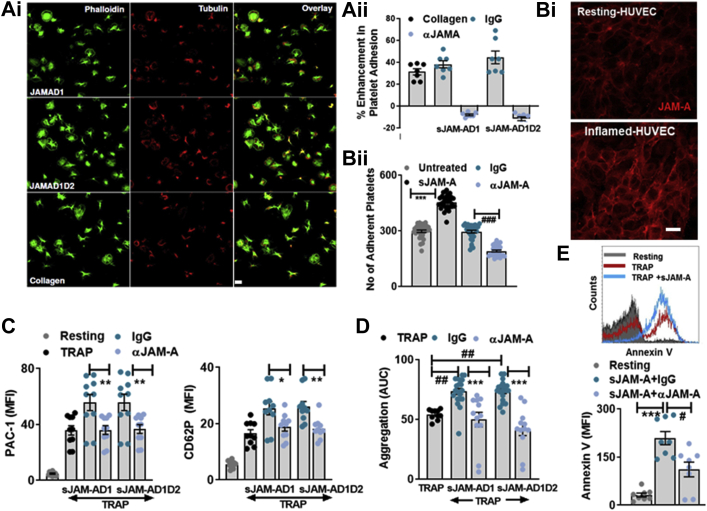
Transmembrane-JAM-A on platelets engages in trans-homophilic interaction with counterparts in endothelial and inflammatory cells.1 Homophilic interaction between 2 transmembrane-JAM-A or sJAM-A molecules occurs through the D1-domains.32 To decipher the molecular features mediating the prothrombotic response, we employed both sJAM-AD1D2 and truncated sJAM-AD132 and verified the possibility of a homophilic interaction between D1 domains of monomeric-sJAM-A and platelet transmembrane-JAM-A using an anti-JAM-A blocking antibody.
Platelet adhesion and spreading on immobilized recombinant full form-sJAM-AD1D2 and truncated-sJAM-AD1 under static conditions were comparable, and counteracted by anti-JAM-A-antibody (Figure 3A, i-ii). Moreover, presence of sJAM-AD1D2 substantiated platelet adhesion on JAM-A-enriched inflamed endothelial layer under dynamic arterial flow conditions (Figure 3B, i-ii), which was also counteracted by anti-JAM-A-antibody, blocking the homophilic interaction. sJAM-AD1D2 and sJAM-AD1 synergistically induced degranulation, αIIbβ3-integrin activation (Figure 3C), aggregation (Figure 3D), and thrombus formation (Supplemental Figure S3A) to a similar extent, which were counteracted by anti-JAM-A-antibody compared with control immunoglobulin G (IgG). Therefore, the D1 domain of sJAM-A is sufficient to engage in a homophilic interaction with platelet transmembrane-JAM-A and execute the prothrombotic effects. Transmembrane-JAM-A deficient Jam-Agt/gt mice show a prothrombotic disposition, as regulatory breaks on platelet-outside-in signaling are abolished.16,17 We observed significantly enhanced basal thrombus formation among megakaryocyte-platelet lineage-specific Pf4-Cre+Jamafl/fl mice ex vivo (Supplemental Figure S3B), confirming these reports. However, unlike control Pf4-Cre-Jamafl/fl littermates, the prothrombotic influence of smJAM-A was absent in Pf4-Cre+Jamafl/fl mice lacking transmembrane-JAM-A on platelets, as the possibility of a homophilic interaction with smJAM-A was prevented (Supplemental Figure S3B).
Figure 3.
Homophilic Interaction Between Transmembrane-JAM-A and sJAM-A Exerts the Prothrombotic Effect
(Ai) Immunofluorescence confocal microscopic images showing phalloidin (green) stained platelets adhered to collagen and immobilized recombinant truncated sJAM-AD1 and full-length sJAM-AD1D2. Bar = 2 μm. (Aii) Fluorescence intensity from platelets adhered to collagen (100 μg/mL), sJAM-AD1 (10 μg/mL), and sJAM-AD1D2 (30 μg/mL), in presence of anti-JAM-A-antibody (10 μg/mL) or IgG control (10 μg/mL) (n = 7). (Bi) Immunofluorescence confocal microscopic images showing enhanced expression of JAM-A (red) on inflamed endothelial cells. Bar = 5 μm. (Bii) sJAM-A (∗∗∗P < 0.001) substantiated platelet adhesion on JAM-A–enriched endothelial surface (n = 5) counteracted by anti-JAM-A-antibody (αJAM-A) (10 μg/mL) (###P < 0.001). (C) Synergistic effect of sJAM-AD1D2 (30 μg/mL) and sJAM-AD1 (10 μg/mL) on TRAP induced αIIbβ3-integrin activation (PAC-1) and platelet degranulation (CD62P) ±anti-JAM-A-antibody (αJAM-A) (10 μg/mL) or IgG control (10 μg/mL). ∗P < 0.05; ∗∗P < 0.01. (D) Synergistic effect of sJAM-AD1D2 (30 μg/mL) and sJAM-AD1 (10 μg/mL) on TRAP-induced platelet aggregation is counteracted by anti-JAM-A-antibody (αJAM-A) (10 μg/mL). ##P < 0.01; ∗∗∗P < 0.001. (C and D) Data represent mean ± SEM from n = 5 experiments with technical replicates. (E) Flow cytometric histogram overlay and bar diagram (n = 4 with technical replicates) showing the synergistic effect of sJAM-AD1D2 (30 μg/mL) on TRAP-induced Annexin-V binding ±anti-JAM-A-antibody (αJAM-A) (10 μg/mL). ∗∗∗P < 0.001; #P < 0.05. Abbreviations as in Figure 1.
sJAM-AD1D2-induced priming of platelets prompted surface externalization of thrombogenic phosphatidylserine, counteracted by anti-JAM-A-antibody (Supplemental Figure S3C). sJAM-AD1D2 also substantiated TRAP-induced Annexin V binding (Supplemental Figure S3C). sJAM-AD1D2-induced phosphatidylserine exposure consequently facilitated thrombin generation (Supplemental Figure S3D) and accelerated clot formation in thromboelastographic assay (Supplemental Figure S3E).
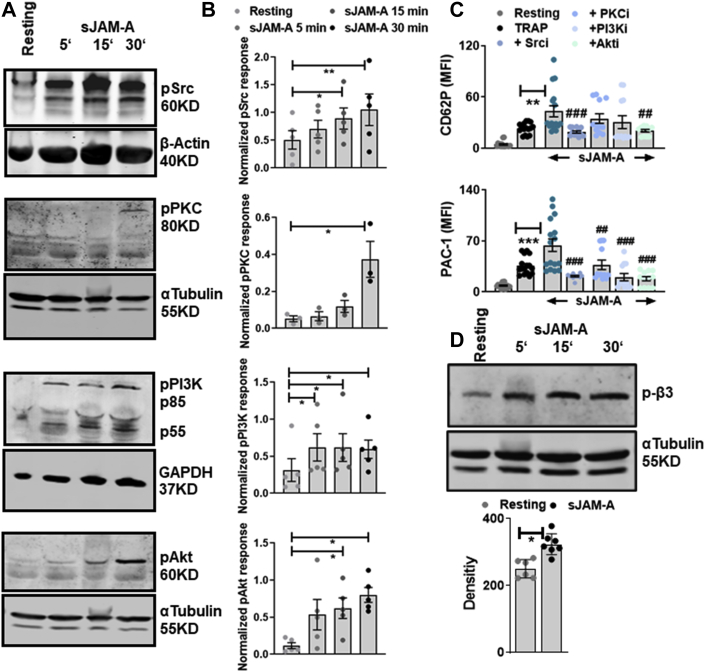
sJAM-A triggers platelet activatory signaling cascade
The endogenous inhibition on platelet outside-in-signaling is dysregulated in the absence of transmembrane-JAM-A in Jam-Agt/gt mice.16,17 However, previous studies did not explore the influence of sJAM-A on platelet response. Currently, priming of platelets with recombinant sJAM-A synergistically induced agonist-driven degranulation, aggregation, and thrombus formation, and accelerated spreading on fibrinogen. Therefore, we explored the influence of sJAM-AD1D2 on platelet activatory signaling cascade. Platelet priming with sJAM-AD1D2 induced phosphorylation of Src, PKC, PI3K, and Akt (Figures 4A and 4B). Pharmacological inhibition of downstream kinases Src (PP2), PKC (Gö6976), PI3K (Ly29004), and Akt (SH6) (Figure 4C) abolished the synergistic effects of sJAM-AD1D2 on TRAP-induced platelet degranulation (CD62P) and αIIbβ3-integrin activation (PAC-1). Interestingly we also observed significant β3-integrin tyrosine (Y733) phosphorylation upon priming of platelets with sJAMA-D1D2 (Figure 4D), which could have facilitated spreading of platelets on fibrinogen (Figure 2D). Thus, sJAM-A, by engaging in a homophilic interaction with transmembrane-JAM-A, may prime platelets, lowering their activation threshold in cardiovascular pathologies whereby circulatory sJAM-A levels are elevated.
Figure 4.
sJAM-A Triggers Activatory Signaling Cascade in Platelets
(A) Representative Western blot and (B) corresponding densitometric analysis (n = 3-5) for phospho-Src, phospho-PKC, phospho-PI3K, and phospho-Akt in platelets treated with sJAM-AD1D2 (30 μg/mL); GAPDH, β-actin, and α-tubulin immunoblots represent loading controls and used for normalization. ∗P < 0.05; ∗∗P < 0.01. (C) Synergistic effect of sJAM-AD1D2 (30 μg/mL) on TRAP (25 μmol/L)-induced CD62P and PAC-1-binding is decreased in the presence of inhibitors of the following kinases Src (Srci-PP2), PKC (PKCi-Gö6976), PI3K (PI3Ki- Ly29004), and Akt (Akti-). ∗∗∗P < 0.001; ∗∗P < 0.01; and ###P < 0.001; ##P < 0.01 compared with sJAM-AD1D2+TRAP. Data are mean ± SEM from n = 6-8 experiments. (D) Representative Western blot (n = 6) and corresponding densitometric analysis for phospho-β3-integrin in platelets treated with sJAM-AD1D2 (30 μg/mL). ∗P < 0.05. Abbreviations as in Figure 1.
sJAM-A enhances thrombo-inflammatory potential of platelets
Because transmembrane-JAM-A is a proinflammatory mediator1 of transcellular associations between vascular endothelium and circulatory cells, we assessed the impact of sJAM-A on thrombo-inflammatory attributes of platelets.33 Although serum sJAM-A levels did not show a significant correlation with serum sP-selectin (Supplemental Table 2), platelet surface-associated JAM-A in the current CAD cohort correlated with platelet surface expression of CD62P (P-selectin) (Figure 1E), which engages in interaction with leukocyte-associated P-selectin glycoprotein ligand and thereby may substantiate platelet-monocyte aggregate (PMA) formation in patients with advanced atherosclerosis.34 Recombinant sJAM-A enhanced mitochondrial transmembrane-potential loss (Δψm), like the platelet activating stimulus-TRAP, which was counteracted by anti-JAM-A-antibody (Figure 5A). Possibly by enhancing platelet CD62P and the “eat-me” signal phosphatidylserine33 (Figure 3G), presence of sJAM-A facilitated CD14+CD42b+-PMA formation (Figure 5B, i) and substantiated phagocytic uptake of phosphatidylserine+-platelets by monocytes (Figure 5B, ii). Consequently, sJAM-A supplementation induced phagocytic clearance of platelets by monocytes, fostering their differentiation32 into macrophages (Figure 5C) and foam cells (Figure 5D) in platelet-monocyte cocultures. The number of macrophages (Figure 5C) and foam cells (Figure 5D) in sJAM-A-supplemented cultures were reduced in the presence of anti-JAM-A-antibody. Platelet-monocyte cocultures predominantly yield CD163+ regenerative M2 macrophages33; however, percentage of CD86+ macrophages were increased in the presence of proinflammatory recombinant sJAM-A (Figure 5E), as were the relative expression of CD11c, CD209, and CD11b on macrophages (Figure 5E). Surface expression of the scavenger receptors CD68 and CD36 were also enhanced in the differentiated macrophages (Figure 5F), possibly aiding in foam cell differentiation (Figure 5D). Analysis of platelet-monocyte culture supernatant revealed elevated levels of proinflammatory mediators, particularly TNF-α and IL-6, upon recombinant sJAM-A supplementation (Figure 5G). These findings taken together with the observation that sJAM-A substantiates platelet adhesion to inflamed endothelium (Figure 3B) suggest its thrombo-inflammatory influence. Data from CAD patients showed that serum proinflammatory sJAM-A levels correlated significantly with other platelet- and leukocyte-derived inflammatory mediators, eg, MCP-1 (CCL2), TARC (CCL17), and high-sensitivity C-reactive protein (Supplemental Table 2). A positive correlation between sJAM-A and inflammatory mediators is also suggestive of its involvement in thrombo-inflammatory actions. Whether or not sJAM-A levels are increased as an acute-phase response in CAD patients can only be assessed in longitudinal cohorts.
Figure 5.
sJAM-A Substantiates Thrombo-inflammation
(A) Flow cytometric histogram overlay showing TMRE fluorescence from resting and TRAP (25 μmol/L) activated/apoptotic platelets ±sJAM-A. Mitochondrial transmembrane potential loss (ΔΨm-decreased TMRE fluorescence) induced by sJAM-AD1D2 (30 μg/mL) is counteracted by anti-JAM-A-antibody (αJAM-A) (10 μg/mL). Data mean ± SEM; n = 5 experiments. ∗∗∗P < 0.001; #P < 0.05. (Bi) Percentage of CD42b+CD14+ platelet-monocyte aggregates in presence of sJAM-A (D1D2 or D1) ±anti-JAM-A-antibody (αJAM-A) (10 μg/mL). ∗P < 0.05;∗∗P < 0.01. (Bii) CD42b+ monocytes that have phagocytosed platelets in presence of sJAM-AD1D2 (30 μg/ml) or sJAM-AD1 (10 μg/ml) ± anti-JAM-A-antibody (αJAM-A) (10 μg/mL) of IgG control (10 μg/mL). ∗∗P < 0.01. (C) Images from platelet-monocyte coculture showing differentiation of monocytes into macrophages ±sJAM-AD1D2 (30 μg/mL) and/or ±anti-JAM-A-antibody (αJAM-A) (10 μg/mL). Bar = 5 μm. ∗∗∗P < 0.0001; ###P < 0.001. (D) Monocyte differentiation into oil red positive foam cells ±sJAM-AD1D2 (30 μg/mL) and/or ±anti-JAM-A-antibody (αJAM-A) (10 μg/mL). ∗∗∗P < 0.001; ###P < 0.001. Bar = 5 μm. (E) Relative percentage of CD11c+, CD11b+, CD68+, CD36+, CD209+, CD86+, macrophages in platelet-monocyte coculture ±sJAM-AD1D2 (30 μg/mL). (F) Flowcytometric histogram overlay and bar diagram showing relative surface expression of CD11b, CD36, and CD68 ±sJAM-AD1D2 (30 μg/mL) in platelet-monocyte cocultures. (G) Presence of proinflammatory mediators in platelet-monocyte cocultures ±sJAM-AD1D2 (30 μg/mL). ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001. (E to G) Data are mean ± SEM from n = 5-6 experiments. Abbreviations as in Figure 1.
Whether or not sJAM-A levels are increased in 72 patients without CAD as main reason of admission (Supplemental Table 3) showed that sJAM-A (median; Q1, Q3) levels were comparable to those of CAD patients (1.52 ng/mL; 1.31, 4.40 ng/mL in non-CAD vs 1.71 ng/mL; 1.18, 4.43 ng/mL in CAD; P = 0.769). A significant number of these non-CAD subjects comprised of elderly, multimorbid patients (myocarditis, hypertensive cardiomyopathy, severe symptomatic aortic stenosis, severe symptomatic mitral regurgitation), explaining high sJAM-A levels possibly associated with a proinflammatory state.
Discussion
The current investigation primarily focused on the thrombotic and thrombo-inflammatory potential of the transmembrane-JAM-A and sJAM-A interaction, revealed the following in CAD patients:
-
1.
F11R-SNVs (rs2774276, rs790056) are significantly and independently (rs2774276) associated with recurrent MI, and that genetic disposition through F11R-SNVs can influence circulatory sJAM-A levels.
-
2.
sJAM-A levels were significantly increased in ACS compared with CCS patients, thereby confirming a previous report.3
-
3.
Higher sJAM-A levels were significantly and independently associated with recurrent MI.
-
4.
Pro-inflammatory1,18,35 sJAM-A correlated significantly with peak troponin I and thrombo-inflammatory mediators.
-
5.
Moreover, platelet surface-associated JAM-A correlated with platelet activation status in CAD patients.
-
6.
Activated platelets shed transmembrane-JAM-A. Considering the hyperactive status of platelets in circulation, platelet-derived sJAM-A could add to sJAM-A from leukocytes and vascular endothelium, accounting for elevated levels in CAD patients.
-
7.
Moreover, we hereby report a novel homophilic interaction between sJAM-A and platelet transmembrane-JAM-A enhancing thrombotic functions and
-
8.
Fostering thrombo-inflammatory platelet-monocyte interactions .
Although concentrations of recombinant sJAM-A required to significantly induce platelet activation were several folds higher than those observed in serum, much of circulatory JAM-A is present in cell or cell-derived microparticle-associated form. Moreover, recombinant sJAM-A that was mostly present in the dimeric form (dimer to monomer ratio 1.7 under pH 7.0 and 150 mmol/L NaCl)36 required a higher concentration, because only the monomeric form of sJAM-A with a free D1 domain can engage in a homophilic interaction with the D1-counterpart in transmembrane-JAM-A. However, because activated platelets are a potential source of sJAM-A, enriched levels of this proinflammatory mediator might be achieved locally at sites of vascular inflammation where platelets adhere, aggregate and form thrombi.
Genetic variations may influence platelet functions29 and their responsiveness to antiplatelet therapies. F11R-SNVs were associated with the risk of recurrent MI during a 3-year follow-up in symptomatic CAD patients, possibly caused by their influence in elevating sJAM-A levels. Genome-wide association studies have extensively identified thousands of genetic variants associated with diverse pathophysiological states, many of which are noncoding. Intronic SNVs may regulate gene expression by influencing alternative splicing of the mRNA or by acting as enhancers. How F11R-SNVs led to elevated sJAM-A levels in patients with cardiovascular risk factors like hypertension8 and obesity7 and in ACS patients needs to be addressed. Further investigations are required to clarify if the observed association of variants is caused by their high linkage with other causative functional F11R variants. Therefore, divergent influence of SNVs leading to phenotypic alterations in protein expression, and in turn favoring platelet-centric thrombotic or thrombo-inflammatory events, are possible. Although observational studies have shown a relevant association of platelet hyper-reactivity and the occurrence of myocardial infarction,37,38 whether platelet response influenced by the F11R-SNVs and sJAM-A axis acts as causal driver for these events warrants further research.
sJAM-A, as a proinflammatory mediator in atheroprogression,2,3 might influence adverse thrombo-ischemic outcome in symptomatic CAD patients. A significant correlation of elevated sJAM-A levels in CAD patients with peak troponin-I indicated potential association with infarction size. Higher sJAM-A levels at baseline were significantly associated with both CE and MI. Therefore, a multimodal approach including clinical risk factors, genetic factors like F11R-SNVs (rs2774276, and potentially rs790056), and inflammatory mediators like sJAM-A may help in better thrombo-ischemic risk stratification and in tailoring therapies for CAD patients. However, before integrating such approaches into clinical decision making, larger validation cohorts, besides inflammatory and genetic marker-guided interventional trials, are necessary. It also needs to be considered that a significant fraction of circulatory JAM-A is associated with the surface of cellular sources or cell-derived microparticles. Although we have demonstrated the prognostic significance of sJAM-A and that platelet surface-associated JAM-A is significantly enriched in ACS patients, we have not explored pMP-associated JAM-A in clinical samples from CAD patients, which could also exert a proinflammatory effect. This remains to be addressed in future investigations. Additionally, sJAM-A levels correlated significantly with other atherothrombotic proinflammatory mediators like high-sensitivity C-reactive protein, MCP-1, and TARC. These observations suggest that JAM-A might have potential therapeutic implications in CAD, as previously demonstrated in atherosclerotic mice.6,35
Vascular inflammation and inflammatory mediators trigger platelet activation, thrombosis, and thrombo-inflammation, driving atherothrombotic progression leading to CAD.39 Transmembrane-JAM-A is associated with αIIbβ3-integrin in resting platelets and acts as an endogenous inhibitor of outside-in-signaling through αIIbβ3-integrin, thereby regulates platelet hypereactivity16,17; but it also mediates inflammatory associations with endothelium and leukocytes.1 Genetic ablation of F11r in murine platelets lowers their activation threshold18 as transmembrane-JAM-A–imposed regulation on c-Src–dependent outside-in-signaling is abolished, enhancing αIIbβ3-integrin activation, platelet spreading, thrombus formation, aggregation, and clot retraction, although inside-out-signaling–driven degranulation, thromboxane A2 production, and fibrinogen binding remain unaffected.16,17 Such gain-of-function in platelets accelerates neointima formation during early stages of atheroprogression and promotes plaque formation in trJAM-A−/−Apoe−/− mice.18,19 However, transmembrane-JAM-A shed from multiple vascular cells, possibly platelets as shown here, circulate as sJAM-A.1 Like recombinant sJAM-A, circulatory sJAM-A may engage in a homophilic interaction with platelet transmembrane-JAM-A to trigger platelet activatory signaling involving Src-PKC-PI3K-Akt to easily activate αIIbβ3-integrin in a synergistic action, and substantiate prothrombotic consequences. Previously, a stimulatory antibody-M.Ab.F11–mediated activation of JAM-A has been shown to increase platelet secretion, and aggregation through dimerizing JAM-A, by promoting its interaction with GPIIb/IIIa and downstream kinase PI3K.15 Trans-homophilic interaction between the membrane distal domains of transmembrane-JAM-A has been suggested in instigating outside-in-signaling involving β1-integrin.40 Currently, observed trans-homophilic interaction between sJAM-A and transmembrane-JAM-A in inducing β3-integrin phosphorylation resembles this scenario. Recombinant sJAM-A also prompted phosphatidylserine exposure on procoagulant platelets, and thereby increased thrombin generation and accelerated clot formation. Although F11r deficiency in mice generates a prothrombotic16,17 and atherosclerotic18 phenotype, these mice are immune to the synergistic stimulation from circulatory sJAM-A, in the absence of platelet transmembrane-JAM-A as a homophilic interaction partner.
Study limitations
Currently, circulatory levels of sJAM-A and platelet surface associated JAM-A were evaluated only at baseline upon admission leaving alternations over time and in response to subsequent anti-thrombotic therapy to speculation. This needs to be addressed in longitudinal studies. Moreover, at present we cannot provide a mechanistic explanation on a causal association between F11R-SNPs and elevated sJAM-A levels in CAD patients which requires further investigation.
Conclusions
Previous16, 17, 18, 19 and current experimental evidence taken together suggest that transmembrane-JAM-A and sJAM-A may differentially influence platelet functions. Although transmembrane-JAM-A acts as an endogenous inhibitor of platelet outside-in-signaling,16,17 sJAM-A in homophilic interaction with transmembrane-JAM-A is prothrombotic. Whereas transmembrane-JAM-A–deficient murine platelets exhibit increased interaction with monocytes, neutrophils, and endothelium, mediated through αIIbβ3-integrin,18 αMβII, or GP Ibα,19 sJAM-A may instigate thrombo-inflammatory platelet-monocyte interactions, as previously documented for IL-6 and sP-selectin in ACS patients.41 Therefore, instead of considering their pathophysiological actions separately, transmembrane-JAM-A and sJAM-A might be considered as a functional duo.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Genetic predisposition is a crucial determinant in influencing thrombotic propensity and even response to antiplatelet therapy. Therefore, such information is fundamental to personalized antithrombotic therapy. Genetic disposition through F11R-SNVs can elevate circulatory levels of sJAM-A in CAD patients, which in turn might substantiate thrombo-inflammatory consequences through homophilic interaction with platelet transmembrane-JAM-A. The current investigation adds to and substantiates previous clinical and experimental investigations that JAM-A might be considered as a potential therapeutic target to avert atherothrombosis.
TRANSLATIONAL OUTLOOK: Thrombo-inflammation exaggerates cardiovascular complications, necessitating the discovery of novel thrombo-inflammatory mediators that may ascertain disease severity and prognosis, and/or can be utilized as therapeutic targets. In the future, transmembrane-JAM-A and sJAM-A, like IL-1β, might be validated as potential therapeutic targets in CAD patients showing elevated sJAM-A levels. However, evaluation of circulatory sJAM-A, and platelet surface-associated JAM-A being limited to baseline at present, subsequent changes under the influence of antiplatelet therapy should be addressed in longitudinal studies. Recently, an F11R/JAM-A antagonistic peptide was shown to interfere with platelet-inflamed endothelium association and reduce atherosclerotic plaque formation in Apoe−/−.6 Interfering with the currently disclosed homophilic interface between transmembrane-JAM-A and sJAM-A may devise therapeutic strategies to regulate thrombotic and thrombo-inflammatory platelet response in cardiovascular pathologies where circulatory sJAM-A levels are elevated.
Funding Support and Author Disclosures
The project was supported by funding from Deutsche Stiftung für Herzforschung (DSHF-F/22/17) to Dr Chatterjee; Deutsche Gesellschaft für Kardiologie-DGK-Otto Hess Promotionstipendium to Dr Rapp; Robert Bosch Stiftung to Drs Winter, Schaeffeler, and Schwab; and Deutsche Forschungsgemeinschaft-190538538, SCHW858/1-2, BO3786/3-1, and DFG-374031971–TRR-240. All other authors have reported that they have no relationships relevant to the contents of this paper.
Acknowledgment
The authors thank Lydia Laptev for her technical assistance with flow cytometry analysis of the clinical samples.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section as well as supplemental figures and tables, please see the online version of this paper.
Contributor Information
Dominik Rath, Email: dominik.rath@med.uni-tuebingen.de.
Madhumita Chatterjee, Email: madhumita.chatterjee@med.uni-tuebingen.de.
Appendix
References
- 1.Weber C., Fraemohs L., Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7:467–477. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- 2.Babinska A., Azari B.M., Salifu M.O., et al. The F11 receptor (F11R/JAM-A) in atherothrombosis: overexpression of F11R in atherosclerotic plaques. Thromb Haemost. 2007;97:272–281. [PubMed] [Google Scholar]
- 3.Cavusoglu E., Kornecki E., Sobocka M.B., et al. Association of plasma levels of F11 receptor/junctional adhesion molecule-A (F11R/JAM-A) with human atherosclerosis. J Am Coll Cardiol. 2007;50:1768–1776. doi: 10.1016/j.jacc.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 4.Babinska A., Kedees M.H., Athar H., et al. F11-receptor (F11R/JAM) mediates platelet adhesion to endothelial cells: role in inflammatory thrombosis. Thromb Haemost. 2002;88:843–850. [PubMed] [Google Scholar]
- 5.Schmitt M.M., Megens R.T., Zernecke A., et al. Endothelial junctional adhesion molecule-a guides monocytes into flow-dependent predilection sites of atherosclerosis. Circulation. 2014;129:66–76. doi: 10.1161/CIRCULATIONAHA.113.004149. [DOI] [PubMed] [Google Scholar]
- 6.Babinska A., Clement C.C., Przygodzki T., et al. A peptide antagonist of F11R/JAM-A reduces plaque formation and prolongs survival in an animal model of atherosclerosis. Atherosclerosis. 2019;284:92–101. doi: 10.1016/j.atherosclerosis.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Ong K.L., Leung R.Y., Wong L.Y., et al. Association of F11 receptor gene polymorphisms with central obesity and blood pressure. J Intern Med. 2008;263:322–332. doi: 10.1111/j.1365-2796.2007.01886.x. [DOI] [PubMed] [Google Scholar]
- 8.Ong K.L., Leung R.Y., Babinska A., et al. Elevated plasma level of soluble F11 receptor/junctional adhesion molecule-A (F11R/JAM-A) in hypertension. Am J Hypertens. 2009;22:500–505. doi: 10.1038/ajh.2009.23. [DOI] [PubMed] [Google Scholar]
- 9.Xu H., Oliveira-Sales E.B., McBride F., et al. Upregulation of junctional adhesion molecule-A is a putative prognostic marker of hypertension. Cardiovasc Res. 2012;96:552–560. doi: 10.1093/cvr/cvs273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salifu M.O., Kolff Q., Murty P., et al. Relationship between the soluble F11 receptor and markers of inflammation in hemodialysis patients. J Investig Med. 2007;55:115–119. doi: 10.2310/6650.2007.06041. [DOI] [PubMed] [Google Scholar]
- 11.Westra H.J., Peters M.J., Esko T., et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45:1238–1243. doi: 10.1038/ng.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Genotype-Tissue Expression (GTEx) project. Broad Institute. https://www.gtexportal.org
- 13.Sobocka M.B., Sobocki T., Banerjee P., et al. Cloning of the human platelet F11 receptor: a cell adhesion molecule member of the immunoglobulin superfamily involved in platelet aggregation. Blood. 2000;95:2600–2609. [PubMed] [Google Scholar]
- 14.Babinska A., Kedees M.H., Athar H., et al. Two regions of the human platelet F11-receptor (F11R) are critical for platelet aggregation, potentiation and adhesion. Thromb Haemost. 2002;87:712–721. [PubMed] [Google Scholar]
- 15.Sobocka M.B., Sobocki T., Babinska A., et al. Signaling pathways of the F11 receptor (F11R; a.k.a. JAM-1, JAM-A) in human platelets: F11R dimerization, phosphorylation and complex formation with the integrin GPIIIa. J Recept Signal Transduct Res. 2004;24:85–105. doi: 10.1081/rrs-120034252. [DOI] [PubMed] [Google Scholar]
- 16.Naik M.U., Stalker T.J., Brass L.F., Naik U.P. JAM-A protects from thrombosis by suppressing integrin alphaIIbbeta3-dependent outside-in signaling in platelets. Blood. 2012;119:3352–3360. doi: 10.1182/blood-2011-12-397398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naik M.U., Caplan J.L., Naik U.P. Junctional adhesion molecule-A suppresses platelet integrin alphaIIbbeta3 signaling by recruiting Csk to the integrin-c-Src complex. Blood. 2014;123:1393–1402. doi: 10.1182/blood-2013-04-496232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karshovska E., Zhao Z., Blanchet X., et al. Hyperreactivity of junctional adhesion molecule A-deficient platelets accelerates atherosclerosis in hyperlipidemic mice. Circ Res. 2015;116:587–599. doi: 10.1161/CIRCRESAHA.116.304035. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Z., Vajen T., Karshovska E., et al. Deletion of junctional adhesion molecule A from platelets increases early-stage neointima formation after wire injury in hyperlipidemic mice. J Cell Mol Med. 2017;21:1523–1531. doi: 10.1111/jcmm.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rath D., Schaeffeler E., Winter S., et al. GPla polymorphisms are associated with outcomes in patients at high cardiovascular risk. Front Cardiovasc Med. 2017;4:52. doi: 10.3389/fcvm.2017.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen B.B., Klopfer S.O. Optimal full matching and related designs via network flows. J Comput Graph Stat. 2006;15:609–627. [Google Scholar]
- 22.R Project for Statistical Computing. https://www.r-project.org/
- 23.Rosner B., Glynn R.J., Lee M.L. Incorporation of clustering effects for the Wilcoxon rank sum test: a large-sample approach. Biometrics. 2003;59:1089–1098. doi: 10.1111/j.0006-341x.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 24.White H. Maximum-likelihood estimation of mis-specified models. Econometrica. 1982;50:1–25. [Google Scholar]
- 25.Lorenz D.J., Levy S., Datta S. Inferring marginal association with paired and unpaired clustered data. Stat Methods Med Res. 2018;27:1806–1817. doi: 10.1177/0962280216669184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 27.Zhou B., Fine J., Latouche A., Labopin M. Competing risks regression for clustered data. Biostatistics. 2012;13:371–383. doi: 10.1093/biostatistics/kxr032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 29.Geisler T., Schaeffeler E., Gawaz M., Schwab M. Genetic variation of platelet function and pharmacology: an update of current knowledge. Thromb Haemost. 2013;110:876–887. doi: 10.1160/TH13-02-0145. [DOI] [PubMed] [Google Scholar]
- 30.Greenwood J., Mason J.C. Statins and the vascular endothelial inflammatory response. Trends Immunol. 2007;28:88–98. doi: 10.1016/j.it.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montoro-Garcia S., Shantsila E., Marin F., Blann A., Lip G.Y. Circulating microparticles: new insights into the biochemical basis of microparticle release and activity. Basic Res Cardiol. 2011;106:911–923. doi: 10.1007/s00395-011-0198-4. [DOI] [PubMed] [Google Scholar]
- 32.Prota A.E., Campbell J.A., Schelling P., et al. Crystal structure of human junctional adhesion molecule 1: implications for reovirus binding. Proc Natl Acad Sci U S A. 2003;100:5366–5371. doi: 10.1073/pnas.0937718100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chatterjee M., von Ungern-Sternberg S.N., Seizer P., et al. Platelet-derived CXCL12 regulates monocyte function, survival, differentiation into macrophages and foam cells through differential involvement of CXCR4-CXCR7. Cell Death Dis. 2015;6 doi: 10.1038/cddis.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gremmel T., Ay C., Riedl J., et al. Platelet-specific markers are associated with monocyte-platelet aggregate formation and thrombin generation potential in advanced atherosclerosis. Thromb Haemost. 2016;115:615–621. doi: 10.1160/TH15-07-0598. [DOI] [PubMed] [Google Scholar]
- 35.Ostermann G., Fraemohs L., Baltus T., et al. Involvement of JAM-A in mononuclear cell recruitment on inflamed or atherosclerotic endothelium: inhibition by soluble JAM-A. Arterioscler Thromb Vasc Biol. 2005;25:729–735. doi: 10.1161/01.ATV.0000157154.14474.3b. [DOI] [PubMed] [Google Scholar]
- 36.Bazzoni G., Martinez-Estrada O.M., Mueller F., et al. Homophilic interaction of junctional adhesion molecule. J Biol Chem. 2000;275:30970–30976. doi: 10.1074/jbc.M003946200. [DOI] [PubMed] [Google Scholar]
- 37.Stone G.W., Witzenbichler B., Weisz G., et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet. 2013;382:614–623. doi: 10.1016/S0140-6736(13)61170-8. [DOI] [PubMed] [Google Scholar]
- 38.Geisler T., Zurn C., Simonenko R., et al. Early but not late stent thrombosis is influenced by residual platelet aggregation in patients undergoing coronary interventions. Eur Heart J. 2010;31:59–66. doi: 10.1093/eurheartj/ehp402. [DOI] [PubMed] [Google Scholar]
- 39.Weber C., Badimon L., Mach F., van der Vorst E.P.C. Therapeutic strategies for atherosclerosis and atherothrombosis: past, present and future. Thromb Haemost. 2017;117:1258–1264. doi: 10.1160/TH16-10-0814. [DOI] [PubMed] [Google Scholar]
- 40.Severson E.A., Parkos C.A. Mechanisms of outside-in signaling at the tight junction by junctional adhesion molecule A. Ann N Y Acad Sci. 2009;1165:10–18. doi: 10.1111/j.1749-6632.2009.04034.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang J., Zhang S., Jin Y., Qin G., Yu L., Zhang J. Elevated levels of platelet-monocyte aggregates and related circulating biomarkers in patients with acute coronary syndrome. Int J Cardiol. 2007;115:361–365. doi: 10.1016/j.ijcard.2006.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.